Abstract
Objective:
To characterize the bladder microbiota of continent adult women.
Design:
Cross-sectional study of adult women who contributed catheterized urine samples, completed validated symptom questionnaires and provided demographic data.
Setting:
US academic medical center.
Population:
Well-characterized continent adult women.
Methods:
Participants contributed symptoms questionnaires, demographic data and catheterized urine samples that were analyzed by enhanced urine culture methodology and 16S rRNA gene sequencing.
Main Outcome Measures:
Associations between demographics and microbial community state structures (urotypes, defined by a specimen’s dominant taxon).
Results:
The bladder microbiota (urobiome) of 224 continent control women were characterized, showing variability in terms of urotype. The most common urotype was Lactobacillus (19%), which did not differ with any demographic. In contrast, the Gardnerella (p<0.001) and Escherichia (p=0.005) urotypes were more common in younger and older women, respectively.
Conclusions:
For urobiome research, enhanced culture methods and/or DNA sequencing are preferred techniques for bacterial detection. Clinical interpretation of clinical tests, such as the standard urine culture, should incorporate knowledge that some women have Gardnerella or Escherichia urotypes without evidence of clinical disorder. Clinical care strategies should preserve or restore the beneficial effects of the native urobiome, as disruption of that microbial community could result in unintended vulnerability to uropathogen invasion or opportunistic pathogen overgrowth. Longitudinal studies of urobiome responses to therapies should be encouraged.
Keywords: Urinary Microbiota, Urinary Microbiome, Urobiome, Female Bladder, Bladder Health
Tweetable abstract:
In continent adult women, bladder microbiome composition differs by age with relevance for clinical practice
INTRODUCTION
Since the discovery and confirmation of live bacteria in the bladders of adult women (urobiome) (1–7), there has been growing interest in the relationship between the urobiome and lower urinary tract symptoms (LUTS), including urinary incontinence (UI). Prior reports of studies that analyzed urine samples obtained from the urinary bladder by transurethral catheterization suggest a difference in urobiome characteristics between women with urgency urinary incontinence (UUI) and unaffected, continent control women (4, 7). Recently, Komesu and co-workers reported on the urobiome, analyzing catheterized urine samples obtained from a well-described cohort of 84 adult women with only slight or no urinary incontinence and significant overactive bladder symptoms (8). However, no comprehensive analysis of continent women has been reported and thus many parameters of the “normal” urobiome are not known and the urobiome characteristics of continent women remains incompletely understood, limiting insights into the relationship between bladder health and LUTS. In this analysis, we describe the urobiome characteristics of a large population of continent adult women.
METHODS
Patient recruitment
Adult women receiving medical care at the Loyola University Medical Center who reported that they were continent of urine were invited to participate. Eligibility was restricted to women, ≥18 years of age without lower urinary tract symptoms. We excluded women who had antibiotic exposure in prior month, systemic immune deficiency, systemic neurologic disease known to affect urinary tract function, current positive urine culture, UTI or recurrent culture-proven urinary tract infection, urogynecologic surgery within the prior 12 weeks, prior intra-vesical onabotulinumtoxin therapy, current or previous bladder stones, urinary incontinence, overactive bladder or pelvic organ prolapse. The long form of the Pelvic Floor Distress Inventory, a validated symptom measure, was used to screen for eligibility. In addition to subjectively reporting no UI, control participants answered “no” to all UI questions (18, 19, 20, 21 and 22).
Enrollment was restricted to women who were willing and able to provide research consent using a current institutional review board approved consent form, complete questionnaires and provide a catheterized urine sample. Consented participants were asked to complete the validated questionnaire, OAB-q, to measure OAB-related symptoms and quality of life (9). Demographic information and clinical characteristics were collected, including age, BMI, parity, medical co-morbidities, hormone status, previous hysterectomy, previous incontinence surgery, and BMI. Physical examination was completed, including evaluation for pelvic organ prolapse with the POP-q examination. 224 participants were pooled from three separate, IRB-approved studies with identical baseline assessment and sample collection procedures. 52 of these participants have been previously described (1, 5, 7). These studies were funded by NIDDK (R01DK104718–01A1, R56DK104718–01, R21DK097435 and P20DK108268) and by the Falk Foundation (LU#202567). The funders did not play a part in the design or conduct of the study.
Urine Collection and Analysis
We collected urine aseptically via transurethral catheter, according to standard clinical protocols. For culture, we added some urine a BD Vacutainer Plus C&S preservative tube. For sequencing, we placed the rest of the urine at 4°C for ≤4 h; we added 10% AssayAssure (Sierra Molecular; Incline Village, NV) before freezing at −80°C.
