Abstract
Calcium (Ca2+) and cyclic AMP (cAMP) signaling cross talk and synergize to stimulate the cardinal functions of exocrine cells, regulated exocytosis, and fluid and electrolyte secretion. This physiological process requires the organization of the two signaling pathways into complexes at defined cellular domains and close placement. Such domains are formed by membrane contact sites (MCS). This review discusses the basic properties of Ca2+ signaling in exocrine cells, the role of MCS in the organization of cell signaling and in cross talk and synergism between the Ca2+ and cAMP signaling pathways and, finally, the mechanism by which the Ca2+ and cAMP pathways synergize to stimulate epithelial fluid and electrolyte secretion.
Calcium (Ca2+) signaling synergizes with other signaling pathways, in particular the cyclic AMP (cAMP) pathway, to mediate numerous cellular functions, including the cardinal functions of exocrine cells, regulated exocytosis, and fluid and electrolyte secretion (Lee et al. 2012). Both functions are vectorial and polarized and thus the signals that regulate them must be generated and function in a polarized and vectorial manner. Moreover, intracellular organelles, mitochondria, lysosomes, endosomes, and secretory granules shape the transduction signals and control the signal localization, propagation pattern, and distance (Raffaello et al. 2016). To achieve this form of communication, signaling proteins and cellular organelles are expressed in a highly polarized manner and signaling proteins are organized in specific cellular microdomains. These microdomains ensure the specificity of signaling to meet physiological needs and allow communication and transfer of material and molecules between organelles.
Each signaling pathway includes multiple proteins with defined function. In the complexes, the proteins are in molecular distances with respect to each other. Compartmentalized signaling complexes are found in the plasma membrane (PM), the endoplasmic reticulum (ER), endosomes, lysosomes, mitochondria, and the nuclear envelope. A significantly improved molecular understanding of cell signaling and communication within signaling pathways emerged with the improved understanding of formation and function of membrane contact sites (MCS). MCS are formed by the ER, which spans the entire cell cytosol, and the membranes of all other cellular membranes. MCS are formed by tethering proteins that form a bridge between the PM and the ER to form the ER/PM junctions (Cao et al. 2015; Henne et al. 2015). There are specific tether proteins that form MCS between the ER/mitochondria (Westermann 2015), ER/endosomes, ER/lysosomes (Penny et al. 2015), ER/Golgi (Mesmin et al. 2019), and ER/nuclear membrane (Prinz 2014). Many MCS serve to communicate and transfer material between membranes, particularly lipids and signaling molecules (Ca2+, cAMP, and possibly others) (Lahiri et al. 2015). In this review, we focus on cell signaling in exocrine glands and the significance of MCS, in particular the ER/PM junction, in exocrine cell signaling. The role of other MCS in cell signaling can be found in Prinz (2014), Penny et al. (2015), Muallem et al. (2017), Tepikin (2018), and Wu et al. (2018).
BASIC PROPERTIES OF Ca2+ SIGNALING IN EXOCRINE CELLS
The receptor-evoked Ca2+ signal is dependent on a combination of Ca2+ pumps that generate Ca2+ gradients across the PM, the ER membrane, and the membrane of all other cellular organelles. The PM Ca2+ ATPase (PMCA) pump (Calì et al. 2017) is the primary Ca2+ extruding mechanism at the PM that forms a 104-fold Ca2+ gradient between the cytoplasm and the cell exterior. The sarco-ER ATPase (SERCA) pump (Chemaly et al. 2018) mediates Ca2+ uptake into the ER to form about a 5 × 103-fold gradient between the ER and the cytoplasm. The secretory pathway Ca2+ ATPase (SPCA) pumps mainly pump Ca2+ into the Golgi (Dang and Rao 2016). Ca2+ uptake into the mitochondria is driven by the mitochondrial membrane potential and is mediated by the mitochondrial Ca2+ uniporter (MCU) complex that includes the pore forming MCU, the gatekeepers MICU1 and MICU2, and the scaffold EMRE (Nemani et al. 2018). The mechanism mediating Ca2+ uptake into lysosomes, endosomes, and secretory granules is not well understood, although a recent study suggested that Ca2+ released from the ER at ER/lysosomes MCS feeds the lysosomes with Ca2+ that is taken into the lysosome by an unknown pathway (Garrity et al. 2016). On the other hand, a transporter proposed to function as Ca2+/H+ exchanger named CAX is expressed in acidic organelles and affect their Ca2+ content, which may be the major Ca2+ uptake mechanism in these organelles and take up Ca2+ released by the ER (Melchionda et al. 2016).
Two Ca2+ sources contribute to the receptor-mediated increase in cytoplasmic Ca2+: Ca2+ release from intracellular stores and Ca2+ influx from the extracellular space into the cell cytosol. These Ca2+ sources are utilized for signaling when exocrine cells are stimulated with a variety of hormones and neurotransmitters. It is only a fraction of the Ca2+ stored in the ER that is released into the cytoplasm during each Ca2+ spike (Petersen and Tepikin 2008), but it is sufficient to activate Ca2+ influx at the PM, at least at Orai1 and stromal interaction molecule 1 (STIM1) clustering domains (see below). Ca2+ release from the ER is mediated by Ca2+ channels called inositol 1,4,5-trisphosphate receptors (IP3 receptors or IP3Rs) (Mikoshiba 2015). Ca2+ is also released from lysosomes and endosomes to increase the Ca2+ concentration in the immediate vicinity of these organelles and regulate their activity. TRPML1 is a primary Ca2+ release channel in lysosomes (Di Paola et al. 2018), while TRPML3 releases Ca2+ from early and late endosomes (Yamaguchi et al. 2011; Grimm et al. 2014). Ca2+ release from lysosomes and endosomes also depends on the two-pore channels (TPCs), TPC1 (mainly endosomes) and TPC2 (mainly lysosomes) (Grimm et al. 2017a,b), because their deletion or inhibition eliminates endosomal and lysosomal Ca2+ release. The TPC channels are activated by phosphatidylinositol 3,5-bisphosphate (PI(3,5)P2) (Xu and Ren 2015) and nicotinic acid adenine dinucleotide phosphate (NAADP) (Jha et al. 2014; Grimm et al. 2017b) to mediate the NAADP-triggered Ca2+ release. Ca2+ is also released from secretory granules and almost all known Ca2+ release channels were found in secretory granules, including IP3Rs, ryanodine receptors (RyRs), and the TPCs (Petersen 2015). It is not clear whether these channels are expressed in different secretory granule populations, or whether all channels are expressed in the same granules. The major source of Ca2+ entry in the cytosol is provided by the PM Ca2+ influx channels that are activated in response to, or shortly after, Ca2+ release. These include the store-operated Orai channels (Prakriya and Lewis 2015), the receptor-stimulated TRPC (Nishida et al. 2015; Chung et al. 2017), and other TRP channels (Nishida et al. 2015), which control Ca2+ influx across the PM.
