Abstract
Background
Several reports on epidemiological and clinical features of the 2019 coronavirus disease (COVID-19) have been published. However, mortality and morbidity analyses, important for better understanding the pathogenesis of this disease, are scarce. We examine the clinical and laboratory features of 14 patients who died of COVID-19.
Methods
The cohort consisted of 11 male and 3 female patients, with 9 patients aged 70 years or above, and nearly all had underlying diseases.
Results
Fever with bilateral pneumonia was the main manifestation. Most patients had consolidations combined with ground glass opacity (GGO) on chest computed tomography scan. Laboratory tests showed lymphocytopenia in 10 patients, high blood glucose in 11, GGT in 5 of the 14 patients, and high LDH in 5 of 6 patients tested. In addition, this cohort had high level of cytokines such as interleukin-6 in all 8 patients tested.
Conclusions
The clinical and laboratory parameters in the cohort of fatal cases may be incorporated into future clinical prognosis models and will be of help in understanding the pathogenesis of this disease.
Keywords: coronavirus, COVID-19, mortality, pneumonia, risk assessment, SARS-CoV-2
The ongoing outbreak of SARS-CoV-2 infection, which causes COVID-19 (2019 coronavirus disease), also known as novel coronavirus pneumonia (NCP), has become a worldwide pandemic. Although the vast majority of infected patients suffer from a mild form of illness and recover, a portion of patients who fall into the severe case category eventually die of the illness [1, 2]. In a recent study of a cohort of 138 patients from our hospital, the overall mortality was 4.6% [1]. Another study with 402 patients showed higher mortality in males than females and a mortality rate of 19% in patients aged 70 years or older [3]. To date, there have been several studies published about COVID-19 on infection transmission and clinical characteristics of hospitalized patients [1, 2, 4–6]. However, pathological data are still scarce, and little is known about the pathogenesis and clinical risk factors of COVID-19. The present study was aimed at identifying common clinical and laboratory features from 14 patients who died of COVID in our hospital to gain insight into the underlying pathogenesis.
METHODS
Data Collection
This study was approved by the Ethics Board of Zhongnan Hospital of Wuhan University. The electronic medical records were retrospectively searched to identify fatal cases of COVID-19 for the month of January 2020. These patients either had the diagnosis confirmed by a positive nucleic acid test [1] or met the clinical diagnostic criteria as provided in the National Health Commission of China Guidelines (http://www.nhc.gov.cn/yzygj/s7652m/202002/e84bd30142ab4d8982326326e4db22ea.shtml). Epidemiological, clinical, laboratory, and radiological characteristics and treatment history were recorded according to the data collection forms and then reviewed and double-checked. Data collection forms included demographic data, medical history, history of exposure to infection, symptoms, signs, laboratory findings, chest computed tomography (CT) scans or chest x-ray results, treatment measures (especially history of corticosteroid use), and duration of illness.
Statistical Analysis
Statistical analyses were performed using Graphpad Prism, version 7.0. Wilcoxon rank-sum tests were used for non–normally distributed data. P values <.05 were considered statistically significant.
RESULTS
Clinical and Radiologic Features of the Cohort
As shown in Table 1, patient ages ranged from 36 to 92 years (median, 71 years). The male-to-female ratio was 3.7 to 1. Medical history included at least 1 underlying disease such as hypertension, cardiovascular disease, diabetes, lymphoma, and carcinoma (cases 6, 13, and 14) in all but 2 patients (case 9, 12). Most patients had a high fever, with the maximal temperature reaching 39.8℃. Two patients were not febrile: 1 had renal failure suggesting an immunocompromised status, and the other had lung cancer (cases 11 and 14). A nuclear acid test for SARS-Cov-2 was positive for 10 patients and unavailable for 4 patients. However, the latter 4 met the criteria for clinical diagnosis of COVID-19. The patients received routine comprehensive treatments including intravenous antibiotics, assisted oxygenation, specific treatment for the underlying diseases, and supportive treatments. In addition, 11 patients had glucocorticoid therapy during the course of their hospitalization (not known in 3 patients). The major complications leading to patient demise were acute respiratory distress syndrome (ARDS), heart and respiratory failure, septic shock, and multiple organ failures. Case 13 died of gastrointestinal bleeding due to underlying natural killer/T-cell lymphoma. The duration of the clinical course from onset of illness to death ranged from 5 days to 28 days, with a mean of 15 days.
