Abstract
Metastatic disease is the leading cause of death in patients with solid cancers. The progression to metastasis is a multistep process that involves detachment of tumor cells from their constraining basement membrane at the primary site, migration and intravasation into the circulation, survival in the circulation, extravasation into the secondary organ, and survival and growth at the secondary site. During these steps, tumor and immune cells interact and influence each other both within the tumor microenvironment and systemically. In particular, myeloid cells such as monocytes, macrophages, neutrophils, and myeloid-derived suppressor cells (myeloid regulatory cells) have been shown to play important roles in the metastatic process. These interactions open new avenues for targeting cancer metastasis, especially given the increasing interest in development of cancer immunotherapies. In this review, we describe the currently reported pathways and mechanisms involved in myeloid cell enhancement of the metastatic cascade.
Cancer metastasis is the major cause of morbidity and mortality in patients. Although improvements have been made for early diagnosis of cancer, and treatment of primary cancers leading to significantly increased survival, limited progress has been made in the treatment of metastasis. In fact, in many cancer types, such as breast and prostate, there has been little change in overall survival of metastatic cancer patients suggesting resistance to the usual standard of care such as chemotherapy and radiation, as well as new targeted biologics. However, significant progress in immunotherapies, particularly checkpoint inhibitors aimed to activate cytotoxic T cells, have shown success in metastatic melanoma, thus adding considerable impetus to develop new immune-oncological treatments. Despite striking success with these therapeutics most metastatic cancers are still resistant, therefore there is a need for a better understanding of the cancer microenvironment in regulating immune cell-mediated attack of cancer.
This renewed interest is based on the realization that the tumor microenvironment is complex and is populated with many immune cell types of both the innate and acquired immune systems. Importantly, this cellular composition evolves as the tumor progresses to malignancy and it is also becoming apparent that different cancers have different immune composition both in the primary and metastatic site. Studies have also indicated that rather than rejecting tumors, the immune cells, particularly those of the innate system (Cotechini et al. 2015) are polarized to be tumor promoting and to suppress cytotoxic cell responses (Condeelis and Pollard 2006; Cassetta and Pollard 2018). Thus, it is imperative that this immune infiltration into tumors is characterized spatially and temporarally and the function and regulation of these cells is defined mechanistically so that new improved therapeutics can be developed.
The majority of solid tumors originate in the epithelial cell population. Successful metastasis requires induction of the ability to break through the basement membrane and invade through the extracellular matrix (ECM) and stromal layers, intravasation into the vasculature or lymphatic system, transport and survival in the circulatory system, extravasation into the parenchyma of distant tissues, seeding at the secondary site, and survival and growth into metastatic tumors, a process termed the metastatic cascade (Fidler 2003; Joyce and Pollard 2009; Lambert et al. 2017). Throughout this process, tumor cells interact with various cells of the immune system, which can modulate every step. A link between tumors and immune cells was first proposed by Virchow, in 1863, when he noticed infiltrating blood cells in malignant tissues (Balkwill and Mantovani 2001). However, the role of the immune system in metastasis is complex and immune responses can play dual, pro- and antitumoral, roles during cancer spread to secondary sites. This review will define the prometastatic roles of innate immune cells particularly those of the myeloid compartment in the metastatic cascade from primary tumors to their final destination in the lung.
LOCAL INVASION AND INTRAVASATION
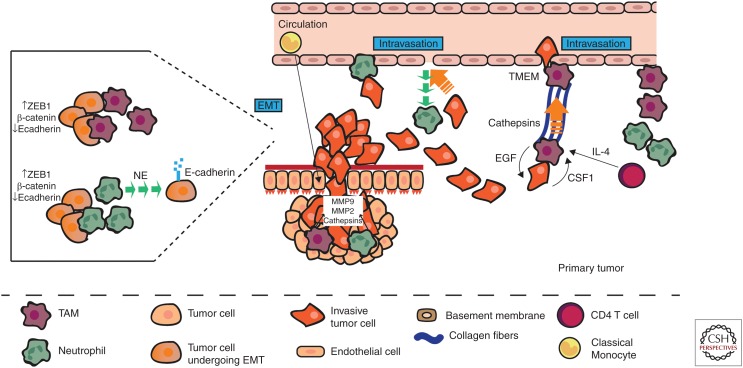
The first step of the metastatic cascade involves invasion of the cancer cells through the primary tumor into surrounding tissues and entry into blood vessels or lymphatic system. To spread out from the primary site, cancer cells need to break through the surrounding extracellular matrix. Epithelial–mesenchymal transition (EMT) has been proposed to be a key mechanism in the acquisition of an invasive phenotype by epithelial cancer cells. During EMT, a polarized epithelial cell transitions to a mesenchymal phenotype. These changes result in increased migratory capacity, invasiveness, resistance to apoptosis, and increased production of ECM components. The final step results in the degradation of the underlying basement membrane and migration of the cell out of the epithelial layer (Lamouille et al. 2014). Although the exact mechanism and definition of this EMT process are debated, for epithelial cells to invade they need to change morphology and gain migratory properties. Activation of these programs is often dependent on a cross-talk between cancer cells and the local microenvironment including immune cells.
In ovarian tumors, tumor associated neutrophils (TANs) were shown to cluster with cells expressing the EMT transcription factor ZEB1, and this was associated with areas of loss of tumor-expressed E-cadherin (Mayer et al. 2016). Similarly, in lung adenocarcinoma, the neutrophil count was negatively associated with E-cadherin expression (Hu et al. 2015). In pancreatic ductal adenocarcinoma (PDAC) biopsies with high TAN numbers, a nuclear accumulation of β-catenin and ZEB1 (markers of EMT) was observed and, in the same study, coculture of neutrophils isolated from healthy donors with pancreatic tumor cells resulted in tumor cell dyshesion, down-regulation of keratins, up-regulation of TWIST, translocation of β-catenin to the nucleus, and appearance of ZEB1 in the nucleus (Große-Steffen et al. 2012). In vitro studies on PDAC, lung adenocarcinoma, and ovarian cancer cell lines showed that neutrophil-derived elastase resulted in digestion of E-cadherin on tumor cells resulting in a transition toward a mesenchymal-like phenotype (Gaida et al. 2012; Hu et al. 2015; Mayer et al. 2016), whereas interactions with TANs have been reported to activate the ERK pathway and induce expression of EMT transcription factors in gastric cancer cells lines (Zhang et al. 2017).
In non-small-cell lung carcinoma (NSCLC), a positive correlation between tumor-associated macrophage (TAM) densities and EMT markers was found (Bonde et al. 2012), and in hepatocellular carcinoma (HCC) EMT markers were found primarily at sites of TAM infiltration (Fu et al. 2015). F9, Hepa1-6, and NMuMG cell line exposure to “TAM”-conditioned medium was shown to decrease expression of E-cadherin, activate the β-catenin pathway, and increase expression of mesenchymal markers (Bonde et al. 2012; Fan et al. 2014), and coculture of macrophages with various cancer cells and cell lines resulted in increased expression of the mesenchymal markers vimentin, SNAIL, and N-cadherin, and down-regulation of E-cadherin (Liu et al. 2013; Su et al. 2014; Fu et al. 2015; Hu et al. 2016; Gao et al. 2018). In human breast cancer tissue, TAMs were found in juxtaposition with cancer stem cells (CSCs) and TAM numbers correlated with numbers of invading CSCs, while co-injection of TAMs with murine breast cancer cells enhanced metastasis, indicating that TAM–CSC interactions induce a tumor-supportive stroma and enhance tumor cell invasiveness (Lu et al. 2014). In support of this mechanism, a study in murine breast cancer found that TAMs provide a permissive niche for CSCs and actively support their maintenance via a paracrine EGF/EGFR/STAT3 signaling loop that was critical for SOX-2 expression in CSCs (Yang et al. 2013). Overall, these data indicate that both TAMs and TANs can induce EMT/stem cell phenotypes in tumor cells and thus promote tumor cell acquisition of an invasive phenotype.
Once the tumor cells have acquired an invasive phenotype, they need to break out of the tumor matrix. ECM degradation is thought to occur primarily through the actions of matrix metalloproteinases (MMPs), in particular MMP-2 and MMP-9, as these proteases preferentially degrade type IV collagen (Clavel et al. 1992). Both TAMs and TANs can act as significant sources of MMPs in primary tumors (Nielsen et al. 1996; Pollard 2008; Bausch et al. 2011). Additionally, TAN-derived MMP-9 is typically not associated with tissue inhibitor of metalloproteinases (TIMPs), and therefore it is more easily activated (Ardi et al. 2007).
TAMs also produce a number of other molecules that promote tumor cell invasion such as osteonectin (SPARC), which increases tumor cell–ECM interactions (Sangaletti et al. 2008), urokinase-type plasminogen activator (uPA), which increases tumor cell invasiveness (Hildenbrand et al. 1999), and cathepsin proteases, which remodel the ECM (Laoui et al. 2011). Similarly, TANs also secrete a number of other proteases capable of ECM remodeling, such as neutrophil elastase (NE), cathepsins, and proteinase-3, which enhance tumor cell invasion (Sato et al. 2006; Tan et al. 2013) and cleave cell surface adhesion molecules on tumor cells (Gaida et al. 2012).