Expanded Quantitative Urine Culture (EQUC)
We quantitatively spread 0.1 mL of urine onto BAP, Chocolate and Colistin, Naladixic Acid (CNA) agars (BD BBL™ Prepared Plated Media), then incubated for 48 hours at 35°C in 5% CO2. We inoculated a second set of BAPs with 0.1 mL of urine and incubated for 48 hours at 30°C and 35°C in room atmosphere. We inoculated 0.1 mL of urine onto two CDC Anaerobe 5% sheep blood agar (ABAP) plates (BD BBL™ Prepared Plated Media), and incubated one in a Campy gas mixture (5% O2, 10% CO2, 85% N) and the other in anaerobic conditions for 48 hours at 35°C. The threshold of detection was 10 CFU/mL, which corresponds to 1 colony of growth on any plate. To prepare a pure culture for organism identification, we isolated each morphologically distinct colony type on a different plate of the same media. To identify the bacterial isolates, we used matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) with the MALDI Biotyper 3.0 software (Bruker Daltonics, Billerica, MA) (1).
16S rRNA gene sequencing and bioinformatics analysis
DNA extraction and isolation, polymerase chain reaction (PCR) amplification, and 16S rRNA gene sequencing of urine samples has been described previously (10). We extracted and isolated genomic DNA in a PCR hood to avoid contamination. From 1 ml of urine, we extracted genomic DNA using previously validated protocols (1, 7, 11). This protocol includes the peptidoglycan degrading enzymes, mutanolysin and lysozyme, to ensure robust lysis of Gram-positive and Gram-negative species (11). We centrifuged 1 ml of urine at 13,500 rpm for 10 min; we resuspended the pellet in 200 μl of filter-sterilized buffer consisting of 20 mM Tris-Cl (pH 8), 2 mM EDTA, 1.2% Triton X-100, and 20 μg/ml lysozyme and supplemented with 30 μl of filter-sterilized mutanolysin (5,000 U/ml; Sigma-Aldrich, St. Louis, MO). We incubated the mixture at 37°C for 1 h; we processed the lysates with the DNeasy blood and tissue kit (Qiagen, Valencia, CA), according to the manufacturer’s protocol. We eluted the DNA into 50 μl of buffer AE (pH 8.0). We stored the isolated genomic DNA at −20° C.
We amplified the hyper-variable region 4 (V4) of the bacterial 16S rRNA gene with a two-step PCR protocol, as described previously (1, 7). We first amplified the V4 region using Illumina MiSeq modified universal primers 515F and 806R. We included extraction negative controls (no urine or swab suspension) and PCR-negative controls (no template) to identify contaminating DNA from reagents. We diluted the reaction mixtures 1:50 and amplified them for 10 more cycles, using primers that include the adapter sequences required for Illumina MiSeq sequencing and an 8-nucleotide sample index. We purified the PCR reaction and then size selected using Agencourt AMPure XP-PCR magnetic beads (Beckman Coulter, Pasadena, CA). We quantified each sample with the Qubit fluorometeric system (Thermo-Fisher, Waltham, MA). We pooled the samples, normalized to a standard volume, and inserted them in the 2 × 250 bp sequencing reagent cartridge, according to the manufacturer’s instructions (Illumina, San Diego, CA).
We removed sample barcodes and sequencing primers after sequencing using the Illumina proprietary MiSeq post-sequencing software. To process the raw sequences, we used the mothur program (v1.37.4), following the recommended MiSeq standard operating procedure (12). Mothur combines paired end reads based on overlapping nucleotides to produce 16S rRNA gene contigs; it removes contigs of incorrect length (<290 bp, >300 bp) and/or contigs containing ambiguous bases. UCHIME in the mothur package removed chimeric sequences (13). To correct for different sequencing depth of each sample, we subsampled to a depth of 5000 sequences. We clustered the sequences into species-level operational taxonomic units (OTUs) using identity threshold of 97% (14). We classified OTUs using RDP classifier (v2.11) at the genus level (14) and BLCA (15) at the species level.
Urine samples generally contain small numbers of bacteria. Because of this low biomass, we amplified and sequenced in duplicate (technical replicates). We classified samples as either sequence-positive or sequence-negative. We defined sequence-positive as a sample that amplified in both technical replicates; if present, the dominant taxon (representing >50% of sequences from the sample) was the same in both replicas. For further analysis, we used both technical replicates for each sequence-positive urine sample. We did not further analyze sequence-negative urines.
