Abstract
Background
Clinical findings indicated that a fraction of coronavirus disease 2019 (COVID-19) patients diagnosed as mild early may progress to severe cases. However, it is difficult to distinguish these patients in the early stage. The present study aimed to describe the clinical characteristics of these patients, analyze related factors, and explore predictive markers of the disease aggravation.
Methods
Clinical and laboratory data of nonsevere adult COVID-19 patients in Changsha, China, were collected and analyzed on admission. A logistic regression model was adopted to analyze the association between the disease aggravation and related factors. The receiver operating characteristic curve (ROC) was utilized to analyze the prognostic ability of C-reactive protein (CRP).
Results
About 7.7% (16/209) of nonsevere adult COVID-19 patients progressed to severe cases after admission. Compared with nonsevere patients, the aggravated patients had much higher levels of CRP (median [range], 43.8 [12.3–101.9] mg/L vs 12.1 [0.1–91.4] mg/L; P = .000). A regression analysis showed that CRP was significantly associated with aggravation of nonsevere COVID-19 patients, with an area under the curve of 0.844 (95% confidence interval, 0.761–0.926) and an optimal threshold value of 26.9 mg/L.
Conclusions
CRP could be a valuable marker to anticipate the possibility of aggravation of nonsevere adult COVID-19 patients, with an optimal threshold value of 26.9 mg/L.
Keywords: biomarkers, COVID-19, C-reactive protein
The current outbreak of coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, in December 2019 [1–5]. As of March 6, 2020, at least 80 651 cases and 3070 deaths have been identified across the mainland of China [6]. Outside China, severe acute respiratory coronavirus 2 (SARS-CoV-2) has spread widely around the world, especially in South Korea, Iran, and Italy [7, 8]. The World Health Organization has raised the assessment of the risk of spread and the risk of impact of COVID-19 to very high at a global level on February 28, 2020 [9]. How to reduce the spread of virus and decrease mortality has become a global problem.
Owing to the rapid increase in the number of COVID-19 patients, the hospitals in many regions face huge challenges. Generally, severe patients are treated in the intensive care unit, while mild patients are hospitalized in the usual isolation ward. However, a small subset of nonsevere patients will develop into severe cases. Therefore, the problem of how to identify this group of patients early and actively monitor and treat them is critical to reducing mortality and improving outcomes of COVID-19 patients. In this study, we found that a few COVID-19 patients diagnosed as nonsevere on admission progressed to severe later in Changsha, China; we analyzed the clinical characteristics of these patients and explored the related factors and predictable markers of aggravation of nonsevere COVID-19 patients as well.
METHODS
Study Design and Participants
This case series was subject to approval by the institutional ethics board of The Second Xiangya Hospital of Central South University (No. 2020001). All laboratory-confirmed adult COVID-19 patients (≥18 years old) admitted to the Public Health Treatment Center of Changsha, China, and diagnosed as nonsevere cases on admission from January 17 to February 20, 2020, were enrolled. Clinical outcomes (severity and mortality) were monitored through February 20, 2020, the final date of follow-up.
Data Collection
Two members of our team carefully collected and reviewed the medical records of patients individually. The detailed information on demographic data, underlying comorbidities, medical history, symptoms, laboratory parameters, and chest computed tomography (CT) scans on admission were recorded. The date of disease onset was defined as the day when the symptoms were noticed. We used one of the following criteria to determine severe cases of COVID-19: (1) respiratory rate ≥30/min; (2) oxygen saturation ≤93%; (3) PaO2/FiO2 ≤300 mmHg; (4) lung lesions progressed >50% within 24–48 hours; (5) mechanical ventilation was implemented; (6) shock; (7) intensive care unit admission [10].
Statistical Analysis
Because all continuous variables in our study were non–normally distributed, we used median with range and the Mann-Whitney test to depict and analyze them. The χ 2 test or Fisher exact test was utilized to compare differences for categorical variables. Univariate and multivariate analyses were carried out using a logistic regression model to analyze the association between the progression of nonsevere COVID-19 cases and related factors. Demographic variables (gender and age) and variables that were significantly associated (P < .05) with disease progression were included in the multivariable analysis. Areas under the curve (AUCs; with 95% confidence intervals) were computed to assess the diagnostic values of C-reactive protein (CRP); AUCs >0.70 were considered clinically relevant [11]. The Kaplan-Meier (KM) curve and the log-rank test were applied to further assess the potential risk factors associated with progression. The Youden index was implemented to set cutoff values. All analyses were performed using IBM SPSS, version 26.
