Abstract
Brain metastases are associated with poor prognosis irrespective of the primary tumor they originate from. Current treatments for brain metastases are palliative, and patients with symptomatic brain metastasis have a one-year survival of <20%. Lung cancer, breast cancer, and melanoma have higher incidences of brain metastases compared with other types of cancers. However, it is not very clear why some cancers metastasize to the brain more frequently than others. Studies thus far suggest that brain-specific tropism of certain types of cancers is defined by a winning combination of the following factors: unique genetic subtypes of primary tumors or its subclones enabling detachment, dissemination, blood–brain barrier penetration, plus proliferation and survival in hypoxic low-glucose microenvironment; specific transcriptomic and epigenetic changes of colony-forming metastatic cells, allowing their outgrowth; favorable metastasis-permissive microenvironment of the brain created by interactions of cancer cells and cells in the brain through triggering inflammation, recruiting myeloid-derived suppressor cells, and promoting metabolic adaptation; immunosuppression resulting in the failure of adaptive immune response to recognize or kill cancer cells in the brain. Here, we briefly review recent advances in understanding brain metastasis organotropism and outline directions for future research.
Distant metastasis is responsible for >90% of cancer-related deaths (Chaffer and Weinberg 2011). Tumors of different origins display unique patterns of dissemination with preferential colonization of a particular set of organs. For example, colon cancer most commonly metastasizes to the liver whereas prostate adenocarcinoma predominantly disseminates to the bone (Nguyen et al. 2009). Breast cancer can metastasize to multiple organs with high success rates in the lungs, bones, brain, liver, and other organ sites (Lowery and Yu 2017). Although this organotropism can be partly explained by anatomical proximity between organs (e.g., the liver collects the venous drainage of the colon system via the portal vein), the past two decades of research provided a strong evidence for the existence of cellular and molecular programs guiding tumor cells to particular organs. Specifically, the concept of premetastatic niche (PMN) postulates that soluble factors and extracellular vesicles (EVs) produced by cells at the primary tumor sites can modify the microenvironment of distant organs in various ways to accommodate traveling cancer cells and facilitate their outgrowth (Kaplan et al. 2005; Hiratsuka et al. 2006; Costa-Silva et al. 2015; Liu and Cao 2016).
The dilemma of site-specific metastasis emerged as early as 1889, when English surgeon Stephen Paget coined his prominent “seed and soil” theory, in which metastasizing cancer cells are the “seeds” and the metastasis-favoring organ microenvironment is the “soil” (Paget 1889). Current research using high-throughput technologies suggests that the relationship between “seeds” and “soil” is complex and bidirectional, with reciprocal interactions between tumor cells and distant organs helping each other to meet. For example, cancer-derived EVs uptaken by host cells, such as resident macrophages, at distant organs can trigger the profibrotic and proinflammatory responses ultimately supporting future metastasis (Costa-Silva et al. 2015). At the same time, certain organs display a milieu intrinsically favoring metastasis; for example, highly fenestrated sinusoidal endothelia of the liver show high permeability to soluble factors and extravasating cells (DeLeve 2007; Brunt et al. 2014; Juza and Pauli 2014). This environment is in striking contrast with brain's highly selective blood–brain barrier (BBB), which is extremely difficult to penetrate by traveling cancer cells because of its tightly adjoined endothelial cells (Wilhelm et al. 2013). This contradiction is puzzling because many patients present with metastases in the brain but not the liver (Budczies et al. 2015; Obenauf and Massagué 2015). Clearly, tumor cells can exploit a specialized set of mechanisms to penetrate the BBB and preferentially seed into the brain.
Brain metastasis is a serious consequence of cancer with no available curative care at the moment (Achrol et al. 2019). The dynamic, complex interplay between the brain microenvironment and disseminating metastatic cells has yet to be fully explored for the purpose of developing new effective targeted therapies for this disease. Understanding tumor cell tropism to the brain may provide an insight on how to tackle metastatic disease to the brain from various primary cancer sites. In this review, we summarize and critically analyze recent discoveries in the field of brain metastases, specifically addressing questions of early metastatic seeding and outgrowth in this organ. Additionally, we discuss the role of host adaptive immunity in a brain metastasis-specific context, including the basic biology behind this process as well as recent clinical trials examining immune checkpoint inhibitors for treatment of brain metastatic disease.
BRAIN METASTASIS REPRESENTS AN IMPOSING CLINICAL CHALLENGE
A diagnosis of brain metastatic disease indicates extremely poor prognosis, and most patients with this diagnosis face a very slim chance of survival. General population-based epidemiology studies reported brain metastasis incidence rates of 2.8 to 14.3 per 100,000 people (Table 1); the width of this range probably reflects difficulties in pathological verification of autopsies and improvement of neuroimaging techniques over time. In the United States, every year 21,000 to 43,000 patients are diagnosed with brain metastases (Fox et al. 2011). Cancer registry data indicate that 5.3% to 9.6% of all newly diagnosed cancer patients will develop brain metastasis (Table 2); however, this could have been underestimated because of the infrequency of autopsies in patients who die from metastatic disease. For example, an autopsy study by Tsukada and colleagues revealed that 309 of 1044 autopsy cases (29.6%) of breast carcinoma showed intracranial metastases (Tsukada et al. 1983). In another study, 2375 autopsies of patients with different cancers revealed brain metastases in 15% of subjects (Posner and Chernik 1978). Another reason to believe that the real incidence of brain metastasis is substantially higher is that established clinical practices do not recommend routine brain magnetic resonance imaging (MRI) in patients without neurological symptoms. Interestingly, metastases to the brain are generally much more prevalent than primary brain malignancies such as gliomas (Gavrilovic and Posner 2005; Davis et al. 2012), which could be explained by the relatively low number of dividing cells in the brain.
Table 1.
Rates of brain metastasis incidence in general population
| Study | Country | Incidence per 100,000 population |
|---|---|---|
| Guõmundsson (1970) | Iceland | 2.8 |
| Fogelholm et al. (1984) | Finland | 3.4 |
| Walker et al. (1985) | United States | 8.3 |
| Materljan et al. (2004) | Croatia | 9.9 |
| Counsell et al. (1996) | Great Britain | 14.3 |
Table 2.
Prevalence of brain metastasis in patients diagnosed with primary cancer
| Study | % | Country |
|---|---|---|
| Davis et al. (2012) | 6.0 | United States |
| Schouten et al. (2002) | 8.5 | Netherlands |
| Barnholtz-Sloan et al. (2004) | 9.6 | United States |
| Nayak et al. (2012) | 8.5–9.6 | Global |
| Matsuda et al. (2018) | 5.3 | Japan |
Brain metastasis generally affects elderly people 50–80 yr old (Fox et al. 2011). Nonetheless, some pediatric cancers, most commonly sarcomas, can also give raise to brain metastases (Graus et al. 1983; Kebudi et al. 2005). Among the primary tumors that most frequently metastasize to the brain are lung cancer, melanoma, breast carcinoma, renal cancer, and colorectal adenocarcinoma (Table 3). Breast cancer is the primary tumor that most commonly metastasizes to the brain in women. Other cancer types rarely metastasize to the brain.
Table 3.
