Abstract
Background:
Guidelines recommend titration of medical therapy for heart failure with reduced ejection fraction (HFrEF) to target doses derived from clinical trials, as tolerated. The degree to which titration occurs in contemporary United States (US) practice is unknown.
Objectives:
This study sought to characterize longitudinal titration of HFrEF medical therapy in clinical practice and identify associated factors and reasons for medication changes.
Methods:
Among 2,588 US outpatients with chronic HFrEF in the CHAMP-HF registry with complete medication data and no contraindications to medical therapy, use and dose of angiotensin-converting enzyme inhibitor (ACEI)/ angiotensin II receptor blocker (ARB), angiotensin receptor neprilysin inhibitor (ARNI), beta-blocker, and mineralocorticoid receptor antagonist (MRA) were examined at baseline and 12-month follow-up. Factors associated with medication changes were examined.
Results:
At baseline, 658 (25%), 525 (20%), 287 (11%), and 45 (2%) patients were receiving target doses of MRA, beta-blocker, ACEI/ARB, and ARNI, respectively. At 12-months, proportions of patients with medication initiation/dosing increase were 6% for MRA, 10% for beta-blocker, 7% for ACEI/ARB, and 10% for ARNI; corresponding proportions with discontinuation/dosing decrease were 4%, 7%, 11%, and 3%. Over 12-months, <1% of patients were simultaneously treated with target doses of ACEI/ARB/ARNI, beta-blocker, and MRA. In multivariate analysis, across the classes of medication, multiple patient characteristics were associated with higher likelihood of initiation/dosing increase (e.g., prior HF hospitalization, higher blood pressure, lower ejection fraction) and discontinuation/dosing decrease (e.g., prior HF hospitalization, impaired quality of life, more severe functional class). Medical reasons were the most common underlying reasons for discontinuations and dosing decreases, but the contributions from patient preference, health team, and systems-based reasons varied by medication.
Conclusions:
In this contemporary US registry, the large majority of eligible HFrEF patients did not receive target doses of medical therapy at any point during follow-up and few patients had doses increased over time. Although most patients had no alterations in medical therapy, multiple clinical factors were independently associated with medication changes. Further quality improvement efforts are urgently needed to improve guideline-directed medication titration.
Keywords: heart failure, reduced ejection fraction, registry, medication, dose
CONDENSED ABSTRACT
In this contemporary United States registry of outpatients with heart failure with reduced ejection fraction (HFrEF), the large majority of eligible patients did not receive target doses of medical therapy at any point during follow-up. Although most patients had no alterations in medication use or dosing, multiple factors were independently associated with dose increases and decreases during follow-up. Medical reasons were the most common reasons for dose decreases and discontinuation across all therapies, but the relative contributions from patient preference, health team, and systems-based reasons varied by medication. Targeted strategies to improve guideline-directed titration of HFrEF medical therapy are needed.
Current management guidelines for patients with heart failure with reduced ejection fraction (HFrEF) strongly support use of multiple medications proven to improve survival and quality of life.(1,2) To achieve maximal benefits, guidelines recommend that each evidence-based HFrEF medication be titrated to the target dose derived from landmark clinical trials, as tolerated.(1–3) Nonetheless, there remain significant gaps in guideline-directed use and dosing of proven HFrEF medical therapies in United States (US) clinical practice.(4,5) Sizeable proportions of patients eligible for specific medications do not receive them, and those that receive therapy generally receive low doses.(4) Given the high levels of morbidity and mortality that remain associated with chronic HFrEF, dose escalation towards recommended target dosing has been consistently emphasized for purposes of improving clinical outcomes.(3,6)
Although prior studies have demonstrated underutilization of guideline-directed medical therapy (GDMT) in clinical practice, such cross-sectional studies cannot account for medication titration over time.(4) In contemporary US practice, granular data regarding practice patterns of medication titration and ultimate doses achieved during longitudinal care are not available. Likewise, barriers to achieving target doses among eligible patients are unclear, as are the precise reasons patients have medications discontinued or doses decreased. Given the importance of GDMT dose optimization as a means for improving patient outcomes, detailed characterization of gaps in longitudinal medication titration and the predictors of dosing changes could be central to future quality improvement initiatives. In this context, the CHAMP-HF (Change the Management of Patients with Heart Failure) registry provides a novel opportunity to describe the practice patterns of longitudinal titration of GDMT in routine US clinical practice, the clinical factors associated with dose increases and decreases, and the underlying reasons for medication changes.
METHODS
Study Design
The design of the CHAMP-HF registry has been previously described.(7) Briefly, CHAMP-HF is a prospective, observational, nonrandomized study that enrolled adult outpatients with HFrEF between December 2015 and October 2017. Eligible patients had a diagnosis of chronic heart failure (HF), a left ventricular ejection fraction (EF) ≤40% on most recent imaging within 12 months of enrollment, and were receiving ≥1 oral medication for HF at study enrollment (including diuretics, angiotensin-converting enzyme inhibitors [ACEI], angiotensin II receptor blockers [ARB], angiotensin receptor-neprilysin inhibitor [ARNI], beta-blockers, mineralocorticoid receptor antagonists [MRA], anti-hypertensives, vasoactive/inotropic agents, or other cardiovascular medications). Key exclusion criteria were current or anticipated participation in a clinical trial, currently receiving comfort care or enrolled in hospice, life expectancy <1 year, or history of or plan for heart transplantation, left ventricular assist device, or dialysis. Patient data were collected at baseline and at 3, 6, 9 and 12 months and recorded in an electronic case report form. Baseline sociodemographic data were self-reported by patients. The registry was conducted in accordance with the Declaration of Helsinki and with institutional review board/ ethics committee approval at all sites. All patients provided written informed consent.
Longitudinal Medication Data
Among patients with complete medication data at baseline and 12-month follow-up and no absolute contraindications to therapy, the following 4 medication classes were examined: ACEI/ARB, ARNI, evidence-based beta-blocker, and MRA. For each patient and each medication, available dosing information was reviewed in reference to guideline recommended target doses (Supplemental Table 1). At baseline and 12-month time points, medication dosing was organized as follows: not receiving medication, receiving <50% target dose, receiving 50 to <100% target dose, and receiving ≥100% target dose. For each medication class, patients were then categorized into 4 mutually exclusive dose trajectory groups defined by the combination of dose category at baseline and 12 months, as follows: 1) stable sub-target dosing or not treated (i.e., dose category remains unchanged and either sub-target or not receiving medication); 2) stable target dosing (i.e., dose category remains unchanged and at target); 3) medication initiation or dose increase (i.e., increase in dose category regardless of achieving target dosing, including medication initiation); and 4) medication discontinuation or dose decrease (i.e., decrease in dose category, including medication discontinuation). Small changes in medication dosing between baseline and 12-month follow-up that did not result in a change in dosing category did not alter dose trajectory group assignment. In addition to 4 dose trajectory categories, separate analyses of medication changes at 3-month intervals over 12-months follow-up were performed, as described below.
Statistical Analysis
For each medication class, baseline characteristics were compared across the 4 dose trajectory groups (defined by baseline and 12-month dosing). Continuous variables were reported as median (quartile 1-quartile 3) and categorical variables were presented as frequencies and percentages. To further characterize detailed patterns of medication dosing for each medication class, Sankey diagrams were constructed to describe the proportions of patients within each dose category and switching categories at multiple time points, including baseline and 3, 6, 9 and 12-month follow-up.
Among practices that contributed ≥10 patients to the study cohort, practice-level variation in medication dosing at baseline and 12 months was assessed using a composite dosing measure. Each patient was defined as having up to 3 total guideline-directed medication opportunities, with 1 opportunity per medication class for which they were eligible without contraindications (i.e., ACEI/ARB/ARNI, evidence-based beta-blocker, MRA). For each practice, the composite dosing measure was defined as the total instances of patients receiving ≥50% of target dose (i.e., maximum of 3 achieved opportunities per patient) divided by the total number of opportunities present in the practice population.
To assess independent associations between patient characteristics and changes in medication dose among eligible patients, hierarchical logistic regression models were constructed for each medication class to assess the probability of medication initiation/dosing increase at 12 months among patients not receiving target dose at baseline. Similar models were constructed to assess probability of medication discontinuation/dosing decrease among patients receiving medication at baseline. To account for clustering of patients receiving care from individual study sites, hierarchical models were used including a random effect for site. Model selection was based on backwards elimination. Variables with a p>0.05 were removed with the highest p value removed first, with subsequent assessment continuing with remaining variables. Model selection continued until all remaining variables had p<0.05. Models were derived from the imputed dataset to eliminate missing patient characteristics; nonetheless, rates of missing data for most variables were <1% with few exceptions (New York Heart Association [NYHA] class 2.1%, systolic blood pressure 4.7%, heart rate 5.9%).
Because reasons for medication initiation versus dosing increase and medication discontinuation versus dosing decrease may differ, a separate organizational framework was used to evaluate reasons for medication changes. All changes to ACEI/ARB, ARNI, beta-blocker, and MRA therapy during 12-month follow-up were identified. Four types of changes were defined as follows: 1) medication initiation – a prescription for medication class that was not prescribed in the preceding 3 months; 2) medication discontinuation - cessation of treatment without a subsequent new prescription during follow-up; 3) dose increase – continued therapy at a new higher relative dose (i.e., higher percent of target dose) and 4) dose decrease - continued therapy at a new lower relative dose. For each type of medication change, the data collection form captured reasons across 4 categories (with precise reasons selected within each category) as follows: 1) patient-centered reasons (e.g., patient decision or request); 2) health team reasons (e.g., provider/practice preference, improve performance metrics); 3) medical reasons (e.g., worsening signs/symptoms, not tolerated as prescribed); and 4) systems-based reasons (e.g., formulary change, insurer-initiated change). Multiple reasons could be selected for each medication change. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Two-tailed p<0.05 was considered statistically significant.
