1. Background
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to more than three million confirmed cases, with over 211,000 deaths globally, as of April 27, 2020. The living and working conditions of billions of people worldwide have been significantly disrupted due to different forms of social distancing and lockdowns in many cities. The world economy has been remarkably weakened as a result of business shutdowns and major restrictions on travel. Widespread availability of accurate and rapid testing procedures is extremely valuable in unraveling the complex dynamics involved in SARS-CoV-2 infection and immunity. To this end, laboratories, universities, and companies around the world have been racing to develop and produce critically needed test kits.
One of the many challenges for containing the spread of COVID-19 is the ability to identify asymptomatic cases that result in spreading of the virus to close contacts. A study of the passengers on a Diamond Princess Cruise ship forced into temporary quarantine from an early outbreak of COVID-19, estimated the asymptomatic proportion (among all infected cases) at 17.9% (95%CrI: 15.5–20.2%).1 Therefore, the actual number of SARS-CoV-2-infected individuals may be much higher than currently accounted for based on positive test results.2 Having accurate, convenient, and rapid testing for widespread deployment can aid in eliminating the silent spread of COVID-19 by asymptomatic viral carriers.
Because COVID-19 exhibits a range of clinical manifestations, from mild flu-like symptoms to life-threatening conditions, it is important to have efficient testing during the early stages of infection to identify COVID-19 patients from those with other illnesses. This avoids unnecessary quarantines of negative individuals and the spread of infection by positive individuals. Early diagnosis permits physicians to provide prompt intervention for patients who are at higher risk for developing more serious complications from COVID-19 illness. More complicated diagnostic testing based on viral genomic sequencing is an essential tool for determining the rate and degree of mutational variability associated with SARS-CoV-2 and for identifying newly emerging strains of the virus for more effective vaccine development. Until a commercial vaccine becomes available, it is important to identify individuals who have been infected with SARS-CoV-2, with or without accompanying symptoms, and who have developed antiviral immunity. This allows for additional analyses of strength and durability of immunity across general populations.
Commercially available COVID-19 tests currently fall into two major categories. The first category includes molecular assays for detection of SARS-CoV-2 viral RNA using polymerase chain reaction (PCR)-based techniques or nucleic acid hybridization-related strategies. The second category includes serological and immunological assays that largely rely on detecting antibodies produced by individuals as a result of exposure to the virus or on detection of antigenic proteins in infected individuals. It is important to reemphasize that these two categories of tests serve overlapping purposes in management of the COVID-19 pandemic. Testing for SARS-CoV-2 viral RNA identifies SARS-CoV-2-infected individuals during the acute phase of infection. Serological testing subsequently identifies individuals who have developed antibodies to the virus and could be potential convalescent plasma donors. It also furthers the ability to conduct contact tracing and monitor the immune status of individuals and groups over time.3
Timely diagnosis, effective treatment, and future prevention are key to management of COVID-19. The current race to develop cost-effective point-of-contact test kits and efficient laboratory techniques for confirmation of SARS-CoV-2 infection has fueled a new frontier of diagnostic innovation. In order to assist ongoing innovation, we developed this report to provide an overview of current COVID-19 diagnostic trends and strategies based on conventional and novel methodologies, including CRISPR. It includes current information on test kits and developers as well as data on COVID-19 diagnostic trends based on journal publication information extracted from the CAS content collections and MEDLINE.
2. Molecular Assays for Detection of Viral Nucleic Acids
SARS-CoV-2 is a single-stranded, positive-sense RNA virus, and since its entire genetic sequence was uploaded to the Global Initiative on Sharing All Influenza Data (GISAID) platform on January 10, 2020, companies and research groups in a matter of weeks have developed a range of diagnostic kits for COVID-19. The availability of sequence data has facilitated the design of primers and probes needed for the development of SARS-CoV-2-specific testing.4
2.1. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR relies on its ability to amplify a tiny amount of viral genetic material in a sample and is considered to be the gold standard for identification of SARS-CoV-2 virus. Currently, RT-PCR tests for COVID-19 generally use samples collected from the upper respiratory system using swabs. In addition, a few studies have also been done using serum, stool, or ocular secretions.5−7 Recently, the Rutgers Clinical Genomics Laboratory developed an RT-PCR assay (TaqPath COVID-19 Combo kit) that uses self-collected saliva samples, which is quicker and less painful than other sample collection methods, lowers the risks to healthcare providers, and may enable higher volume testing.8,9
As illustrated in Figure 1, RT-PCR starts with laboratory conversion of viral genomic RNA into DNA by RNA-dependent DNA polymerase (reverse transcriptase). This reaction relies on small DNA sequence primers designed to specifically recognize complementary sequences on the RNA viral genome and the reverse transcriptase to generate a short complementary DNA copy (cDNA) of the viral RNA. In real-time RT-PCR, the amplification of DNA is monitored in real time as the PCR reaction progresses. This is done using a fluorescent dye or a sequence-specific DNA probe labeled with a fluorescent molecule and a quencher molecule, as in the case of TaqMan assays. An automated system then repeats the amplification process for about 40 cycles until the viral cDNA can be detected, usually by a fluorescent or electrical signal.10
Figure 1.
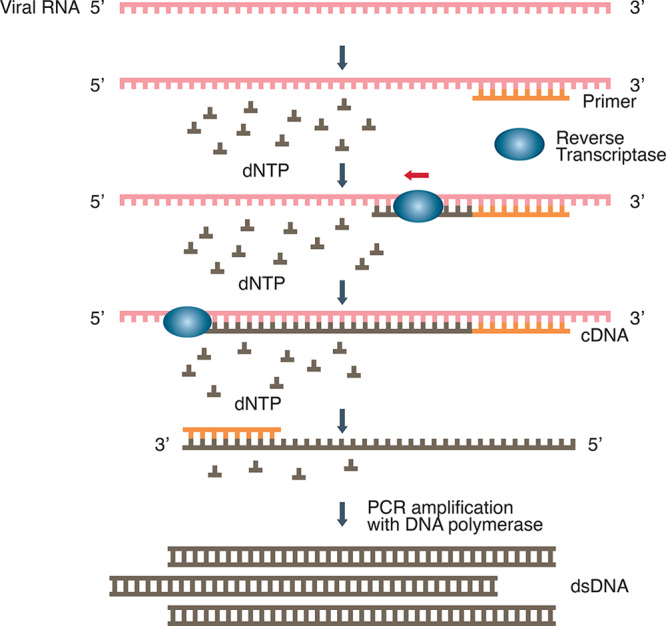
Reverse transcription-polymerase chain reaction (RT-PCR). The RT-PCR creates a cDNA copy of a specific segment of the viral RNA, which is converted to dsDNA that is exponentially amplified.
RT-PCR has traditionally been carried out as a one-step or a two-step procedure. One-step real-time RT-PCR uses a single tube containing the necessary primers to run the entire RT-PCR reaction. Two-step real-time RT-PCR involves more than one tube to run the separate reverse transcription and amplification reactions, but offers greater flexibility and higher sensitivity than the one-step procedure. It requires less starting material and allows for the ability to stock cDNA for quantification of multiple targets.11 The one-step procedure is generally the preferred approach for detection of SARS-CoV-2 because it is quick to set up and involves limited sample handling and reduced bench time, decreasing chances for pipetting errors and cross-contamination between the RT and real-time PCR steps.
To date, the majority of molecular diagnostic tests have utilized the real-time RT-PCR technology targeting different SARS-CoV-2 genomic regions, including the ORF1b or ORF8 regions, and the nucleocapsid (N), spike (S) protein, RNA-dependent RNA polymerase (RdRP), or envelope (E) genes (see Table 1 in section 2.5 and Supporting Information Table S1).12−15
Table 1. Examples of Molecular Diagnostic Tests Used to Detect Viral Genetic Material in SARS-CoV-2.