Table 1.
Demographic and Clinical Features in 14 Fatal Cases of SARS-CoV-2 Pneumonia
| Patient | Age, y | Sex | Medical History | CT | Nuclear Acid Test | Maximal Temperature, ºC | Glucocorticoid Therapy | Complication | Duration of Clinical Course, d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | Hypertension | Bilateral, 2nd week confirmed, 3rd week alleviated | Positive | 39.3 | Yes | Cardiac and respiratory arrest | 28 |
| 2 | 78 | M | Diabetes | Positive (outside hospital) | Positive | 39.1 | Yes | Cardiac and respiratory arrest | 10 |
| 3 | 88 | M | Encephalotrophy, smoking | Bilateral positive | Positive | 39 | Yes | Acute respiratory distress syndrome | 9 |
| 4 | 84 | F | Hypertension and diabetes | Bilateral positive | Positive | 39 | Yes | Heart and respiratory failure, severe pneumonia | 6 |
| 5 | 78 | M | Hypertension and heart failure | Bilateral positive | Positive | 38.7 | Yes | Cardiac and respiratory arrest | 5 |
| 6 | 67 | F | Chronic bronchitis, hypertension, GI ulceration, liver cirrhosis, esophagus carcinoma | Right side positive | Positive | 38.3 | Yes | Respiratory failure | 28 |
| 7 | 72 | M | Chronic bronchitis | Bilateral positive | Positive | 39.4 | Yes | Heart failure | 13 |
| 8 | 92 | M | Brain infarction | Bilateral positive | NA | 38.5 | Yes | Acute respiratory distress syndrome | 20 |
| 9 | 36 | M | Unknown | Bilateral positive | NA | 39.5 | Yes | Acute respiratory distress syndrome, septic shock | 14 |
| 10 | 81 | M | Cardiac disease | Bilateral positive | NA | 38.9 | Yes | Septic shock | 7 |
| 11 | 66 | M | Chronic renal failure, hypertension | Bilateral positive | Positive | 36.6 | NA | Cardiac and respiratory arrest | 8 |
| 12 | 70 | M | Unknown | Bilateral positive | NA | 39 | Yes | Cardiac and respiratory arrest | 18 |
| 13 | 38 | M | Lymphoma | Right lung positive | Positive | 39.8 | NA | GI bleeding | 19 |
| 14 | 84 | F | Lung cancer, hypertension, diabetes | Bilateral positive | Positive | Normal | NA | Multiple organ failure | 28 |
Abbreviations: CT, computed tomography; d, day; GI, gastrointestinal; NA, not applicable; y, year.
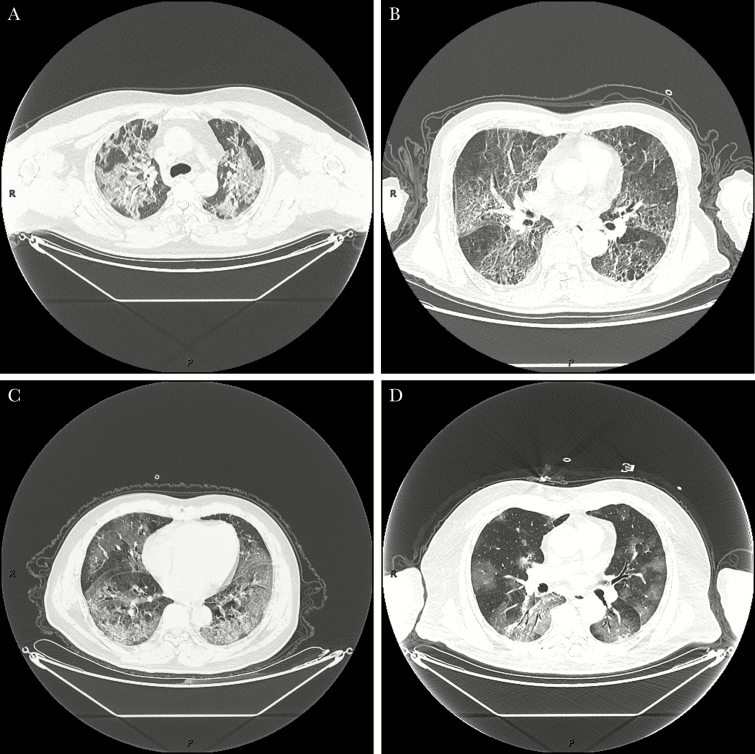
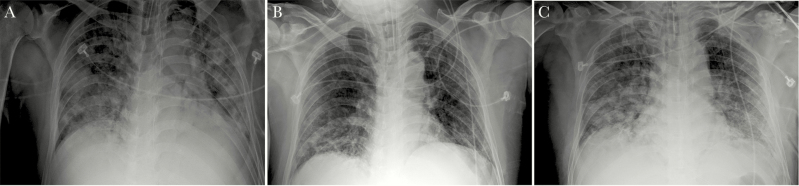
