Abstract
For years, clinical and basic researchers have been aware of the presence of PTEN in the nucleus in cell culture, animal models, and both healthy and diseased human tissues. Despite the early recognition of nuclear PTEN, the understanding of the mechanisms of its nuclear localization, function in the nucleus, and importance in biology and human disease has been lacking. Over the last decade, emerging concepts for the complex involvement of nuclear PTEN in a variety of processes, including genome maintenance and DNA repair, cell-cycle control, gene expression, and DNA replication, are illuminating what could prove to be the key path toward a full understanding of PTEN function in health and disease. Dysregulation of nuclear PTEN is now considered an important aspect of the etiology of many pathologic conditions, prompting reconsideration of the therapeutic approaches aimed at countering the consequences of PTEN deficiency. This new knowledge is fueling the development of innovative therapeutic modalities for a broad spectrum of human conditions, from cancer and metabolic diseases, to neurological disorders and autism.
DETECTION OF PTEN IN THE NUCLEUS
Some of the earliest detection of PTEN in tissue, using immunohistochemical staining of patient samples, revealed PTEN's cytoplasmic and nuclear localization and potential differential distribution of the protein in healthy and diseased cells. In addition to cytoplasmic localization, PTEN was found in the nuclei of normal breast epithelium (Perren et al. 1999), neuronal and endothelial cells (Sano et al. 1999), proliferating endometrium (Mutter et al. 2000), normal pancreatic islet cells (Perren et al. 2000), vascular smooth muscle cells (Déléris et al. 2003), and follicular thyroid cells (Gimm et al. 2000). These reports described PTEN in normal tissue nuclei, but absence in neoplastic tissue. Nuclear localization of PTEN in tissue corroborated the initial observations on nuclear and cytoplasmic localization of transfected PTEN in various cell lines (Furnari et al. 1997; Li and Sun 1997). The first study to examine the cytoplasmic nuclear partitioning of PTEN in melanoma reported that lack of nuclear PTEN was associated with a high mitotic index (Whiteman et al. 2002). Coupled with the previous findings of loss of nuclear PTEN in neoplastic tissue, these observations raised a possibility for a contribution of nuclear PTEN to tumor suppression. Nevertheless, the discovery that PTEN acts as a phosphatidylinositol-(3,4,5) and -(3,4) phosphatase and opposes PI3K signaling from the plasma membrane (Maehama and Dixon 1998; Myers et al. 1998; Stambolic et al. 1998), and the existence of these substrates within the plasma membrane, overshadowed PTEN nuclear localization as the potential mean of tumor suppression. The emergence of PIK3CA as a major oncogene and the PI3K signaling pathway as one of the most frequently activated signaling cascades in a variety of cancers further fueled this notion (Samuels et al. 2004). However, practically universal failure of PI3K inhibitors in the treatment of PTEN-deficient cancers (Juric et al. 2015; Le et al. 2016) renewed the interest in the role of nuclear PTEN in tumor suppression over the last decade, resulting in considerable insight into nuclear PTEN biology, with broad implications for management and treatment of PTEN loss-related pathologies.
MECHANISMS OF PTEN NUCLEAR LOCALIZATION
PTEN amino acid sequence lacks defined nuclear import (nuclear localization signal [NLS]) and nuclear export (nuclear export signal [NES]) motifs. While simple PTEN nuclear diffusion is possible (Liu et al. 2005a), a number of active, more complex mechanisms have been identified that may facilitate PTEN shuttling to and from the nuclear compartment. For example, in MCF-7 breast cancer cells, the major vault protein (MVP) appears involved in transport of PTEN into the nucleus via four noncanonical NLS motifs (NLS1-4) (Chung et al. 2005). The MVP is a ribonucleoprotein that functions in general nuclear cytoplasmic transport and interacts with the C2 domain of PTEN in a process facilitated by Ca2+ (Minaguchi et al. 2006). Mutation of any one of the PTEN NLS1-4 motifs individually does not affect nuclear localization, but modification of any two NLSs in combination reduces PTEN binding to MVP and nuclear accumulation (Minaguchi et al. 2006). The small GTPase Ran can also mediate PTEN nuclear transport, in the context of cell death by apoptosis. Coexpression of PTEN and a dominant negative, GTPase function-deficient form of the Ran protein (Q69L) in U87MG cells led to reduction in nuclear PTEN, whereas wild-type (WT) Ran overexpression had no effect on PTEN localization (Gil et al. 2006). A cytoplasmic localization sequence (CLS) has also been found within the PTEN amino terminus and mutations of this region have been shown to affect nuclear accumulation of the protein (Denning et al. 2007).
Nuclear transport of PTEN appears to be further regulated by posttranslational modifications. PTEN ubiquitylation has been implicated as an important mediator of its localization. Namely, ubiquitylation of K289 in PTEN was found to promote nuclear import, while a mutation of this residue led to nuclear exclusion of the protein (Trotman et al. 2007). PTEN monoubiquitylation is thought to facilitate nuclear import, while polyubiquitylation of the protein is believed to induce proteasome-mediated degradation (Trotman et al. 2007; Planchon et al. 2008).