Statistical methods
Descriptive statistics for baseline demographics and clinical characteristics were presented. Continuous variables were compared using Wilcoxon rank sum tests; categorical variables were compared using Chi-squared or Fisher’s exact tests when appropriate. One-way analysis of variance (ANOVA) tests were used to compare categorical variables among groups. Post-hoc comparisons were performed if necessary. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Catheterized urine specimens from 224 participants were collected and analyzed via EQUC and 16S rRNA gene sequencing: 52 have been reported previously (1, 5, 7). The 224 participants had a mean age of 48 years (SD=14), most were White/Caucasian (66%, N=148) and were overweight [mean BMI of 29.96 (SD=7.73)]. About half were pre-menopausal (51%, N=115) (Table 1).
Table 1.
Patient/Clinical Variables by EQUC Urotype.
| Patient and Clinical Variables | Total Cohort (N=224) |
Lacto-bacillus Urotype (N = 42) |
Gardnerella Urotype (N=12) |
Streptococcus Urotype (N=21) |
Escherichia Urotype (N=8) |
Other* Urotype (N=19) |
Mixed Urotype (N=13) |
Culture Negative Urotype (N=109) |
|---|---|---|---|---|---|---|---|---|
| Age (years), mean (SD)a | 48 (14) | 45 (14) | 36 (13) [<0.001] | 49 (14) | 60 (13) [0.005] |
49 (12) | 46 (15) | 49 (13) |
| Race: | ||||||||
| -White/Caucasian | 148 (66%) | 31 (74%) | 7 (58%) | 14 (67%) | 7 (88%) | 13 (68%) | 6 (46%) | 70 (64%) |
| -Black/African American | 53 (24%) | 8 (19%) | 4 (33%) | 7 (33%) | 0 (0%) | 6 (32%) | 6 (46%) | 22 (20%) |
| -Asian | 9 (4%) | 2 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (6%) |
| -NHPI | 1 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| -Other | 13 (6%) | 1 (2%) | 1 (8%) | 0 (0%) | 1 (12%) | 0 (0%) | 1 (8%) | 9 (8%) |
| Ethnicity: | ||||||||
| -Hispanic or Latina | 28 (12%) | 6 (14%) | 2 (17%) | 1 (5%) | 1 (12%) | 0 (0%) | 3 (23%) | 15 (14%) |
| -Not Hispanic or Latina | 196 (88%) | 36 (86%) | 10 (83%) | 20 (95%) | 7 (88%) | 19 (100%) | 10 (77%) | 94 (86%) |
| BMI (kg/m2), mean (SD)a | 29.96 (7.73) | 30.24 (7.82) | 33.44 (11.92) [0.04] | 29.62 (5.95) | 33.24 (8.14) | 31.05 (9.19) | 31.25 (7.87) | 28.94 (7.09) [0.03] |
| Vaginal Parity, median (IQR)c | 2 (0–3) | 2 (0–2) | 0 (0–0) [0.001] | 2 (1–2) | 2 (2–3) | 2 (1–3) | 2 (0–3) | 2 (0–3) |
| Engage in Vaginal Intercourse (N=146) | 92 (63%) | (N=27) 19 (70%) |
(N=9) 7 (78%) |
(N=13) 8 (62%) |
(N=6) 0 (0%) [0.001]b |
84(N=7) 4 (57%) |
(N=9) 8 (89%) |
(N=75) 46 (61%) |
| Menopausal Status: | [0.02]d | |||||||
| - Pre-menopausal | 115 (51%) | 22 (52%) | 8 (66%) | 10 (48%) | 0 (0%) [0.003]b | 9 (47%) | 7 (54%) | 59 (54%) |
| - Post-menopausal | 72 (32%) | 13 (31%) | 2 (17%) | 8 (38%) | 6 (75%) [0.008] | 7 (37%) | 3 (23%) | 33 (30%) |
| - Not Sure | 37 (17%) | 7 (17%) | 2 (17%) | 3 (14%) | 2 (25%) | 3 (16%) | 3 (23%) | 17 (16%) |
| Use of Hormone Replacement Therapy | 12 (6%) | 4 (10%) | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (6%) |
| Diabetes | 15 (7%) | 3 (7%) | 1 (8%) | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 10 (9%) |
| Heart Disease | 1 (0%) | 0 (0%) | 0 (0%) | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypertension | 50 (22%) | 8 (19%) | 1 (8%) | 7 (33%) | 2 (25%) | 2 (11%) | 3 (23%) | 27 (25%) |
| Urine Dipstick: (N=223) | (N=41) | |||||||
| - White Blood Cells (WBC) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| - Nitrites | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| - Red Blood Cells (RBC) | 11 (5%) | 4 (10%) | 1 (8%) | 2 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (4%) |
| Standard Urine Culture (SUC)-Positive | 13 (6%) | 1 (2%) | 1 (8%) | 0 (0%) | 7 (88%) [<0.001] | 2 (11%) | 2 (15%) | 0 (0%) [<0.001]b |
Chi-Square Test used unless otherwise indicated. Fisher’s Exact Test used when appropriate. SD = Standard Deviation. IQR = Interquartile Range.