Radiologically, this cohort showed severe radiologic abnormalities. The majority had bilateral pneumonia, and 13 had consolidations combined with ground glass opacity (GGO), interstitial thickening, air bronchogram, pleural puckering, and parenchymal band, which were different from the pure GGO seen in mild cases (Figure 1). Seven patients had pleural effusion at the same time. For case 1, it was unusual that both CT scan and x-ray showed improvement in his lungs during the third week compared with the second week, but this middle-aged patient’s condition deteriorated toward the end (Figure 2).
Figure 1.
Consolidations combined with ground glass opacity (GGO), interstitial thickening, air bronchogram, pleural puckering, and parenchymal band in fatal cases (A, Case 1; B, Case 5; C, Case 7; D, Case 12).
Figure 2.
Chest x-ray images from Case 1. Compared with the second week (A), there is improvement in the lungs in the third week (B), but worsened changes in the fourth week (C).
Laboratory Findings
Hematological findings are summarized in Table 2. Abnormalities in complete blood count (CBC) included marked lymphocytopenia (10/14, 71%), leukocytosis (6/14, 43 %), or occasional leukopenia. Neutrophil count had the same tendency as white blood cell count (WBC). Compared with the WBC and neutrophil count from the group of patients reported in the Wang et al. study [1], the present group showed higher counts than the non–intensive care unit (ICU) group and counts similar to those of the ICU group (Table 3). As for subsets of peripheral lymphocyte, both CD4+ and CD8+ T lymphocyte subsets decreased significantly, and the ratio of CD4+ to CD8+ T cells decreased in some patients (data not shown). The eosinophil count of most patients (12/14, 86%) was 0. Although red blood cell counts ranged from normal to a mild decrease, hematocrit decreased obviously.
Table 2.
Laboratory Tests in 14 Fatal Cases of SARS-CoV-2
| Patient | WBC, ×109/L | Neutrophil, ×109/L | Lymphocyte, ×109/L | HCT, % | Eosinophil, ×109/L | AST, U/L | ALT, U/L | GGT, U/L | LDH, U/L | FBG, mmol/L | C1q, mg/L | IL-10, pg/mL | IL-6, pg/mL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal range | 3.5–9.5 | 1.8–6.3 | 1.1–3.2 | 35–45 | 0.02–0.52 | 13–35 | 7–45 | 8–57 | 125–243 | 3.9–6.1 | 159–233 | 0.1–5.0 | 0.1–2.9 |
| 1 | 16.5 | 15.3 | 0.6 | 34.8 | 0.0 | 49.0 | 33.5 | 232.5 | 402.0 | 7.4 | 246.1 | 33.1 | 101.2 |
| 2 | 4.3 | 3.9 | 0.3 | 35.2 | 0.0 | 46.0 | 30.5 | 45.0 | 23.0 | 129.1 | |||