The E3 ubiquitin ligase NEDD4-1 (neural precursor cell–expressed developmentally down- regulated protein 4-1) was originally reported to be responsible for PTEN polyubiquitylation and subsequent degradation (Wang et al. 2007). However, ubiquitylation and stability of PTEN were not affected in cells and mice deficient for NEDD4-1, implicating other E3 ligases in the regulation of PTEN ubiquitylation and stability (Fouladkou et al. 2008). More recent studies have identified WWP2 (a NEDD4-like E3 ubiquitin ligase) and X-linked inhibitor of apoptosis protein (XIAP) as possible candidates (Worby and Dixon 2014). The WWP2 protein interacts with PTEN causing subsequent polyubiquitylation and degradation, while XIAP can directly ubiquitylate PTEN in vitro and mouse embryonic fibroblasts (MEFs) deficient for XIAP exhibit increased PTEN protein levels, reduced PTEN mono- and polyubiquitylation, and reduced nuclear PTEN levels when compared to WT MEFs (Van Themsche et al. 2009; Maddika et al. 2011). PTEN is also subject to deubiquitylation and nuclear exclusion by the herpes-associated ubiquitin-specific protease (HAUSP), which is a process promoted by the activity of the BCR–ABL fusion oncoprotein (Song et al. 2008; Morotti et al. 2014) and opposed by the promyelocytic leukemia protein (PML), through a mechanism involving the death domain–associated adaptor protein (DAXX) (Song et al. 2008). Considering the relatively long half-life of the PTEN protein (7–8 hours) in both normal and transformed cells (Tamguney and Stokoe 2007; Trotman et al. 2007; Xu et al. 2010), its ubiquitylation-dependent rapid turnover does not seem to be a dominant regulatory mechanism. However, mutations that drive PTEN polyubiquitylation have been discovered, which reduce protein stability and nuclear localization in glioblastoma cells (Yang et al. 2017). In cell culture, monoubiquitylation of these mutant PTEN proteins increased stability and rescued nuclear localization, suggesting agents that target protein polyubiquitylation may be useful in treating some brain cancers.
Another posttranslational modification that influences PTEN nuclear trafficking is SUMOylation. PTEN is SUMOylated on lysine 254 and this modification promotes nuclear accumulation of PTEN (Bassi et al. 2013). A non-SUMOylated PTEN mutant harboring an arginine substitution at this residue (K254R) fails to accumulate in the nucleus (Bassi et al. 2013). SUMOylation does not appear to be a factor in PTEN nuclear import, but rather provides a nuclear retention signal (Bassi et al. 2013). On exposure to genotoxic stress, including ionizing radiation (IR) and cisplatin, SUMOylated PTEN is excluded from the nucleus in an ataxia telangiectasia-mutated (ATM)-dependent manner, resulting from direct phosphorylation of PTEN on T398 by ATM (Bassi et al. 2013). The SUMO-mediated nuclear localization of PTEN is functionally important, as cells lacking nuclear PTEN are hypersensitive to DNA damage (as described below). PTEN is also SUMOylated on lysine 266, which facilitates interactions with the plasma membrane and is important for the inhibitory effects of PTEN on cell growth in soft agar, tumor growth in mice, and Akt activation (Huang et al. 2012). SUMOylation of PTEN is believed to increase its association with the plasma membrane either through electrostatic contacts between the positively charged SUMO-1 and negatively charged phospholipids or interactions with membrane-bound proteins that have SUMO-binding motifs (Huang et al. 2012; Matunis and Guzzo 2012). Regardless of the mechanism, it is likely that SUMOylation acts as a localization anchor, facilitating PTEN accumulation at a variety of cellular membranes including those of the cell surface, nucleus, vesicles, and mitochondria, where it may mediate diverse localization-specific effects (Matunis and Guzzo 2012).
Additional mechanisms for nuclear transport and localization of PTEN have been proposed and warrant further characterization. For example, interactions of PTEN with nuclear-targeting proteins may affect PTEN localization. The PNUTS (protein phosphatase-1 nuclear targeting subunit, PPP1R10) protein interacts with the C2 domain of PTEN and sequesters it in the nucleus (Kavela et al. 2013). Reduction of cellular PNUTS levels led to induction of apoptosis and decreased proliferation of cervical and prostate cancer cells in a PTEN-dependent manner, underscoring the functional significance of this interaction and exposing additional layers of PTEN nuclear function. Other protein modifications, such as oxidation, can also impact PTEN nuclear shuttling. Increased PTEN oxidation favors nuclear accumulation through processes mediated by altered succinate dehydrogenase subunit D (SDHD) function and SRC activation (Yu et al. 2015). These observations reveal the diverse mechanisms implicated in PTEN subcellular localization and highlight the need for additional research that may uncover approaches for modifying PTEN localization for the purposes of disease treatment.
NUCLEAR FUNCTIONS OF PTEN
The function of PTEN in countering the activity of the oncogenic PI3K signaling pathway offers an obvious molecular mechanism to explain the function of PTEN in tumor suppression (Maehama and Dixon 1999). Nevertheless, various anti-PI3K agents have universally failed to counter the growth of PTEN-deficient tumors, implicating non-PI3K aspects of PTEN function in tumor suppression (Juric et al. 2015; Le et al. 2016). Despite frequent detection of nuclear PTEN loss in numerous cancer cell types, the possibility of the involvement of PTEN nuclear function in tumor suppression has only been recently realized. Many components of the PI3K pathway are found in the nucleus, such as PI3K, AKT, and PDK1; thus, it was reasonable to hypothesize that PTEN may act as a lipid phosphatase in the nucleus (Déléris et al. 2006). Nevertheless, while nuclear pools of PIP3 also exist, they have been found to be insensitive to the lipid phosphatase activity of PTEN and are thus unlikely to feature in PTEN's nuclear functions (Lindsay et al. 2006).
NUCLEAR PTEN AND GENOME INTEGRITY
Multiple lines of evidence implicate nuclear PTEN in the maintenance of genome integrity. Early reports have shown that in the absence of PTEN, hyperactive Akt leads to impaired CHK1 function (Puc et al. 2005). Metaphase spreads of PTEN-deficient MEFs uncovered a variety of chromosomal alterations (Shen et al. 2007). A range of molecular mechanisms for PTEN involvement in genome maintenance and damage repair have been put forward.