Only p-values ≤ 0.05 are shown (bold and highlighted).
– Independent T test;
– Fisher’s Exact Test;
– Wilcoxon Rank Sum Test;
– One-way ANOVA
– “Other” Urotype includes any additional urotypes present in < 5 samples in the Total Cohort
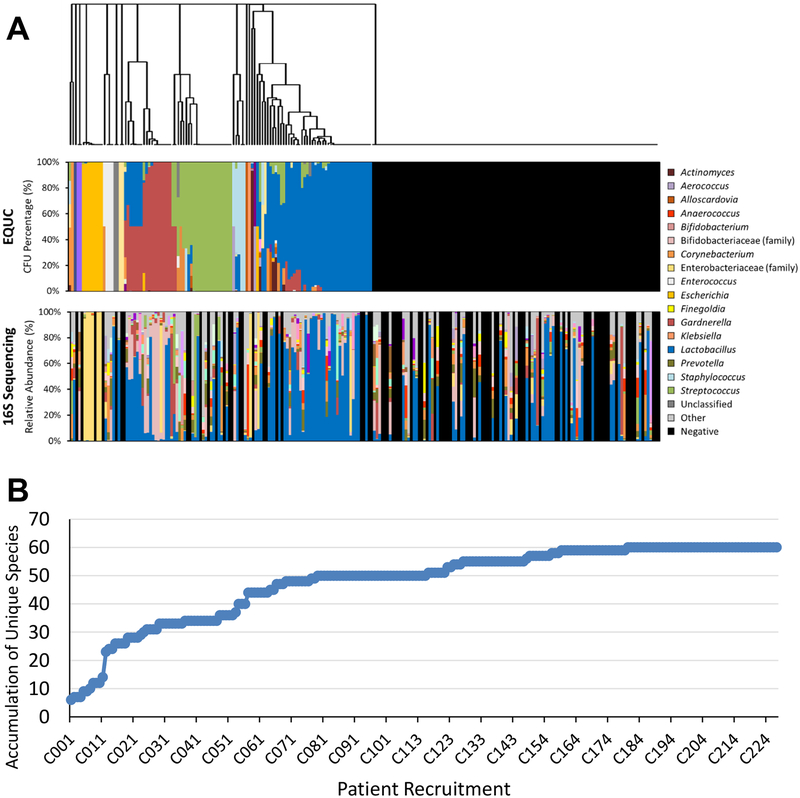
EQUC detected bacteria in 115 (51%) urine specimens, whereas the standard clinical urine culture (SUC) method detected bacteria in only 13 (6%) (Table 1). 16S rRNA gene sequencing detected bacterial DNA in 141 (63%) urine specimens. Microbiota histograms are shown in Figure 1A. Species level identification was obtained by EQUC. Figure 1B displays a species accumulation curve (rarefaction) based on EQUC data; the plateau indicates that we have identified almost all the species that can be detected by EQUC in this participant population.
Figure 1. Urinary Microbiota Assessed by EQUC and 16S rRNA Sequencing.
(A) Microbiota profiles are shown as stacked bar graphs depicting the relative abundance (y-axes) of various taxa from each participant (x-axes). Data were obtained using EQUC (top) and 16S rRNA gene sequencing (bottom). EQUC-obtained data were sorted using hierarchical clustering of the the Euclidean distance between samples (dendrogram). Subsequently, the data obtained by 16S rRNA gene sequencing were sorted according to the EQUC data. A legend containing the most common taxa is shown on the left. ‘Other’ refers to the combined relative abundance for all taxa not included in the 20 most abundant taxa obtained by 16S rRNA gene sequencing. Note that the genera Escherichia (gold) and Gardnerella (maroon) are members of the families Enterobacteriaceae (yellow) and Bifidobacteriaceae (pink), respectively. (B) Species accumulation curve of the bacterial species culture and identified by EQUC.
