Abstract
Inflammasomes assemble in the cytosol of myeloid and epithelial cells on sensing of cellular stress and pathogen-associated molecular patterns and serve as scaffolds for recruitment and activation of inflammatory caspases. Inflammasomes play beneficial roles in host and immune responses against diverse pathogens but may also promote inflammatory tissue damage if uncontrolled. Gasdermin D (GSDMD) is a recently identified substrate of murine caspase-1 and caspase-11, and human caspases-1, -4, and -5 that mediates a regulated lytic cell death mode termed pyroptosis. Recent studies have identified pyroptosis as a critical inflammasome effector mechanism that controls inflammasome-dependent cytokine secretion and contributes to antimicrobial defense and inflammasome-mediated autoinflammatory diseases. Here, we review recent developments on inflammasome-associated effector functions with an emphasis on the emerging roles of gasdermin pores and pyroptosis.
CANONICAL AND NONCANONICAL INFLAMMASOME PATHWAYS
Although frequently providing protection against bacterial, viral, and fungal pathogens, inflammasome activation is often detrimental in a diverse range of (auto)inflammatory, autoimmune, metabolic, neurodegenerative, and malignant diseases (Mangan et al. 2018; Van Gorp et al. 2019; Voet et al. 2019).
Inflammasome pathways are traditionally divided into the canonical and noncanonical pathways, referring to which inflammatory caspase initiates signaling that critically contributes to inflammation and host–defense responses (Fig. 1). The canonical inflammasomes by definition assemble a multiprotein complex that activates caspase-1, and their signaling is initiated by engagement of cytosolic pattern recognition receptors (PRRs) mostly from the nucleotide-binding domain, leucine-rich repeat containing (NLR) protein family (Lamkanfi and Dixit 2014; Broz and Dixit 2016). NLRP3 scavenges the cytosol of myeloid and epithelial cells for a plethora of danger- and pathogen-associated molecular patterns (DAMPs and PAMPs); thus, it is speculated that this inflammasome senses cellular stress and imbalance. Other well-defined canonical inflammasome-forming NLRs include NLRC4, human NLRP1, and murine Nlrp1a and Nlrp1b, which respond to stimuli such as flagellin, inhibition of dipeptidyl peptidases (DPPs)8/9, and Bacillus anthracis–derived lethal toxin (LeTx) (Franchi et al. 2006; Miao et al. 2006; Okondo et al. 2018; Zhong et al. 2018; de Vasconcelos et al. 2019b). Akin to NLRs, the HIN200 family member AIM2 and the TRIM protein family member pyrin are well-defined inflammasome-forming PRRs. AIM2 directly binds to cytosolic DNA via its carboxy-terminal HIN200 domain, and pyrin indirectly senses inactivating RhoA GTPase modifications by microbial pathogens (Bürckstümmer et al. 2009; Fernandes-Alnemri et al. 2009; Xu et al. 2014). Once active, these inflammasome receptors recruit the bipartite inflammasome adaptor ASC, which bridges the interaction with caspase-1, although direct engagement of caspase-1 is also possible in the context of the caspase recruitment domain (CARD)-containing NLRs including NLRP1, Nlrp1a, Nlrp1b, and NLRC4 (Broz et al. 2010; Masters et al. 2012; Van Opdenbosch et al. 2014; Broz and Dixit 2016). The noncanonical inflammasome is engaged on sensing of cytosolic lipopolysaccharide (LPS) by murine caspase-11 and its human orthologs, caspase-4 and -5 (Kayagaki et al. 2011; Shi et al. 2014; Schmid-Burgk et al. 2015). Inflammasomes perform several cell-intrinsic and extrinsic functions, and highlighting these are complex machineries integrating several cellular signaling pathways (Fig. 1). Yet, defining the contribution of the different effector functions initiated on inflammasome activation is only now emerging. This review will discuss recent progress in understanding of inflammasome functions and how these may affect disease outcomes.
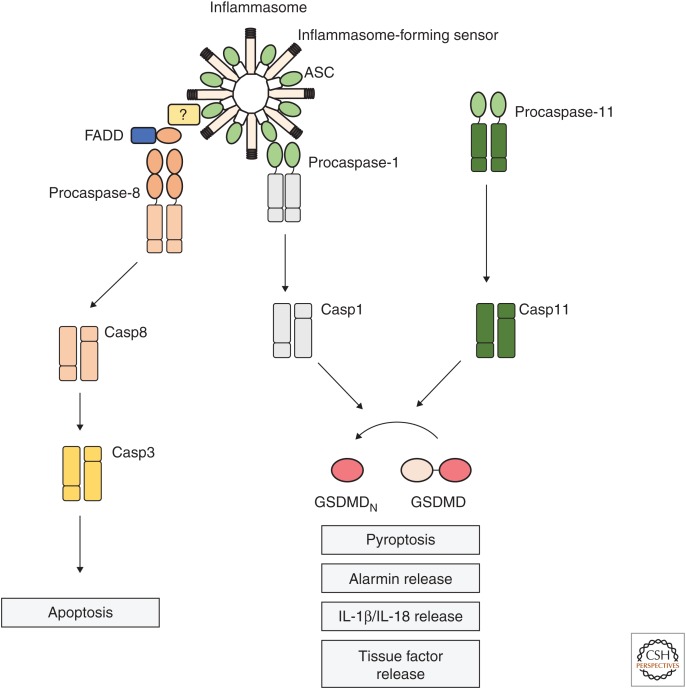
Figure 1.
Inflammasome platforms activate caspase-1, -11, and -8 for multiple downstream effector functions. Intracellular lipopolysaccharide (LPS) precipitates the oligomerization of caspase-11, in the so-called noncanonical inflammasome (right), and its automaturation. Cytosolic presence of danger- and pathogen-associated molecular patterns (DAMPs or PAMPs) can also trigger the canonical inflammasome pathway, which recruits procaspase-1 for proximity-induced autoactivation (left). Caspase-1 and caspase-11 cleave the common substrate, gasdermin D (GSDMD), releasing an amino-terminal fragment (GSDMDN), which initiates cell death by pyroptosis. This lytic cell death mode is associated with alarmin release to the extracellular space and discharge of tissue factor–containing microvesicles. Inflammasomes also mediate the activation and secretion of the proinflammatory cytokines interleukin (IL)-1β and IL-18, either through caspase-1-directed cleavage of the procytokines or through indirect NLRP3 activation. At the canonical inflammasome, ASC also recruits FADD and caspase-8, through a still-undefined mechanism. Caspase-8 activation at inflammasomes leads to apoptosis in caspase-1-deficient cells.
INFLAMMASOME EFFECTOR FUNCTIONS: PYROPTOSIS AND MATURATION OF INTERLEUKIN-1 CYTOKINES
Both canonical and noncanonical inflammasome pathways converge on secretion of proinflammatory cytokines and initiation of a lytic cell death mode named pyroptosis, both of which are unequivocally linked to inflammasome-driven antimicrobial functions (Fig. 1). Interleukin (IL)-1β and IL-18 are key proinflammatory cytokines of the IL-1 family that are produced and stored in the cytosol as functionally inactive proteins (Dinarello 2009). Caspase-1, originally named IL-1β converting enzyme (ICE), cleaves both IL-1β and IL-18 after specific aspartate residues in their linker regions, allowing the mature cytokines to bind their cognate receptors on their extracellular release (Black et al. 1989; Kostura et al. 1989; Ghayur et al. 1997; Gu et al. 1997). IL-1β is a pyrogenic cytokine, and its binding to IL-1 receptor 1 (IL-1R1) triggers assembly of a tertiary receptor complex with the IL-1 receptor accessory protein (IL-1RAcP) on effector cells that promotes secretion of inflammatory mediators, local infiltration of immune cells, antibody production by B cells, and development of a T helper (Th)17 response. IL-18 is a costimulatory factor for interferon γ (IFN-γ) production and consequently a stimulator of natural killer (NK) cell function, polarizing the T-cell response to either a Th1 or Th2 pattern according to the context (Dinarello 2009). Protein expression of pro-IL-1β is mainly confined to cells of the myeloid lineage and further regulated by the requirement of NF-κB and MAPK signaling for its transcriptional up-regulation (often named signal 1), whereas pro-IL-18 is constitutively present in cells throughout the body (Van Opdenbosch et al. 2017; Van Gorp et al. 2019). Only caspase-1 can directly cleave IL-1β and IL-18; meanwhile, caspase-11 must activate the NLRP3 inflammasome for cytokine secretion in the context of noncanonical inflammasome signaling (Kayagaki et al. 2011, 2015).
In contrast to maturing cytokines, murine caspase-1 and -11 (and human caspases -1, -4, and -5) are equally capable of autonomously inducing pyroptosis (Kayagaki et al. 2011). This is accomplished through caspase-1/-11-directed cleavage of a common pyroptosis substrate, gasdermin D (GSDMD), at Asp275 (corresponding to Asp276 in human GSDMD), releasing a cytotoxic GSDMD amino-terminal fragment (GSDMDN) (Kayagaki et al. 2015; Shi et al. 2015). GSDMDN assembles pores in the plasma membrane for execution of pyroptosis (Aglietti et al. 2016; Ding et al. 2016; Liu et al. 2016; Sborgi et al. 2016).
In addition to pyroptosis, inflammasomes were also described to initiate an alternative form of cell death, namely, NETosis. The term NETosis refers to a neutrophil-associated cell death mode, accompanied by extrusion of DNA webs, called NETs (Papayannopoulos 2018). Histones and antimicrobial proteins aggregate in the decondensed DNA structure, serving as a scaffold to hold pathogens and facilitate antimicrobial responses. In the context of noncanonical inflammasome activation, caspase-11 was shown to control DNA extrusion and cell death by cleaving and activating GSDMD, after sensing LPS or Citrobacter rodentium in the cytosol of neutrophils (Chen et al. 2018). In vivo, DNase treatment, known to dissolve NETs, prevented the host response to the vacuole destabilizing and caspase-11-activating ΔsifA mutant of Salmonella enterica serovar Typhimurium (S. Typhimurium) strain, but it had no effect in the absence of caspase-11 or GSDMD. These data suggest that inflammasomes have an ability to regulate the initiation of cell death, which would take on different morphological characteristics depending on the cell type. However, understanding the specific contributions of each form of inflammasome-mediated cell death during antimicrobial defenses awaits the use of targeted conditional knockouts.