RESULTS
There were 209 adult patients with laboratory-confirmed COVID-19 diagnosed as nonsevere cases on admission by February 20, 2020, in Changsha, and all of them were included in our study; 16 patients (7.7%) progressed to severe cases after admission by February 20, 2020, including 2 patients who eventually developed into critically ill cases.
The clinical characteristics of the 16 aggravated patients are summarized in Table 1. The median age (range) was 54 (35–68) years, and 10 (62.5%) were men. The median duration from the onset of symptoms to disease progression (range) was 9.5 (3–12) days. Of the 16 patients, hypertension (5, 31.3%), cerebrovascular disease (2, 12.5%), and cardiovascular disease (1, 6.3%) were the most common comorbidities. The most common symptoms were fever (14, 87.5%), cough (9, 56.3%), anorexia (9, 56.3%), fatigue (9, 56.3%). All 16 patients showed pulmonary exudative lesions on chest CT scans, of whom 10 (62.5%) showed ground glass opacity in the lungs.
Table 1.
Baseline Characteristics of the Aggravated Nonsevere COVID-19 Patients
| Nonsevere to Severe (n = 16) | Nonsevere (n = 193) | P Valuea | |
|---|---|---|---|
| Sex (male/female), No. | 10/6 | 95/98 | .307 |
| Age, median (range), y | 54 (35–68) | 42 (19–84) | .021 |
| Comorbidity | |||
| Hypertension, No. (%) | 5 (31.3) | 22 (11.4) | .059 |
| Cardiovascular disease, No. (%) | 1 (6.3) | 4 (2.1) | .331 |
| Diabetes, No. (%) | 0 (0.0) | 11 (5.7) | .690 |
| Cerebrovascular disease, No. (%) | 2 (12.5) | 4 (2.0) | .105 |
| No signs and symptoms, No. (%) | 0 (0.0) | 17 (8.8) | .446 |
| Symptoms | |||
| Fever, No. (%) | 14 (87.5) | 122 (63.2) | .050 |
| Fatigue, No. (%) | 9 (56.3) | 54 (28.0) | .037 |
| Cough, No. (%) | 9 (56.3) | 105 (54.4) | .887 |
| Anorexia, No. (%) | 9 (56.3) | 60 (31.1) | .040 |
| Chills, No. (%) | 5 (31.3) | 19 (9.8) | .030 |
| Myalgia, No. (%) | 1 (6.3) | 16 (8.3) | 1.000 |
| Dyspnea, No. (%) | 4 (25.0) | 9 (4.7) | .007 |
| Expectoration, No. (%) | 4 (25.0) | 45 (23.3) | 1.000 |
| Pharyngalgia, No. (%) | 1 (6.3) | 29 (15.0) | .554 |
| Diarrhea, No. (%) | 1 (6.3) | 13 (6.7) | 1.000 |
| Nausea, No. (%) | 0 (0.0) | 5 (2.6) | 1.000 |
| Dizziness, No. (%) | 2 (12.5) | 11 (5.7) | .587 |
| Headache, No. (%) | 5 (31.3) | 9 (4.7) | .000 |
| Vomiting, No. (%) | 1 (6.3) | 4 (2.1) | .331 |
| Abdominal pain, No. (%) | 0 (0.0) | 2 (1.0) | 1.000 |
| Chest CT positive rate, No. (%) | 16 (100.0) | 183 (94.8) | .746 |
| Chest CT with ground glass change, No. (%) | 10 (62.5) | 81 (42.0) | .111 |
Abbreviation: CT, computed tomography.
a P values indicate differences between nonsevere COVID-19 patients and COVID-19 patients who progressed from nonsevere to severe. P < .05 was considered statistically significant. Continuous variables were described as median with range and analyzed by Mann-Whitney test. Categorical variables were described as percentages and analyzed by the χ 2 test or Fisher exact test.