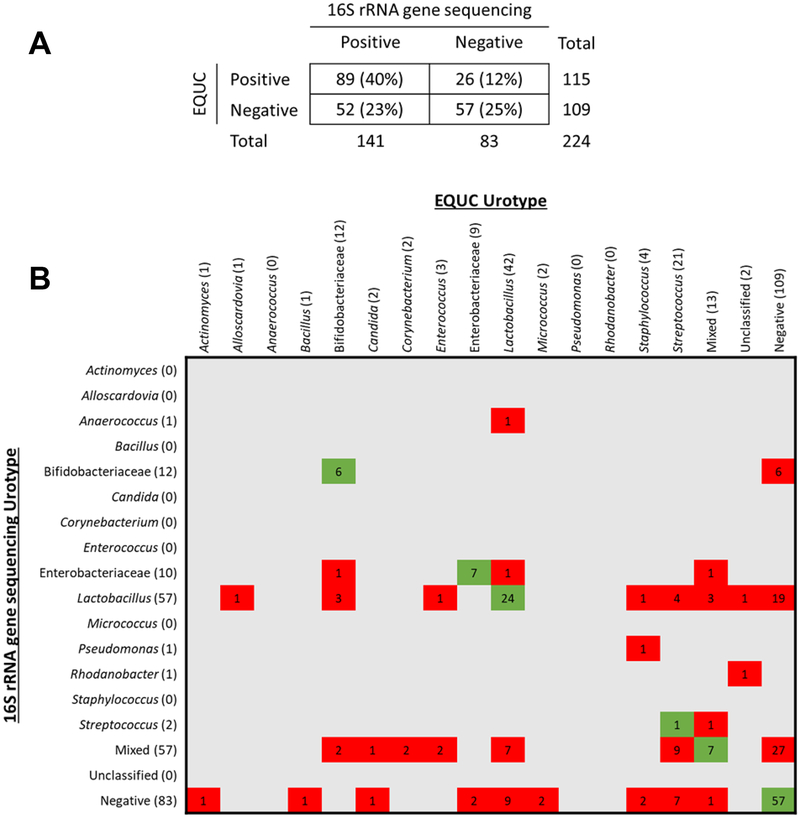
16S rRNA gene sequencing detected bacteria in 141 (63%) urine samples. The total number of sequences generated was 16039173 and the median reads per sample was 51426 (IQR:14158–122254). Figure 2A shows the comparison of bacteria detected bacteria by EQUC and sequencing: 89 (40%) were positive by both methods, 57 (25%) were negative by both methods, and the remaining 78 (35%) were positive by only one method. EQUC and sequencing agreed for the majority of the double positive specimens.
Figure 2. Comparison of EQUC and 16S rRNA Gene Sequencing Data.
Comparison of taxa detected by EQUC and 16S rRNA gene sequencing. (A) Comparison of EQUC and sequence status. (B) Comparison of the urotypes determined by EQUC and sequencing. Urotypes were assigned based on the presence of a taxa at >50% relative abundance. Numbers depict the number of urine specimens with the corresponding urotypes. Cells in red indicate that the two methods did not match; cells in green indicated that the two methods matched.
Figure 2B shows the comparison of urotypes defined by EQUC and sequencing. For both detection methods, urine specimens were classified into urotypes, defined by the predominant (>50% abundance) taxon present. If no taxon reached 50% abundance, the urotype was classified as “mixed.” 102 urine specimens had matching urotypes by both methods (i.e., green squares). Of the 122 urine specimens that did not match, most (n=78) resulted primarily from bacterial detection by only one method.