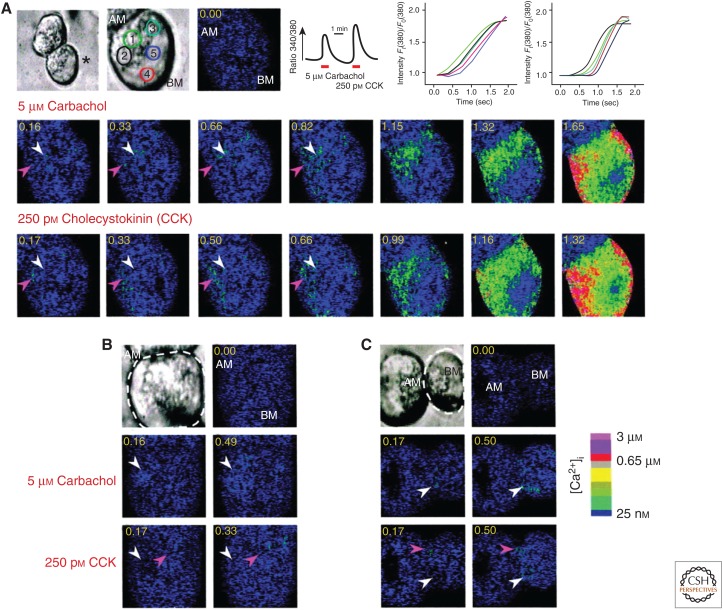
A characteristic feature of exocrine cells is the polarized expression of receptors and signaling proteins with their prominent concentration at the apical pole. This localization results in the generation of specific Ca2+ signals with respect to initiation site, propagation pattern, and ultimately a physiological response. The localized Ca2+ signal is shown in Figure 1 (taken from Shin et al. 2001). The same pancreatic acinar cells were stimulated consecutively with the M3 agonist carbachol and then with cholecystokinin (CCK). The receptor-evoked Ca2+ signal starts with generation of IP3, which then binds to the IP3 receptors that are expressed at a high level at the apical pole (Lee et al. 1997b; Yule et al. 1997), thereby causing Ca2+ release within the apical region of the ER. Ca2+ released at the apical region either remains confined to the apical pole (Thorn et al. 1993; Tinel et al. 1999), or propagates in the form of a Ca2+ wave to the basal pole along the lateral membrane (Hong et al. 2011; Sneyd et al. 2017). Several (but not all) G-protein-coupled receptors (GPCRs) generate NAADP simultaneously with IP3 to trigger additional Ca2+ release from endosomes, lysosomes, and possibly secretory granules at the apical pole (Cancela et al. 2002; Gerasimenko et al. 2006; Gerasimenko et al. 2015). Indeed, the receptors that generate NAADP, and those that do not, appear to engage different Ca2+ pools. This is evident from the generation of distinctive agonist-specific Ca2+ signals (Shin et al. 2001). Ca2+ release from the ER is followed by activation of PM Ca2+ influx channels that involves clustering of the ER Ca2+ sensor STIM1 (Liou et al. 2005; Roos et al. 2005) at the ER/PM junctions where it interacts with and activates Orai1 (Zhang et al. 2006; Prakriya and Lewis 2015) and TRPC channels (Bodnar et al. 2017). Interaction of STIM1-Orai1 and likely STIM1-TRPC channels take place at phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-rich MCS (Maléth et al. 2014).
Figure 1.
Receptor-specific initiation site and propagation pattern of Ca2+ waves. Cells were sequentially stimulated with 5 μm carbachol and 0.25 nm cholecystokinin (CCK), as indicated in the traces in the upper panel. (A) Initiation site and pattern of the Ca2+ wave evoked by activation of the M3 and CCK receptors in the same cell. (B,C) Additional examples of the different Ca2+ wave initiation sites evoked by carbachol (white arrowhead) and CCK (magenta arrowhead) stimulation in the same cells. AM, Apical membrane; BM, basal membrane. (From Shin et al. 2001; adapted, with permission, from the American Society for Biochemistry and Molecular Biology © 2001.)
Long-lasting elevation of cytoplasmic Ca2+ concentration is toxic. As a protection, cells express Ca2+-regulated Ca2+ clearance mechanisms, including SERCA, PMCA, and SPCA, and negative feedback loops to restrict the cytosolic Ca2+ concentration from being continually elevated. A high cytoplasmic Ca2+ concentration partially inhibits the IP3 receptors to restrict ER Ca2+ release (Mak and Foskett 2015), and the Orai (Prakriya and Lewis 2015; Parekh 2017) and TRPC channels (Beech 2013) to reduce Ca2+ influx. Some of the cytoplasmic Ca2+ is taken up by mitochondria to activate energy production and regulate other mitochondrial functions (Gunter et al. 2004; Petersen 2012; Giorgi et al. 2018). However, the bulk of the Ca2+ is reincorporated into the ER by the SERCA pumps, and part of it is extruded out of the cells by the PMCA pumps (Bruce 2018). The cycle of Ca2+ release, clearance, and Ca2+ store refilling is repeated during the period of cell stimulation, resulting in Ca2+ oscillations.
SIGNALING AT APICAL POLE MEMBRANE CONTACT SITES IN EXOCRINE CELLS
The cardinal functions of all epithelial cells are vectorial in nature. This is achieved by polarized cell structure with basolateral and apical membranes that are structurally and functional separated by the tight junctions. The basolateral membrane receives signals from the circulating hormones and neurotransmitters secreted by nerve endings to varicosities, whereas the apical membrane receives signals secreted by neighboring cells (hormones, proteases, and chemicals such as ATP). Signals received by receptors on one membrane (i.e., basolateral or apical) generate messengers that transmit the signals to the cell interior and to the other membrane to ensure coordination and fidelity of cellular function. This process is achieved by the signaling complexes at MCS in all cellular membranes. The ER plays a vital role in the formation of MCS as it spans the entire cytoplasm and thus establishes contacts with all cellular organelles. Moreover, signals like Ca2+ diffuse within the ER to deliver messages to sites where it cannot be delivered by diffusion through the cytosol because of cytoplasmic Ca2+ buffers (Chen et al. 2015).
The best example of signaling at MCS is Ca2+ signaling. Ca2+ signaling complexes are formed at the ER/PM junction (Muallem et al. 2017), ER/mitochondria (Tepikin 2018), ER/lysosomes (Penny et al. 2015), ER/endosomes (Kilpatrick et al. 2017), and ER/phagosomes (Nunes-Hasler and Demaurex 2017) contacts. Below, we illustrate Ca2+ signaling at MCS by describing signaling at the ER/PM MCS. The ER/PM junctions in secretory cells are formed mostly at the apical pole. Receptors at these junctions include several GPCRs, including the CCK, muscarinic M3, and the vasoactive intestinal peptide (VIP) receptors (Hodges et al. 1998; Shin et al. 2001). High levels of all three IP3 receptor isoforms at the apical pole have been demonstrated by immunolocalization and functional assays (Lee et al. 1997b; Yule et al. 1997), with IP3R2 and IP3R3 being the dominant receptors in exocrine cells (Yule et al. 2010). The PMCA1 is localized at the lateral and basal membranes (Lee et al. 1997a), whereas the PMCA4 that respond to agonist stimulation is confined to the ER/PM junctions at the apical pole (Shin et al. 2001). Orai1 is markedly enriched at the apical pole, and the ER Ca2+ sensor STIM1 clusters at the apical pole upon depletion of ER Ca2+ (Hong et al. 2011). Exocrine cells also express the TRPC1, TRPC3, and TRPC6 channels at the apical pole (Kim et al. 2006; Hong et al. 2011). The scaffolding protein Homer1 is localized at the apical pole, binds IP3Rs, TRPC channels, GPCRs (Worley et al. 2007; Yuan et al. 2012), and PMCA (Yang et al. 2014), and aids in the assembly of the complexes localized to the apical pole. The components of the cAMP pathway are also found at the apical pole ER/PM junctions. The membrane localization and function of Ca2+-dependent adenylyl cyclases (ACs) (Nlend et al. 2007), the soluble AC (Kolodecik et al. 2012), AKAP proteins, PKA (Zinn et al. 2015), and phosphodiesterase 4D (Barnes et al. 2005) have been demonstrated in the apical pole of exocrine cells.
The assembly of Ca2+ signaling components at ER/PM junctions in exocrine cells results in polarized Ca2+ signals that are initiated at the apical pole when cells are stimulated with physiological agonist concentrations (Kasai and Augustine 1990; Thorn et al. 1993; Lee et al. 1997b; Shin et al. 2001). In this manner, an agonist-induced Ca2+ increase occurs at the site where exocytosis of secretory granules take place and fluid and electrolyte secretion is initiated (Lee et al. 2012). For example, granule exocytosis by pancreatic acinar cells is triggered by increases in free cytoplasmic Ca2+ concentration (Messenger et al. 2014). Exocytosis by salivary gland acinar cells is triggered by increases in cytoplasmic cAMP concentration (Proctor and Carpenter 2014), which is likely caused by cAMP generated at the apical pole. Fluid secretion by pancreatic, salivary, and sweat gland acinar cells follows Ca2+ oscillations at the apical pole.
