Abstract
Although there is growing interest in the use of repetitive Transcranial Magnetic Stimulation (TMS) as a treatment for suicidality, efficacy data in this area, and knowledge of potential treatment mechanisms, remains limited. The first objective of this study was to systematically review clinical trial data examining the effectiveness of TMS as a treatment for suicidal ideation. Our secondary objective was to investigate the extent to which changes in suicidality are independent of improvements in depression in a clinical sample of veterans who received TMS treatment. In Study 1, we searched the Pubmed and biRxiv databases from inception until July 2019 to identify studies that examined the efficacy of TMS for suicidal thoughts and/or behaviors. Data regarding sample characteristics, treatment parameters, and results were synthesized from six randomized controlled trials and five unblinded trials (total n=593). Our systematic review indicated that while TMS was consistently associated with reduced depression, its impact on suicidality is unclear. Interpretation of results related to suicidality were complicated by study design elements and modest sample sizes. In Study 2, we conducted a retrospective analysis of 43 patients who received care for depression in a neuromodulation clinic at a Veteran’s Affairs hospital. Results found significant decreases in suicidal ideation, and depressive symptom change did not always account for improvements in ideation. Taken together, our literature review and clinic study indicate preliminary promise of TMS for suicide, and underscore the need for more fine-grained, suicide-specific TMS research.
Keywords: Suicide, Depression, Brain stimulation, Transcranial magnetic stimulation, Theta burst stimulation
1. Introduction
Suicide is a significant public health problem worldwide, resulting in over 800,000 deaths each year (Fleischmann and De Leo, 2014). Although various interventions are being developed to reduce suicide through prevention and post-attempt interventions, suicide rates remain relatively unchanged over the last fifty years (National Action Alliance for Suicide Prevention, 2014). Furthermore, rates appear to have increased over the last decade in certain demographic cohorts and particularly in veterans (US Department of Veterans Affairs, 2019). Currently available psychological and pharmacological (e.g., lithium) interventions yield modest improvements in suicide-related outcomes (Hawton et al., 2016; Zalsman et al., 2016); however, they are less effective among patients with more complex symptom presentations, who are also at greater risk for suicide (Brown et al., 2016). Thus, innovative treatment strategies, including those that could augment existing treatments for suicide, are critically needed.
There is a growing interest in the use of noninvasive brain stimulation to reduce suicidal thoughts and behaviors. Repetitive Transcranial Magnetic Stimulation (rTMS; hereafter TMS) is a nonconvulsive intervention that uses rapidly fluctuating magnetic fields to induce neuronal depolarization (Rowny and Lisanby, 2008), and can be administered in outpatient settings without anesthesia or significant side effects (Rossi et al., 2009). TMS is currently cleared by the US FDA for pharmacoresistant major depressive disorder, and, when coupled with symptom provocation, obsessive compulsive disorder (Food and Drug Administration, 2018). Growing evidence also supports its use in other psychiatric disorders, including posttraumatic stress disorder (Koek et al., 2019) and schizophrenia (e.g., Osoegawa et al., 2018). While the mechanism of action of TMS remains an area of active study, neurons appear to adapt to repeated stimulation by changing their connection strengths (George & Aston-Jones, 2010), which can have downstream effects on large scale brain networks (Philip et al., 2018) that are also relevant to both suicide thoughts and behaviors (Barredo et al., 2019). Furthermore, TMS targets regions of the brain implicated in cognitive and affective control (Bredemeier and Miller, 2015; Davidson et al., 2002; Luber and Lisanby, 2014), functions that are impaired among patients with suicidal thoughts and behaviors (Allen et al., 2019). As such, TMS appears to be a logical choice for further development as a novel intervention to reduce suicide risk. Importantly, given the nature of the population, patients are unable to overdose on the intervention, unlike pharmacotherapy. Furthermore, it is likely that the nonspecific effects associated with TMS, such as regular scheduled daily visits to a clinic, provide additional opportunities for monitoring and likely have anti-suicidal effects as well.
Thus, there appears to be an empirical argument for the use of TMS to reduce suicide, but little systematic review and naturalistic data to support this use or to provide insight into how best to develop this intervention. This study sought to address these gaps in the literature through two aims. First, we reviewed existing research examining the efficacy of TMS and related noninvasive approaches (e.g., transcranial direct current stimulation) in treatment for suicide ideation and behaviors, especially in relation to the improvement of depressive symptoms. We conducted a qualitative synthesis because the dearth of studies and heterogeneity procedures precluded the application of meta-analytic methods. Second, we evaluated naturalistic outcomes in our VA-based TMS Clinic, since this patient group is at higher risk than community samples (US Department of Veterans Affairs, 2019), and examined the relationships between different features of suicide risk (e.g., the frequency and severity of suicide ideation) and depression outcomes.
2. Focused Review of TMS for Suicide
2.1. Methods
Studies in English of any design that investigated the clinical efficacy of transcranial electromagnetic stimulation (operationally defined as transcranial magnetic stimulation, transcranial alternating current stimulation and transcranial direct current stimulation for suicide ideation and/or behaviors were eligible for inclusion in this review. The Pubmed and bioRxiv databases were searched from inception until July 2019 by crossing suicide-related terms (suicid*) with key terms related to TMS, tACS and tDCS (transcranial magnetic stimulation, TMS, rTMS, dTMS, theta burst stimulation, TBS, iTBS, transcranial direct current stimulation, tDCS, transcranial alternating current stimulation, tACS). These search terms were derived from keywords used in other reviews of TMS and other neurostimulation approaches (J. Cole et al., 2019). We searched for additional articles by examining related literature reviews, searching the reference lists of relevant papers, and citation tracking in Web of Science. All abstracts were reviewed, and papers that did not involve a TMS, tACS, or tDCS intervention with a suicide-specific outcome among humans were excluded. The full-text versions of the remaining articles were then obtained and screened for inclusion (see Figure 1). A total of eleven studies were included in this review; no tDCS or tACS studies were identified. Of note, the several large, multisite studies of TMS for major depressive disorder (e.g., George et al., 2010; O’Reardon et al., 2007; Levkovitz et al., 2015) were excluded because suicide outcomes were qualitatively, but not quantitatively reported.
