Abstract
Standfirst | In 2018, the acute myeloid leukaemia treatment landscape expanded notably, with the findings of several trials leading to the approval of novel targeted therapies. Furthermore, comprehensive sequencing of patient samples revealed the effects of co-occurring mutations and gene- expression patterns on drug sensitivity, providing hope that future treatment options will become increasingly precise and personalized.
For approximately 50 years, the treatment of patients with acute myeloid leukaemia (AML) has consisted of a combination of cytarabine and anthracycline, in a standard intensive regimen termed ‘7+3’ chemotherapy. This approach is effective in a small subset of patients with AML, although the majority of patients are not cured and long-term disease-free survival has remained <30% with this ‘standard intensive therapy’. Outcomes are particularly poor in older patients (often, and somewhat arbitrarily, defined as >65 years of age) who comprise the majority of those diagnosed with AML, with current expectations remaining a median overall survival (OS) duration of <1 year from diagnosis.(1)
The advent of rapid and affordable genome sequencing has ushered in a decade of improved scientific understanding of the pathophysiology of AML. Awareness of the AML genomic landscape initially improved our ability to classify and prognosticate, such as the improved ability to determine which patients will benefit most from allogeneic stem cell transplantation (SCT) in first remission. Now, at last, the AML community is experiencing long-awaited clinical progress in AML-directed therapies. Building upon a successful 2017, which saw landmark approvals including midostaurin in combination with 7+3 chemotherapy for patients with FLT3-mutated AML, enasidenib for patients with isocitrate dehydrogenase 2 (IDH2)-mutated AML, the (re)approval of the anti-CD33 monoclonal antibody–drug conjugate gemtuzumab ozogamicin, and liposomal daunorubicin plus cytarabine for adults with newly diagnosed secondary or therapy-related AML, 2018 was another banner year for advances in AML therapy.
Mutations in fms-related tyrosine kinase 3 (FLT3) occur in approximately one-third of patients with AML, and are associated with younger age, proliferative disease, and — for internal tandem duplication mutations (FLT3–ITD) — inferior outcomes with standard therapy. The approval of the multikinase inhibitor midostaurin, in combination with 7+3 chemotherapy, for younger patients (<60 years of age) with newly diagnosed FLT3-mutant AML in late 2017 was a seminal advance; however, the need for more-potent and more-selective FLT3 inhibitors is readily apparent.(2) Results from a phase II trial of the second-generation FLT3 inhibitor, quizartinib, were published in May 2018, and demonstrated an overall response rate (ORR) of >70% and a composite complete remission rate of ~50% in patients receiving quizartinib as a single agent for relapsed and/or refractory (R/R) FLT3-mutant AML.(3) Furthermore, over a third of younger patients with R/R disease were able to proceed to SCT, thus confirming not only the efficacy but also the safety and tolerability of this approach. In June 2018, results from the successive randomized phase III trial of quizartinib (QuANTUM-R) were presented in abstract form, confirming that this agent provides a significant improvement in overall survival in patients with R/R FLT3-mutant AML versus best-available therapy (median OS 6.2 versus 4.7 months).(4) The AML community eagerly awaits the FDA’s decision on quizartinib, as well as on gilteritinib, another potent, selective FLT3 inhibitor for which enrollment of patients with R/R FLT3-mutated AML into a phase III trial has been completed (NCT02421939).
Mutations in IDH1 occur in ~8% of patients with AML. The first targeted inhibitor of mutant IDH1, ivosidenib, entered clinical trials in 2014 in a phase I, first-in-human dose-finding study. Over 250 patients were treated in this study, with striking levels of activity at all dose levels seen in patients with R/R IDH1-mutant AML (n= 179) including a CR rate of 22%, CR/CR with partial haematological recovery (CRh) rates of 30%, and median OS of 9 months.(5) This first-in-class IDH1 inhibitor was approved by the FDA in July 2018 based on these frequent and durable responses and limited toxicities observed in this population. Similar to the IDH2 inhibitor enasidenib, which was approved in 2017 for patients with R/R IDH2-mutant AML,(6) the distinctive mechanism of action of ivosidenib involves leukemic differentiation and maturation without cytotoxicity, which leads to an IDH differentiation syndrome in ~10% of patients that can be managed using corticosteroids, hydroxyurea, and dose interruption in severe cases.