In acinar cells, the initial increase in [Ca2+]i as a result of Ca2+ release from the ER activates the Ca2+-activated Cl− channel anoctamin 1 (TMEM16A) at the apical membrane (Park et al. 2001; Catalán et al. 2015; Concepcion et al. 2016; Concepcion and Feske 2017) and K+ channels at both the apical and basolateral (Romanenko et al. 2006, 2007; Almassy et al. 2012) membranes. However, ER Ca2+ content is limited and requires continuous replenishment by Orai1-mediated Ca2+ influx, without which anoctamin 1 (ANO1) activation and secretion stops, both in mouse models and in human with nonfunctional mutations, or deletion, of Orai1 (Concepcion and Feske 2017). Ca2+- dependent activation of ANO1 and K+ channels results in Cl− and K+ efflux and cell shrinkage. Electrical neutrality is maintained by paracellular Na+ flow through the tight junctions. NaCl secretion dives fluid secretion through the water channel AQP5 (Zeng et al. 2017). During a Ca2+ spike, cell shrinkage inhibits Ca2+ signaling by an unknown mechanism, causing reduction in [Ca2+]i that leads to reduced ANO1 and K+ channel activity, and consequently less fluid secretion. Cell shrinkage activates the basolateral Na+/K+/2Cl− cotransporter NKCC1 (Melvin et al. 2005; Lee et al. 2012) to replenish K+ and Cl− and cell volume. The Na+ entering the cells through NKCC1 is exchanged with K+ by the Na+ pump. A second Ca2+ spike is now initiated to trigger another fluid and electrolyte secretory cycle. Hence, acinar cells function as fluid and electrolyte secretory pumps driven by repetitive Ca2+ spikes (also termed Ca2+ oscillations).
As indicated above, all components of the cAMP signaling pathway are also localized at the apical pole ER/PM junctions in exocrine cells. Although measurement of the dynamics of receptor-stimulated cAMP generation was not reported as yet in exocrine cells, the localization of the cAMP signaling pathway at the apical pole and the cross talk and synergism between the Ca2+ and cAMP pathways suggest that cAMP is generated at the apical pole next to the Ca2+ signal. The best understanding of localized cAMP signaling in mammalian cells is in striated muscle cells. All components of the cAMP pathway are expressed at the sarcoplasmic reticulum (SR)/PM junctions (Gorelik et al. 2013; Filadi and Pozzan 2015). Receptor stimulation increases cAMP at the SR/PM junction and activation of PKA (Rudolf et al. 2006; Filadi and Pozzan 2015). cAMP is also generated at the mitochondria surface and matrix (Lefkimmiatis et al. 2013; Filadi and Pozzan 2015). cAMP increase at the mitochondrial surface and cytoplasm were similar, but cAMP at the mitochondrial surface lasted longer and activated PKA more robustly (Lefkimmiatis et al. 2013; Burdyga et al. 2018). Matrix generation of cAMP is mediated by the soluble AC and regulates ATP synthesis (Di Benedetto et al. 2013) and aldosterone secretion in glomerulosa cells (Katona et al. 2015).
SIGNALING CROSS TALK AND SYNERGISM
The localization of the Ca2+ and cAMP signaling pathways at the same MCS allows for their functional interaction. Cross talk between the Ca2+ and cAMP occurs on many levels. cAMP/PKA phosphorylates the IP3Rs to increase their affinity to IP3 (Straub et al. 2004; Betzenhauser et al. 2009). PKA phosphorylates and activates PMCA (Baggaley et al. 2007) and regulates the activity of TRPC channels (Shen et al. 2011; Sung et al. 2011). PKA-mediated phosphorylation of the STIM-Orai-activating-region (SOAR) domain of STIM1 specifies the activation of the arachidonic acid-regulated Ca2+ selective channel rather than Orai1 (Thompson et al. 2018). Regulation of the cAMP pathway by Ca2+ is well documented through activation by Ca2+ of the Ca2+-dependent ACs by Ca2+. AC8, and perhaps other Ca2+-dependent ACs, are activated specifically by Ca2+ entering through the Orai1 (Willoughby et al. 2012) and TRPC1 channels (Willoughby et al. 2014). The regulation requires the proximity of all components in the same ER/PM junctions and is dependent on the direct interaction of AC8 with Orai1 (Willoughby et al. 2012).
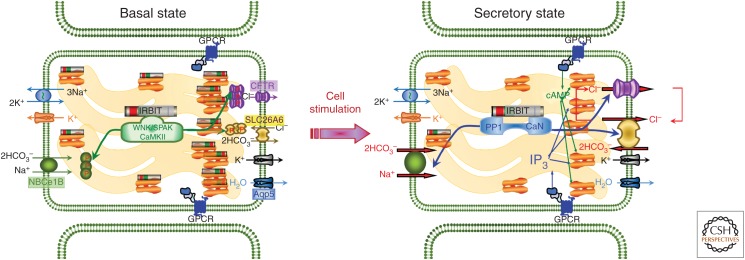
A prominent interaction between the Ca2+ and cAMP signaling pathways is the synergy between the pathways in activation of many cellular functions. In fact, most cellular functions are activated by synergism between signaling pathways to avoid overactivation that often leads to pathology (Ahuja et al. 2014). A molecular mechanism of synergy between Ca2+ and cAMP signaling involves PKA modulation of an IP3R-binding protein released with inositol 1,4,5-trisphosphate (IRBIT), which interacts with the IP3 receptors and other target proteins (shown in Fig. 2). IRBIT was identified as an IP3 receptor-binding protein released with IP3 (thus the name IRBIT), which interacts with the IP3-binding site of the IP3Rs to inhibit their activity (Ando et al. 2006). Subsequent studies have shown that IRBIT activates Na+-HCO3− cotransporter (NBCe1-B), the Cl− and HCO3− channel (CFTR), and the Cl−/2HCO3− exchanger (Slc26a6) (Shirakabe et al. 2006; Yang et al. 2009; Park et al. 2013). The function of these transporters is regulated by the With No Lysine (WNK) (Huang et al. 2007), STE20/SPS1-related proline-alanine-rich protein (SPAK) (Gagnon and Delpire 2012) kinases and the PP1 and CaMKII phosphatases (Yang et al. 2011; Vachel et al. 2018). In the resting state, IRBIT interacts with the unphosphorylated IP3Rs and inhibits Ca2+ release. Upon cell stimulation with physiological concentrations of agonists that trigger cAMP production and Ca2+ mobilization, PKA phosphorylates the IP3Rs, which increases their affinity for IP3, while reducing its affinity for IRBIT, thus releasing IRBIT from the IP3Rs (Park et al. 2013). The released IRBIT interacts with the Cl− and HCO3− transporters to increase their surface expression, modulate their inhibition by intracellular Cl−, and activates their transport function (Yang et al. 2009; Park et al. 2013; Shcheynikov et al. 2015).
Figure 2.
Molecular mechanism of synergism in cyclic AMP (cAMP) and Ca2+ signaling in epithelial fluid and HCO3− secretion. In the resting state, IRBIT recruits the WNK/SPAK and CaMKII kinases to the transporters to phosphorylate NBCe1-B autoinhibitory domain, Slc26a6 and CFTR to sequester most of them in intracellular compartments. Al low cytoplasmic IP3, most IRBIT is bound to the IP3 receptors, which in secretory glands are expressed at high levels at the apical pole. When the cells are stimulated with a combination of physiological concentrations of IP3 and cAMP-generating agonists, PKA phosphorylates the IP3Rs to facilitate the IP3-mediated release of IRBIT from the IP3Rs. IRBIT recruits protein phosphatase 1 (PP1) and calcineurin (CaN) to the transporters to dephosphorylate them and fuse the intracellular transporters pool with the PM. IRBIT remains bound to the transporters to relieve their constitutive inhibition and expose their intracellular Cl− regulatory sites. The IRBIT-activated transporters mediate fluid and electrolyte secretion by CFTR-expressing epithelia.