| 3 | 11.5 | 10.2 | 0.8 | 30.2 | 0.0 | 680.5 | 276.5 | 44.0 | 1592.5 | 7.0 | 130.9 | ||
| 4 | 5.4 | 3.4 | 1.7 | 36.0 | 0.0 | 58.0 | 44.0 | 40.0 | 9.6 | ||||
| 5 | 12.7 | 11.8 | 0.4 | 43.7 | 0.0 | 54.0 | 22.5 | 30.0 | 683.0 | 6.8 | 172.2 | ||
| 6 | 7.5 | 5.9 | 1.1 | 32.7 | 0.0 | 18.5 | 12.5 | 15.0 | 168.0 | 6.0 | 176.9 | 54.8 | |
| 7 | 5.5 | 4.8 | 0.6 | 29.7 | 0.0 | 101.0 | 40.0 | 133.0 | 5.8 | ||||
| 8 | 2.6 | 2.0 | 0.4 | 29.0 | 0.0 | 57.0 | 26.0 | 36.0 | 254.0 | 6.0 | 441.5 | 119.7 | |
| 9 | 13.7 | 10.3 | 2.7 | 43.8 | 0.0 | 81.5 | 52.0 | 106.5 | 18.3 | 54.5 | 260.6 | ||
| 10 | 4.2 | 3.3 | 0.6 | 39.1 | 0.0 | 18.0 | 13.0 | 12.0 | 10.8 | 100.1 | 128.3 | ||
| 11 | 4.1 | 3.6 | 0.3 | 40.5 | 0.0 | 66.0 | 38.0 | 81.0 | 277.0 | 11.6 | 274.5 | 167.0 | |
| 12 | 4.3 | 3.9 | 0.3 | 38.4 | 0.0 | 46.0 | 30.5 | 45.0 | 9.9 | 138.9 | |||
| 13 | 15.4 | 11.7 | 2.7 | 18.7 | 0.1 | 28.0 | 32.0 | 43.0 | 6.3 | 173.4 | 23.5 | ||
| 14 | 23.5 | 21.7 | 1.2 | 39.8 | 0.1 | 52.5 | 38.5 | 67.5 | 10.4 | 140.5 | |||
| Mean | 9.4 | 8.0 | 1.0 | 35.1 | 0.0 | 96.9 | 49.3 | 66.5 | 562.8 | 9.9 | 185.4 | 33.1 | 124.2 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; FBG, fasting blood glucose; GGT, gamma-glutamyl transpeptidase; HCT, hematocrit; IL, interleukin; LDH, lactate dehydrogenase; WBC, white blood cell.
Table 3.
Comparison of Laboratory Parameters Between This Cohort and Groups in the Wang et al. [1] Study
| Normal Range | ICU (n = 36) | Non-ICU (n = 102) | Alive (n = 138) | Fatal (n = 14) | P Value (Overall) | P Value (ICU) | P Value (Non-ICU) | |
|---|---|---|---|---|---|---|---|---|
| White blood cell count, ×109/L | 3.5–9.5 | 6.6 (3.6–9.8) | 4.3 (3.3–5.4) | 4.5 (3.3–6.2) | 9.4 (2.6–23.5) | .0243* | .3098 | .0125* |
| Neutrophil count, ×109/L | 1.8–6.3 | 4.6 (2.6–7.9) | 2.7 (1.9–3.9) | 3.0 (2.0–4.9) | 8.0 (2.0–21.7) | .0183* | .2177 | .0066* |
| Lymphocyte count, ×109/L | 1.1–3.2 | 0.8 (0.5–0.9) | 0.9 (0.6–1.2) | 0.8 (0.6–1.1) | 1.0 (0.3–2.7) | .5948 | .3489 | .7863 |
| Alanine aminotransferase, U/L | 9–50 | 24 (16–40) | 35 (19–57) | 23 (15–36) | 49.3 (12.5–276.5) | .2097 | .2549 | .5675 |
| Aspartate aminotransferase, U/L | 15–40 | 31 (24–51) | 52 (30–70) | 29 (21–38) | 96.9 (18–680.5) | .1616 | .2028 | .3394 |
| Lactate dehydrogenase, U/L | 125–243 | 261 (182–403) | 435 (302–596) | 212 (171–291) | 562.8 (168–1592.5) | .1845 | .2657 | .6309 |
*P < .05.