In a seminal study, it was found that TANs increased the invasion of tumor cells in vitro and increased rat mammary tumor metastasis to lung in vivo compared with normal and inflammatory neutrophils (Welch et al. 1989). In the PyMT mammary tumor model, increased number of TAMs were observed at sites of basement membrane breakdown (Lin et al. 2001, 2003) and an enrichment of extracellular proteases has been reported in the tumor stroma of PyMT animals in areas corresponding to TAM localization (Pedersen et al. 2005; Wyckoff et al. 2007). In human gastric cancer, both TAMs and TANs have been found to concentrate at the invasive front of the tumor (Liu et al. 2009, 2017), and in human fibrosarcoma and prostate tumors, TANs expressing MMP-9 were associated with increased tumor cell intravasation (Bekes et al. 2011).
TAM-derived cathepsins, (CTs) B and S, have been shown to be critical for tumor invasion in mouse models of pancreatic islet cancers and mammary tumors. Tumor-derived interleukin 4 (IL-4) was responsible for inducing cathepsin activity in TAMs and thus a model was proposed whereby tumor-derived IL-4 stimulates CTs B and S production in TAMs at the tumor–stromal interface, which degrade ECM substrates and create a path for the migration/invasion of cancer cells (Gocheva et al. 2010). In addition, CD4+ T cell-derived IL-4 has been reported to induce a protumoral phenotype in TAMs, which then enhance tumor cell invasion via production of proinvasive factors such as EGF (DeNardo et al. 2009).
TAMs not only promote tumor cell invasion via ECM breakdown but have also been shown to promote directional tumor cell migration. This occurs via a paracrine loop consisting of tumor cell-derived colony stimulating factor (CSF)-1 and macrophage-derived EGF that induces tumor cells and macrophages to move in conjunction along collagen fibers ending in tumor cell clustering around blood vessels (Condeelis and Pollard 2006; Wyckoff et al. 2007). Furthermore, a structure consisting of macrophages, endothelial, and tumor cells, termed the tumor microenvironment for metastasis (TMEM) that predicts metastasis in human breast cancers (Rohan et al. 2014), has been associated with tumor cell intravasation in mouse models of breast cancer (Wyckoff et al. 2007; Roh-Johnson et al. 2014). Within the TMEM, macrophage and tumor cell contact induces the formation of invadopodia (actin-rich matrix degrading protrusions) in tumor cells via a Notch1/MenaINV signaling pathway (Roh-Johnson et al. 2014; Pignatelli et al. 2016), and transient vascular permeability has been reported at TMEM sites resulting in increased tumor cell intravasation into the circulation (Harney et al. 2015). Thus, TAMs alone, or as part of the TMEM complex, attract invasive tumor cells to blood vessels where they increase vascular permeability and allow for tumor cell escape into the circulation.
TAMs can also increase the density of blood vessels (De Palma et al. 2003; Lin et al. 2006, 2007; Riabov et al. 2014). TAM-induced angiogenesis has been reported to be promoted, at least in part, through vascular endothelial growth factor (VEGF)A expression in the PyMT model of breast cancer (Lin et al. 2007), and inhibition of angiogenesis in PyMT primary tumors reduces metastasis (Zabuawala et al. 2010; Yeo et al. 2014). A subset of TAMs that are Tie2+ can migrate to blood vessels where they differentiate into perivascular macrophages, promote vascular leakiness, and cancer cell intravasation (Wyckoff et al. 2007; Mazzieri et al. 2011; Arwert et al. 2018).
TANs have also been reported to boost tumor cell intravasation, although via different mechanisms. In a normal inflammatory response, neutrophils extravasate from the circulation into affected tissues. It has been proposed that neutrophil migration into the tumor creates a channel in the ECM through which tumor cells can more easily escape into the vasculature, this has been termed the countercurrent model (Opdenakker and Van Damme 2004; Piccard et al. 2012). It has also been proposed that tumor cells may in fact attach to neutrophils and traverse the endothelium in conjunction with neutrophils as they exit the primary tumor site (Wu et al. 2001; Strell et al. 2010). (See Figure 1 for a summary of the roles of myeloid cells in local invasion and intravasation.)
Figure 1.
Myeloid cells promote tumor cell escape from the primary tumor. Tumor-associated macrophages and neutrophils (TAMs and TANs, respectively) induce an invasive phenotype in tumor cells by up-regulating mesenchymal markers (ZEB1, β-catenin) and down-regulating epithelial markers (E-cadherin). TAMs and TANs also secrete proteases and cathepsins, which degrade the extracellular matrix (ECM) and allow the tumor cells to invade through their basement membrane. The tumor cells can then attach to neutrophils and use them to intravasate into the circulation, or the tumor cells can use channels left by extravasating neutrophils to more easily escape into the blood vessels. TAMs guide tumor cells to blood vessels along collagen fibers via an EGF/CSF1 signaling loop, induced by CD4 T cell-derived interleukin 4 (IL-4). Once at the vessel, the TAMs, either alone or as part of a TMEM complex, induce vascular leakiness thus enhancing tumor cell escape into the circulation.
SURVIVAL AND DISSEMINATION THE CIRCULATION
Once the tumor cells have successfully invaded into the blood vessels, they need to survive a number of stresses to reach distant organs; (1) they are deprived of the integrin-dependent adhesion to ECM components that is normally essential for epithelial cell survival, (2) they must survive shear forces and, (3) they must evade killing by immune cells.
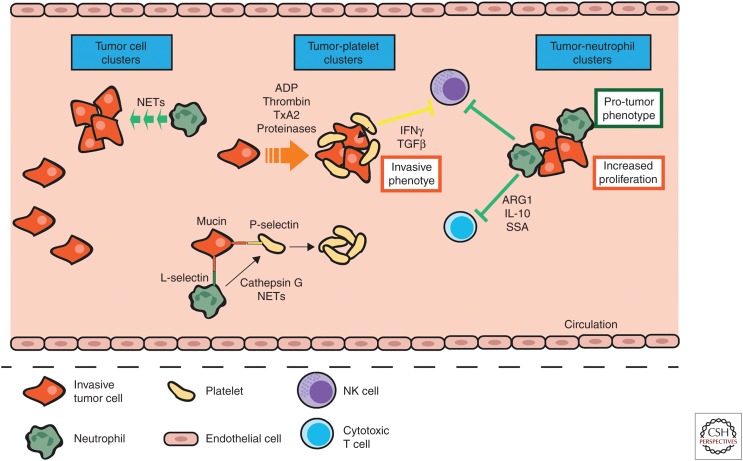
Loss of cell–cell and cell–ECM contact and shear forces constitute significant challenges for cancer cells entering the circulation. One of the ways that the cancer cells overcome this problem is by forming cell aggregates (Choi et al. 2015). In vitro, neutrophils have been shown to promote breast cancer and colorectal carcinoma cell clustering (Jadhav et al. 2001; Yui et al. 2005; Morimoto-Kamata et al. 2012), and in a mammary cancer model NETosis was associated with the appearance of venous thrombi in the lung (Demers et al. 2012). In the 4T1 mammary tumor model, as well as in breast cancer patients, circulating tumor cells (CTCs) formed clusters with neutrophils and this induced a protumor gene expression profile in the neutrophils. Pathway analysis showed that the CTCs from these clusters were enriched in positive regulators of cell cycle and DNA replication programs when compared with CTCs alone. Further, these neutrophil-associated CTCs showed higher levels of Ki67, a marker of DNA synthesis, which was retained in disseminated tumor cells. Blockade of neutrophils ablated these clusters and reduced metastasis, whereas overexpression of granulocyte-colony stimulating factor (G-CSF) led to earlier release of CTCs, more CTC–neutrophil clusters and enhanced metastasis (Szczerba et al. 2019). Thus, CTC–neutrophil clustering induces a protumor phenotype in the neutrophils, which then support tumor cell cycle progression in the circulation leading to enhanced metastatic seeding.
The formation of platelet-rich thrombi around the tumor cells has also been proposed to shield these cells from stresses in the circulation. In the vascular circulation, tumor cells activate platelets either via direct contact or through the release of mediators such as ADP, thrombin, TxA2, or proteinases, which in turn induce tumor cell–platelet aggregation (Schlesinger 2018). Coculture of tumor cells induces aggregation of platelets in vitro (Heinmöller et al. 1995; Medina et al. 2012; Lian et al. 2013) and the ability to do so increases with increasing metastatic potential (Honn et al. 1992; Zarà et al. 2017). Inhibition of platelet aggregation resulted in reduced bone and visceral metastasis in a model of melanoma (Bakewell et al. 2003). Platelet–tumor cell aggregates may also protect CTCs by conferring resistance against detachment-induced death. Coculture of platelets with human ovarian cancer cell lines prevented anoikis via activation of the YAP1 signaling pathway in vitro (Haemmerle et al. 2017). However, this has yet to be confirmed in vivo.
Platelets may act as more than just a shield for tumor cells and actively manipulate tumor cells in the circulation to enhance metastatic phenotypes. Coculture of colon carcinoma cells with platelets before tumor cell injection into mice increased the number of metastatic foci via transforming growth factor β (TGFβ) and nuclear factor (NF)κB signaling-mediated induction of a prometastatic phenotype in tumor cells (Labelle et al. 2011). Coculture of ovarian cancer cells and ascites with platelets induced stromal-like phenotypic changes in the tumor cells (Orellana et al. 2015). Addition of washed thrombin-activated membranes to breast cancer, melanoma, and lymphoma cell cultures enhanced the ability of tumor cells to degrade matrigel, and stimulation of cancer cells with platelet activated membranes before injection into mice increased tumor cell infiltration into the lung and metastatic nodule numbers (Pang et al. 2015). Hence, platelets may induce an invasive phenotype in circulating cancer cells.