TETHERING MCS
Signaling and synergism requires the formation and stabilization of MCS. This is achieved by tether proteins that facilitate the interaction of the ER with various cellular membranes. The tether proteins were initially described in yeast studies of lipid transfer between membranes and organelles. The ER/PM junctions are tethered by three tricalbins, which are anchored in the ER and interact with PI(4,5)P2 in the PM (Toulmay and Prinz 2012). Another tether protein found in yeast is Ist2, which has transmembrane domains in the ER and a polybasic domain that interacts with PM PI(4,5)P2 (Maass et al. 2009; Wolf et al. 2012). In addition, lipid transfer proteins localize to the ER/PM junctions, some of which do not have ER-interacting domains. Instead, these proteins have two phenylalanine in acidic tract motifs that interact with the ER-localized vesicle-associated membrane protein-associated proteins (VAPs) (Stefan et al. 2011).
The mammalian homologs of the tricalbins are the three extended synaptotagmins (E-Syts) (Min et al. 2007; Giordano et al. 2013). The E-Syts are anchored in the ER and interact with PM PI(4,5)P2 through their C2 domains (Giordano et al. 2013). Although all the E-Syts tether the ER to the PM (Giordano et al. 2013), they have distinct functions. E-Syt1, but not E-Syt2 and E-Syt3, affect STIM1-Orai1 function at the ER/PM junctions (Maléth et al. 2014; Cao et al. 2015), while E-Syt2 and E-Syt3, but not E-Syt1, mediate receptor endocytosis (Jean et al. 2010) and E-Syt2, but not E-Syt1 and E-Syt3, recruits phosphatidylinositol 4-phosphate (PI4P) phosphatase to the ER/PM junctions to control PI(4,5)P2 levels (Dickson et al. 2016). Sequence similarity suggests that the mammalian homolog of Ist2 is a member of the 10 anoctamins family (ANOs, also known as TMEM16A-J) (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008). The mammalian VAPs bind the lipid exchange Nir proteins that maintain the PM level of PI(4,5)P2 by exchanging phosphatidic acid with PI(4,5)P2 (Kim et al. 2013, 2015). Other important proteins in the ER/PM junctions are ORP5 and ORP8, which play a role in lipid transfer and control the PM level of phosphatidylserine (PS) by exchanging PM PI4P with ER synthesized PS (Chung et al. 2015). ORP5 and ORP8 have also been found at the ER-mitochondria MCS and are proposed to have a role in mitochondrial function (Galmes et al. 2016).
The ER Ca2+ sensors, STIM1 and STIM2 (hereafter denoted STIMs), are clustered at the ER/PM junctions in response to Ca2+ release from the ER, after which they have key roles in ER/PM junction stabilization. The STIMs have a single transmembrane domain that spans the ER membrane and a carboxy-terminal polybasic domain, which, in response to ER Ca2+ depletion, binds to PM PI(4,5)P2 and to Orai1 (Liou et al. 2005; Roos et al. 2005; Zhang et al. 2006; Brandman et al. 2007). The STIMs have a Ca2+-binding EF-hand and an SAM domain that reside in the ER lumen. When Ca2+ is bound to the EF-hand, the STIMs do not tether the ER and PM. The cytoplasmic portion of the STIMs starts with three coiled-coil domains that regulate unfolding of STIM1 (Korzeniowski et al. 2010; Muik et al. 2011) to release the SOAR domain (Yuan et al. 2009), also known as CAD (Park et al. 2009) and CCb9 (Kawasaki et al. 2009). The STIM1 SOAR domain interacts with the carboxy- and amino-termini of Orai1 to activate the channel (Park et al. 2009; Yuan et al. 2009). SOAR is followed by the carboxyl-terminus inhibitory domain (CTID) (Jha et al. 2013), serine/proline rich, and polybasic domains (Liou et al. 2005; Roos et al. 2005). STIM2 is a poor activator of Orai1 (relative to STIM1) but affects Ca2+ signaling by functioning as a tether to enhance the efficiency of receptor-stimulated Ca2+ signaling (Ong et al. 2015) by facilitating STIM1 conformational change required for STIM1 interaction with and activation of Orai1 (Subedi et al. 2018).
Ca2+ SIGNALING AT THE ER/PM JUNCTIONS IN PATHOLOGY
Excessive or prolonged increases in cytoplasmic Ca2+ concentration occurs in many pathological states, leading to cell death by disrupting several cellular processes, including mitochondrial function (Pizzo et al. 2012; Dorn and Kitsis 2015), apoptosis (Dong et al. 2006), gene regulation (Parekh 2010), and membrane transport (Lee et al. 2012; Maléth and Hegyi 2014). Uncontrolled Ca2+ influx is the result of sustained activation of the Ca2+ influx channels, which can be caused either by excessive Ca2+ release from the ER, or lack of Ca2+ influx inactivation. In exocrine cells TRPC1, TRPC3, and Orai1 are the Ca2+ influx channels activated by GPCR stimulation (Choi et al. 2014; Cao et al. 2015). Excessive epithelial Ca2+ influx causes several pathologies (Hecquet and Malik 2009; Eisenhut and Wallace 2011; Gerasimenko et al. 2014; Ilatovskaya and Staruschenko 2015).
Acute pancreatitis is a life-threatening disease with multiple etiologies and no cure, and is caused by ductal obstruction, bile acid reflux, or excessive alcohol consumption. Sustained Ca2+ increase, because of excessive Ca2+ influx, is the nodal point in triggering the various forms of acute pancreatitis (Gerasimenko et al. 2014; Maléth and Hegyi 2014). Indeed, in animal models, genetic or pharmacological inhibition of Ca2+ influx channels can prevent, and even reverse, the disease after its initiation. However, unfortunately, inhibition of the two principal Ca2+ influx channels, TRPC and Orai1, has significant drawbacks. The advantage of inhibiting TRPC channels is that minor phenotypes were observed in the deletion of TRPC1 (Liu et al. 2007), TRPC3 (Kim et al. 2009), TRPC6 (Dietrich et al. 2005), and a combination of these channels in mice (Nilius and Flockerzi 2014; Sexton et al. 2016). Indeed, knockout of TRPC3 or inhibition of TRPC3 with Pyrazol 3 in mice markedly reduced the tissue damage caused by induction of acute pancreatitis (Kim et al. 2009, 2011). Unfortunately, inhibition of TRPC3 protected against acute pancreatitis only if the channel was inhibited prior to induction of the disease and was not effective in reversing the damage when applied after initiation of the disease (Kim et al. 2009, 2011).