During mitosis, accurate chromosomal segregation is essential to cell survival and breakdown of this process can result in chromosomal instability. The role of the centromere in chromosome segregation is to provide a platform for kinetochore assembly, through the binding of centromere-specific binding proteins such as CENP-A, CENP-B, and CENP-C (Westhorpe and Straight 2013). PTEN was shown to interact with CENP-C at centromeres in a phosphatase-independent manner, whereas expression of PTEN189, a cancer-associated truncation mutant lacking the carboxyl terminus and unable to bind CENP-C, caused chromosomal aberrations (Shen et al. 2007). PTEN-CENP-C association may be essential to centromere organization and could explain why PTEN loss results in chromosomal instability.
Maintenance of genomic stability requires DNA repair mechanisms to protect cellular DNA from genotoxic agents such as reactive oxygen species (ROS), IR or ultraviolet (UV) radiation. Lesions produced by such agents are repaired by mechanisms such as base excision repair, nucleotide excision repair, mismatch repair, and DNA double-strand break (DSB) repair (Hoeijmakers 2001). These repair mechanisms are regulated by the DNA damage response (DDR) signaling pathway and coordinated with the cell cycle, DNA replication, and apoptosis. Two members of the PI3K-like family of protein kinases (PIKKs), ATM and ataxia telangiectasia and Rad3-related (ATR) kinases, are master regulators of the DDR. The two distinct pathways, ATM-Chk2 and ATR-Chk1, are activated by DSBs and single-stranded DNA (ssDNA) lesions, respectively (Smith et al. 2010). DSBs are initially detected by the MRN complex (MRE11, Rad50, NBS1), which recruits ATM monomers and provides a platform for ATM activation via trans-autophosphorylation leading to phosphorylation of DNA repair proteins such as 53BP1, BRCA1, CHK2, and histone H2AX. DSBs can be repaired either through nonhomologous end joining (NHEJ) or homologous recombination (HR). NHEJ can occur at any stage of the cell cycle, whereas HR is restricted to S and G2 phases because of the requirement of a sister chromatid template. Phosphorylation of H2AX (γH2AX) serves as a marker of DSBs and leads to changes in chromatin marks that recruit 53BP1 to sites of DNA damage. When sites of DSBs are undergoing repair via HR, proteins such as BRCA1, BRCA2, Rad51, and Rad52 are recruited. BRCA1 is required for generating ssDNA (Schlegel et al. 2006) while BRCA2 is necessary for HR by facilitating the loading of Rad51 recombinase onto DNA (Thorslund and West 2007).
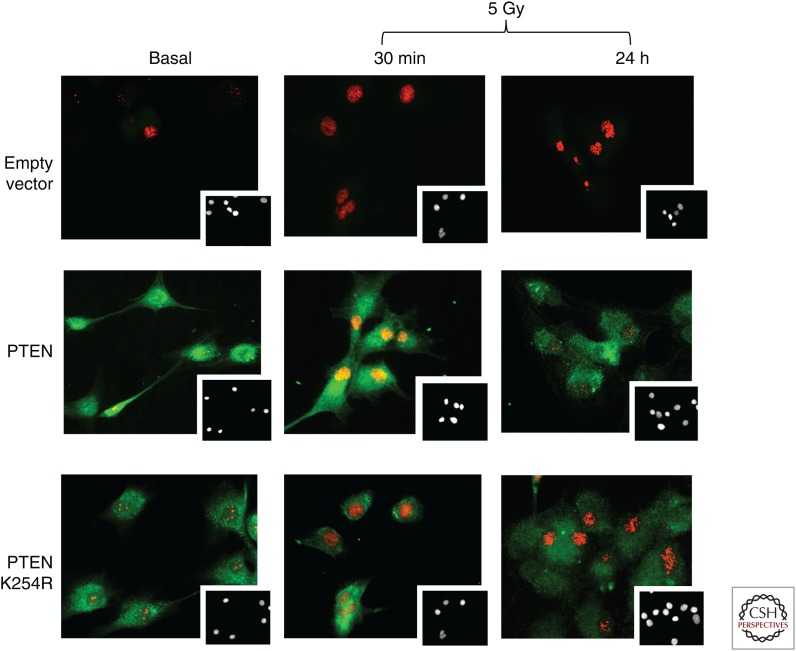
In addition to chromosomal abnormalities, PTEN loss was found to be associated with an accumulation of DNA DSBs as evidenced by positive γH2AX staining, suggesting a defect in DNA DSB repair (Bassi et al. 2013). Expression of Rad51, a critical component of the DSB DNA repair pathway, appears to be regulated by PTEN, although this may be restricted to only certain cell types (He et al. 2016). Phosphorylation on S366 and T370 facilitates translocation of PTEN to chromatin following genotoxic stress, where it colocalizes with Rad52 to regulate its SUMOylation (Choi et al. 2013). It was later shown that PTEN plays a more direct role in the DDR, as cells lacking nuclear PTEN were found to be sensitive to genotoxic stress (Bassi et al. 2013). When cells expressing a nuclear-excluded SUMOylation mutant of PTEN, K254R, were exposed to γ irradiation, they failed to recruit Rad51 to sites of DNA damage (Bassi et al. 2013). Monitoring PTEN localization by immunofluorescence and biochemical fractionation, it was shown that nuclear PTEN and more specifically, SUMOylated PTEN, was reduced within hours following γ irradiation (Bassi et al. 2013). 53BP1 foci detected in immunofluorescence can be used as an indicator of DSBs following γ irradiation. In U87MG cells reconstituted with WT PTEN or a mutant G129E lacking lipid phosphatase activity, 53BP1 foci were mostly resolved by 24 hours following IR (Fig. 1; Bassi et al. 2013). However, in PTEN-deficient parental U87MG cells, or lines reconstituted with a C124S PTEN mutant lacking all phosphatase activity, 53BP1 foci persisted 24 hours post-IR, indicative of defective DNA repair and a potential requirement for PTEN's protein phosphatase activity (Fig. 1; Bassi et al. 2013). Further supporting a defect in HR-based repair, cells lacking PTEN or expressing mutants K254R or C124S, failed to recruit RAD51 to sites of DNA damage and were defective in resolving γH2AX and BRCA1 foci (Bassi et al. 2013). PTEN was also found to be phosphorylated by ATM on residue 398 following IR, further implicating PTEN in the DDR (Bassi et al. 2013). More recently, it has been shown