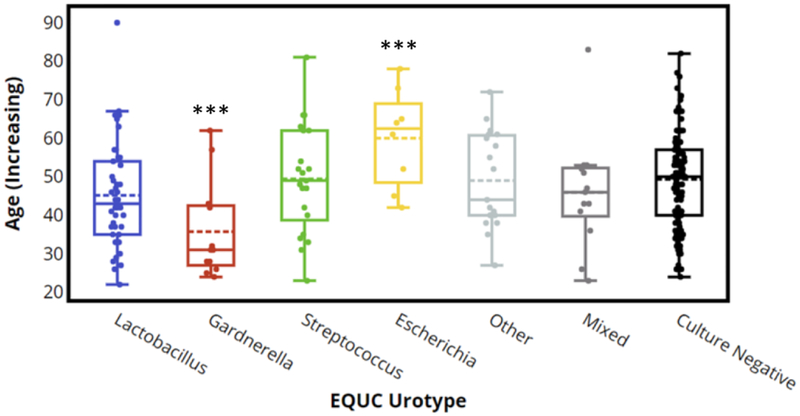
Table 1 displays the demographic data of participants grouped by EQUC urotype, using only urotypes that were found in at least 5 specimens. The 19 specimens whose urotypes were found in less than 5 specimens were pooled together and grouped as “other.” The most common urotype in the 115 EQUC-positive participants was Lactobacillus (n=42), followed by Streptococcus (n=21), Other (n=19), Mixed (n=13), Gardnerella (12) and Escherichia (n=8). The 12 participants with a Gardnerella were younger (mean age 36 (SD=13), p<0.001), heavier (mean BMI 33.44 (SD=11.92), p=0.04), and had a lower median vaginal parity (0, p<0.001). The 8 participants with an Escherichia urotype were older (mean age 60 (SD=13), p=0.005), more likely to be post-menopausal (6/8 (75%), p=0.008), less likely to engage in vaginal intercourse([0/6 (0%), p=0.002), and more likely to be SUC-positive (7/8 (88%), p<0.001). The 13 participants with the Mixed urotype were more likely to be Black/African American (6/13 (46%), p=0.05). Finally, the 109 participants with a Culture Negative urotype had a lower mean BMI (28.94 (SD=7.09), p=0.03). Similar results were observed when urotypes were defined by sequence data (Table S1).
Both Table 1 and Figure 3 display the association of age with EQUC urotype. The Gardnerella urotype (n=12) was more common in younger women, whereas the Escherichia urotype (n=8) was more common in older women. These differences were also associated with three demographic factors: menopausal status, vaginal parity and engaging in vaginal intercourse. Women who reported being post-menopausal were more likely to have the Escherichia urotype (p=0.002) (Table S2). Women who had given birth were unlikely to be in the Gardnerella urotype (vaginal parity=0; p=0.001) (Table S3). Finally, women who reported not engaging in vaginal intercourse were more likely to be in the Escherichia urotype (p=0.002) (Table S4).
Figure 3. EQUC Urotype by Age Distribution.
Box plots depict the range of ages of the participants according to EQUC urotype. Median scores are depicted by the solid lines in each box.
DISCUSSION
Main Findings
The urobiome of this well-characterized cohort of continent women varied amongst individuals. Individual urobiomes most often were dominated by the members of the genera Lactobacillus, Streptococcus, Gardnerella and Escherichia.
The size of this cohort was large enough to detect nearly all bacterial species contained in the urine specimens of this population, as shown by the species accumulation curve. Our use of two detection methods confirms that EQUC and 16S rRNA gene sequencing provide confirmatory and complementary information. Each technique has advantages. 16S rRNA gene sequencing provides depth and detects some taxa that EQUC does not. In contrast, EQUC provides species level identification and allows storage of isolates for subsequent testing. Rather than assuming that samples that were negative by both techniques are sterile, we recognize the possibility that these specimen have bacterial loads that are below the threshold for current detection techniques.
Standard urine culture had a false negative rate of 89% in this cohort, consistent with previous reports (1, 5, 7, 16). Based on its inferior ability for bacterial detection, we do not recommend standard culture for urobiome research.
The variability of the urobiome, in terms of urotype, has been reported previously (4, 7, 8, 17). The most common urotype, Lactobacillus, did not show strong correlations with specific demographic features, including age, menopausal status, parity, and vaginal intercourse. This lack of correlation differs from common knowledge concerning Lactobacillus in the vagina, which is thought to be less common in post-menopausal women (18–20). Some urotypes were associated with age, with Escherichia and Gardnerella more common in older and younger women, respectively. Most women with the Escherichia urotype had a positive standard urine culture, consistent with the standard method’s preferential detection of Escherichia. These findings related to increasing concern about the clinical algorighm for management of women who do not have symptoms of UTI, yet have a positive urine culture. Prior to the discovery of the urobiome, the term “asymptomatic bacteriuria” was broadly applied to such patients; however, the utility of this term is being reconsidered in order to avoid inappropriate antibiotic use and optimize antibiotic stewardship (21, 22). Although Gardnerella urotypes have been reported in the adult female bladder (4, 5, 7, 8, 16, 17, 23), this is the first report that the Gardnerella urotype is common in young but not older women. Note that none of these women had symptoms of bacterial vaginosis.
The detected differences in urotype may relate to hormonal influences in the genituourinary tract, including the beneficial effect on Lactobacillus growth in both the vagina and lower urinary tract. The biological purpose for these various urotypes is not known. It is possible that urotypes confer protection or predisposition for various lower urinary conditions, including urinary incontinence, overactive bladder or even urinary tract infection. Longitudinal studies in large, well-characterized cohorts will be needed to detect these associations.