Figure 1.
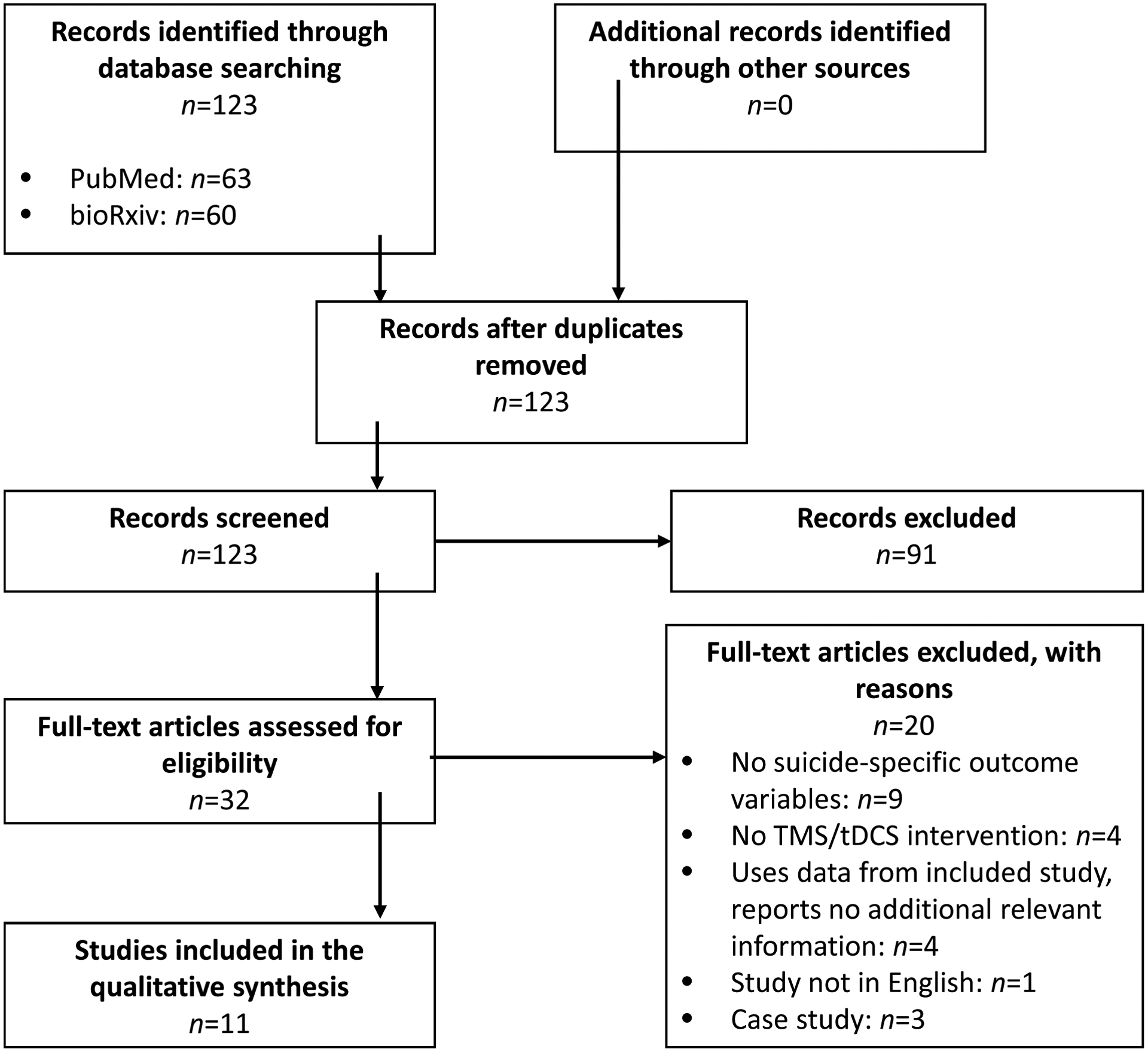
Flowchart of study selection process.
2.2. Results
2.2.1. Characteristics of included studies
Eleven studies were identified from the literature search (see Table 1), and most (k=9) studies were conducted in adults (Berlim et al., 2014; Cole et al., 2019; Desmyter et al., 2016; George et al., 2014; Hadley et al., 2011; Keshtkar et al., 2011; Rao et al., 2019; Weissman et al., 2018; Yesavage et al., 2018), and in adolescents (Bloch et al., 2008; Croarkin et al., 2018). Of these studies, 9 were studies of outpatient participants with treatment-resistant depression (Berlim et al., 2014; Bloch et al., 2008; Cole et al., 2019; Croarkin et al., 2018; Desmyter et al., 2016; Hadley et al., 2011; Keshtkar et al., 2011; Weissman et al., 2018; Yesavage et al., 2018), one was conducted on suicidal inpatients (George et al., 2014) and one focused on participants with traumatic brain injuries and associated depressive and co-occurring neuropsychiatric disorders (George et al., 2014; Rao et al., 2019).