Pyroptotic cell lysis releases intracellular contents that act in neighboring cells for initiation of inflammatory responses (Fig. 1). One of these components, called alarmins, is IL-1α, an IL-1 family member that, akin to IL-1β, binds to IL-1R1 (Dinarello 2009). However, different from IL-1β, IL-1α is present in the nucleus of resting cells and is biologically active both as a full-length or matured cytokine (Mosley et al. 1987; Dinarello 2009). Caspase-1/-11-initiated pyroptosis controls IL-1α release on several inflammasome triggers (Kayagaki et al. 2011). Another alarmin, HMGB1, is an abundant nuclear protein involved in chromatin maintenance at steady-state conditions. Yet, extracellular HMGB1 signals through RAGE to initiate a chemotactic response (Bertheloot and Latz 2017). It may also engage TLRs when bound to dsDNA or LPS to promote proinflammatory signaling. Studies have shown that HMGB1 is released on inflammasome activation both in vitro and in vivo in mice challenged with LPS or LeTx (Lamkanfi et al. 2010; Kayagaki et al. 2011; Lu et al. 2012; Van Opdenbosch et al. 2014). Notably, recent studies have clarified the role of hepatocyte-derived HMGB1 in promoting caspase-11-dependent lethality during LPS-induced endotoxemia, revealing that initial contact between hepatocytes and LPS triggers the observed systemic HMGB1 release seen in this model (Deng et al. 2018). In a second step, HMGB1 binding to extracellular LPS promotes RAGE-dependent translocation to the cytosol of the HMGB1–LPS complex, where LPS activates caspase-11. Indeed, impairing HMGB1 signaling confers protection to septic shock and endotoxemia, similar to the resistance seen in caspase-11-deficient mice (Lamkanfi et al. 2010; Deng et al. 2018). Pyroptotic cells also release microvesicles containing tissue factor (TF), which promotes intravascular blood clotting and further instigates LPS-induced lethality in vivo (Wu et al. 2019). Initiation of an eicosanoid storm is another inflammasome effector mechanism. Different from pyroptosis induction and IL-1β/IL-18 release; however, resident peritoneal macrophages are selectively capable of releasing eicosanoids, which contributed to hypothermia on systemic Nlrp1b and NLRC4 inflammasome activation in vivo (von Moltke et al. 2012).
Although caspase-1 is an absolute requirement for initiation of pyroptosis during canonical inflammasome signaling, deletion of caspase-1 in murine macrophages and epithelial cells does not prevent cell death per se after triggering of the NLRP3, AIM2, NLRC4, and Nlrp1b inflammasomes (Puri et al. 2012; Sagulenko et al. 2013; Rauch et al. 2017; Schneider et al. 2017; Van Opdenbosch et al. 2017; Lee et al. 2018a). Instead, caspase-1 knockout cells switch to an apoptotic phenotype that is driven by ASC-mediated caspase-8 activation, albeit frequently delayed by a few hours (Sagulenko et al. 2013; Rauch et al. 2017; Van Opdenbosch et al. 2017; Lee et al. 2018a). Accordingly, knockin of the C284A amino acid substitution in the catalytic site of caspase-1 inactivates not only its protease activity, but also suffices for triggering ASC- and caspase-8-mediated apoptosis on activation of the NLRC4 and Nlrp1b inflammasome pathways (Van Opdenbosch et al. 2017; Lee et al. 2018a). Moreover, activation of caspase-8 by inflammasomes was fully dependent on ASC (Man et al. 2013; Sagulenko et al. 2013; Rauch et al. 2017; Van Opdenbosch et al. 2017; Lee et al. 2018a), and genetic deletion of ASC in caspase-1-deficient macrophages fully rescued these cells from inflammasome-initiated apoptosis (Van Opdenbosch et al. 2017; Lee et al. 2018a). Furthermore, inflammasome-induced caspase-8 activation and apoptosis by the Nlrp1b and NLRC4 inflammasomes was negatively regulated by TLR-induced up-regulation of the caspase-8-regulatory protein cFLIP (Van Opdenbosch et al. 2017; Lee et al. 2018a). Whether cFLIP is also incorporated in ASC specks remains unknown. Overall, these findings suggest that inflammasome assembly and ASC specks might function as a critical checkpoint that integrates cellular signals committing an infected cell to die by redundant regulated cell death pathways.
Interestingly, recruitment of caspase-8 to ASC specks is also observed in wild-type macrophages, suggesting that it is a native aspect of inflammasome signaling and does not only occur in the absence of caspase-1 signaling (Fig. 1; Man et al. 2013; Gurung et al. 2014; Van Opdenbosch et al. 2017). Ultrastructure modeling of the NLRP3 and AIM2 inflammasomes suggests these receptors oligomerize in star-like structures to expose their pyrin (PYD) domains for ASC recruitment (Lu et al. 2014). Additionally, a recent cryo-electron microscopic (cryo-EM) analysis of NLRP3 complexed with NEK7 suggests that the latter stabilizes NLRP3 complexes by bridging the interaction between leucine-rich repeats (LRRs) of adjacent NLRP3 molecules in the inflammasome (Sharif et al. 2019). PYDAIM2/NLRP3/PYDASC interactions act as a seeding mechanism for rapidly assembling ASC molecules and forming PYD-based filaments, which are cross-linked by CARDASC to form the speck and recruit caspase-1 (Lu et al. 2014; Dick et al. 2016; Schmidt et al. 2016). Although the mechanisms governing caspase-8 recruitment to the ASC speck are not fully clarified, FADD, a death effector domain (DED)-containing protein that recruits caspase-8 to death receptors during extrinsic apoptosis (Hughes et al. 2016), colocalizes with ASC following NLRC4 activation, which suggests that this adaptor protein may mediate caspase-8 recruitment to the ASC speck (Fig. 1; Van Opdenbosch et al. 2017). Consistently, FADD deficiency was shown to hamper caspase-8 maturation on canonical and noncanonical NLRP3 inflammasome activation (Gurung et al. 2014). Because FADD does not have either a CARD or a PYD, its interaction with ASC may be indirect. Thus, more work is needed to clarify how FADD is recruited to ASC specks.
Remarkably, genetic deletion of GSDMD in murine bone marrow–derived macrophages (BMDMs) elicits apoptosis in canonical inflammasome-triggered cells (He et al. 2015; de Vasconcelos et al. 2019a). A recent report suggests that caspase-1-mediated cleavage of the proapoptotic Bcl-2 family member BID contributed to caspase-1-induced apoptosis induction in GSDMD-deficient BMDMs (Tsuchiya et al. 2019). Notably, caspase-1-dependent apoptosis might drive cell death in primary cortical neurons on oxygen/glucose deprivation (Zhang et al. 2003) because these cells express caspase-1 and have low levels of GSDMD protein expression (Tsuchiya et al. 2019).
A recent study has shown that full autoprocessing of caspase-1, which releases the soluble p20/p10 form from the complex, inactivates the protease, thus suggesting that only caspase-1 associated with the inflammasome is active and that the inflammasome platform together with the polymerized caspase functions as a holoenzyme (Boucher et al. 2018). In agreement, inflammasome receptors that contain a CARD domain, such as NLRC4 and Nlrp1b, were previously shown to potently induce pyroptosis in the absence of ASC, by activating caspase-1 in the apparent absence of caspase-1 autoprocessing (Broz et al. 2010; Guey et al. 2014; Van Opdenbosch et al. 2014). Consistently, other studies show that the full-length form of caspase-1 becomes catalytically active and cleaves GSDMD during NLRC4 activation in Asc−/− cells (Dick et al. 2016; Boucher et al. 2018). However, maturation of IL-1β is less effective when CARD-containing inflammasome receptors are engaged in ASC-deficient macrophages (Broz et al. 2010; Van Opdenbosch et al. 2014). This might suggest that GSDMD is a better caspase-1 substrate than pro-IL-1β and/or that it can be efficiently recruited to complexes composed of NLRC4/Nlrp1b and caspase-1, whereas pro-IL-1β recruitment would occur more efficiently to ASC-containing complexes such as the ASC speck. Notably, also GSDMD and pro-IL-1β (Man et al. 2013; He et al. 2015) have been localized to inflammasome platforms alongside caspase-8 and FADD (Man et al. 2013; Van Opdenbosch et al. 2017).
Although alternative downstream cell death pathways have not been described for the noncanonical inflammasome, substantial progress has been made in understanding its upstream signaling requirements. Guanylate-binding proteins (GBPs)—small GTPases that are transcriptionally up-regulated in response to IFN stimulation—act upstream of caspase-11 in activation of the noncanonical inflammasome pathway by vacuolar pathogens (Meunier et al. 2014; Pilla et al. 2014; Santos et al. 2018). Several GBP family members accumulate at endosomal membranes and bacterial outer membrane vesicles, in which GBP2 and GBP5 are prominently involved in the release of PAMPs from the endolysosomal compartment or unmasking intramembrane domains of LPS for detection by caspase-11 (Meunier et al. 2014; Pilla et al. 2014; Shi et al. 2014; Santos et al. 2018). This process promotes caspase-11 dimerization and automaturation that is essential for it to gain catalytic activity and cleave GSDMD (Lee et al. 2018b; Ross et al. 2018). However, GBPs appear to be dispensable for caspase-11-mediated host defense and in vivo sensing of cytosol-invasive bacterial pathogens that deliberately access the cytosol in their course of infection such as Burkholderia thailandensis (Aachoui et al. 2015).