Strengths and Limitations
Strengths of this study include the large cohort size; this is the largest group of well-characterized continent controls reported to date. The study was further strengthened through the use of validated symptoms assessment. Another important strength of this study is the exclusive use of catheterized urine samples. There are ample data to demonstrate that the voided urine sample represents a broad genitourinary sample that variably includes microbes from the labial and/or vulvar skin. Improved urinary collection techniques are available for voided samples, although the interpretation of the voided urine samples requires diligence to account for the genital (non-urinary) contribution (24). The use of catheterized urine specimens allowed us to interpret our data in terms of the bacterial communities that inhabit the bladders of this cohort. Prior work has addressed the issue of potential contamination from other pelvic niches during urethral catheterization (3), although clinicians may remain concerned about other sources of bacterial introduction into the bladder. Finally, our use of two high-quality detection methods (EQUC and 16S rRNA gene sequencing) that vastly outperform standard urine culture methods provides superior compilation of the urinary species in continent adult women. While these two techniques do not provide identical results (for example, EQUC does a poor job of detecting some strict anaerobes, while sequencing underdetects Streptococcus species because the tough cell wall of Streptococcus limits DNA extraction), the complementary information from EQUC and sequencing allowed us to verify the results of each and provided us with a broader and deeper view of the bladder microbiota/microbiome.
A limitation of our study is the use of a single urine specimen, which represents a single point in time. We fully expect that the urobiome changes over time; longitudinal data is needed especially over times of biologically relevant changes in nearby adjacent pelvic microbial niches (e.g., vagina, anus). Specific urotypes may have different community characteristics, with some being more stable and/or less likely to change when nearby niches, such as the vagina, experience transient microbial shifts.
Interpretation
This study confirms prior studies that suggest that EQUC or other enhanced urine culture methods and/or DNA sequencing are effective techniques for bacterial detection and characterization for urobiome research purposes. Well over half of this cohort of well characterized continent adult women exhibited a detectable urobiome, most commonly of the Lactobacillus urotype. This is consistent with other studies that have shown that Lactobacillus is common in the bladders of adult women with and without lower urinary symptoms (4, 5, 7, 8, 10, 17, 23). It is not surprising that Lactobacillus predominates the urobiome of many adult women, given the beneficial role that Lactobacillus generally plays in other microbial niches (20, 25) and evidence that certain Lactobacillus species are associated with the lack of lower urinary tract symptoms (7) and reduce risk of post-surgical UTI (26). Injudicious antibiotic use may have collateral effects that reduce the Lactobacillus population, causing an unintended, subsequent vulnerability to the urobiome; a vulnerable urobiome may be more susceptible to invasion and/or overgrowth of opportunistic pathogens that are or may be associated with lower urinary tract symptoms. What is surprising is the lack of any association between the Lactobacillus urotype and race, as evidence exists that the vaginal microbiome of Black/African American women is less likely to be Lactobacillus-dominant (27).
The relationship between specific urobiome members and specific urinary symptoms is poorly understood. It is well known that Escherichia coli and a few other uropathogens are associated with the clinical diagnosis of UTI (28). However, most of the participants in this study did not have evidence of urinary E. coli by standard urine culture testing. Strategies for detection and clinical interpretation of clinical tests, such as the standard urine culture, should incorporate knowledge that some women have an Escherichia urotype without evidence of clinical disorder, such as UTI. A subset of bacterial species, including emerging uropathogens Actinotignum schaalii, Atopoboium vaginae and Aerococcus urinae, have been attributed to overactive bladder and/or urinary urgency incontinence (7, 10). Further studies are needed to clarify the contributions of individual microbes or entire microbial communities to common lower urinary symptoms.
CONCLUSION
The EQUC technique documented a urobiome in over half of the well characterized continent adult women in this study; the most common urotype was Lactobacillus. Given this finding, clinical care strategies should preserve or restore the beneficial effects of the Lactobacillus population, especially in these women. Details regarding longitudinal changes of the urobiome, especially in response to therapeutic interventions, such as antibiotics, are likely to improve clinical care for adult women affected by a wide variety of lower urinary tract symptoms.
Supplementary Material
ACKNOWLEDGMENTS:
We thank Mary Tulke RN for her assistance with participant recruitment and sample collection, and Cynthia Brincat MD who greatly assisted with recruitment efforts of the study population. We also thank Roberto Limeira of the Loyola Genomics Facility for performing the DNA sequencing.