| test name | test type | manufacturer/organization name | sample source | gene or region detected | test result time/additional information | throughput information | EUAb | country of approval |
|---|---|---|---|---|---|---|---|---|
| ID NOW COVID-19 | isothermal nucleic acid amplification technology | Abbott Diagnostics Scarborough, Inc. | nasal, nasopharyngeal and throat swabs | RdRP gene | positive results <5 min and negative results in 13 min | 1 sample/run | US FDA 3/27/2020 | United States |
| iAMP COVID-19 detection kit | real-time RT isothermal amplification test | Atila BioSystems, Inc. D11 | nasal, nasopharyngeal, and oropharyngeal swabs | ORF1ab and/or N gene | results <1.5 h | high throughputa | US FDA 4/10/2020 | United States |
| BioFire COVID-19 test | multiplex real-time RT-PCR | BioFire Defense, LLC | nasopharyngeal swabs | ORF1ab and ORF8 | results in ∼45 min | 94 samples/run | US FDA 3/23/2020 | United States |
| CDC 2019- Novel Coronavirus Real-Time RT-PCR Diagnostic Panel | real-time RT-PCR | CDC-US | nasopharyngeal or oropharyngeal aspirates/washes/swabs and bronchoalveolar lavage fluid, tracheal aspirates, sputum | N gene | human RNase P gene used as control | 264 samples/day | US FDA 2/4/2020 | United States |
| Xpert Xpress SARS-CoV-2 test | real-time RT-PCR | Cepheid | nasopharyngeal, nasal, and midturbinate swabs | N2 and E genes | results in ∼45 min with <1 min of hands-on time | high throughputa | US FDA 3/20/2020 | Australia, Canada, Singapore, United States |
| CRISPR-based tests for SARS-CoV-2 | CRISPR-based lateral flow assay isothermal amplification | Cepheid Sherlock Biosciences | respiratory samples | viral RNA | combines Sherlock’s Cas12 and Cas13 enzymes for nucleic acid detection with Cepheid’s GeneXpert test-processing instruments | US FDA 3/20/2020 | United States | |
| VitaPCR SARS-CoV-2 assay | real-time PCR | Credo Diagnostics Biomedical Pte Ltd. | nasal and oropharyngeal swabs | viral RNA | results in 20 min with 1 min of hands-on time | 2000 samples/day | CE mark 3/2020 | Singapore |
| LYRA SARS-CoV-2 assay | real-time RT-PCR | Diagnostic Hybrids, Inc. Quidel Corporation | nasopharyngeal and oropharyngeal swabs | pp1ab | results in <75 min after extraction | US FDA 3/17/2020 | Canada | |
| SARS-CoV-2 assay | real-time RT-PCR | Diagnostic Molecular Laboratory – Northwestern Medicine | nasopharyngeal, oropharyngeal, nasal, and midturbinate nasal swabs and bronchoalveolar lavage fluid | N1 and RdRP genes | results in <1 h without manual RNA extraction | US FDA 4/2/2020 | United States | |
| Simplexa COVID-19 Direct | real-time RT-PCR | DiaSorin Molecular LLC | nasopharyngeal swabs | OFR1ab and S gene | results in ∼1 h with no RNA extraction | high throughputa | US FDA 3/19/2020 | United States |
| ePlex SARS-CoV-2 test | RT-PCR | GenMark Diagnostics, Inc. | nasopharyngeal swabs | RNA | <2 min hands-on time and results in ∼2 h | US FDA 3/19/2020 | United States | |
| Panther Fusion SARS-CoV-2 assay (Panther Fusion System) | real time RT-PCR | Hologic Inc. | nasopharyngeal and oropharyngeal swabs | ORF1ab regions 1 and 2 | each Panther Fusion system can provide results in <3 h and process up to 1150 coronavirus tests in 24-h period | 1 sample/run | Australia 3/20/2020, US FDA 3/16/2020 | Australia, United States |
| COVID-19 RT-PCR test | real-time RT-PCR | LabCorp Laboratory Corporation of America | nasopharyngeal and oropharyngeal swabs/washes/aspirates and sputum, bronchoalveolar lavage fluid | N gene | results in 2–4 days | 24 samples/run | US FDA 3/16/2020 | United States |
| ARIES SARS-CoV-2 assay | real-time RT-PCR | Luminex Corporation | nasopharyngeal swabs | ORF1ab and N gene | minimal hands-on time and an automated workflow delivers results in ∼2 h | high throughputa | US FDA 4/3/2020 | United States |
| SARS-CoV-2 DETECTR | CRISPR-based lateral flow assay isothermal amplification | Mammoth Biosciences | respiratory samples | E and N genes | CRISPR Cas12a-based lateral flow assay results in 30–40 min | high throughputa | filed for US FDA 4/16/2020 | United States |
| Accula SARS-CoV-2 test | PCR and lateral flow technologies | Mesa Biotech Inc. | throat and nasal swabs | N gene | results in 30 min, the palm-sized device can be used in physician office or patients’ home | 144 tests/day | US FDA 3/23/2020 | United States |
| MiRXES FORTITUDE KIT 2.0 | real-time RT-PCR | MiRXES Pte Ltd. | nasopharyngeal swabs | viral RNA genes less prone to mutation | results in 90 min, produces 100,000 test kits/wk | 96 samples in 1 run | Singapore HSA 3/2020 | Singapore |
| QIAstat-Dx Respiratory SARS-CoV-2 panel | multiplex real-time RT-PCR | Qiagen GmbH | nasopharyngeal swabs | E and RdRP genes | results in ∼1 h, by differentiating novel coronavirus from 21 other bacterial and viral respiratory pathogens | 1 sample/run | US FDA 3/30/2020 | United States |
| cobas SARS-CoV-2 | real-time RT-PCR | Roche Molecular Systems, Inc. | nasopharyngeal and oropharyngeal swabs | viral RNA | results in ∼3.5 h, instruments can process up to 384 results (cobas 6800 System) and 1056 results (cobas 8800 System) in 8 h | high throughputa | Australia 2/20/20, CE mark 2020, US FDA 3/12/20 | Australia, Brazil, Canada, Japan, Singapore, United States |
| TaqPath COVID-19 combo kit | multiplex real-time RT-PCR | Rutgers Clinical Genomics Laboratory ThermoFisher-Applied Biosystems | oropharyngeal, nasopharyngeal, anterior nasal, midturbinate nasal swabs and saliva specimens | ORF1b and N and S genes | high throughputa | US FDA 3/13/2020 | United States | |
| Novel Coronavirus (2019-nCoV) Nucleic Acid diagnostic kit (PCR-fluorescence probing) | real-time RT-PCR | Sansure Biotech Inc. | nasopharyngeal and oropharyngeal swab, serum, blood and feces | ORF1ab and N gene | results in 30 min | China NMPA 4/2020 | China | |
| STANDARD M nCoV RT detection kit | real-Time RT-PCR | SD BIOSENSOR | oropharyngeal swabs | E and RdRP genes | results within 90 min | Korea MFDS 2/27/2020 | South Korea | |
| Allplex 2019-nCoV assay | multiplex real-time RT-PCR | Seegene | nasopharyngeal, oropharyngeal, or anterior nasal swabs, midturbinate and sputum specimens | E, N, and RdRP genes | results in <2 h after extraction | CE mark 2/2020, Korea MFDS 2/12/2020, US FDA 4/21/2020 | Australia, South Korea, Singapore, United States | |
| Viracor SARS-CoV-2 assay | real-time RT-PCR | Viracor Eurofins Clinical Diagnostics | nasopharyngeal, nasal, oropharyngeal washes/swabs and bronchoalveolar lavage fluid | N gene | results the same day, 12–18 h from receipt of specimen | US FDA 4/6/2020 | United States |
Information from FDA https://www.fda.gov/media/136702/download.
Emergency Use Authorization by U.S. FDA or other drug regulatory authorities.
The earliest COVID-19 RT-PCR diagnostic tests to come on the scene included (1) COVID-19 RT-PCR (LabCorp),16 (2) 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel [U.S. Centers for Disease Control and Prevention (CDC)],17 (3) TaqPath COVID-19 Combo kit (ThermoFisher-Applied Biosystems),18 (4) Allplex 2019-nCoV Assay (Seegene),19 and (5) cobas SARS-CoV-2 (Roche).20 Detailed information about these tests is provided in Table 1 in section 2.5 and Supporting Information I.
RT-PCR tests are constantly evolving with improved detection methods and more automated procedures. For example, the ePlex SARS-CoV-2 test developed by GenMark Diagnostics, Inc.21 uses “The True Sample-to-Answer Solution” ePlex instrument to detect SARS-CoV-2 in nasopharyngeal swabs. Each test cartridge contains reagents for magnetic solid-phase extraction of viral RNA, cDNA amplification, and detection combining electrowetting and GenMark’s eSensor technology. Target DNA is mixed with ferrocene-labeled signal probes complementary to specific targets. The target DNA hybridizes to the signal and capture probes which are both bound to gold-plated electrodes. The presence of a target is determined using voltammetry which generates specific electrical signals from the ferrocene-labeled signal probe.
Although RT-PCR is the most widely used method for detecting SARS-CoV-2 infections, it has the disadvantage of requiring expensive laboratory instrumentation highly skilled laboratory personnel, and can take days to generate results. As a result, a number of companies and laboratories around the globe are working to further improve the efficiency and timeliness of the RT-PCR technologies and develop various other techniques.
2.2. Isothermal Nucleic Acid Amplification
RT-PCR requires multiple temperature changes for each cycle, involving sophisticated thermal cycling equipment.22 Isothermal nucleic acid amplification is an alternative strategy that allows amplification at a constant temperature and eliminates the need for a thermal cycler. Therefore, several methods based on this principle have been developed.
2.2.1. Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP)
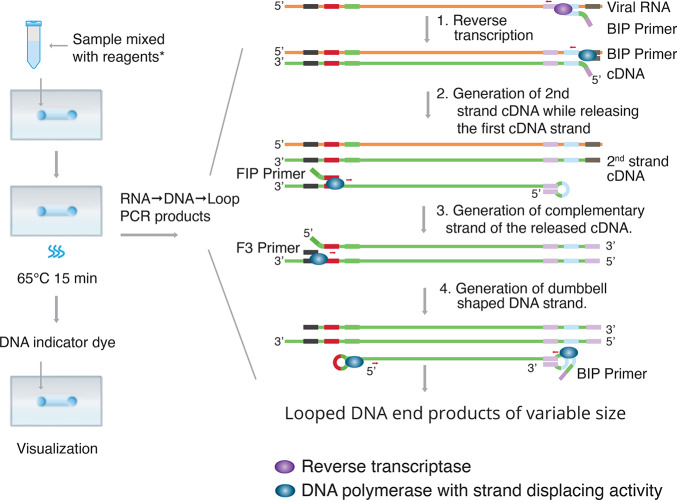
RT-LAMP has been developed as a rapid and cost-effective testing alternative for SARS-CoV-2. As shown in Figure 2, RT-LAMP requires a set of four primers specific for the target gene/region to enhance the sensitivity and combines LAMP with a reverse transcription step to allow for the detection of RNA. The amplification product can be detected via photometry, measuring the turbidity caused by magnesium pyrophosphate precipitate in solution as a byproduct of amplification. The reaction can be followed in real time either by measuring the turbidity or by fluorescence using intercalating dyes. Since real-time RT-LAMP diagnostic testing requires only heating and visual inspection, its simplicity and sensitivity make it a promising candidate for virus detection.23
Figure 2.