Ranking of primary tumors by frequency of associated brain metastasis
| Study | Barnholtz-Sloan et al. (2004) | Cagney et al. (2017) | Schouten et al. (2002) | Smedby et al. (2009) | ||||
|---|---|---|---|---|---|---|---|---|
| Rank | Type of cancer | % | Type of cancer | % | Type of cancer | % | Type of cancer | %a |
| 1 | Lung cancer (all) | 19.9 | Melanoma | 28.2 | Lung cancer (all) | 16.3 | Lung cancer (all) | 44.1 (33.5) |
| 2 | Melanoma | 6.9 | Lung Adenocarcinoma | 26.8 | Renal cancer | 9.8 | Melanoma | 12.3 (5.8) |
| 3 | Renal cancer | 6.5 | Non-small-cell lung cancer | 25.6 | Melanoma | 7.4 | Colorectal cancer | 9.0 (7.4) |
| 4 | Breast carcinoma | 5.1 | Small cell lung cancer | 23.5 | Breast carcinoma | 5.0 | Prostate cancer | 8.6 |
| 5 | Colorectal cancer | 1.8 | Squamous cell carcinoma of the lung | 15.9 | Colorectal cancer | 1.2 | Renal cancer | 7.8 (4.7) |
| 6 | Bronchioloalveolar carcinoma | 15.5 | Other solid tumors | 18.2 (10.5) | ||||
| 7 | Renal cancer | 10.8 | Breast cancer | (32) | ||||
aData shown for men (no parentheses) and women (parentheses).
Treatment of brain metastasis is generally done with palliative intent. It includes treatments such as chemotherapy using BBB-penetrating drugs (Freilich et al. 1995; Lesser 1996; Korfel and Thiel 1999; Tawbi et al. 2018), surgical resection when the number of brain lesions is limited (Alvarez-Breckenridge et al. 2019; Olesrud et al. 2019), whole-brain radiotherapy (Brown et al. 2017; Sun et al. 2018; Or et al. 2019), and stereotactic radiosurgery (Badiyan et al. 2016). These treatments may lead to a partial response or short-term stable disease, but are unable to extend patient survival substantially (Kotecha et al. 2018). Individuals with a single cranial metastasis have more treatment options available such as surgery, yet their median survival is only ∼60% longer (35.6 vs 22.6 wk) (Patchell 2003). Untreated brain metastases lead to death in ∼2 mo after diagnosis (Markesbery et al. 1978).
Thus, metastases to the brain are a global public health burden, and unfortunately, many decades of cancer research have offered little, if any, therapeutic benefit to patients with this disease.
BRAIN-SPECIFIC METASTASES DISPLAY A UNIQUE BIOLOGICAL PHENOTYPE
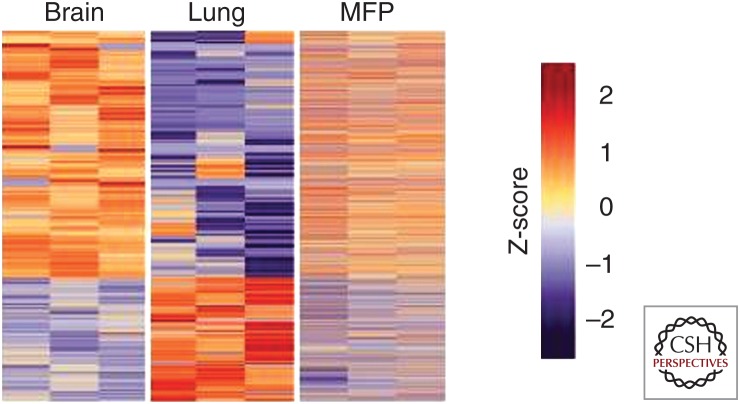
Early metastatic colonization of the brain requires cancer cells to trigger the expression of a specific gene set and communicate with host cells to succeed in penetrating the BBB and propagating under conditions of hypoxia and glucose shortage (Peters et al. 2004; Winkler 2015; Lowery and Yu 2017). The gene alterations unique of brain metastatic cancer cells were elegantly shown in a recent study by the Massagué group (Basnet et al. 2019), who used a novel method for in situ transcriptomic profiling of rare cell populations. In mice, early micrometastases in the brain and lungs derived from intracardiacally injected MDA-MB-231 cells showed a remarkable difference in gene expression patterns, also displaying a substantial divergence with the transcriptome of cells injected orthotopically into the mammary fat pad inducing primary tumor (Fig. 1). Compared with those of lung colonies and mammary tumors, the transcriptome of brain-seeking cancer cells had lower levels of oxidative stress and antioxidative response, suggesting adaptation of metastasizing cells to peculiar metabolic conditions of the brain (Flavahan et al. 2013). These in vivo findings are consistent with findings from patients, whose brain metastatic lesions harbor mutations undetectable in matched primary tumors, regional lymph nodes, or extracranial metastases (Brastianos et al. 2015). Interestingly, many such mutations represent clinically actionable targets such as ERBB2, BRAF, MYC, and BRCA2 (Brastianos et al. 2015), potentially opening new avenues for targeted therapies.
Figure 1.
Heatmap representation of differentially expressed genes identified by Flura-seq in MDA231 cells. Cells residing in the brain, lung, or mammary fat pad (MFP), compared with the expression of these genes in the third organ. (Reproduced from Figure 4 in Basnet et al. 2019, under the Creative Commons Attribution 4.0 International Public License [CC BY 4.0; see creativecommons.org/licenses/by/4.0/].)
In line with general mRNA changes occurring in early metastatic colonies, disseminating tumor cells were also shown to produce a specific set of cancer-derived microRNAs (miRNAs) that can shape the surrounding milieu to facilitate metastasis (Zhou et al. 2014). It was found that EVs from metastatic MDA-MB-231 breast cancer cells are highly enriched in miR-105, an miRNA targeting a migration-related protein zonula occludens 1 (ZO-1). Via microvesicular transport, cancer cell-derived miR-105 transferred into endothelial cells, regulating their migration properties. By targeting ZO-1, miRNA-producing cancer cells diminished tight junctions and destroyed the barrier function of endothelial monolayers; this led to increased vascular permeability and promoted metastasis in this experimental model (Zhou et al. 2014).
In addition to differences at a transcriptional level, brain metastases show a unique epigenetic phenotype. To this end, analysis of 425 tumor-specific, differentially methylated loci showed higher overall genome methylation in 35 breast cancer brain metastases compared with 50 nonmatched primary mammary carcinoma samples (Salhia et al. 2014). This finding highlights the fundamental epigenome alterations in brain metastatic cells.
It is also important to note that following BBB penetration, many disseminated cancer cells can undergo dormancy by adopting a slow-cycling state and gaining stem-like characteristics through expression of SOX2 and SOX9 (Malladi et al. 2016). The key determinants of cancer cells fate, specifically, whether they should proliferate or stay latent, are yet to be fully recognized. Although the quiescent state may last for years or even decades, the consensus is that dormant cells will eventually awake under influence of unidentified factors and give rise to outgrowing metastatic foci (Sosa et al. 2014). Collectively, these studies point at a distinctly altered genetic and epigenetic program activated in cancer cells on their dissemination into the brain, which largely determines cancer cells homing to this organ and facilitates their subsequent outgrowth.
Altered transcription programs of metastatic cancer cells that facilitate organotropism to the brain are significantly dependent on genetic subtypes of primary tumors. For example, ∼50% of non-small-cell lung cancer patients with epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) rearrangement develop metastases in the central nervous system, strongly implicating these two genes in determining brain organotropism (Rangachari et al. 2015). Additionally, brain metastases derived from triple-negative or basal-type breast cancers were reported to effectively disrupt the BBB and therefore subsequently colonize the brain, whereas HER2/neu-positive breast cancer cells failed to alter BBB permeability (Yonemori et al. 2010). Counterintuitively, patients with HER2-positive primary breast cancer and brain metastasis have a 30.8% probability of having metastases in other organs, such as the liver and lung (Kim et al. 2018). Interestingly, spatial distribution of metastases within the brain is also affected by the molecular subtype of primary tumor (Takano et al. 2016). Evidence from these studies indicates that molecular subtypes of primary tumors not only define overall survival, risks of relapse, and response to therapies but also predetermine the tropism of disseminating cells to seek particular organs, including the brain.