Table 1.
TMS and Suicide Studies
| TMS Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TMS Type | Target | Frequency | Intensity (%MT) | Total Sessions | Number of Pulses per Session | ||||
| Berlim et al., 2014 | 17 | Adults with TRD | Unblinded | dTMS | L DLPFC | 20 Hz | 120% | 20 sessions over 4 weeks | 3000 |
| Bloch et al., 2008 | 9 | Adolescents with TRD | Unblinded | rTMS | L DLPFC | 10 Hz | 80% | 14 sessions over 2 weeks | 400 |
| Cole et al., 2019 | 31 | Adults with TRD | Unblinded | iTBS | L DLPFC | 50 Hz | 90% | 50 sessions (10 daily sessions over 5 days) | 1800 |
| Croarkin et al., 2018 | 19 | Adolescents with TRD | Unblinded | rTMS | L DLPFC | 10 Hz | 120% | 30 sessions over 6–8 weeks | 3000 |
| Desmyter et al., 2016 | Sham>Active: 18 Active>Sham: 14 |
Adults with TRD | Sham-controlled (crossover) | iTBS | L DLPFC | 50 Hz | 110% | 20 sessions (5 daily sessions over 4 days) | 1620 |
| George et al., 2014 | Sham: 21 Active: 20 |
Depressed Adults hospitalized for suicidality with PTSD and/or mild TBI | Sham-controlled (parallel) | rTMS | L DLPFC | 10 Hz | 120% | 9 sessions (3 times daily for 3 days) | 6000 |
| Hadley et al., 2011 | 21 | Depressed Adults | Unblinded | rTMS | L PFC | 10 Hz | 120% | At least 5 sessions | 6800 |
| Keshtar et al., 2011 | ECT: 40 adults Active TMS: 33 adults |
Adults with TRD | ECT-controlled (parallel group) | rTMS | L DLPFC | Not reported | 90% | 10 sessions | 4080 |
| Rao et al., 2019 | Active: 13 Sham: 17 |
Adults with comorbid TBI and depression | Sham-controlled (parallel) | rTMS | R DLPFC | 1 Hz | 110% | 400 sessions over 4 weeks (20 daily sessions over 4 weeks) | 1200 |
| Weissman et al., 2018 | Sham: 48 Unilateral: 56 Bilateral: 52 |
Adults with TRD | Sham-controlled (parallel) | rTMS | Left unilateral versus Bilateral | Left unilateral: 10 Hz Bilateral R: 1 Hz L: 10 Hz |
100(<60 yrs) or 120% (>60 yrs) of RMT | 15 sessions over 3 weeks |
Study 1 L unilateral: 1,450 Bilateral R: 465, L: 750 Study 2 Left Unilateral: 2,100 Bilateral R:600, L: 1,500 |
| Yesavage et al., 2018 | Active: 81 Sham: 83 |
Adults with TRD | Sham-controlled (parallel) | rTMS | L PFC | 10 Hz | 120% | Up to 30 sessions | 4000 |
Note. DLPFC = Dorsolateral prefrontal cortex; dTMS = Deep transcranial magnetic stimulation; iTBS = Intermittent Theta Burst Stimulation; PFC = Prefrontal Cortex; L= Left; R= Right; MT = Motor Threshold; rTMS = Repetitive TMS; TMS = Transcranial magnetic stimulation; TRD = Treatment-resistant depression.
A common feature across all studies was the use of varied treatment study design or TMS parameter. For example, there were five sham-controlled (Desmyter et al., 2016; George et al., 2014; Rao et al., 2019; Weissman et al., 2018; Yesavage et al., 2018) and five open-label (Berlim et al., 2014; Bloch et al., 2008; E. J. Cole et al., 2019; Croarkin et al., 2018; Hadley et al., 2011) studies. Most studies delivered high frequency stimulation to the left dorsolateral prefrontal cortex (Berlim et al., 2014; Bloch et al., 2008; Cole et al., 2019; Croarkin et al., 2018; Desmyter et al., 2016; George et al., 2014; Hadley et al., 2011; Keshtkar et al., 2011; Yesavage et al., 2018), one delivered low frequency stimulation to the right dorsolateral prefrontal cortex (Rao et al., 2019), and one examined left unilateral and bilateral stimulation (Weissman et al., 2018). Studies were nearly evenly split with regards to frequency of stimulation, with seven studies conducting daily sessions (Berlim et al., 2014; Bloch et al., 2008; Croarkin et al., 2018; Hadley et al., 2011; Keshtkar et al., 2011; Weissman et al., 2018; Yesavage et al., 2018), and four conducting multiple sessions per day (Cole et al., 2019; Desmyter et al., 2016; George et al., 2014; Rao et al., 2019). Eight studies utilized figure-of-eight TMS coils with parameters used in the treatment of depression (Croarkin et al., 2018; George et al., 2014; Hadley et al., 2011; Keshtkar et al., 2011; Rao et al., 2019; Weissman et al., 2018; Yesavage et al., 2018), two utilized intermittent theta burst stimulation (iTBS) (Cole et al., 2019; Desmyter et al., 2016), and one utilized H-coil dTMS (Berlim et al., 2014).
2.2.2. Controlled Studies
Sham-controlled studies.