As for biochemical parameters or organ functional parameters, a common finding was elevated LDH in most patients tested (5/6). Eleven (79%) showed elevated AST, 2 (14%) showed elevated ALT, and 5 (36%) showed elevated GGT, which reflects function of biliary epithelial cells. Eleven (79%) showed elevated fasting blood glucose.
Of interest, analyses of cytokines and complement showed increased release in tested patients. Specifically, interleukin (IL)-6 and IL-10 were elevated in all the patients tested, and C1q was elevated in 3 patients tested. Other chemokines or cytokines such as IL-4 and tumor necrosis factor decreased (not shown).
Culture Results
Pharyngeal swab test for mycoplasma infection was positive in some patients. It was common to detect bacterial colonies in urine samples. These may suggest secondary infections due to impaired immunity and the use of antibiotics (not shown).
Discussion
SARS-CoV-2 is the seventh member of the family Coronaviridae that infects human [6]. It shares similarity with MERS-CoV and SARS-CoV in gene sequences but is different from the latter in many regards [5]. It is believed that SARS-CoV-2 is less pathogenic than SARS-CoV but stronger in transmission competence [7]. The pathogenic mechanism remains poorly understood. We attempted to gain insight into the pathogenesis through an analysis of available clinical data on fatal cases and literature review, partly relying on the homology of SARS-CoV-2 with SARS.
From this cohort of fatal cases, it is evident that male gender and advanced age predominate (12/14, 86%). This is consistent with previous reports [2]. Most of the patients had underlying diseases before the infection, which likely contribute to risk for death. Radiographically, these patients presented with bilateral pneumonia and severe abnormalities on chest CT images, indicating another hint for a worse prognosis. Of note, some patients showed improvements radiographically during the clinical course, but deteriorated abruptly later on, as in case 1.
Elevation of WBC indicates bacterial infection, and viral infection causes lymphocytopenia. Some patients in this cohort exhibited both elevated WBC and lowered lymphocyte count. A number of potential mechanisms may have been involved. First, SARS-CoV-2 infection may affect hematopoietic stem/progenitor cells via CD13 or CD66a directly or through development of auto-antibodies or immune complexes, as seen in SARS [8]. Atrophy of bone marrow hematopoietic tissue has been seen in SARS and COVID-19 [9, 10]. Second, glucocorticoid treatment can induce lymphocytopenia in some patients [8]. Interestingly, case 14 had no fever during her stay in the hospital. The CBC profile from the first day postop showed high WBC counts and lymphocytopenia before the positive CT and nuclear acid test, suggesting that this CBC profile may serve as a good clue for early diagnosis in the future. Third, as seen in another study of ours [11], superimposed bacterial infection can be seen a portion of patients, which can lead to elevation of WBC.
Homeostasis and a normal immune system are critical in controlling acute viral infection. By dynamically screening 14 cytokines/chemokines in blood, Jiang et al. found that levels of the proinflammatory cytokines IL-6, IL-8, and monocyte chemoattractant protein–1 increased in the patients with superinfection, and the mRNAs for these cytokines were also increased in lung tissues [12]. These observations suggest that elevation of these cytokines/chemokines is a sign of superinfection and may be related to a high risk for death [12]. Complement is also a major component of innate immunity. In a mouse model, mice deficient in complement C3 had less weight loss and reduced pathology, improved respiratory function, and lower levels of inflammatory cytokines/chemokines in the lung and peripheral blood after SARS-CoV infection, as compared with wild-type mice [13]. Therefore, it is suggested that inhibiting complement signaling may serve as a strategy for immune therapy. Other theories suggest that lack of antiviral cytokine response such as interferon alpha, IFN-beta, IFN-gamma, and interleukin 12p40 may represent a mechanism of immune evasion by SARS-CoV [14]. The elevated IL-6 and a component of complement C1q in our study suggest that innate immunity play an important role in COVID-19 pathogenesis. Dynamic screening of the level of these cytokines and component of the complement system will be helpful for evaluating prognosis and clinical treatment.