that PTEN phosphorylated on S/T398 is recognized and bound by MDC1, a DNA repair scaffold protein, to promote an interaction with the methyltransferase NSD2 (Zhang et al. 2019). PTEN is subsequently dimethylated by NSD2 on K349, which is recognized by the tudor domain of 53BP1 to mediate an interaction (Zhang et al. 2019). Another possible site of ATM-mediated PTEN phosphorylation, S113, was identified in A549 and HeLa cells treated with the genotoxic agent topotecan, and found to direct nuclear translocation (Chen et al. 2015). In addition to SUMOylation and lysine methylation, recent work has identified another posttranslational modification critical to the function of nuclear PTEN in DNA repair, tyrosine phosphorylation at Y240 (Ma et al. 2019). PTEN is recruited to chromatin through an interaction with Ki67 and is dependent on FGFR2-mediated phosphorylation of PTEN Y240 (Ma et al. 2019). Once on chromatin, PTEN facilitates the loading of RAD51 to promote DNA repair following IR (Ma et al. 2019).
Figure 1.
Impaired DNA damage response in the absence of nuclear PTEN. PTEN-deficient U87MG glioblastoma cells were stably reconstituted with either empty vector (control), wild-type (WT) PTEN or PTEN K254R mutant. The resulting cell pools were exposed to 5 Gy of γ irradiation and monitored at 30 minutes and 24 hours for PTEN expression (green) and 53BP1 foci (red) indicative of damaged DNA ends. Note the abundance of 53BP1 foci at 24 hours postirradiation in the control and PTEN K254R-reconstituted cells. (Inset) DAPI staining of nuclei.
NUCLEAR PTEN IN CONTROL OF DNA REPLICATION, CHROMATIN ORGANIZATION, AND THE CELL CYCLE
Nuclear PTEN functions extend beyond DNA repair pathways, to include roles related to cell-cycle progression (Song et al. 2011), DNA replication (Feng et al. 2015), DNA decatenation (Kang et al. 2015), and maintenance of heterochromatin structure (Chen et al. 2014; Gong et al. 2015; Benitez et al. 2017).
Nuclear PTEN levels appear to be elevated in the G0-G1 phase and lower in the S-phase of the cell cycle (Ginn-Pease and Eng 2003), suggesting PTEN's involvement in cell-cycle transitions. Indeed, nuclear PTEN was shown to inhibit nuclear localization of cyclin D1, a protein required for progression through the G1 phase of the cell cycle, during the G1 to S-phase transition (Radu et al. 2003). Moreover, overexpression of WT PTEN in MCF7 cells, but not its nuclear-excluded mutant, led to slower growth and an increase in the number of cells in G0-G1, revealing that nuclear PTEN can induce G0-G1 cell-cycle arrest (Chung and Eng 2005). Similarly, ectopic expression of an NLS-PTEN fusion protein in U251 glioblastoma cells induced cell-cycle G1 arrest and suppressed anchorage-independent growth (Liu et al. 2005b). Finally, nuclear PTEN was found to interact with components of the anaphase-promoting complex/cyclosome (APC/C), the major ubiquitin ligase required for progression through mitosis, and promote its association with CDH1 and drive progression through the cell cycle (Song et al. 2011).
Cellular DNA replication is highly coordinated with cell-cycle progression and DNA repair. Nuclear PTEN associates with MCM2, a DNA replication licensing factor, in response to replication stress (Feng et al. 2015). Acting as a protein phosphatase, PTEN dephosphorylates MCM2 on S41 at stalled replication forks to restrict progression of DNA replication (Feng et al. 2015). PTEN also seems to be involved in regulating another aspect of DNA replication, DNA decatenation, via direct interaction with the DNA topoisomerase IIA (TOP2A) and its stabilization, by preventing TOP2A ubiquitin-dependent proteasomal degradation (Kang et al. 2015).
PTEN status directs general changes in gene expression (Hong et al. 2000; Matsushima-Nishiu et al. 2001; Mulholland et al. 2012). Considering PTEN's engagement of multiple cellular pathways, it is difficult to discern between direct and indirect effects on regulating transcription. Nevertheless, PTEN contribution to the higher-order chromatin structure has also been observed. PTEN interacts with the core histone H1 to maintain heterochromatin organization, through repression of H4K16 acetylation (Chen et al. 2014). Heterochromatin structure is also regulated by PTEN binding to HP1α, a heterochromatin constituent, whose stability is supported by PTEN in a phosphatase activity-independent manner (Gong et al. 2015). Finally, independent of its phosphatase activity, PTEN interacts with the histone chaperone DAXX and modulates the capacity of the DAXX complex with the histone variant H3.3 to control transcription, and restrict expression of a number of genes implicated in oncogenesis, including Myc and cyclin D1 (Benitez et al. 2017).