RESULTS
Patient Cohort and Baseline Medication Use
From the total sample of 4,982 CHAMP-HF patients, patients were excluded due to death before 12-month follow-up (n=293), early study termination (n=272), lack of sufficient follow-up/medication data (n=1,763), and contraindication to medical therapy (n=66) (Supplemental Figure 1). The present analysis included the remaining 2,588 HFrEF patients enrolled between December 2015 and July 2017 with complete medication data and eligible for ACEI/ARB, ARNI, beta-blocker, and MRA therapy at baseline and 12-months. At baseline, 2,076 (80.2%), 1,717 (66.3%), 873 (33.7%), and 353 (13.6%) patients were treated with beta-blocker, ACEI/ARB, MRA, and ARNI therapy, respectively. Proportions of patients receiving target doses at baseline were 20.3% for beta-blocker, 11.1% for ACEI/ARB, 25.4% for MRA, and 1.7% for ARNI. At the practice-level, the median percent of medication opportunities successfully achieved (i.e., ≥50% target dose achieved) at baseline was 37.2%, and ranged from 10.0% to 66.7% across practices (Supplemental Figure 2).
Medication Titration Through 12-month Follow-up
For each class of medical therapy, proportions of patients with 1) stable sub-target dosing or not treated; 2) stable target dosing; 3) medication initiation or dose increase; and 4) medication discontinuation or dose decrease are displayed in the Central Illustration. Proportions of patients receiving stable target dosing at both baseline and 12-months ranged from 1.5% for ARNI to 22.0% for MRA; 0.7% (n=17) patients simultaneously received target doses of ACE/ARB/ARNI, beta-blocker, and MRA at baseline and 12 months. At 12-months, rates of initiation/dose increase were 7.0% for ACEI/ARB, 9.9% for ARNI, 9.9% for beta-blocker, and 6.3% for MRA; corresponding rates of discontinuation/dose decrease were 11.5%, 2.5%, 6.6%, and 4.4%. Among 297 patients with ACEI/ARB discontinuation/dose decrease, 138 (46.5%) patients had discontinuation followed by ARNI initiation within the 12-month period. Evaluation of medication use and dose at 3-month follow-up intervals showed minimal patient movement between dosing categories over time (Central Illustration). For all therapies, the majority of patients remained on stable sub-target doses of medication throughout follow-up, with the exception of a modest gradual increase in overall ARNI use (19.8% at 12 months) and target dosing (3.4% at 12 months), and modest decrease in ACEI/ARB use (61.7% at 12-months). Overall, 577 (22.3%) patients were simultaneously treated with any dose of ACEI/ARB/ARNI, beta-blocker, and MRA at baseline and 12 months. At the practice-level, the median percent of medication opportunities successfully achieved (i.e., ≥50% target dose achieved) at 12 months was 39.6%, and ranged from 9.5% to 72.2% across practices (Supplemental Figure 2).
Central Illustration. Changes in use and dose of guideline-directed medical therapy over 12-month follow-up among patients with chronic HFrEF in contemporary US outpatient practice.
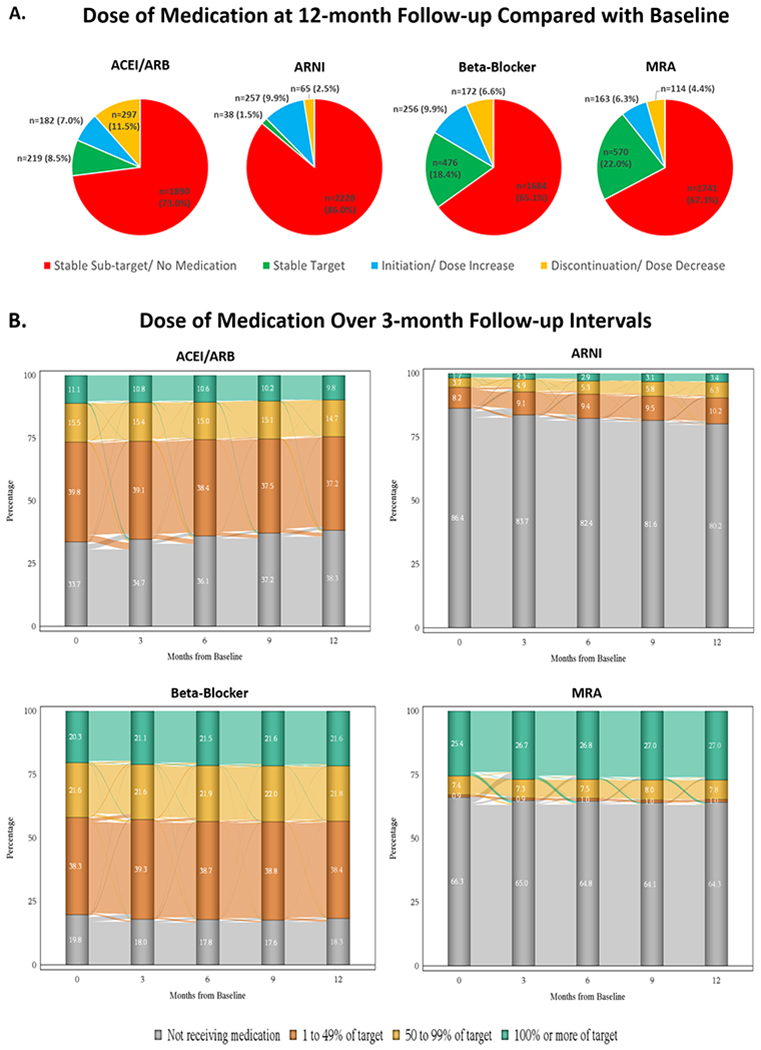
Panel A: For each class of medication, data shown reflect the relative proportions of eligible patients by trajectory of medication use and dose from baseline to 12-month follow-up. Panel B: Sankey diagrams displaying detailed longitudinal trajectories of how patients moved between dosing groups over 3-month intervals of follow-up. ARB, angiotensin II receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor-neprilysin inhibitor; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist.
Patient and Practice Characteristics by Change in Medication Dosing
Angiotensin-Converting Enzyme Inhibitor or Angiotensin II Receptor Blocker
Patients with stable sub-target dosing and discontinuation/dose decrease were more likely to be white, less likely to have full-time employment, and tended to have lower blood pressure and worse renal function (Table 1). In contrast, patients receiving stable target doses of ACEI/ARB therapy tended to have higher blood pressure and EF, better renal function, less severe NYHA functional class, better quality of life, and were less likely to have had a HF hospitalization within 12 months of enrollment. Patients with either initiation/dose increase or discontinuation/dose decrease tended to have greatest impairments in baseline functional status and quality of life, and the highest rates of prior HF hospitalization. The proportion of patients receiving care from a HF specialist was highest among patients with dose discontinuation/dose decrease and lowest among those with stable target dosing (Supplemental Table 2).
Table 1.
Baseline Patient Characteristics by ACEI/ARB Dosing Through 12-month Follow-up
| Stable Sub-target or Not Treated (n=1890) | Stable Target (n=219) | Medication Initiation/Dose Increase (n=182) | Medication Discontinuation/Dose Decrease (n=297) | P Value | |
|---|---|---|---|---|---|
| Age (years) | 68 (60-76) | 66 (56-73) | 67 (59-74) | 66 (59-75) | 0.030 |
| Female | 567 (30.0) | 55 (25.1) | 52 (28.6) | 81 (27.3) | 0.404 |
| Race | 0.019 | ||||
| White | 1449 (76.7) | 158 (72.1) | 126 (69.2) | 220 (74.1) | |
| Black/ African American | 264 (14.0) | 51 (23.3) | 40 (22.0) | 55 (18.5) | |
| Other | 177 (9.4) | 10 (4.6) | 16 (8.8) | 22 (7.4) | |
| Hispanic ethnicity | 369 (19.5) | 44 (20.1) | 30 (16.5) | 31 (10.4) | 0.002 |
| Ejection fraction (%) | 30 (24-26) | 32 (25-37) | 30 (23-35) | 30 (21-33) | <0.001 |
| NYHA class | 0.025 | ||||
| I | 186 (10.0) | 33 (15.3) | 23 (12.9) | 26 (9.0) | |
| II | 1093 (59.0) | 132 (61.4) | 90 (50.6) | 171 (59.4) | |
| III | 541 (29.2) | 43 (20.0) | 60 (33.7) | 84 (29.2) | |
| IV | 34 (1.8) | 7 (3.3) | 5 (2.8) | 7 (2.4) | |
| KCCQ-os Score | 68 (48-85) | 73 (56-90) | 66 (46-84) | 67 (42-80) | 0.001 |
| Vital sign and laboratory findings | |||||
| Systolic blood pressure (mmHg) | 120 (110-130) | 130 (119-142) | 122 (110-138) | 118 (106-130) | <0.001 |
| Diastolic blood pressure (mmHg) | 72 (65-80) | 78 (70-85) | 74 (65-80) | 70 (63-80) | <0.001 |
| Heart rate (bpm) | 72 (65-81) | 72 (64-80) | 71 (65-80) | 75 (67-84) | 0.006 |
| Body mass index (kg/m2) | 29.2 (25.4-33.7) | 30.4 (27.1-34.5) | 29.6 (26.2-33.6) | 30.8 (26.3-35.1) | 0.005 |
| Hemoglobin (g/dL) * | 13.3 (12.0-14.4) | 13.5 (12.6-14.6) | 13.5 (12.0-14.7) | 13.2 (11.9-14.3) | 0.142 |
| Serum sodium (mmol/L) † | 139 (137-141) | 140 (139-142) | 139 (138-141) | 139 (137-141) | 0.002 |
| BUN (mg/dL) ‡ | 20 (15-27) | 18 (15-23) | 20 (15-28) | 20 (16-26) | 0.313 |
| eGFR (mL/min/1.73m2) § | 0.055 | ||||
| <30 | 61 (5.1) | 4 (3.0) | 10 (8.2) | 6 (2.9) | |
| 30-44 | 151 (12.6) | 15 (11.1) | 20 (16.4) | 29 (14.2) | |
| 45-59 | 279 (23.3) | 30 (22.2) | 22 (18.0) | 64 (31.4) | |
| ≥60 | 708 (59.0) | 86 (63.7) | 70 (57.4) | 105 (51.5) | |
| NT-proBNP (pg/mL) ‖ | 1832 (804-4534) | 970 (464-2309) | 1484 (531-2565) | 1955 (719-5680) | 0.054 |
| Hemoglobin A1c (%) # | 6.4 (5.9-7.5) | 6.2 (5.8-7.5) | 6.6 (6.0-7.7) | 6.9 (6.1-8.0) | 0.132 |
| Medical history | |||||
| HF hospitalization within 12 months prior to enrollment | 665 (35.2) | 56 (25.6) | 88 (48.4) | 126 (42.4) | <0.001 |
| Coronary artery disease | 1184 (62.6) | 132 (60.3) | 117 (64.3) | 192 (64.6) | 0.748 |
| Hypertension | 1539 (81.4) | 201 (91.8) | 152 (83.5) | 250 (84.2) | 0.002 |
| Hyperlipidemia | 1463 (77.4) | 181 (82.6) | 132 (72.5) | 232 (78.1) | 0.112 |