We identified three randomized, double-blind, sham-controlled TMS studies, all conducted in samples with a current depressive episode. Two of these studies administered high frequency TMS to the left dorsolateral prefrontal cortex. One study was conducted among veterans psychiatrically admitted for high suicide risk, with comorbid PTSD and/or a mild traumatic brain injury (TBI) (George et al., 2014). Three daily sessions of TMS were conducted adjunctively to ongoing pharmacological and therapeutic interventions during hospitalization. Suicide ideation scores improved over the course of treatment regardless of treatment condition (Active n=20; Sham n=21). In a second study by Yesavage et al. (2018), up to 30 daily TMS sessions were conducted in a sample of veterans with treatment-resistant depression but were not high risk for suicide (e.g., denied current suicidal ideation with plan, and had not attempted suicide in the past six months). Although results showed that suicide ideation improved over time, similar to George et al. (2014), changes in ideation did not differ across the active (n=81) or sham (n=83) conditions; results remained the same at a 6-month follow-up. The third sham-controlled study administered 20 sessions of low frequency rTMS (1 Hz) to the right dorsolateral prefrontal cortex in patients with a TBI and comorbid moderate-to-severe depression (Rao et al., 2019). It yielded modest improvements in suicide ideation (Hedges g=.20) that did not differ across sham (n=17) and active (n=13) treatment conditions. None of these studies examined whether changes in depression severity accounted for improvements in suicide ideation.
Crossover studies.
Desmyter et al., (2016) used a randomized, double-blind, sham-controlled cross-over design to deliver accelerated iTBS (i.e., five sessions per day) for four days in participants with treatment-resistant depression. Individuals who attempted suicide in the prior six months were excluded from participation, and all participants discontinued psychotropic medications two weeks before stimulation. They found significant reductions in suicide ideation two weeks after completion of iTBS that were unrelated to active or sham stimulation (active-to-sham n=14; sham-to-active n=18), and change in suicide ideation were not associated with clinical response (i.e., decrease by 50% in depression scores) two weeks after completion of iTBS.
Other controlled studies.
Two studies compared TMS against an active treatment condition. Weissman et al. (2018) pooled data from two randomized, sham-controlled clinical trials to compare the effects of bilateral (n=52), left unilateral (n=56), and sham (n=48) rTMS on suicide ideation. All participants had treatment-resistant depression; individuals with acute suicidality (e.g., current suicide ideation or a history of suicide attempts) were excluded. Participants generally received fifteen sessions of TMS over three weeks, repeated if necessary; stimulation delivered in daily sessions were generally below current recommended standards (Perera et al., 2016). Reductions in suicide ideation were observed across conditions. Bilateral, but not unilateral TMS outperformed sham rTMS in resolution of suicide ideation. Although analyses did not control for change in depressive symptoms, change in suicidal ideation and change in depression were moderately correlated (r=.38).
A separate, unblinded study compared TMS to electroconvulsive therapy (ECT; Keshtar et al., 2011) in patients with refractory depression. Patients completed 10 sessions of either treatment over a three-week period. Although both treatment groups (TMS n=33; ECT n=40) showed significant decreases in suicide ideation, effects were significantly greater for ECT versus TMS. This study did not examine if decreases in suicidality were related to changes in depression severity.
2.2.3. Unblinded studies
A total of 5 unblinded (i.e., “open-label”) studies examined changes in suicidal thoughts following a course of TMS, all in patients with treatment-resistant depression. Two of these studies were conducted with adolescents; one study recruited from inpatient and outpatient settings (n=9) found no changes in suicide ideation one month after completing a course of 14 daily TMS sessions (Bloch et al., 2008). A second study (n=19) conducted 30 daily TMS sessions as an adjunctive treatment to antidepressant medication (Croarkin et al., 2018). Although it found a significant reduction suicide ideation after six weeks of treatment, this was no longer significant after adjusting for change in depression symptom severity.
Three studies were conducted in adults, all administered as adjunctive treatments to antidepressant and/or mood-stabilization medication regimens in patients with treatment-resistant depression. Hadley et al. (2011) found TMS was associated with reduced suicidal ideation (n=21) that received an average of 38 sessions of rTMS (range: 3–116 sessions), and Berlim et al. (2014) conducted 20 daily sessions of dTMS (n=17), and found moderate decreases in suicide ideation approximately 1 week after treatment ended. Most recently, Cole et al. (2019) delivered 10 sessions of iTBS per day over five days, and reported a 100% reduction in suicide ideation immediately following treatment (n=31), with effects lasting up to one month.
2.3. Interim Discussion
Overall, the extent literature suggests that suicidal ideation improves over a course of TMS, however due to the limited number of sham-controlled studies, it is unclear whether decreases can be attributed to TMS or potentially to non-specific treatment effects. The extent to which effects of TMS on suicide ideation are driven by improvement in depressive symptoms also remains unclear. Moreover, no studies to-date have examined the effects of TMS on suicidal behavior. Although several studies found changes in depression correlated with changes in ideation following TMS, only one study statistically controlled for depressive symptom improvement (Croarkin et al., 2018).
Importantly, the exclusion of patients at higher risk for suicide was a shared element in most studies with obvious impacts for the generalizability of findings to the clinical milieu. This limitation in conjunction with the lack of naturalistic clinic data highlight the urgent need for studies explicitly designed to evaluate the impact of TMS on suicidal thoughts and behaviors. To address the need for naturalistic data, we performed a retrospective analysis of data from our VA-based TMS clinic to examine whether improvements in particular features of suicidal ideation (e.g., frequency, severity, endorsement of ideation at baseline) persist after controlling for change in depressive symptoms, using elements obtained from standard rating scales of depression.