Natural killer (NK) cells are the predominant immune cell type involved in immune surveillance and destruction of circulating cancer cells (Morvan and Lanier 2016). Platelets inhibit NK-mediated lysis of target cells in vitro and disruption of platelet aggregation subverts this protection (Honn et al. 1992). Depletion of platelets results in reduced tumor cell seeding in metastatic organs but only in tumors typically sensitive to NK cell-mediated killing (Honn et al. 1992). In another study, disruption of platelet function significantly reduced CTC survival and metastasis. This was associated with increased NK cell killing of CTCs and inhibition of NK cell function reversed this (Palumbo et al. 2005). Platelet–tumor cell aggregates can also secrete various factors such as interferon (IFN)γ and TGFβ, which down-regulate NKG2D receptors on NK cells, thus impairing their ability to become activated and kill tumor cells (Kopp et al. 2009). Furthermore, platelet coating can confer major histocompatibility complex (MHC) class I expression onto tumor cells, which also protects cancer cells against NK cell-mediated killing (Placke et al. 2012).
Neutrophils too protect CTCs against immune-mediated destruction. In a mammary tumor model, neutrophils suppressed NK cell ability to respond to signaling via cell surface receptors and hence as a consequence suppressed NK-cell activation leading to enhanced survival of D2A1 mammary tumor cells (Spiegel et al. 2016). In addition to shielding CTCs against NK cell killing, neutrophils can also protect tumor cells against antitumor T-cell responses. In a mammary tumor model a systemic expansion of T-cell suppressive neutrophils was observed (Casbon et al. 2015), and neutrophils isolated from melanoma and renal cell carcinoma (RCC) patients showed increased expression of ARG1 when compared with neutrophils isolated from normal controls (Zea et al. 2005; De Santo et al. 2010). ARG1 has been shown to inhibit T-cell responses by inhibiting re-expression of the ζ-chain of CD3 after TCR-signaling–induced internalization (Rodriguez et al. 2004). A negative correlation was observed between arginase activity and CD3ζ-chain expression in the RCC patient blood, and depletion of neutrophils resulted in recovery of CD3ζ-chain expression and T-cell proliferation (Zea et al. 2005). The neutrophils from the melanoma patients also suppressed lymphocyte proliferation and were associated with a reduction in tumor-specific CD8+ T cells, although in this case the T-cell suppression was linked to IL-10 and serum amyloid A (SAA) (De Santo et al. 2010).
Recently, it was proposed that CTCs instigate a reciprocal activation loop between neutrophils and platelets. Tumor cell-derived mucins bind to P-selectin on platelets and L-selectin on neutrophils initiating cell–cell contact. Interaction of P-selectin with PSGL-1 on neutrophils stimulates release of cathepsin G from the neutrophils, which in turn activates platelets (Shao et al. 2011). Neutrophil extracellular traps (NETs) have also been shown to stimulate platelet aggregation (Fuchs et al. 2010). Thus, platelets and neutrophils may work together to protect CTCs from mechanical and immune-regulated destruction in the vasculature. (See Figure 2 for a summary of the roles of myeloid cells in tumor cell survival and dissemination in the circulation.)
Figure 2.
Myeloid cells protect tumor cells in the circulation. Circulating tumor cells have a higher chance of survival when in clusters. Neutrophil-derived neutrophil extracellular traps (NETs) induce tumor cell aggregation in the circulation and circulating tumor cells release various factors such as ADP, thrombin, TxA2, and proteinases to stimulate platelet–tumor cell aggregation. This tumor cell–platelet clustering induces an invasive phenotype in the tumor cells and the release of IFNγ and TGFβ from platelets, which then suppress antitumor NK cell responses. Additionally, neutrophils themselves cluster with circulating tumor cells and this induces a protumor phenotype in the neutrophils and increases tumor cell proliferation within these clusters. This protects the tumor cells against shear forces and immune-mediated destruction by cytotoxic T and NK cells via neutrophil release of ARG1, IL-10, and SSA. Tumor cells also engage L-selectin on neutrophils and P-selectin on platelets, which causes these cells to interact and neutrophils to release cathepsin G and NETs, which in turn activate platelets.
ARREST AND EXTRAVASATION AT DISTANT ORGANS
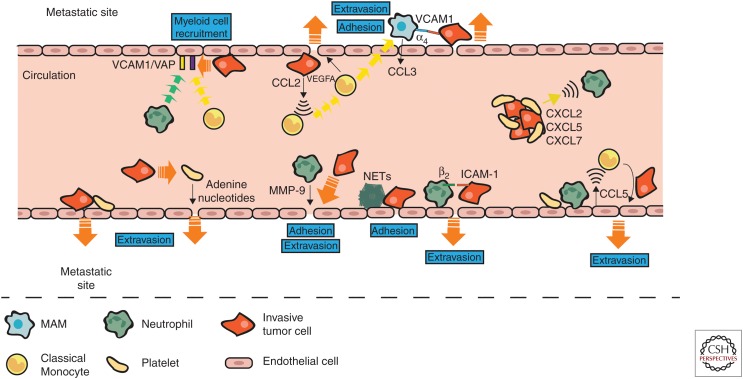
Having successfully disseminated and survived the intravascular environment tumor cells must now arrest and transmigrate from the vessel at the metastatic tissue site. Myeloid cells have been shown to support circulating tumor cell adhesion and transmigration into metastatic sites. For example, murine mammary tumor cell attachment to vessels at metastatic sites induces the endothelial activation markers vascular cell adhesion protein-1 (VCAM-1) and vascular adhesion protein-1 (VAP-1), which in turn recruits myeloid cells. These myeloid cells were found to be essential to tumor cell survival during early metastatic outgrowth (Ferjančič et al. 2013).
Neutrophils have been shown to colocalize with tumor cells in, or near, vessels at sites of metastasis (McDonald et al. 2009; Spicer et al. 2012) and it has been proposed that direct interactions between adherent neutrophils and CTCs increases tumor cell adhesion in the lung (Spicer et al. 2012) and tumor cell arrest in the liver (McDonald et al. 2009). Addition of neutrophils to melanoma cells in a flow migration chamber significantly enhanced tumor cell extravasation when compared with melanoma cells alone and blocking neutrophil tethering reduced melanoma cell adhesion efficacy (Dong et al. 2005). Similar results were seen in a murine model in which it was shown that presence of neutrophils enhanced retention of melanoma cells in the lung. Neutrophils-bound tumor cells and interaction of ICAM-1 and β2 integrin promoted anchoring to the vascular endothelium (Liang and Dong 2008; Huh et al. 2010). This data indicates that on tethering to the vessel, neutrophils capture and/or trap tumor cells and subsequently allow for more efficient tumor cell arrest at distant sites.
Neutrophil-derived NETs may also augment tumor cell adhesion to vessels at distant sites. Indeed, more aggressive mammary tumor models were found to have higher numbers of NETs in the lungs when compared with less aggressive models, and in human breast cancer higher numbers of NETs were seen in TNBC primary tumors, when compared with luminal and ER positive (Park et al. 2016). Induction of NETosis enhanced tumor cell adhesion to neutrophil monolayers in a flow chamber, and inhibition of NET formation reduced this. Further, the NETs appeared to be wrapped around the adherent tumor cells (Cools-Lartigue et al. 2013). Tumor cell trapping by NETs was also visualized in murine lung and liver in real time and NETosis was associated with an increase in metastatic burden in these models (Cools-Lartigue et al. 2013; Najmeh et al. 2017). Moreover, inhibition of NET formation has been shown to reduce metastasis in a number of in vivo models (Cools-Lartigue et al. 2013; Park et al. 2016; Najmeh et al. 2017). Thus, it is possible that it is the neutrophil-derived NETs that trap CTCs at the vessel.
Neutrophils have also been shown to assist tumor cell extravasation via endothelial activation. Neutrophils from tumor-bearing mice increased transendothelial migration of mammary tumor cells in vitro, and this was attenuated on inhibition of MMP-8 and MMP-9 (Spiegel et al. 2016). In vivo, adoptive transfer of neutrophils from MMP-9 knockout mice showed reduced metastasis when compared with neutrophils from WT mice (Spiegel et al. 2016). In this study, the increase in tumor cell extravasation was attributed to neutrophil-mediated effects on the endothelial cells as pretreatment of endothelial cells with neutrophil conditioned medium enhanced transendothelial migration rate in vitro and also induced MMP-9 expression and secretion (Spiegel et al. 2016).
Two circulating monocyte populations have been described: classical “inflammatory” monocytes (IMo, CCR2highLy6C+ in mouse; CCR2highCD14++CD16− in human) and nonclassical “patrolling” monocytes (PMo, CX3CR1highLy6C− in mouse; CXRCR1high CD14+CD16+ in human). Monocytes in cancer have been typically studied as one total population. However, more studies are now assessing the roles of monocyte subpopulations. IMo are typically recruited to sites of inflammation whereas PMo are localized in the microvasculature where they patrol the capillaries (Auffray et al. 2007; Saha and Geissmann 2011). PMo have not been well studied in cancer, however a recent report indicates they may have antitumor functions. In murine models of Lewis ling carcinoma (LLC), melanoma, and mammary tumors, PMo were found to establish early interactions with metastatic tumor cells in the lung, scavenge tumor debris from the lung vasculature, and recruit and activate NK cells leading to increased tumor cell killing (Hanna et al. 2015). The antitumor activity of PMo was only evident during the early stages of metastatic seeding, and not after metastases were already established.