The noncanonical inflammasome has long been recognized to rely on the NLRP3 inflammasome for cytokine secretion, a process in which caspase-11-mediated plasma membrane damage allows K+ efflux to activate NLRP3 (Kayagaki et al. 2011; Rühl and Broz 2015). In agreement, Gsdmd−/− macrophages autoprocess caspase-11 following LPS transfection, but fail to undergo pyroptosis, activate caspase-1, or mature IL-1β/IL-18 (Kayagaki et al. 2015; Shi et al. 2015; Gonzalez Ramirez et al. 2018). NLRP3 activation downstream from GSDMD has also been shown in the context of caspase-8-mediated GSDMD cleavage, and this mechanism promotes IL-1β secretion in parallel to direct caspase-8-mediated IL-1β maturation in the context of extrinsic and intrinsic apoptosis induction in macrophages (Yabal et al. 2014; Lawlor et al. 2015; Chauhan et al. 2018; Malireddi et al. 2018; Orning et al. 2018; Sarhan et al. 2018). Caspase-8-mediated control of the NLRP3 inflammasome was shown in various conditions, such as TLR4 or TNFR1 signaling combined with inhibition of IAPs, after TAK1 inhibition and following Yersinia pestis infection (Yabal et al. 2014; Lawlor et al. 2015; Malireddi et al. 2018; Orning et al. 2018; Sarhan et al. 2018), and thus suggesting a role in diverse pathophysiological settings.
Gasdermin Proteins: A Conserved Family of Pore-Forming Proteins
Gasdermin proteins were initially identified as proteins with high expression levels in skin and the upper gastrointestinal tract (Saeki et al. 2000). There are four gasdermin family members in humans, namely, gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), and gasdermin D (GSDMD) (Tamura et al. 2007). The mouse genome does not encode homologs of GSDMB, but GSDMA and GSDMC are represented by three (Gsdma1-3) and four (Gsdmc1-4) paralogs, respectively. Mice further express a single GSDMD ortholog. All gasdermin family members are able to induce necrotic cell death through their amino-terminal domains (Fig. 2; Ding et al. 2016). Interestingly, gasdermin-induced cytotoxicity was shown to be shared even with the distant gasdermin relative, deafness-associated tumor suppressor (DFNA5). Identified as the gene bearing a mutation causative of nonsyndromic hearing impairment, DFNA5 was initially described as a separate branch of the gasdermin phylogenetic tree, but recently renamed gasdermin E (GSDME) (Van Laer et al. 1998; Tamura et al. 2007; Ding et al. 2016; Wang et al. 2017).
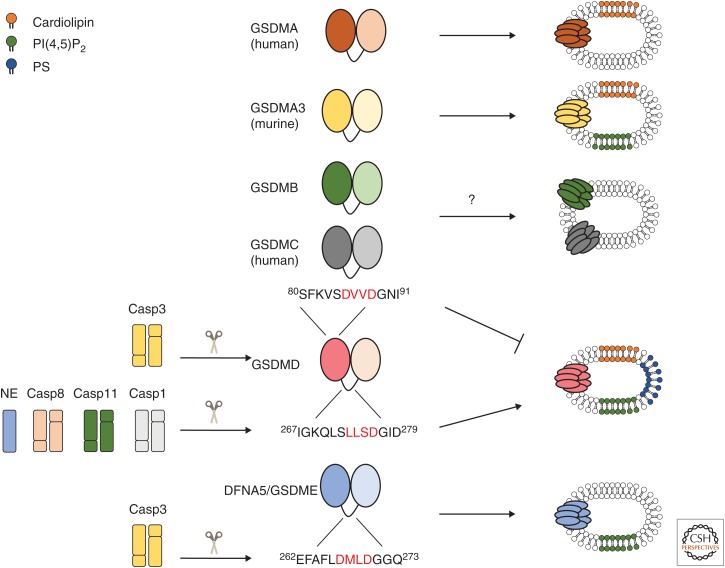
Figure 2.
The gasdermin family encompasses pore-forming proteins. All gasdermin family members are composed of a pore-forming amino-terminal domain, which is kept autoinhibited by the carboxy-terminal domain, both connected by an unstructured flexible linker. Caspase-1 (Casp1), caspase-8 (Casp8), caspase-11 (Casp11), and neutrophil elastase (NE) cleave gasdermin D (GSDMD) in the linker region (the caspase recognition sequences are marked in red), releasing the pore forming amino-terminal fragment (GSDMDN). GSDMDN is able to perforate liposomes containing complex mixtures, which include phosphatidylserine (PS), phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and cardiolipin-containing preparations. Conversely, caspase-3 (Casp3) cleavage of deafness-associated tumor suppressor/gasdermin E (DFNA5/GSDME) in the linker region releases an amino-terminal fragment that perforates liposomes containing PI(4,5)P2. Caspase-3 (Casp3) can also cleave GSDMD, generating an amino-terminal fragment that is unable to perforate plasma membranes. Although amino-terminal fragments of human gasdermin A (GSDMA) and murine gasdermin A3 (GSDMA3) forms pores in lipososomes containing cardiolipin and/or PI(4,5)P2, their activation mechanism remains unknown. It is also unclear how gasdermin B (GSDMB) and gasdermin C (GSDMC) could be activated and, although overexpression of their amino-terminal fragments triggers a lytic form of cell death, pore formation by these proteins has not yet been confirmed.
The amino-terminal domains of GSDMD, GSDMA, GSDMA3, and DFNA5/GSDME associate with phospholipids in vitro and perforate liposomes (Fig. 2). There seems to be a level of specialization because GSDMD, GSDMA3, DFNA5/GSDME, and GSDMB all bind to phosphoinositides, but only GSDMD, GSDMA3, and GSDMA efficiently interact with cardiolipin, a lipid found in the inner leaflet of the mitochondrial membrane (Aglietti et al. 2016; Ding et al. 2016; Liu et al. 2016; Chao et al. 2017; Wang et al. 2017). Uniquely, GSDMB efficiently binds to sulfatide, a lipid present in specialized areas such as the apical face of epithelial cells (Chao et al. 2017). Together with their differential tissue distribution profiles (Tamura et al. 2007), these findings suggest that perforation of cellular membranes is a common mechanism of gasdermin-induced cytotoxicity (Aglietti et al. 2016; Ding et al. 2016; Liu et al. 2016; Sborgi et al. 2016; Wang et al. 2017).
In all gasdermins, the amino-terminal pore-forming domain is kept inhibited by intramolecular interactions with the regulatory carboxy-terminal domain (Ding et al. 2016). However, other aspects of gasdermin biochemistry may be different between family members. For GSDMD, GSDMA3, GSDME, and GSDMA, the carboxy-terminal domain also prevents binding to lipids, in addition to preventing cell death (Shi et al. 2015; Ding et al. 2016). Conversely, GSDMB full-length protein is already able to bind lipids, but toxicity remains prevented by the presence of the carboxy-terminal domain (Ding et al. 2016; Chao et al. 2017).The regulatory interfaces in GSDMD and GSDMA3 were mapped to two main points of contact: (interface 1) the amino-terminal α1 helix and β1-β2 hairpin that intramolecularly interact with the carboxy-terminal domain; and (interface 2) the amino-terminal α4 helix, which interacts with the carboxy-terminal α9 and α11 helices at the upper part of the carboxy-terminal structure (Shi et al. 2015; Ding et al. 2016; Liu et al. 2018). Interface 1 is the lipid-binding component of GSDMA3, GSDMD, and DFNA5/GSDME, and this region is kept hidden by the carboxy-terminal domain in the full-length GSDMA3 and GSDMD proteins (Rogers et al. 2017; Liu et al. 2018; Ruan et al. 2018). Mutations that distress these molecular interfaces, particularly at interface 1, or that shorten the linker region destabilize the interdomain inhibitory interaction and are sufficient to promote cell death by full-length GSDMD and GSDMA3 (Ding et al. 2016; Kuang et al. 2017; Liu et al. 2018). Although the tertiary structure of DFNA5/GSDME has not been solved, mutations that cause hearing impairment in patients drive production of a truncated protein that lacks the last exon (Op de Beeck et al. 2012). Overexpression in HEK293T cells of these deafness-associated DFNA5/GSDME mutants triggers cell death (Wang et al. 2017). Therefore, it is likely that DFNA5/GSDME pathogenicity is associated with conformational changes that weaken the interface between the amino- and carboxy-terminal domains, although it remains unresolved why only cochlea cells are affected in patients expressing these mutant forms of DFNA5/GSDME.
Notwithstanding the considerable insights that have been reported on the secondary and tertiary structure of gasdermin family members (Ding et al. 2016), the mechanisms that govern dissociation of the amino- and carboxy-terminal domains are still unclear for most gasdermin proteins. GSDMD was initially discovered as a substrate of the inflammatory caspases -1, -11, -4, and -5, and the long-awaited executor of plasma membrane lysis during inflammasome-mediated pyroptosis (Fig. 2; Kayagaki et al. 2015; Shi et al. 2015). The cleavage site of inflammatory caspases lies at an unstructured loop that segregates the amino- and carboxy-terminal domains. Caspase-3 also cleaves GSDMD at Asp87, which destroys the amino-terminal domain and inactivates GSDMD (Rogers et al. 2017; Taabazuing et al. 2017; Orning et al. 2018), but further analysis is required to show that this regulation mechanism operates under physiological conditions. Furthermore, recent studies suggest that regulation of GSDMD activity is not restricted to caspase-3 and the inflammatory caspases because neutrophil elastase and caspase-8 also cleave GSDMD at the caspase-1/-11 cleavage site or at adjacent sites in the same linker (Fig. 2; Kambara et al. 2018; Orning et al. 2018; Sollberger et al. 2018). Notably, gasdermins have substantially less sequence similarity in the linker region, suggesting that each gasdermin may potentially use a different mechanism to disrupt the inhibitory interface (Tamura et al. 2007). Indeed, DFNA5/GSDME is cleaved in this same region by caspase-3 (Fig. 2; Rogers et al. 2017; Wang et al. 2017), a process that has been suggested to promote secondary necrosis of cells that follows apoptosis in cell culture settings (Rogers et al. 2017), although this has been contested by others (Lee et al. 2018a; Tixeira et al. 2018; Vince et al. 2018). Interestingly, cell types with high expression level of DFNA5/GSDME do not display features of apoptosis, such as cell shrinkage and blebbing, but instead undergo lytic cell death on activation of death receptors (Wang et al. 2017). This form of DFNA5/GSDME-mediated regulated necrosis was suggested to drive adverse inflammation in the lung and intestine in response to chemotherapy (Wang et al. 2017).