Reverse transcription loop-mediated isothermal amplification (RT-LAMP). Step 1: At the 3′-end of the viral RNA, reverse transcriptase and BIP primer initiate conversion of RNA to cDNA. Step 2: At the same end, DNA polymerase and B3 primer continue to generate the second cDNA strand to displace and release the first cDNA strand. Step 3: The FIP primer binds to the released cDNA strand and DNA polymerase generates the complementary strand. Step 4: F3 primer binds to the 3′ end, and DNA polymerase then generates a new strand while displacing the old strand. LAMP cycling produces various sized double-stranded looped DNA structures containing alternately inverted repeats of the target sequence as detected by a DNA indicator dye. Reagents*: Primers and master mix containing reverse transcriptase, DNA polymerase with strand displacing activity, dNTPs, and buffers.
As shown in Table 1 in section 2.5, a few of the currently available molecular assays for detecting SARS-CoV-2 utilize real-time RT-LAMP technology, such as the ID NOW COVID-19 test from Abbott Diagnostics (see Supporting Information I for details). This point-of-care test is rapid (13 min or less) and is used to detect SARS-CoV-2 viral RNA in upper respiratory swabs, but is limited to one sample per run.24,25
The RT-LAMP test prepared by Zhang et al. uses reverse transcriptase (WarmStart RTx from BioLabs) to convert the viral RNA to cDNA, which is subsequently amplified by the DNA-dependent DNA polymerase (Bst2.0 Warmstart) for rapid colorimetric detection with a DNA-binding dye (SYTO-9, ThermoFisher S34854).26 The enzyme is a unique in silico designed RNA-directed DNA polymerase coupled with a reversibly bound aptamer that inhibits RTx activity below 40 °C. It is particularly well-suited for use in LAMP. The colorimetric LAMP has been shown to be effective at detecting viral RNA in cell lysates at levels of approximately 480 RNA copies, without interference, providing an alternative to RT-PCR for rapid and simple detection of SARS-CoV-2 RNA.
2.2.2. Transcription-Mediated Amplification (TMA)
TMA is a patented single tube, isothermal amplification technology modeled after retroviral replication which can be used to amplify specific regions of either RNA or DNA much more efficiently than RT-PCR.27 It uses a retroviral reverse transcriptase and T7 RNA polymerase and has been used for detection of nucleic acids from multiple pathogens. On the basis of this principle, Hologic’s Panther Fusion platform has the capability to perform both RT-PCR and TMA.28 The Panther fusion platform is distinctive because of its high testing throughput (up to 1000 tests in 24 h) and its capability to simultaneously screen for other common respiratory viruses whose symptoms overlap with COVID-19 using the same patient sample and collection vial.
The initial step involves hybridization of the viral RNA target to a specific capture probe and an additional oligonucleotide containing a T7 promoter primer, which are captured onto magnetic microparticles by application of a magnetic field. Then, the captured RNA target hybridized to the T7 promoter primer is reverse transcribed into a complementary cDNA. The RNase H activity of the reverse transcriptase subsequently degrades the target RNA strand from the hybrid RNA–cDNA duplex, leaving a single-stranded cDNA, which includes the T7 promoter. An additional primer is used to generate a double-stranded DNA, which is subsequently transcribed into RNA amplicons by T7 RNA polymerase. These new RNA amplicons then reenter the TMA process allowing this exponential amplification process to generate billions of RNA amplicons in less than 1 h. The detection process involves the use of single-stranded nucleic acid torches that hybridize specifically to the RNA amplicon in real time. Each torch is conjugated to a fluorophore and a quencher. When the torch hybridizes to the RNA amplicon, the fluorophore is able to emit a signal upon excitation.
2.2.3. CRISPR-Based Assays
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) represents a family of nucleic acid sequences found in prokaryotic organisms, such as bacteria. These sequences can be recognized and cut by a set of bacterial enzymes, called CRISPR-associated enzymes, exemplified by Cas9, Cas12, and Cas13. Certain enzymes in the Cas12 and Cas13 families can be programmed to target and cut viral RNA sequences.29
Two companies, Mammoth Biosciences and Sherlock Biosciences, established by the CRISPR pioneer scientists, are independently exploring the possibility of using the gene-editing CRISPR methodology for detection of SARS-CoV-2. The SHERLOCK method developed by Sherlock Biosciences uses Cas13 that is capable of excising reporter RNA sequences in response to activation by SARS-CoV-2-specific guide RNA.30 The DETECTR assay by Mammoth Biosciences relies on the cleavage of reporter RNA by Cas12a to specifically detect viral RNA sequences of the E and N genes, followed by isothermal amplification of the target, resulting in a visual readout with a fluorophore.31 These CRISPR-based methods, as depicted in Figure 3, do not require complex instrumentation and can be read using paper strips to detect the presence of the SARS-CoV-2 virus without loss of sensitivity or specificity. These tests are both low-cost and can be performed in as little as 1 h. These tests have great potential for point-of-care diagnosis.4,32,33
Figure 3.
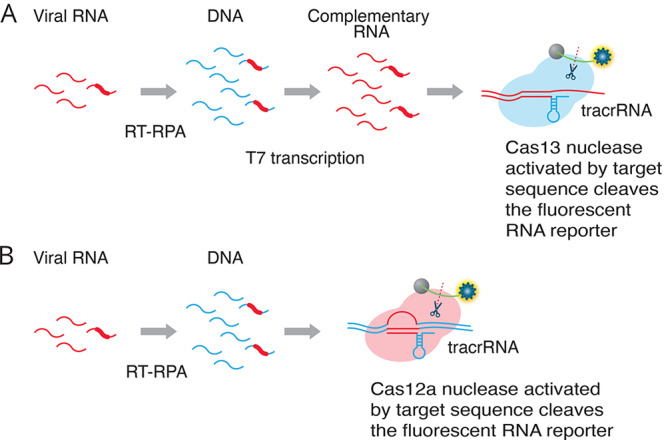
Two alternative CRISPR methods for detecting viral RNA. Method A (SHERLOCK assay30): RT-RPA (recombinase polymerase amplification) converts viral RNA to dsDNA. T7 transcription generates complementary RNA from the dsDNA template. The Cas13–tracrRNA complex binds to the target sequence, which activates the general nuclease enzyme activity of Cas13 to cleave the target sequence and the fluorescent RNA reporter. Method B (DETECTR assay31): RT-RPA (recombinase polymerase amplification) converts viral RNA to dsDNA. The Cas12a–tracrRNA complex binds to the target sequence, which activates the general nuclease enzyme activity of Cas12a to cleave the target sequence and the fluorescent RNA reporter.
2.2.4. Rolling Circle Amplification
An alternative method of isothermal nucleic acid amplification known as rolling circle amplification (RCA) has attracted considerable attention for nucleic acid detection, since in isothermal conditions, it is capable of 109-fold signal amplification of each circle within 90 min. RCA is advantageous in that it can be performed under isothermal conditions with minimal reagents and avoids the generation of false-positive results frequently encountered in PCR-based assays. An efficient assay for the detection of SARS-CoV by RCA was previously performed in both liquid and solid phases and used to test clinical respiratory specimens.34 This method, however, has not been deployed for detection of SARS-CoV-2 at this point.
2.3. Nucleic Acid Hybridization Using Microarray
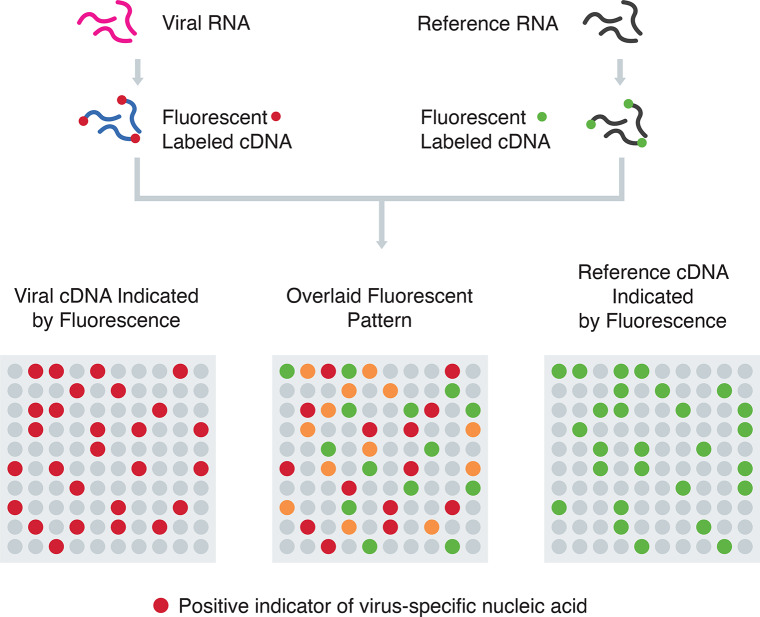
Microarray assays have been used for rapid high-throughput detection of SARS-CoV nucleic acids. As shown in Figure 4, they rely on the generation of cDNA from viral RNA using reverse transcription and subsequent labeling of cDNA with specific probes. The labeled cDNAs are loaded into the wells of microarray trays containing solid-phase oligonucleotides fixed onto their surfaces. If they hybridize, they will remain bound after washing away the unbound DNA, thus signaling the presence of virus-specific nucleic acid.35 The microarray assay has proven useful in identifying mutations associated with SARS-CoV and has been used to detect up to 24 single nucleotide polymorphisms (SNP) associated with mutations in the spike (S) gene of SARS-CoV with 100% accuracy.36
Figure 4.
