Abstract
Understanding how coronary blood vessels form and regenerate during development and progression of cardiac diseases will shed light on the development of new treatment options targeting coronary artery diseases. Recent studies with the state-of-the-art technologies have identified novel origins of, as well as new, cellular and molecular mechanisms underlying the formation of coronary vessels in the postnatal heart, including collateral artery formation, endocardial-to-endothelial differentiation and mesenchymal-to-endothelial transition. These new mechanisms of coronary vessel formation and regeneration open up new possibilities targeting neovascularization for promoting cardiac repair and regeneration. Here, we highlight some recent studies on cellular mechanisms of coronary vessel formation, and discuss the potential impact and significance of the findings on basic research and clinical application for treating ischemic heart disease.
The heart pumps blood through blood vessels of the circulatory system and provides the body with oxygen, nutrients, hormones, and immune cells. As a pumping muscular organ, the heart itself needs specialized blood vessels, namely, coronary vessels, to supply blood for the beating heart muscle cells or cardiomyocytes. Defects in coronary vessels can lead to myocardial ischemia or even heart failure after severe myocardial infarction. Coronary artery disease (CAD) remains the leading cause of morbidity and mortality worldwide (Benjamin et al. 2018). Once coronary arteries are obstructed in diseases such as atherosclerosis, billions of cardiomyocytes die because of deprivation of oxygen and nutrients after acute myocardial infarction. As the adult heart has minimal regenerative capacity, the injured myocardium is eventually replaced by scar tissue to maintain the structural integrity. However, the cardiac function is permanently damaged and cannot be restored in the remodeled heart, leading to heart failure and possibly death. Therefore, development of new treatment strategies for cardiovascular diseases is urgently needed, and this requires more basic knowledge about how coronary arteries are formed.
During early embryonic stages, the heart muscle is oxygenated by passive diffusion of the blood in the heart chambers. Later, as the heart grows and the ventricular wall becomes thicker, oxygen supply through passive diffusion is insufficient to supply the thickening myocardial wall. The developing heart requires its own blood vessels that populate and supply blood to the myocardium. There has been significant progress in our knowledge of coronary vessel development in recent years, with important findings that the coronary endothelium has multiple cellular origins during development. Multiple signaling pathways have been discovered for the process. In addition, the preexisting endothelial cells, endocardium, and fibroblasts of the adult heart have been proposed to contribute to neovascularization after cardiac injury. The new mechanisms underlying collateral artery formation have also been proposed. In this review, we focus on the cellular mechanisms of coronary vessel formation in development and in response to injury, with some discussion on the signaling pathways involved in the process.
CORONARY VASCULAR FORMATION IN FETAL HEART
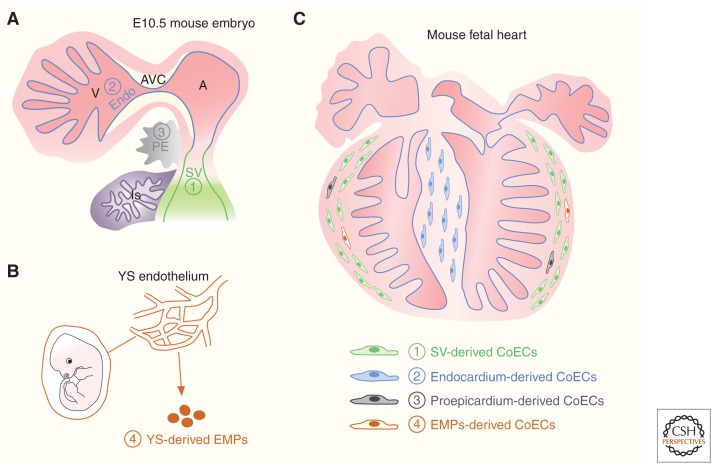
The coronary vascular plexus first emerges at embryonic day 11.5 (E11.5), and subsequently migrates and spreads over the surface of ventricle under the epicardium to form a primitive coronary vascular network. The origins of the coronary endothelium have been a topical subject for debate, with at least four different developmental origins of coronary vascular endothelial cells identified in the embryonic heart, namely, the proepicardium/epicardium, sinus venosus, endocardium, and circulating progenitors from the yolk sac (Fig. 1).
Figure 1.
Cellular origins of coronary endothelial cells in embryonic mouse heart. (A) The cartoon figure shows the structures of sinus venous (SV), endocardium (Endo), and proepicardium (PE) in the E10.5 mouse embryo. (V), ventricle; (A), atrium; (AVC), atrioventricular canal; (LS), liver sinusoid. (B) Erythromyeloid progenitors (EMPs) generated in the yolk sac (YS) endothelium. (C) There are four cell sources for coronary endothelial cells (CoECs) in the fetal heart: SV, endocardium, proepicardium, and EMPs.
Proepicardium/Epicardium Origin
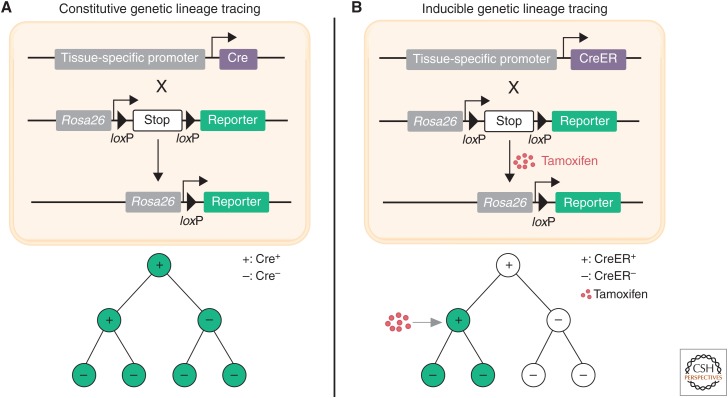
The proepicardium is a transient extracardiac embryonic tissue that originates at the juncture between the sinus venosus and the septum transversum in mice between E9–E11 (Fig. 1; Gittenberger-de Groot et al. 1998). Proepicardial cells attach to the heart and spread over the surface of the myocardium to form the epicardium (Männer et al. 2001). The proepicardium was assumed to be the cellular origin of coronary endothelial cells based on the seminal studies in chick models (Mikawa and Fischman 1992; Mikawa and Gourdie 1996; Dettman et al. 1998; Pérez-Pomares et al. 2002). In these cell-tagging and quail-chick chimera experiments, the labeled proepicardial cells or cells from the quail proepicardium were found to undergo an epithelial-to-endothelial transition, and then contribute to the vascular endothelial cells of the chicken heart. Following these studies, it had been widely accepted in the field that coronary vessels arise from the proepicardium. In recent years, genetic lineage tracing using the Cre-loxP system has been used in mice as a powerful tool to study cell origin and cell-fate mapping during tissue development, homeostasis, and disease (Fig. 2; Kretzschmar and Watt 2012; Tian et al. 2015). To study the cell-fate decision of the proepicardial/epicardial cells in vivo, genetic lineage tracing tools that mark the proepicardial/epicardial cells, such as Gata5-Cre (Merki et al. 2005), Wt1-Cre (Zhou et al. 2008; Wessels et al. 2012), Tbx18-Cre (Cai et al. 2008), and Tcf21-MerCreMer (Acharya et al. 2011), were generated for fate-mapping studies of the proepicardium/epicardium by several groups. Unexpectedly, the genetically labeled proepicardial/epicardial cells give rise to minimal or no coronary endothelial cells and instead they were observed to contribute to the majority of fibroblasts and smooth muscle cells (Cai et al. 2008; Zhou et al. 2008; Wessels et al. 2012). One explanation could be that the proepicardium is a molecularly compartmentalized structure, and these mouse genetic tools target a subset, but not all of the proepicardial cells. In a subsequent study, two new genetic mouse lines (Scx-Cre and Sema3D-Cre) were used to label the proepicardial cells, which are largely distinct from those of Tbx18-Cre and WT1-Cre labeled cells. Scx-Cre and Sema3D-Cre labeled proepicardial cells were found to contribute coronary endothelial cells (Katz et al. 2012). In addition, a recent report based on the G2-Gata4Cre transgene also showed that proepicardium/epicardium contributes at least 20% of the coronary arterial and capillary endothelial cells of the embryonic mouse heart (Cano et al. 2016). However, in these studies, constitutive rather than inducible Cre mouse lines were used, confounded by the fact that Cre labels any cells with history of its expression from the emergence of coronary vessels to the time point of analysis and, as such, if any endothelial cell expresses the transgene/Cre during the period of fate mapping, even transiently, the results of constitutive Cre-based lineage tracing would be overinterpreted. Genetic lineage tracing analysis using inducible CreER is required to inform on the contribution of proepicardium to the formation of the coronary endothelium.