Overall, despite great recent advances in understanding the function of several gasdermin proteins, the field is still left with major questions. Most studies so far have relied on overexpression systems, which might overlook fine-tuned regulations and functions of these proteins. Therefore, there should be a focus in establishing in vivo physiological roles of gasdermins going forward. Further studies on the tissue and cell type–associated expression of gasdermin proteins and their regulation and activation mechanisms will allow a better understanding of different necrotic cell death pathways and their potential contributions to homeostasis and pathology.
Gasdermin Pores: Structural and Biochemical Features
Plasma membrane damage is one of the most outspoken features of pyroptosis. Yet, mechanistic understanding of pyroptotic cell lysis only gained significant traction with the discovery of GSDMD. In the context of inflammasome activation, cleavage of GSDMD by caspase-1, -11, -4, and -5 releases the pore-forming amino-terminal domain (GSDMDN) from its inhibitory carboxy-terminal fragment (Kayagaki et al. 2015; Shi et al. 2015). GSDMDN monomers oligomerize in part through disulfide bonds, precipitated mainly by Cys39 and Cys192 of mouse GSDMD (Liu et al. 2016; Rathkey et al. 2018), and integration of GSDMD units into membranes promotes GSDMDN-mediated pyroptotic cell lysis (Aglietti et al. 2016; Ding et al. 2016; Liu et al. 2016; Sborgi et al. 2016). Cryo-EM-based structural analysis indicated that GSDMA3 pores are formed by 26–28 monomers (Ruan et al. 2018). This ultrastructure suggests that, on membrane insertion, GSDMA3N undergoes significant conformational changes in which its unstructured loops reorganize into four β-strands. The final pore is composed of the adjoining of several β-strands from GSDMA3N subunits to form a β-barrel domain inserted in the lipid bilayer, with their globular domains emerging at the surface of the membrane.
The observation of a GSDMA3N oligomer formed outside of the lipid bilayer, in which GSDMA3N still adopted its monomeric conformation, instigated the suggestion that this was a soluble prepore assembly, normally formed before membrane insertion (Ruan et al. 2018). Interestingly, in this same study, an attempt to analyze GSDMD pores was not successful, owing to the fact that these formed a heterogeneous population. Indeed, prior observations of the GSDMDN pore in overexpression systems had shown that its size ranges from 10 to 20 nm in internal diameter (Fig. 3; Ding et al. 2016; Liu et al. 2016; Sborgi et al. 2016). This pore assembly could potentially allow even large proteins to flux through, which appears to conflict with the observation that pyroptotic cells are subjected to osmotic pressure that drives cell lysis. However, further work is needed to understand how plasma membrane insertion of endogenous GSDMDN units proceeds in macrophages and other cell types that are triggered to undergo pyroptosis.
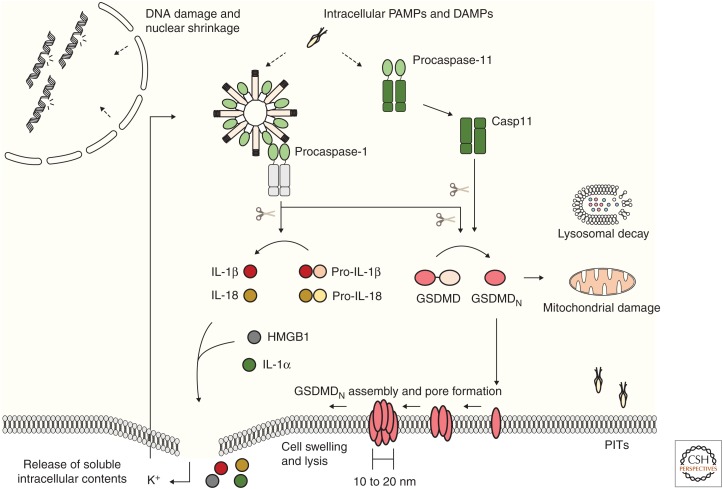
Figure 3.
Amino-terminal gasdermin D (GSDMD) determines several morphological and biochemical features during pyroptosis. Presence of intracellular danger- and pathogen-associated molecular patterns (DAMPs and PAMPs) initiates signaling through canonical and noncanonical inflammasomes. Both caspase-1 and caspase-11 direct the cleavage of GSDMD, releasing its pore-forming amino-terminal fragment (GSDMDN). Evidence suggests that GSDMDN inserts into the cellular plasma membrane in lower magnitude structures and assembles the pore through oligomerization within membranes until a higher magnitude final pore is formed. Finally, pyroptotic cellular swelling and plasma membrane rupture release soluble intracellular contents, including the proinflammatory cytokines IL-1β and IL-18, matured by caspase-1. During the noncanonical signaling, pyroptotic-mediated K+ efflux causes cell-intrinsic NLRP3 inflammasome activation, accounting for cytokine maturation. GSDMDN also damages mitochondria during execution of cell death, and might be responsible for the organellar damage seen in lysosomes and nuclei. The pyroptotic cell corpse further maintains intracellular pathogens, in the pore-induced intracellular trap (PIT), facilitating their clearance by infiltrating immune cells.
Early studies in this regard have shown that PEG-based osmoprotectants of only 2.4 nm prevented release of soluble cytosolic factors, including lactate dehydrogenase (LDH), from pyroptotic J774A.1 macrophages (Fink and Cookson 2006). Accordingly, osmoprotection with glycine allowed pyroptotic cells to be stained by a nuclear dye impermeable to intact membranes, named propidium iodide (PI), while bigger contents, tracked with LDH, were still retained in the cytoplasm (Russo et al. 2016). From this, the hypothesis emerged that caspase-1 initially triggers small pores at the plasma membrane that selectively allow ions to flux, with the associated osmotic pressure accounting for the ensuing total cell lysis. Consistent herewith, time-lapse analysis by atomic force microscopy of GSDMD pore formation showed that recombinant GSDMDN can initially form arc- or slit-shaped structures in liposomes, which grow over time into ring-shaped assemblies through the insertion of additional GSDMDN units at one end of the expanding oligomer (Mulvihill et al. 2018). Furthermore, GSDMDN monomers seem to require membranes to oligomerize, as the investigators could not detect any pore formation in the absence of lipids. Accordingly, a single-cell analysis of pyroptosis induction in primary macrophages showed that Ca2+ fluxes and osmotic increase in cell volume is detected before uptake of membrane-impermeable dyes (de Vasconcelos et al. 2019a). Furthermore, small plasma membrane–impermeant DNA dyes (molecular weight ∼400 Da) accessed the nucleus of pyroptotic cells earlier than slightly larger ones (molecular weight ∼600 Da). GSDMD-deficiency prevented these responses, suggesting that GSDMDN pores in the plasma membrane may gradually expand to promote plasma membrane permeability to larger cytosolic factors under physiological conditions (de Vasconcelos et al. 2019a). The concept that GSDMDN oligomerizes within the plasma membrane is further supported by the fact that GSDMDN higher magnitude assemblies are only found in membrane extracts (Ding et al. 2016; Liu et al. 2016). Therefore, the large pore GSDMD assemblies seen in cryo-EM and atomic force microscopy studies might be favored by the high concentrations of recombinant GSDMDN required for these analyses and may correspond to the final stages of in vivo pore formation, which may proceed more gradually at physiological concentrations (Fig. 3).
Pyroptosis: Morphological and Biochemical Hallmarks
In addition to the plasma membrane alterations and leakage promoted by GSDMD, pyroptotic cells undergo a series of intracellular changes during cell death, which is thought to be directed by inflammatory caspase activity. Of note, caspases were first recognized as mediators of apoptotic cell death, and their ability to cleave a large set of substrates generates the morphological and biochemical characteristics of this program of cellular demise (Taylor et al. 2008). Shigella- and Salmonella-induced cell death of infected macrophages was initially postulated to proceed through apoptosis (Zychlinsky et al. 1992; Chen et al. 1996; Monack et al. 1996; Hilbi et al. 1998; Hersh et al. 1999). However, these conclusions were debunked by the observation that caspase-1-initiated cell death triggered by these intracellular pathogens was accompanied by the release of cytosolic proteins, and the term pyroptosis—alluding to the inflammatory nature of this cell death—was coined (Cookson and Brennan 2001).
Although pyroptotic and apoptotic cells are morphologically distinct, they nevertheless have several biochemical features in common, which might be associated with their shared dependency on caspases. During canonical inflammasome activation, caspase-1 cleaves and activates caspase-7 (Lamkanfi et al. 2008). Caspase-7 activation does not contribute to inflammasome-mediated plasma membrane permeabilization or cytokine release, but it assists caspase-1 in the cleavage of PARP1, a nuclear protein involved in DNA repair (Lamkanfi et al. 2008; Malireddi et al. 2010). Interestingly, PARP1 cleavage during pyroptosis has been linked to an increased NF-κB response (Erener et al. 2012). Moreover, caspase-7 activation downstream from NLRC4 was shown to aid bacterial control by promoting phagolysosomal transport of bacteria-containing vacuoles in infected cells (Akhter et al. 2009). Activation of inflammasomes was also found to promote cleavage of several glycolytic enzymes, such as GAPDH, α-enolase, and pyruvate kinase (Shao et al. 2007), although future studies should investigate how these proteolytic events impact inflammasome responses. Fewer proteins have been reported to be cleaved during noncanonical inflammasome signaling. Caspase-11 cleaves TRPC1, a component of cation-permeable pores, in vitro (Py et al. 2014). Absence of TRPC1 facilitates IL-1β release, but whether and how this protein is involved in pyroptosis is still unclear. The difference between the extensive list of caspase-1 putative substrates and the few proteins directly cleaved by caspase-11 might be related to enzyme substrate specificity. Indeed, in vitro comparisons of these two inflammatory caspases towards tetrapeptidic substrates suggested caspase-1 has a broader recognition spectrum than caspase-11 (Gonzalez Ramirez et al. 2018). Overall, the findings on alternative inflammatory substrates suggest that there might exist unidentified signaling pathways that contribute to the execution or consequences of pyroptosis.