With a median age of 68 at diagnosis, older patients make up the majority of all patients with AML. In older adults, and in patients with secondary AML (that is AML evolving from an antecedent haematological disorder or arising as a complication of previous chemotherapy or radiotherapy), standard 7+3 is associated with poor outcomes, in terms of both increased toxicities and lower response and survival. Results of the phase III trial of 7+3 versus CPX-351, a liposomal encapsulation of cytarabine plus daunorubicin at a fixed 5:1 molar ratio, demonstrated improved response (CR/CR with incomplete neutrophil or platelet recovery (CRi) 48% versus 33%) and survival (median OS 9.56 versus 5.95 months; HR 0.69, 95% CI 0.52–0.90; one-sided P= 0.003) in intensive-chemotherapy eligible patients 60–75 years of age with secondary AML or AML with MDS-related features.(7)
The sizeable cohort of older patients who are ineligible for intensive therapy has remained a population of critical unmet medical need. The frequent use of DNA methyltransferase inhibitors (azacitidine and decitabine, also referred to as hypomethylating agents or HMAs) or low-dose cytarabine (LDAC) provides only modest levels of benefit. In this setting, dramatic early results from trials of combinations containing the selective BCL-2 inhibitor venetoclax, or the Sonic hedgehog pathway smoothened receptor inhibitor glasdegib, have the potential to transform the treatment paradigm for this population of IC-ineligible older patients with AML. Updated results from a phase I combination trial of venetoclax with HMA therapy for older patients with treatment-naïve AML is particularly notable.(8) In 145 patients with a median age of 74, CR/CRi rates were 67%, with a duration of CR/CRi lasting >11 months and a median OS of 17.5 months. In the cohort receiving 400 mg venetoclax in combination with azacitidine, the CR/CRi rate was 73% and median OS was not reached. The FDA recently provided accelerated approval of this combination based on the phase I data, and a Phase III placebo-controlled confirmatory trial of azacitidine +/− venetoclax is ongoing. Improved results with LDAC combinations for older IC-ineligible AML patients are also noteworthy 2018 advances, including the FDA approval of LDAC + glasdegib based on the randomized Phase II trial demonstrating benefit of the combination (CR/CRi rate 24% vs 5% with LDAC alone) and median OS 8.3 vs 4.3 mo (HR 0.5, 1-sided p= 0.0002). The combination of venetoclax plus LDAC also received accelerated FDA approval, reporting a CR/CRh rate of 42% and median OS 11.4 months with a confirmatory Phase III trial ongoing, providing another treatment option for this historically difficult-to-treat patient population.(9)
In addition to major advances in treatment (TABLE 1), 2018 also saw the emergence of a deeper understanding of AML biology. Owing to the substantial genomic heterogeneity of AML, previous analyses have been underpowered and have failed to provide clear associations between complex mutational patterns and treatment responses. In 2018, a valuable advance emerged in the form of the Vizome Beat AML data viewer, providing data on 672 tumour samples from 562 patients with AML treated as part of the multi-institutional Beat AML program.(10) Detailed investigations of patient level data, including treatment outcomes and data from whole-exome sequencing, RNA sequencing, and ex vivo drug sensitivity to >100 agents are now freely accessible, and will undoubtedly accelerate and enable profound future discoveries.
Table 1.