Another important player in the pathogenesis of SARS-CoV-2 is angiotensin-converting enzyme (ACE2), which has been recognized as the functional receptor for both SARS-CoV and SARS-CoV-2 [5, 7, 15]. ACE2 mRNA and protein are known to be present in virtually all organs (respiratory system, gastrointestinal tract, skin, lymph nodes, thymus, bone marrow, spleen, liver, kidney, and brain) [15]. The surface expression of ACE2 protein in alveolar epithelial cells and gastrointestinal epithelial cells provides the route of viral entry [16]. These routes are also related to symptoms such as nonproductive cough, dyspnea, and diarrhea. We suspect that there are higher densities of ACE2 in lung alveolar epithelial cells in male patients than in female patients, which may be the underlying reason for higher morbidity in males. In addition, elevated fasting blood glucose, GGT, and LDH can be closely related to impairment of the microenvironment of the infected tissue and the damage to target cells such as beta cells in the islets, biliary epithelial cells in the liver, and myocardium cells in the heart through ACE2 and the subsequent disruption of different feedback loops [17, 18]. The presence of ACE2 in bone morrow tissue can be one of the causes of the hematological changes described above. SARS-CoV-2 may also aggravate the condition of patients with hypertension through the ACE2 in human vascular endothelia and lead to a worse prognosis [16].
Of note, patient 9 was a 36-year-old male with no medical history. He had a rather short course of illness (14 days) before he died of septic shock. Although no underlying disease was identified, he did have high FBG of 18.3, suggesting an unrecognized diabetic status. He also tested 260.6 for IL-6.
In conclusion, we analyzed a cohort of patients who died of severe COVID-19 for clinical and laboratory features. Male gender, advanced age, and underlying diseases appear to be most common associations with fatal outcome of this disease. Most of the patients had lymphopenia and elevated levels of IL-6 in their blood. Further studies including more cases and ancillary tests and animal models are necessary to elucidate the mechanism of disease progression and fatal outcome.
Acknowledgments
Potential conflicts of interest. We declare that no author has a potential conflict of interest or funding source. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yingjie Wu WG, Liu H, Qi B, et al. . Clinical outcomes of 402 patients with COVID-2019 from a single center in Wuhan, China. medRxiv. doi: 10.1101/2020.03.07.20032672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan JF, Yuan S, Kok KH, et al. . A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou P, Yang XL, Wang XG, et al. . A pneumonia outbreak associated with a new coronavirus of probable bat origin Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu N, Zhang D, Wang W, et al. . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian HY. 2019-nCoV: new challenges from coronavirus [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi 2020; 54:E001. [DOI] [PubMed] [Google Scholar]
- 8. Yang M, Hon KL, Li K, et al. . The effect of SARS coronavirus on blood system: its clinical findings and the pathophysiologic hypothesis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2003; 11:217–21. [PubMed] [Google Scholar]
- 9. Ding Y, Wang H, Shen H, et al. . The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003; 200:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Xie J, Zhao L, et al. . Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Researchsquare. doi: 21203/rs.3.rs-19346/v1. [Google Scholar]
- 11. Tian S, Xiong Y, Liu H, et al. . Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Mod Pathol. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang Y, Xu J, Zhou C, et al. . Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med 2005; 171:850–7. [DOI] [PubMed] [Google Scholar]
- 13. Gralinski LE, Sheahan TP, Morrison TE, et al. . Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 2018; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Law HK, Cheung CY, Ng HY, et al. . Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 2005; 106:2366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamming I, Timens W, Bulthuis ML, et al. . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ge XY, Li JL, Yang XL, et al. . Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013; 503:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xuan X, Gao F, Ma X, et al. . Activation of ACE2/angiotensin (1–7) attenuates pancreatic beta cell dedifferentiation in a high-fat-diet mouse model. Metabolism 2018; 81:83–96. [DOI] [PubMed] [Google Scholar]
- 18. Yuan L, Wang Y, Lu C, Li X. Angiotensin-converting enzyme 2 deficiency aggravates glucose intolerance via impairment of islet microvascular density in mice with high-fat diet. J Diabetes Res 2013; 2013:405284. [DOI] [PMC free article] [PubMed] [Google Scholar]