Figure 2.
Genetic lineage tracing by Cre-loxP recombination. (A) Diagram of constitutive genetic lineage tracing strategy. Cre is under the control of a tissue-specific promoter and reporter is on Rosa26 locus where there is STOP sequence flanked by loxP before the reporter. In Cre+ cells, Cre-loxP-mediated recombination removes the STOP sequence and leads to labeling (e.g., GFP) of Cre+ cells and all their descendants (with or without Cre expression). (B) Diagram of inducible genetic lineage tracing strategy. CreER, a fusion protein of Cre and estrogen receptor (ER), is located in the cytoplasm. In the presence of tamoxifen, CreER is translocated to the nucleus, where CreER-loxP recombination removes the STOP sequence to label the CreER+ cells and their descendants.
Sinus Venosus Origin
A recent study has reported that the coronary arteries are derived from endothelial sprouts of the sinus venosus, a transient structure of venous inflow track that returns the blood to the embryonic heart (Fig. 1; Mommersteeg et al. 2010). By using whole heart histology and cardiac organ culture, Red-Horse and colleagues reported that venous endothelial cells dedifferentiate to form progenitor cells first as they migrate onto the myocardium and then redifferentiate into arterial endothelial cells, and also veins and capillaries that form the entire functional vascular network (Red-Horse et al. 2010). Clonal analysis via VE-cadherin-CreER transgene for labeling endothelial cells showed that most labeled coronary endothelial cells are clonally related to sinus venous, suggesting sinus venosus as the major origin of coronary vessels. Of note, a few coronary endothelial clones that are located near the interventricular groove have sister cells of the endocardium but not sinus venosus, indicating that the endocardium is an alternative cellular origin of coronary vessels (Red-Horse et al. 2010). Although this elegant clonal study supports that the coronary vessels mainly arise from the sinus venosus and endocardium, direct lineage tracing evidence is lacking. A subsequent study employing Apln-CreER or Apj-CreER demonstrated that most intramyocardial coronary arteries in the ventricle wall are primarily derived from the subepicardial endothelial precursors that originate from the sinus venous (Tian et al. 2013; Chen et al. 2014). Relatively few coronary vessels of the interventricular septum were derived from Apln-CreER or Apj-CreER labeled subepicardial endothelial precursors (Tian et al. 2013; Chen et al. 2014), indicating that the coronary vessels of the interventricular septum are derived from other sources, such as the endocardium.
Endocardium Origin
The endocardium was first described as a source of coronary vessels by clonal analysis using VE-cadherin-CreER (Red-Horse et al. 2010), which showed some, but not most, of the coronary endothelial clones are associated with endocardium, and, moreover, blood islands were proposed as the endocardium-derived vasculatures. Because VE-cadherin is more weakly expressed in the endocardium than coronary vascular endothelial cells, the clonal analysis based on VE-cadherin could shift toward labeling of vascular endothelial cells rather than endocardium. To address whether ventricular endocardium gives rise to coronary vessels, a more specific marker is required to distinguish ventricular endocardium, sinus venosus, and coronary vascular endothelial cells. Nfatc1 was supposed to be expressed strongly in endocardium (Zhou et al. 2005). Wu and colleagues took advantage of Nfatc1-Cre to trace the cell fate of endocardium, and found that Nfatc1-Cre-labeled cells give rise to the majority of the coronary vessels (Wu et al. 2012). This is in contrast with the conclusion that sinus venosus is the major cellular origin of coronary vessels (Red-Horse et al. 2010; Tian et al. 2013; Chen et al. 2014). Indeed, Nfatc1-Cre-labeled cells were also observed in a subset of sinus venous cells (Zhou et al. 2005). As Nfatc1-Cre is constitutively expressed, if Nfatc1 is expressed in the sinus venous or the coronary endothelial cells (Combs et al. 2011) at any time, even transiently during development, the lineage tracing results by Nfatc1-Cre are likely to be overinterpreted. To circumvent the limitation of Nfatc1-Cre-based lineage tracing, Zhang et al. identified a novel endocardial gene Npr3 by single-cell gene expression analysis. Npr3 is expressed in the ventricular endocardium but not the sinus venosus or coronary vasculature (Zhang et al. 2016). Taking advantage of this unique expression pattern, an inducible Npr3-CreER was generated to reexamine the cell fate of the ventricular endocardium in the developing heart (Zhang et al. 2016). Npr3-CreER-labeled-endocardium minimally contributed to coronary vascular endothelial cells of the embryonic ventricular free wall, but contributed to the majority of the vascular endothelial cells of the interventricular septum (Zhang et al. 2016). Unlike mammals, the coronary vessels of the zebrafish heart are proposed to arise from the endocardium rather than sinus venosus (Harrison et al. 2015), suggesting some evolutionary differences in the origins of coronary endothelial cells exists between fish, chicken, and mouse.