3. Study 2: Research Study
3.1. Methods
3.1.1. Participants and Procedure
The Providence VA IRB approved this retrospective chart review. Participants were 43 veterans who provided informed consent to receive care at the neuromodulation clinic at the Providence VA Medical Center in Providence, RI from October 2013 to December 2017. Veterans were 55 years old on average (SD=12.5, Range 27–73), and 89% were men. All veterans met standard eligibility criteria for TMS for MDD (i.e., failed at least one antidepressant in the current episode), and were on stable treatment (medications and/or pharmacotherapy) for at least six weeks prior to TMS initiation. Individuals with greater than mild TBI, current or past significant neurological disorder (e.g., seizure disorder, stroke), primary psychotic disorder, bipolar I disorder, uncontrolled substance use disorders, or acute suicidality requiring hospitalization were excluded from participation. Over half of the sample had been previously psychiatrically hospitalized (n=22), and several participants had previously received electroconvulsive therapy (n=4). All veterans received 5 or 10 Hz rTMS delivered to the left dorsolateral prefrontal cortex (Carpenter et al., 2018; Philip et al., 2015), targeted via the Beam/F3 method (Beam et al., 2009), at 120% of motor threshold for up to 40 sessions (M=31.5, SD =6.93, Range = 15–40), using the Neuronetics Neurostar (Malvern, PA). Only patients that received an adequate “dose” of rTMS, operationally defined as at least 15 sessions, were included in this analysis. Details of the sample treatment parameters are provided in Table 2. Following standard clinical practice, measures of depressive symptoms and suicide ideation were measured at the first and last TMS treatment session. This study was carried out in accordance with the Declaration of Helsinki.
Table 2.
TMS treatment parameters for the sample
| Frequency | Number of Pulses/Session | n |
|---|---|---|
| 5Hz | 3000 | 8 |
| 5Hz | 3000–4000 | 30 |
| 5Hz–10Hz | 5Hz: 3000, 10Hz: 4000 | 2 |
| 5Hz–10Hz | 5Hz: 3000–4000, 10Hz: 4000 | 3 |
Note. Each row in this table represents a distinct treatment period.
3.1.2. Clinical Assessments
Depressive Symptoms.
The Patient Health Questionnaire-9 (PHQ-9; Kroenke et al., 2001) and Inventory of Depressive Symptomatology, Self-Report (IDSSR; Rush et al., 1986) was used to assess depression severity. Both measures were administered at baseline and following the last TMS treatment. We calculated the percentage of change in depressive symptoms (excluding suicide items) following TMS for both measures.
Suicide Ideation.
The suicide item from the PHQ-9 was used to examine changes in suicide ideation (“Thoughts that you would be better off dead or of hurting yourself in some way,” with responses ranging from 0, “Not at all” to 3, “Nearly every Day”) over the prior two weeks. The suicide item from the IDSSR was used as a second marker of self-reported suicidality (“Thoughts of death or suicide” ranging from 0, “I do not think of suicide or death,” to 3, “I think of suicide or death several times a day in some detail, or I have made specific plans for suicide or have actually tried to take my life”) in the past week. These items were also used to create an Ideation Group variable for each measure (i.e., No ideation at baseline (item score =0) or Any ideation at baseline (item score ≥1)) for analyses.
3.1.3. Data Analyses
All data were screened for violations of normality prior to conducting analyses. First, we examined whether suicide ideation improved following treatment using paired t-tests. Then, for each of our mixed-model repeated measures ANOVAs (RMANOVAs), we evaluated the interaction (Symptom Change × Time) between the between-subjects factor of change in depressive symptoms and the within-subjects factor of Time (SI Pre- versus post-TMS) (2)). We subsequently examined the potential effects of TMS for patients who had suicide ideation at baseline using the same RMANOVA model. Finally, we computed regression analyses to examine the size of the relationship (using R2 change) between change in depressive symptoms and change in ideation after controlling for baseline ideation severity and baseline depression severity.
3.2. Results
3.2.1. Sample Characteristics
Within the sample (n=43), the majority of patients completed both indices of suicidality (PHQ-9 n=42, IDSSR n=41). More than half of participants endorsed any suicide ideation at baseline (55% endorsed ideation on the PHQ-9; 71% endorsed ideation on the IDSSR). Few participants endorsed ideation at follow-up (remission rates of 58% on the PHQ-9 and 62% on the IDSSR; remission was defined as a score of zero). Two participants without ideation at baseline endorsed SI on either the PHQ- 9 or IDSSR at the treatment endpoint.
3.2.2. PHQ-9 Analysis (Item: Frequency of Suicidal Ideation)
Paired t-tests indicated that suicidal ideation improved after stimulation (t(41)=4.46, p<.001). We did not observe significant change in suicide ideation following treatment (F(1,40)=2.61, p=.11, ηp2=.06), nor did we observe a significant Depressive Symptom Change x Time interaction (F(1,40)=1.39, p=.25, ηp2=.03). As the main effect of Time became nonsignificant after incorporating Depressive Symptom Change into the model, these results suggest that that Depressive Symptom Change explains at least some of the variance in changes in ideation frequency over time, although it is likely we were underpowered to detect the main effect of Time. There was also not a significant between-subjects effect of changes in depression on suicide ideation (F(1,39)=1.04, p=.31, ηp2=.03). The lack of a significant interaction effect indicated that changes in the suicide ideation over time did not vary as a function of changes in depressive symptoms.