MICROENVIRONMENT IN SUPPORT OF BRAIN METASTASIS
Organotropism to the brain is a highly complicated phenomenon and is also regulated by interactions of metastatic cancer cells with the brain tumor microenvironment. The brain microenvironment has a unique composition and also consists of multiple cell populations with a diverse array of functions (Table 4). In the light of the “seed and soil” hypothesis, some of these cell populations could, under certain conditions, be supportive of metastasizing cancer cells and facilitate their seeding and propagation. Some of these cells could elicit their prometastatic action long before the arrival of tumor cells (brain PMN concept), whereas another may create a growth- and proliferation-permissive microenvironment for the newly extravasated cancer cells or their clusters.
Table 4.
Major cell populations in the brain
| Cell type | Main function | Approximate percentage (Pelvig et al. 2008; von Bartheld et al. 2016) |
|---|---|---|
| Neurons | Receive, conduct and transmit electrical signals | 33% of all brain cells |
| Glial cells | Supporting neurons | 66% of all brain cells |
| Oligodendrocyte (macroglia) | Creating the myelin sheath (Bradl and Lassmann 2010) | 75.6% macroglia |
| Astrocyte (macroglia) | Nutrient supplementation of the nervous tissue and tissue repair | 17.3% of macroglia |
| Glioblast (macroglia) | Undifferentiated macroglial cells | Unknown |
| Microglia | Resident immune populations of the brain | 6.5% of glial cells |
| Endothelial cells and pericytes | Blood supply | 10%–30% of nonneuronal cells |
| Infiltrating (nonresident) immune cells | Elimination of pathogens, PAMPs and DAMPs | Varies, unknown |
Abbreviations: DAMPs, damage-associated molecular patterns; PAMPs, pathogen-associated molecular patterns.
Extracellular Vesicles and Exosomes
Recently, tumor-derived EVs were found to be in control of shaping the PMN of different organs (Costa-Silva et al. 2015; Hoshino et al. 2015; Plebanek et al. 2017). Under physiological conditions, EVs serve as means of intercellular communication by transporting various biomolecules (Isola and Chen 2017). However, Lyden's group showed that EVs isolated from the brain-seeking subline of breast cancer cells traveled exclusively to the brain when injected retro-orbitally into tumor-free mice (Hoshino et al. 2015). Similarly, breast cancer sublines metastasizing to other organs secrete EVs that traveled exclusively to the corresponding organs. Importantly, 98% of mouse EV-harboring cells were brain endothelial cells, suggesting endothelium as a major determinant of BBB penetration and subsequent brain metastasis (Hoshino et al. 2015). These EVs may awaken dormant disseminated tumor cells, which are localized in tight proximity with brain microvascular endothelium (Carbonell et al. 2009; Ghajar et al. 2013; Bentolila et al. 2016). Alternatively, tumor-derived EVs may promote microvascular hyperpermeability, which has been described as a feature of brain micrometastases (Schwartz et al. 2016), thus enhancing extravasation. Studies of human disease and experimental mouse models in our laboratory revealed a loss of tumor suppressor “phosphatase and tensin homolog deleted on chromosome ten” (PTEN) in brain metastases from PTEN-positive primary breast tumors. Intriguingly, such PTEN down-regulation was reversed after in vitro culture of cancer cells isolated from brain metastasis lesions in mice, suggesting a brain microenvironment-induced PTEN loss. We discovered that brain metastatic tumor cells uptake the astrocyte-derived EVs carrying PTEN-targeting miR-19a that down-regulates PTEN in tumor cells, leading to NF-κB activation and up-regulation of CCL2 to facilitate metastasis through recruitment of myeloid-derived suppressor cells (Zhang et al. 2015). Taken together, these findings suggest that both tumor- and stroma-derived EVs may help disseminating cancer cells to form distant metastases in the brain.
Neuroinflammation
There is accumulating evidence that brain neuroinflammation orchestrated under physiological conditions by response to pathogen-associated molecular patterns or tissue damage (damage-associated molecular pattern) can promote metastatic outgrowth in the brain (Doron et al. 2019). In a model of spontaneous melanoma brain metastasis, early micrometastatic brain lesions recruited activated Glial fibrillary acidic protein (GFAP)-positive astrocytes, which in turn produced proinflammatory molecules such as CCL2, IL-1β, and IL-6, thus enhancing tumor growth (Schwartz et al. 2016). Interestingly, the activation of astrocytes was at least in part mediated by disseminating tumor cells, which up-regulated the expression of an astrogliosis-related wound-healing gene signature in these cells through unidentified secreted factors. Intracranial coinjection of melanoma cells with astrocytes resulted in a substantial growth advantage (ninefold) of tumor cells as compared with tumor cells injected alone, implicating that astrocytes could modulate cancer cell proliferation in the tumor microenvironment (Schwartz et al. 2016). Consistently, astrocyte-driven cancer cell proliferation through release of proinflammatory cytokines was also documented by several other groups (Sierra et al. 1997; Seike et al. 2011).
Like astrocytes, microglial cells are actively recruited to metastatic brain lesions (Bowman et al. 2016; Qiao et al. 2019), and their elimination by targeting colony stimulating factor-1 receptor (CSF-1R) resulted in a reduction of both the size and number of melanoma-derived brain metastases in mice injected with tumor cells via internal carotid artery (Qiao et al. 2019). Several attempts have been made to explore the underlying mechanisms of these reductions. Metastasis-promoting effects of microglia, such as tumor cell invasion, were negated by pharmacological inhibition of PI3K in vitro and in vivo. On the other hand, small-molecule PI3K inhibitor administration reduced PDL-1 and CTLA-4 levels in mouse brain lesions induced by stereotactical intracortical injection of 4T1 breast cancer cells (Blazquez et al. 2018). Another study provided evidence that activated microglia may control invasion and colonization of the brain by metastasizing cancer cells via the Wnt pathway (Pukrop et al. 2010). However, these findings have an observational nature, and the in-depth mechanism underlying the function of activated microglia on brain metastasis development remains to be elucidated.
Altogether, these studies indicate that activated glial cells (macroglia and microglia) actively infiltrate the brain metastasis lesions (possibly because of cancer-derived chemo-attractants) and further stimulate tumor outgrowth via release of proinflammatory molecules that trigger neuroinflammation.
Extracellular Matrix in Control of Metastatic Outgrowth
In recent years, multiple lines of evidence strongly implicated the noncellular component of tumor microenvironment, the extracellular matrix (ECM), in promotion of metastatic seeding and outgrowth (Yuzhalin et al. 2018a,b; Zhang et al. 2019). Genes encoding major ECM proteins are associated with epithelial-to-mesenchymal transition and poor prognosis across many cancer types (Yuzhalin et al. 2018c). Furthermore, physical stiffness of tumor ECM directly correlates with metastasis (Wei et al. 2015), and orientation of collagen fibers in relation to the tumor is an independent prognostic indicator in breast carcinoma (Conklin et al. 2011). However, the role of ECM in brain metastasis is not well understood, primarily because the brain lacks structurally well-defined stromal space (Bonneh-Barkay and Wiley 2009). Unlike ECM in other parenchymal organs, brain ECM predominantly localizes near vascular basement membranes and therefore is likely to have a different composition of collagens, proteoglycans, and glycoproteins (Quail and Joyce 2017). Because of these structural differences, the functional role of brain ECM could be unique. In-depth comprehensive proteomic or functional analyses of brain ECM would provide critical insights in this direction.