Nucleic acid hybridization using microarray. Viral cDNA and reference cDNA with different fluorescent labels are mixed and applied to the microarray wells coated with specific DNA probes.
The ability to detect different emergent strains of SARS-CoV-2 may become necessary as the COVID-19 pandemic evolves, and microarray assays provide a platform for rapid detection of those strains as a result of mutational variation. Although one of the drawbacks of microarray testing has been the high cost generally associated with it, a nonfluorescent, low-cost, low-density oligonucleotide array test has been developed to detect multiple coronavirus strains with a sensitivity equal to that of individual real-time RT-PCR.37 In addition, a portable diagnostic platform based on the microarray chip has been used to identify nucleic acids specific to the MERS coronavirus as well as to influenza and respiratory syncytial viruses.38
2.4. Amplicon-Based Metagenomic Sequencing
This diagnostic technique for identification of SARS-CoV-2 relies on a dual approach involving the use of amplicon-based sequencing in addition to metagenomics sequencing. Metagenomics sequencing is used primarily to address the background microbiome of infected individuals. It allows for the ability to rapidly identify both the SARS-CoV-2 virus and other pathogens contributing to secondary infections influencing the severity of COVID-19 symptoms. Amplicon-based sequencing of SARS-CoV-2 allows for potential contact tracing, molecular epidemiology, and studies of viral evolution. Metagenomics approaches such as sequence-independent single primer amplification (SISPA) provide additional checks on sequence divergence. This dual technique is particularly relevant to SARS-CoV-2 in assessment of its rate of mutation and to detect its possible recombination with other human coronaviruses, both of which have implications for vaccine development and antiviral efficacy.
Amplicon and metagenomics MinION based sequencing were used by Moore et al. (2020) to rapidly (within 8 h) sequence the genome of SARS-CoV-2 and the other microbiome in nasopharyngeal swabs obtained from patients with COVID-19 by the ISARIC 4C consortium.39 For the amplicon-based system, the group chose 16 primer binding sites from conserved regions in the SARS-CoV-2 genome to sequentially amplify roughly 1000 bp fragments with an approximately 200 bp overlapping region. These primer sets were then used to generate 30 amplicons from the cDNA, which were subsequently sequenced using MinION.
A next-generation shotgun metagenomics sequencing platform has been developed by Illumina with the ability not only to detect the presence of multiple strains of coronaviruses but also to comprehensively examine multiple pathogenic organisms present in a complex sample. The Illumina metagenomics workflow involves sample preparation using their TruSeq Ribo-Zero Gold rRNA depletion kit, library preparation using TruSeq stranded total RNA, sequencing using the Illumina benchtop sequencing system, and final data analysis using their LRM Resequencing module or IDbyDNA Explify Platform.40
2.5. High-Level Overview of Current Molecular Genetic Assays on SARS-CoV-2 Detection
While the scientific literature has enumerated many different molecular genetic assays that have been used to detect viral nucleic acids over the years, most recent assays developed to detect the SARS-CoV-2 virus rely on RT-PCR and isothermal nucleic acid amplification technologies. Because of the urgent need for assays for COVID-19 diagnosis, manufacturers, commercial laboratories, and molecular-based laboratories can request EUA for their tests from the FDA or other regulatory agencies for diagnostic purposes. New tests approved by the FDA for emergency use are based on analytic validity and developed under idealized conditions using verified positive samples and negative controls without a requirement for demonstrated clinical validity.41 EUA approval for tests from manufacturers and commercial laboratories limits the testing to be done in clinical laboratories that have been certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA). On the other hand, the EUA approval of tests from molecular-based laboratories (laboratory developed tests) limits the testing to be done at the single laboratory that developed the test and is certified under CLIA.
Of 112 currently available molecular assays for detecting SARS-CoV-2 (detailed in Supporting Information Table S1), 90% utilize PCR or RT-PCR technologies, 6% utilize isothermal amplification technologies, 2% utilize hybridization technologies, and 2% utilize CRISPR-based technologies. Some of these tests use high-throughput platforms or have short time to results, and therefore are in high demand. Table 1 provides an overview of over 20 diagnostic tests that employ molecular genetic assays and includes information on test type, developer/manufacturer, throughput, time to results, and other related features. More molecular genetic assay-based tests are listed in Supporting Information Table S1.
3. Serological and Immunological Assays
While RT-PCR-based viral RNA detection has been widely used in diagnosis of COVID-19, it cannot be used to monitor the progress of the disease stages and cannot be applied to broad identification of past infection and immunity.
Serological testing is defined as an analysis of blood serum or plasma and has been operationally expanded to include testing of saliva, sputum, and other biological fluids for the presence of immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies. This test plays an important role in epidemiology and vaccine development, providing an assessment of both short-term (days to weeks) and long-term (years or permanence) trajectories of antibody response, as well as antibody abundance and diversity. IgM first becomes detectable in serum after a few days and lasts a couple of weeks upon infection and is followed by a switch to IgG. Thus, IgM can be an indicator of early stage infection, and IgG can be an indicator of current or prior infection. IgG may also be used to suggest the presence of post-infection immunity. In recent years, the sophistication and sensitivity of immunological assays have increased not only for the detection of antibodies themselves but also for the application of antibodies (primarily monoclonal antibodies) to the detection of pathogen-derived antigens. These tests have a huge potential for the epidemiology of COVID-19,32,42−45 but test results can be impacted by at least three situations: (1) a subset of subjects with a positive result from molecular genetic assays for SARS-CoV-2 infection are seronegative due to the lag in antibody production following infection, (2) the subjects may be seropositive yet negative for molecular genetic assay results reflecting clearance of an earlier, milder infection, and (3) limitation in sensitivity and specificity of the assays. The last issue is particularly important because even a small percentage of false positive results due to low specificity (cross reaction) may lead to misleading predictive antibody prevalence among a given population, which may have undesirable impact on the socioeconomic decisions and overall public confidence in the results.46,47
The determination of SARS-CoV-2 exposure relies largely on the detection of either IgM or IgG antibodies that are specific for various viral antigens including, but not exclusively, the spike glycoprotein (S1 and S2 subunits, receptor-binding domain) and nucleocapsid protein. The methodology for these determinations includes the traditional enzyme-linked immunosorbent assay (ELISA), immunochromatographic lateral flow assay, neutralization bioassay, and specific chemosensors. Each of these formats brings advantages (speed, multiplexing, automation) and disadvantages (trained personnel, dedicated laboratories). Complementary to these antibody-detecting methods are the rapid antigen tests wherein antibodies are used to detect the presence of viral antigen(s) in serological samples. Development of high-throughput serology tests is a current focus of major diagnostic companies.45 The FDA granted EUA status to the first serology test, qSARS-CoV-2 IgG/IgM Rapid Test, manufactured by Cellex Inc., on April 1, 2020,48 but continues to allow clinical laboratories and commercial manufacturers to launch serology tests without an EUA.
3.1. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is a microwell, plate-based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies, and hormones. The test can be qualitative or quantitative, and the time to results is typically 1–5 h. In the case of SARS-CoV-2 as shown in Figure 5A, the plate wells are typically coated with a viral protein. If present, antiviral antibodies in the patient samples will bind specifically, and the bound antibody–protein complex can be detected with an additional tracer antibody to produce a colorimetric or fluorescent-based readout. ELISA is speedy, has the ability to test multiple samples, and is adaptable to automation for increased throughput but can be variable in sensitivity and is suitable for point-of-care determinations.
Figure 5.
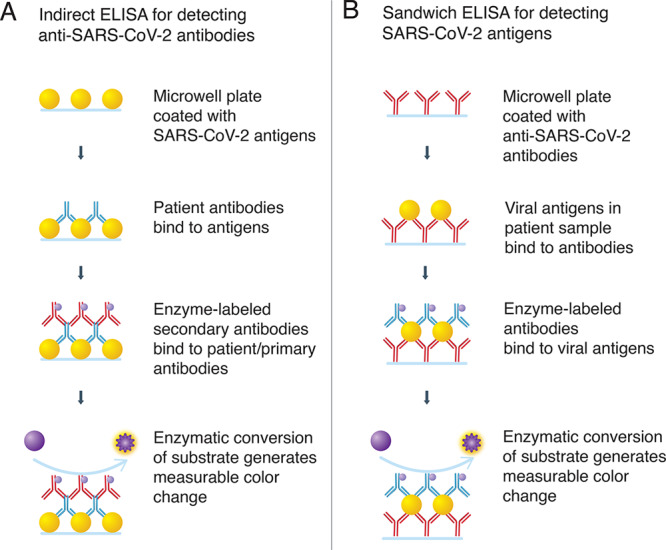
ELISA assays detecting antibodies (A) or antigens (B).