Conversely, IMo appear to have a protumor function. Coculture of human breast cancer cells with monocytes increased cancer cell MMP-9, TNFα, and tissue factor production in vitro (Blot et al. 2003), indicating that monocytes can induce an invasive phenotype in tumor cells. Indeed, studies of lung metastasis have shown that tumor cell arrest at distant tissue vessels induces CCL2 production by the tumor cells, which in turn generates a chemoattractive gradient that recruits CCR2+Ly6C+ monocytes (IMos). The IMos then promote the extravasation of breast carcinoma cells into the lung tissue via VEGFA production (Qian et al. 2011). Additionally, IMo promote tumor cell extravasation via production of MMP-9 (Hiratsuka et al. 2002). Given that VEGF and MMP9 can increase vascular permeability it is possible that IMo may induce vascular leakiness leading to enhanced tumor cell extravasation into the lung. It also appears that IMo can manipulate the permeability of the endothelium at metastatic sites. In a murine model of colorectal cancer (CRC), IMos were recruited to lungs in which they promoted transendothelial migration of tumor cells via induction of E-selectin–dependent endothelial retractions and modulation of tight junctions through dephosphorylation of VE-cadherin (Häuselmann et al. 2016). In another study, Gr-1+CD11b+ cells (which contain neutrophils and monocytes/macrophages) secreted MMP-9, which induced a disruption and diffusion of VE–cadherin expression on endothelial cell monolayers thus increasing permeability (Yan et al. 2010). These data indicated that both neutrophils and monocytes may increase CTC extravasation possibly by modifying the permeability of endothelial cell barrier and/or inducing vascular leakiness.
A unique population of macrophages, termed metastasis-associated macrophages (MAMs) have been found in metastatic lungs that are distinct from resident lung macrophages, and interact with CTCs at lung vessels during extravasation (Qian et al. 2009). MAMs are derived from circulating Ly6C+ monocytes whose accumulation is increased during metastatic growth (Qian et al. 2011; Kitamura et al. 2018). Tumor cells interact with MAMs at the site of the vessel, which promotes tumor cell extravasation and loss of MAMs significantly reduced the number of extravasated tumor cells (Qian et al. 2009). MAMs are activated by CCL2 to produce CCL3, and this enhances the interaction between VCAM-1 on tumor cells and α4 integrin on MAMs resulting in a reciprocal augmented retention and extravasation of macrophages and CTCs (Kitamura et al. 2018). Other “metastasis-associated macrophage” populations have been reported in the liver and are required for colorectal cancer metastasis (Zhao et al. 2013) and PDAC metastasis (Nielsen et al. 2016).
Platelets can also mediate tumor cell arrest and adhesion to the vascular endothelium, as well as transmigration of CTCs. In vitro platelets were shown to enhance colon adenocarcinoma cell tethering to the endothelium (Burdick and Konstantopoulos 2004). Pretreatment of mouse prostate and mammary tumor cells with platelets increased their invasiveness and transendothelial migration in vitro and increased vascular permeability and metastatic burden in vivo. Disruption of platelet–tumor cell interactions ablated this (Ward et al. 2018). Platelet-derived adenine nucleotides can also increase permeability of the endothelium thus allowing for enhanced transendothelial migration of tumor cells (Schumacher et al. 2013). This indicates that platelets enhance CTC transendothelial migration via direct effects on the tumor cells themselves, but also by modifying the endothelial barrier. In P-selectin deficient mice, platelets failed to adhere to tumor cells resulting in fewer platelet–tumor cell complexes in the lungs and fewer metastases (Kim et al. 1998; Becker et al. 2017).
Platelets, neutrophils, and monocytes can all regulate and recruit each other. Hence, it is likely that reciprocal signaling occurs between these cells during metastasis that can enhance or promote CTC adhesion and transmigration into the metastatic site. In support of this, in a murine model of colon cancer, platelet–tumor aggregates were observed in the vicinity of neutrophils. Platelet–tumor cell aggregates were found in the lungs 1 min after intravenous injection of tumors cells, whereas neutrophils progressively increased in number over the next 30 min indicating that the platelet–tumor aggregates recruit neutrophils. Indeed, platelets were shown to produce CXCL2, CXCL5, and CXCL7 on contact with tumor cells and blockade of the CXCL5/7 receptor, CXCR2, or depletion of platelets or neutrophils significantly reduced metastatic seeding and progression (Labelle et al. 2014). In another study, human microvascular endothelial cells were activated by colorectal cancer cells only when in the presence of platelets and neutrophils, and this activation resulted in up-regulation of CCL5 and an increase in monocyte migration and adherence to the endothelial cells (Läubli et al. 2009). In a mouse model of colorectal cancer, CCL5 also recruited monocytes to the metastatic lung and the majority of CCL5 expression in metastatic lungs was from CD31+ endothelial cells suggesting that in the presence of tumor cells platelets and neutrophils initiate activation of endothelium and this in turn recruits monocytes. Blockade of this monocyte recruitment significantly reduced metastasis (Läubli et al. 2009). (See Figure 3 for a summary of the roles of myeloid cells in tumor cell arrest and extravasation at metastatic sites.)
Figure 3.
Myeloid cells promote tumor cell extravasation into metastatic sites. Tumor cell attachment to vessels induces expression of vascular cell adhesion protein-1 (VCAM-1) and vascular adhesion protein (VAP) on the endothelium and secretion of CCL2 from the tumor cells, which then recruits classical monocytes (IMo) and neutrophils. IMo produce vascular endothelial growth factor (VEGF), which stimulates vascular leakiness and enhances tumor cell extravasation. Tumor cell/platelet aggregates release neutrophil and monocyte recruitment factors such as CXCL2, CXCL5, CXCL7, and CCL5, which direct these myeloid cells to sites of tumor cell extravasation where they promote tumor cell adhesion to vessel walls and extravasation via production of proteases such as MMP-9. Neutrophils also help circulating tumor cells to adhere to vessels by tethering them via β2/ICAM-1 binding and NETs trap circulating tumor cells at vessel walls. Platelets also trap circulating tumor cells at vessels and enhance their extravasation via release of adenine nucleotides, which enhance permeability of the endothelium. IMo extravasate and differentiate into metastasis-associated macrophages (MAMs). MAM α4 integrin-binding to VCAM-1 on tumor cells promotes extravasation of tumor cells into metastatic sites.
SURVIVAL AND METASTATIC GROWTH
For successful metastatic growth, extravasated tumor cells must survive in the foreign microenvironment of the distant tissue, one that usually differs greatly from the primary site. It has been proposed that cancers overcome this problem by establishment of a “premetastatic niche,” whereby primary tumors release systemic signals that induce changes at the secondary site to make these distant microenvironments favorable for metastatic cell seeding (Psaila and Lyden 2009). For example, by the LOX-mediated up-regulation of fibronectin from resident fibroblasts and recruitment of myeloid cells (Erler et al. 2009), or through recruitment of VEGFR1+ hematopoietic progenitor cells to metastatic sites (Kaplan et al. 2005).
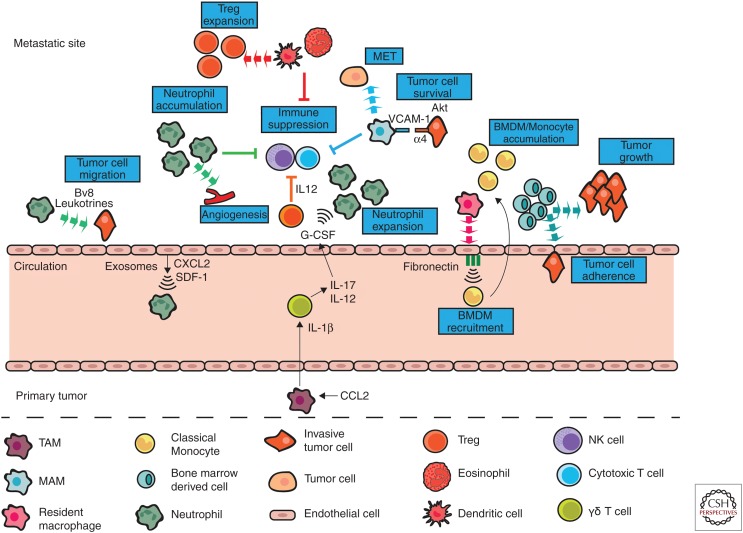
A study in the KEP mammary tumor model showing the importance of neutrophils during early steps of the metastatic cascade, and the fact that these neutrophils produced Bv8, a protein involved in tumor cell migration, led the investigators to speculate that neutrophils may be involved in establishing the premetastatic niche (Coffelt et al. 2015). Indeed, primary tumor-derived exosomal RNAs induced epithelial cell-mediated chemokine secretion in the premetastatic lung that resulted in recruitment of neutrophils in models of LLC and melanoma (Liu et al. 2016). In the PyMT mammary tumor model, neutrophils were found to accumulate in the lungs before infiltration by tumor cells and it was proposed that neutrophil-derived leukotrienes altered the cancer cells to favor metastasis-initiating cells (Wculek and Malanchi 2015). Neutrophils have also been shown to accumulate in premetastatic livers of mice bearing colorectal tumors (Wang et al. 2017) and neutrophil accumulation in the premetastatic liver has been shown to be required for pancreatic cancer metastasis (Steele et al. 2016). SDF-1/CXCR4-mediated neutrophil recruitment to the liver was shown to be essential for TIMP-1-induced premetastatic niche formation in a model of colon cancer (Seubert et al. 2015).