| Diabetes mellitus | 765 (40.5) | 98 (44.7) | 65 (35.7) | 126 (42.4) | 0.288 |
| Atrial fibrillation | 670 (35.4) | 62 (28.3) | 73 (40.1) | 109 (36.7) | 0.079 |
| Chronic renal insufficiency | 359 (19.0) | 26 (11.9) | 32 (17.6) | 61 (20.5) | 0.055 |
| Asthma/ COPD | 597 (31.6) | 58 (26.5) | 54 (29.7) | 92 (31.0) | 0.468 |
| History of ventricular tachycardia/fibrillation | 354 (18.7) | 30 (13.7) | 27 (14.8) | 71 (23.9) | 0.013 |
| Depression | 494 (26.1) | 41 (18.7) | 53 (29.1) | 67 (22.6) | 0.040 |
| Active cigarette smoking | 382 (20.2) | 32 (14.6) | 36 (19.8) | 62 (20.9) | 0.248 |
| Heart failure device therapy | |||||
| Implantable cardioverter-defibrillator | 813 (43.0) | 89 (40.6) | 51 (28.0) | 139 (46.8) | <0.001 |
| Cardiac resynchronization therapy | 119 (6.3) | 7 (3.2) | 11 (6.0) | 33 (11.1) | 0.002 |
| Social and economic characteristics | |||||
| Insurance status | 0.230 | ||||
| Private Insurance/ Managed care (HMO, PPO) | 495 (26.2) | 57 (26.0) | 47 (25.8) | 81 (27.3) | |
| Medicare | 1130 (59.8) | 123 (56.2) | 100 (54.9) | 162 (54.5) | |
| Medicaid | 162 (8.6) | 18 (8.2) | 23 (12.6) | 34 (11.4) | |
| Other | 72 (3.8) | 15 (6.8) | 9 (4.9) | 17 (5.7) | |
| Uninsured | 31 (1.6) | 6 (2.7) | 3 (1.6) | 3 (1.0) | |
| Highest level of education | 0.005 | ||||
| Less than high school | 260 (13.8) | 25 (11.4) | 21 (11.5) | 20 (6.7) | |
| High school/ GED | 630 (33.3) | 67 (30.6) | 74 (40.7) | 98 (33.0) | |
| Some college | 585 (31.0) | 84 (38.4) | 43 (23.6) | 103 (34.7) | |
| Four-year college (Bachelor’s Degree) | 225 (11.9) | 30 (13.7) | 25 (13.7) | 45 (15.2) | |
| Graduate or other professional degree | 190 (10.1) | 13 (5.9) | 19 (10.4) | 31 (10.4) | |
| Total household income | 0.317 | ||||
| <$25,000 | 593 (31.4) | 69 (31.5) | 55 (30.2) | 84 (28.3) | |
| $25,000-$49,999 | 362 (19.2) | 39 (17.8) | 37 (20.3) | 65 (21.9) | |
| $50,000-$74,999 | 224 (11.9) | 20 (9.1) | 17 (9.3) | 35 (11.8) | |
| $75,000-$99,999 | 111 (5.9) | 10 (4.6) | 17 (9.3) | 22 (7.4) | |
| $100,000-$149,999 | 96 (5.1) | 15 (6.8) | 5 (2.7) | 22 (7.4) | |
| ≥$150,000 | 40 (2.1) | 7 (3.2) | 7 (3.8) | 8 (2.7) | |
| Prefer not to answer | 464 (24.6) | 59 (26.9) | 44 (24.2) | 61 (20.5) | |
| Employment status | 0.003 | ||||
| Full-time employee (≥35 hours/week) | 254 (13.4) | 43 (19.6) | 31 (17.0) | 36 (12.1) | |
| Part-time employee (<35 hours/week) | 131 (6.9) | 16 (7.3) | 7 (3.8) | 28 (9.4) | |
| Disability for medical reasons | 452 (23.9) | 58 (26.5) | 46 (25.3) | 94 (31.6) | |
| Not employed for other reasons (e.g., retired, student, unemployed) | 1053 (55.7) | 102 (46.6) | 98 (53.8) | 139 (46.8) | |
Data represent median (quartile 1 – quartile 3) or n (%).
There were 1034, 112, 109, 175 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1339, 149, 133, and 226 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1291, 145, 132, and 224 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1199, 135, 122, and 204 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 178, 22, 28, and 49 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 396, 49, 37, and 61 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GED, general equivalency diploma; HF, heart failure; HMO, health maintenance organization; KCCQ-os, Kansas City Cardiomyopathy Questionnaire overall summary score; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PPO, preferred provider organization.
Angiotensin Receptor-Neprilysin Inhibitor
Patients with stable sub-target dosing and discontinuation/dose decrease were significantly older with lower body mass index and hemoglobin and least likely to have full-time employment (Table 2). Patients prescribed stable target doses had higher EF and less severe functional class. There were no significant differences in sex, race, blood pressure, renal function, quality of life, comorbidities, or insurance status across dose trajectory groups. Patients with stable target dosing and initiation/dose increase were most likely to be receiving care from a HF specialist and least likely to be in a rural practice setting (Supplemental Table 3).
Table 2.
Baseline Patient Characteristics by ARNI Dosing Through 12-month Follow-up
| Stable Sub-target or Not Treated (n=2228) | Stable Target (n=38) | Medication Initiation/Dose Increase (n=257) | Medication Discontinuation/Dose Decrease (n=65) | P Value | |
|---|---|---|---|---|---|
| Age (years) | 68 (60-76) | 61 (52-67) | 63 (55-71) | 65 (56-73) | <0.001 |
| Female | 638 (28.6) | 16 (42.1) | 76 (29.6) | 25 (38.5) | 0.106 |
| Race | 0.122 | ||||
| White | 1692 (75.9) | 22 (57.9) | 187 (72.8) | 52 (80.0) | |
| Black/ African American | 339 (15.2) | 13 (34.2) | 47 (18.3) | 11 (16.9) | |
| Other | 197 (8.8) | 3 (7.9) | 23 (8.9) | 2 (3.1) | |
| Hispanic ethnicity | 439 (19.7) | 2 (5.3) | 29 (11.3) | 4 (6.2) | <0.001 |
| Ejection fraction (%) | 30 (24-36) | 30 (25-35) | 28 (20-33) | 28 (20-33) | <0.001 |
| NYHA class | 0.004 | ||||
| I | 238 (10.9) | 6 (16.2) | 22 (8.8) | 2 (3.1) | |
| II | 1287 (59.0) | 23 (62.2) | 149 (59.6) | 27 (41.5) | |
| III | 615 (28.2) | 7 (18.9) | 72 (28.8) | 34 (52.3) | |
| IV | 43 (2.0) | 1 (2.7) | 7 (2.8) | 2 (3.1) | |
| KCCQ-os Score | 68 (48-85) | 74 (49-90) | 68 (47-82) | 63 (44-83) | 0.326 |
| Vital sign and laboratory findings | |||||
| Systolic blood pressure (mmHg) | 120 (110-132) | 120 (110-134) | 120 (110-130) | 117 (108-130) | 0.354 |
| Diastolic blood pressure (mmHg) | 72 (66-80) | 77 (70-80) | 72 (65-80) | 73 (62-79) | 0.627 |
| Heart rate (bpm) | 72 (65-81) | 74 (65-80) | 73 (66-84) | 75 (65-80) | 0.641 |
| Body mass index (kg/m2) | 29.2 (25.5-33.6) | 32.4 (29.4-35.8) | 31.5 (26.9-36.2) | 29.5 (26.2-34.2) | <0.001 |
| Hemoglobin (g/dL) * | 13.3 (11.9-14.4) | 13.9 (12.7-14.5) | 13.6 (12.3-14.9) | 13.3 (11.9-14.2) | 0.030 |
| Serum sodium (mmol/L) † | 139 (137-141) | 139 (138-142) | 139 (137-141) | 140 (137-142) | 0.260 |
| BUN (mg/dL) ‡ | 20 (16-27) | 19 (16-25) | 19 (15-24) | 18 (15-24) | 0.067 |
| eGFR (mL/min/1.73m2) § | 0.424 | ||||
| <30 | 75 (5.3) | 1 (3.4) | 3 (1.8) | 2 (4.3) | |
| 30-44 | 189 (13.4) | 2 (6.9) | 17 (9.9) | 7 (15.2) | |
| 45-59 | 330 (23.3) | 6 (20.7) | 49 (28.7) | 10 (21.7) | |
| ≥60 | 820 (58.0) | 20 (69.0) | 102 (59.6) | 27 (58.7) | |
| NT-proBNP (pg/mL) ‖ | 1789 (714-4288) | 2780 (1015-6289) | 1741 (619-4016) | 1610 (660-1891) | 0.698 |
| Hemoglobin A1c (%) # | 6.4 (5.9-7.6) | 7.6 (6.4-9.3) | 6.6 (5.9-7.8) | 7.0 (6.2-7.8) | 0.279 |
| Medical history | |||||
| HF hospitalization within 12 months prior to enrollment | 784 (35.2) | 14 (36.8) | 109 (42.4) | 28 (43.1) | 0.085 |
| Coronary artery disease | 1413 (63.4) | 18 (47.4) | 156 (60.7) | 38 (58.5) | 0.155 |
| Hypertension | 1838 (82.5) | 33 (86.8) | 216 (84.0) | 55 (84.6) | 0.799 |
| Hyperlipidemia | 1733 (77.8) | 27 (71.1) | 196 (76.3) | 52 (80.0) | 0.692 |
| Diabetes mellitus | 901 (40.4) | 19 (50.0) | 106 (41.2) | 28 (43.1) | 0.658 |
| Atrial fibrillation | 774 (34.7) | 17 (44.7) | 104 (40.5) | 19 (29.2) | 0.120 |
| Chronic renal insufficiency | 415 (18.6) | 3 (7.9) | 49 (19.1) | 11 (16.9) | 0.388 |
| Asthma/ COPD | 690 (31.0) | 11 (28.9) | 77 (30.0) | 23 (35.4) | 0.852 |
| History of ventricular tachycardia/fibrillation | 409 (18.4) | 7 (18.4) | 55 (21.4) | 11 (16.9) | 0.674 |
| Depression | 557 (25.0) | 10 (26.3) | 68 (26.5) | 20 (30.8) | 0.720 |
| Active cigarette smoking | 433 (19.4) | 8 (21.1) | 56 (21.8) | 15 (23.1) | 0.728 |
| Heart failure device therapy | |||||