Subsequent analyses conducting this RMANOVA model within patients that reported baseline SI (n=23) revealed several findings. First, patients with SI demonstrated reduced ideation following TMS (F(1,21)=8.63, p<.01, ηp2=.29; large effect), suggesting that depressive symptom change did not account for improvements in ideation. The Depression Symptom Change x Time (F(1,21)=1.31, p=.26, ηp2=.06), and between-subjects Depressive Symptom Change (Ideation: F(1,21)=2.71, p=.11, ηp2=.11) effects were nonsignificant. Finally, results of regression analyses indicated that improvement in depressive symptom severity explained a nearly moderate effect in suicide ideation improvement (change in R2=0.08, p<.01), after controlling for baseline ideation and depressive symptom severity.
3.2.3. IDSSR Analysis (Item: Frequency and Severity of Suicidal Ideation)
Paired t-tests indicated that suicidal ideation improved after stimulation (t(40)=3.82, p<.001). Similar to the PHQ9 analyses, after controlling for change in depression we did not observe a significant decrease in the suicide item on the IDSSR) (F(1,39)=0.94, p=.76, ηp2=.002) or a between-subjects effect of changes in depression on the overall frequency and severity of ideation (F(1,39)=2.40, p=.13, ηp2=.06). However, there was a significant Depression Change x Time interaction (F(1,39)=6.74, p<.05, ηp2=.15; medium effect), such that improvements in depression were related to improvements over time (r=.38, p<.05).
When evaluating the group with baseline SI (n=29), patients showed reduced SI following TMS (F(1,27)=8.63, p<.01, ηp2=.12). The group showed significant Depressive Symptom Change × Time interactions (Ideation: F(1,27)=4.86, p<.05, ηp2=.15), such that improvements in depression were associated with improvements in ideation. Results of regression analyses indicated that improvement in depressive symptom severity explained a nearly large effect in suicide ideation improvement (change in R2=0.18, p<.01), after controlling for baseline ideation and depressive symptom severity.
4. Discussion
Overall, our review of the extant literature indicates that research examining the effectiveness of TMS for the treatment of suicide risk is promising, but in its early stages. Results preliminarily link improvements in suicidal ideation with TMS treatment. However, since many of the sham-controlled studies reviewed did not find consistent benefits of TMS on suicide ideation, the extent to which changes in ideation are from verum TMS remains an important area for further study. Furthermore, whether the benefits of TMS are independent of those observed in depression is also not well understood. Those caveats aside, the results from our review of naturalistic data provide some indication that changes in frequency of suicidal ideation are partially independent of changes in depressive symptoms. Although additional, sham-controlled research is required to confirm this finding, it may be that TMS improves suicide risk via multiple pathways that are not fully linked with depression. Moreover, there may be differences in measurement among assessment instruments used to evaluate ideation improvement that contributed to these findings. Understanding the mechanisms underlying improvement in different features of suicidal ideation is clinically relevant since both the frequency (Miranda et al., 2014) and severity (Bryan et al., 2015) of suicidal ideation have been linked with subsequent suicide attempts.
In addition, several themes emerged from our literature review. First, few sham-controlled studies have examined suicide-specific effects of TMS, and the majority of these studies examined suicidal ideation as a secondary outcome during efficacy studies for depression, and none examined effects of TMS on suicidal behavior. Second, the varied design of available studies (e.g., modest sample sizes, measurement of suicide ideation as a single item), combined with the exclusion of high-risk individuals, likely reduced power to detect active treatment effects. Within the same theme, the vast parameter space of TMS likely complicates detecting verum effects. It remains largely unknown whether the site of stimulation, frequency, and type of TMS, differentially influence suicide outcomes; mechanistic guidance, likely from imaging studies (e.g., Barredo et al., 2019; Philip et al., 2018) may help refine potential approaches.
Moreover, since suicide is a transdiagnostic, low incidence phenomenon, studies in this area should address it as a primary outcome in research, and design treatment studies accordingly (e.g., recruiting patients with a range of suicide ideation and behaviors, especially individuals who are high-risk for suicide; including a range of diagnostic profiles; measuring the spectrum of suicidal thoughts and behaviors). Furthermore, to our knowledge, there are no published studies using the combination of stimulation with evidence-based psychotherapy for suicide, which conceptually could leverage the effects of both interventions (as is being developed in other areas, e.g., Sathappan et al., 2019; van ‘t Wout-Frank et al., 2019), although efforts to this end are ongoing (e.g., NCT03952468).
4.1. Limitations
The primary limitations of our clinic study are those inherent to retrospective studies of naturalistic treatment data, although our study has the added benefit of including patients who might otherwise be too ill to participate in randomized controlled studies. Furthermore, suicide was not the primary focus of treatment, our measure of suicide ideation was limited to single items that largely indexed ideation and not behavior, and ideation was assessed in the context of clinical factors that can lead to under-reporting of symptoms (e.g., fear of hospitalization). Finally, given the small sample size used in our naturalistic study, results are preliminary and require replication in larger samples.
4.2. Conclusions
Taken together, our literature review and clinic study indicate preliminary promise of TMS for suicide, and underscore the need for more fine-grained, suicide-specific TMS research. There is a clear role for development of mechanistic targets, such as brain regions and cognitive processes that may be improved by TMS. Furthermore, there remains a paucity of data to inform how best to combine TMS with other modalities. Further research addressing these areas is likely to have a substantive impact on a critical public health problem and meaningfully improve the lives of patients suffering from a broad range of psychiatric illnesses.