Monocytes and macrophages are also recruited to premetastatic sites. Ly6C+ monocytes increased in the premetastatic lungs in a melanoma model (van Deventer et al. 2013) and monocyte/macrophage recruitment to premetastatic lungs was shown to be essential for circulating melanoma cell homing and survival in a process involving coagulation (Gil-Bernabé et al. 2012). It has been reported that bone marrow-derived cells form clusters in the premetastatic lung and these clusters then enhance the adherence and promote the growth of tumor cells (Kaplan et al. 2005). An increase in macrophages has also been observed in premetastatic livers (Wang et al. 2017) and exosomes derived from human pancreatic cancer cell lines expressing macrophage migration inhibitory factor (MIF) were shown to influence resident Kupffer cells to produce TGFβ that then induced up-regulation of fibronectin production leading to recruitment of bone marrow-derived macrophages (Costa-Silva et al. 2015).
TAMs can even influence the premetastatic site from the primary tumor. For example, TAMs can produce systemic factors that then affect neutrophil recruitment to sites of metastasis. In the KEP mammary tumor model it was found that CCL2 induces IL1β expression by TAMs that contributes to mammary tumor-induced immunosuppression at distant sites. TAM-derived IL1β led to the systemic induction of IL-17 and IL-12 by γδ T cells, G-CSF mediated expansion of immunosuppressive neutrophils in the lung and the suppression of CD8+ T cells (Coffelt et al. 2015; Kersten et al. 2017). Furthermore, TAM-derived CXCL1 has been shown to induce accumulation of neutrophils at pre-metastatic sites in a colorectal cancer model (Wang et al. 2017).
Although we know that myeloid cells are important for the formation of metastasis in preclinical studies, little is known about the mechanisms by which they support metastatic cell growth. Nonetheless, some recent studies have shed light on how myeloid cells enhance growth of metastases. MAMs are important for metastatic growth as depletion of macrophages resulted in a reduced metastatic load (Qian et al. 2009). One possible mechanism for this is MAM-induced enhanced survival via induction of Akt activation that protects cancer cells from proapoptotic cytokines. This activation was reported to be triggered by VCAM-1 tethering of MAMs to circulating mammary tumor cells via α4 integrins and subsequent VCAM-1 clustering on the cell surface (Chen et al. 2011; Kitamura et al. 2018). FLT1 (VEGFR1) signaling in MAMs has also been shown to support metastatic tumor cell survival through the induction of CSF1 after metastatic seeding (Qian et al. 2015). Similarly, in murine models of colorectal and pancreatic cancer metastasis neutrophil depletion significantly reduced microvessel density and vascular branching and this was associated with reduced metastatic colony growth (Gordon-Weeks et al. 2017). Gr-1+CD11b+ cells have also been shown to support metastatic tumor cell growth via PDGF-BB–mediated angiogenesis in the lung of mammary tumor bearing mice (Hsu et al. 2019), and the production of CCL9, which supported tumor cell survival in the lungs of mammary and melanoma tumor models (Yan et al. 2015). Unfortunately, the monocytic or granulocytic identity of these cells was not defined.
One very important step for metastatic tumor cell survival is the return to an epithelial phenotype via mesenchymal–epithelial transition (MET) to regain the ability of proliferation and differentiation. Myeloid cell conditioned media can induce up-regulation of the epithelial cell markers E-cadherin and inhibition of the mesenchymal marker vimentin on tumor cells in vitro. In vivo depletion of myeloid cells was associated with a switch from vimentinlow/E-cadherin+ microscopic lesions to vimentinhigh/E-cadherin lesions indicating a failure of MET. This was also associated with a significant reduction in metastatic tumor growth (Gao et al. 2012). Macrophages in metastatic lungs have been implicated in inducing MET in tumor cells in a mammary tumor model via IL-35 activated JAK-STAT6 signaling, although much of this work is yet to be confirmed in vivo (Lee et al. 2018).
Finally, tumor cells at metastatic sites must also avoid immune-mediated destruction. In a model of PDAC, neutrophil depletion resulted in reduced metastasis and a marked increase of T lymphocytes. CXCR2 inhibition showed a similar result and also substantially enhanced sensitivity to anti-PD-1 immunotherapy (Steele et al. 2016) suggesting that neutrophils were suppressing T-cell recruitment to the metastatic site. Metastasis-associated neutrophils also suppress proliferation of cytotoxic CD8+ T cells (Coffelt et al. 2015), and in a model of cecal cancer, neutrophils isolated from the premetastatic liver were able to inhibit proliferation of and production of IFNγ by CD8+ T cells in vitro (Wang et al. 2017). MAM precursor cells (MAMPC), a transient stage between recruited monocytes and fully mature MAMS, suppress T-cell cytotoxicity in models of mammary metastasis to the lung. Mechanistically, this is via a ROS-and CTLA4- mediated mechanism, in MAMPC and MAMs, respectively, (Kitamura et al. 2018). Gr-1+CD11b+ myeloid cells create an immunosuppressive environment at metastatic sites via the secretion of suppressive factors such as b-FGF, IGF-1, IL-10, IL-4, MMP-9, and S100A8/A9; but again the identity of these cells has not been elucidated (Hiratsuka et al. 2006; Yan et al. 2010). In breast cancer models, it was reported that increased frequency of Gr-1+CD115+CCR2highCX3CR1low cells (likely IMos) in the lungs of tumor-bearing mice was associated with an increase of CD25+FoxP3+ Tregs and a decrease in NK cell numbers. Furthermore, it was shown that NK cell activity was suppressed in the presence of these myeloid cells and Tregs (Eisenblaetter et al. 2017).
Recently, an immunosuppressive metastases-associated dendritic cell (DC) subpopulation has been reported. In mice bearing PDAC tumors, an enrichment of DCs was seen bordering micrometastases and these expressed various immunosuppressive molecules. These DCs were shown to expand Treg numbers around the micrometastases and suppress CD8+ T cells via the PD-L2 pathway (Kenkel et al. 2017). It has also been proposed that tumor-derived exosomes can influence DCs toward a protumor phenotype (Shen et al. 2017). A recent study suggest that eosinophils may also suppress antitumor immune responses in the metastatic lung, and hence promote metastatic growth. Inhibition of IL-5 (a key eosinophil factor) reduced experimental lung metastasis of LLC, melanoma, and adenocarcinoma and this was associated with a reduction in eosinophil number in the lung. In this study, eosinophil-derived CCL22 recruited Tregs to the lung, which then suppressed NK cell activation and altered macrophage phenotypes toward protumor (Zaynagetdinov et al. 2015). (See Figure 4 for a summary of the roles of myeloid cells in tumor survival and growth at metastatic sites.)
Figure 4.
Myeloid cells promote tumor cell survival and growth at metastatic sites. Tumor-derived exosomes recruit neutrophils to metastatic sites by inducing CXCL2 and SDF-1 secretion by epithelial cells. Once recruited, neutrophils secrete Bv8 and leukotrienes, which enhance tumor cell migration into the tissue. Neutrophils also stimulate new blood vessel formation, which promotes growth of metastatic tumor cells by giving them access to nutrients and oxygen. Neutrophils, metastasis-associated DCs, eosinophils, and MAMs suppress antitumor NK and cytotoxic T-cell responses. Dendritic cells (DCs) and eosinophils also expand Treg populations leading to further suppression of antitumor immune responses. Monocytes (IMo) and BMDMs are recruited to sites of metastasis via resident macrophage-induced up-regulation of fibronectin on endothelial cells. IMos then differentiate into MAMs, which support tumor cell survival by inducing AKT expression in the tumor cells on binding of VCAM-1 to α4 integrins. MAMs also induce transition from a mesenchymal state to an epithelial one in the tumor cells thus allowing them to proliferate. Lastly TAM-derived IL-1β (released from the primary tumor into the circulation) instigates IL-17 and IL-12 production in circulating γδ T cells resulting in G-CSF-mediated neutrophil recruitment to metastatic sites and suppression of antitumor immune responses.
Not all disseminated tumor cells grow into overt metastases immediately after colonization of the metastatic site. Some will become dormant and remain as either circulating tumor cells, individual disseminated cells, or micrometastases, where the cancer cells are present, but not proliferating (Aguirre-Ghiso 2007). Dormant cells can be present for months or even decades before they awaken. The mechanisms behind the awakening and growth of dormant cells are still largely unknown. However, inflammation has been implicated in this process (De Cock et al. 2016). In a model of melanoma, neutrophil-derived MMP-9 was found to be essential for triggering angiogenesis during the growth of dormant micrometastases (Luo et al. 2016). In a recent study, sustained inflammation was found to increase neutrophil infiltration and NET formation at metastatic sites in mouse models of breast and prostate cancer. These neutrophil-derived NETs concentrated netrophil elastase (NE) and MMP-9 at laminin sites, leading to remodeling of the laminin, which revealed a epitope that triggered awakening and proliferation of dormant cancer cells via integrin activation and FAK/ERK/MLCK/YAP signaling (Albrengues et al. 2018).
CONCLUSIONS
Significant advances have been made in treating cancer but sadly metastasis is still essentially untreatable. The studies described above, in a number of different animal models, have now established that myeloid cells are critical in metastatic spread of tumors. It is clear that myeloid cells, in particular TAMs, play a key role in tumor invasion and egress into the circulation from the primary tumor site. However, the mechanisms by which myeloid cells support and promote metastasis in the circulation and at the metastatic site remain largely unknown. Preclinical data suggest that in some cases, but not all, myeloid cells cluster with tumor cells in the circulation where they protect tumor cells from destruction by shear forces and immune-mediated attacks, as well as provide survival signals. Furthermore, it appears that platelets, neutrophils, and monocytes work together to assist with tumor cell arrest and extravasation into metastatic sites. Myeloid cells are also recruited early to sites of metastasis, often before the arrival of tumor cells, in which they can create a permissive niche for metastatic cells to grow. Some of the currently reported functions of myeloid cells at metastatic sites include induction of fibrin deposition, suppression of immune cells, induction of MET, protection against apoptosis, induction of blood vessel development and suppression of antitumor immune responses. However, many of these functions have yet to be established in vivo and there is still much to be discovered about the mechanisms by these cells promote and/or enhance metastatic growth.