| Implantable cardioverter-defibrillator | 921 (41.3) | 15 (39.5) | 122 (47.5) | 34 (52.3) | 0.092 |
| Cardiac resynchronization therapy | 134 (6.0) | 3 (7.9) | 24 (9.3) | 9 (13.8) | 0.018 |
| Social and economic characteristics | |||||
| Insurance status | 0.133 | ||||
| Private Insurance/ Managed care (HMO, PPO) | 567 (25.4) | 15 (39.5) | 83 (32.3) | 15 (23.1) | |
| Medicare | 1333 (59.8) | 17 (44.7) | 126 (49.0) | 39 (60.0) | |
| Medicaid | 196 (8.8) | 4 (10.5) | 31 (12.1) | 6 (9.2) | |
| Other | 96 (4.3) | 2 (5.3) | 11 (4.3) | 4 (6.2) | |
| Uninsured | 36 (1.6) | 0 (0.0) | 6 (2.3) | 1 (1.5) | |
| Highest level of education | 0.390 | ||||
| Less than high school | 294 (13.2) | 4 (10.5) | 23 (8.9) | 5 (7.7) | |
| High school/ GED | 742 (33.3) | 15 (39.5) | 95 (37.0) | 17 (26.2) | |
| Some college | 705 (31.6) | 10 (26.3) | 74 (28.8) | 26 (40.0) | |
| Four-year college (Bachelor’s Degree) | 272 (12.2) | 4 (10.5) | 40 (15.6) | 9 (13.8) | |
| Graduate or other professional degree | 215 (9.6) | 5 (13.2) | 25 (9.7) | 8 (12.3) | |
| Total household income | 0.005 | ||||
| <$25,000 | 692 (31.1) | 14 (36.8) | 78 (30.4) | 17 (26.2) | |
| $25,000-$49,999 | 444 (19.9) | 11 (28.9) | 41 (16.0) | 7 (10.8) | |
| $50,000-$74,999 | 234 (10.5) | 3 (7.9) | 44 (17.1) | 15 (23.1) | |
| $75,000-$99,999 | 132 (5.9) | 3 (7.9) | 22 (8.6) | 3 (4.6) | |
| $100,000-$149,999 | 119 (5.3) | 1 (3.8) | 16 (6.2) | 2 (3.1) | |
| ≥$150,000 | 50 (2.2) | 1 (2.6) | 8 (3.1) | 3 (4.6) | |
| Prefer not to answer | 557 (25.0) | 5 (13.2) | 48 (18.7) | 18 (27.7) | |
| Employment status | <0.001 | ||||
| Full-time employee (≥35 hours/week) | 303 (13.6) | 12 (31.6) | 44 (17.1) | 5 (7.7) | |
| Part-time employee (<35 hours/week) | 152 (6.8) | 2 (5.3) | 25 (9.7) | 3 (4.6) | |
| Disability for medical reasons | 525 (23.6) | 13 (34.2) | 88 (34.2) | 24 (36.9) | |
| Not employed for other reasons (e.g., retired, student, unemployed) | 1248 (56.0) | 11 (28.9) | 100 (38.9) | 33 (50.8) |
Data represent median (quartile 1 – quartile 3) or n (%).
There were 1223, 22, 147, and 38 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1571, 33, 190, and 53 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1521, 32, 186, and 53 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1414, 29, 171, and 46 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 215, 9, 40, and 13 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 466, 10, 54, and 13 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
ARNI, angiotensin receptor-neprilysin inhibitor; body mass index; bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GED, general equivalency diploma; HF, heart failure; HMO, health maintenance organization; KCCQ-os, Kansas City Cardiomyopathy Questionnaire overall summary score; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PPO, preferred provider organization.
Evidence-based Beta-Blocker
Patients with medication discontinuation/dose decrease tended to have lower blood pressure and EF and more significant impairments in renal function and quality of life (Table 3). These patients were least likely to have private insurance or full-time employment. Compared with other patients, patients with initiation/dose increase tended to have better renal function and were most likely to have private insurance and full-time employment. Patients with initiation/dose increase or discontinuation/dose decrease were most likely to have prior HF hospitalization in the 12 months prior to enrollment. Patients receiving stable target dosing were most likely to be enrolled from a cardiology practice (i.e., HF specialist or other cardiologist) and patients receiving stable sub-target dosing were least likely (Supplemental Table 4).
Table 3.
Baseline Patient Characteristics by Beta-Blocker Dosing Through 12-month Follow-up
| Stable Sub-target or Not Treated (n=1684) | Stable Target (n=476) | Medication Initiation/Dose Increase (n=256) | Medication Discontinuation/Dose Decrease (n=172) | P Value | |
|---|---|---|---|---|---|
| Age (years) | 69 (61–76) | 65 (56–73) | 63 (56–72) | 67 (59–75) | <0.001 |
| Female | 508 (30.2) | 125 (26.3) | 80 (31.3) | 42 (24.4) | 0.159 |
| Race | <0.001 | ||||
| White | 1334 (79.2) | 314 (66.0) | 175 (68.4) | 130 (75.6) | |
| Black/ African American | 205 (12.2) | 118 (24.8) | 56 (21.9) | 31 (18.0) | |
| Other | 145 (8.6) | 44 (9.2) | 25 (9.8) | 11 (6.4) | |
| Hispanic ethnicity | 369 (21.9) | 54 (11.3) | 38 (14.8) | 13 (7.6) | <0.001 |
| Ejection fraction (%) | 30 (25-36) | 30 (23-35) | 30 (22-35) | 28 (22-33) | 0.001 |
| NYHA class | 0.245 | ||||
| I | 161 (9.7) | 59 (12.6) | 32 (13.2) | 16 (9.5) | |
| II | 989 (59.8) | 268 (57.3) | 140 (57.6) | 89 (52.7) | |
| III | 466 (28.2) | 134 (28.6) | 68 (28.0) | 60 (35.5) | |
| IV | 39 (2.4) | 7 (1.5) | 3 (1.2) | 4 (2.4) | |
| KCCQ-os Score | 68 (48-85) | 71 (49-87) | 69 (48-84) | 55 (39-76) | <0.001 |
| Vital sign and laboratory findings | |||||
| Systolic blood pressure (mmHg) | 120 (110-130) | 122 (112-136) | 120 (110-132) | 116 (105-130) | <0.001 |
| Diastolic blood pressure (mmHg) | 72 (65-80) | 74 (67-80) | 73 (68-80) | 70 (62-80) | 0.008 |
| Heart rate (bpm) | 72 (65-80) | 72 (65-80) | 76 (68-87) | 74 (64-82) | <0.001 |
| Body mass index (kg/m2) | 28.9 (25.3-33.3) | 31.5 (27.6-36.5) | 30.2 (25.6-35.1) | 29.3 (25.5-33.9) | <0.001 |
| Hemoglobin (g/dL) * | 13.3 (12.1-14.5) | 13.3 (12.2-14.4) | 13.3 (11.9-14.4) | 13.0 (11.4-14.3) | 0.272 |
| Serum sodium (mmol/L) † | 139 (137-141) | 140 (138-142) | 139 (137-141) | 139 (137-142) | 0.435 |
| BUN (mg/dL) ‡ | 20 (15-27) | 20 (15-27) | 19 (15-25) | 22 (16-28) | 0.227 |
| eGFR (mL/min/1.73m2) § | 0.009 | ||||
| <30 | 46 (4.4) | 20 (6.5) | 5 (2.8) | 10 (7.5) | |
| 30-44 | 135 (12.9) | 40 (13.1) | 21 (11.8) | 19 (14.3) | |
| 45-59 | 242 (23.2) | 76 (24.8) | 32 (18.0) | 45 (33.8) | |
| ≥60 | 620 (59.4) | 170 (55.6) | 120 (67.4) | 59 (44.4) | |
| NT-proBNP (pg/mL) ‖ | 1880 (811-4534) | 1045 (638-3020) | 2016 (801-5143) | 1137 (571-4135) | 0.141 |
| Hemoglobin A1c (%) # | 6.4 (5.8-7.4) | 6.9 (6.1-8.6) | 6.5 (5.9-7.9) | 6.3 (5.7-7.0) | 0.005 |
| Medical history | |||||
| HF hospitalization within 12 months prior to enrollment | 588 (34.9) | 137 (28.8) | 122 (47.7) | 88 (51.2) | <0.001 |
| Coronary artery disease | 1077 (64.0) | 274 (57.6) | 149 (58.2) | 125 (72.7) | 0.001 |
| Hypertension | 1388 (82.4) | 403 (84.7) | 203 (79.3) | 148 (86.0) | 0.187 |
| Hyperlipidemia | 1334 (79.2) | 363 (76.3) | 174 (68.0) | 137 (79.7) | <0.001 |
| Diabetes mellitus | 644 (38.2) | 228 (47.9) | 108 (42.2) | 74 (43.0) | 0.002 |
| Atrial fibrillation | 588 (34.9) | 155 (32.6) | 89 (34.8) | 82 (47.7) | 0.004 |
| Chronic renal insufficiency | 290 (17.2) | 104 (21.8) | 40 (15.6) | 44 (25.6) | 0.006 |
| Asthma/ COPD | 539 (32.0) | 127 (26.7) | 79 (30.9) | 56 (32.6) | 0.161 |
| History of ventricular tachycardia/ fibrillation | 295 (17.5) | 109 (22.9) | 32 (12.5) | 46 (26.7) | <0.001 |
| Depression | 436 (25.9) | 111 (23.3) | 65 (25.4) | 43 (25.0) | 0.727 |
| Active cigarette smoking | 343 (20.4) | 80 (16.8) | 54 (21.1) | 35 (20.3) | 0.343 |
| Heart failure device therapy | |||||
| Implantable cardioverter-defibrillator | 672 (39.9) | 254 (53.4) | 78 (30.5) | 88 (51.2) | <0.001 |
| Cardiac resynchronization therapy | 101 (6.0) | 37 (7.8) | 18 (7.0) | 14 (8.1) | 0.423 |
| Social and economic characteristics | |||||
| Insurance status | <0.001 | ||||