Similarly, inhibitors of Orai1 prevented the tissue damage due to acute pancreatitis in both in vitro assays and in mice (Gerasimenko et al. 2013; Wen et al. 2015). Pharmacological inhibition of Orai1 partially protected against all models of acute pancreatitis, induced by excessive agonist stimulation, infusion of bile acids or of alcohol metabolites, even after initiation of the disease (Wen et al. 2015). Another significant advantage of Orai1 inhibitors is that they will likely inhibit the inflammation associated with all forms of acute pancreatitis (Sah et al. 2013) as Orai1-mediated Ca2+ influx is critical for activation of various inflammatory cells (Shaw and Feske 2012). However, one serious problem with the use of Orai1 inhibitors is that Ca2+ influx mediated by Orai1 is essential for many cellular functions in all cell types. Thus, inhibitors of Orai1 are expected to have serious adverse side effects. For example, in the pancreas, targeted induced deletion of Orai1 only in acinar cells resulted in 70% mortality because of bacteremia and sepsis (Ahuja et al. 2017). Pancreatic acinar cells synthesize and secrete large quantities of antibacterial agents to the gut to control the gut microbiota. Deletion of acinar cells’ Orai1 inhibited synthesis and secretion of the antibacterial agents to the gut, resulting in dysbiosis that proved to be lethal (Ahuja et al. 2017). Hence, caution must be used in the application of Orai1 inhibitors to treat diseases resulting from excessive Ca2+ influx. One possible approach is to use a combination of TRPC channel blockers and low-dose Orai1 inhibitors to treat diseases like acute pancreatitis. Another is to use small molecules to control the function of the Ca2+ influx regulators store-operated calcium entry (SOCE)-associated regulatory factor (SARAF) (Jha et al. 2013) and the STIM-activating enhancer (STIMATE) (Jing et al. 2015; Quintana et al. 2015) that controls the function of STIM1.
CONCLUDING REMARKS
Aberrant Ca2+ signals have been known to be the root cause of many cell damage– and inflammation-associated diseases. Other, less appreciated, aspects of signaling associated with pathology is aberrant synergy between signaling pathways, in particular, cross talk and synergy between the Ca2+ and cAMP/PKA pathways. Recent evidence highlights the importance of organizing signaling complexes at MCS to mediate the synergy and the role of tether proteins in the formation of MCS. Therefore, a more complete understanding of the formation of MCS and their role in the organization of cell signaling and in cross talk and synergism is essential for understanding basic aspects of cell regulation and, importantly, the alterations that occur in disease states. This should allow development of a more precise and perhaps better targeted intervention to treat aberrant cell signaling in disease states.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) intramural Grant DE000735.
Footnotes
Editors: Geert Bultynck, Martin D. Bootman, Michael J. Berridge, and Grace E. Stutzmann
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Ahuja M, Jha A, Maléth J, Park S, Muallem S. 2014. cAMP and Ca2+ signaling in secretory epithelia: Crosstalk and synergism. Cell Calcium 55: 385–393. 10.1016/j.ceca.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja M, Schwartz DM, Tandon M, Son A, Zeng M, Swaim W, Eckhaus M, Hoffman V, Cui Y, Xiao B, et al. 2017. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab 25: 635–646. 10.1016/j.cmet.2017.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almassy J, Won JH, Begenisich TB, Yule DI. 2012. Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J Gen Physiol 139: 121–133. 10.1085/jgp.201110718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. 2006. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell 22: 795–806. 10.1016/j.molcel.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Baggaley E, McLarnon S, Demeter I, Varga G, Bruce JI. 2007. Differential regulation of the apical plasma membrane Ca2+-ATPase by protein kinase A in parotid acinar cells. J Biol Chem 282: 37678–37693. 10.1074/jbc.M703416200 [DOI] [PubMed] [Google Scholar]
- Barnes AP, Livera G, Huang P, Sun C, O'Neal WK, Conti M, Stutts MJ, Milgram SL. 2005. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem 280: 7997–8003. 10.1074/jbc.M407521200 [DOI] [PubMed] [Google Scholar]
- Beech DJ. 2013. Characteristics of transient receptor potential canonical calcium-permeable channels and their relevance to vascular physiology and disease. Circ J 77: 570–579. 10.1253/circj.CJ-13-0154 [DOI] [PubMed] [Google Scholar]
- Betzenhauser MJ, Fike JL, Wagner LE II, Yule DI. 2009. Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. J Biol Chem 284: 25116–25125. 10.1074/jbc.M109.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar D, Chung WY, Yang D, Hong JH, Jha A, Muallem S. 2017. STIM-TRP pathways and microdomain organization: Ca2+ influx channels: The Orai-STIM1-TRPC complexes. Adv Exp Med Biol 993: 139–157. 10.1007/978-3-319-57732-6_8 [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. 2007. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131: 1327–1339. 10.1016/j.cell.2007.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce JIE. 2018. Metabolic regulation of the PMCA: Role in cell death and survival. Cell Calcium 69: 28–36. 10.1016/j.ceca.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga A, Surdo NC, Monterisi S, Di Benedetto G, Grisan F, Penna E, Pellegrini L, Zaccolo M, Bortolozzi M, Swietach P, et al. 2018. Phosphatases control PKA-dependent functional microdomains at the outer mitochondrial membrane. Proc Natl Acad Sci 115: E6497–E6506. 10.1073/pnas.1806318115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T, Brini M, Carafoli E. 2017. Regulation of cell calcium and role of plasma membrane calcium ATPases. Int Rev Cell Mol Biol 332: 259–296. 10.1016/bs.ircmb.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Cancela JM, Van Coppenolle F, Galione A, Tepikin AV, Petersen OH. 2002. Transformation of local Ca2+ spikes to global Ca2+ transients: The combinatorial roles of multiple Ca2+ releasing messengers. EMBO J 21: 909–919. 10.1093/emboj/21.5.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Choi S, Maleth JJ, Park S, Ahuja M, Muallem S. 2015. The ER/PM microdomain, PI(4,5)P2 and the regulation of STIM1-Orai1 channel function. Cell Calcium 58: 342–348. 10.1016/j.ceca.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. 2008. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594. 10.1126/science.1163518 [DOI] [PubMed] [Google Scholar]
- Catalán MA, Kondo Y, Peña-Munzenmayer G, Jaramillo Y, Liu F, Choi S, Crandall E, Borok Z, Flodby P, Shull GE, et al. 2015. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc Natl Acad Sci 112: 2263–2268. 10.1073/pnas.1415739112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaly ER, Troncone L, Lebeche D. 2018. SERCA control of cell death and survival. Cell Calcium 69: 46–61. 10.1016/j.ceca.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Van Hook MJ, Thoreson WB. 2015. Ca2+ diffusion through endoplasmic reticulum supports elevated intraterminal Ca2+ levels needed to sustain synaptic release from rods in darkness. J Neurosci 35: 11364–11373. 10.1523/jneurosci.0754-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Maleth J, Jha A, Lee KP, Kim MS, So I, Ahuja M, Muallem S. 2014. The TRPCs-STIM1-Orai interaction. Handb Exp Pharmacol 223: 1035–1054. 10.1007/978-3-319-05161-1_13 [DOI] [PubMed] [Google Scholar]
- Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. 2015. Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349: 428–432. 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WY, Jha A, Ahuja M, Muallem S. 2017. Ca2+ influx at the ER/PM junctions. Cell Calcium 63: 29–32. 10.1016/j.ceca.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion AR, Feske S. 2017. Regulation of epithelial ion transport in exocrine glands by store-operated Ca2+ entry. Cell Calcium 63: 53–59. 10.1016/j.ceca.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion AR, Vaeth M, Wagner LE II, Eckstein M, Hecht L, Yang J, Crottes D, Seidl M, Shin HP, Weidinger C, et al. 2016. Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J Clin Invest 126: 4303–4318. 10.1172/JCI89056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang D, Rao R. 2016. Calcium-ATPases: Gene disorders and dysregulation in cancer. Biochim Biophys Acta 1863: 1344–1350. 10.1016/j.bbamcr.2015.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. 2013. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab 17: 965–975. 10.1016/j.cmet.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Dickson EJ, Jensen JB, Vivas O, Kruse M, Traynor-Kaplan AE, Hille B. 2016. Dynamic formation of ER-PM junctions presents a lipid phosphatase to regulate phosphoinositides. J Cell Biol 213: 33–48. 10.1083/jcb.201508106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, et al. 2005. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989. 10.1128/MCB.25.16.6980-6989.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola S, Scotto-Rosato A, Medina DL. 2018. TRPML1: The Ca2+ retaker of the lysosome. Cell Calcium 69: 112–121. 10.1016/j.ceca.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. 2006. Calcium in cell injury and death. Annu Rev Pathol 1: 405–434. 10.1146/annurev.pathol.1.110304.100218 [DOI] [PubMed] [Google Scholar]
