Abstract
Background
During the ongoing COVID-19 pandemic, contact with the health care system for cancer treatment can increase risk of infection and associated mortality. Treatment recommendations must consider this risk for elderly and vulnerable cancer patients. We reanalyzed trials in elderly glioblastoma (GBM) patients, incorporating COVID-19 risk, in order to provide a quantitative framework for comparing different radiation (RT) fractionation schedules on patient outcomes.
Methods
We extracted individual patient-level data for 1321 patients from Kaplan–Meier curves from 5 randomized trials on treatment of elderly GBM patients including available subanalyses based on O6-methylguanine-DNA methyltransferase (MGMT) methylation status. We simulated trial data with incorporation of COVID-19–associated mortality risk in several scenarios (low, medium, and high infection and mortality risks). Median overall survival and hazard ratios were calculated for each simulation replicate.
Results
Our simulations reveal how COVID-19–associated risks affect survival under different treatment regimens. Hypofractionated RT with concurrent and adjuvant temozolomide (TMZ) demonstrated the best outcomes in low and medium risk scenarios. In frail elderly patients, shorter courses of RT are preferable. In patients with methylated MGMT receiving single modality treatment, TMZ-alone treatment approaches may be an option in settings with high COVID-19–associated risk.
Conclusions
Incorporation of COVID-19–associated risk models into analysis of randomized trials can help guide clinical decisions during this pandemic. In elderly GBM patients, our results support prioritization of hypofractionated RT and highlight the utility of MGMT methylation status in decision making in pandemic scenarios. Our quantitative framework can serve as a model for assessing COVID-19 risk associated with treatment across neuro-oncology.
Key Points
• Re-analysis of randomized controlled trials in COVID-19 era gives insight on optimal treatment of GBM.
• Hypofractionated RT or temozolomide alone may be reasonable options in high risk pandemic settings.
• A quantitative framework incorporating COVID-19 risks can be applied across neuro-oncology.
Keywords: COVID-19, elderly, glioblastoma , randomized controlled trials
Importance of the Study.
During the COVID-19 global pandemic, cancer care remains an essential clinical need, especially for patients with aggressive tumors such as glioblastoma. For vulnerable elderly cancer patients, there is also a risk of infection associated with treatment and contact with the health care system. We have developed a quantitative framework for incorporating COVID-19–associated risk into results from seminal randomized trials in management of glioblastoma in the elderly. Our results provide guidance on the optimal treatment options under various pandemic scenarios and provide an approach that can be applied to guide treatment decisions across neuro-oncology during this pandemic.
With rapid spread of COVID-19 cases around the world, the World Health Organization declared a worldwide pandemic on March 11, 2020.1 During this global pandemic, cancer care remains an essential clinical need, but cancer patients are particularly vulnerable to infections due to their immunocompromised state.2 Initial reports of COVID-19 suggest that, relative to the general population, patients with a history of cancer have a higher incidence of infection with severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) and higher risk of severe COVID-19–associated events.3
Radiation therapy is an important component of cancer therapy, and standard treatments require daily visits to a health care environment over several weeks. Radiation oncology departments have therefore quickly developed and implemented protocols to minimize risks of COVID-19 to patients.4,5 While the use of the shortest possible course of radiation for patients has been advocated by the American Society for Radiation Oncology,6 abbreviated courses of treatment may require trade-offs in cancer control or toxicity compared with standard courses. In this study we develop a quantitative framework for incorporating COVID-19–associated risk to characterize the effects of this trade-off on patient outcomes and applied this framework to elderly patients with glioblastoma (GBM).
GBM is an incurable primary brain tumor, and there is no consensus on the optimal adjuvant treatment of elderly GBM patients given worse outcomes and concerns of treatment-related toxicity.7,8 During a pandemic, treatment recommendations require careful thought, as these older patients are also at highest risk of COVID-19–associated mortality.9 Prospective, randomized trials provide evidence for treatment regimens that incorporate hypofractionated radiation (higher dose per fraction for fewer fractions) or chemotherapy alone with omission of radiation.10 An emerging challenge has been understanding the risks and benefits of these regimens and possible effects on clinical outcomes of patients in the setting of a pandemic.