Acknowledgments
Effort on this manuscript was supported in part by the VA RR&D Center for Neurorestoration and Neurotechnology, Department of Veterans Affairs grants I01 RX002450 (NSP), IK2 CX001824 (JB) and I01 HX002572 (NSP, JP, MLB); and P20 GM130452 (NSP). The funders had no role in the conduct of the study, manuscript preparation, or the decision to submit for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors report no biomedical conflicts of interest related to this work. In the past three years, Dr. Philip has received grant support from Neuronetics and Neosync through clinical trial contracts and has been an unpaid scientific advisory board member for Neuronetics. Other coauthors report no conflicts of interest.
References
- Allen KJD, Bozzay ML, Edenbaum ER, 2019. Neurocognition and Suicide Risk in Adults. Curr. Behav. Neurosci. Reports 10.1007/s40473-019-00189-y [DOI] [Google Scholar]
- Baeken C, Wu G-R, van Heeringen K, 2019. Placebo aiTBS attenuates suicidal ideation and frontopolar cortical perfusion in major depression. Transl. Psychiatry 9, 38 10.1038/s41398-019-0377-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J, Aiken E, van ‘t Wout-Frank M, Greenberg BD, Carpenter LL, Philip NS, 2019. Neuroimaging Correlates of Suicidality in Decision-Making Circuits in Posttraumatic Stress Disorder. Front. Psychiatry 10, 44 10.3389/fpsyt.2019.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam W, Borckardt JJ, Reeves ST, George MS, 2009. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2, 50–54. 10.1016/j.brs.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim MT, Van den Eynde F, Tovar-Perdomo S, Chachamovich E, Zangen A, Turecki G, 2014. Augmenting antidepressants with deep transcranial magnetic stimulation (DTMS) in treatment-resistant major depression. World J. Biol. Psychiatry 15, 570–578. 10.3109/15622975.2014.925141 [DOI] [PubMed] [Google Scholar]
- Bloch Y, Grisaru N, Harel EV, Beitler G, Faivel N, Ratzoni G, Stein D, Levkovitz Y, 2008. Repetitive Transcranial Magnetic Stimulation in the Treatment of Depression in Adolescents: An Open-Label Study. J. ECT 24. [DOI] [PubMed] [Google Scholar]
- Bredemeier K, Miller IW, 2015. Executive function and suicidality: A systematic qualitative review. Clin. Psychol. Rev 40, 170–183. 10.1016/J.CPR.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GK, Karlin BE, Trockel M, Gordienko M, Yesavage J, Taylor CB, 2016. Effectiveness of Cognitive Behavioral Therapy for Veterans with Depression and Suicidal Ideation. Arch. Suicide Res 20, 677–682. 10.1080/13811118.2016.1162238 [DOI] [PubMed] [Google Scholar]
- Bryan CJ, Clemans TA, Leeson B, Rudd MD, 2015. Acute vs. Chronic Stressors, Multiple Suicide Attempts, and Persistent Suicide Ideation in US Soldiers, in: Journal of Nervous and Mental Disease VO - 203. Lippincott Williams and Wilkins, United States, p. 48. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Conelea C, Tyrka AR, Welch ES, Greenberg BD, Price LH, Niedzwiecki M, Yip AG, Barnes J, Philip NS, 2018. 5Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J. Affect. Disord 235, 414–420. 10.1016/j.jad.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Debattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR, 2019. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression (SAINT-TRD). bioRxiv 581280 10.1101/581280 [DOI] [PubMed] [Google Scholar]
- Cole J, Bright K, Gagnon L, McGirr A, 2019. A systematic review of the safety and effectiveness of repetitive transcranial magnetic stimulation in the treatment of peripartum depression. J. Psychiatr. Res 115, 142–150. 10.1016/J.JPSYCHIRES.2019.05.015 [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Nakonezny PA, Deng Z-D, Romanowicz M, Voort JL Vande, Camsari DD, Schak KM, Port JD, Lewis CP, 2018. High-frequency repetitive TMS for suicidal ideation in adolescents with depression. J. Affect. Disord 239, 282–290. 10.1016/J.JAD.2018.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS, 2002. Neural and behavioral substrates of mood and mood regulation. Biol. Psychiatry 52, 478–502. 10.1016/S0006-3223(02)01458-0 [DOI] [PubMed] [Google Scholar]
- Desmyter S, Duprat R, Baeken C, Van Autreve S, Audenaert K, van Heeringen K, 2016. Accelerated Intermittent Theta Burst Stimulation for Suicide Risk in Therapy-Resistant Depressed Patients: A Randomized, Sham-Controlled Trial. Front. Hum. Neurosci 10, 480 10.3389/fnhum.2016.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann A, De Leo D, 2014. The World Health Organization’s report on suicide: A fundamental step in worldwide suicide prevention. Cris. J. Cris. Interv. Suicide Prev 10.1027/0227-5910/a000293 [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer III PE, Schwartz T, Sackeim HA, 2010. Daily Left Prefrontal Transcranial Magnetic Stimulation Therapy for Major Depressive Disorder: A Sham-Controlled Randomized Trial. Arch. Gen. Psychiatry 67, 507–516. 10.1001/archgenpsychiatry.2010.46 [DOI] [PubMed] [Google Scholar]
- George MS, Raman R, Benedek DM, Pelic CG, Grammer GG, Stokes KT, Schmidt M, Spiegel C, DeAlmeida N, Beaver KL, Borckardt JJ, Sun X, Jain S, Stein MB, 2014. A Two-site Pilot Randomized 3 Day Trial of High Dose Left Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS) for Suicidal Inpatients. Brain Stimul. 7, 421–431. 10.1016/J.BRS.2014.03.006 [DOI] [PubMed] [Google Scholar]