Circulating Progenitors from the Yolk Sac
After endothelial cells arise from mesenchymal precursors, termed angioblasts, at early embryonic stages, these endothelial cells proliferate, sprout into avascular tissue regions, and coalesce to form vessels (Coultas et al. 2005). The current view holds that these endothelial cells are a self-contained cell lineage, which proliferate and expand without significant contribution from circulating precursors (Potente et al. 2011). Likewise, the coronary vasculature is thought to arise from proximal to or within the developing heart and not via external precursors. However, a recent study by Ruhrberg's group unexpectedly showed that erythromyeloid progenitors (EMPs) (Fig. 1) from the yolk sac could migrate to the early developing heart and integrate into the preexisting vasculatures of the heart (Plein et al. 2018), emphasizing further the multiple cellular origins and heterogeneity of the coronary vasculature. Red blood cells and immune cells arise early in development from their common precursors called EMPs before hematopoietic stem cells emerge in the embryo. These EMPs emerge from endothelial cells of the yolk sac. Using genetic lineage tracing tools, Ruhrberg and colleagues labeled EMPs specifically with fluorescent proteins at early stages and followed their fate at later stages. EMPs, migrating from the yolk sac into the embryo, reverted to their endothelial cell fate, and incorporated into the vasculature of different organs, including the heart (Plein et al. 2018). The EMP-derived endothelial cells were interspersed in the mesoderm-derived endothelium, and their coexistence and coordination for functional vessels maintained into adult stages. Whereas the contribution of EMP-derived endothelial cells constituted about 60% of the liver vasculature, particularly the liver sinusoidal endothelium, the EMP-derived endothelial cells contributed about ∼10%–20% of the coronary vasculatures (Plein et al. 2018). Mechanistically, these EMP-derived endothelial cells express high level of Hoxa, and specific deletion of Hoxa in EMPs reduced their contribution to endothelial cells. The EMP source of vessels is not only important to our understanding of the developmental biology that endothelial cells of the organs could have dual origins (Iruela-Arispe 2018), but also may provide insights into how the coronary vascularizes after ischemia. Whether these EMP-derived coronary endothelial cells behave differently from mesoderm-derived vessels, whether EMPs derived from ES or iPS cells could be transplanted for adult vasculogenesis, and whether modulating Hoxa gene in EMPs could be explored in the future to support vessel growth in the ischemic myocardium are interesting and important questions awaiting further investigation.
Molecular Mechanism Regulating Coronary Vessel Development
Because coronary endothelial cells of the embryonic heart have multiple developmental origins (for now, at least four sources identified), it is important to delineate the distinct molecular signaling pathways underlying their differentiation, migration, and formation of cells from different sources. Development of the sinus venous-derived coronary vessels requires VEGFC, which is highly expressed in the cardiomyocytes of the outflow tract at E13.5 in mice. VEGFC depletion impaired sinus venous vessel sprouting and growth, while the endocardium-derived coronary vessels develops rather normally in VEGFC-deficient hearts (Chen et al. 2014). The sinus venosus is itself heterogeneous, composed of APJ-positive and -negative endothelial cells (Arita et al. 2014). Interestingly, myocardium-derived angiopoietin-1 (Ang1) is essential for the formation of the surface coronary veins, which are derived from APJ-negative endothelial cells (Arita et al. 2014). The ELABELA-APJ signaling axis is required for sinus venosus–derived vessel growth, while endocardium-derived vessels are not dependent on ELA-APJ and can expand to compensate for impaired SV-derived vessel growth in Apj mutants, resulting in normal adult heart function (Sharma et al. 2017b). In a recent study from Red-Horse's group, the chromatin remodeler Ino80 was shown to be essential for coronary vessel growth from both SV and endocardium sources (Rhee et al. 2018). Deletion of Ino80 not only impairs coronary angiogenesis but also leads to left ventricular noncompaction (Rhee et al. 2018), indicating that defective angiogenesis may contribute to noncompaction heart defects. Furthermore, CXCL12 was found to promote the maturation of the coronary vascular plexus in a paracrine manner: absence of CXCL12/CXCR4 signaling resulted in defective coronary vessel formation and late-gestation lethality (Cavallero et al. 2015; Harrison et al. 2015). The upstream signal, DACH1 was been shown to stimulate coronary artery growth through the CXCL12/CXCR4 signaling axis (Chang et al. 2017) and CXCL12/CXCR4 signaling also plays an important role in coronary artery stem formation (Ivins et al. 2015). With respect to the molecular mechanism that specifically regulates endocardial-derived coronary vessels, VEGFA was reported to play a pivotal role in this process. Myocardial VEGFA signals through VEGFR1 and VEGFR2 to inhibit and stimulate endocardial cells to undergo angiogenesis, respectively (Wu et al. 2012; Zhang and Zhou 2013). More comprehensive reviews on signaling pathways regulating coronary vessel development, can be found elsewhere (Tian et al. 2015; Sharma et al. 2017a; Red-Horse and Siekmann 2019).
CORONARY VESSEL FORMATION IN THE POSTNATAL HEART
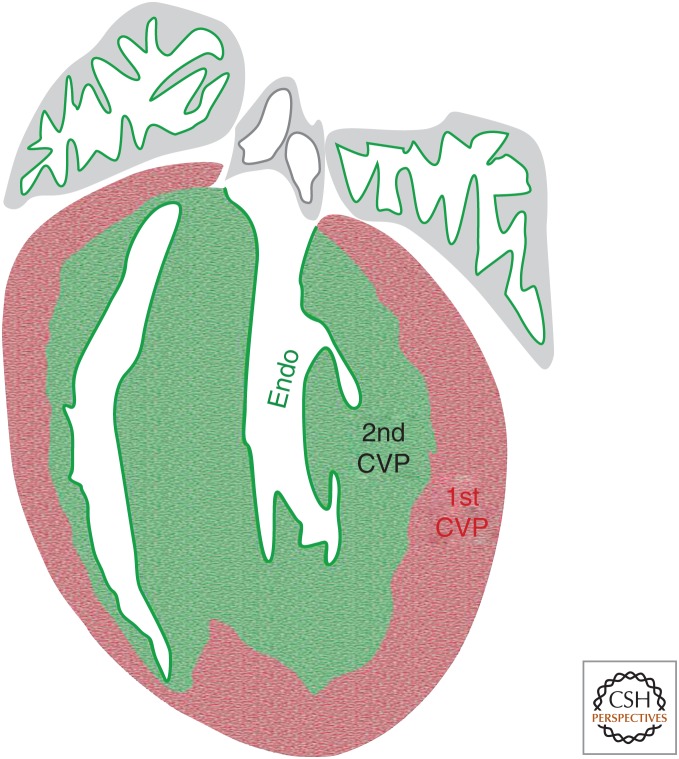
There are two distinct layers that comprise the myocardial wall of the ventricle in embryonic stages: the outer compact layer and the inner trabecular layer (Sedmera et al. 2000). The compact myocardium receives oxygen from the coronary vessels, while the trabecular myocardium is oxygenated by chamber blood through diffusion. After birth, the trabecular myocardium undergoes compaction, and new coronary vessels emerge in this region to supply oxygen to the newly formed inner compacted myocardium. For a long time, the coronary vessels of the inner myocardial wall had been thought to arise from angiogenic expansion of fetal coronary vessels (Bernanke and Velkey 2002; Munoz-Chapuli et al. 2002; Tomanek 2005). Recently, Tian et al. found that the inner myocardial coronary vessels arise de novo in the neonatal mouse heart, rather than expansion of preexisting coronary vasculature (Tian et al. 2014). As Apln is expressed in coronary vascular endothelial cells, but not endocardium (Sheikh et al. 2008; Red-Horse et al. 2010), Apln-CreER (with tamoxifen induction at E10.5) labels virtually all the embryonic vascular endothelial cells of the ventricular free wall permanently. If the postnatal coronary vessels are mainly generated by expansion of the preexisting vessels, most, if not all, of the new coronary vessels of the postnatal heart should be genetically labeled by Apln-CreER. Surprisingly, Apln-CreER labeled coronary vessels are mainly restricted to the outer compact layer of the postnatal heart, while the inner myocardial coronary vessels remain unlabeled (Tian et al. 2014), indicating that they form de novo from an alternative source. The endocardium is closely associated with the inner myocardium, and