| Private Insurance/ Managed care (HMO, PPO) | 423 (25.1) | 134 (28.2) | 86 (33.6) | 37 (21.5) | |
| Medicare | 1033 (61.3) | 259 (54.4) | 116 (45.3) | 107 (62.2) | |
| Medicaid | 139 (8.3) | 45 (9.5) | 33 (12.9) | 20 (11.6) | |
| Other | 69 (4.1) | 24 (5.0) | 14 (5.5) | 6 (3.5) | |
| Uninsured | 20 (1.2) | 14 (2.9) | 7 (2.7) | 2 (1.2) | |
| Highest level of education | 0.542 | ||||
| Less than high school | 224 (13.3) | 53 (11.1) | 30 (11.7) | 19 (11.0) | |
| High school/ GED | 552 (32.8) | 172 (36.1) | 85 (33.2) | 60 (34.9) | |
| Some college | 528 (31.4) | 155 (32.6) | 82 (32.0) | 50 (29.1) | |
| Four-year college (Bachelor’s Degree) | 211 (12.5) | 62 (13.0) | 33 (12.9) | 19 (11.0) | |
| Graduate or other professional degree | 169 (10.0) | 34 (7.1) | 26 (10.2) | 24 (14.0) | |
| Total household income | 0.080 | ||||
| <$25,000 | 534 (31.7) | 130 (27.3) | 87 (34.0) | 50 (29.1) | |
| $25,000-$49,999 | 321 (19.1) | 109 (22.9) | 37 (14.5) | 36 (20.9) | |
| $50,000-$74,999 | 191 (11.3) | 55 (11.6) | 35 (13.7) | 15 (8.7) | |
| $75,000-$99,999 | 108 (6.4) | 26 (5.5 ) | 14 (5.5) | 12 (7.0) | |
| $100,000-$149,999 | 92 (5.5) | 21 (4.4) | 13 (5.1) | 12 (7.0) | |
| ≥$150,000 | 42 (2.5) | 7 (1.5) | 12 (4.7) | 1 (0.6) | |
| Prefer not to answer | 396 (23.5) | 128 (26.9) | 58 (22.7) | 46 (26.7) | |
| Employment status | <0.001 | ||||
| Full-time employee (≥35 hours/week) | 218 (12.9) | 70 (14.7) | 58 (22.7) | 18 (10.5) | |
| Part-time employee (<35 hours/week) | 115 (6.8) | 39 (8.2) | 18 (7.0) | 10 (5.8) | |
| Disability for medical reasons | 380 (22.6) | 150 (31.5) | 64 (25.0) | 56 (32.6) | |
| Not employed for other reasons (e.g., retired, student, unemployed) | 971 (57.7) | 217 (45.6) | 116 (45.3) | 88 (51.2) |
Data represent median (quartile 1 – quartile 3) or n (%).
There were 916, 249, 146, and 119 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1175, 335, 190, and 147 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1135, 323, 192, and 142 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1043, 306, 178, and 133 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 159, 52, 43, and 23 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 362, 97, 51, and 33 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
BMI, body mass index; bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GED, general equivalency diploma; HF, heart failure; HMO, health maintenance organization; KCCQ-os, Kansas City Cardiomyopathy Questionnaire overall summary score; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PPO, preferred provider organization.
Mineralocorticoid Receptor Antagonist
Patients with stable sub-target dosing were generally older with higher EF and blood pressure, superior functional status and quality of life, and most likely to have public insurance. Patients with discontinuation/dose decrease tended to have lower blood pressure and lower likelihood of full-time employment (Table 4). Renal function was similar across groups. Prior HF hospitalization in past 12 months, NYHA III/IV status, and impaired quality of life were all more common among patients with initiation/dose increase or discontinuation/dose decrease. Patients with discontinuation/dose decrease were most likely to be receiving care from a HF specialist and least likely from a non-HF cardiology or primary care practice (Supplemental Table 5).
Table 4.
Baseline Patient Characteristics by MRA Dosing Through 12-month Follow-up
| Stable Sub-target or Not Treated (n=1741) | Stable Target (n=570) | Medication Initiation/Dose Increase (n=163) | Medication Discontinuation/Dose Decrease (n=114) | P Value | |
|---|---|---|---|---|---|
| Age (years) | 69 (61-77) | 63 (54-71) | 65 (56-73) | 64 (55-72) | <0.001 |
| Female | 491 (28.2) | 187 (32.8) | 47 (28.8) | 30 (26.3) | 0.180 |
| Race | <0.001 | ||||
| White | 1370 (78.7) | 383 (67.2) | 116 (71.2) | 84 (73.7) | |
| Black/ African American | 224 (12.9) | 130 (22.8) | 31 (19.0) | 25 (21.9) | |
| Other | 147 (8.4) | 57 (10.0) | 16 (9.8) | 5 (4.4) | |
| Hispanic ethnicity | 378 (21.7) | 68 (11.9) | 21 (12.9) | 7 (6.1) | <0.001 |
| Ejection fraction (%) | 33 (25-37) | 28 (20-33) | 28 (20-33) | 28 (20-33) | <0.001 |
| NYHA class | <0.001 | ||||
| I | 179 (10.5) | 57 (10.2) | 18 (11.4) | 14 (12.6) | |
| II | 1061 (62.2) | 303 (54.1) | 79 (50.0) | 43 (38.7) | |
| III | 434 (25.4) | 187 (33.4) | 58 (36.7) | 49 (44.1) | |
| IV | 32 (1.9) | 13 (2.3) | 3 (1.9) | 5 (4.5) | |
| KCCQ-os Score | 69 (49-85) | 67 (48-87) | 62 (40-84) | 64 (44-82) | 0.008 |
| Vital sign and laboratory findings | |||||
| Systolic blood pressure (mmHg) | 122 (110-132) | 118 (106-129) | 119 (106-128) | 118 (104-130) | <0.001 |
| Diastolic blood pressure (mmHg) | 72 (67-80) | 71 (64-80) | 71 (62-80) | 72 (64-80) | 0.037 |
| Heart rate (bpm) | 72 (65-80) | 73 (66-83) | 76 (68-84) | 75 (64-83) | 0.001 |
| Body mass index (kg/m2) | 29.1 (25.6-33.5) | 30.7 (26.2-35.5) | 30.6 (24.5-34.7) | 29.1 (26.4-33.8) | 0.001 |
| Hemoglobin (g/dL) * | 13.3 (12.0-14.5) | 13.3 (12.2-14.5) | 13.2 (11.7-14.1) | 13.5 (11.7-14.7) | 0.573 |
| Serum sodium (mmol/L) † | 140 (138-142) | 139 (137-141) | 139 (136-141) | 139 (137-141) | <0.001 |
| BUN (mg/dL) ‡ | 20 (15-27) | 20 (15-26) | 20 (16-28) | 23 (17-28) | 0.084 |
| eGFR (mL/min/1.73m2) § | 0.272 | ||||
| <30 | 58 (5.4) | 13 (3.4) | 4 (3.4) | 6 (6.7) | |
| 30-44 | 150 (14.0) | 37 (9.8) | 13 (10.9) | 15 (16.7) | |
| 45-59 | 250 (23.3) | 92 (24.3) | 30 (25.2) | 23 (25.6) | |
| ≥60 | 615 (57.3) | 236 (62.4) | 72 (60.5) | 46 (51.1) | |
| NT-proBNP (pg/mL) ‖ | 1880 (818-4299) | 1234 (627-3125) | 2421 (919-5680) | 1664 (635-5580) | 0.205 |
| Hemoglobin A1c (%) # | 6.4 (5.9-7.6) | 6.5 (5.8-7.9) | 6.7 (6.2-7.9) | 6.7 (5.6-7.0) | 0.651 |
| Medical history | |||||
| HF hospitalization within 12 months prior to enrollment | 532 (30.6) | 247 (43.3) | 88 (54.0) | 68 (59.6) | <0.001 |
| Coronary artery disease | 1126 (64.7) | 330 (57.9) | 96 (58.9) | 73 (64.0) | 0.022 |
| Hypertension | 1467 (84.3) | 454 (79.6) | 132 (81.0) | 89 (78.1) | 0.033 |
| Hyperlipidemia | 1399 (80.4) | 415 (72.8) | 110 (67.5) | 84 (73.7) | <0.001 |
| Diabetes mellitus | 710 (40.8) | 233 (40.9) | 65 (39.9) | 46 (40.4) | 0.996 |
| Atrial fibrillation | 613 (35.2) | 195 (34.2) | 58 (35.6) | 48 (42.1) | 0.454 |
| Chronic renal insufficiency | 316 (18.2) | 102 (17.9) | 37 (22.7) | 23 (20.2) | 0.494 |
| Asthma/ COPD | 548 (31.5) | 178 (31.2) | 43 (26.4) | 32 (28.1) | 0.516 |
| History of ventricular tachycardia/ fibrillation | 297 (17.1) | 126 (22.1) | 24 (14.7) | 35 (30.7) | <0.001 |
| Depression | 437 (25.1) | 145 (25.4) | 47 (28.8) | 26 (22.8) | 0.684 |
| Active cigarette smoking | 325 (18.7) | 122 (21.4) | 42 (25.8) | 23 (20.2) | 0.112 |
| Heart failure device therapy | |||||
| Implantable cardioverter-defibrillator | 680 (39.1) | 297 (52.1) | 64 (39.3) | 51 (44.7) | <0.001 |
| Cardiac resynchronization therapy | 94 (5.4) | 44 (7.7) | 15 (9.2) | 17 (14.9) | <0.001 |
| Social and economic characteristics | |||||
| Insurance status | <0.001 | ||||
| Private Insurance/ Managed care (HMO, PPO) | 418 (24.0) | 178 (31.2) | 50 (30.7) | 34 (29.8) | |
| Medicare | 1100 (63.2) | 279 (48.9) | 77 (47.2) | 59 (51.8) | |
| Medicaid | 133 (7.6) | 75 (13.2) | 19 (11.7) | 10 (8.8) | |
| Other | 65 (3.7) | 31 (5.4) | 12 (7.4) | 5 (4.4) | |
| Uninsured | 25 (1.4) | 7 (1.2) | 5 (3.1) | 6 (5.3) | |
| Highest level of education | 0.095 | ||||