Both apoptosis and pyroptosis are associated with DNA fragmentation, as evidenced by terminal deoxynucleotidyl transferase dUTP nick end (TUNEL) labeling, although a different staining pattern distinguishes these distinct regulated cell death modes (Fig. 3; Brennan and Cookson 2000). The mechanism for pyroptotic-initiated DNA damage is unclear, but the apoptotic caspase-3–ICAD/CAD pathway seems dispensable for pyroptosis as ICAD is not degraded in Salmonella-infected macrophages (Fink and Cookson 2006). Intriguingly, in addition to DNA fragmentation, the whole nuclear compartment changes during pyroptosis, taking a more roundish appearance (Fig. 3; de Vasconcelos et al. 2019a).
Mitochondrial damage has been observed on activation of canonical and noncanonical inflammasomes, suggesting this is a common feature of pyroptosis (Fig. 3; Allam et al. 2014; Yu et al. 2014; de Vasconcelos et al. 2019a). BID, a member of the proapoptotic Bcl2 family, is also cleaved during pyroptosis (Yu et al. 2014; de Vasconcelos et al. 2019a). However, transgenic overexpression of antiapoptotic Bcl2 and genetic deletion of mitochondrial Bax/Bak pores does not impair permeabilization of the mitochondrial outer membrane (MOMP) and pyroptosis-associated cell permeabilization by the Nlrp1b and NLRP3 inflammasomes (Allam et al. 2014; de Vasconcelos et al. 2019a). Therefore, pyroptotic mitochondrial damage does not seem to rely on Bax/Bak pores. Instead, mitochondrial depolarization in the context of pyroptosis driven by the noncanonical inflammasome pathway is abolished in GSDMD-deficient macrophages (de Vasconcelos et al. 2019a), suggesting that GSDMD may also target intracellular membrane-bound organelles in addition to the plasma membrane (Aglietti et al. 2016; Ding et al. 2016; Liu et al. 2016; Sborgi et al. 2016). Consistently, pyroptotic organelle damage is not restricted to mitochondria, but also affects lysosomes and possibly other organelles (Fig. 3; de Vasconcelos et al. 2019a).
Release of the proinflammatory cytokines, IL-1β and IL-18, has long been associated with pyroptotic cell death (Fig. 3; Lamkanfi 2011). Several other soluble intracellular proteins, whether cytosolic or organellar, are also retrieved extracellularly following activation of the Nlrp1b and NLRC4 inflammasomes (de Vasconcelos et al. 2019a). Contrastingly, pyroptosis-induced membrane permeabilization does not allow the passage of organelles and phagocytosed pathogens (Jorgensen et al. 2016), suggesting there is a size limit for intracellular components that can be released during pyroptosis. Biologically, keeping larger intracellular agents, such as pathogenic bacteria, inside the pyroptotic corpse, which has been named the pore-induced intracellular trap (PIT), prevents the spread of such pathogens and could facilitate their clearance by infiltrating neutrophils and other professional phagocytes (Fig. 3; Jorgensen et al. 2016). Conversely, unspecific leakage in the extracellular milieu of soluble intracellular proteins and DAMPs may contribute to chemotactic and proinflammatory responses.
The Role of GSDMD in Inflammasome Effector Responses
IL-1β and IL-18 are cytosolic proteins that lack targeting sequences for secretion through the classical ER–Golgi secretion pathway (Rubartelli et al. 1990; Gu et al. 1997). Thus, a longstanding central question in the field has been how these cytokines are released from myeloid cells when their biological activity as a cytokine is required. Casp1−/− mice are impaired in both cytokine maturation and secretion (Kuida et al. 1995). Because caspase-1 also controls pyroptosis initiation, cell death has long been postulated to be the secretion mechanism for IL-1β and IL-18, although several alternative modes of IL-1β secretion from live cells have also been put forward. In support of a key role for pyroptosis in secretion of IL-1β and DAMPs, single-cell studies in the myeloid leukemia cell line THP-1 and in murine peritoneal macrophages have shown that extracellular release of IL-1β was only detected in the vicinity of dying cells (Liu et al. 2014; Cullen et al. 2015). In mouse BMDMs—the gold standard for inflammasome studies—and epithelial cells, most inflammasome triggers promote cell death coupled to IL-1β secretion (Kayagaki et al. 2011, 2015; Gurung et al. 2014; Zhong et al. 2016; Rauch et al. 2017; Van Opdenbosch et al. 2017). Accordingly, Gsdmd−/− BMDMs are impaired in LDH release and maintain matured IL-1β in their cytosol, failing to secrete the cytokine when shortly stimulated (He et al. 2015; Kayagaki et al. 2015; Shi et al. 2015). More importantly, GSDMD seems to be an essential controller of cytokine release in vivo, as Gsdmd−/− animals are rescued from the increase in circulating IL-1β caused by familial Mediterranean fever (FMF) and neonatal-onset multisystem inflammatory disease (NOMID)-associated mutations in mice (Kanneganti et al. 2018; Xiao et al. 2018). Thus, GSDMD is a critical determinant of inflammasome-mediated cytokine release in vitro and in vivo.
Nonetheless, several reports have shown a dissociation between cell death and cytokine secretion on inflammasome activation with specific stimuli and/or in different cell types. For example, activation of BMDMs with certain lipids of the mixture of oxidized phospholipids, oxPAPC, commonly released by necrotic cells, and with peptidoglycan from O-acetyltransferase A–deficient Staphylococcus aureus triggers IL-1β release in the absence of detectable LDH release (Shimada et al. 2010; Zanoni et al. 2017). In human primary monocytic cells, TLR4 signaling is sufficient to initiate IL-1β release, which is not accompanied by cell death above background levels (Gaidt et al. 2016). Similarly, IL-1β, but not LDH, was recently shown to be secreted by oxPAPC-triggered dendritic cells (DCs), although a recent publication shows that bone marrow–derived DCs are contaminated with BMDMs prompts a reevaluation of these conclusions (Zanoni et al. 2016; Erlich et al. 2019). Finally, a similar dissociation between cell death and cytokine release has been reported for S. Typhimurium–infected neutrophils (Chen et al. 2014; Heilig et al. 2018).
However, the molecular mechanisms driving cytokine secretion from live cells have so far remained unresolved. Alternative mechanisms for the active secretion of IL-1β could play a role in the absence of pyroptosis, as reviewed elsewhere (Monteleone et al. 2015). In some situations, macrophages and neutrophils were suggested to rely on GSDMD for IL-1β release without inducing LDH release (Evavold et al. 2018; Heilig et al. 2018), which could be explained by the rapid shedding of GSDMD pores in the plasma membrane by the endosomal sorting complexes required for transport (ESCRT) machinery (Rühl et al. 2018). It remains unclear whether this membrane recycling mechanism is sufficiently efficient and rapid to shed all GSDMD pores that appear in the plasma membrane to prevent cell death, and its relevance for controlling IL-1β secretion from activated monocytes and macrophages in vivo also warrants further analysis. Notably, delayed IL-1β release is detected in Gsdmd−/− BMDMs cultured ex vivo (Kayagaki et al. 2015). Careful analysis of GSDMD-deficient macrophages has revealed that they undergo apoptosis on activation of caspase-1, with morphological alterations appearing within the same time frame as pyroptosis is detected in wild-type cells through a pathway recently suggested to involve cleavage of BID by caspase-1 (He et al. 2015; de Vasconcelos et al. 2019a; Tsuchiya et al. 2019).
ROLE OF PYROPTOSIS IN INFECTIONS AND INFLAMMATORY DISEASE
Inflammasomes have long been implicated in host defense against microbial pathogens. Studies in knockout mice have firmly established the critical role of inflammasome activation in protection against in vivo infection with B. anthracis spores (Moayeri et al. 2010; Terra et al. 2010), Burkholderia spp. (Ceballos-Olvera et al. 2011; Aachoui et al. 2015), S. Typhimurium (Karki et al. 2018), Francisella tularensis (Fernandes-Alnemri et al. 2010; Jones et al. 2010), murine cytomegalovirus (Rathinam et al. 2010), Candida albicans (Joly et al. 2009), and many other pathogens. Remarkably, certain effector functions of inflammasomes—that is, IL-1β or IL-18 secretion, pyroptosis, and DAMP release—may each exert specific functions in in vivo models of inflammasome activation that together contribute to host and immune responses during infection. As a result, genetic ablation of the inflammasome sensor not always phenocopies animals that lack expression of specific inflammasome effector proteins. Il18−/− mice are more susceptible to lethality when infected with Burkholderia pseudomallei or B. thailandensis compared with wild-type mice, whereas deletion of IL-1β did not alter host defense to the infections (Ceballos-Olvera et al. 2011; Aachoui et al. 2015). Additionally, IL-18 was shown to be essential for clearing Chromobacterium violaceum from the liver of infected mice (Maltez et al. 2015). Distinctively, IL-1-mediated signaling is important for resistance to B. anthracis infection, and deletion of IL-1R1 renders inbred mouse strains that express the LeTx-sensitive Nlrp1b allele susceptible to B. anthracis infection (Moayeri et al. 2010).
Another prominent example is that co-ablation of IL-1β and IL-18 signaling prevents lethality to lower LPS doses in the mouse endotoxemia model of sepsis, but this protective effect is not sustained at higher LPS doses (Lamkanfi et al. 2010; Berghe et al. 2014). However, Casp11−/− and Gsdmd−/− mice are highly resistant to LPS-induced lethality (Lamkanfi et al. 2010; Kayagaki et al. 2011, 2015; Berghe et al. 2014). This decisive role for pyroptosis in LPS-induced endotoxic shock is at least partially related to the release of microvesicles containing TF by dying cells, thus initiating an overt systemic coagulation response (Wu et al. 2019). Similarly, on C. violaceum infection, IL-1β/IL-18 signaling contributes partially to host defense against this pathogen, whereas caspase-1/-11 double knockout mice succumb to infection (Maltez et al. 2015). Furthermore, caspase-1 and -11 are essential to control bacterial loads in the spleen, with no role found for inflammasome-produced cytokines. In an in vivo sterile model of inflammasome activation, intracellular flagellin delivery causes fast hypothermia in wild-type animals that is fully rescued by ablation of NLRC4 (von Moltke et al. 2012; Rauch et al. 2017). In contrast, Casp1−/− and Gsdmd−/− animals experience an intermediate phenotype. The hematocrit increases seen in wild-type animals was only observed in Casp1−/− at later time points, whereas Nlrc4−/− animals remained protected (von Moltke et al. 2012). A similar intermediate phenotype is seen on Legionella spp. infection, in which caspase-1/-11-deficient mice present with higher colony-forming unit (CFU) counts than wild-type controls, and Nlrc4−/− mice are even more susceptible (Mascarenhas et al. 2017).