Key trials involving patients with AML with results reported in 2018
| Agent | Comparator | Trial Population | Outcome | Follow - up |
|---|---|---|---|---|
| Quizartinib (3) | None | R/R FLT3 – ITD AML | CRc 46%, median OS 31.1 weeks (FLT3 – ITD cohort 2) | Phase III QuANTUM – R (4); CRc 48% vs 27%; median OS 6.2 months vs 4.7 months |
| Ivosidenib (5) | None | R/R IDH1 – mutant AML | CR/CRh 30%; ORR 40%; median OS 9 months | - |
| CPX – 351 (7) | 7 + 3 chemotherapy | CPX – 351 – eligible treatment – naïve, older (60 −75 years of age) patients with secondary AML or AMLwith MDS – related changes | CR/CRi; 48% vs 33%; median OS 9.5 vs 5.9 months; EFS 2.5 vs 1.3 months | - |
| HMA + venetoclax (8) | None | Unfit, treatment naïve elderly patients with AML | CR/Cri 67%; median OS 17.5 months | Phase III ongoing (NCT02993523) |
2018 will indeed be remembered as a landmark year in AML. The approval of multiple targeted therapies offers new hope and increasingly effective treatment options, and also reinforces the importance of genomic profiling for the optimal care of patients. We look forward to future results of trials investigating the efficacy of targeted therapies in upfront, combination settings. Additionally, the results of large-scale sequencing efforts have demonstrated true progress, not only in the recognition of the genomic complexity of AML, but also in providing tangible hope for a future of increasingly individualized and precision-based AML therapy.
Fig. 1 |. Developments in the treatment of AML.
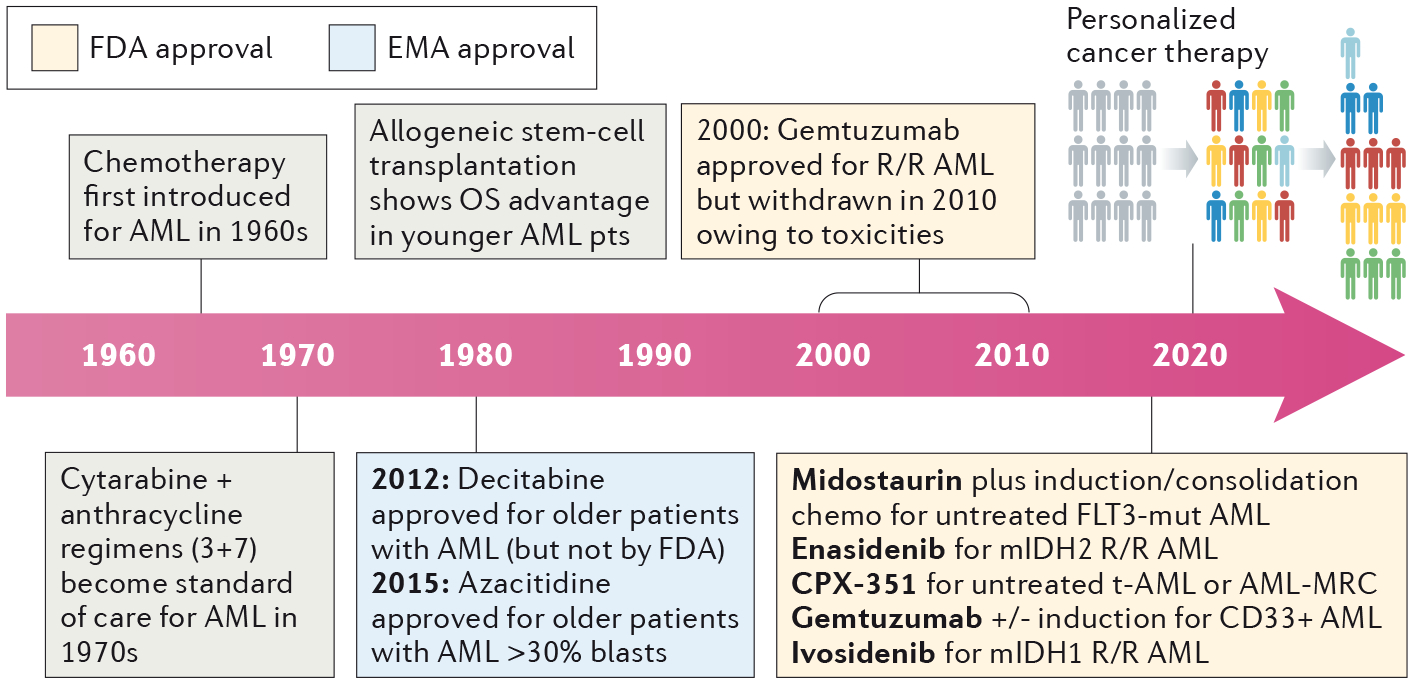
AML, acute myeloid leukaemia; CR, complete remission; CRc, composite complete remission; CRh, CR with incomplete haematological recovery; CRi, complete remission with incomplete neutrophil or platelet recovery; EFS, event-free survival; HMA, hypomethylating agent; ITD, internal tandem duplication; MDS, myelodysplastic syndrome; OS, overall survival; ORR, overall response rate; R/R, relapsed and/or refractory.