- Dorn GW II, Kitsis RN. 2015. The mitochondrial dynamism-mitophagy-cell death interactome: Multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res 116: 167–182. 10.1161/circresaha.116.303554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M, Wallace H. 2011. Ion channels in inflammation. Pflugers Arch 461: 401–421. 10.1007/s00424-010-0917-y [DOI] [PubMed] [Google Scholar]
- Filadi R, Pozzan T. 2015. Generation and functions of second messengers microdomains. Cell Calcium 58: 405–414. 10.1016/j.ceca.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Gagnon KB, Delpire E. 2012. Molecular physiology of SPAK and OSR1: Two Ste20-related protein kinases regulating ion transport. Physiol Rev 92: 1577–1617. 10.1152/physrev.00009.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmes R, Houcine A, van Vliet AR, Agostinis P, Jackson CL, Giordano F. 2016. ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO Rep 17: 800–810. 10.15252/embr.201541108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. 2016. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife 5: e15887 10.7554/eLife.15887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV. 2006. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci 119: 226–238. 10.1242/jcs.02721 [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Gryshchenko O, Ferdek PE, Stapleton E, Hebert TO, Bychkova S, Peng S, Begg M, Gerasimenko OV, Petersen OH. 2013. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc Natl Acad Sci 110: 13186–13191. 10.1073/pnas.1300910110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Gerasimenko OV, Petersen OH. 2014. The role of Ca2+ in the pathophysiology of pancreatitis. J Physiol 592: 269–280. 10.1113/jphysiol.2013.261784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Charlesworth RM, Sherwood MW, Ferdek PE, Mikoshiba K, Parrington J, Petersen OH, Gerasimenko OV. 2015. Both RyRs and TPCs are required for NAADP-induced intracellular Ca2+ release. Cell Calcium 58: 237–245. 10.1016/j.ceca.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. 2013. PI(4,5)P2-dependent and Ca2+-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153: 1494–1509. 10.1016/j.cell.2013.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Marchi S, Pinton P. 2018. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 19: 713–730. 10.1038/s41580-018-0052-8 [DOI] [PubMed] [Google Scholar]
- Gorelik J, Wright PT, Lyon AR, Harding SE. 2013. Spatial control of the βAR system in heart failure: the transverse tubule and beyond. Cardiovasc Res 98: 216–224. 10.1093/cvr/cvt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Barthmes M, Wahl-Schott C. 2014. Trpml3. Handb Exp Pharmacol 222: 659–674. 10.1007/978-3-642-54215-2_26 [DOI] [PubMed] [Google Scholar]
- Grimm C, Butz E, Chen CC, Wahl-Schott C, Biel M. 2017a. From mucolipidosis type IV to ebola: TRPML and two-pore channels at the crossroads of endo-lysosomal trafficking and disease. Cell Calcium 67: 148–155. 10.1016/j.ceca.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Grimm C, Chen CC, Wahl-Schott C, Biel M. 2017b. Two-pore channels: Catalyzers of endolysosomal transport and function. Front Pharmacol 8: 45 10.3389/fphar.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. 2004. Calcium and mitochondria. FEBS Lett 567: 96–102. 10.1016/j.febslet.2004.03.071 [DOI] [PubMed] [Google Scholar]
- Hecquet CM, Malik AB. 2009. Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb Haemost 101: 619–625. 10.1160/TH08-10-0641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Liou J, Emr SD. 2015. Molecular mechanisms of inter-organelle ER-PM contact sites. Curr Opin Cell Biol 35: 123–130. 10.1016/j.ceb.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Hodges RR, Zoukhri D, Lightman JP, Dartt DA. 1998. Identification and cellular localization of the components of the VIP signaling pathway in the lacrimal gland. Adv Exp Med Biol 438: 169–176. 10.1007/978-1-4615-5359-5_24 [DOI] [PubMed] [Google Scholar]
- Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. 2011. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic 12: 232–245. 10.1111/j.1600-0854.2010.01138.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Cha SK, Wang HR, Xie J, Cobb MH. 2007. WNKs: Protein kinases with a unique kinase domain. Exp Mol Med 39: 565–573. 10.1038/emm.2007.62 [DOI] [PubMed] [Google Scholar]
- Ilatovskaya DV, Staruschenko A. 2015. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am J Physiol Renal Physiol 309: F393–F397. 10.1152/ajprenal.00186.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S, Mikryukov A, Tremblay MG, Baril J, Guillou F, Bellenfant S, Moss T. 2010. Extended-synaptotagmin-2 mediates FGF receptor endocytosis and ERK activation in vivo. Dev Cell 19: 426–439. 10.1016/j.devcel.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Maléth J, Moreno CM, Yuan JP, Kim MS, Muallem S. 2013. The STIM1 CTID domain determines access of SARAF to SOAR to regulate Orai1 channel function. J Cell Biol 202: 71–79. 10.1083/jcb.201301148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. 2014. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J 33: 501–511. 10.1002/embj.201387035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, et al. 2015. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca2+ influx. Nat Cell Biol 17: 1339–1347. 10.1038/ncb3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Augustine GJ. 1990. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature 348: 735–738. 10.1038/348735a0 [DOI] [PubMed] [Google Scholar]
- Katona D, Rajki A, Di Benedetto G, Pozzan T, Spät A. 2015. Calcium-dependent mitochondrial cAMP production enhances aldosterone secretion. Mol Cell Endocrinol 412: 196–204. 10.1016/j.mce.2015.05.002 [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Lange I, Feske S. 2009. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun 385: 49–54. 10.1016/j.bbrc.2009.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Hockey LN, Yates E, Futter CE, Patel S. 2017. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep 18: 1636–1645. 10.1016/j.celrep.2017.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Zeng W, Kiselyov K, Yuan JP, Dehoff MH, Mikoshiba K, Worley PF, Muallem S. 2006. Homer 1 mediates store- and inositol 1,4,5-trisphosphate receptor-dependent translocation and retrieval of TRPC3 to the plasma membrane. J Biol Chem 281: 32540–32549. 10.1074/jbc.M602496200 [DOI] [PubMed] [Google Scholar]
- Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. 2009. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology 137: 1509–1517. 10.1053/j.gastro.2009.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. 2011. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology 140: 2107–2115.e4. 10.1053/j.gastro.2011.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kedan A, Marom M, Gavert N, Keinan O, Selitrennik M, Laufman O, Lev S. 2013. The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signalling. EMBO Rep 14: 891–899. 10.1038/embor.2013.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. 2015. Phosphatidylinositol-phosphatidic acid exchange by Nir2 at ER-PM contact sites maintains phosphoinositide signaling competence. Dev Cell 33: 549–561. 10.1016/j.devcel.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodecik TR, Shugrue CA, Thrower EC, Levin LR, Buck J, Gorelick FS. 2012. Activation of soluble adenylyl cyclase protects against secretagogue stimulated zymogen activation in rat pancreaic acinar cells. PLoS ONE 7: e41320 10.1371/journal.pone.0041320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Manjarres IM, Varnai P, Balla T. 2010. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal 3: ra82 10.1126/scisignal.2001122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Toulmay A, Prinz WA. 2015. Membrane contact sites, gateways for lipid homeostasis. Curr Opin Cell Biol 33: 82–87. 10.1016/j.ceb.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Xu X, Zeng W, Diaz J, Kuo TH, Wuytack F, Racymaekers L, Muallem S. 1997a. Polarized expression of Ca2+ pumps in pancreatic and salivary gland cells. Role in initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15771–15776. 10.1074/jbc.272.25.15771 [DOI] [PubMed] [Google Scholar]
- Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. 1997b. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15765–15770. 10.1074/jbc.272.25.15765 [DOI] [PubMed] [Google Scholar]
- Lee MG, Ohana E, Park HW, Yang D, Muallem S. 2012. Molecular mechanism of pancreatic and salivary gland fluid and HCO3− secretion. Physiol Rev 92: 39–74. 10.1152/physrev.00011.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K, Leronni D, Hofer AM. 2013. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J Cell Biol 202: 453–462. 10.1083/jcb.201303159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241. 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, et al. 2007. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc Natl Acad Sci 104: 17542–17547. 10.1073/pnas.0701254104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass K, Fischer MA, Seiler M, Temmerman K, Nickel W, Seedorf M. 2009. A signal comprising a basic cluster and an amphipathic α-helix interacts with lipids and is required for the transport of Ist2 to the yeast cortical ER. J Cell Sci 122: 625–635. 10.1242/jcs.036012 [DOI] [PubMed] [Google Scholar]
- Mak DO, Foskett JK. 2015. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view. Cell Calcium 58: 67–78. 10.1016/j.ceca.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maléth J, Hegyi P. 2014. Calcium signaling in pancreatic ductal epithelial cells: An old friend and a nasty enemy. Cell Calcium 55: 337–345. 10.1016/j.ceca.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Maléth J, Choi S, Muallem S, Ahuja M. 2014. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat Commun 5: 5843 10.1038/ncomms6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda M, Pittman JK, Mayor R, Patel S. 2016. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J Cell Biol 212: 803–813. 10.1083/jcb.201510019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. 2005. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol 67: 445–469. 10.1146/annurev.physiol.67.041703.084745 [DOI] [PubMed] [Google Scholar]
- Mesmin B, Kovacs D, D'Angelo G. 2019. Lipid exchange and signaling at ER-Golgi contact sites. Curr Opin Cell Biol 57: 8–15. 10.1016/j.ceb.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Messenger SW, Falkowski MA, Groblewski GE. 2014. Ca2+-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell Calcium 55: 369–375. 10.1016/j.ceca.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. 2015. Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul 57: 217–227. 10.1016/j.jbior.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Min SW, Chang WP, Sudhof TC. 2007. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci 104: 3823–3828. 10.1073/pnas.0611725104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S, Chung WY, Jha A, Ahuja M. 2017. Lipids at membrane contact sites: Cell signaling and ion transport. EMBO Rep 18: 1893–1904. 10.15252/embr.201744331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, et al. 2011. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J 30: 1678–1689. 10.1038/emboj.2011.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani N, Shanmughapriya S, Madesh M. 2018. Molecular regulation of MCU: Implications in physiology and disease. Cell Calcium 74: 86–93. 10.1016/j.ceca.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Flockerzi V. 2014. What do we really know and what do we need to know: Some controversies, perspectives, and surprises. Handbook Exp Pharmacol 223: 1239–1280. 10.1007/978-3-319-05161-1_20 [DOI] [PubMed] [Google Scholar]
- Nishida M, Kuwahara K, Kozai D, Sakaguchi R, Mori Y. 2015. TRP channels: Their function and potentiality as drug targets. In Innovative medicine: Basic research and development (ed. Nakao K, Minato N, Uemoto S), pp. 195–218. Springer, Tokyo. [PubMed] [Google Scholar]
- Nlend MC, Schmid A, Sutto Z, Ransford GA, Conner GE, Fregien N, Salathe M. 2007. Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS Lett 581: 3241–3246. 10.1016/j.febslet.2007.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Hasler P, Demaurex N. 2017. The ER phagosome connection in the era of membrane contact sites. Biochim Biophys Acta Mol Cell Res 1864: 1513–1524. 10.1016/j.bbamcr.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Ong HL, de Souza LB, Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS. 2015. STIM2 enhances receptor-stimulated Ca2+ signaling by promoting recruitment of STIM1 to the endoplasmic reticulum-plasma membrane junctions. Sci Signal 8: ra3 10.1126/scisignal.2005748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. 2010. Store-operated CRAC channels: Function in health and disease. Nat Rev Drug Discov 9: 399–410. 10.1038/nrd3136 [DOI] [PubMed] [Google Scholar]
- Parekh AB. 2017. Regulation of CRAC channels by Ca2+-dependent inactivation. Cell Calcium 63: 20–23. 10.1016/j.ceca.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Park MK, Lomax RB, Tepikin AV, Petersen OH. 2001. Local uncaging of caged Ca2+ reveals distribution of Ca2+-activated Cl− channels in pancreatic acinar cells. Proc Natl Acad Sci 98: 10948–10953. 10.1073/pnas.181353798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890. 10.1016/j.cell.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Shcheynikov N, Hong JH, Zheng C, Suh SH, Kawaai K, Ando H, Mizutani A, Abe T, Kiyonari H, et al. 2013. Irbit mediates synergy between Ca2+ and cAMP signaling pathways during epithelial transport in mice. Gastroenterology 145: 232–241. 10.1053/j.gastro.2013.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Kilpatrick BS, Eden ER, Patel S. 2015. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium 58: 387–396. 10.1016/j.ceca.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Petersen OH. 2012. Specific mitochondrial functions in separate sub-cellular domains of pancreatic acinar cells. Pflugers Arch 464: 77–87. 10.1007/s00424-012-1099-6 [DOI] [PubMed] [Google Scholar]
- Petersen OH. 2015. Ca2+ signalling in the endoplasmic reticulum/secretory granule microdomain. Cell Calcium 58: 397–404. 10.1016/j.ceca.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Petersen OH, Tepikin AV. 2008. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol 70: 273–299. 10.1146/annurev.physiol.70.113006.100618 [DOI] [PubMed] [Google Scholar]
- Pizzo P, Drago I, Filadi R, Pozzan T. 2012. Mitochondrial Ca2+ homeostasis: Mechanism, role, and tissue specificities. Pflugers Arch 464: 3–17. 10.1007/s00424-012-1122-y [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. 2015. Store-operated calcium channels. Physiol Rev 95: 1383–1436. 10.1152/physrev.00020.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA. 2014. Bridging the gap: Membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol 205: 759–769. 10.1083/jcb.201401126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. 2014. Salivary secretion: Mechanism and neural regulation. Monogr Oral Sci 24: 14–29. 10.1159/000358781 [DOI] [PubMed] [Google Scholar]
- Quintana A, Rajanikanth V, Farber-Katz S, Gudlur A, Zhang C, Jing J, Zhou Y, Rao A, Hogan PG. 2015. TMEM110 regulates the maintenance and remodeling of mammalian ER-plasma membrane junctions competent for STIM-ORAI signaling. Proc Natl Acad Sci 112: E7083–E7092. 10.1073/pnas.1521924112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, Rizzuto R. 2016. Calcium at the center of cell signaling: Interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci 41: 1035–1049. 10.1016/j.tibs.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko V, Nakamoto T, Srivastava A, Melvin JE, Begenisich T. 2006. Molecular identification and physiological roles of parotid acinar cell maxi-K channels. J Biol Chem 281: 27964–27972. 10.1074/jbc.M603871200 [DOI] [PubMed] [Google Scholar]