IMPLICATIONS FOR HUMAN DISEASE
Considering the central role of PTEN in regulation of numerous cellular processes, and the lack of either protein sequence or functional PTEN homologs in the animal kingdom, expectedly, PTEN deficiency is implicated in the etiology of a variety of human conditions and diseases, including cancer, metabolic disorders, inflammation, neurodegeneration, and autism (Worby and Dixon 2014). While a plethora of research has been conducted to delineate the specific molecular mechanisms of PTEN involvement in these diseases, PTEN nuclear function has been largely overlooked in most studies. The majority of clinical, as well as experimental studies, have made the presence or absence of PTEN protein a critical, driving variable, ignoring subcellular distribution as a factor in PTEN biology. Given the complexities of PTEN subcellular localization, these aspects of PTEN control and their implications in biology and human disease research have remained largely uncharacterized and underappreciated.
In the context of brain injury, subcellular localization of PTEN, rather than its global protein levels, associates with neuronal survival, suggesting that PTEN may participate in recovery from injury (Goh et al. 2014). In mice, brain trauma led to increased nuclear PTEN accumulation in the surviving neurons. This change in localization was under the control of Ndfip1 (Nedd4 family interacting protein 1), an adaptor protein and activator of protein ubiquitination by Nedd4 E3 ligases (Goh et al. 2014). Additional support for the importance of nuclear PTEN in brain functions comes from nuclear PTEN-deficient mice generated using CRISPR/Cas9 technology. Mice lacking nuclear PTEN mostly present with neuronal and brain defects, suffering from seizures and microcephaly, because of reduced neuronal soma size in the cerebellum, cerebral cortex, and the hippocampus (Igarashi et al. 2018). Alterations in nuclear localization and neuronal soma size also accompany PTEN mutations that are found in patients with autism spectrum disorder (ASD) (Fricano-Kugler et al. 2018), further supporting the importance of nuclear PTEN in neuronal development and health. Specifically, ASD-associated PTEN mutations near the CBR3 loop within the C2 domain (including F241S, D252G, and W274L) may affect its conformation and accessibility to ubiquitylases/deubiquitylases, thus impacting PTEN stability and nuclear transport (Fricano-Kugler et al. 2018).
PTEN status, preferably determined by immunohistochemistry, is frequently used as a biomarker for prognosis and patient stratification strategies in a variety of human cancers. PTEN subcellular localization is emerging as an additional important pathologic feature in many human tumor types. For example, in a cohort of colorectal cancer patients, 50% of colorectal adenocarcinomas (241/482 cases) and 54% of metastatic lymph nodes (30/56 cases) exhibited reduced nuclear PTEN accumulation (Jang et al. 2010). In contrast, none of the 19 cases of normal colonic tissue displayed loss of nuclear PTEN (Jang et al. 2010). Similar findings have been reported for thyroid tumors, where reduced nuclear PTEN levels were found in follicular thyroid adenomas and thyroid carcinomas, as compared to normal follicular thyroid cells (Gimm et al. 2000). Nuclear PTEN status also associates with disease outcome, with reduced nuclear PTEN in colorectal adenocarcinomas predictive of poor overall and disease-free survival (Jang et al. 2010) and low nuclear PTEN levels in human prostate tumors correlation with earlier relapse and shorter patient survival (McCall et al. 2008).
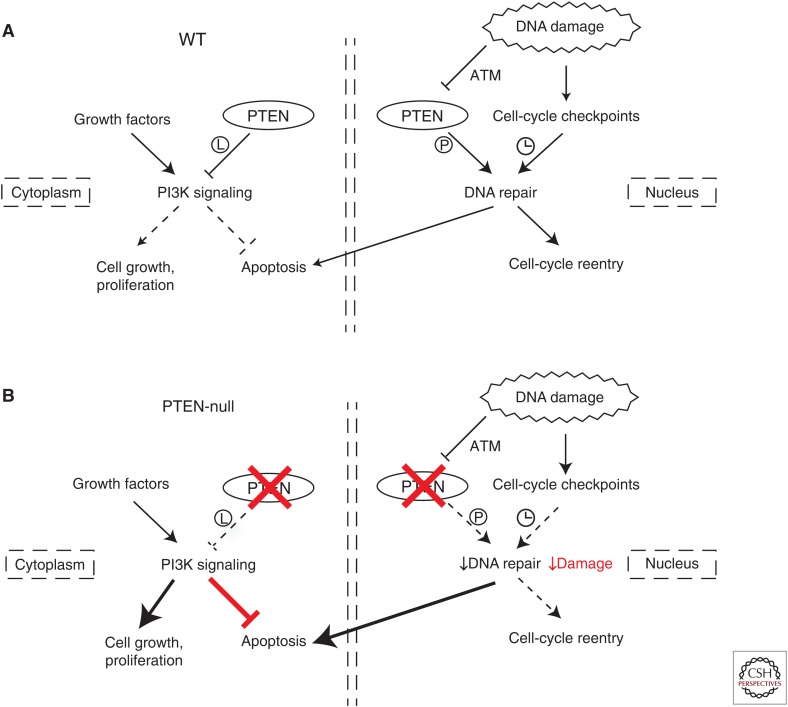
Such observations raise the possibility that interventional approaches that take PTEN localization into consideration may improve therapeutic strategies for certain types of cancer. As described above, cells lacking nuclear PTEN are hypersensitive to DNA damage (Bassi et al. 2013), implicating numerous chemotherapeutic agents and IR as potential effective treatment options for tumors exhibiting reduced nuclear PTEN. Because the most frequent presentation in cancer is complete loss of PTEN, coordinate lack of its cytoplasmic function is likely to drive increased survival as a result of hyperactive PI3K signaling, and possible accumulation of further spontaneous or treatment-driven mutations (Fig. 2). Treatments targeting DNA damage vulnerability or PI3K pathway activation of PTEN-deficient cancers in isolation would likely only result in increased selective pressure for propagation of cells acquiring further protumorigenic genetic events and increased treatment resistance. Possible efficacy could be achieved by parallel administration of genotoxic and anti-PI3K agents at first presentation of PTEN deficiency, although general toxicity may be a concern. Work in mouse models supports the feasibility of such a concept, where a combination of genotoxic agents with inhibitors of PI3K signaling has demonstrated efficacy in the treatment of PTEN-deficient cancer xenografts (Bassi et al. 2013).