High expression of collagens, proteoglycans, and hyaluronic acid binding proteins is associated with poor prognosis in lung cancer (Stevens et al. 2017). Importantly, multiple cell surface receptors that bind to ECM molecules are also overexpressed in these tumors, indicating a reciprocal interaction between cancer cells and the tumor microenvironment (Stevens et al. 2017). One such receptor, hyaluronan-mediated motility receptor (HMMR), was shown to correlate with lung cancer brain metastasis and mediate colonization of the brain in vivo, specifically affecting early survival and outgrowth of metastatic lesions. Remarkably, genetic knockdown of HMMR reduced the activation of mitogen-activated protein kinase/ERK and AKT in H2030 lung cancer cells cultured as ECM-embedded organoids but not in cells cultured in an ECM-free environment. The expression of HMMR in cancer cells was negatively regulated by miR-34a (Stevens et al. 2017), an miR linked to ECM deposition in cardiac fibroblasts (Zhang et al. 2018). Overall, this study showed that tumor initiation and propagation of early metastasis can be mechanistically connected through activation of the ECM receptor HMMR, and thus therapeutic inhibition of HMMR-hyaluronic acid interactions in the ECM may lead to clinical advances in treating brain metastases from lung cancer.
In an experimental brain metastasis model, the in-depth characterization of tumor-derived and stromal proteolytic networks has uncovered a role of cathepsin S (a member of a large family of proteases involved in cleavage of multiple targets, including the ECM) in metastasis formation (Sevenich et al. 2014). Highlighting the clinical relevance of this molecule, these investigators documented a negative association between cathepsin S levels and metastasis-free survival in a cohort of patients with brain metastases. In the same study, genetic depletion of cathepsin S in cancer and stromal cells diminished brain metastatic burden in mice. Follow-up mechanistic studies showed that cathepsin S promoted the transmigration of disseminating tumor cells through the BBB by inducing a cleavage of intercellular tight junctions (Sevenich et al. 2014). It is unclear, however, what upstream signals induce cathepsin S expression in metastasizing tumors and whether these signals are attributable to activity of host cell populations in the brain.
Interestingly, there seem to be a correlation between reelin (a major brain ECM glycoprotein) and HER2 expression in human brain metastatic lesions derived from mammary carcinoma (Jandial et al. 2017). Reelin was localized near tumor-associated astrocytes, which induced reelin expression in breast cancer cells on their coculture (Jandial et al. 2017). Importantly, astrocyte-derived factors promoted proliferation of HER2-positive breast cancer cells through enforcing HER2 phosphorylation, and knockdown of the reelin gene, RELN, in breast cancer cells reduced astrocyte-driven HER2-dependent cell proliferation.
Collectively, these studies point to the specific contribution of ECM to brain PMN development; in particular, extracellular signaling in the brain can promote growth of disseminated cancer cells as well as enable their entry into the brain parenchyma through destruction of BBB integrity. More systematic research along this line would identify the true significance of extracellular cues in the context of brain metastasis.
ADAPTIVE IMMUNE RESPONSE IN BRAIN METASTASES
The microenvironment of brain metastases is to a large extent shaped by infiltrating lymphocytes exerting the adaptive immune response program. CD3+ and CD8+ cytotoxic T-cells can infiltrate brain metastasis lesions and be found in both stromal and epithelial compartments, with higher abundance in the former (Harter et al. 2015; Duchnowska et al. 2016). Interestingly, distribution of T cells in brain lesions may be observed in three distinct histological patterns: perivascular, stromal, and diffuse (Harter et al. 2015). However, it remains unclear what signals control such different patterns of infiltration. The highest numbers of CD3+ and CD8+ T cells were found in brain metastases from renal cell carcinoma and melanoma, whereas brain lesions from the lung, breast, and colon cancers displayed considerably fewer of these T cells (Harter et al. 2015). Higher counts of tumor-infiltrating T cells correlated with smaller brain metastasis size without affecting overall survival (Harter et al. 2015), whereas subjects whose tumor-infiltrating T cells expressed higher levels of PD-1 had longer overall survival after resection of metastases (Duchnowska et al. 2016). Along similar lines, another study confirmed that elevated peritumoral CD3+ T-cell density positively correlated with brain metastasis-associated survival, and 52% of peritumoral T cells expressed PD-1 on their surface (Zakaria et al. 2018). It is important to note that infiltration of T cells into brain lesions can be restricted by certain ECM components, such as tenascin C (Huang et al. 2010), suggesting a link between the noncellular component of tumor stroma with T-cell–mediated killing of cancer cells.
Immune checkpoint blockade can promote CD8+ T-cell numbers in mouse experimental brain metastasis; however, such an increase was shown to be caused by CD8+ cell trafficking from outside of the brain rather than their proliferation in situ (Taggart et al. 2018). Notably, preclinical immune checkpoint blockade treatment of brain metastasis-bearing mice did not alter the activation/exhaustion status of T cells, suggesting that this therapy regimen can attract lymphocytes to the metastatic site but is unable to unleash their cytotoxic potential (Taggart et al. 2018).
Despite the higher mutation burden of human brain metastases, they seem to contain substantially fewer T cell clones compared with paired primary lung tumors (Mansfield et al. 2018). Various mechanisms may contribute to the observed lower diversity of T cell clones. For example, it could be because of the difficulty of T cells to penetrate the BBB, thus only few T-cell clones infiltrating within the brain lesions. In this respect, T-cell migration through the BBB is not a passive process and can be controlled by soluble factors such as IFN-γ (Sonar et al. 2017). However, some studies suggest that T-cell trafficking into the brain may occur directly via migration through choroid plexus epithelial cells of the brain–cerebrospinal fluid barrier (Strazielle et al. 2016), rather than the BBB. How immune cells respond to brain–tropic cancer cells and through what means these cancer cells manage to escape the immune surveillance is not well known. Better understanding of these important questions could bring new insights on how to mobilize immune response to control brain metastasis.
IMMUNOTHERAPY FOR TREATMENT OF BRAIN METASTASIS
Despite the knowledge gap described above, there have been remarkable efforts to therapeutically stimulate the immune response to control brain metastasis. Recent advances in immune checkpoint blockade therapies have led to a breakthrough in enhancing the survival outcome and quality of life of patients with melanoma (Carreau and Pavlick 2019), advanced renal cell carcinoma (Motzer et al. 2018), bladder cancer (Konala et al. 2019), non-small-cell lung cancer (Nadal et al. 2019), and other cancer types (Wei et al. 2018). However, clinical trials of immune checkpoint blockade therapies frequently exclude patients with brain metastatic disease. Thus, there is only a handful of studies that explored the effect of immune checkpoint blockade therapies in patients with brain metastases.