- Romanenko VG, Nakamoto T, Srivastava A, Begenisich T, Melvin JE. 2007. Regulation of membrane potential and fluid secretion by Ca2+-activated K+ channels in mouse submandibular glands. J Physiol 581: 801–817. 10.1113/jphysiol.2006.127498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169: 435–445. 10.1083/jcb.200502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R, Magalhães PJ, Pozzan T. 2006. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol 173: 187–193. 10.1083/jcb.200601160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah RP, Dawra RK, Saluja AK. 2013. New insights into the pathogenesis of pancreatitis. Curr Opin Gastroenterol 29: 523–530. 10.1097/MOG.0b013e328363e399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. 2008. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029. 10.1016/j.cell.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JE, Desmonds T, Quick K, Taylor R, Abramowitz J, Forge A, Kros CJ, Birnbaumer L, Wood JN. 2016. The contribution of TRPC1, TRPC3, TRPC5 and TRPC6 to touch and hearing. Neurosci Lett 610: 36–42. 10.1016/j.neulet.2015.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Feske S. 2012. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J Physiol 590: 4157–4167. 10.1113/jphysiol.2012.233221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Son A, Hong JH, Yamazaki O, Ohana E, Kurtz I, Shin DM, Muallem S. 2015. Intracellular Cl− as a signaling ion that potently regulates Na+/HCO3− transporters. Proc Natl Acad Sci 112: E329–E337. 10.1073/pnas.1415673112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Kwan HY, Ma X, Wong CO, Du J, Huang Y, Yao X. 2011. cAMP activates TRPC6 channels via the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (PKB)-mitogen-activated protein kinase kinase (MEK)-ERK1/2 signaling pathway. J Biol Chem 286: 19439–19445. 10.1074/jbc.M110.210294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Luo X, Wilkie TM, Miller LJ, Peck AB, Humphreys-Beher MG, Muallem S. 2001. Polarized expression of G protein-coupled receptors and an all-or-none discharge of Ca2+ pools at initiation sites of [Ca2+]i waves in polarized exocrine cells. J Biol Chem 276: 44146–44156. 10.1074/jbc.M105203200 [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. 2006. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1). Proc Natl Acad Sci 103: 9542–9547. 10.1073/pnas.0602250103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J, Means S, Zhu D, Rugis J, Won JH, Yule DI. 2017. Modeling calcium waves in an anatomically accurate three-dimensional parotid acinar cell. J Theor Biol 419: 383–393. 10.1016/j.jtbi.2016.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. 2011. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144: 389–401. 10.1016/j.cell.2010.12.034 [DOI] [PubMed] [Google Scholar]
- Straub SV, Wagner LE II, Bruce JI, Yule DI. 2004. Modulation of cytosolic calcium signaling by protein kinase A-mediated phosphorylation of inositol 1,4,5-trisphosphate receptors. Biol Res 37: 593–602. 10.4067/S0716-97602004000400013 [DOI] [PubMed] [Google Scholar]
- Subedi KP, Ong HL, Son GY, Liu X, Ambudkar IS. 2018. STIM2 induces activated conformation of STIM1 to control Orai1 function in ER-PM junctions. Cell Rep 23: 522–534. 10.1016/j.celrep.2018.03.065 [DOI] [PubMed] [Google Scholar]
- Sung TS, Jeon JP, Kim BJ, Hong C, Kim SY, Kim J, Jeon JH, Kim HJ, Suh CK, Kim SJ, et al. 2011. Molecular determinants of PKA-dependent inhibition of TRPC5 channel. Am J Physiol Cell Physiol 301: C823–832. 10.1152/ajpcell.00351.2010 [DOI] [PubMed] [Google Scholar]
- Tepikin AV. 2018. Mitochondrial junctions with cellular organelles: Ca2+ signalling perspective. Pflugers Arch 470: 1181–1192. 10.1007/s00424-018-2179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Zhao Y, Stathopulos PB, Grossfield A, Shuttleworth TJ. 2018. Phosphorylation-mediated structural changes within the SOAR domain of stromal interaction molecule 1 enable specific activation of distinct Orai channels. J Biol Chem 293: 3145–3155. 10.1074/jbc.M117.819078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. 1993. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell 74: 661–668. 10.1016/0092-8674(93)90513-P [DOI] [PubMed] [Google Scholar]
- Tinel H, Cancela JM, Mogami H, Gerasimenko JV, Gerasimenko OV, Tepikin AV, Petersen OH. 1999. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J 18: 4999–5008. 10.1093/emboj/18.18.4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmay A, Prinz WA. 2012. A conserved membrane-binding domain targets proteins to organelle contact sites. J Cell Sci 125: 49–58. 10.1242/jcs.085118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachel L, Shcheynikov N, Yamazaki O, Fremder M, Ohana E, Son A, Shin DM, Yamazaki-Nakazawa A, Yang CR, Knepper MA, et al. 2018. Modulation of Cl− signaling and ion transport by recruitment of kinases and phosphatases mediated by the regulatory protein IRBIT. Sci Signal 11: eaat5018 10.1126/scisignal.aat5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Voronina S, Javed MA, Awais M, Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J, et al. 2015. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology 149: 481–492.e7. 10.1053/j.gastro.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. 2015. The mitochondria-plasma membrane contact site. Curr Opin Cell Biol 35: 1–6. 10.1016/j.ceb.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM. 2012. Direct binding between Orai1 and AC8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal 5: ra29 10.1126/scisignal.2002299 [DOI] [PubMed] [Google Scholar]
- Willoughby D, Ong HL, De Souza LB, Wachten S, Ambudkar IS, Cooper DM. 2014. TRPC1 contributes to the Ca2+-dependent regulation of adenylate cyclases. Biochem J 464: 73–84. 10.1042/BJ20140766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf W, Kilic A, Schrul B, Lorenz H, Schwappach B, Seedorf M. 2012. Yeast Ist2 recruits the endoplasmic reticulum to the plasma membrane and creates a ribosome-free membrane microcompartment. PLoS ONE 7: e39703 10.1371/journal.pone.0039703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley PF, Zeng W, Huang G, Kim JY, Shin DM, Kim MS, Yuan JP, Kiselyov K, Muallem S. 2007. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 42: 363–371. 10.1016/j.ceca.2007.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Carvalho P, Voeltz GK. 2018. Here, there, and everywhere: The importance of ER membrane contact sites. Science 361: eaan5835 10.1126/science.aan5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ren D. 2015. Lysosomal physiology. Annu Rev Physiol 77: 57–80. 10.1146/annurev-physiol-021014-071649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Jha A, Li Q, Soyombo AA, Dickinson GD, Churamani D, Brailoiu E, Patel S, Muallem S. 2011. Transient receptor potential mucolipin 1 (TRPML1) and two-pore channels are functionally independent organellar ion channels. J Biol Chem 286: 22934–22942. 10.1074/jbc.M110.210930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, et al. 2008. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215. 10.1038/nature07313 [DOI] [PubMed] [Google Scholar]
- Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. 2009. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest 119: 193–202. 10.1172/JCI36983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. 2011. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest 121: 956–965. 10.1172/JCI43475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Lee J, Jo H, Park S, Chang I, Muallem S, Shin DM. 2014. Homer2 protein regulates plasma membrane Ca2+-ATPase-mediated Ca2+ signaling in mouse parotid gland acinar cells. J Biol Chem 289: 24971–24979. 10.1074/jbc.M114.577221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. 2009. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11: 337–343. 10.1038/ncb1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Lee KP, Hong JH, Muallem S. 2012. The closing and opening of TRPC channels by Homer1 and STIM1. Acta Physiol 204: 238–247. 10.1111/j.1748-1716.2011.02319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ. 1997. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem 272: 9093–9098. 10.1074/jbc.272.14.9093 [DOI] [PubMed] [Google Scholar]
- Yule DI, Betzenhauser MJ, Joseph SK. 2010. Linking structure to function: Recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell Calcium 47: 469–479. 10.1016/j.ceca.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M, Szymczak M, Ahuja M, Zheng C, Yin H, Swaim W, Chiorini JA, Bridges RJ, Muallem S. 2017. Restoration of CFTR activity in ducts rescues acinar cell function and reduces inflammation in pancreatic and salivary glands of mice. Gastroenterology 153: 1148–1159. 10.1053/j.gastro.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. 2006. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci 103: 9357–9362. 10.1073/pnas.0603161103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn VZ, Khatri A, Mednieks MI, Hand AR. 2015. Localization of cystic fibrosis transmembrane conductance regulator signaling complexes in human salivary gland striated duct cells. Eur J Oral Sci 123: 140–148. 10.1111/eos.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]