is considered to be a possible source of the newly emerged coronary vessels. To address this question, a new mouse line, Nfatc1-CreER, was generated to exclusively label endocardial cells prior to coronary vessel formation. This mouse line is different from the previously reported constitutively active Nfatc1-Cre (Wu et al. 2012), which also labels a subset of sinus venous endothelial cells in addition to ventricular endocardium. In the neonatal heart, Nfatc1-CreER labeled the majority of the de novo formed coronary vessels of the inner myocardial wall, while few coronary vessels in the outer myocardial wall are labeled by Nfatc1-CreER, demonstrating that the endocardial cells transdifferentiated to coronary endothelial cells during trabecular compaction in the neonatal stage. This study indicates that there are two distinct coronary vascular populations (CVPs) in the postnatal mouse heart, the first CVP labeled by Apln-CreER activation at E10.5, and the second CVP, originating from the Nfatc1-CreER-labeled endocardial cells (Fig. 3). In addition, genetic lineage tracing using Fabp4-CreER confirmed the existence of two unique CVPs in the postnatal heart (He et al. 2014). It is likely that the endocardial cells are trapped during the compaction of trabecular myocardium (Tian et al. 2017). Hypoxia and VEGFA are up-regulated during this process, and induce these endocardial cells to adopt the vascular endothelial cell fate (Tian et al. 2014). It would be intriguing to know whether entrapment is required for endocardial cells to switch into vascular endothelial cells, or whether endocardial cells could migrate into myocardium directly and form coronary vessels. Because there is no trabecular compaction in the adult stage, it would be important that the endocardial cells could be stimulated again to migrate into myocardium to form new coronary vessels after cardiac injuries. Further studies are required to delineate the molecular mechanisms that orchestrate the development of coronary vessels in the neonatal stage, providing better understanding of adult neovascularization by remodeling and reprogramming endocardial cells.
Figure 3.
Coronary vascular populations (CVPs) in the postnatal mouse heart. Cartoon figure shows there are two CVPs in the postnatal mouse heart. The first CVP formed in the embryonic stage and expanded to populate the outer myocardial wall (red area). The second CVP arose de novo from endocardium (Endo) in the later embryonic and neonatal stages, and populates the ventricular septum and inner myocardial wall (green area).
CELLULAR ORIGINS OF CORONARY ENDOTHELIUM AFTER CARDIAC INJURY
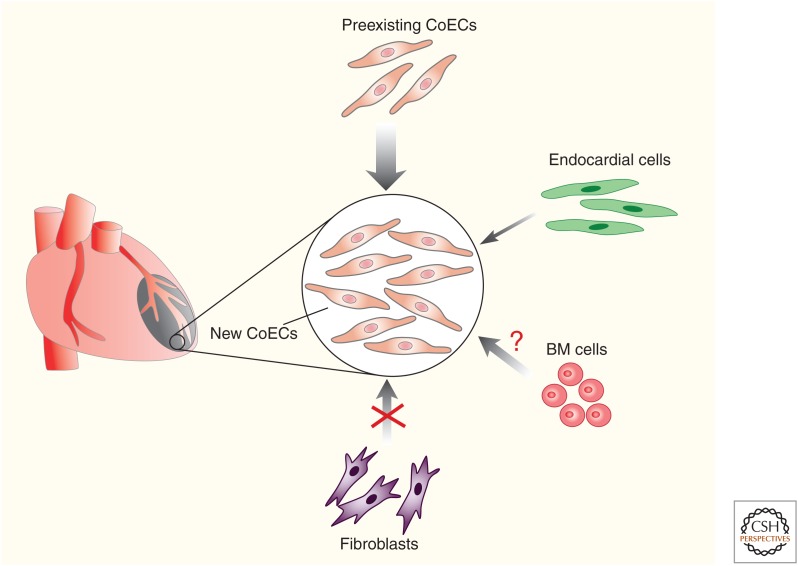
In the adult heart, angiogenic expansion of the preexisting coronary vessels is thought to be the main mechanism of coronary revascularization following injury. Many studies propose different sources of endothelial cells to mediate neovascularization, although some of them are still under debate in the field (Fig. 4). Recently, it was reported that cardiac fibroblasts, including mesenchymal stromal cells, can undergo mesenchymal-to-endothelial transition (MEndoT) to generate vascular endothelial cells after heart injuries (Ubil et al. 2014). Cardiac fibroblasts play an important role in maintaining normal heart function (Davis and Molkentin 2014). Following injury, they proliferate (Moore-Morris et al. 2016) and are activated to produce extracellular matrix proteins to maintain the integrity of the heart and prevent rupture, a process known as fibrosis. Whereas fibrosis is crucial for maintaining and restoring cardiac function after cardiac injuries, excessive fibrosis is considered to have adverse effects, including pathological remodeling leading to heart failure. Endothelial cells have been shown to give rise to cardiac fibroblast formation during heart development (Moore-Morris et al. 2014; Chen et al. 2016). In adult stages, endothelial cells have also been reported to contribute to cardiac fibroblasts through endothelial-to-mesenchymal transition (EndoMT) after cardiac pressure overload in adult mice (Zeisberg et al. 2007). Whether mesenchymal cells give rise to endothelial cells (MEndoT) remains largely unexplored. In a recent report by Deb and colleagues, cardiac fibroblasts give rise to coronary endothelial cells after cardiac injury (Ubil et al. 2014). In this study, the transgenic mouse line Col1a2-CreER was used for fibroblast lineage tracing studies. After myocardial ischemia-reperfusion cardiac injury, about 30%–40% of Col1a2-CreER-labeled cardiac fibroblasts were shown to contribute to coronary endothelial cells in the injured site and this process was p53-dependent. These exciting data, if correct, suggest entirely new therapeutic strategies to target patients with deficient coronary blood supply, as recently highlighted (Miyake and Kalluri 2014). However, the significant contribution of fibroblasts to endothelial cells does not reconcile with previous studies that suggest fibroblasts mainly adopt a myofibroblast cell fate after cardiac injury (Moore-Morris et al. 2014, 2016). A more rigorous assessment of MEndoT is required beyond previous studies (Miyake and Kalluri 2014; Ubil et al. 2014), to determine the potential for revascularization and therapeutic manipulation after cardiac injury. Cardiac fibroblasts are a heterogenous population (Ali et al. 2014; Kanisicak et al. 2016; Kaur et al. 2016; Fu et al. 2018); hence, to reassess MEndoT, multiple genetic mouse tools (Col1a2-CreER, Col1a2-2a-CreER, PDGFRa-DreER, Sox9-CreER, Postn-MerCreMer, and Tcf21-MerCreMer) have been required to label distinct subpopulations and trace their cell fate after cardiac ischemia-reperfusion injury (He et al. 2017a). Surprisingly, fate-mapping results based on these genetic tools revealed that cardiac fibroblasts expand substantially after injury, but do not contribute to vascular endothelial cells. To independently explore whether nonendothelial cell sources such as fibroblasts contribute to a substantial number of new endothelial cells after injury, a series of lineage dilution experiments were performed (He et al. 2017a). The preexisting coronary endothelial cells were prelabeled by Cdh5-CreER, Apln-CreER, and Fabp4-CreER. If the proportion of labeled endothelial cells were significantly reduced after cardiac injury, this suggests new coronary endothelial cells may arise from nonendothelial cells; if not, coronary endothelial cells must be derived from preexisting coronary endothelium. The three lineage dilution experiments unanimously demonstrated that most, if not all, new coronary vessels of the injured heart are derived from preexisting coronary endothelial cells, without significant contribution from other cell lineages (He et al. 2017a). Nevertheless, the lineage dilution experiment has a caveat that if a very small number of nonendothelial cells contribute (e.g., 0.1%), then it is not sensitive enough to reveal this low-level lineage conversion event. To simultaneously track nonendothelial cells and endothelial cells in one heart, a dual recombinase (Cre and Dre)-mediated lineage-tracing approach (He et al. 2017b; Li et al. 2018) could be considered for future studies to definitively address whether nonendothelial cells adopt an endothelial cell fate after cardiac injury.