| Less than high school | 238 (13.7) | 69 (12.1) | 13 (8.0) | 6 (5.3) | |
| High school/ GED | 567 (32.6) | 206 (36.1) | 64 (39.3) | 32 (28.1) | |
| Some college | 552 (31.7) | 168 (29.5) | 52 (31.9) | 43 (37.7) | |
| Four-year college (Bachelor’s Degree) | 213 (12.2) | 75 (13.2) | 19 (11.7) | 18 (15.8) | |
| Graduate or other professional degree | 171 (9.8) | 52 (9.1) | 15 (9.2) | 15 (13.2) | |
| Total household income | 0.522 | ||||
| <$25,000 | 552 (31.7) | 178 (31.2) | 41 (25.2) | 30 (26.3) | |
| $25,000-$49,999 | 328 (18.8) | 119 (20.9) | 32 (19.6) | 24 (21.1) | |
| $50,000-$74,999 | 198 (11.4) | 65 (11.4) | 17 (10.4) | 16 (14.0) | |
| $75,000-$99,999 | 106 (6.1) | 36 (6.3) | 13 (8.0) | 5 (4.4) | |
| $100,000-$149,999 | 100 (5.7) | 28 (4.9) | 4 (2.5) | 6 (5.3) | |
| ≥$150,000 | 38 (2.2) | 12 (2.1) | 7 (4.3) | 5 (4.4) | |
| Prefer not to answer | 419 (24.1) | 132 (23.2) | 49 (30.1) | 28 (24.6) | |
| Employment status | <0.001 | ||||
| Full-time employee (≥35 hours/week) | 222 (12.8) | 99 (17.4) | 31 (19.0) | 12 (10.5) | |
| Part-time employee (<35 hours/week) | 126 (7.2) | 31 (5.4) | 15 (9.2) | 10 (8.8) | |
| Disability for medical reasons | 366 (21.0) | 198 (34.7) | 47 (28.8) | 39 (34.2) | |
| Not employed for other reasons (e.g., retired, student, unemployed) | 1027 (59.0) | 242 (42.5) | 70 (42.9) | 53 (46.5) | |
Data represent median (quartile 1 – quartile 3) or n (%).
There were 932, 314, 110, and 74 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1193, 429, 129, and 96 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1163, 413, 124, and 92 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 1073, 378, 119, and 90 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 159, 72, 26, and 20 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
There were 371, 118, 36, and 18 patients with available data for stable sub-target, stable target, medication initiation/dose increase, and medication discontinuation/dose decrease, respectively.
BMI, body mass index; bpm, beats per minute; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GED, general equivalency diploma; HF, heart failure; HMO, health maintenance organization; KCCQ-os, Kansas City Cardiomyopathy Questionnaire overall summary score; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PPO, preferred provider organization; PRO, patient-reported outcome.
Factors Associated with Medication Changes
Independent associations between baseline patient characteristics and medication initiation/dose increase and discontinuation/dose decrease are displayed in Table 5.
Table 5.
Independent Associations Between Patient Characteristics and Medication Dosing Changes at 12-month Follow-up*
| Dose Increase or Medication Initiation | Dose Decrease or Medication Discontinuation | |||||
|---|---|---|---|---|---|---|
| Odds ratio (95% confidence interval) | P value | Odds ratio (95% confidence interval) | P value | |||
| ACEI/ARB | ||||||
| Systolic blood pressure (per 10 mmHg increase) | 1.13 (1.04-1.22) | 0.006 | Hispanic ethnicity | 0.59 (0.37-0.93) | 0.024 | |
| HF hospitalization within 12 months prior to enrollment | 1.61 (1.17-2.21) | 0.004 | Ejection fraction (per 10% increase) | 0.73 (0.61-0.86) | <0.001 | |
| Cardiac resynchronization therapy | 1.70 (1.07-2.71) | 0.026 | ||||
| KCCQ (per 10 point increase) | 0.89 (0.84-0.94) | <0.001 | ||||
| High school education or higher | 1.76 (1.06-2.92) | 0.028 | ||||
| ARNI | ||||||
| Age (per 10 year increase) | 0.84 (0.74-0.94) | 0.003 | Systolic blood pressure (per 10 mmHg increase) | 1.18 (1.01-1.38) | 0.041 | |
| Ejection fraction (per 10% increase) | 0.68 (0.57-0.81) | <0.001 | NYHA Class III/IV | 2.55 (1.43-4.54) | 0.002 | |
| BMI (per 10 kg/m2) | 1.21 (1.01-1.46) | 0.040 | Working full or part-time | 0.25 (0.11-0.57) | 0.001 | |
| Annual household income <$50,000 | 0.69 (0.52-0.91) | 0.009 | Annual household income <$50,000 | 0.48 (0.26-0.87) | 0.016 | |
| Beta-blocker | ||||||
| Age (per 10 year increase) | 0.84 (0.75-0.95) | 0.006 | Hispanic ethnicity | 0.45 (0.24-0.84) | 0.012 | |
| Systolic blood pressure (per 10 mmHg increase) | 1.09 (1.01-1.18) | 0.026 | HF hospitalization within 12 months prior to enrollment | 1.56 (1.12-2.19) | 0.009 | |
| Heart rate (per 10 beat/min increase) | 1.21 (1.09-1.34) | <0.001 | KCCQ (per 10 point increase) | 0.84 (0.78-0.90) | <0.001 | |
| Working full or part-time | 1.41 (1.01-1.96) | 0.042 | Coronary artery disease | 1.66 (1.15-2.38) | 0.007 | |
| History of ventricular tachycardia/fibrillation | 0.60 (0.37-0.96) | 0.032 | ||||
| MRA | ||||||
| Ejection fraction (per 10% increase) | 0.68 (0.55-0.85) | <0.001 | NYHA Class III/IV | 2.00 (1.32-3.03) | 0.001 | |
| HF hospitalization within 12 months prior to enrollment | 1.90 (1.33-2.70) | <0.001 | HF hospitalization within 12 months prior to enrollment | 1.97 (1.30-3.00) | 0.001 | |
| KCCQ (per 10 point increase) | 0.91 (0.85-0.98) | 0.009 | BMI (per 10 kg/m2) | 0.74 (0.56-0.97) | 0.029 | |
| Hyperlipidemia | 0.56 (0.39-0.81) | 0.002 | Cardiac resynchronization therapy | 1.93 (1.05-3.57) | 0.036 | |
| Atrial fibrillation | 1.97 (1.06-3.67) | 0.032 | ||||
| High school education or higher | 2.54 (1.06-6.09) | 0.036 | ||||
Model selection was based on backwards elimination and variables with a p>0.05 were removed based on highest p value first, with subsequent assessment completed using the remaining variables. Variables considered in the model were age, female sex, race (white versus non-white), Hispanic ethnicity, employment (full or part-time), insurance (private or managed care), education (high school or higher), household income <$50,000, atrial fibrillation, COPD, coronary artery disease, depression, diabetes mellitus, hypertension, dyslipidemia, current smoker, ventricular tachycardia/fibrillation, chronic renal insufficiency, heart failure hospitalization in 12 months prior to enrollment, cardiac resynchronization therapy, ventricular arrhythmia, systolic blood pressure, heart rate, left ventricle ejection fraction (%), KCCQ-12, and NYHA class (I or II versus III or IV).
ARB, angiotensin II receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor-neprilysin inhibitor;
BMI, body mass index; COPD, chronic obstructive pulmonary disease; HF, heart failure; KCCQ-os, Kansas City Cardiomyopathy Questionnaire overall summary score; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association
Angiotensin-Converting Enzyme Inhibitor or Angiotensin II Receptor Blocker
HF hospitalization in the prior 12 months and higher systolic blood pressure were strongly associated with higher likelihood of initiation/dose increase. Higher EF, better quality of life, and Hispanic ethnicity were associated with lower likelihood of discontinuation/dose decrease, whereas presence of cardiac resynchronization therapy and ≥high school education was associated with higher likelihood of discontinuation/dose decrease.
Angiotensin Receptor-Neprilysin Inhibitor
Younger age and lower EF were strongly associated with initiation/dose increase, whereas severe functional class was associated with higher probability of discontinuation/dose decrease. Low annual household income was associated with decreased likelihood of both initiation/dose increase and discontinuation/dose decrease. Higher systolic blood pressure was associated with higher likelihood of discontinuation/dose decrease with nominal statistical significance.