Figure 2.
PTEN deficiency drives a progrowth, antiapoptotic and DNA damage-prone phenotype. (A) In PTEN wild-type (WT) cells exposed to genotoxic stress, PTEN coordinates a cellular response that determines the balance between “DNA repair and survival” and “DNA damage and apoptosis” outcomes via lipid phosphatase-dependent regulation of PI3K signaling in the cytoplasm and protein phosphatase activity-dependent regulation of the DNA damage response (DDR) in the nucleus. (B) In PTEN-null cells exposed to genotoxic stress, loss of cytoplasmic PTEN function drives hyperactivation of the progrowth and antiapoptotic PI3K/Akt signaling pathway while loss of PTEN nuclear function leads to accumulation of DNA damage. The increase in apoptotic signals emanating from failure to repair DNA is countered by constitutive activation of PI3K/Akt survival signaling, allowing unrepaired cells to reenter the cycle despite accumulating irreparable DNA damage and further contributing to the diseased state. (L) lipid phosphatase activity, (P) protein phosphatase activity.
CONCLUSIONS AND FUTURE OUTLOOK
Over the last several years, PTEN nuclear functions have emerged as important determinants of PTEN biology, both in healthy and diseased state. PTEN protein phosphatase activity, as well as its phosphatase activity-independent protein–protein interactions, appear to underlie the molecular mechanisms of PTEN action in the nucleus. This new knowledge is fueling renewed interest in the development of therapeutics aimed at the molecular and pathological features of PTEN-deficient disease, from cancer predisposition syndromes and cancer, to ASD and metabolic diseases. Considering that the cytoplasmic and nuclear functions of PTEN impact disparate processes, it is likely that PTEN deficiency will need to be countered by combination treatments directed at the key aspects of PTEN biology in these two cellular compartments. Recent progress in the development of cell and animal model systems designed to uncouple the cytoplasmic and nuclear PTEN functions, together with the recognition of the importance of PTEN subcellular localization status in patient material and its impact on disease outcome, will make a significant contribution to the understanding of the complexity of PTEN's action in health and disease.
Footnotes
Editors: Charis Eng, Joanne Ngeow, and Vuk Stambolic
Additional Perspectives on The PTEN Family available at www.perspectivesinmedicine.org
REFERENCES
- Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. 2013. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341: 395–399. 10.1126/science.1236188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez JA, Ma J, D'Antonio M, Boyer A, Camargo MF, Zanca C, Kelly S, Khodadadi-Jamayran A, Jameson NM, Andersen M, et al. 2017. PTEN regulates glioblastoma oncogenesis through chromatin-associated complexes of DAXX and histone H3.3. Nat Commun 8: 15223 10.1038/ncomms15223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Zhu M, Yang J, Liang H, He J, He S, Wang P, Kang X, McNutt MA, Yin Y, et al. 2014. PTEN interacts with histone H1 and controls chromatin condensation. Cell Rep 8: 2003–2014. 10.1016/j.celrep.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Zhang P, Chen WD, Li DD, Wu XQ, Deng R, Jiao L, Li X, Ji J, Feng GK, et al. 2015. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy 11: 239–252. 10.1080/15548627.2015.1009767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Chen Y, Dai W. 2013. Chromatin PTEN is involved in DNA damage response partly through regulating Rad52 sumoylation. Cell Cycle 12: 3442–3447. 10.4161/cc.26465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Eng C. 2005. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res 65: 8096–8100. 10.1158/0008-5472.CAN-05-1888 [DOI] [PubMed] [Google Scholar]
- Chung JH, Ginn-Pease ME, Eng C. 2005. Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has nuclear localization signal-like sequences for nuclear import mediated by major vault protein. Cancer Res 65: 4108–4116. 10.1158/0008-5472.CAN-05-0124 [DOI] [PubMed] [Google Scholar]
- Déléris P, Bacqueville D, Gayral S, Carrez L, Salles JP, Perret B, Breton-Douillon M. 2003. SHIP-2 and PTEN are expressed and active in vascular smooth muscle cell nuclei, but only SHIP-2 is associated with nuclear speckles. J Biol Chem 278: 38884–38891. 10.1074/jbc.M300816200 [DOI] [PubMed] [Google Scholar]
- Déléris P, Gayral S, Breton-Douillon M. 2006. Nuclear Ptdlns(3,4,5)P3 signaling: an ongoing story. J Cell Biochem 98: 469–485. 10.1002/jcb.20695 [DOI] [PubMed] [Google Scholar]
- Denning G, Jean-Joseph B, Prince C, Durden DL, Vogt PK. 2007. A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene 26: 3930–3940. 10.1038/sj.onc.1210175 [DOI] [PubMed] [Google Scholar]
- Feng J, Liang J, Li J, Li Y, Liang H, Zhao X, McNutt MA, Yin Y. 2015. PTEN controls the DNA replication process through MCM2 in response to replicative stress. Cell Rep 13: 1295–1303. 10.1016/j.celrep.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, Stambolic V, Rotin D. 2008. The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc Natl Acad Sci 105: 8585–8590. 10.1073/pnas.0803233105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricano-Kugler CJ, Getz SA, Williams MR, Zurawel AA, DeSpenza T Jr, Frazel PW, Li M, O'Malley AJ, Moen EL, Luikart BW. 2018. Nuclear excluded autism-associated phosphatase and tensin homolog mutations dysregulate neuronal growth. Biol Psychiatry 84: 265–277. 10.1016/j.biopsych.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari FB, Lin H, Huang HS, Cavenee WK. 1997. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci 94: 12479–12484. 10.1073/pnas.94.23.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Andrés-Pons A, Fernández E, Valiente M, Torres J, Cervera J, Pulido R. 2006. Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell 17: 4002–4013. 10.1091/mbc.e06-05-0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, et al. 2000. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 156: 1693–1700. 10.1016/S0002-9440(10)65040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn-Pease ME, Eng C. 2003. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res 63: 282–286. [PubMed] [Google Scholar]