Figure 4.
Cellular sources of coronary endothelial cells after cardiac injury. In the adult heart, preexisting coronary endothelial cells (CoECs) is the major cell source of new CoECs after cardiac injury. Endocardial cells minimally contribute to CoECs. It remains unclear whether bone marrow (BM) cells contribute to CoECs. Fibroblasts do not transdifferentiate into CoECs after cardiac injury.
Bone marrow (BM) cells in the adult have been shown to become activated and recruited to ischemic tissues and differentiate into endothelial cells that incorporate into blood vessels (Asahara et al. 1997; Takahashi et al. 1999; Kwon et al. 2008). This was termed postnatal vasculogenesis for de novo formation of new vessels; however, it remains controversial as to whether BM cells can physically incorporate into vessels or promote tissue angiogenesis through secretion of growth factors (Rehman et al. 2003; Bautch 2011). Recent studies utilized the irradiation-induced endothelial cell injury model, and found BM-derived cells were recruited to incorporate into blood vessels of the liver (Singhal et al. 2018). In contrast, BM-derived cells do not give rise to any liver endothelial cells after partial hepatectomy, indicating that the source of regenerating vasculature largely depends on the status of residual endothelial cells. This elegant study reconciles many previous discrepancies on the endothelial cell fate of BM cells, highlighting that the fitness of in situ endothelial cells dictates the sources of regenerating vasculature. Whether BM cells give rise to coronary endothelial cells after cardiac injury requires further investigation.
Endocardium is a major source of coronary endothelial cells during embryonic and neonatal stages (Wu et al. 2012; Tian et al. 2014); it is unclear whether endocardial cells of the adult heart have the potential to give rise to coronary endothelial cells. Recently, the endocardium was reported as an important source of coronary arteries to promote vascularization after myocardial infarction (Miquerol et al. 2015). To investigate the vascular remodeling of coronary arteries after cardiac injury, Miquerol et al. performed myocardial infarction on Connexin40-GFP mice, which express GFP in endothelial cells of coronary arteries, but not in veins, capillaries, and endocardium. They found new arterial endothelial foci, termed “endocardial flowers,” within the injured site of the endocardium, which acquired artery features (Cx40+, VEGFR2+, endoglin–) and were distinct from the surrounding endocardium (Cx40–, VEGFR2–, endoglin+). Genetic lineage tracing using Cx40-CreER demonstrated that the endocardial flowers develop by arteriogenesis of Cx40– endocardial cells and by outgrowth of preexisting coronary artery endothelial cells. However, the evidence for endocardial contribution to arteries is indirect, and endocardium-specific lineage tracing is required to validate this interesting finding. More recently, another study by Dubé and colleague also suggested that the endocardium contributes to neovascularization following myocardial infarction (Dube et al. 2017). Following myocardial infarction, hypertrabeculation was observed in the endocardial surface, and medium-sized vessels subsequently increased below the endocardial surface. This morphological change of the endocardium was interpreted as an endocardial contribution to coronary endothelial cells after myocardial injury (Dube et al. 2017). To directly assess the coronary endothelial potential of the endocardium after cardiac injury, separate genetic lineage tracing of the endocardium, based on Npr3-CreER, has been performed. Four cardiac injury models (myocardial infarction, myocardial ischemia reperfusion, cryoinjury, and transverse aortic constriction) were included (Tang et al. 2018). Npr3-CreER-labeled endocardial cells minimally contribute to coronary endothelial cells. Interestingly, following endocardial cell transplantation, donor cells acquired the properties of coronary endothelial cells. In addition, the endocardium was found to give rise to coronary endothelial cells in a purse-string, suture-like cardiac injury model in which endocardial cells were trapped in the underlying myocardium (Tang et al. 2018), indicating that entrapment or location of endocardial cells in the myocardium is required for adult endocardium to transdifferentiate into coronary vascular endothelial cells. In the future, it will be important to explore any growth factors that could stimulate the migration of endocardial cells and promote a vascular contribution in the injured adult heart. With more cutting-edge technology and novel approaches (including more refined cell-fate mapping tools combined with single-cell RNA sequencing), an improved understanding of the cellular sources and process of neovascularization may provide novel therapeutic approaches for treating ischemic heart diseases.