Key advances.
The addition of the multikinase inhibitor midostaurin to 7+3 chemotherapy for newly diagnosed patients, and the use of second-generation FLT3 inhibitors (such as quizartinib) for those with relapsed and/or refractory (R/R) FLT3-mutated AML demonstrate improved overall survival compared with standard-of-care therapy.2,3,4
The oral targeted mutant IDH1 inhibitor ivosidenib is now approved for the treatment of patients with relapsed/refractory AML with an IDH1 mutation, as a result of the substantial activity and durable responses seen in a phase 1 trial.5
In adults with newly diagnosed secondary or therapy-related AML, treatment with the liposomal encapsulation of cytarabine plus daunorubicin at a fixed 5:1 molar ratio demonstrated improved response rates and median survival durations compared with standard 7+3 therapy in a randomized phase III trial.7
The addition of the BCL2-inhibitor venetoclax to lower-intensity AML therapy such as hypomethylating agents or low-dose cytarabine, for older patients unsuitable for intensive chemotherapy approaches suggests markedly improved patient outcomes; confirmatory randomized trials are ongoing.8,9
Clinical and genomic data from patients with AML, including whole-exome and RNA sequencing, and analysis of ex vivo sensitivity to >100 agents is now freely available, and is likely to accelerate and enable future discovery.10
Acknowledgements
C.D.D. is supported by The V Foundation for Cancer Research and the MD Anderson Khalifa Clinical Scholar Award.
Footnotes
Competing interests
C.D.D. is a consultant of Abbvie, Agios, and Celgene and has received honoraria from Bayer, Jazz, Karyopharm, Medimmune, and Syros as an advisory board member. A.E.P. is a consultant for Abbvie, Arog, Astellas, and Daiichi Sankyo. Additionally he has received honoraria from Actinium Pharmaceuticals, Agios, Jazz Pharmaceuticals, NewLink Genetics, Novartis, and Takeda as an advisory board member.
Contributor Information
Courtney D. DiNardo, Department of Leukemia, UT MD Anderson Cancer Center, Houston, TX, USA.
Alexander E. Perl, Division of Hematology-Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2015. 2018. [Google Scholar]
- 2.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. The New England journal of medicine. 2017;377(5):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes J, Perl AE, Dohner H, Kantarjian H, Martinelli G, Kovacsovics T, et al. Quizar – ITD Cohort 2)tinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology. 2018;19(7):889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes JKS, Martinelli G, Perl AE, Ganguly S, Russell N, Kramer A, Dombret H, Hogge D, Jonas BA, Leung AY, Mehta P, Montesinos P, Radsak M, Sica S, Arunachalam M, Holmes M, Kobayashi K, Namuyinga R, Ge N, Yver A, Zhang Y, Levis MJ. QUIZARTINIB SIGNIFICANTLY PROLONGS OVERALL SURVIVAL IN PATIENTS WITH FLT3-INTERNAL TANDEM DUPLICATION–MUTATED (MUT) RELAPSED/REFRACTORY AML IN THE PHASE 3, RANDOMIZED, CONTROLLED QUANTUM-R TRIAL. EHA 2018;LB2600. [Google Scholar]
- 5.DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. The New England journal of medicine. 2018;378(25):2386–98. [DOI] [PubMed] [Google Scholar]
- 6.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(26):2684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei A, Strickland SA, Roboz GJ, Hou J-Z, Fiedler W, Lin TL, et al. Phase 1/2 Study of Venetoclax with Low-Dose Cytarabine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia Unfit for Intensive Chemotherapy: 1-Year Outcomes. Blood. 2017;130(Suppl 1):890-. [Google Scholar]
- 10.Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562(7728):526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