In a retrospective analysis of 146 melanoma patients treated with ipilimumab (an anti-CTLA4 antibody) for their brain metastases, a modest global immune-related overall response rate of 11% was achieved, and four patients (3%) showed immune-related complete responses (Queirolo et al. 2014). Most patients (73%) experienced progressive disease. In another study, melanoma patients with brain metastases benefited from ipilimumab therapy if they had small and asymptomatic lesions and were not taking corticosteroids (Margolin et al. 2012), showing a median overall survival of 7.0 mo compared with 3.7 mo for those who had symptomatic large brain metastases and were taking corticosteroids. These findings were corroborated by recent experimental studies in mice that revealed an involvement of corticosteroids in metastasis seeding and outgrowth (Obradović et al. 2019).
The combination of ipilimumab with nivolumab (an anti-PD1 antibody) showed a much more promising clinical benefit, with 24 patients (26%) having a complete response and 28 patients (30%) having a partial response in brain metastases from melanoma, thus resulting in a 56% intracranial objective response (Tawbi et al. 2018). A better clinical benefit from this therapy was achieved in patients with tumor PD-L1 expression (at least 5%), which is similar to findings obtained for extracranial disease (Larkin et al. 2015). These results are consistent with those of another study in which a combination of ipilimumab and nivolumab yielded a 46% intracranial response in patients with melanoma brain metastases (Long et al. 2018). In this study, patients receiving nivolumab alone had a significantly lower rate of intracranial progression-free survival after 6 mo of treatment compared with patients receiving dual therapy (20% vs. 53%) (Long et al. 2018). Interestingly, the intracranial response and survival rates in this study were lower in patients who previously had BRAF and MEK inhibitors therapy (Long et al. 2018); suggesting that evolution of BRAF and MEK inhibitors-resistant clones toward an immune resistance phenotype (Hugo et al. 2015).
In a trial of pembrolizumab (an anti-PD1 antibody) monotherapy in patients with brain metastases from melanoma, four of 18 patients had a partial intracranial response ranging from 4 to 10 mo (Goldberg et al. 2016). In the same study, six of 18 patients with brain metastatic disease from non-small-cell lung cancer responded to pembrolizumab, including partial and complete intracranial responses in two and four patients, respectively (Goldberg et al. 2016).
Findings from these clinical efforts show that current immune checkpoint blockade treatments have a modest effect on survival of patients with brain metastases, largely for a lack of response in a significant proportion of patients. Therefore, future studies should focus on understanding principal genetic and phenotypic differences between responders and nonresponders to immune therapies to gain novel insights on how brain–tropic cancer cells succeed in immune escape, which could guide the development of new strategies to optimize and amend current immunotherapy regimens to more effectively control brain metastasis.
CONCLUDING REMARKS
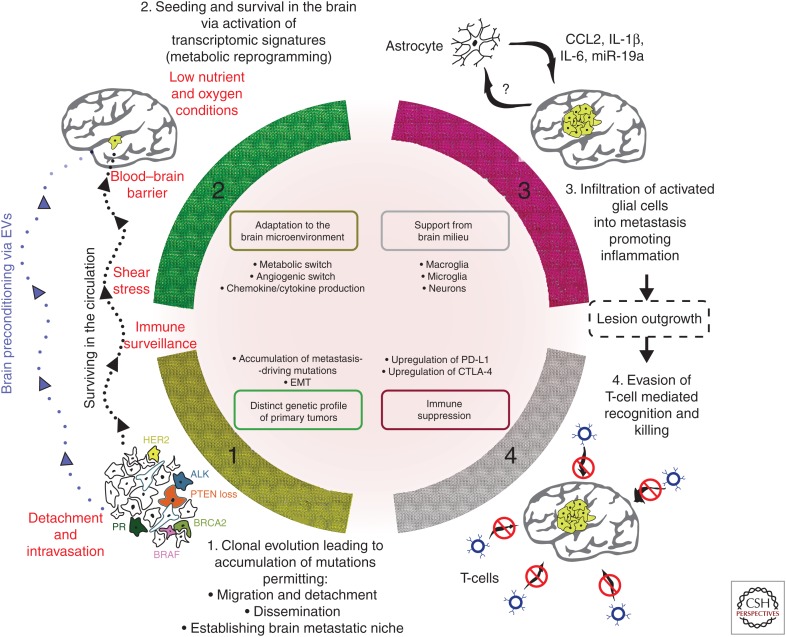
Patients with metastases to the brain have a poor prognosis. The key to combating this disease is understanding its etiology and pathogenesis at a molecular level. The emergence of novel high-throughput technologies and their applications in biology and medicine have substantially advanced the “seed and soil” concept put forward more than a century ago. Currently, organotropism and subsequent development of brain metastasis can be defined as a successful combination of a multitude of factors, which can be conditionally pooled into several groups (Fig. 2):
The distinctive genetic profile of primary tumors enabling detachment, metastatic dissemination (as a prerequisite for metastasis in general), followed by destruction of the BBB and subsequent seeding within the brain followed by propagation under low-glucose and hypoxic conditions. Accumulation of certain genetic alterations as a result of clonal evolution (McGranahan and Swanton 2017) that is influenced by the mutation rate and doubling time of individual tumors.
The emergence of transcriptomic and epigenetic changes in the newly established colonies, enabling successful growth of micrometastases through metabolic adaptation (Fischer et al. 2019) and activation of an angiogenic switch program (Baeriswyl and Christofori 2009). These changes include production of chemokines and cytokines to disarm both resident and recruited immune cells with their subsequent shift into immune-suppressive phenotype (Condamine et al. 2015).
The brain microenvironment accommodates cancer cells, and the brain positively responds to biochemical and biomechanical signals from cancer cells, enforces activation of proinflammatory response (Quail and Joyce 2013) followed by ECM remodeling (Yuzhalin et al. 2018b), and attracts myeloid cells with their subsequent transformation into myeloid-derived suppressor cells (Ouzounova et al. 2017). These events can be influenced by susceptibility of host cells to obey cancer signals as well as the biological phenotype (i.e., aggressiveness) of tumor cells themselves.
The inability of tumor-infiltrating lymphocytes to recognize and successfully eliminate brain–tropic metastatic cancer cells, resulting from failure of patrolling immune cells (Vinay et al. 2015) to kill disseminating tumor cells in the circulation; failed T-cell infiltration through the dense tumor ECM (Caruana et al. 2015); T-cell dysfunction/exhaustion caused by the up-regulation of PD-L1, CTLA-4, and other immune inhibitory receptors (Wherry and Kurachi 2015); or reprogramming by myeloid-derived suppressor cells (Ouzounova et al. 2017).
Figure 2.
Schematic representation of key phases enabling brain organotropism of cancer cells and leading to establishment of metastatic colonies. Factors limiting brain organotropism are in red color. Abbreviations: EVs, extracellular vesicles; EMT, epithelial–mesenchymal transition; CCL2, C-C motif chemokine ligand 2; IL, interleukin; miR, micro RNA; CTLA4, cytotoxic T-lymphocyte-associated protein 4; PD-L1, programmed death-ligand 1.
The requirement for a combination of many different events limits the efficiency of the metastatic process, with <0.01% of tumor cells injected into the circulation forming metastatic foci (Fidler 1970). Importantly, the individual contribution of each event to the onset and progression of metastasis is obscure; it is likely that some of these events are substantially more important than others, and dissecting individual roles of every element will be a key to fully understanding brain organotropism. Future research in the field should encompass the investigation of these key features of brain organotropism: (1) a unique ability of brain-seeking cancer cells to penetrate the BBB; (2) peculiarities of cancer cell metabolic adaptation in the brain; (3) interaction of cancer cells with resident cells in the brain; and (4) brain-specific immune suppression. Importantly, to boost antitumor immunity in the brain, it is needed to develop novel strategies, including innovative immune checkpoint blockade regimens as well as more effective chimeric antigen receptor T cells. In addition, future investigations should use high-throughput technologies such as mass spectrometry, mass cytometry, next-generation sequencing, etc. to unbiasedly search for new molecular targets for brain metastasis therapies. More in-depth studies of brain metastasis organotropism are required to warrant successful treatment of this dreadful disease and open new horizons in improving patients’ quality of life.