Funding: This work was supported by R01 DK104718, R56 DK104718, R21 DK097435 and P20 DK108268 and a grant from the Falk Foundation (LU#202567)
DISCLOSURE STATEMENT: Dr. Mueller discloses research support from NIH, Astellas and Boston Scientific, Dr. Wolfe discloses research support from NIH, Astellas and Kimberly Clark, Dr. Brubaker discloses editorial stipends from Female Pelvic Medicine and Reconstructive Surgery, UpToDate and JAMA; she also discloses research funding from NIH. The remaining authors (Price, Hilt, Thomas-White) report no disclosures. Completed disclosure of interest forms are available to view online as supporting information.
Footnotes
REFERENCES
- 1.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol. 2012;50(4):1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karstens L, Asquith M, Davin S, Stauffer P, Fair D, Gregory WT, et al. Does the Urinary Microbiome Play a Role in Urgency Urinary Incontinence and Its Severity? Front Cell Infect Microbiol. 2016;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas-White KJ, Hilt EE, Fok C, Pearce MM, Mueller ER, Kliethermes S, et al. Incontinence medication response relates to the female urinary microbiota. Int Urogynecol J. 2016;27:723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brubaker L, Nager CW, Richter HE, Visco A, Nygaard I, Barber MD, et al. Urinary bacteria in adult women with urgency urinary incontinence. Int Urogynecol J. 2014;25:1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce MM, Hilt EE, Rosenfeld AB, Zilliox MJ, Thomas-White K, Fok C, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5(4):e01283–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komesu YM, Richter HE, Carper B, Dinwiddie DL, Lukacz ES, Siddiqui NY, et al. The urinary microbiome in women with mixed urinary incontinence compared to similarly aged controls. Int Urogynecol J. 2018; 29(12):1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–74. [DOI] [PubMed] [Google Scholar]
- 10.Fok CS, Gao X, Lin H, Thomas-White KJ, Mueller ER, Wolfe AJ, et al. Urinary symptoms are associated with certain urinary microbes in urogynecologic surgical patients. Int Urogynecol J. 2018;29(12):1765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan S, Cohen DB, Ravel J, Abdo Z, Forney LJ. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS One. 2012;7(3):e33865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Lin H, Revanna K, Dong Q. A Bayesian taxonomic classification method for 16S rRNA gene sequences with improved species-level accuracy. BMC Bioinformatics. 2017;18(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price TK, Dune T, Hilt EE, Thomas-White KJ, Kliethermes S, Brincat C, et al. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J Clin Microbiol. 2016;54:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce MM, Zilliox MJ, Rosenfeld AB, Thomas-White KJ, Richter HE, Nager CW, et al. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol. 2015;213(3):347 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cauci S, Driussi S, De Santo D, Penacchioni P, Iannicelli T, Lanzafame P, et al. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol. 2002;40(6):2147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brotman RM, Shardell MD, Gajer P, Fadrosh D, Chang K, Silver MI, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014;21(5):450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol. 2017;595(2):451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finucane TE. “Urinary Tract Infection”-Requiem for a Heavyweight. J Am Geriatr Soc. 2017;65(8):1650–5. [DOI] [PubMed] [Google Scholar]
- 22.Price TK, Hilt EE, Dune TJ, Mueller ER, Wolfe AJ, Brubaker L. Urine trouble: should we think differently about UTI? Int Urogynecol J. 2017;29:205–10. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs KM, Thomas-White KJ, Hilt EE, Wolfe AJ, Waters TP. Microorganisms Identified in the Maternal Bladder: Discovery of the Maternal Bladder Microbiota. AJP Rep. 2017;7(3):e188–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Southworth E, Hochstedler B, Price TK, Joyce C, Wolfe AJ, Mueller ER. A Cross-sectional Pilot Cohort Study Comparing Standard Urine Collection to the Peezy Midstream Device for Research Studies Involving Women. Female Pelvic Med Reconstr Surg. 2019;25(2):e28–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeney DD, Gareau MG, Marco ML. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr Opin Biotechnol. 2018;49:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas-White KJ, Gao X, Lin H, Fok CS, Ghanayem K, Mueller ER, et al. Urinary microbes and postoperative urinary tract infection risk in urogynecologic surgical patients. Int Urogynecol J. 2018;29(12):1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivick KE, Mobley HL. Waging war against uropathogenic Escherichia coli: winning back the urinary tract. Infect Immun. 2010;78(2):568–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.