These midway results obtained with Casp1−/− mice have been associated with caspase-8 activation at the ASC speck. Co-ablation of either ASC or caspase-8 (in RIPK3 knockout animals to prevent caspase-8-driven embryonic lethality [Kaiser et al. 2011; Oberst et al. 2011]) in the caspase-1/-11-deficient background phenocopies NLRC4 deletion on flagellin challenge in vivo or infection with Legionella spp. (Mascarenhas et al. 2017; Rauch et al. 2017). Therefore, in some disease settings, inflammasome signaling to caspase-8-dependent apoptosis suffices to clear the infected cell, resulting in a partial containment of the bacteria. Cell death also plays a role in the in vivo eicosanoid storm observed on NLRC4 triggering in epithelial cells, as ablation of both caspase-1 and -8 is required to protect mice from eicosanoid increase (Rauch et al. 2017).
In conclusion, it appears that there is an intricate relationship between inflammasome effector functions within several tissue compartments, which might determine susceptibility to specific pathogens. In B. thailandensis infection, IL-18 is required to up-regulate caspase-11 through IFN-γ production, and caspase-11-driven signaling is essential for mice to survive the infection (Aachoui et al. 2015). In addition, absence of caspase-11 does not alter S. Typhimurium bacterial loads in vivo, but Casp1−/− Casp11−/− mice in which caspase-11 expression was reconstituted from a C57BL/6 bacterial artificial chromosome (BAC) have higher CFUs in their organs than caspase-1/-11-codeleted controls (Broz et al. 2012). This surprisingly shows that caspase-11-mediated pyroptosis might be essential in the absence of caspase-1-driven signaling, and suggests proinflammatory cytokines and cell death may be needed simultaneously to promote full innate immune activation and protection.
Although the role of GSDMD in inflammasome-associated diseases remains less understood, much progress has been made in recent years. GSDMD was defined as an important effector to clear Francisella novicida in vivo infection (Banerjee et al. 2018; Zhu et al. 2018). However, similar to what has been observed with Casp1−/− mice, GSDMD-deficient animals have an intermediate phenotype on in vivo challenge with flagellin (Rauch et al. 2017). Defining the molecular mechanisms by which GSDMD-deficient cells switch to an apoptotic cell death mode on canonical inflammasome stimulation would help elucidate why Gsdmd−/− mice are not fully rescued on flagellin challenge. Remarkably, Gsdmd−/− animals were shown to clear Escherichia coli infection better than wild-type counterparts (Kambara et al. 2018). E. coli is sensed by caspase-11 in macrophages, triggering GSDMD-mediated pyroptosis (Kayagaki et al. 2011, 2015). Mice require caspase-11 to mount an immune response and survive in vivo delivery of E. coli outer membrane vesicles, showed to mediate LPS intracellular delivery (Vanaja et al. 2016; Santos et al. 2018). Although the protection observed in Gsdmd−/− mice was associated with lower cell death induction by neutrophils, this was not shown to be the case in vivo.
Inflammasome-initiated effector functions may play specific roles not only for infectious disease resolution, but also during development of sterile inflammasomopathies. These inflammasome-driven autoinflammatory diseases are caused by activating mutations in inflammasome-coding genes, and most of their symptoms recollect features of generalized inflammation, such as recurrent fever and body rash (Van Gorp et al. 2019). CAPS collectively represents three diseases—NOMID, Muckle–Wells syndrome (MWS), and familial cold autoinflammatory syndrome (FCAS)—which represent a spectrum of severity and are all caused by mutations in NLRP3 (Kuemmerle-Deschner 2015). Deletion of the IL-18R in mice carrying the Nlrp3 A350V allele, associated with MWS, is sufficient to rescue their survival (Brydges et al. 2013). In agreement with an important role for IL-18 in NLRC4-mediated autoinflammatory disease progression, a patient carrying the autoinflammatory NLRC4 variant V341A was successfully treated with recombinant IL-18-binding protein, which prevents IL-18 binding to its receptor, while blocking IL-1 alone did not provide a benefit to this patient (Romberg et al. 2014; Canna et al. 2017). Development of FMF is in most patients linked to mutations in the MEFV gene, which encodes the inflammasome receptor pyrin. A knockin mouse model that expresses a chimeric humanized version of Pyrin with an FMF-associated mutation, V726A, develops systemic autoinflammatory pathology that is fully dependent on IL-1β signaling (Sharma et al. 2017). Interestingly, deletion of GSDMD completely rescued circulating IL-1β levels and provided the same level of protection as seen in Il1b−/− mice, highlighting the role of pyroptosis for cytokine secretion in vivo (Sharma et al. 2017; Kanneganti et al. 2018). GSDMD-deletion also rescued all signs of autoinflammation seen in the Nlrp3 D301N mice, a mutation that causes NOMID in humans (Xiao et al. 2018). As deletion of GSDMD in these mice abolished circulating IL-1β levels, it would be of interest to determine the relative contribution of this cytokine to inflammatory pathology in Nlrp3 D301N mice. A critical role for cell death in driving NLRP3-mediated pathology has also been suggested in mice bearing the FCAS-linked L351P mutation in Nlrp3 (Brydges et al. 2013). Post-birth lethality seen in these animals is fully rescued by the absence of caspase-1, whereas co-ablation of IL-1R/IL-18 is only partially protective. Thus, pyroptosis appears to contribute to inflammasome-dependent cytokine secretion and other inflammasome-associated effector pathways. Modulating GSDMD activation may therefore hold promise for the treatment of inflammasome-driven diseases in the clinic.
CONCLUSIONS
Inflammasome activation is emerging as an increasingly complex mechanism for regulated activation of inflammatory caspases and induction of inflammatory and host defense responses. In addition to canonical proinflammatory cytokine maturation and secretion, GSDMD-driven pyroptosis is rapidly emerging as another key function associated with inflammasome activation that intercrosses with cytokine and DAMP release and other immune effector mechanisms. Functional analysis of additional caspase substrates that are cleaved during pyroptosis may reveal unexpected and diverse roles in intracellular signaling that contribute to how pyroptotic cells regulate immune activation and host defense. A better understanding of the diverse roles pyroptosis plays in the immune system could aid in understanding how inflammasome activation signals to neighbor cells to control pathogen spread and potentiates sterile tissue damage in inflammatory diseases. Furthermore, addressing these mechanisms could reveal novel therapeutic approaches to benefit patients suffering from a broad suite of inflammasome-mediated diseases.
ACKNOWLEDGMENTS
We apologize to colleagues whose work was not cited because of space constraints. This work was supported by European Research Council Grant 683144 (PyroPop) to M.L.