On a cautionary note, many studies, particularly at the metastatic site, use selected homogenous cell lines that do not resemble truly autochthonous tumor metastasis. These tumors will contain heterogeneous populations of cells with different ranges of phenotype from dormant to aggressively growing cells. It is important, therefore to validate results using cell lines in spontaneously evolving tumor models (Qian et al. 2009,2015; Kitamura et al. 2015, 2018).
Because of technical reasons, most of the studies described above have focused on a particular cell type, or a group of cell types defined by a single marker such as the generic Gr-1. These studies have been informative but they cannot dissect the complexity of different subpopulations nor their dynamic behavior in response to tumor cells or damage induced signals. This is particularly apparent with macrophages in which both pro- and antitumoral types existing within the tumor (Zhu et al. 2017). Indeed, recent evidence shows the need to target TAM protumoral roles while preserving their antitumoral activities to induce an effective antitumor immune response through checkpoint inhibition (Hoves et al. 2018). Advances in single-cell sequencing, more sophisticated multichannel fluorescence-activated cell sorting (FACS) or similar methods using mass cytometry such as CyTOF, together with high-resolution multiplex immunohistochemical/fluorescent imaging will help define cancer-associated immune cell heterogeneity, their evolution as tumors become metastatic, and the differences between metastases and primary tumors. The response of these immune populations in their “niches” to immunotherapy or conventional therapies defined by these methods may very well lead to identification of unique subpopulations that could be specifically targeted therapeutically (Cassetta and Pollard 2018).
Experimentally, and ultimately clinically, real-time imaging will be enormously beneficial to understand these changing microenvironments, particularly with refinements in MRI, PET, and use of smart-enzyme activated probes or probes for specific biomarkers (Condeelis and Weissleder 2010). In mouse models, advanced multiphoton microscopy will expand the ability to visualize many different cell types in real-time and the use of indwelling windows (Entenberg et al. 2018) will allow their visualization over tumor progression and in response to therapy. In this case, seeing-is-believing and the true interaction between cells can be determined. For example, it will be able us to attribute whether the education of macrophages to promote tumor cell invasion by IL-4 produced from CD4+ T cells results from direct interaction or through long-term signals (DeNardo et al. 2009). Similarly, these methods will show whether cells in the circulation are truly chaperoned by platelets or neutrophils or that these observations are the result of isolation artifacts. Indeed, immune cells never act alone and there is dynamic interplay between the innate and acquired immune systems, which is only recently being appreciated and yet to be visualized.
Several mechanisms have been described whereby macrophages and neutrophil promote steps in extravasation through different mechanisms. In evolving tumors, all these mechanism might be in play with some dominating whereas others might be minor unless the dominant one is blocked. Similar problems arise for overcoming immunosuppression, for example, we showed that recruited IMos and MAMs suppress antitumor T-cell killing using different mechanisms dependent on stage of differentiation (Kitamura et al. 2018). And this is only one cell type! Furthermore, most systems use redundancy and resilience to prosper. Tumor cells are masters at these activities, mutating to exploit compensatory or alternative mechanisms to thrive.
Immunotherapy is a beacon of hope for many patients with startlingly beneficial results in some cases. These methods do bring the promise of treating established metastases from which most patients die, but current data suggests that multipronged approaches will be required to enhance immunotherapies. We would contend that to be successful a greater understanding of the complexity of myeloid-suppression of cytotoxic cells in the metastatic environment will be needed in mouse and nonhuman primates and then translation of these data into human clinical trials.
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Aguirre-Ghiso JA. 2007. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7: 834–846. 10.1038/nrc2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, et al. 2018. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361: eaao4227 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. 2007. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci 104: 20262–20267. 10.1073/pnas.0706438104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, Condeelis JS. 2018. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep 23: 1239–1248. 10.1016/j.celrep.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, et al. 2003. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc Natl Acad Sci 100: 14205–14210. 10.1073/pnas.2234372100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. 2001. Inflammation and cancer: back to Virchow? Lancet 357: 539–545. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-Del-Castillo C, Warshaw AL, Thayer SP, Keck T. 2011. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 14: 235–243. 10.1007/s10456-011-9207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KA, Beckmann N, Adams C, Hessler G, Kramer M, Gulbins E, Carpinteiro A. 2017. Melanoma cell metastasis via P-selectin-mediated activation of acid sphingomyelinase in platelets. Clin Exp Metastasis 34: 25–35. 10.1007/s10585-016-9826-6 [DOI] [PubMed] [Google Scholar]
- Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. 2011. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 179: 1455–1470. 10.1016/j.ajpath.2011.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot E, Chen W, Vasse M, Paysant J, Denoyelle C, Pillé JY, Vincent L, Vannier JP, Soria J, Soria C. 2003. Cooperation between monocytes and breast cancer cells promotes factors involved in cancer aggressiveness. Br J Cancer 88: 1207–1212. 10.1038/sj.bjc.6600872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. 2012. Intratumoral macrophages contribute to epithelial–mesenchymal transition in solid tumors. BMC Cancer 12: 35 10.1186/1471-2407-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MM, Konstantopoulos K. 2004. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol 287: C539–C547. 10.1152/ajpcell.00450.2003 [DOI] [PubMed] [Google Scholar]
- Casbon A-J, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, Passegué E, Werb Z. 2015. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci 112: E566–E575. 10.1073/pnas.1424927112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassetta L, Pollard JW. 2018. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17: 887–904. 10.1038/nrd.2018.169 [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang XHF, Massagué J. 2011. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20: 538–549. 10.1016/j.ccr.2011.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Kim JK, Yang YJ, Kim P, Yoon KH, Yun SH. 2015. Urokinase exerts antimetastatic effects by dissociating clusters of circulating tumor cells. Cancer Res 75: 4474–4482. 10.1158/0008-5472.CAN-15-0684 [DOI] [PubMed] [Google Scholar]
- Clavel C, Polette M, Doco M, Binninger I, Birembaut P. 1992. Immunolocalization of matrix metallo-proteinases and their tissue inhibitor in human mammary pathology. Bull Cancer 79: 261–270. [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, et al. 2015. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522: 345–348. 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. 2006. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124: 263–266. 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Condeelis J, Weissleder R. 2010. In vivo imaging in cancer. Cold Spring Harb Perspect Biol 2: a003848 10.1101/cshperspect.a003848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. 2013. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 123: 3446–3458. 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. 2015. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17: 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotechini T, Medler TR, Coussens LM. 2015. Myeloid cells as targets for therapy in solid tumors. Cancer J 21: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cock JM, Shibue T, Dongre A, Keckesova Z, Reinhardt F, Weinberg RA. 2016. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res 76: 6778–6784. 10.1158/0008-5472.CAN-16-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. 2012. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci 109: 13076–13081. 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. 2009. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16: 91–102. 10.1016/j.ccr.2009.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Roca C, Naldini L. 2003. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 9: 789–795. [DOI] [PubMed] [Google Scholar]
- De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, Salio M, Middleton M, Cerundolo V. 2010. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol 11: 1039–1046. 10.1038/ni.1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Slattery MJ, Liang S, Peng HH. 2005. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol Cell Biomech 2: 145–159. [PMC free article] [PubMed] [Google Scholar]
- Eisenblaetter M, Flores-Borja F, Lee JJ, Wefers C, Smith H, Hueting R, Cooper MS, Blower PJ, Patel D, Rodriguez-Justo M, et al. 2017. Visualization of tumor-immune interaction—target-specific imaging of S100A8/A9 reveals pre-metastatic niche establishment. Theranostics 7: 2392–2401. 10.7150/thno.17138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, Jones J, Baccay F, Oktay M, Condeelis J. 2018. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat Methods 15: 73–80. 10.1038/nmeth.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. 2009. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15: 35–44. 10.1016/j.ccr.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, et al. 2014. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-β1-induced epithelial–mesenchymal transition in hepatocellular carcinoma. Cancer Lett 352: 160–168. 10.1016/j.canlet.2014.05.008 [DOI] [Google Scholar]
- Ferjančič Š, Gil-Bernabé AM, Hill SA, Allen PD, Richardson P, Sparey T, Savory E, McGuffog J, Muschel RJ. 2013. VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood 121: 3289–3297. 10.1182/blood-2012-08-449819 [DOI] [PubMed] [Google Scholar]
- Fidler IJ. 2003. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3: 453–458. 10.1038/nrc1098 [DOI] [PubMed] [Google Scholar]
- Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al. 2015. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol 46: 587–596. 10.3892/ijo.2014.2761 [DOI] [PubMed] [Google Scholar]
- Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. 2010. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci 107: 15880–15885. 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaida MM, Steffen TG, Günther F, Tschaharganeh DF, Felix K, Bergmann F, Schirmacher P, Hänsch GM. 2012. Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. Eur J Immunol 42: 3369–3380. 10.1002/eji.201242628 [DOI] [PubMed] [Google Scholar]
- Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, et al. 2012. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res 72: 1384–1394. 10.1158/0008-5472.CAN-11-2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM, Yu ZL, Zhao YF. 2018. Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma. Oncol Rep 40: 2558–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Bernabé AM, Ferjančič Š, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Amirkhosravi A, Francis JL, et al. 2012. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 119: 3164–3175. 10.1182/blood-2011-08-376426 [DOI] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. 2010. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24: 241–255. 10.1101/gad.1874010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks AN, Lim SY, Yuzhalin AE, Jones K, Markelc B, Kim KJ, Buzzelli JN, Fokas E, Cao Y, Smart S, et al. 2017. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology 65: 1920–1935. 10.1002/hep.29088 [DOI] [PubMed] [Google Scholar]
- Große-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hänsch GM, Gaida MM. 2012. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol 2012: 720768, 1–12 10.1155/2012/720768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, et al. 2017. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun 8: 310 10.1038/s41467-017-00411-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, et al. 2015. Patrolling monocytes control tumor metastasis to the lung. Science 350: 985–990. 10.1126/science.aac9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. 2015. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov 5: 932–943. 10.1158/2159-8290.CD-15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, Bauer AT, Schneider SW, Borsig L. 2016. Monocyte induction of E-selectin–mediated endothelial activation releases VE–cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res 76: 5302–5312. 10.1158/0008-5472.CAN-16-0784 [DOI] [Google Scholar]
- Heinmöller E, Schropp T, Kisker O, Simon B, Seitz R, Weinel RJ. 1995. Tumor cell-induced platelet aggregation in vitro by human pancreatic cancer cell lines. Scand J Gastroenterol 30: 1008–1016. 10.3109/00365529509096346 [DOI] [PubMed] [Google Scholar]
- Hildenbrand R, Wolf G, Böhme B, Bleyl U, Steinborn A. 1999. Urokinase plasminogen activator receptor (CD87) expression of tumor-associated macrophages in ductal carcinoma in situ, breast cancer, and resident macrophages of normal breast tissue. J Leukoc Biol 66: 40–49. 10.1002/jlb.66.1.40 [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. 2002. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2: 289–300. 10.1016/S1535-6108(02)00153-8 [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. 2006. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8: 1369–1375. 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- Honn KV, Tang DG, Crissman JD. 1992. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev 11: 325–351. 10.1007/BF01307186 [DOI] [PubMed] [Google Scholar]
- Hoves S, Ooi C-H, Wolter C, Sade H, Bissinger S, Schmittnaegel M, Ast O, Giusti AM, Wartha K, Runza V, et al. 2018. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med 215: 859–876. 10.1084/jem.20171440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YL, Yen MC, Chang WA, Tsai PH, Pan YC, Liao SH, Kuo PL. 2019. CXCL17-derived CD11b+Gr-1+ myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res 21: 23 10.1186/s13058-019-1114-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Shen M, Zhang P, Zheng C, Pang Z, Zhu L, Du J. 2015. Intratumoral neutrophil granulocytes contribute to epithelial–mesenchymal transition in lung adenocarcinoma cells. Tumor Biol 36: 7789–7796. 10.1007/s13277-015-3484-1 [DOI] [PubMed] [Google Scholar]
- Hu Y, He MY, Zhu LF, Yang CC, Zhou ML, Wang Q, Zhang W, Zheng YY, Wang DM, Xu ZQ, et al. 2016. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J Exp Clin Cancer Res 35: 12 10.1186/s13046-015-0281-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. 2010. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70: 6071–6082. 10.1158/0008-5472.CAN-09-4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S, Bochner BS, Konstantopoulos K. 2001. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J Immunol 167: 5986–5993. 10.4049/jimmunol.167.10.5986 [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. 2009. Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252. 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. 2005. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827. 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel JA, Tseng WW, Davidson MG, Tolentino LL, Choi O, Bhattacharya N, Seeley ES, Winer DA, Reticker-Flynn NE, Engleman EG. 2017. An immunosuppressive dendritic cell subset accumulates at secondary sites and promotes metastasis in pancreatic cancer. Cancer Res 77: 4158–4170. 10.1158/0008-5472.CAN-16-2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten K, Coffelt SB, Hoogstraat M, Verstegen NJM, Vrijland K, Ciampricotti M, Doornebal CW, Hau CS, Wellenstein MD, Salvagno C, et al. 2017. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. Oncoimmunology 6: e1334744 10.1080/2162402X.2017.1334744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. 1998. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci 95: 9325–9330. 10.1073/pnas.95.16.9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Qian B-Z, Soong D, Cassetta L, Noy R, Sugano G, Kato Y, Li J, Pollard JW. 2015. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med 212: 1043–1059. 10.1084/jem.20141836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Doughty-Shenton D, Cassetta L, Fragkogianni S, Brownlie D, Kato Y, Carragher N, Pollard JW. 2018. Monocytes differentiate to immune suppressive precursors of metastasis-associated macrophages in mouse models of metastatic breast cancer. Front Immunol 8: 2004 10.3389/fimmu.2017.02004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Placke T, Salih HR. 2009. Platelet-derived transforming growth factor-β down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 69: 7775–7783. 10.1158/0008-5472.CAN-09-2123 [DOI] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. 2011. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590. 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. 2014. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci 111: E3053–E3061. 10.1073/pnas.1411082111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, Weinberg RA. 2017. Emerging biological principles of metastasis. Cell 168: 670–691. 10.1016/j.cell.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. 2014. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoui D, Movahedi K, van Overmeire E, van den Bossche J, Schouppe E, Mommer C, Nikolaou A, Morias Y, de Baetselier P, van Ginderachter JA. 2011. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 55: 861–867. 10.1387/ijdb.113371dl [DOI] [PubMed] [Google Scholar]
- Läubli H, Spanaus KS, Borsig L. 2009. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood 114: 4583–4591. 10.1182/blood-2008-10-186585 [DOI] [PubMed] [Google Scholar]
- Lee CC, Lin JC, Hwang WL, Kuo YJ, Chen HK, Tai SK, Lin CC, Yang MH. 2018. Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat Commun 9: 3763 10.1038/s41467-018-06268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L, Li W, Li ZY, Mao YX, Zhang YT, Zhao YM, Chen K, Duan WM, Tao M. 2013. Inhibition of MCF-7 breast cancer cell-induced platelet aggregation using a combination of antiplatelet drugs. Oncol Lett 5: 675–680. 10.3892/ol.2012.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Dong C. 2008. Integrin VLA-4 enhances sialyl-Lewisx/a-negative melanoma adhesion to and extravasation through the endothelium under low flow conditions. Am J Physiol Cell Physiol 295: C701–C707. 10.1152/ajpcell.00245.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. 2001. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193: 727–740. 10.1084/jem.193.6.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. 2003. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol 163: 2113–2126. 10.1016/S0002-9440(10)63568-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Li J, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue X, Pollard JW. 2006. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66: 11238–11246 [DOI] [PubMed] [Google Scholar]
- Lin EY, Li Jf, Bricard G, Wang W, Deng Y, Sellers R, Porcelli SA, Pollard JW. 2007. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol 1: 288–302. 10.1016/j.molonc.2007.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ubukata H, Tabuchi T, Takemura A, Motohashi G, Nishimura M, Satani T, Hong J, Katano M, Nakada I, et al. 2009. It is possible that tumour-infiltrating granulocytes promote tumour progression. Oncol Rep 22: 29–33. [DOI] [PubMed] [Google Scholar]
- Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. 2013. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 93: 844–854. 10.1038/labinvest.2013.69 [DOI] [PubMed] [Google Scholar]
- Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X. 2016. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 30: 243–256. 10.1016/j.ccell.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Liu JY, Peng C, Yang GF, Hu WQ, Yang XJ, Huang CQ, Xiong B, Li Y. 2017. Distribution pattern of tumor associated macrophages predicts the prognosis of gastric cancer. Oncotarget 8: 92757–92769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, et al. 2014. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol 16: 1105–1117. 10.1038/ncb3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Feng XX, Luo C, Wang Y, Li D, Shu Y, Wang SS, Qin J, Li YC, Zou JM, et al. 2016. 14,15-EET induces the infiltration and tumor-promoting function of neutrophils to trigger the growth of minimal dormant metastases. Oncotarget 7: 43324–43336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Darb-Esfahani S, Meyer AS, Hübner K, Rom J, Sohn C, Braicu I, Sehouli J, Hänsch GM, Gaida MM. 2016. Neutrophil granulocytes in ovarian cancer—induction of epithelial-to-mesenchymal-transition and tumor cell migration. J Cancer 7: 546–554. 10.7150/jca.14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, et al. 2011. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19: 512–526. 10.1016/j.ccr.2011.02.005 [DOI] [PubMed] [Google Scholar]
- McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. 2009. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer 125: 1298–1305. 10.1002/ijc.24409 [DOI] [PubMed] [Google Scholar]
- Medina C, Harmon S, Inkielewicz I, Santos-Martinez MJ, Jones M, Cantwell P, Bazou D, Ledwidge M, Radomski MW, Gilmer JF. 2012. Differential inhibition of tumour cell-induced platelet aggregation by the nicotinate aspirin prodrug (ST0702) and aspirin. Br J Pharmacol 166: 938–949. 10.1111/j.1476-5381.2011.01794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto-Kamata R, Mizoguchi S, Ichisugi T, Yui S. 2012. Cathepsin G induces cell aggregation of human breast cancer MCF-7 cells via a 2-step mechanism: catalytic site-independent binding to the cell surface and enzymatic activity-dependent induction of the cell aggregation. Mediators Inflamm 2012: 456462 10.1155/2012/456462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan MG, Lanier LL. 2016. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16: 7–19. 10.1038/nrc.2015.5 [DOI] [PubMed] [Google Scholar]
- Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE, et al. 2017. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer 140: 2321–2330. 10.1002/ijc.30635 [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Danø K. 1996. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer 65: 57–62. [DOI] [PubMed] [Google Scholar]
- Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, et al. 2016. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol 18: 549–560. 10.1038/ncb3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G, Van Damme J. 2004. The countercurrent principle in invasion and metastasis of cancer cells. Recent insights on the roles of chemokines. Int J Dev Biol 48: 519–527. 10.1387/ijdb.041796go [DOI] [PubMed] [Google Scholar]
- Orellana R, Kato S, Erices R, Bravo ML, Gonzalez P, Oliva B, Cubillos S, Valdivia A, Ibañez C, Brañes J, et al. 2015. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer 15: 290 10.1186/s12885-015-1304-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. 2005. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105: 178–185. 10.1182/blood-2004-06-2272 [DOI] [PubMed] [Google Scholar]
- Pang JH, Coupland LA, Freeman C, Chong BH, Parish CR. 2015. Activation of tumour cell ECM degradation by thrombin-activated platelet membranes: potentially a P-selectin and GPIIb/IIIa-dependent process. Clin Exp Metastasis 32: 495–505. 10.1007/s10585-015-9722-5 [DOI] [PubMed] [Google Scholar]
- Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, et al. 2016. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 8: 361ra138 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TX, Pennington CJ, Almholt K, Christensen IJ, Nielsen BS, Edwards DR, Rømer J, Danø K, Johnsen M. 2005. Extracellular protease mRNAs are predominantly expressed in the stromal areas of microdissected mouse breast carcinomas. Carcinogenesis 26: 1233–1240. 10.1093/carcin/bgi065 [DOI] [PubMed] [Google Scholar]
- Piccard H, Muschel RJ, Opdenakker G. 2012. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 82: 296–309. 10.1016/j.critrevonc.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Pignatelli J, Bravo-Cordero JJ, Roh-Johnson M, Gandhi SJ, Wang Y, Chen X, Eddy RJ, Xue A, Singer RH, Hodgson L, et al. 2016. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/Mena INV-initiated invadopodium formation. Sci Rep 6: 37874 10.1038/srep37874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placke T, Örgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, Salih HR. 2012. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72: 440–448. 10.1158/0008-5472.CAN-11-1872 [DOI] [PubMed] [Google Scholar]
- Pollard JW. 2008. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol 84: 623–630. 10.1189/jlb.1107762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. 2009. The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293. 10.1038/nrc2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. 2009. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 4: e6562 10.1371/journal.pone.0006562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. 2011. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475: 222–225. 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DYH, Carragher NO, Munro A, Chang A, Bresnick AR, et al. 2015. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med 212: 1433–1448. 10.1084/jem.20141555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. 2014. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol 5: 75 10.3389/fphys.2014.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. 2004. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 64: 5839–5849. 10.1158/0008-5472.CAN-04-0465 [DOI] [PubMed] [Google Scholar]
- Rohan TE, Xue X, Lin HM, D'Alfonso TM, Ginter PS, Oktay MH, Robinson BD, Ginsberg M, Gertler FB, Glass AG, et al. 2014. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst 106: dju136 10.1093/jnci/dju136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. 2014. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 33: 4203–4212. 10.1038/onc.2013.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, Geissmann F. 2011. Toward a functional characterization of blood monocytes. Immunol Cell Biol 89: 2–4. 10.1038/icb.2010.130 [DOI] [PubMed] [Google Scholar]
- Sangaletti S, Di Carlo E, Gariboldi S, Miotti S, Cappetti B, Parenza M, Rumio C, Brekken RA, Chiodoni C, Colombo MP. 2008. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Res 68: 9050–9059. 10.1158/0008-5472.CAN-08-1327 [DOI] [PubMed] [Google Scholar]
- Sato T, Takahashi S, Mizumoto T, Harao M, Akizuki M, Takasugi M, Fukutomi T, Yamashita Ji. 2006. Neutrophil elastase and cancer. Surg Oncol 15: 217–222. 10.1016/j.suronc.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Schlesinger M. 2018. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol 11: 125 10.1186/s13045-018-0669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher D, Strilic B, Sivaraj K, Wettschureck N, Offermanns S. 2013. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 24: 130–137. 10.1016/j.ccr.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N, et al. 2015. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 61: 238–248. 10.1002/hep.27378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP. 2011. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 118: 4015–4023. 10.1182/blood-2011-07-368514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang Y, Wang P, Wang J, Cai Z. 2017. Tumor-derived exosomes educate dendritic cells to promote tumor metastasis via HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology 6: e1362527 10.1080/2162402X.2017.1362527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE. 2012. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72: 3919–3927. 10.1158/0008-5472.CAN-11-2393 [DOI] [PubMed] [Google Scholar]
- Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, Decock J, Zervantonakis IK, et al. 2016. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov 6: 630–649. 10.1158/2159-8290.CD-15-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K, Wilson Z, et al. 2016. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29: 832–845. 10.1016/j.ccell.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strell C, Lang K, Niggemann B, Zaenker KS, Entschladen F. 2010. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp Cell Res 316: 138–148. 10.1016/j.yexcr.2009.09.003 [DOI] [PubMed] [Google Scholar]
- Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, et al. 2014. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 25: 605–620. 10.1016/j.ccr.2014.03.021 [DOI] [PubMed] [Google Scholar]
- Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, et al. 2019. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566: 553–557. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- Tan GJ, Peng ZK, Lu JP, Tang FQ. 2013. Cathepsins mediate tumor metastasis. World J Biol Chem 4: 91–101. 10.4331/wjbc.v4.i4.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer HW, Palmieri DA, Wu QP, McCook EC, Serody JS. 2013. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J Immunol 190: 4861–4867. 10.4049/jimmunol.1202857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun H, Wei J, Cen B, DuBois RN. 2017. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res 77: 3655–3665. 10.1158/0008-5472.CAN-16-3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Y, Lake R, Faraji F, Sperger J, Martin P, Gilliard C, Ku KP, Rodems T, Niles D, Tillman H, et al. 2018. Platelets promote metastasis via binding tumor CD97 leading to bidirectional signaling that coordinates transendothelial migration. Cell Rep 23: 808–822. 10.1016/j.celrep.2018.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wculek SK, Malanchi I. 2015. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528: 413–417. 10.1038/nature16140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch DR, Schissel DJ, Howrey RP, Aeed PA. 1989. Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci 86: 5859–5863. 10.1073/pnas.86.15.5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP. 2001. Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol 280: C814–C822. 10.1152/ajpcell.2001.280.4.C814 [DOI] [PubMed] [Google Scholar]
- Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. 2004. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64: 7022–7029. 10.1158/0008-5472.CAN-04-1449 [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. 2007. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 67: 2649–2656. 10.1158/0008-5472.CAN-06-1823 [DOI] [PubMed] [Google Scholar]
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. 2010. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 70: 6139–6149. 10.1158/0008-5472.CAN-10-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Jiang J, Pang Y, Achyut BR, Lizardo M, Liang X, Hunter K, Khanna C, Hollander C, Yang L. 2015. CCL9 induced by TGFβ signaling in myeloid cells enhances tumor cell survival in the premetastatic organ. Cancer Res 75: 5283–5298. 10.1158/0008-5472.CAN-15-2282-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y. 2013. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine egfr/stat3/sox-2 signaling pathway. Stem Cells 31: 248–258. 10.1002/stem.1281 [DOI] [PubMed] [Google Scholar]
- Yeo EJ, Cassetta L, Qian BZ, Lewkowich I, Li JF, Stefater JA, Smith AN, Wiechmann LS, Wang Y, Pollard JW, et al. 2014. Myeloid wnt7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res 74: 2962–2973. 10.1158/0008-5472.CAN-13-2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S, Tomita K, Kudo T, Ando S, Yamazaki M. 2005. Induction of multicellular 3-D spheroids of MCF-7 breast carcinoma cells by neutrophil-derived cathepsin G and elastase. Cancer Sci 96: 560–570. 10.1111/j.1349-7006.2005.00097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K, Leone G, et al. 2010. An Ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res 70: 1323–1333. 10.1158/0008-5472.CAN-09-1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarà M, Guidetti G, Boselli D, Villa C, Canobbio I, Seppi C, Visconte C, Canino J, Torti M. 2017. Release of prometastatic platelet-derived microparticles induced by breast cancer cells: a novel positive feedback mechanism for metastasis. TH Open 1: e155–e163. 10.1055/s-0037-1613674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, Connelly L, Dulek D, Peebles RS, Fingleton B, et al. 2015. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res 75: 1624–1634. 10.1158/0008-5472.CAN-14-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, et al. 2005. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65: 3044–3048. 10.1158/0008-5472.CAN-04-4505 [DOI] [PubMed] [Google Scholar]
- Zhang W, Gu J, Chen J, Zhang P, Ji R, Qian H, Xu W, Zhang X. 2017. Interaction with neutrophils promotes gastric cancer cell migration and invasion by inducing epithelial-mesenchymal transition. Oncol Rep 38: 2959–2966. 10.3892/or.2017.5942 [DOI] [PubMed] [Google Scholar]
- Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier TT, Im JH, Cao Y, Beech J, Allen D, Smart S, Muschel RJ. 2013. Recruitment of a myeloid cell subset (CD11b/Gr1mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57: 829–839. 10.1002/hep.26094 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, et al. 2017. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47: 323–338.e6. 10.1016/j.immuni.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]