Laboratory findings for the aggravated patients on admission are shown in Table 2. On admission, the median white blood cell count, lymphocyte count, and lymphocyte percentage were all in the normal range. Decreased number of white blood cells, lymphocytes, and lymphocyte percentage occurred in 25.0%, 37.5%, and 25.0% of patients, respectively. CRP (100.0%) was elevated in all 16 patients, and erythrocyte sedimentation rate (81.3%) increased in most patients on admission. Additionally, median levels of enzymatic indicators of liver, kidney, and myocardial damage were all within the normal range.
Table 2.
Laboratory Findings of Aggravated and Nonsevere COVID-19 Patients
| Normal Range | Nonsevere to Severe (n = 16) | Nonsevere (n = 193) | P Valuea | |
|---|---|---|---|---|
| White blood cell count, median (range), ×109/L | 4–10 | 4.8 (2.2–7.2) | 4.5 (1.9–13.4) | .683 |
| Lymphocyte count, median (range), ×109/L | 0.8–4.0 | 1.0 (0.4–1.9) | 1.2 (0.4–3.7) | .110 |
| Lymphocyte, median (range), % | 20–40 | 23.9 (10.4–31.2) | 27.7 (6.8–61.1) | .021 |
| Alanine aminotransferase, median (range), U/L | 0–42 | 23.9 (13.7–37.1) | 19.0 (2.6–87.7) | .068 |
| Aspartate aminotransferase, median (range), U/L | 0–37 | 29.2 (18.3–49.4) | 23.2 (2.0–78.8) | .010 |
| Total bilirubin, median (range), μmol/L | 3.4–20.5 | 15.0 (5.3–22.9) | 10.9 (4.0–40.2) | .653 |
| C-reactive protein, median (range), mg/L | 0–8 | 43.8 (12.3–101.9) | 12.1 (0.1–91.4) | .000 |
| Erythrocyte sedimentation rate, median (range), mm/h | 0–15 | 49.0 (.0–106.0) | 38.0 (1.0–143.0) | .222 |
| Procalcitonin ≥0.05 nmol/L, No. (%) | ≤0.05 | 6 (37.5) | 48 (24.9) | .417 |
| Creatinine, median (range), μmol/L | 21.5–104 | 55.4 (27.2–68.1) | 51.5 (20.6–124.1) | .783 |
| Creatine kinase, median (range), U/L | 10–190 | 81.2 (19.3–436.3) | 68.5 (11.3–365.3) | .594 |
| Creatine kinase-MB, median (range), U/L | 0–24 | 6.5 (1.1–19.4) | 9.5 (0.3–221.7) | .034 |
a P values indicate differences between nonsevere COVID-19 patients and COVID-19 patients who progressed from nonsevere to severe. P < .05 was considered statistically significant. Continuous variables were described as median with range and analyzed by Mann-Whitney test. Categorical variables were described as percentages and analyzed by the χ 2 test.
Among the 16 aggravated patients, noninvasive ventilation, high-flow oxygen therapy, and invasive mechanical ventilation were adopted in 6.3%, 18.8%, and 6.3% of patients, respectively. One patient (6.3%) received extracorporeal membrane oxygenation as rescue therapy but eventually died.
As shown in Table 1, compared with nonsevere patients, the aggravated patients were significantly older (median age, 54 vs 42 years; P = .021). They also showed higher ratios of symptoms than nonsevere cases, including fatigue (56.3% vs 28.0%; P = 0.037), chills (31.3% vs 9.8%; P = .030), anorexia (56.3% vs 31.1%; P = .040), dyspnea (25.0% vs 4.7%; P = .007), and headache (31.3% vs 4.7%; P = .000). Additionally, the aggravated patients had a lower lymphocyte proportion (median, 23.9% vs 27.7%; P = .021) and higher levels of CRP (median, 43.8 vs 12.1 mg/L; P = .000) and aspartate aminotransferase (median, 29.2 vs 23.2 U/L; P = .010) (Table 2).
The associations between the progression of nonsevere COVID-19 patients and their clinical characteristics are presented in Table 3. In the univariate analysis, the progression was associated with hypertension (odds ratio [OR], 3.533; 95% confidence interval [CI], 1.123–11.120; P = .031), creatine kinase (OR, 1.006; 95% CI, 1.000–1.012; P = .047), lymphocyte proportion (OR, 0.932; 95% CI, 0.876–0.991; P = .025), aspartate aminotransferase (OR, 1.044; 95% CI, 1.001–1.088; P = .044), and CRP (OR, 1.049; 95% CI, 1.028–1.070; P = .000). Only CRP was significantly associated with the progression of nonsevere COVID-19 patients (OR, 1.056; 95% CI, 1.025–1.089; P = .000) as determined by multivariate analysis, which suggested that for every 1-unit increase in CRP level, the risk of developing severe events increased by about 5%.