Footnotes
Editors: Kim Newton, James M. Murphy, and Edward A. Miao
Additional Perspectives on Cell Survival and Cell Death available at www.cshperspectives.org
REFERENCES
- Aachoui Y, Kajiwara Y, Leaf IA, Mao D, Ting JP, Coers J, Aderem A, Buxbaum JD, Miao EA. 2015. Canonical inflammasomes drive IFN-γ to prime caspase-11 in defense against a cytosol-invasive bacterium. Cell Host Microbe 18: 320–332. 10.1016/j.chom.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. 2016. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci 113: 7858–7863. 10.1073/pnas.1607769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, et al. 2009. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 5: e1000361 10.1371/journal.ppat.1000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, Ke F, Mason KD, White MJ, Stacey KJ, et al. 2014. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep 15: 982–990. 10.15252/embr.201438463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee I, Behl B, Mendonca M, Shrivastava G, Russo AJ, Menoret A, Ghosh A, Vella AT, Vanaja SK, Sarkar SN, et al. 2018. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49: 413–426.e5. 10.1016/j.immuni.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghe TV, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, Vuylsteke M, Roelandt R, Van Wonterghem E, Vandenbroecke J, et al. 2014. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 189: 282–291. 10.1164/rccm.201308-1535OC [DOI] [PubMed] [Google Scholar]
- Bertheloot D, Latz E. 2017. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol 14: 43–64. 10.1038/cmi.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. 1989. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1β. J Biol Chem 264: 5323–5326. [PubMed] [Google Scholar]
- Boucher D, Monteleone M, Coll RC, Chen KW, Ross CM, Teo JL, Gomez GA, Holley CL, Bierschenk D, Stacey KJ, et al. 2018. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J Exp Med 215: 827–840. 10.1084/jem.20172222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MA, Cookson BT. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol 38: 31–40. 10.1046/j.1365-2958.2000.02103.x [DOI] [PubMed] [Google Scholar]
- Broz P, Dixit VM. 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16: 407–420. 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. 2010. Differential requirement for caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8: 471–483. 10.1016/j.chom.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. 2012. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490: 288–291. 10.1038/nature11419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. 2013. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest 123: 4695–4705. 10.1172/JCI71543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10: 266–272. 10.1038/ni.1702 [DOI] [PubMed] [Google Scholar]
- Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, et al. 2017. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 139: 1698–1701. 10.1016/j.jaci.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Olvera I, Sahoo M, Miller MA, Barrio L, Re F. 2011. Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1β is deleterious. PLoS Pathog 7: e1002452 10.1371/journal.ppat.1002452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao KL, Kulakova L, Herzberg O. 2017. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci 114: E1128–E1137. 10.1073/pnas.1616783114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Bartok E, Gaidt MM, Bock FJ, Herrmann J, Seeger JM, Broz P, Beckmann R, Kashkar H, Tait SWG, et al. 2018. BAX/BAK-induced apoptosis results in caspase-8-dependent IL-1β maturation in macrophages. Cell Rep 25: 2354–2368.e5. 10.1016/j.celrep.2018.10.087 [DOI] [PubMed] [Google Scholar]
- Chen LM, Kaniga K, Galan JE. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol 21: 1101–1115. 10.1046/j.1365-2958.1996.471410.x [DOI] [PubMed] [Google Scholar]
- Chen KW, Groß CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, Schroder K. 2014. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8: 570–582. 10.1016/j.celrep.2014.06.028 [DOI] [PubMed] [Google Scholar]
- Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. 2018. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 3: eaar6676 10.1126/sciimmunol.aar6676 [DOI] [PubMed] [Google Scholar]
- Cookson BT, Brennan MA. 2001. Pro-inflammatory programmed cell death. Trends Microbiol 9: 113–114. 10.1016/S0966-842X(00)01936-3 [DOI] [PubMed] [Google Scholar]
- Cullen SP, Kearney CJ, Clancy DM, Martin SJ. 2015. Diverse activators of the NLRP3 inflammasome promote IL-1β secretion by triggering necrosis. Cell Rep 11: 1535–1548. 10.1016/j.celrep.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, et al. 2018. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity 49: 740–753.e7. 10.1016/j.immuni.2018.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M. 2019a. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ 26: 146–161. 10.1038/s41418-018-0106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos NM, Vliegen G, Gonçalves A, De Hert E, Martín-Pérez R, Van Opdenbosch N, Jallapally A, Geiss-Friedlander R, Lambeir A-M, Augustyns K, et al. 2019b. DPP8/DPP9 inhibition elicits canonical Nlrp1b inflammasome hallmarks in murine macrophages. Life Sci Alliance 2: e201900313 10.26508/lsa.201900313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick MS, Sborgi L, Rühl S, Hiller S, Broz P. 2016. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun 7: 11929 10.1038/ncomms11929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27: 519–550. 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. 2016. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535: 111–116. 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- Erener S, Pétrilli V, Kassner I, Minotti R, Castillo R, Santoro R, Hassa Paul O, Tschopp J, Hottiger Michael O. 2012. Inflammasome-activated caspase 7 cleaves PARP1 to enhance the expression of a subset of NF-κB target genes. Mol Cell 46: 200–211. 10.1016/j.molcel.2012.02.016 [DOI] [PubMed] [Google Scholar]
- Erlich Z, Shlomovitz I, Edry-Botzer L, Cohen H, Frank D, Wang H, Lew AM, Lawlor KE, Zhan Y, Vince JE, et al. 2019. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat Immunol 20: 397–406. 10.1038/s41590-019-0313-5 [DOI] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. 2018. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48: 35–44.e6. 10.1016/j.immuni.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458: 509–513. 10.1038/nature07710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, et al. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11: 385–393. 10.1038/ni.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. 2006. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol 8: 1812–1825. 10.1111/j.1462-5822.2006.00751.x [DOI] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Özören N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat Immunol 7: 576–582. 10.1038/ni1346 [DOI] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. 2016. Human monocytes engage an alternative inflammasome pathway. Immunity 44: 833–846. 10.1016/j.immuni.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. 1997. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature 386: 619–623. 10.1038/386619a0 [DOI] [PubMed] [Google Scholar]
- Gonzalez Ramirez ML, Poreba M, Snipas SJ, Groborz K, Drag M, Salvesen GS. 2018. Extensive peptide and natural protein substrate screens reveal that mouse caspase-11 has much narrower substrate specificity than caspase-1. J Biol Chem 293: 7058–7067. 10.1074/jbc.RA117.001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. 1997. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275: 206–209. 10.1126/science.275.5297.206 [DOI] [PubMed] [Google Scholar]
- Guey B, Bodnar M, Manié SN, Tardivel A, Petrilli V. 2014. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc Natl Acad Sci 111: 17254–17259. 10.1073/pnas.1415756111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RKS, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. 2014. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192: 1835–1846. 10.4049/jimmunol.1302839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 25: 1285–1298. 10.1038/cr.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. 2018. The gasdermin-D pore acts as a conduit for IL-1β secretion in mice. European J Immunol 48: 584–592. 10.1002/eji.201747404 [DOI] [PubMed] [Google Scholar]
- Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci 96: 2396–2401. 10.1073/pnas.96.5.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell RA, Yuan J, Sansonetti PJ, Zychlinsky A. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem 273: 32895–32900. 10.1074/jbc.273.49.32895 [DOI] [PubMed] [Google Scholar]
- Hughes MA, Powley IR, Jukes-Jones R, Horn S, Feoktistova M, Fairall L, Schwabe JW, Leverkus M, Cain K, MacFarlane M. 2016. Co-operative and hierarchical binding of c-FLIP and caspase-8: a unified model defines how c-FLIP isoforms differentially control cell fate. Mol Cell 61: 834–849. 10.1016/j.molcel.2016.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. 2009. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183: 3578–3581. 10.4049/jimmunol.0901323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O'Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, et al. 2010. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci 107: 9771–9776. 10.1073/pnas.1003738107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, Zhang Y, Krantz BA, Miao EA. 2016. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med 213: 2113–2128. 10.1084/jem.20151613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. 2011. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471: 368–372. 10.1038/nature09857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, Zhao L, Zhou S, Yu H, Zhou W, et al. 2018. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep 22: 2924–2936. 10.1016/j.celrep.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti A, Malireddi RKS, Saavedra PHV, Vande Walle L, Van Gorp H, Kambara H, Tillman H, Vogel P, Luo HR, Xavier RJ, et al. 2018. GSDMD is critical for autoinflammatory pathology in a mouse model of familial Mediterranean fever. J Exp Med 215: 1519–1529. 10.1084/jem.20172060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, Lee E, Place D, Samir P, Mavuluri J, Sharma BR, Balakrishnan A, Malireddi RKS, Geiger R, Zhu Q, et al. 2018. IRF8 regulates transcription of Naips for NLRC4 inflammasome activation. Cell 173: 920–933.e13. 10.1016/j.cell.2018.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121. 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. 2015. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526: 666–671. 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, Schmidt JA. 1989. Identification of a monocyte specific pre-interleukin 1β convertase activity. Proc Natl Acad Sci 86: 5227–5231. 10.1073/pnas.86.14.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, Ji C, Gan J, Xu XW, Li J. 2017. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci 114: 10642–10647. 10.1073/pnas.1708194114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle-Deschner JB. 2015. CAPS—pathogenesis, presentation and treatment of an autoinflammatory disease. Semin Immunopathol 37: 377–385. 10.1007/s00281-015-0491-7 [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. 1995. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science 267: 2000–2003. 10.1126/science.7535475 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M. 2011. Emerging inflammasome effector mechanisms. Nat Rev Immunol 11: 213–220. 10.1038/nri2936 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. 2014. Mechanisms and functions of inflammasomes. Cell 157: 1013–1022. 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Van Damme P, Vanden Berghe T, Vanoverberghe I, Vandekerckhove J, Vandenabeele P, Gevaert K, Núñez G. 2008. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol Cell Proteomics 7: 2350–2363. 10.1074/mcp.M800132-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. 2010. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 185: 4385–4392. 10.4049/jimmunol.1000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, et al. 2015. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun 6: 6282 10.1038/ncomms7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Mirrashidi KM, Stowe IB, Kummerfeld SK, Watanabe C, Haley B, Cuellar TL, Reichelt M, Kayagaki N. 2018a. ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci Rep 8: 3788 10.1038/s41598-018-21998-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Stowe IB, Gupta A, Kornfeld OS, Roose-Girma M, Anderson K, Warming S, Zhang J, Lee WP, Kayagaki N. 2018b. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med 215: 2279–2288. 10.1084/jem.20180589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Yamaguchi Y, Shirasaki Y, Shikada K, Yamagishi M, Hoshino K, Kaisho T, Takemoto K, Suzuki T, Kuranaga E, et al. 2014. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep 8: 974–982. 10.1016/j.celrep.2014.07.012 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. 2016. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535: 153–158. 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Rathkey JK, Yang J, Dubyak GR, Abbott DW, Xiao TS. 2018. Structures of the gasdermin D C-terminal domains reveal mechanisms of autoinhibition. Structure 26: 778–784.e3. 10.1016/j.str.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, et al. 2012. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488: 670–674. 10.1038/nature11290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA, Wu H, Egelman EH. 2014. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156: 1193–1206. 10.1016/j.cell.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RK, Ippagunta S, Lamkanfi M, Kanneganti TD. 2010. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol 185: 3127–3130. 10.4049/jimmunol.1001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, Kanneganti T-D. 2018. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med 215: 1023–1034. 10.1084/jem.20171922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltez VI, Tubbs AL, Cook KD, Aachoui Y, Falcone EL, Holland SM, Whitmire JK, Miao EA. 2015. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity 43: 987–997. 10.1016/j.immuni.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. 2013. Salmonella infection induces recruitment of caspase-8 to the inflammasome to modulate IL-1β production. J Immunol 191: 5239–5246. 10.4049/jimmunol.1301581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. 2018. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17: 688 10.1038/nrd.2018.149 [DOI] [PubMed] [Google Scholar]