Evidence-based Beta-Blocker
Younger age, higher systolic blood pressure, and higher heart rate were all associated with increased likelihood of initiation/dose increase. Presence of coronary artery disease and HF hospitalization in the prior 12 months were associated with higher probability of discontinuation/dose decrease, whereas better quality of life and Hispanic ethnicity were associated with lower likelihood of discontinuation/dose decrease.
Mineralocorticoid Receptor Antagonist
Higher EF and quality of life score were associated with decreased likelihood of initiation/dose increase. Severe functional class was strongly associated with increased probability of discontinuation/dose decrease, and body mass index, cardiac resynchronization therapy, atrial fibrillation, and ≥ high-school education were associated with nominal statistical significance. HF hospitalization in the prior 12 months was strongly associated with increased likelihood of both initiation/dose increase and discontinuation/dose decrease.
Reasons for Medication Changes
Reasons for medication changes are displayed in Figure 1 and Supplemental Tables 6–9.
Figure 1. Reasons for change in guideline-directed medical therapy over 12-month follow-up among patients with chronic HFrEF in contemporary US outpatient practice.
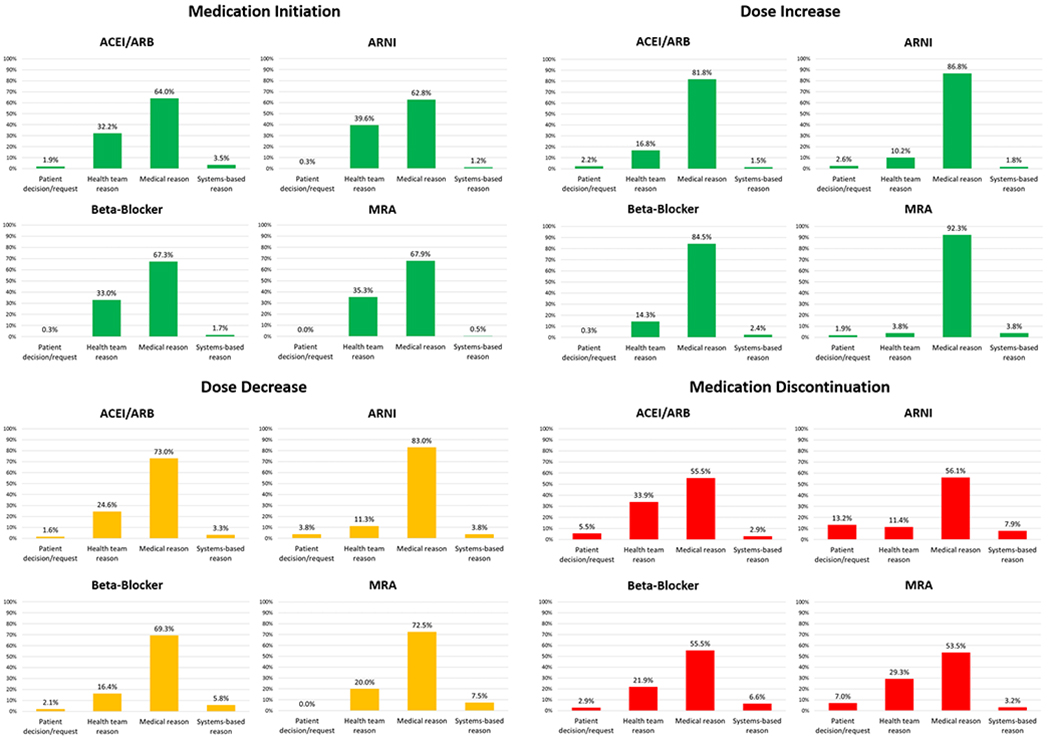
For each class of medication and for each type of medication change, reasons for medication change were classified as either patient decision/request, health team reason, medical reason, and systems-based reasons. Data regarding specific reasons within each category are provided in Supplementary Tables 6–9. ARB, angiotensin II receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor-neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist.
Medication Initiation
ARNI (n=336) and MRA therapy (n=215) were the most and least frequently initiated medications, respectively. For all therapies, the most frequent reasons for initiation were medical reasons and health team reasons, whereas the least frequent reasons to start medication were patient decision/request.
Dose Increase
Dose of beta-blocker therapy (n=335) was most frequently increased and MRA was least frequently increased (n=52). Medical reasons were the most common reasons for dosing increases, ranging from 81.8% for ACEI/ARB to 92.3% for MRA. Frequency of health team reasons varied substantially by therapy, from 16.8% for ACEI/ARB to 3.8% in MRA. A similar degree of variation existed across therapies for patient-centered and systems-based reasons.
Dose Decrease
Beta-blocker therapy had the highest number of dose decreases (n=189) and MRA therapy had the lowest (n=40). Medical reasons were the most frequent reasons for dose decreases, ranging from 69.3% for beta-blocker to 83.0% for ARNI. ARNI had the highest number of dose decreases per patient decision/refusal (3.8%), whereas there were no instances of patient decision/refusal for MRA. Health team reasons for dose decreases ranged from 24.6% for ACEI/ARB to 11.3% for ARNI.
Medication Discontinuation
ACEI/ARB (n=384) was had the highest number of discontinuations and ARNI had the lowest (n=114). For all therapies, the most common reason for discontinuation were medical reasons (54-56%). In contrast, other reasons for discontinuation differed by therapy. ARNI had the highest rate of discontinuation due to patient decision/refusal at 13.2%, whereas beta-blocker therapy had the lowest at 2.9%. ARNI therapy had the markedly higher rate of discontinuation due to systems-based reasons and markedly lower rate discontinuation due to health-team reasons.
DISCUSSION
In this large contemporary US registry of outpatients with HFrEF, the large majority of eligible patients did not receive target doses of medical therapy and few patients had doses increased over time. For all HFrEF medical therapies studied, >80% of patients had stable dosing at 12-month follow-up, with the large majority of these patients receiving sub-target doses. By comparison, rates of medication initiation/dose increase and discontinuation/dose decrease were ≤12% across all therapies. Over 12 months, <1% of patients simultaneously received stable target doses of ACEI/ARB/ARNI, beta-blocker, and MRA therapy. In multivariate analysis, across the classes of medication, multiple patient characteristics were repeatedly associated with higher likelihood of dose increase/initiation (e.g., prior HF hospitalization, higher blood pressure, lower EF) and dose decrease/discontinuation (e.g., prior HF hospitalization, impaired quality of life, more severe functional class). Medical reasons (e.g., new or worsening signs/symptoms, intolerance) were the most common underlying reason for changes in all therapies, but the relative contributions from patient-centered, health team, and systems-based reasons varied across medications.
To our knowledge, we present the most comprehensive and contemporary analysis of longitudinal outpatient medication dosing and titration in US clinical practice. In this respect, several strengths and novel features of this analysis warrant mention. First, the CHAMP-HF registry included pre-specified, detailed, and serial collection of medication dosing across a wide range of outpatient practices for the duration of study follow-up. Notably, data capture included ARNI therapy to be reflective of the most recent HF treatment guidelines.(2) Second, aside from medication use and dosing data, the registry collected underlying reasons for medication changes. To better reflect the realities and complexities of modern care, potential reasons extended beyond medical reasons to include health team, systems-based, and patient reasons for medication changes. Third, recognizing the potential impact of social factors on longitudinal medication use, patients were characterized across several socioeconomic domains, including insurance status, household income, employment status, and level of education. Fourth, practice-level variation in medication dosing among eligible patients was characterized using a novel composite measure and identified wide variability in GDMT dosing across US practices. Fifth, rigorous multivariate hierarchical models were used to provide this first description of patient factors independently associated with longitudinal increases and decreases of GDMT dosing in current US practice.
Prior data regarding longitudinal changes in HFrEF medical therapy and dose in US practice come largely from the IMPROVE HF program, a prospective study evaluating the effectiveness of a practice-specific quality improvement intervention on use of GDMT.(8) The study found rates of target dosing among treated patients to increase modestly over 24 months for ACEI/ARB (36.1% to 37.9%) and MRA (74.4% to 78.4%), and substantially for beta-blocker therapy (20.5% to 30.3%). In this context and to the extent that optimal dosing of GDMT improves patient-centered outcomes, the current CHAMP-HF findings reflecting care nearly a decade later are disappointing. Rates of overall use and target dosing showed only minimal gains over 12-month follow-up. Moreover, final 12-month rates of target dosing among treated patients in CHAMP-HF (ACEI/ARB, 15.9%; beta-blocker, 26.4%; MRA, 75.5%) were lower than those observed at the conclusion of IMPROVE HF.
Clinical Implications
Clinical guidelines and the recent American College of Cardiology Expert Consensus document strongly recommend clinicians make every possible effort to achieve target doses of GDMT among eligible patients.(1–3) These target doses were derived from landmark clinical trials where protocols employed gradual, tolerance-limited, up-titration over several weeks until target dose was achieved. Lower doses were prescribed only when target doses could not be tolerated. Using these protocols, 49-84% of patients in ACEI/ARB trials and 43%-65% of patients in beta-blocker trials achieved target dosing.(9–15) More recently, 80% of patients in the PARADIGM-HF (Angiotensin Receptor–Neprilysin Inhibitor with Angiotensin-Converting–Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial successfully completed a run-in phase of several weeks, defined as no unacceptable side effects to target doses of enalapril and sacubitril/valsartan.(16) Reconciling trial data with persistently lower rates of target dosing in clinical practice, some have reasoned that key differences in patient characteristics (e.g., older age and more comorbidities in routine practice) may explain these gaps. However, accumulating data suggests that some gaps between trial and registry populations may be narrower than previously thought, including a recent analysis demonstrating remarkably similar patient characteristics and risk between patients enrolled in the PARADIGM-HF trial and those routinely seen in US practice.(17,18) Although some degree of persistent difference between trial and real-world populations may contribute, the magnitude of the dosing gap in routine practice strongly supports suboptimal quality of care and clinical inertia as prominent factors. Concerns over quality of care are further supported by recent data demonstrating high rates of medication underuse and underdosing irrespective of systolic blood pressure.(5) Wide practice-level variation in levels of GDMT dosing observed in this analysis further suggests substantial improvements in routine practice are indeed possible. Moving forward, it may be reasonable to consider use of one or more performance measures that evaluate dosing of GDMT achieved in HFrEF care, such as the composite measure utilized in this study (i.e., composite of ≥50% target dose in absence of documented contraindication or intolerance) or alternative measure construct.