ACKNOWLEDGMENTS
We thank members of the Yu laboratory for helpful discussions. We thank Bryan Tutt in the Department of Scientific Publications of MD Anderson Cancer Center for editing the manuscript. This work was supported by National Institutes of Health grants R01-CA112567-06 (D.Y.), R01CA184836 (D.Y.), R01 CA208213 (D.Y.), R21CA223102 (D.Y.), and the China Medical University Research Fund (D.Y.). D.Y. is the Hubert L. & Olive Stringer Distinguished Chair in Basic Science of MD Anderson Cancer Center. We apologize for not being able to cite all the relevant original research and review articles because of space limitation.
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS, et al. 2019. Brain metastases. Nat Rev Dis Prim 5: 5 10.1038/s41572-018-0055-y [DOI] [PubMed] [Google Scholar]
- Alvarez-Breckenridge C, Giobbie-Hurder A, Gill CM, Bertalan M, Stocking J, Kaplan A, Nayyar N, Lawrence DP, Flaherty KT, Shih HA, et al. 2019. Upfront surgical resection of melanoma brain metastases provides a bridge toward immunotherapy-mediated systemic control. Oncologist 24: 671–679. 10.1634/theoncologist.2018-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiyan SN, Regine WF, Mehta M. 2016. Stereotactic radiosurgery for treatment of brain metastases. J Oncol Pract 12: 703–712. 10.1200/JOP.2016.012922 [DOI] [PubMed] [Google Scholar]
- Baeriswyl V, Christofori G. 2009. The angiogenic switch in carcinogenesis. Semin Cancer Biol 19: 329–337. 10.1016/j.semcancer.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. 2004. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22: 2865–2872. 10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- Basnet H, Tian L, Ganesh K, Huang Y-H, Macalinao DG, Brogi E, Finley LW, Massagué J. 2019. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. eLife 8: e43627 10.7554/eLife.43627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, Péault B, Barnhill RL, Lugassy C. 2016. Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci Rep 6: 23834 10.1038/srep23834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez R, Wlochowitz D, Wolff A, Seitz S, Wachter A, Perera-Bel J, Bleckmann A, Beißbarth T, Salinas G, Riemenschneider MJ, et al. 2018. PI3K: a master regulator of brain metastasis-promoting macrophages/microglia. Glia 66: 2438–2455. 10.1002/glia.23485 [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wiley CA. 2009. Brain extracellular matrix in neurodegeneration. Brain Pathol 19: 573–585. 10.1111/j.1750-3639.2008.00195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, Dhara S, Simpson K, Gardner EE, Iacobuzio-Donahue CA, et al. 2016. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep 17: 2445–2459. 10.1016/j.celrep.2016.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. 2010. Oligodendrocytes: biology and pathology. Acta Neuropathol 119: 37–53. 10.1007/s00401-009-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, et al. 2015. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5: 1164–1177. 10.1158/2159-8290.CD-15-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NNI, Ashman JB, et al. 2017. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18: 1049–1060. 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt EM, Gouw ASH, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, Callea F, Clouston AD, Dienes HP, Goodman ZD, et al. 2014. Pathology of the liver sinusoids. Histopathology 64: 907–920. 10.1111/his.12364 [DOI] [PubMed] [Google Scholar]
- Budczies J, von Winterfeld M, Klauschen F, Bockmayr M, Lennerz JK, Denkert C, Wolf T, Warth A, Dietel M, Anagnostopoulos I, et al. 2015. The landscape of metastatic progression patterns across major human cancers. Oncotarget 6: 570–583. 10.18632/oncotarget.2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, Wen PY, Dunn IF, Bi WL, Weiss SE, et al. 2017. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19: 1511–1521. 10.1093/neuonc/nox077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell WS, Ansorge O, Sibson N, Muschel R. 2009. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4: e5857 10.1371/journal.pone.0005857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau N, Pavlick A. 2019. Revolutionizing treatment of advanced melanoma with immunotherapy. Surg Oncol. 10.1016/j.suronc.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, Ittmann MM, Marchetti D, Dotti G. 2015. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 21: 524–529. 10.1038/nm.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science 331: 1559–1564. 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. 2015. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66: 97–110. 10.1146/annurev-med-051013-052304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. 2011. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 178: 1221–1232. 10.1016/j.ajpath.2010.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell CE, Collie DA, Grant R. 1996. Incidence of intracranial tumours in the Lothian region of Scotland, 1989-90. J Neurol Neurosurg Psychiatry 61: 143–150. 10.1136/jnnp.61.2.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FG, Dolecek TA, McCarthy BJ, Villano JL. 2012. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14: 1171–1177. 10.1093/neuonc/nos152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeve L. 2007. Hepatic microvasculature in liver injury. Semin Liver Dis 27: 390–400. 10.1055/s-2007-991515 [DOI] [PubMed] [Google Scholar]
- Doron H, Pukrop T, Erez N. 2019. A blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res 79: 423–436. 10.1158/0008-5472.CAN-18-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchnowska R, Pęksa R, Radecka B, Mandat T, Trojanowski T, Jarosz B, Czartoryska-Arłukowicz B, Olszewski WP, Och W, Kalinka-Warzocha E, et al. 2016. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res 18: 43 10.1186/s13058-016-0702-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ. 1970. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 45: 773–782. [PubMed] [Google Scholar]
- Fischer GM, Jalali A, Kircher DA, Lee W-C, McQuade JL, Haydu LE, Joon AY, Reuben A, de Macedo MP, Carapeto FCL, et al. 2019. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov 9: 628–645. 10.1158/2159-8290.CD-18-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, Weil RJ, Nakano I, Sarkaria JN, Stringer BW, et al. 2013. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci 16: 1373–1382. 10.1038/nn.3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelholm R, Uutela T, Murros K. 1984. Epidemiology of central nervous system neoplasms. A regional survey in Central Finland. Acta Neurol Scand 69: 129–136. 10.1111/j.1600-0404.1984.tb07791.x [DOI] [PubMed] [Google Scholar]
- Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. 2011. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am 22: 1–6. 10.1016/j.nec.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Freilich RJ, Seidman AD, DeAngelis LM. 1995. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer 76: 232–236. [DOI] [PubMed] [Google Scholar]
- Gavrilovic IT, Posner JB. 2005. Brain metastases: epidemiology and pathophysiology. J Neurooncol 75: 5–14. 10.1007/s11060-004-8093-6 [DOI] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DYR, et al. 2013. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15: 807–817. 10.1038/ncb2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L, et al. 2016. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17: 976–983. 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F, Walker RW, Allen JC. 1983. Brain metastases in children. J Pediatr 103: 558–561. 10.1016/S0022-3476(83)80583-6 [DOI] [PubMed] [Google Scholar]
- Guõmundsson KR. 1970. A survey of tumors of the central nervous system in Iceland during the 10-year period 1954–1963. Acta Neurol Scand 46: 538–552. 10.1111/j.1600-0404.1970.tb05811.x [DOI] [PubMed] [Google Scholar]