- Mascarenhas DPA, Cerqueira DM, Pereira MSF, Castanheira FVS, Fernandes TD, Manin GZ, Cunha LD, Zamboni DS. 2017. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog 13: e1006502 10.1371/journal.ppat.1006502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O'Donnell JA, McArthur K, Baldwin TM, Chevrier S, Nowell CJ, et al. 2012. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity 37: 1009–1023. 10.1016/j.immuni.2012.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al. 2014. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509: 366–370. 10.1038/nature13157 [DOI] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol 7: 569–575. 10.1038/ni1344 [DOI] [PubMed] [Google Scholar]
- Moayeri M, Crown D, Newman ZL, Okugawa S, Eckhaus M, Cataisson C, Liu S, Sastalla I, Leppla SH. 2010. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog 6: e1001222 10.1371/journal.ppat.1001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monack DM, Raupach B, Hromockyj AE, Falkow S. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci 93: 9833–9838. 10.1073/pnas.93.18.9833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone M, Stow JL, Schroder K. 2015. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 74: 213–218. 10.1016/j.cyto.2015.03.022 [DOI] [PubMed] [Google Scholar]
- Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. 1987. The interleukin-1 receptor binds the human interleukin-1α precursor but not the interleukin-1β precursor. J Biol Chem 262: 2941–2944. [PubMed] [Google Scholar]
- Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Müller DJ. 2018. Mechanism of membrane pore formation by human gasdermin-D. EMBO J 37: e98321 10.15252/embj.201798321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. 2011. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471: 363–367. 10.1038/nature09852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okondo MC, Rao SD, Taabazuing CY, Chui AJ, Poplawski SE, Johnson DC, Bachovchin DA. 2018. Inhibition of Dpp8/9 activates the Nlrp1b inflammasome. Cell Chem Biol 25: 262–267.e5. 10.1016/j.chembiol.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Beeck K, Van Laer L, Van Camp G. 2012. DFNA5, a gene involved in hearing loss and cancer: a review. Ann Otol Rhinol Laryngol 121: 197–207. 10.1177/000348941212100310 [DOI] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, et al. 2018. Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 362: 1064–1069. 10.1126/science.aau2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V. 2018. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18: 134–147. 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J. 2014. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci 111: 6046–6051. 10.1073/pnas.1321700111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri AW, Broz P, Shen A, Monack DM, Bogyo M. 2012. Caspase-1 activity is required to bypass macrophage apoptosis upon Salmonella infection. Nat Chem Biol 8: 745–747. 10.1038/nchembio.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Jin M, Desai BN, Penumaka A, Zhu H, Kober M, Dietrich A, Lipinski MM, Henry T, Clapham DE, et al. 2014. Caspase-11 controls interleukin-1β release through degradation of TRPC1. Cell Rep 6: 1122–1128. 10.1016/j.celrep.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11: 395–402. 10.1038/ni.1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR, et al. 2018. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3: eaat2738 10.1126/sciimmunol.aat2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch I, Deets KA, Ji DX, von Moltke J, Tenthorey JL, Lee AY, Philip NH, Ayres JS, Brodsky IE, Gronert K, et al. 2017. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46: 649–659. 10.1016/j.immuni.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. 2017. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun 8: 14128 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, Overton J, Meffre E, Khokha MK, Huttner AJ, et al. 2014. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet 46: 1135–1139. 10.1038/ng.3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C, Chan AH, Von Pein J, Boucher D, Schroder K. 2018. Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome. Life Sci Alliance 1: e201800237 10.26508/lsa.201800237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, Wu H. 2018. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557: 62–67. 10.1038/s41586-018-0058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. 1990. A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J 9: 1503–1510. 10.1002/j.1460-2075.1990.tb08268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl S, Broz P. 2015. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux. Eur J Immunol 45: 2927–2936. 10.1002/eji.201545772 [DOI] [PubMed] [Google Scholar]
- Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. 2018. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362: 956–960. 10.1126/science.aar7607 [DOI] [PubMed] [Google Scholar]
- Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR. 2016. Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. J Immunol 197: 1353–1367. 10.4049/jimmunol.1600699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. 2000. Gasdermin (Gsdm) localizing to mouse chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome 11: 718–724. 10.1007/s003350010138 [DOI] [PubMed] [Google Scholar]
- Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, et al. 2013. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20: 1149–1160. 10.1038/cdd.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JC, Dick MS, Lagrange B, Degrandi D, Pfeffer K, Yamamoto M, Meunier E, Pelczar P, Henry T, Broz P. 2018. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J 37: e98089 10.15252/embj.201798089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, Rongvaux A, Bunnell SC, Shao F, Green DR, et al. 2018. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci 115: E10888 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, Hiller S. 2016. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35: 1766–1778. 10.15252/embj.201694696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. 2015. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol 45: 2911–2917. 10.1002/eji.201545523 [DOI] [PubMed] [Google Scholar]
- Schmidt FI, Lu A, Chen JW, Ruan J, Tang C, Wu H, Ploegh HL. 2016. A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. J Exp Med 213: 771–790. 10.1084/jem.20151790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider KS, Groß CJ, Dreier RF, Saller BS, Mishra R, Gorka O, Heilig R, Meunier E, Dick MS, Ćiković T, et al. 2017. The Inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep 21: 3846–3859. 10.1016/j.celrep.2017.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. 2007. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 282: 36321–36329. 10.1074/jbc.M708182200 [DOI] [PubMed] [Google Scholar]
- Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, Hauenstein AV, Wu Z, Núñez G, Mao Y, et al. 2019. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570: 338–343. 10.1038/s41586-019-1295-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Sharma BR, Vogel P, Kanneganti TD. 2017. IL-1β and caspase-1 drive autoinflammatory disease independently of IL-1α or caspase-8 in a mouse model of familial Mediterranean fever. Am J Pathol 187: 236–244. 10.1016/j.ajpath.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192. 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Götz F, et al. 2010. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1β secretion. Cell Host Microbe 7: 38–49. 10.1016/j.chom.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, et al. 2018. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3: eaar6689 10.1126/sciimmunol.aar6689 [DOI] [PubMed] [Google Scholar]
- Taabazuing CY, Okondo MC, Bachovchin DA. 2017. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol 24: 507–514.e4. 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K, Sumiyama K, Sagai T, Shiroishi T. 2007. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89: 618–629. 10.1016/j.ygeno.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. 2008. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231–241. 10.1038/nrm2312 [DOI] [PubMed] [Google Scholar]
- Terra JK, Cote CK, France B, Jenkins AL, Bozue JA, Welkos SL, LeVine SM, Bradley KA. 2010. Cutting edge: resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol 184: 17–20. 10.4049/jimmunol.0903114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixeira R, Shi B, Parkes MAF, Hodge AL, Caruso S, Hulett MD, Baxter AA, Phan TK, Poon IKH. 2018. Gasdermin E does not limit apoptotic cell disassembly by promoting early onset of secondary necrosis in Jurkat T cells and THP-1 monocytes. Front Immunol 9: 2842 10.3389/fimmu.2018.02842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, Hori O, Mahib MR, Yamaguchi Y, Miura M, et al. 2019. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun 10: 2091 10.1038/s41467-019-09753-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK. 2016. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165: 1106–1119. 10.1016/j.cell.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp H, Van Opdenbosch N, Lamkanfi M. 2019. Inflammasome-dependent cytokines at the crossroads of health and autoinflammatory disease. Cold Spring Harb Perspect Biol 11: a028563 10.1101/cshperspect.a028563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laer L, Huizing EH, Verstreken M, van Zuijlen D, Wauters JG, Bossuyt PJ, Van de Heyning P, McGuirt WT, Smith RJ, Willems PJ, et al. 1998. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet 20: 194–197. 10.1038/2503 [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N, Gurung P, Vande Walle L, Fossoul A, Kanneganti TD, Lamkanfi M. 2014. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun 5: 3209 10.1038/ncomms4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Opdenbosch N, Van Gorp H, Verdonckt M, Saavedra PHV, de Vasconcelos NM, Goncalves A, Vande Walle L, Demon D, Matusiak M, Van Hauwermeiren F, et al. 2017. Caspase-1 engagement and TLR-induced c-FLIP expression suppress ASC/caspase-8-dependent apoptosis by inflammasome sensors NLRP1b and NLRC4. Cell Rep 21: 3427–3444. 10.1016/j.celrep.2017.11.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, Simpson D, Vijayaraj S, Lindqvist LM, Bouillet P, et al. 2018. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep 25: 2339–2353.e4. 10.1016/j.celrep.2018.10.103 [DOI] [PubMed] [Google Scholar]
- Voet S, Srinivasan S, Lamkanfi M, van Loo G. 2019. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med 11: e10248 10.15252/emmm.201810248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, et al. 2012. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490: 107–111. 10.1038/nature11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. 2017. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547: 99–103. 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- Willingham SB, Bergstralh DT, O'Connor W, Morrison AC, Taxman DJ, Duncan JA, Barnoy S, Venkatesan MM, Flavell RA, Deshmukh M, et al. 2007. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2: 147–159. 10.1016/j.chom.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Lu W, Zhang Y, Zhang G, Shi X, Hisada Y, Grover SP, Zhang X, Li L, Xiang B, et al. 2019. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 50: 1401–1411.e4. 10.1016/j.immuni.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, Civitelli R, Abu-Amer Y, Kanneganti TD, Link DC, et al. 2018. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol 16: e3000047 10.1371/journal.pbio.3000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, et al. 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513: 237–241. 10.1038/nature13449 [DOI] [PubMed] [Google Scholar]
- Yabal M, Müller N, Adler H, Knies N, Groß Christina J, Damgaard Rune B, Kanegane H, Ringelhan M, Kaufmann T, Heikenwälder M, et al. 2014. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep 7: 1796–1808. 10.1016/j.celrep.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. 2014. Inflammasome activation leads to caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci 111: 15514–15519. 10.1073/pnas.1414859111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, et al. 2016. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352: 1232–1236. 10.1126/science.aaf3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Springstead JR, Kagan JC. 2017. By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity 47: 697–709.e3. 10.1016/j.immuni.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WH, Wang X, Narayanan M, Zhang Y, Huo C, Reed JC, Friedlander RM. 2003. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci 100: 16012–16017. 10.1073/pnas.2534856100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong FL, Mamaï O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverényi I, Takeichi T, Balaji R, Lau A, et al. 2016. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell 167: 187–202.e17. 10.1016/j.cell.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Zhong FL, Robinson K, Teo DET, Tan KY, Lim C, Harapas CR, Yu CH, Xie WH, Sobota RM, Au VB, et al. 2018. Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding. J Biol Chem 293: 18864–18878. 10.1074/jbc.RA118.004350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti T-D. 2018. Gasdermin D promotes AIM2 inflammasome activation and is required for host protection against Francisella novicida. J Immunol 201: 3662–3668. 10.4049/jimmunol.1800788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358: 167–169. 10.1038/358167a0 [DOI] [PubMed] [Google Scholar]