- Goh CP, Putz U, Howitt J, Low LH, Gunnersen J, Bye N, Morganti-Kossmann C, Tan SS. 2014. Nuclear trafficking of Pten after brain injury leads to neuron survival not death. Exp Neurol 252: 37–46. 10.1016/j.expneurol.2013.11.017 [DOI] [PubMed] [Google Scholar]
- Gong L, Govan JM, Evans EB, Dai H, Wang E, Lee SW, Lin HK, Lazar AJ, Mills GB, Lin SY. 2015. Nuclear PTEN tumor-suppressor functions through maintaining heterochromatin structure. Cell Cycle 14: 2323–2332. 10.1080/15384101.2015.1044174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhang Z, Ouyang M, Yang F, Hao H, Lamb KL, Yang J, Yin Y, Shen WH. 2016. PTEN regulates EG5 to control spindle architecture and chromosome congression during mitosis. Nat Commun 7: 12355 10.1038/ncomms12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH. 2001. Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374. 10.1038/35077232 [DOI] [PubMed] [Google Scholar]
- Hong TM, Yang PC, Peck K, Chen JJ, Yang SC, Chen YC, Wu CW. 2000. Profiling the downstream genes of tumor suppressor PTEN in lung cancer cells by complementary DNA microarray. Am J Respir Cell Mol Biol 23: 355–363. 10.1165/ajrcmb.23.3.4002 [DOI] [PubMed] [Google Scholar]
- Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, et al. 2012. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun 3: 911 10.1038/ncomms1919 [DOI] [PubMed] [Google Scholar]
- Igarashi A, Itoh K, Yamada T, Adachi Y, Kato T, Murata D, Sesaki H, Iijima M. 2018. Nuclear PTEN deficiency causes microcephaly with decreased neuronal soma size and increased seizure susceptibility. J Biol Chem 293: 9292–9300. 10.1074/jbc.RA118.002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KS, Song YS, Jang SH, Min KW, Na W, Jang SM, Jun YJ, Lee KH, Choi D, Paik SS. 2010. Clinicopathological significance of nuclear PTEN expression in colorectal adenocarcinoma. Histopathology 56: 229–239. 10.1111/j.1365-2559.2009.03468.x [DOI] [PubMed] [Google Scholar]
- Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, Ebbesen SH, Ainscough BJ, Ramu A, Iyer G, et al. 2015. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature 518: 240–244. 10.1038/nature13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Song C, Du X, Zhang C, Liu Y, Liang L, He J, Lamb K, Shen WH, Yin Y. 2015. PTEN stabilizes TOP2A and regulates the DNA decatenation. Sci Rep 5: 17873 10.1038/srep17873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavela S, Shinde SR, Ratheesh R, Viswakalyan K, Bashyam MD, Gowrishankar S, Vamsy M, Pattnaik S, Rao S, Sastry RA, et al. 2013. PNUTS functions as a proto-oncogene by sequestering PTEN. Cancer Res 73: 205–214. 10.1158/0008-5472.CAN-12-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le X, Antony R, Razavi P, Treacy DJ, Luo F, Ghandi M, Castel P, Scaltriti M, Baselga J, Garraway LA. 2016. Systematic functional characterization of resistance to PI3K inhibition in breast cancer. Cancer Discov 6: 1134–1147. 10.1158/2159-8290.CD-16-0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DM, Sun H. 1997. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor β. Cancer Res 57: 2124–2129. [PubMed] [Google Scholar]
- Lindsay Y, McCoull D, Davidson L, Leslie NR, Fairservice A, Gray A, Lucocq J, Downes CP. 2006. Localization of agonist-sensitive PtdIns(3,4,5)P3 reveals a nuclear pool that is insensitive to PTEN expression. J Cell Sci 119: 5160–5168. 10.1242/jcs.000133 [DOI] [PubMed] [Google Scholar]
- Liu F, Wagner S, Campbell RB, Nickerson JA, Schiffer CA, Ross AH. 2005a. PTEN enters the nucleus by diffusion. J Cell Biochem 96: 221–234. 10.1002/jcb.20525 [DOI] [PubMed] [Google Scholar]
- Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. 2005b. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol 25: 6211–6224. 10.1128/MCB.25.14.6211-6224.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Benitez JA, Li J, Miki S, Ponte de Albuquerque C, Galatro T, Orellana L, Zanca C, Reed R, Boyer A, et al. 2019. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell 35: 504–518.e507. 10.1016/j.ccell.2019.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen J. 2011. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol 13: 728–733. 10.1038/ncb2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378. 10.1074/jbc.273.22.13375 [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. 1999. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol 9: 125–128. 10.1016/S0962-8924(99)01519-6 [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiu M, Unoki M, Ono K, Tsunoda T, Minaguchi T, Kuramoto H, Nishida M, Satoh T, Tanaka T, Nakamura Y. 2001. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res 61: 3741–3749. [PubMed] [Google Scholar]
- Matunis MJ, Guzzo CM. 2012. SUMO, PTEN and tumor suppression. Pigment Cell Melanoma Res 10.1111/pcmr.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J. 2008. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J Cancer 99: 1296–1301. 10.1038/sj.bjc.6604680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaguchi T, Waite KA, Eng C. 2006. Nuclear localization of PTEN is regulated by Ca2+ through a tyrosil phosphorylation-independent conformational modification in major vault protein. Cancer Res 66: 11677–11682. 10.1158/0008-5472.CAN-06-2240 [DOI] [PubMed] [Google Scholar]