3.2. Lateral Flow Immunoassay
This test is typically a qualitative (positive or negative) chromatographic assay that is small, portable, and used at the point-of-care. The test is a type of rapid diagnostic test (RDT) as the result can be obtained in 10–30 min. In practice, fluid samples are applied to a substrate material that allows the sample to flow past a band of immobilized viral antigen. If present, anti-CoV antibodies are collected at the band, where, along with co-collected tracer antibodies, a color develops to indicate the results as shown in Figure 6. The test is inexpensive and requires no trained personnel, but provides only qualitative results. When used in conjunction with symptomology, a diagnosis of infection may be feasible. Rapid antigen tests (section 3.6), where anti-CoV antibodies are used in place of immobilized viral antigen, allow for a more direct assessment of ongoing infection.
Figure 6.
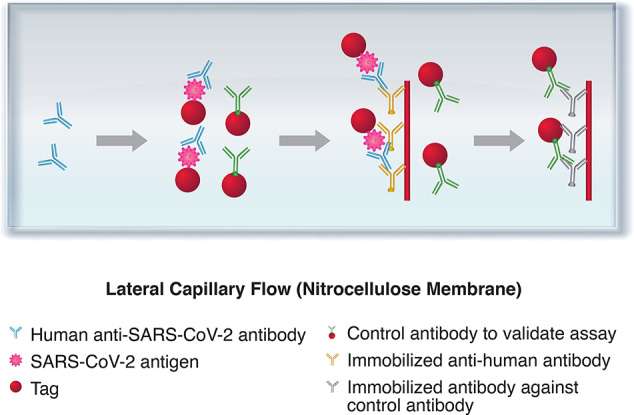
Lateral flow immunoassay for detection of anti-SARS-CoV-2 antibodies. Samples move via capillary flow on the nitrocellulose membrane. When anti-SARS-CoV-2 antibodies are present, they bind to the labeled antigen and continue to move until they are captured by the immobilized antihuman antibodies. The presence of the captured antibody–antigen complex is visualized as a colored test band. The labeled control antibodies comigrate until they are captured at the control band.
3.3. Neutralization Assay
Neutralization assays determine the ability of an antibody to inhibit virus infection of cultured cells and the resulting cytopathic effects of viral replication. For this assay, patient samples of whole blood, serum, or plasma are diluted and added at decreasing concentrations to the cell cultures. If neutralizing antibodies are present, their levels can be measured by determining the threshold at which they are able to prevent viral replication in the infected cell cultures. The time to results for neutralization assays is typically 3–5 days, but recent advances have reduced this to hours.49,50 This type of testing requires cell culture facilities, and in the case of SARS coronavirus, Biosafety Level 3 (BSL3) laboratories. Despite these limitations, determination of neutralizing antibodies is important in the short term for the therapeutic application of convalescent plasma and, in the long term, for vaccine development.
3.4. Luminescent Immunoassay
Luminescent immunoassays comprise methods that lower the limits of detection for antibody-based reagents. Generally they involve chemiluminescence and fluorescence. Cai et al. have developed a peptide-based magnetic chemiluminescence enzyme immunoassay for diagnosis of COVID-19, and Diazyme Laboratories, Inc. (San Diego, California) announced the availability of two new fully automated serological tests for SARS-CoV-2 that are run on the fully automated Diazyme DZ-lite 3000 Plus chemiluminescence analyzer.51,52
3.5. Biosensor Test
Biosensor tests rely on converting the specific interaction of biomolecules into a measurable readout via optical, electrical, enzymatic, and other methods. Surface plasmon resonance (SPR) is a technique that measures interference with incident light at a solid boundary due to local disturbances such as the adsorption of antibody or antigen. An SPR-based biosensor was developed for the diagnosis of SARS using coronaviral surface antigen (SCVme) anchored onto a gold substrate.53 The SPR chip had a lower limit of detection of 200 ng/mL for anti-SCVme antibodies within 10 min. Most recently, PathSensors Inc. announced a CANARY biosensor to detect the novel SARS coronavirus. This platform utilizes a cell-based immunosensor that couples capture of the virus with signal amplification to provide a result in 3–5 min. The biosensor is slated to be available for research purposes in May 2020.54
3.6. Rapid Antigen Test
Complementary to molecular genetic assays are the rapid antigen tests that allow detection of viral antigens.55,56 As shown in Figure 5B, these tests rely on specific monoclonal antibodies to provide a mechanism for the capture of viral antigens from an analytical sample. These assays are not restricted to a particular format. Examples include a colorimetric enzyme immunoassay for SARS-CoV in 2004,57 an enhanced chemiluminescent immunoassay for SARS-CoV in 2005,58 and more recently a fluorescence lateral flow assay59 for the detection of SARS-CoV-2 nucleocapsid protein.
3.7. High-Level Overview of Current Serological and Immunological Assays for COVID-19 Diagnosis
Table 2 provides a collection of current available serological and immunological assays for COVID-19 diagnosis, and a complete list of similar assays is provided in Supporting Information Table S2.12−15 Finally, while serological and immunological tests have a huge potential for tracing the SARS-CoV-2 virus, most of these tests are still in the development phase. Serological testing has been used to a limited extent to determine infection status (in combination with molecular genetic assays), seroprevalence, and immune protection status for healthcare workers. This testing is impacted by the fact that only a subset of patients with positive molecular genetic assay results for SARS-CoV-2 infections are seropositive, due to the delay in antibody production. There is currently no clear or strong evidence correlating seropositivity with immune protection.
Table 2. Examples of Serological and Immunological Tests Used to Detect Viral Protein or Antibodies to SARS-CoV-2 Virus.
| test name | test type | manufacturer/organization name | sample source | Ig or protein detected | test result time/additional information | EUAa | country of approval |
|---|---|---|---|---|---|---|---|
| m2000 SARS-CoV-2 assay | chemiluminescent microparticle immunoassay | Abbott Core Laboratory | serum/plasma/whole blood | IgG | runs up to 100–200 tests/h | United States | |
| COVID-19 IgG/IgM LF | lateral flow immunoassay | Advagen Biotech | serum/plasma/whole blood | IgG/IgM | results in 10 min | Brazil | |
| COVID-19 IgG/IgM Point of Care Rapid test | lateral flow immunoassay | Aytu Biosciences/Orient Gene Biotech | serum/plasma/whole blood | IgG/IgM | results in 2–10 min | China, United States | |
| COVID-19 IgM/IgG rapid test | lateral flow immunoassay | BioMedomics | serum/plasma/whole blood | IgG/IgM | results in 15 min | United States | |
| IgG antibody test kit for novel coronavirus 2019-nCoV | magnetic particle-based chemiluminescence immunoassay | Bioscience (Chongqing) Diagnostic Technology Co., Ltd. | serum | IgG | NMPA | China | |
| One-Step COVID-2019 test | lateral flow immunoassay | Celer Biotechnologia | serum/plasma/whole blood | IgG/IgM | results in 15 min | Brazil | |
| qSARS-CoV-2 IgG/IgM rapid test | lateral flow immunoassay | Cellex Inc. | serum/plasma/whole blood | IgG/IgM | results in 15–20 min, antibodies specific for N protein | Australia 3/31/2020, US FDA 4/01/2020 | Australia, United States |
| COVID-19 Ag Respi-Strip | lateral flow immunoassay (dipstick) | Coris Bioconcept | nasal mucus swabs | viral antigen | results in 15 min | Belgium | |
| DPP COVID-19 IgM/IgG system | lateral flow immunoassay | Chembio Diagnostics | serum/plasma/whole blood | IgG/IgM | results in 15 min | US FDA 4/14/2020 | Brazil |
| DEIASL019/020 SARS-CoV-2 IgG ELISA kit | ELISA | Creative Diagnostics | serum/plasma | IgG/IgM | IgG specific for N protein | United States | |
| OnSite COVID-19 IgG/IgM rapid test | lateral flow immunoassay | CTK Biotech Inc. (USA) | serum/plasma/whole blood | IgG/IgM | results in 10 min | Australia | |
| Diazyme DZ-Lite SARS-CoV-2 IgG/IgM test | luminescent immunoassay | Diazyme Laboratories | blood sample | IgG/IgM | EUA not required | United States | |
| KT-1033 EDI Novel Coronavirus COVID-19 ELISA kit | ELISA | Epitope Diagnostics | serum | IgG/IgM | United States | ||
| VivaDiag COVID-19 IgM/IgG rapid test | lateral flow immunoassay | Everest Links Pte Ltd. | serum/plasma/whole blood | IgG/IgM | results in 15 min | Singapore | |
| COVID-19 IgG/IgM rapid test cassette | lateral flow immunoassay | Hangzhou Biotest Biotech Co. Ltd. | serum/whole blood | IgG/IgM | results in 15–20 min | Australia 4/4/2020 | Australia |
| VITROS-Immunodiagnostics Products Anti-SARS-CoV-2 total reagent pack | ELISA | Ortho-Clinical Diagnostics | blood serum/plasma | IgG/IgM | cannot distinguish between IgG/IgM | US FDA 4/14/2020 | United States |
| SARS-CoV-2 rapid test | lateral flow immunoassay | PharmACT | whole blood/serum | IgG/IgM | results in 20 min, N protein, S1 and S2 subunits used as antigens | Germany | |
| Standard Q COVID-19 IgM/IgG Duo | lateral flow immunoassay | SD Biosensor | serum/plasma/whole blood | IgG/IgM | results in 10 min | EUA not required | South Korea |
| Standard Q COVID-19 Ag | chromatographic immunoassay | SD Biosensor | nasopharyngeal swabs | viral antigen | results in 30 min | South Korea | |
| iFLASH-SARS-CoV-2-IgG/IgM | immunoassay | Shenzhen Yhlo Biotech Company | serum/plasma/whole blood | IgG/IgM | China | ||
| MAGLUMI IgG/IgM de 2019-nCoV (CLIA) | chemiluminescence immunoassay | Snibe Diagnostic (China) | blood serum/plasma | IgG/IgM | results in 30 min | CE mark 2/2020 | Brazil |
Emergency Use Authorization by US FDA or other drug regulatory authorities.