- Harter PN, Bernatz S, Scholz A, Zeiner PS, Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B, et al. 2015. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 6: 40836–40849. 10.18632/oncotarget.5696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. 2006. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8: 1369–1375. 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature 527: 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JY, Cheng YJ, Lin YP, Lin HC, Su CC, Juliano R, Yang B-C. 2010. Extracellular matrix of glioblastoma inhibits polarization and transmigration of T cells: The role of tenascin-C in immune suppression. J Immunol 185: 1450–1459. 10.4049/jimmunol.0901352 [DOI] [PubMed] [Google Scholar]
- Hugo W, Shi H, Sun L, Piva M, Song C, Kong X, Moriceau G, Hong A, Dahlman KB, Johnson DB, et al. 2015. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 162: 1271–1285. 10.1016/j.cell.2015.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola AL, Chen S. 2017. Exosomes: the messengers of health and disease. Curr Neuropharmacol 15: 157–165. 10.2174/1570159X14666160825160421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandial R, Choy C, Levy DM, Chen MY, Ansari KI. 2017. Astrocyte-induced Reelin expression drives proliferation of Her2+ breast cancer metastases. Clin Exp Metastasis 34: 185–196. 10.1007/s10585-017-9839-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juza RM, Pauli EM. 2014. Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat 27: 764–769. 10.1002/ca.22350 [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827. 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebudi R, Ayan İ, Görgün Ö, Ağaoğlu FY, Vural S, Darendeliler E. 2005. Brain metastasis in pediatric extracranial solid tumors: survey and literature review. J Neurooncol 71: 43–48. 10.1007/s11060-004-4840-y [DOI] [PubMed] [Google Scholar]
- Kim Y-J, Kim J-S, Kim IA. 2018. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol 144: 1803–1816. 10.1007/s00432-018-2697-2 [DOI] [PubMed] [Google Scholar]
- Konala VM, Adapa S, Aronow WS. 2019. Immunotherapy in bladder cancer. Am J Ther. 10.1097/MJT.0000000000000934 [DOI] [PubMed] [Google Scholar]
- Korfel A, Thiel E. 1999. Chemotherapy of brain metastases. Front Radiat Ther Oncol 33: 343–348. 10.1159/000061217 [DOI] [PubMed] [Google Scholar]
- Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. 2018. Recent advances in managing brain metastasis. F1000Res 7: 1772 10.12688/f1000research.15903.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. 2015. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser GJ. 1996. Chemotherapy of cerebral metastases from solid tumors. Neurosurg Clin N Am 7: 527–536. 10.1016/S1042-3680(18)30378-4 [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao X. 2016. Characteristics and significance of the pre-metastatic niche. Cancer Cell 30: 668–681. 10.1016/j.ccell.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, et al. 2018. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19: 672–681. 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- Lowery FJ, Yu D. 2017. Brain metastasis: unique challenges and open opportunities. Biochim Biophys Acta Rev Cancer 1867: 49–57. 10.1016/j.bbcan.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massagué J. 2016. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165: 45–60. 10.1016/j.cell.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield AS, Ren H, Sutor S, Sarangi V, Nair A, Davila J, Elsbernd LR, Udell JB, Dronca RS, Park S, et al. 2018. Contraction of T cell richness in lung cancer brain metastases. Sci Rep 8: 2171 10.1038/s41598-018-20622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF, et al. 2012. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13: 459–465. 10.1016/S1470-2045(12)70090-6 [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Brooks WH, Gupta GD, Young AB. 1978. Treatment for patients with cerebral metastases. Arch Neurol 35: 754–756. 10.1001/archneur.1978.00500350058012 [DOI] [PubMed] [Google Scholar]
- Materljan E, Materljan B, Sepcić J, Tuskan-Mohar L, Zamolo G, Erman-Baldini I. 2004. Epidemiology of central nervous system tumors in Labin area, Croatia, 1974-2001. Croat Med J 45: 206–212. [PubMed] [Google Scholar]
- Matsuda Y, Seki A, Nonaka K, Kakizaki M, Wang T, Aida J, Ishikawa N, Nakano Y, Kaneda D, Takata T, et al. 2018. Clinicopathological characteristics of distant metastases of adenocarcinoma, squamous cell carcinoma and urothelial carcinoma: An autopsy study of older Japanese patients. Geriatr Gerontol Int 18: 211–215. 10.1111/ggi.13165 [DOI] [PubMed] [Google Scholar]
- McGranahan N, Swanton C. 2017. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168: 613–628. 10.1016/j.cell.2017.01.018 [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al. 2018. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378: 1277–1290. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal E, Massuti B, Dómine M, García-Campelo R, Cobo M, Felip E. 2019. Immunotherapy with checkpoint inhibitors in non-small cell lung cancer: insights from long-term survivors. Cancer Immunol Immunother 68: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak L, Lee EQ, Wen PY. 2012. Epidemiology of brain metastases. Curr Oncol Rep 14: 48–54. 10.1007/s11912-011-0203-y [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massagué J. 2009. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9: 274–284. 10.1038/nrc2622 [DOI] [PubMed] [Google Scholar]
- Obenauf AC, Massagué J. 2015. Surviving at a distance: organ-specific metastasis. Trends Cancer 1: 76–91. 10.1016/j.trecan.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović MMS, Hamelin B, Manevski N, Couto JP, Sethi A, Coissieux M-M, Münst S, Okamoto R, Kohler H, Schmidt A, et al. 2019. Glucocorticoids promote breast cancer metastasis. Nature 567: 540–544. 10.1038/s41586-019-1019-4 [DOI] [PubMed] [Google Scholar]
- Olesrud IC, Schulz MK, Marcovic L, Kristensen BW, Pedersen CB, Kristiansen C, Poulsen FR. 2019. Early postoperative MRI after resection of brain metastases—complete tumour resection associated with prolonged survival. Acta Neurochir (Wien) 161: 555–565. 10.1007/s00701-019-03829-0 [DOI] [PubMed] [Google Scholar]
- Or M, Jayamanne D, Guo L, Stevens M, Parkinson J, Cook R, Little N, Back M. 2019. Focal radiation therapy for limited brain metastases is associated with high rates of local control and low subsequent whole brain radiation therapy. ANZ J Surg 89: 418–422. [DOI] [PubMed] [Google Scholar]
- Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, Marasco D, Asm I, Chadli A, Hassan KA, et al. 2017. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun 8: 14979 10.1038/ncomms14979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S. 1889. The distribution of secondary growths in cancer of the breast. Lancet 133: 571–573. 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- Patchell RA. 2003. The management of brain metastases. Cancer Treat Rev 29: 533–540. 10.1016/S0305-7372(03)00105-1 [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. 2008. Neocortical glial cell numbers in human brains. Neurobiol Aging 29: 1754–1762. 10.1016/j.neurobiolaging.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, Schultes B, Born J, Fehm HL. 2004. The selfish brain: competition for energy resources. Neurosci Biobehav Rev 28: 143–180. 10.1016/j.neubiorev.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, Filleur S, Bhowmick R, Henkin J, Miller SD, et al. 2017. Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun 8: 1319 10.1038/s41467-017-01433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner JB, Chernik NL. 1978. Intracranial metastases from systemic cancer. Adv Neurol 19: 579–592. [PubMed] [Google Scholar]
- Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M, et al. 2010. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58: 1477–1489. 10.1002/glia.21022 [DOI] [PubMed] [Google Scholar]