- Morotti A, Panuzzo C, Crivellaro S, Pergolizzi B, Familiari U, Berger AH, Saglio G, Pandolfi PP. 2014. BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP. Leukemia 28: 1326–1333. 10.1038/leu.2013.370 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. 2012. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res 72: 1878–1889. 10.1158/0008-5472.CAN-11-3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Eng C. 2000. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab 85: 2334–2338. [DOI] [PubMed] [Google Scholar]
- Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. 1998. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci 95: 13513–13518. 10.1073/pnas.95.23.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren A, Weng LP, Boag AH, Ziebold U, Thakore K, Dahia PL, Komminoth P, Lees JA, Mulligan LM, Mutter GL, et al. 1999. Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 155: 1253–1260. 10.1016/S0002-9440(10)65227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren A, Komminoth P, Saremaslani P, Matter C, Feurer S, Lees JA, Heitz PU, Eng C. 2000. Mutation and expression analyses reveal differential subcellular compartmentalization of PTEN in endocrine pancreatic tumors compared to normal islet cells. Am J Pathol 157: 1097–1103. 10.1016/S0002-9440(10)64624-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planchon SM, Waite KA, Eng C. 2008. The nuclear affairs of PTEN. J Cell Sci 121: 249–253. 10.1242/jcs.022459 [DOI] [PubMed] [Google Scholar]
- Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, et al. 2005. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 7: 193–204. 10.1016/j.ccr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. 2003. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol 23: 6139–6149. 10.1128/MCB.23.17.6139-6149.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. 2004. High frequency of mutations of the PIK3CA gene in human cancers. Science 304: 554 10.1126/science.1096502 [DOI] [PubMed] [Google Scholar]
- Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, Hess KR, Myers JN, Hong YK, Yung WK, et al. 1999. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res 59: 1820–1824. [PubMed] [Google Scholar]
- Schlegel BP, Jodelka FM, Nunez R. 2006. BRCA1 promotes induction of ssDNA by ionizing radiation. Cancer Res 66: 5181–5189. 10.1158/0008-5472.CAN-05-3209 [DOI] [PubMed] [Google Scholar]
- Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. 2007. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157–170. 10.1016/j.cell.2006.11.042 [DOI] [PubMed] [Google Scholar]
- Smith J, Tho LM, Xu N, Gillespie DA. 2010. The ATM–Chk2 and ATR–Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res 108: 73–112. 10.1016/B978-0-12-380888-2.00003-0 [DOI] [PubMed] [Google Scholar]
- Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. 2008. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455: 813–817. 10.1038/nature07290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP. 2011. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144: 187–199. 10.1016/j.cell.2010.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95: 29–39. 10.1016/S0092-8674(00)81780-8 [DOI] [PubMed] [Google Scholar]
- Tamguney T, Stokoe D. 2007. New insights into PTEN. J Cell Sci 120: 4071–4079. 10.1242/jcs.015230 [DOI] [PubMed] [Google Scholar]
- Thorslund T, West SC. 2007. BRCA2: a universal recombinase regulator. Oncogene 26: 7720–7730. 10.1038/sj.onc.1210870 [DOI] [PubMed] [Google Scholar]
- Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, et al. 2007. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128: 141–156. 10.1016/j.cell.2006.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Themsche C, Leblanc V, Parent S, Asselin E. 2009. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem 284: 20462–20466. 10.1074/jbc.C109.009522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, et al. 2007. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128: 129–139. 10.1016/j.cell.2006.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhorpe FG, Straight AF. 2013. Functions of the centromere and kinetochore in chromosome segregation. Curr Opin Cell Biol 25: 334–340. 10.1016/j.ceb.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C. 2002. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer 99: 63–67. 10.1002/ijc.10294 [DOI] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. 2014. PTEN. Annu Rev Biochem 83: 641–669. 10.1146/annurev-biochem-082411-113907 [DOI] [PubMed] [Google Scholar]
- Xu D, Yao Y, Jiang X, Lu L, Dai W. 2010. Regulation of PTEN stability and activity by Plk3. J Biol Chem 285: 39935–39942. 10.1074/jbc.M110.166462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JM, Schiapparelli P, Nguyen HN, Igarashi A, Zhang Q, Abbadi S, Amzel LM, Sesaki H, Quiñones-Hinojosa A, Iijima M. 2017. Characterization of PTEN mutations in brain cancer reveals that pten mono-ubiquitination promotes protein stability and nuclear localization. Oncogene 36: 3673–3685. 10.1038/onc.2016.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, He X, Ni Y, Ngeow J, Eng C. 2015. Cowden syndrome-associated germline SDHD variants alter PTEN nuclear translocation through SRC-induced PTEN oxidation. Hum Mol Genet 24: 142–153. 10.1093/hmg/ddu425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lee YR, Dang F, Gan W, Menon AV, Katon JM, Hsu CH, Asara JM, Tibarewal P, Leslie NR, et al. 2019. PTEN methylation by NSD2 controls cellular sensitivity to DNA damage. Cancer Discov 9: 1306–1323. 10.1158/2159-8290.CD-18-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]