- Hadley D, Anderson BS, Borckardt JJ, Arana A, Li X, Nahas Z, George MS, 2011. Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. J. ECT 27, 18–25. 10.1097/YCT.0b013e3181ce1a8c [DOI] [PubMed] [Google Scholar]
- Hawton K, Witt KG, Salisbury TLT, Arensman E, Gunnell D, Hazell P, Townsend E, van Heeringen K, 2016. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. The Lancet Psychiatry 3, 740–750. 10.1016/S2215-0366(16)30070-0 [DOI] [PubMed] [Google Scholar]
- Keshtkar M, Ghanizadeh A, Firoozabadi A, 2011. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for the treatment of major depressive disorder, a randomized controlled clinical trial. J. ECT 27, 310–314. 10.1097/YCT.0b013e318221b31c [DOI] [PubMed] [Google Scholar]
- Koek RJ, Roach J, Athanasiou N, van ‘t Wout-Frank M, Philip NS, 2019. Neuromodulatory treatments for post-traumatic stress disorder (PTSD). Prog. Neuropsychopharmacol. Biol. Psychiatry 92, 148–160. 10.1016/j.pnpbp.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2001. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med 16, 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B, Lisanby SH, 2014. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 85, 961–970. 10.1016/J.NEUROIMAGE.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Ortin A, Scott M, Shaffer D, 2014. Characteristics of suicidal ideation that predict the transition to future suicide attempts in adolescents. J. Child Psychol. Psychiatry 55, 1288–1296. 10.1111/jcpp.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA, 2007. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216. 10.1016/j.biopsych.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Osoegawa C, Gomes JS, Grigolon RB, Brietzke E, Gadelha A, Lacerda ALT, Dias ÁM, Cordeiro Q, Laranjeira R, de Jesus D, Daskalakis ZJ, Brunelin J, Cordes J, Trevizol AP, 2018. Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizophr. Res 197, 34–44. 10.1016/J.SCHRES.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS, 2016. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul. 9, 336–346. 10.1016/j.brs.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Barredo J, Aiken E, Carpenter LL, 2018. Neuroimaging Mechanisms of Therapeutic Transcranial Magnetic Stimulation for Major Depressive Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 211–222. 10.1016/J.BPSC.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Carpenter SL, Ridout SJ, Sanchez G, Albright SE, Tyrka AR, Price LH, Carpenter LL, 2015. 5Hz Repetitive transcranial magnetic stimulation to left prefrontal cortex for major depression. J. Affect. Disord 186, 13–17. 10.1016/j.jad.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V, Bechtold K, McCann U, Roy D, Peters M, Vaishnavi S, Yousem D, Mori S, Yan H, Leoutsakos J, Tibbs M, Reti I, 2019. Low-Frequency Right Repetitive Transcranial Magnetic Stimulation for the Treatment of Depression After Traumatic Brain Injury: A Randomized Sham-Controlled Pilot Study. J. Neuropsychiatry Clin. Neurosci 31, 306–318. 10.1176/appi.neuropsych.17110338 [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120, 2008–2039. 10.1016/J.CLINPH.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowny S, Lisanby SH, 2008. Brain Stimulation in Psychiatry. Psychiatry, Wiley Online Books; 10.1002/9780470515167.ch109 [DOI] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C, 1986. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 18, 65–87. 10.1016/0165-1781(86)90060-0 [DOI] [PubMed] [Google Scholar]
- Sathappan AV, Luber BM, Lisanby SH, 2019. The Dynamic Duo: Combining noninvasive brain stimulation with cognitive interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 89, 347–360. 10.1016/j.pnpbp.2018.10.006 [DOI] [PubMed] [Google Scholar]
- van ‘t Wout-Frank M, Shea MT, Larson VC, Greenberg BD, Philip NS, 2019. Combined transcranial direct current stimulation with virtual reality exposure for posttraumatic stress disorder: Feasibility and pilot results. Brain Stimul. 12, 41–43. 10.1016/j.brs.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman CR, Blumberger DM, Brown PE, Isserles M, Rajji TK, Downar J, Mulsant BH, Fitzgerald PB, Daskalakis ZJ, 2018. Bilateral Repetitive Transcranial Magnetic Stimulation Decreases Suicidal Ideation in Depression. J. Clin. Psychiatry 79 10.4088/JCP.17m11692 [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS, Forman SD, Thase M, Williams LM, Etkin A, O’Hara R, Georgette G, Beale T, Huang GD, Noda A, George MS, 2018. Effect of Repetitive Transcranial Magnetic Stimulation on Treatment-Resistant Major Depression in US Veterans: A Randomized Clinical Trial. JAMA psychiatry 75, 884–893. 10.1001/jamapsychiatry.2018.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G, Hawton K, Wasserman D, van Heeringen K, Arensman E, Sarchiapone M, Carli V, Höschl C, Barzilay R, Balazs J, Purebl G, Kahn JP, Sáiz PA, Lipsicas CB, Bobes J, Cozman D, Hegerl U, Zohar J, 2016. Suicide prevention strategies revisited: 10-year systematic review. The Lancet Psychiatry 3, 646–659. 10.1016/S2215-0366(16)30030-X [DOI] [PubMed] [Google Scholar]