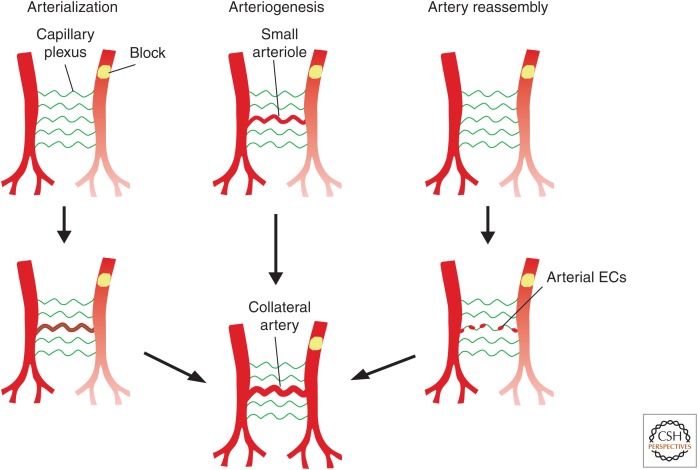
CELLULAR ORIGINS OF COLLATERAL ARTERIES AFTER ISCHEMIC INJURY
In addition to new endothelial cells (vasculogenesis), revascularization can be achieved through angiogenesis (Carmeliet 2003). However, large artery formation by angiogenesis is not fast enough to revascularize the injured myocardium after acute, yet severe, myocardial infarction. One alternative approach is to induce the development of collateral vessels after ischemic injury (Seiler et al. 2013; Faber et al. 2014). Collateral arteries are extra blood vessels that bridge two arteries, forming an alternate blood circulation route to sustain blood supply downstream of an occluded artery (Herzog et al. 2002; Helisch and Schaper 2003). Currently, there are two models for collateral arteries formation: arteriogenesis and arterialization (Fig. 5). Arteriogenesis is the process by which the preexisting small arterioles, with very low or no blood flow, enlarge to form large conducting arteries. In contrast, arterialization is remodeling of the capillary vessels into new arteries (White et al. 1998; Rocic et al. 2007; Belmadani et al. 2009; Faber et al. 2014). The proposed models of collateral artery formation are based on angiography. Because of the limited resolution of angiography, it has been challenging to determine which of the two models underpins collateral artery formation. With development of genetic technology and more molecular markers specific for subsets of endothelial cells, there has been recent progress in elucidating the mechanisms underlying the formation of collaterals (He et al. 2016; Das et al. 2019). Here it is important to distinguish capillaries from arteries with unique genetic markers. As Apln is specifically expressed in coronary capillary endothelial cells but not in arterial endothelial cells, Apln-CreER (tamoxifen induction at P1) was used to specifically label coronary capillaries for studying collateral artery formation following myocardial infarction in the neonatal or adult stage (He et al. 2016). Apln-CreER-based genetic lineage tracing revealed that collateral arteries were not labeled by Apln-CreER, indicating that coronary capillaries do not contribute to collateral arteries, that is, collateral arteries are not formed by arterialization (He et al. 2016). Lineage tracing studies supported that collateral arteries are mainly derived from remodeling of preexisting arteries (He et al. 2016).
Figure 5.
Models of collateral artery formation in mouse heart. Schematic figure showing three models for collateral artery formation in mouse heart: arterialization, arteriogenesis, and artery reassembly. Arterialization is the process of remodeling of capillary vessels into new collateral arteries to compensate the blood supply downstream of the obstruction. Arteriogenesis indicates small arterioles enlarge to form larger functional collateral arteries. Artery reassembly is the process that arterial endothelial cells (ECs) migrate away from arteries along existing capillaries and reassemble into collateral arteries.
Recently, a novel collateral artery formation model was proposed in the neonatal mouse heart after injury (Das et al. 2019). Myocardial infarction was performed at P2 and collateral arteries analyzed at P6 by whole-mount confocal imaging of the intact heart. After injury, numerous collateral arteries appeared, particularly in the watershed area that receives blood from distal branches of two large arteries and where no preexisting artery exists, suggesting that they are not developed through arterialization. Then APJ-CreER (tamoxifen induction at P0) and Cx40-CreER (tamoxifen induction at P0) lines were used to label capillary endothelial cells and artery endothelial cells, respectively, to analyze collateral artery formation. Consistent with the previous study (He et al. 2016), APJ-CreER-labeled capillary endothelial cells rarely present in collateral arteries, suggesting that collateral arteries are not derived from arterialization in neonates. Surprisingly, the forming collateral arteries were primarily derived from Cx40-CreER-labeled preexisting artery endothelial cells (Das et al. 2019), which is distinct from arteriogenesis because they appear from where there is no preexisting artery. To explore the mechanism of the formation of collateral arteries in neonates, earlier time points were examined and endothelial cell morphology at higher magnification was analyzed. Single arterial endothelial cells were found to proliferate and migrate out from the capillary endothelial layer and then coalesce with each other to make collateral arteries (Fig. 5; Das et al. 2019). This collateral artery formation model was termed “artery reassembly” (Fig. 5), and was shown to be guided by CXCL12-CXCR4 signaling. Nevertheless, there is still a lack of direct evidence for the artery reassembly model, notably in the absence of time-lapse imaging, which is not currently feasible in the mouse heart. It is still a possibility that a subset of pre-artery endothelial cells, expressing Cx40, may assemble into new coronary arteries after injury, recapitulating the developmental program of artery formation (Red-Horse et al. 2010), and it is also possible that the process is different again in adult stages. It will be important to develop improved genetic tracing approaches with more advanced 3D imaging to study the mechanisms of neonate collateral artery formation and to extrapolate to the adult heart in the future.
CONCLUDING REMARKS
Unraveling the sources of coronary endothelial cells in heart development and disease provides new insights into therapeutic neovascularization of ischemic diseased or injured hearts. With more advanced technologies, we are at a stage of understanding most, if not all, cell sources and mechanisms for coronary vessel formation in the developing and adult heart. The new cellular origins and sources advance our understanding on how different programs are employed for blood vessel formation and these can be exploited for therapeutic gain in the future. More effort is required to dissect the signaling pathways that distinctly regulate discrete programs across the forming and injury-responsive coronary vasculature, including endocardium-to-vessel formation and artery reassembly, to better inform approaches to instigate therapeutic revascularization.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2018YFA0108100, 2017YFC1001303), Strategic Priority Research Program of the Chinese Academy of Sciences (CAS, XDB19000000, XDA16010507), National Science Foundation of China (31730112, 91639302, 31625019, 91849202, 81761138040, 81872241, 31701292), Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), Youth Innovation Promotion Association of CAS (2060299), Sanofi Fellowship, Astrazeneca, and the Royal Society-Newton Advanced Fellowship.