- Qiao S, Qian Y, Xu G, Luo Q, Zhang Z. 2019. Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J Neuroinflammation 16: 4 10.1186/s12974-018-1389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. 2013. Microenvironmental regulation of tumor progression and metastasis. Nat Med 19: 1423–1437. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. 2017. The microenvironmental landscape of brain tumors. Cancer Cell 31: 326–341. 10.1016/j.ccell.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirolo P, Spagnolo F, Ascierto PA, Simeone E, Marchetti P, Scoppola A, Del Vecchio M, Di Guardo L, Maio M, Di Giacomo AM, et al. 2014. Efficacy and safety of ipilimumab in patients with advanced melanoma and brain metastases. J Neurooncol 118: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, Uhlmann EJ, Wong ET, Dahlberg SE, Huberman MS, et al. 2015. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 88: 108–111. 10.1016/j.lungcan.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhia B, Kiefer J, Ross JTD, Metapally R, Martinez RA, Johnson KN, DiPerna DM, Paquette KM, Jung S, Nasser S, et al. 2014. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS ONE 9: e85448 10.1371/journal.pone.0085448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. 2002. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94: 2698–2705. 10.1002/cncr.10541 [DOI] [PubMed] [Google Scholar]
- Schwartz H, Blacher E, Amer M, Livneh N, Abramovitz L, Klein A, Ben-Shushan D, Soffer S, Blazquez R, Barrantes-Freer A, et al. 2016. Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Res 76: 4359–4371. 10.1158/0008-5472.CAN-16-0485 [DOI] [PubMed] [Google Scholar]
- Seike T, Fujita K, Yamakawa Y, Kido MA, Takiguchi S, Teramoto N, Iguchi H, Noda M. 2011. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis 28: 13–25. 10.1007/s10585-010-9354-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, et al. 2014. Analysis of tumor- and stroma-supplied proteolytic networks reveals a brain metastasis-promoting role for cathepsin S. Nat Cell Biol 16: 876–888. 10.1038/ncb3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Price JE, García-Ramirez M, Méndez O, López L, Fabra A. 1997. Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest 77: 357–368. [PubMed] [Google Scholar]
- Smedby KE, Brandt L, Bäcklund ML, Blomqvist P. 2009. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer 101: 1919–1924. 10.1038/sj.bjc.6605373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonar SA, Shaikh S, Joshi N, Atre AN, Lal G. 2017. IFN-γ promotes transendothelial migration of CD4+ T cells across the blood-brain barrier. Immunol Cell Biol 95: 843–853. 10.1038/icb.2017.56 [DOI] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. 2014. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14: 611–622. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LE, Cheung WKC, Adua SJ, Arnal-Estapé A, Zhao M, Liu Z, Brewer K, Herbst RS, Nguyen DX. 2017. Extracellular matrix receptor expression in subtypes of lung adenocarcinoma potentiates outgrowth of micrometastases. Cancer Res 77: 1905–1917. 10.1158/0008-5472.CAN-16-1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazielle N, Creidy R, Malcus C, Boucraut J, Ghersi-Egea JF. 2016. T-lymphocytes traffic into the brain across the blood-CSF barrier: evidence using a reconstituted choroid plexus epithelium. PLoS ONE 11: e0150945 10.1371/journal.pone.0150945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Xu L, Wang Y, Zhao J, Xu K, Qi J, Yuan Z, Zhao L, Wang P. 2018. Additional radiation boost to whole brain radiation therapy may improve the survival of patients with brain metastases in small cell lung cancer. Radiat Oncol 13: 250 10.1186/s13014-018-1198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart D, Andreou T, Scott KJ, Williams J, Rippaus N, Brownlie RJ, Ilett EJ, Salmond RJ, Melcher A, Lorger M. 2018. Anti-PD-1/anti–CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc Natl Acad Sci 115: E1540–E1549. 10.1073/pnas.1714089115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Kinoshita M, Takagaki M, Sakai M, Tateishi S, Achiha T, Hirayama R, Nishino K, Uchida J, Kumagai T, et al. 2016. Different spatial distributions of brain metastases from lung cancer by histological subtype and mutation status of epidermal growth factor receptor. Neuro Oncol 18: 716–724. 10.1093/neuonc/nov266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, Lewis K, Lao CD, Postow MA, et al. 2018. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379: 722–730. 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Fouad A, Pickren JW, Lane WW. 1983. Central nervous system metastasis from breast carcinoma autopsy study. Cancer 52: 2349–2354. [DOI] [PubMed] [Google Scholar]
- Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, et al. 2015. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 35: S185–S198. 10.1016/j.semcancer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Bahney J, Herculano-Houzel S. 2016. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524: 3865–3895. 10.1002/cne.24040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Robins M, Weinfeld FD. 1985. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 35: 219–226. 10.1212/WNL.35.2.219 [DOI] [PubMed] [Google Scholar]
- Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, et al. 2015. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat Cell Biol 17: 678–688. 10.1038/ncb3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Duffy CR, Allison JP. 2018. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8: 1069–1086. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486–499. 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. 2013. Role of the blood–brain barrier in the formation of brain metastases. Int J Mol Sci 14: 1383–1411. 10.3390/ijms14011383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler F. 2015. The brain metastatic niche. J Mol Med 93: 1213–1220. 10.1007/s00109-015-1357-0 [DOI] [PubMed] [Google Scholar]
- Yonemori K, Tsuta K, Ono M, Shimizu C, Hirakawa A, Hasegawa T, Hatanaka Y, Narita Y, Shibui S, Fujiwara Y. 2010. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 116: 302–308. 10.1002/cncr.24735 [DOI] [PubMed] [Google Scholar]
- Yuzhalin AE, Gordon-Weeks AN, Tognoli ML, Jones K, Markelc B, Konietzny R, Fischer R, Muth A, O'Neill E, Thompson PR, et al. 2018a. Colorectal cancer liver metastatic growth depends on PAD4-driven citrullination of the extracellular matrix. Nat Commun 9: 4783 10.1038/s41467-018-07306-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzhalin AE, Lim SY, Kutikhin AG, Gordon-Weeks AN. 2018b. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim Biophys Acta Rev Cancer 1870: 207–228. 10.1016/j.bbcan.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Yuzhalin AE, Urbonas T, Silva MA, Muschel RJ, Gordon-Weeks AN. 2018c. A core matrisome gene signature predicts cancer outcome. Br J Cancer 118: 435–440. 10.1038/bjc.2017.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria R, Platt-Higgins A, Rathi N, Radon M, Das S, Das K, Bhojak M, Brodbelt A, Chavredakis E, Jenkinson MD, et al. 2018. T-cell densities in brain metastases are associated with patient survival times and diffusion tensor MRI changes. Cancer Res 78: 610–616. 10.1158/0008-5472.CAN-17-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang W-C, Li P, Li M, Wang X, Zhang C, et al. 2015. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527: 100–104. 10.1038/nature15376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang Y, Zhu H, Hu J, Xie Z. 2018. MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat cardiac fibroblasts and extracellular matrix deposition in vivo and in vitro. Cell Signal 46: 145–153. 10.1016/j.cellsig.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Zhang H, Pan Y, Cheung M, Cao M, Yu C, Chen L, Zhan L, He Z, Sun C. 2019. LAMB3 mediates apoptotic, proliferative, invasive, and metastatic behaviors in pancreatic cancer by regulating the PI3 K/Akt signaling pathway. Cell Death Dis 10: 230 10.1038/s41419-019-1320-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor STF, Chin AR, et al. 2014. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25: 501–515. 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]