4. Literature Trends and Notable Journal Articles Related to Diagnostics for COVID-19
4.1. Trends in Literature Publications Related to Diagnostic Tests for COVID-19
A significant research effort has been devoted to the development of new COVID-19 diagnostic tests that are faster and more reliable. Since the outbreak of the COVID-19 crisis, over 500 journal articles related to laboratory testing have been published. Figure 7 shows that the number of journal articles focused on COVID-19 diagnostics has significantly increased over the past few months, indicating a continuous research and development effort in this area.
Figure 7.
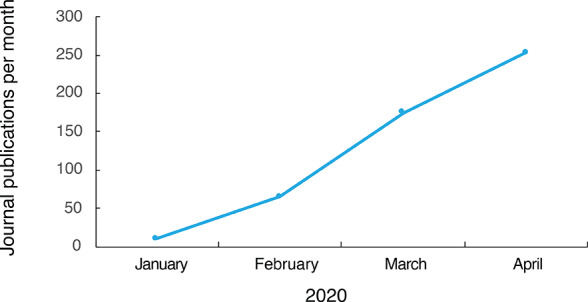
Monthly trend of journal publications related to COVID-19 diagnostics in 2020.
Table 3 lists 10 notable journal articles published from January 31, 2020 through April 10, 2020. These articles that highlight a variety of COVID-19 diagnostic methods were selected based on a number of factors, including journal impact factor and number of citations/downloads.
Table 3. Ten Notable Journal Articles Published from January 31, 2020 through April 10, 2020 Related to COVID-19 Diagnostic Testing.
| title | source | organization | type of test | digital object identifier |
|---|---|---|---|---|
| Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases | Radiology (February 26, 2020) | Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China | chest CT and RT-PCR | https://dx.doi. org/10.1148/radiol.2020200642 |
| Development and Clinical Application of a Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis | Journal of Medical Virology (February 27, 2020) | State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China | lateral flow immunoassay, POC | https://dx.doi. org/10.1002/jmv.25727 |
| Fecal Specimen Diagnosis 2019 Novel Coronavirus-Infected Pneumonia | Journal of Medical Virology (March 3, 2020) | Department of Respiration, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, China | RT-PCR | https://dx.doi. org/10.1002/jmv.25742 |
| Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-Polymerase Chain Reaction Assay Validated in Vitro and with Clinical Specimens | Journal of Clinical Microbiology (March 4, 2020) | The University of Hong Kong-Shenzhen Hospital, Shenzhen, Guangdong, The University of Hong Kong, Pokfulam, Hong Kong, and Hainan Medical University, Haikou, Hainan, all in China | real-time RT-PCR | https://dx.doi. org/10.1128/JCM.00310-20 |
| Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia | Clinical Chemistry (April 1, 2020), Vol. 66 (4), pp. 549–555 | School of Public Health, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong, China | real-time RT-PCR | https://dx.doi. org/10.1093/clinchem/hvaa029 |
| Comparative Performance of SARS-CoV-2 Detection Assays Using Seven Different Primer/Probe Sets and One Assay Kit | Journal of Clinical Microbiology (April 8, 2020) | Department of Laboratory Medicine, University of Washington, Seattle, WA, and Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA | real-time RT-PCR | https://dx.doi. org/10.1128/JCM.00557-20 |
| Analytical Sensitivity and Efficiency Comparisons of SARS-CoV-2 qRT-PCR Assays | medRxiv (2020), 1–18 | Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, CT, USA | qRT-PCR | https://dx.doi. org/10.1101/2020.03.30.20048108 |
| ddPCR: A More Sensitive and Accurate Tool for SARS-CoV-2 Detection in Low Viral Load Specimens | medRxiv (2020), 1–24 | State Key Laboratory of Virology, Modern Virology Research Center, College of Life Sciences, Wuhan University, Wuhan, China | droplet digital PCR | https://dx.doi. org/10.1101/2020.02.29.20029439 |
| Evaluating the Accuracy of Different Respiratory Specimens in the Laboratory Diagnosis and Monitoring the Viral Shedding of 2019-nCoV Infections | medRxiv (2020) 1–17 | Shenzhen Key Laboratory of Pathogen and Immunity, National Clinical Research Center for Infectious Disease, State Key Discipline of Infectious Disease, Shenzhen Third People’s Hospital, Second Hospital Affiliated to Southern University of Science and Technology, Shenzhen, China | qRT-PCR | https://dx.doi. org/10.1101/2020.02.11.20021493 |
| Rapid Detection of 2019 Novel Coronavirus SARS-CoV-2 Using a CRISPR-Based DETECTR Lateral Flow Assay | medRxiv (2020), 1–27 | Mammoth Biosciences, Inc.; University of California, San Francisco, California, USA | CRISPR, lateral flow, RT-LAMP | https://dx.doi. org/10.1101/2020.03.06.20032334 |
Many of the diagnostic methods discussed are based on patented technologies developed over the past 20 years and scientific journal publications related to diagnosis of viral infections. SARS-CoV-2 belongs to the genus Betacoronavirus that also includes SARS-CoV and MERS-CoV. Therefore, diagnostic studies on those closely related coronaviruses may shed light on the ongoing efforts to develop effective diagnostic assays for SARS-CoV-2 infection. More information about patents related to detection of SARS-CoV and MERS-CoV is provided in Supporting Information II.
4.2. SARS-CoV-2 Sequences in Journal Publications with Application for Diagnosis
Nucleic acid and amino acid sequences in CAS REGISTRY obtained from SARS-CoV-2-related journals were analyzed for potential applications in diagnostics. Table 4 lists selected journal articles and corresponding numbers of sequences related to SARS-CoV-2. These sequence records include primers and probes, gene and genomic sequences, protein domains and epitopes, and viral protein sequences with potential use for strain differentiation. Additional sequence information from SARS-CoV-related patents is provided in the Supporting Information Table S3.
Table 4. Journal Articles with SARS-CoV-2-Related Sequences for Potential Applications in Diagnostics.
| publication date | title | journal | nucleic acids | proteins |
|---|---|---|---|---|
| 2020 | Nanopore Target Sequencing for Accurate and Comprehensive Detection of SARS-CoV-2 and Other Respiratory Viruses | medRxiv | 40 primers | |
| 2020 | A Single and Two-Stage, Closed-Tube, Molecular Test for the 2019 Novel Coronavirus (COVID-19) at Home, Clinic, and Points of Entry | ChemRxiv | 6 COVID-19 LAMP primers | |
| 2020 | Transmission and Clinical Characteristics of Coronavirus Disease 2019 in 104-Outside-Wuhan Patients, China | medRxiv | 6 primers and probes | |
| 2020 | A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin | Nature | 4 | 50 |
| 2020 | A New Coronavirus Associated with Human Respiratory Disease in China | Nature | 1 | 10 |
| 2020 | A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2 | Cell Host & Microbe | 51 | |
| 2020 | Comparative Analysis of Primer-Probe Sets for the Laboratory Confirmation of SARS-CoV-2 | bioRxiv | 20 primers, 10 probes | |
| 2020 | Spike Protein Binding Prediction with Neutralizing Antibodies of SARS-CoV-2 | bioRxiv | 3 | |
| 2020 | SARS-CoV-2 Proteome Microarray for Mapping COVID-19 Antibody Interactions at Amino Acid Resolution | bioRxiv | 11 | |
| 2020 | Evaluation of Recombinant Nucleocapsid and Spike Proteins for Serological Diagnosis of Novel Coronavirus Disease 2019 (COVID-19) | medRxiv | 12 primers | |
| 2020 | RBD Mutations from Circulating SARS-CoV-2 Strains Enhance the Structure Stability and Infectivity of the Spike Protein | bioRxiv | 8 | |
| 2020 | Teicoplanin Potently Blocks the Cell Entry of 2019-nCoV | bioRxiv | 14 | 134 |
| 2020 | Differential Antibody Recognition by SARS-CoV-2 and SARS-CoV Spike Protein Receptor Binding Domains: Mechanistic Insights and Implications for the Design of Diagnostics and Therapeutics | bioRxiv | 7 | |
| 2020 | A Proposal of an Alternative Primer for the ARTIC Network’s Multiplex PCR to Improve Coverage of SARS-CoV-2 Genome Sequencing | bioRxiv | 2 | |
| 2020 | First 12 Patients with Coronavirus Disease 2019 (COVID-19) in the United States | medRxiv | 12 | 109 |
5. Summary and Perspectives
While the past few months have witnessed rapid progress in diagnostic kit development for COVID-19, the race continues to develop even more efficient laboratory techniques and cost-effective, point-of-care test kits that can be deployed in mass quantities. This report provides a broad survey of molecular genetic assays, and serological and immunological tests for identification of COVID-19 infection. While RT-PCR has been the dominant technique for detection of viral RNA, other nucleic acid assays including isothermal amplification assays, hybridization microarray assays, amplicon-based metagenomics sequencing, and the cutting-edge CRISPR-related technologies are also under development or have resulted in approved tests.60 The efficiency of such testing has also been significantly improved. This report also provides trend analysis of journal articles related to COVID-19 diagnostic techniques. The number of FDA EUA-approved tests available for COVID-19 diagnosis keeps growing, but the many new tests are still in various stages of development. Ultrarapid test kits and point-of-care tests are a major focus of development in order to speed up the response time for treatment and eliminate the need for elaborate laboratory equipment and waiting time involved with testing in approved laboratories.