Table 3.
Analysis of Factors Related to the Progression of Nonsevere COVID-19 Patients
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Gender | 1.719 (0.601–4.917) | .312 | 0.757 (0.203–2.818) | .678 |
| Age | 1.035 (1.000–1.071) | .050 | 0.998 (0.953–1.046) | .946 |
| Hypertension | 3.533 (1.123–11.120) | .031* | 0.357 (0.078–1.639) | .185 |
| Creatine kinase | 1.006 (1.000–1.012) | .047* | 1.005 (0.998–1.013) | .182 |
| Lymphocyte % | 0.932 (0.876–0.991) | .025* | 1.015 (0.941–1.093) | .705 |
| Aspartate aminotransferase | 1.044 (1.001–1.088) | .044* | 0.982 (0.919–1.050) | .598 |
| C-reactive protein | 1.049 (1.028–1.070) | .000* | 1.056 (1.025–1.089) | .000* |
Univariate and multivariate analyses were carried out using a logistic regression model.
Abbreviation: CI, confidence interval.
*Statistically significant.
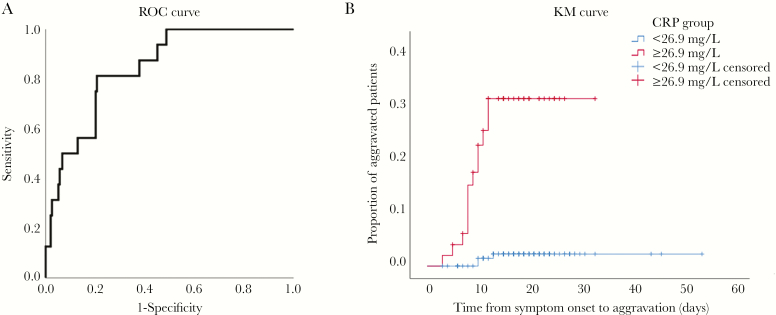
Next, the ROC curve analysis indicated moderate accuracy, with an area under the curve (AUC) of 0.844 (95% CI, 0.761–0.926; P < .001) for CRP to predict the possibility of aggravation of nonsevere COVID-19 patients (Figure 1A). Defined by the ROC curve, the optimal threshold value was 26.9 mg/L and was associated with a sensitivity of 81.3% and specificity of 79.3%. Furthermore, the KM curve showed that patients with high levels of CRP (≥26.9 mg/L) had significantly elevated risks of developing into severe cases when compared with patients with low levels (24.5% vs 1.9%; log-rank P < .001) (Figure 1B).
Figure 1.
The relationship between CRP and exacerbations of nonsevere COVID-19 patients and CRP’s predictive effect. A, Receiver operating characteristics curve of CRP for the diagnosis of aggravation of nonsevere COVID-19 patients. B, The time-dependent risk of reaching aggravation between nonsevere COVID-19 patients with lower (<26.9 mg/L) and higher (≥26.9 mg/L) levels of CRP. Abbreviations: CRP, C-reactive protein; KM, Kaplan-Meier; ROC, receiver operating characteristics.
DISCUSSION
To our knowledge, this is the first article concerning mild COVID-19 patients who develop into severe cases. We presented the clinical characteristics of those aggravated patients, compared their clinical features with those of nonsevere cases, and analyzed the possible factors associated with progression. Moreover, we further demonstrated the prognostic value of CRP in the progression of mild COVID-19 patients.
In this study, a small fraction of nonsevere patients developed into severe cases in the first 2 weeks after symptom onset. Therefore, health care institutions should also pay close attention to the mild patients, identify progressors early, and provide appropriate treatment to reduce mortality. As reported in a recent article [12], severe COVID-19 patients were older and were more likely to have underlying disease, which is consistent with our study. Nonsevere patients who progressed to severe cases were older and had a higher probability of having underlying diseases. In terms of the symptoms, patients who progressed from nonsevere to severe were more likely to have shortness of breath. Several published articles have also reported a higher rate of shortness of breath in severe COVID-19 patients [5, 12]. Although these exacerbated patients felt dyspnea at an early stage, their blood oxygen saturation did not reach the standard of severe cases, which may be explained by the compensatory effect of faster breathing.