Footnotes
Editors: Benoit G. Bruneau and Paul R. Riley
Additional Perspectives on Heart Development and Disease available at www.cshperspectives.org
REFERENCES
- Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. 2011. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis 49: 870–877. 10.1002/dvg.20750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Müller AM, Volz KS, Tang Z, et al. 2014. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115: 625–635. 10.1161/CIRCRESAHA.115.303794 [DOI] [PubMed] [Google Scholar]
- Arita Y, Nakaoka Y, Matsunaga T, Kidoya H, Yamamizu K, Arima Y, Kataoka-Hashimoto T, Ikeoka K, Yasui T, Masaki T, et al. 2014. Myocardium-derived angiopoietin-1 is essential for coronary vein formation in the developing heart. Nat Commun 5: 4552 10.1038/ncomms5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. 1997. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967. 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- Bautch VL. 2011. Stem cells and the vasculature. Nat Med 17: 1437–1443. 10.1038/nm.2539 [DOI] [PubMed] [Google Scholar]
- Belmadani S, Matrougui K, Kolz C, Pung YF, Palen D, Prockop DJ, Chilian WM. 2009. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol 29: 802–808. 10.1161/ATVBAHA.109.186189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. 2018. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137: e67–e492. 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Bernanke DH, Velkey JM. 2002. Development of the coronary blood supply: changing concepts and current ideas. Anat Rec 269: 198–208. 10.1002/ar.10139 [DOI] [PubMed] [Google Scholar]
- Cai C-L, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, et al. 2008. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454: 104–108. 10.1038/nature06969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E, Carmona R, Ruiz-Villalba A, Rojas A, Chau Y-Y, Wagner KD, Wagner N, Hastie ND, Muñoz-Chápuli R, Pérez-Pomares JM. 2016. Extracardiac septum transversum/proepicardial endothelial cells pattern embryonic coronary arterio-venous connections. Proc Natl Acad Sci 113: 656–661. 10.1073/pnas.1509834113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. 2003. Angiogenesis in health and disease. Nat Med 9: 653–660. 10.1038/nm0603-653 [DOI] [PubMed] [Google Scholar]
- Cavallero S, Shen H, Yi C, Lien C-L, Kumar SR, Sucov HM. 2015. CXCL12 Signaling is essential for maturation of the ventricular coronary endothelial plexus and establishment of functional coronary circulation. Dev Cell 33: 469–477. 10.1016/j.devcel.2015.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AH, Raftrey BC, D'Amato G, Surya VN, Poduri A, Chen HI, Goldstone AB, Woo J, Fuller GG, Dunn AR, et al. 2017. DACH1 stimulates shear stress-guided endothelial cell migration and coronary artery growth through the CXCL12–CXCR4 signaling axis. Genes Dev 31: 1308–1324. 10.1101/gad.301549.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HI, Sharma B, Akerberg BN, Numi HJ, Kivela R, Saharinen P, Aghajanian H, McKay AS, Bogard PE, Chang AH, et al. 2014. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 141: 4500–4512. 10.1242/dev.113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang H, Liu Y, Adams S, Eilken H, Stehling M, Corada M, Dejana E, Zhou B, Adams RH. 2016. Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells. Nat Commun 7: 12422 10.1038/ncomms12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs MD, Braitsch CM, Lange AW, James JF, Yutzey KE. 2011. NFATC1 promotes epicardium-derived cell invasion into myocardium. Development 138: 1747–1757. 10.1242/dev.060996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. 2005. Endothelial cells and VEGF in vascular development. Nature 438: 937–945. 10.1038/nature04479 [DOI] [PubMed] [Google Scholar]
- Das S, Goldstone AB, Wang H, Farry J, D'Amato G, Paulsen MJ, Eskandari A, Hironaka CE, Phansalkar R, Sharma B, et al. 2019. A unique collateral artery development program promotes neonatal heart regeneration. Cell 176: 1128–1142.e18. 10.1016/j.cell.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Molkentin JD. 2014. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70: 9–18. 10.1016/j.yjmcc.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettman RW, Denetclaw W Jr, Ordahl CP, Bristow J. 1998. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 193: 169–181. 10.1006/dbio.1997.8801 [DOI] [PubMed] [Google Scholar]
- Dube KN, Thomas TM, Munshaw S, Rohling M, Riley PR, Smart N. 2017. Recapitulation of developmental mechanisms to revascularize the ischemic heart. JCI Insight 2: e96800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. 2014. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol 34: 1854–1859. 10.1161/ATVBAHA.114.303929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, et al. 2018. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 128: 2127–2143. 10.1172/JCI98215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. 1998. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res 82: 1043–1052. 10.1161/01.RES.82.10.1043 [DOI] [PubMed] [Google Scholar]
- Harrison MR, Bussmann J, Huang Y, Zhao L, Osorio A, Burns CG, Burns CE, Sucov HM, Siekmann AF, Lien CL. 2015. Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev Cell 33: 442–454. 10.1016/j.devcel.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Tian X, Zhang H, Wythe JD, Zhou B. 2014. Fabp4-CreER lineage tracing reveals two distinctive coronary vascular populations. J Cell Mol Med 18: 2152–2156. 10.1111/jcmm.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Liu Q, Hu T, Huang X, Zhang H, Tian X, Yan Y, Wang L, Huang Y, Miquerol L, et al. 2016. Genetic lineage tracing discloses arteriogenesis as the main mechanism for collateral growth in the mouse heart. Cardiovasc Res 109: 419–430. 10.1093/cvr/cvw005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Huang X, Kanisicak O, Li Y, Wang Y, Li Y, Pu W, Liu Q, Zhang H, Tian X, et al. 2017a. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest 127: 2968–2981. 10.1172/JCI93868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L, et al. 2017b. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med 23: 1488–1498. 10.1038/nm.4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helisch A, Schaper W. 2003. Arteriogenesis: the development and growth of collateral arteries. Microcirculation 10: 83–97. 10.1080/mic.10.1.83.97 [DOI] [PubMed] [Google Scholar]
- Herzog S, Sager H, Khmelevski E, Deylig A, Ito WD. 2002. Collateral arteries grow from preexisting anastomoses in the rat hindlimb. Am J Physiol Heart Circ Physiol 283: H2012–H2020. 10.1152/ajpheart.00257.2002 [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML. 2018. A dual origin for blood vessels. Nature 562: 195–197. 10.1038/d41586-018-06199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins S, Chappell J, Vernay B, Suntharalingham J, Martineau A, Mohun TJ, Scambler PJ. 2015. The CXCL12/CXCR4 axis plays a critical role in coronary artery development. Dev Cell 33: 455–468. 10.1016/j.devcel.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, J Lin S-C, Aronow BJ, Tallquist MD, et al. 2016. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7: 12260 10.1038/ncomms12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, Tabin CJ. 2012. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell 22: 639–650. 10.1016/j.devcel.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, Poetsch A, Hoelper S, Conway SJ, Mollmann H, et al. 2016. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ Res 118: 1906–1917. 10.1161/CIRCRESAHA.116.308643 [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Watt FM. 2012. Lineage tracing. Cell 148: 33–45. 10.1016/j.cell.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Kwon S-M, Eguchi M, Wada M, Iwami Y, Hozumi K, Iwaguro H, Masuda H, Kawamoto A, Asahara T. 2008. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation 118: 157–165. 10.1161/CIRCULATIONAHA.107.754978 [DOI] [PubMed] [Google Scholar]
- Li Y, He L, Huang X, Bhaloo SI, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, et al. 2018. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 138: 793–805. 10.1161/CIRCULATIONAHA.118.034250 [DOI] [PubMed] [Google Scholar]