Quality Improvement Strategies
Although widespread improvements in use and dose of GDMT are needed, the patient factors identified in CHAMP-HF as independently associated with medication changes highlight subpopulations worthy of particular attention in future quality improvement programs. For example, efforts targeting NYHA class III/IV patients and those with recent HF hospitalization may be highest yield in mitigating against medication discontinuation or decreased dosing.(19) Likewise, these data suggest a bi-directional response to a recent HF hospitalization, with such events independently associated with both medication initiation/dose increases and medication discontinuation/dose decreases. Although the explanation for this bi-directional relationship is unclear, it is plausible that some clinicians and patients may appropriately interpret HF hospitalization as a call for escalated medical therapy, while others may view as a sign of patient fragility and reason for apprehension towards medications with possible hemodynamic and renal effects.(20) Future dedicated efforts targeting GDMT optimization during and soon after HF hospitalizations are needed to more consistently couple these high-risk events with impetus to augment (rather than reduce) GDMT in efforts to prevent future clinical events.(19,21,22) In contrast, withdrawal or intolerance to medical therapy in the setting of recent HF hospitalization should be understood as an exceptionally high-risk scenario for which advanced HF therapies or palliative care may be considered. Similar rationale and future steps may apply to patients with poor quality of life, where bi-directional relationships with medication increases and decreases were also seen.
Aside from identifying patients subsets in particular need of quality improvement strategies, the current data from CHAMP-HF also inform the expected yield of various types of interventions. Although medical reasons were the most frequent trigger for medication changes, the significant proportion of non-medical reasons represents modifiable factors likely most conducive to targeted study. For instance, interventions targeting patient perceptions and systems-based barriers may be most effective in preventing dosing decreases or discontinuation of ARNI therapy, whereas strategies targeting health team education may be higher yield for other therapies.
Limitations
First, despite rigorous multivariate modeling techniques, these observational data cannot definitively determine cause-effect relationships. While observed relationships between most factors and dosing changes were clinically plausible, some are not easily understood. For example, the finding of higher systolic blood pressure predicting greater likelihood of ARNI discontinuation/dose decrease is curious, and highlights the possibility of residual confounding or collinearity with unmeasured factors. Second, this analysis should be interpreted in the context of patients with complete medication dosing information at baseline and 12-months, thus excluding patients with interval death or lost-to-follow-up. Third, CHAMP-HF data capture was derived from documentation within the medical record. Despite pre-specified features designed to lessen any potential influence of documentation quality and completeness on registry data, inherent limitations remain. For example, contraindications to therapy may have been present in some instances but not documented. Likewise, some patients receiving lower doses may be individuals where dose increases were previously attempted but not tolerated. Fourth, although study sites reflected a diverse set of cardiology and primary care outpatient practices, data reflect sites and patients that elected to participate in the registry and thus may not be generalizable to all outpatient facilities and HFrEF patients. Fifth, the registry permitted multiple reasons for each medication change. Although this approach may be most reflective of clinical practice where multiple factors may contribute, these data do not discern the primary reason for each medication change or associate objective vital signs or laboratory values with medication changes.
Conclusions
In this contemporary US outpatient HFrEF registry, the large majority of eligible patients did not receive target doses of medical therapy at any point during longitudinal follow-up and few patients had doses increased over time. Although most patients had no alterations in medication use or dosing, multiple clinical factors were independently associated with dose increases and decreases during follow-up. Among the modest proportion of patients where GDMT use or dosing changed, medical reasons were the most common underlying reasons for dose decreases and discontinuation across all therapies, but the relative contributions from patient preference, health team, and systems-based reasons varied by medication. Further quality improvement efforts are urgently needed to improve guideline-directed medication titration in routine practice. These findings may inform future targeted strategies to improve the medical care of outpatients with HFrEF.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge:
There are major gaps in guideline-directed titration of GDMT for HFrEF in contemporary US outpatient practice. Most patients eligible for therapy never receive target doses of medications at any point in time during longitudinal clinical care.
Competency in Medical Knowledge:
Several high-risk patient features are paradoxically associated with higher likelihood of medication discontinuation or dosing decrease, such as prior HF hospitalization, impaired quality of life, and more severe functional class. Medical reasons (e.g., new or worsening signs/symptoms, intolerance) are the most common underlying reasons for discontinuations and dosing decreases, but the relative contributions from patient preference, health team, and systems-based reasons vary by medication.
Competency in Patient Care:
Clinicians should recognize the tendency to not uptitrate medical therapy during longitudinal care. Many eligible patients who would benefit from escalation of the medical therapy receive no therapy or stable sub-target doses. To improve patient outcomes, every effort should be taken to maximize GDMT among eligible patients, as tolerated.
Translational Outlook:
Future targeted quality improvement efforts are needed to improve longitudinal titration of HFrEF medical therapy and increase the rates of target dosing. Such efforts are particularly needed for high-risk patients with severe symptoms or recent HF hospitalization. Interventions targeting patient perceptions and systems-based barriers may be most effective in preventing dosing decreases or discontinuation of ARNI therapy, whereas strategies targeting health team education may be higher yield for other therapies.
ACKNOWLEDGEMENTS
FUNDING SOURCES
The CHAMP-HF registry is funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA).
DISCLOSURES
Dr. Greene is supported by the National Heart Lung and Blood Institute T32 postdoctoral training grant (T32HL069749-14), a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis, and has received research support from Amgen and Novartis. Dr Fonarow reports research funding from the NIH and serving as a consultant for Amgen, Bayer, Medtronic, and Novartis. Dr. DeVore reports receiving research funding from Akros Medical, the American Heart Association, Amgen, Bayer, Intra-Cellular Therapies, Luitpold Pharmaceuticals, the NHLBI, Novartis, and PCORI and serving as a consultant for Novartis. Drs. Sharma and Duffy and Mr. McCague report being an employee of Novartis. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), and has served on advisory boards or received research funding from AstraZeneca, Bayer AG, and Baxter Healthcare. Dr. Albert reports serving as a consultant for Novartis and Boston Scientific. Dr. Patterson reports research funding from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Otsuka and Novartis and serves as a consultant to Novartis. Dr. Spertus reports that, relevant to this work, he serves as a consultant for Novartis and owns the copyright to the Kansas City Cardiomyopathy Questionnaire. Dr. Hernandez reports consulting fees from AstraZeneca, Bayer, Boston Scientific, Merck, Novartis, Sanofi, and research support from AstraZeneca, GlaxoSmithKline, Luitpold, Merck, Novartis. Dr. Butler has received research support from the National Institutes of Health, PCORI and the European Union; and serves as a consultant for Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmacautical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, and ZS Pharma. All other authors report no disclosures.
ABBREVIATIONS
- ACEI
Angiotensin-converting enzyme inhibitor
- ARB
Angiotensin II receptor blocker
- ARNI
Angiotensin receptor-neprilysin inhibitor
- GDMT
Guideline-directed medical therapy
- HF
Heart failure
- HFrEF
Heart failure with reduced ejection fraction
- MRA
Mineralocorticoid receptor antagonist
- NYHA
New York Heart Association
Footnotes
Twitter handles: @SJGreene_md, @gcfmd, @JavedButler1, @DCRINews, @UMMCMedicine
Tweet: The large majority of eligible #heartfailure patients in the US never receive target doses of GDMT at any point in routine follow-up. A major opportunity to improve care and outcomes.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Januzzi JL, Jr., Allen LA et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;71:201–230. [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Albert NM et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 5.Peri-Okonny PA, Mi X, Khariton Y et al. Target Doses of Heart Failure Medical Therapy and Blood Pressure: Insights From the CHAMP-HF Registry. J Am Coll Cardiol HF 2019. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Albert NM, Curtis AB et al. Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail 2012;18:9–17. [DOI] [PubMed] [Google Scholar]
- 7.DeVore AD, Thomas L, Albert NM et al. Change the management of patients with heart failure: Rationale and design of the CHAMP-HF registry. Am Heart J 2017;189:177–183. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Albert NM, Curtis AB et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation 2010;122:585–96. [DOI] [PubMed] [Google Scholar]
- 9.SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Johnson G, Ziesche S et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303–10. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–75. [DOI] [PubMed] [Google Scholar]
- 12.Young JB, Dunlap ME, Pfeffer MA et al. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: results of the CHARM low-left ventricular ejection fraction trials. Circulation 2004;110:2618–26. [DOI] [PubMed] [Google Scholar]
- 13.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 14.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed] [Google Scholar]
- 15.Packer M, Coats AJ, Fowler MB et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. [DOI] [PubMed] [Google Scholar]
- 16.McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 17.Tahhan AS, Vaduganathan M, Greene SJ et al. Enrollment of Older Patients, Women, and Racial and Ethnic Minorities in Contemporary Heart Failure Clinical Trials: A Systematic Review. JAMA Cardiol 2018;3:1011–1019. [DOI] [PubMed] [Google Scholar]
- 18.DeVore AD, Mi X, Thomas L et al. Characteristics and Treatments of Patients Enrolled in the CHAMP-HF Registry Compared With Patients Enrolled in the PARADIGM-HF Trial. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, Continuation, Switching, and Withdrawal of Heart Failure Medical Therapies During Hospitalization. J Am Coll Cardiol HF 2019;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson PN, Rumsfeld JS, Liang L et al. Treatment and risk in heart failure: gaps in evidence or quality? Circ Cardiovasc Qual Outcomes 2010;3:309–15. [DOI] [PubMed] [Google Scholar]
- 21.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220–9. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez EJ, Morrow DA, DeVore AD et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2018;380:539–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