CRP is a type of protein produced by the liver that is elevated in response to inflammation [13–15]. Generally, CRP level is much higher in bacterial infections than in viral infections [16–18]. To our surprise, many COVID-19 patients in this study showed elevated CRP levels, which is in agreement with other studies [12, 19]. Moreover, aggravated cases in this study showed significantly higher levels of CRP than nonsevere patients, which suggested that CRP may be a serum marker of disease aggravation in COVID-19 patients. The logistic regression analysis demonstrated that CRP was indeed related to disease progression, and ROC analysis and a KM curve confirmed that CRP was a valuable predictor of disease progression in nonsevere COVID-19 patients. What’ s more, the elevated CRP levels occurred before the disease progressed. Therefore, we believe that COVID-19 patients with high levels of CRP should be adequately monitored and treated even though their respiratory function indicators do not meet the criteria for severe cases.
This study has several limitations. First, the small number of patients limits the conclusions of this study. For example, several indicators may be related to disease progression in univariate regression analysis, while only CRP remains significant in the multivariate analysis. In the future research, these indicators need more attention and analysis. Second, exacerbation of the condition is generally a dynamic process; thus the data at a certain time point may not accurately reflect change in the condition. Third, exacerbation of the remaining mild patients may still occur, because approximately half of the patients were still hospitalized as of February 20, 2020. Thus, the clinical characteristics of COVID-19 patients who may progress from nonsevere to severe still need to be further studied and followed up.
CONCLUSIONS
In summary, we present for the first time the clinical features of COVID-19 patients who progressed from nonsevere to severe cases. Mild patients with an older age and underlying diseases were more likely to exacerbate. Elevated CRP level could be a valuable marker to predict the possibility of aggravation of nonsevere COVID-19 patients, which could help health care workers identify those patients at an early stage for early treatment. However, a large sample size and multicenter studies are needed in order to confirm these results.
Acknowledgments
Financial support. This work was not supported by any funded projects.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol 2020; 92:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, Azhar EI, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020; 91:264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Health Commission of the People’s Republic of China. Update on the COVID-19 outbreak as of March 6.2020. Available at: http://www.nhc.gov.cn/wjw/index_gzbd.shtml. Accessed 7 March 2020. [DOI] [PMC free article] [PubMed]
- 7. Yoon SH, Lee KH, Kim JY, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol 2020; 21:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albarello F, Pianura E, Di Stefano F, et al. ; COVID 19 INMI Study Group 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis 2020; 93:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19.2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---28-february-2020. Accessed 28 February 2020.
- 10. National Health Commission, National Administration of Traditional Chinese Medicine. Diagnosis and treatment of new coronavirus pneumonia (trial sixth edition). Chin J Viral Dis. In press. [Google Scholar]
- 11. Tape TG. Interpreting Diagnostic Tests. University; of Nebraska Medical Center; 2016. Available at: http://gim.unmc.edu/dxtests/. Accessed 7 March 2020. [Google Scholar]
- 12. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res 2001; 24:163–76. [DOI] [PubMed] [Google Scholar]
- 14. Nehring SM, Goyal A, Patel BC.. C Reactive Protein (CRP). Treasure Island, FL: StatPearls; 2020. [Google Scholar]
- 15. Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol 2005; 117:104–11. [DOI] [PubMed] [Google Scholar]
- 16. Coster D, Wasserman A, Fisher E, et al. Using the kinetics of C-reactive protein response to improve the differential diagnosis between acute bacterial and viral infections. Infection 2020; 48:241–8. [DOI] [PubMed] [Google Scholar]
- 17. Ahn S, Kim WY, Kim SH, et al. Role of procalcitonin and C-reactive protein in differentiation of mixed bacterial infection from 2009 H1N1 viral pneumonia. Influenza Other Respir Viruses 2011; 5:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lubell Y, Blacksell SD, Dunachie S, et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect Dis 2015; 15:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]