- Männer J, Pérez-Pomares JM, Macías D, Muñoz-Chápuli R. 2001. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs 169: 89–103. 10.1159/000047867 [DOI] [PubMed] [Google Scholar]
- Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, Burch J, Kubalak SW, Kaliman P, Izpisua Belmonte JC, et al. 2005. Epicardial retinoid X receptor α is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci 102: 18455–18460. 10.1073/pnas.0504343102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. 1992. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci 89: 9504–9508. 10.1073/pnas.89.20.9504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Gourdie RG. 1996. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 174: 221–232. 10.1006/dbio.1996.0068 [DOI] [PubMed] [Google Scholar]
- Miquerol L, Thireau J, Bideaux P, Sturny R, Richard S, Kelly RG. 2015. Endothelial plasticity drives arterial remodeling within the endocardium after myocardial infarction. Circ Res 116: 1765–1771. 10.1161/CIRCRESAHA.116.306476 [DOI] [PubMed] [Google Scholar]
- Miyake T, Kalluri R. 2014. Cardiac biology: cell plasticity helps hearts to repair. Nature 514: 575–576. 10.1038/nature13928 [DOI] [PubMed] [Google Scholar]
- Mommersteeg MT, Domínguez JN, Wiese C, Norden J, de Gier-de Vries C, Burch JB, Kispert A, Brown NA, Moorman AF, Christoffels VM. 2010. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc Res 87: 92–101. 10.1093/cvr/cvq033 [DOI] [PubMed] [Google Scholar]
- Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, et al. 2014. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 124: 2921–2934. 10.1172/JCI74783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Morris T, Cattaneo P, Puceat M, Evans SM. 2016. Origins of cardiac fibroblasts. J Mol Cell Cardiol 91: 1–5. 10.1016/j.yjmcc.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Chapuli R, Gonzalez-Iriarte M, Carmona R, Atencia G, Macias D, Perez-Pomares JM. 2002. Cellular precursors of the coronary arteries. Tex Heart Inst J 29: 243–249. [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pomares JM, Phelps A, Sedmerova M, Carmona R, González-Iriarte M, Muñoz-Chápuli R, Wessels A. 2002. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Dev Biol 247: 307–326. 10.1006/dbio.2002.0706 [DOI] [PubMed] [Google Scholar]
- Plein A, Fantin A, Denti L, Pollard JW, Ruhrberg C. 2018. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 562: 223–228. 10.1038/s41586-018-0552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887. 10.1016/j.cell.2011.08.039 [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Siekmann AF. 2019. Veins and arteries build hierarchical branching patterns differently: bottom-up versus top-down. Bioessays 41: e1800198 10.1002/bies.201800198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman I, Krasnow M. 2010. Coronary arteries form by developmental reprogramming of venous cells. Nature 464: 549–553. 10.1038/nature08873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman J, Li J, Orschell CM, March KL. 2003. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164–1169. 10.1161/01.CIR.0000058702.69484.A0 [DOI] [PubMed] [Google Scholar]
- Rhee S, Chung JI, King DA, D'Amato G, Paik DT, Duan A, Chang A, Nagelberg D, Sharma B, Jeong Y, et al. 2018. Endothelial deletion of Ino80 disrupts coronary angiogenesis and causes congenital heart disease. Nat Commun 9: 368 10.1038/s41467-017-02796-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocic P, Kolz C, Reed R, Potter B, Chilian WM. 2007. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736. 10.1152/ajpheart.01330.2006 [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH. 2000. Developmental patterning of the myocardium. Anat Rec 258: 319–337. [DOI] [PubMed] [Google Scholar]
- Seiler C, Stoller M, Pitt B, Meier P. 2013. The human coronary collateral circulation: development and clinical importance. Eur Heart J 34: 2674–2682. 10.1093/eurheartj/eht195 [DOI] [PubMed] [Google Scholar]
- Sharma B, Chang A, Red-Horse K. 2017a. Coronary artery development: progenitor cells and differentiation pathways. Annu Rev Physiol 79: 1–19. 10.1146/annurev-physiol-022516-033953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Ho L, Ford GH, Chen HI, Goldstone AB, Woo YJ, Quertermous T, Reversade B, Red-Horse K. 2017b. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and Apj-deficient hearts. Dev Cell 42: 655–666.e3. 10.1016/j.devcel.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AY, Chun HJ, Glassford AJ, Kundu RK, Kutschka I, Ardigo D, Hendry SL, Wagner RA, Chen MM, Ali ZA, et al. 2008. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol 294: H88–H98. 10.1152/ajpheart.00935.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal M, Liu X, Inverso D, Jiang K, Dai J, He H, Bartels S, Li W, Abdul Pari AA, Gengenbacher N, et al. 2018. Endothelial cell fitness dictates the source of regenerating liver vasculature. J Exp Med 215: 2497–2508. 10.1084/jem.20180008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. 1999. Ischemia- and cytokine-induced mobilization of bone marrow–derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438. 10.1038/7434 [DOI] [PubMed] [Google Scholar]
- Tang J, Zhang H, He L, Huang X, Li Y, Pu W, Yu W, Zhang L, Cai D, Lui KO, et al. 2018. Genetic fate mapping defines the vascular potential of endocardial cells in the adult heart. Circ Res 122: 984–993. 10.1161/CIRCRESAHA.117.312354 [DOI] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z, et al. 2013. Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res 23: 1075–1090. 10.1038/cr.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Yan Y, et al. 2014. Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science 345: 90–94. 10.1126/science.1251487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Pu WT, Zhou B. 2015. Cellular origin and developmental program of coronary angiogenesis. Circ Res 116: 515–530. 10.1161/CIRCRESAHA.116.305097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Li Y, He L, Zhang H, Huang X, Liu Q, Pu W, Zhang L, Li Y, Zhao H, et al. 2017. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat Commun 8: 87 10.1038/s41467-017-00118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ. 2005. Formation of the coronary vasculature during development. Angiogenesis 8: 273–284. 10.1007/s10456-005-9014-9 [DOI] [PubMed] [Google Scholar]
- Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, et al. 2014. Mesenchymal–endothelial transition contributes to cardiac neovascularization. Nature 514: 585–590. 10.1038/nature13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels A, van den Hoff MJ, Adamo RF, Phelps AL, Lockhart MM, Sauls K, Briggs LE, Norris RA, van Wijk B, Perez-Pomares JM, et al. 2012. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev Biol 366: 111–124. 10.1016/j.ydbio.2012.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FC, Bloor CM, McKirnan MD, Carroll SM. 1998. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol 85: 1160–1168. 10.1152/jappl.1998.85.3.1160 [DOI] [PubMed] [Google Scholar]
- Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O'Rourke BP, Sharp DJ, et al. 2012. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151: 1083–1096. 10.1016/j.cell.2012.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. 2007. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961. 10.1038/nm1613 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhou B. 2013. Accelerated coronary angiogenesis by VEGFR1-knockout endocardial cells. PLoS ONE 8: e70570 10.1371/journal.pone.0070570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pu W, Li G, Huang X, He L, Tian X, Liu Q, Zhang L, Wu SM, Sucov HM, et al. 2016. Endocardium minimally contributes to coronary endothelium in the embryonic ventricular free walls. Circ Res 118: 1880–1893. 10.1161/CIRCRESAHA.116.308749 [DOI] [PubMed] [Google Scholar]
- Zhou B, Wu B, Tompkins KL, Boyer KL, Grindley JC, Baldwin HS. 2005. Characterization of Nfatc1 regulation identifies an enhancer required for gene expression that is specific to pro-valve endocardial cells in the developing heart. Development 132: 1137–1146. 10.1242/dev.01640 [DOI] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, et al. 2008. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454: 109–113. 10.1038/nature07060 [DOI] [PMC free article] [PubMed] [Google Scholar]