The urgent need for accurate and rapid diagnosis of SARS-CoV-2 infection remains critical as global healthcare systems continue to operate during the course of the COVID-19 pandemic. In particular, serological and immunological testing of infected asymptomatic and symptomatic individuals, and their close contacts, is expected to be in high demand. In addition to its role complementary to molecular genetic testing to confirm suspected cases, this type of testing would provide valuable information about the course and degree of immune response as well as the durability of immunity in both infected individuals and participants in vaccine clinical trials. The results from these tests may assist epidemiological assessment and can be used to manage the return to normal activities. However, many questions regarding serological tests remain to be addressed, including their degree of sensitivity and specificity. Finally, it remains to be confirmed that the presence of antibodies against SARS-CoV-2 indeed correlates with immunity to the virus.
In summary, significant progress has been made in the development of diagnostic tests despite all the remaining questions and challenges. Ongoing global efforts are working to communicate and facilitate new diagnostic assay development and worldwide test kit delivery. To promote more accurate and faster diagnostic solutions, a number of organizations are supporting these efforts by inviting assay developers to submit their test products for independent evaluation or by providing huge investments for greater collaboration. As similar initiatives and knowledge sharing become available, including collaborative technological advancements, it is likely that the COVID-19 diagnostic market will continue to thrive well into the future.
Acknowledgments
We sincerely appreciate Manuel S. Guzman, Dr. Gilles P. George, and Dana Albaiu for their encouragement and support for this work, especially Dana’s effort in critical review. We thank Steve P. Watkins for his assistance in the preparation of this paper and Cristina Tomeo for her support in the publication process.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00501.
(1) Descriptions of selected molecular genetic tests for COVID-19 diagnosis, (2) Tables S1 and S2 highlight over 200 of the currently available molecular, serological, and immunological diagnostic tests, (3) highlights of patented technology for diagnosis of related coronavirus such as SARS-CoV and MERS-CoV, and (4) selected patents with SARS-CoV-related sequences for potential applications in diagnostics (PDF)
Author Contributions
# L.J.C. and L.V.G. contributed equally.
This paper was published ASAP on May 1, 2020, with an incorrect reference 34. This reference has now been corrected in this current version.
Supplementary Material
References
- Mizumoto K.; Kagaya K.; Zarebski A.; Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020, 25 (10), 2000180. 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendavid E.; Mulaney B.; Sood N.; Shah S.; Ling E.; Bromley-Dulfano R.; Lai C.; Weissberg Z.; Saavedra R.; Tedrow J.; Tversky D.; Bogan A.; Kupiec T.; Eichner D.; Gupta R.; Ioannidis J.; Bhattacharya J.. COVID-19 Antibody Seroprevalence in Santa Clara County, California medRxiv 2020, 2020.04.14.20062463. 10.1101/2020.04.14.20062463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H.The Importance of diagnostic testing for COVID-19. Infectious Diseases Hub; April 2, 2020. www.id-hub.com/2020/04/02/the-importance-of-diagnostic-testing-for-covid-19/.
- Tan R.COVID-19 Diagnostics Explained. Asian Scientist; April 8, 2020. www.asianscientist.com/2020/04/features/covid-19-diagnostics-explained/.
- Xia J.; Tong J.; Liu M.; Shen Y.; Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Physicians. COVID-19 found in sputum and feces samples after pharyngeal specimens no longer positive. Science Daily; March 30, 2020. sciencedaily.com/releases/2020/03/200330110348.htm.
- Kujawski S. A.; Wong K. K.; Collins J. P.; et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat. Med. 2020, 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- Rutgers University; New Rutgers Saliva Test for Coronavirus Gets FDA Approval: Emergency Use Authorization Granted for New Biomaterial Collection Approach. Rutgers Today, April 2020. www.rutgers.edu/news/new-rutgers-saliva-test-coronavirus-gets-fda-approval.
- U.S. Food & Drug Administration. Accelerated emergency use authorization (EUA) summary SARS-CoV-2 ASSAY (Rutgers Clinical Genomics Laboratory). April 10, 2020, pp 1–8. www.fda.gov/media/136875/download.
- VanGuilder H. D.; Vrana K. E.; Freeman W. M. Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques 2008, 44 (5), 619–626. 10.2144/000112776. [DOI] [PubMed] [Google Scholar]
- Wong M. L.; Medrano J. F. Real-time PCR for mRNA quantitation. BioTechniques 2005, 39 (1), 75–85. 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Coronavirus Test Tracker: Commercially Available COVID-19 Diagnostic Tests. 360Dx, April 21, 2020. www.360dx.com/coronavirus-test-tracker-launched-covid-19-tests.
- COVID-19 test kits included on the ARTG for legal supply in Australia. Australian Government, Department of Health, Therapeutic Goods Administration, Newsroom, April 21, 2020. www.tga.gov.au/covid-19-test-kits-included-artg-legal-supply-australia.
- Emergency Use Authorization: Emergency Use Authorization (EUA) information, and list of all current EUAs. U.S. Food & Drug Administration. www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#LDTs, accessed on 04/26/2020.
- Find Evaluation Update: SARS-CoV-2 Molecular Diagnostics, COVID-10 Diagnostics Resource Centre; Foundation for Innovative New Diagnostics (FIND). www.finddx.org/covid-19/sarscov2-eval-molecular/, accessed on 04/26/2020.
- LabCorp launches test for coronavirus disease 2019 (COVID-19). Laboratory Corporation of America, News release. March 5, 2020. ir.labcorp.com/news-releases/news-release-details/labcorp-launches-test-coronavirus-disease-2019-covid-19.
- Hinton D. M. Emergency use authorization for the 2019-nCoV Real-Time RT-PCR Diagnostic Panel (Centers for Disease Control and Prevention). U.S. Food & Drug Administration, March 15, 2020, pp 1–12. www.fda.gov/media/134919/download.
- TaqPath COVID-19 Multiplex Diagnostic Solution. ThermoFisher Scientific. www.thermofisher.com/us/en/home/clinical/clinical-genomics/pathogen-detection-solutions/coronavirus-2019-ncov/genetic-analysis/taqpath-rt-pcr-covid-19-kit.html, accessed on 04/29/2020.
- AllplexTM 2019-nCoV Assay. Seegene. www.seegene.com/assays/allplex_2019_ncov_assay, accessed on 04/29/2020.
- cobas SARS-CoV-2 Test (for the COVID-19 Coronavirus). Roche Diagnostics. diagnostics.roche.com/us/en/products/params/cobas-sars-cov-2-test.html, accessed on 04/29/2020.
- GenMark Responds to the SARS-CoV-2 Outbreak. GenMark Diagnostics Inc. www.genmarkdx.com/solutions/panels/eplex-panels/eplex-sars-cov-2-test/, accessed on 04/29/2020.
- Notomi T.; Okayama H.; Masubuchi H.; Yonekawa T.; Watanabe K.; Amino N.; Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28 (12), E63–7. 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai H. T. C.; Le M. Q.; Vuong C. D.; Parida M.; Minekawa H.; Notomi T.; Hasebe F.; Morita K. Development and Evaluation of a novel loop-mediated isothermal amplification method for rapid detection of Severe Acute Respiratory Syndrome coronavirus. J. Clin. Microbiol. 2004, 42 (5), 1956–1961. 10.1128/JCM.42.5.1956-1961.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Update on Abbott’s Work on COVID-19 Testing. Abbott Laboratories. April 15, 2020. www.abbott.com/corpnewsroom/product-and-innovation/an-update-on-abbotts-work-on-COVID-19-testing.html.
- ID NOW COVID-19, Abbott Laboratories. www.alere.com/en/home/product-details/id-now-covid-19.html.
- Zhang Y.; Odiwuor N.; Xiong J.; Sun L.; Nayaruaba R. O.; Wei H.; Tanner N.. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimeteric LAMP. medRxiv 2020, 2020.02.26.20028373, 10.1101/2020.02.26.20028373. [DOI] [Google Scholar]
- Kacian D. L.; Fultz T. J. Kits for nucleic acid sequence amplification methods. U.S. Patent 4888779, 1999. patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5888779.PN.&OS=PN/5888779&RS=PN/5888779.
- Hologic’s Molecular Test for the Novel Coronavirus, SARS-CoV-2, Receives FDA Emergency Use Authorization. Coronavirus Update, 2020. Hologic, Inc.hologic.com/coronavirus-test.
- What is CRISPR? Ask the Brain, January 1, 2019. The McGovern Institute for Brain Research, Massachusetts Institute of Technology. mcgovern.mit.edu/2019/01/01/crispr-in-a-nutshell/.
- Zhang F.; Abudayyeh O. O.; Gootenberg J. S. A protocol for detection of COVID-19 using CRISPR diagnostics (v.20200321). Broad Institute of MIT and Harvard. www.broadinstitute.org/files/publications/special/COVID-19%20detection%20(updated).pdf.
- Broughton J. P.; et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serology-based tests for COVID-19. Johns Hopkins Center for Health Security. www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html, accessed on 04/26/2020.
- Pryor J. 3 Questions: How COVID-19 tests work and why they’re in short supply. MIT News: On Campus and around the World, April 10, 2020. Massachusetts Institute of Technology. news.mit.edu/2020/how-covid-19-tests-work-why-they-are-in-short-supply-0410.
- Wang B.; Potter S. J.; Lin Y.; Cunningham A. L.; Dwyer D. E.; Su Y.; Ma X.; Hou Y.; Saksena N. K. Rapid and sensitive detection of severe acute respiratory syndrome coronavirus by rolling circle amplification. J. Clin. Microbiol. 2005, 43 (5), 2339–2344. 10.1128/JCM.43.5.2339-2344.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Li J.; Deng Z.; Xiong W.; Wang Q.; Hu Y. Q. Comprehensive detection and identification of seven animal coronaviruses and human respiratory coronavirus 229E with a microarray hybridization assay. Intervirology 2010, 53 (2), 95–104. 10.1159/000264199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Geng P.; Wang Q.; Cao B.; Liu B. Development of a single nucleotide polymorphism DNA microarray for the detection and genotyping of the SARS coronavirus. J. Microbiol. Biotechnol. 2014, 24 (10), 1445–1454. 10.4014/jmb.1404.04024. [DOI] [PubMed] [Google Scholar]
- de Souza Luna L. K.; Heiser V.; Regamey N.; Panning M.; Drexler J. F.; Mulangu S.; Poon L.; Baumgarte S.; Haijema B. J.; Kaiser L.; Drosten C. Generic detection of coronaviruses and differentiation at the prototype strain level by reverse-transcription-PCR and nonfluorescent low-density microarray. J. Clin. Microbiol. 2007, 45 (3), 1049–1052. 10.1128/JCM.02426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardick J.; Metzgar D.; Risen L.; Myers C.; Balansay M.; Malcom T.; Rothman R.; Gaydos C. Initial performance evaluation of a spotted array Mobile Analysis Platform (MAP) for the detection of influenza A/B, RSV, and MERS coronavirus. Diagn. Microbiol. Infect. Dis. 2018, 91 (3), 245–247. 10.1016/j.diagmicrobio.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. C.; Penrice-Randal R.; Alruwaili M.; Dong X.; Pullan S. T.; Carter D. P.; Bewley K.; Zhao Q.; Sun Y.; Hartley C.; Zhou E.-M.; Solomon T.; Beadsworth M. B. J.; Cruise J.; Bogaert D.; Crook D. W.; Matthews D. A.; Davidson A. D.; Mahmood Z.; Aljabr W.; Druce J.; Vipond R. T.; Ng L. F. P.; Renia L.; Openshaw P. J. M.; Baillie J. K.; Carroll M. W.; Semple M. G.; Turtle L.; Hiscox J. A. Amplicon based MinION sequencing of SARS-CoV-2 and metagenomics characterisation of nasopharyngeal swabs from patients with COVID-19. medRxiv 2020, 2020.03.05.20032011. 10.1101/2020.03.05.20032011. [DOI] [Google Scholar]
- Comprehensive Workflow for Detecting Coronavirus Using Illumina Benchtop Systems – A Shotgun Metagenomics Sequencing Workflow for Effective Detection and Characterization of Coronavirus Strains. Infectious Disease Surveillance, 2020. www.illumina.com/content/dam/illumina-marketing/documents/products/appnotes/ngs-coronavirus-app-note-1270-2020-001.pdf.
- Emergency Use Authorizations. U.S. Food & Drug Administration. www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd, accessed on 04/25/2020.
- Loeffelholz M. J.; Tang Y.-W. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerging Microbes Infect. 2020, 9 (1), 747–756. 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama B.; Kadhiresan P.; Kozlowski H. N.; Malekjahani A.; Osborne M.; Li V. Y. C.; Chen H.; Mubareka S.; Gubbay J. B.; Chan W. C. W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822. 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Zou L.; Ruan F.; Huang M.; Liang L.; Huang H.; Hong Z.; Yu J.; Kang M.; Song Y.; Xia J.; Guo Q.; Song T.; He J.; Yen H.-L.; Peiris M.; Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serology testing for COVID-19. Johns Hopkins Center for Health Security. https://www.centerforhealthsecurity.org/resources/COVID-19/COVID-19-fact-sheets/200228-Serology-testing-COVID.pdf, accessed on 4/21/2020.
- FDA Fact Sheet: Serological testing for antibodies to SARS-CoV-2 infection. U.S. Food & Drug Administration, April 17, 2020, pp 1–2. www.fda.gov/media/137111/download.
- Maxim D. L.; Niebo R.; Utell M. J. Screening tests: a review with examples. Inhalation Toxicol. 2014, 26 (13), 811–828. 10.3109/08958378.2014.955932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton D. M. Emergency Use Authorization for qSARS-CoV-2 IgG/IgM Rapid Test (Cellex Inc.). U.S. Food & Drug Administration, April 1, 2020, 1–8. www.fda.gov/media/136622/download.
- Postnikova E. N.; Pettitt J.; Van Ryn C. J.; Holbrook M. R.; Bollinger L.; Yu S.; Cai Y.; Liang J.; Sneller M. C.; Jahrling P. B.; Hensley L. E.; Kuhn J. H.; Fallah M. P.; Bennett R. S.; Reilly C. Scalable, semi-automated fluorescence reduction neutralization assay for qualitative assessment of Ebola virus-neutralizing antibodies in human clinical samples. PLoS One 2019, 14 (8), e0221407. 10.1371/journal.pone.0221407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman M. C.; Antonello J. M.; Bogardus L. A.; Giacone D. G.; Rubinstein L. J.; Sun D.; Tou A. H. M.; Gurney K. B. A virus neutralization assay utilizing imaging cytometry. WO 2020/036811. World Intellectual Property Organization, 20 February 2020. Patentscope, patentscope.wipo.int/search/en/detail.jsf?docId=WO2020036811&_cid=P11-K98Z94-66129-1.
- Cai X.; Chen J.; Hu J.; Long Q.; Deng H.; Fan K.; Liao P.; Liu B.; Wu G.; Chen Y.; Li Z.; Wang K.; Zhang X.; Tian W.; Xiang J.; Du H.; Wang J.; Hu Y.; Tang N.; Lin Y.; Ren J.; Huang L.; Wei J.; Gan C.; Chen Y.; Gao Q.; Chen A.; He C.; Wang D.; Hu P.; Zhou F.; Huang A.; Liu P.; Wang D.. A Peptide-based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Corona Virus Disease 2019 (COVID-19) medRxiv 2020, 2020.02.22.20026617. 10.1101/2020.02.22.20026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazyme Laboratories, Inc. Announces Availability of COVID-19 Antibody Tests, News & Media. Diazyme Laboratories, Inc. March 23, 2020. www.diazyme.com/diazyme-laboratories-inc-announces-availability-of-covid-19-antibody-tests.
- Park T. J.; Hyun M. S.; Lee H. J.; Lee S. Y.; Ko S. A self-assembled fusion protein-based surface plasmon resonance biosensor for rapid diagnosis of severe acute respiratory syndrome. Talanta 2009, 79 (2), 295–301. 10.1016/j.talanta.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PathSensors, Inc. Announced the Development of a SARS-CoV-2 Biosensor. PathSensors Inc, PathSensors News and Press, March 24, 2020. www.pathsensors.com/psi-sars-cov-2-biosensor/.
- Analysis of serologic cross-reactivity between common human coronaviruses and SARS-CoV-2 using coronavirus antigen microarray. bioRxiv 2020, 2020.03.24.006544. 10.1101/2020.03.24.006544. [DOI] [Google Scholar]
- Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. medRxiv 2020, 2020.03.07.20032524. 10.1101/2020.03.07.20032524. [DOI] [Google Scholar]
- Che X. Y.; Qiu L. W.; Pan Y. X.; Wen K.; Hao W.; Zhang L. Y.; Wang Y. D.; Liao Z. Y.; Hua X.; Cheng V. C.; Yuen K. Y. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 2004, 42 (6), 2629–2635. 10.1128/JCM.42.6.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B.; Wen K.; Chen J.; Liu Y.; Yuan Z.; Han C.; Chen J.; Pan Y.; Chen L.; Dan Y.; Wang J.; Chen Y.; Deng G.; Zhou H.; Wu Y.. Diagnosis of Acute Respiratory Syndrome Coronavirus 2 Infection by Detection of Nucleocapsid Protein, medRxiv 2020, 2020.03.07.20032524. 10.1101/2020.03.07.20032524. [DOI] [Google Scholar]
- Yang X.; Sun X. Chemiluminescent immunometric detection of SARS-CoV in sera as an early marker for the diagnosis of SARS. Bioluminescence and Chemiluminescence; Progress and Perspectives 2005, 491–494. 10.1142/9789812702203_0117. [DOI] [Google Scholar]
- Service R. F. Standard coronavirus test, if available, works well—but can new diagnostics help in this pandemic? Science, March 22, 2020. www.sciencemag.org/news/2020/03/standard-coronavirus-test-if-available-works-well-can-new-diagnostics-help-pandemic. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.