The development of a mathematical framework allows for a quantitative analysis to weigh competing risks and inform treatment decision making. With this in mind, we reviewed seminal trials in elderly GBM patients and re-analyzed trials with extracted individual patient-level data (IPLD) and incorporation of the risk of SARS-CoV-2 infection and COVID-19 mortality to evaluate how decisions on radiation regimen may affect clinical outcomes.
Methods
Study Inclusion
We included published randomized trials identified by recent meta-analyses as randomized trials that inform the treatment of elderly GBM patients.8,10 To be included, publications required Kaplan–Meier survival curves with at-risk tables and reporting of total number of death events. We included the Nordic randomized trial (International Standard Randomised Controlled Trial #ISRCTN81470623),11 the Canadian Cancer Trials Group (CCTG)/European Organisation for Research Treatment of Cancer (EORTC) trial (NCT00482677),12 the German NOA-08 trial (NCT01502241),13 a French trial evaluating radiation versus supportive care (NCT00430911),14 and a Canadian hypofractionation trial (Table 1).15 We did not include the International Atomic Energy Agency hypofractionation trial16 because the published Kaplan–Meier survival curves did not include at-risk tables. We did not include EORTC/National Cancer Institute of Canada Clinical Trials Group trial sub-analysis of elderly GBM patients17 because Kaplan–Meier figures were not included for this subset of patients in the publication. For each trial, trial population, design, sample size, primary endpoint(s), reported hazard ratio (HR) and outcome measures were extracted.
Table 1.
Randomized trials evaluating adjuvant therapy in elderly GBM patients included in analysis
| Trial Registration | NCT00482677 | ISRCTN81470623 | NCT01502241# | NCT00430911 | |
|---|---|---|---|---|---|
| Publication | Perry et al. 2017 (NEJM) | Malmstrom et al. 2012 (Lancet Onc) | Wick et al. 2012 (Lancet Onc) | Keime-Guibert et al. 2007 (NEJM) | Roa et al. 2004 (JCO) |
| PubMed ID | 28296618 | 22877848 | 22578793 | 17429084 | 15051755 |
| Study years | 2007–2013 | 2000–2009 | 2005–2009 | 2001–2007 | 1996–2001 |
| Patients (n) | 562 | 291 | 373 | 81 | 95 |
| Sex | |||||
| Male | 61% | 59% | 47% | 63% | 58% |
| Female | 39% | 41% | 53% | 37% | 42% |
| Age, minimum | 65 | 60 | 65 | 70 | 60 |
| Age, median (range) | 73 (65–90)+ | 70 (60–83)* | 72 (66–84) | 73 (70–85) | 72 (mean) |
| Performance status, min | ECOG 2 | ECOG 2 | KPS 60 | KPS 70 | KPS 50 |
| Performance status, median | ECOG 1 (0–1, 77%) | ECOG 1 (0–1, 77%) | KPS 80 | KPS 70 | KPS 70 |
| MGMT status | |||||
| MGMT methylated | 47% (165/354) | 45% (91/203) | 35% (73/209) | N/A | N/A |
| MGMT unmethylated | 53% (189/354) | 55% (112/203) | 65% (136/209) | N/A | N/A |
| Unknown | 208 (37%) | 88 (30%) | 164 (44%) | 81 (100%) | 95 (100%) |
| Extent of resection | |||||
| Biopsy | 32% | 26% | 39% | 52% | 39% |
| Partial or complete resection | 68% | 74% | 61% | 48% | 61% |
| Randomization | RT-15 + TMZ vs RT-15 | RT-30 vs RT-10 vs TMZ | RT-30 vs TMZ | RT-28 versus supportive care | RT-30 vs RT-15 |
#Included GBM and anaplastic astrocytoma.
+Age 65–70: n = 82; age 71–75: n = 114; age 76+: n = 85.
*Age 60–70: n = 125; age 70+: n = 117.
RT-30 = 6 weeks [30 fractions] of RT (total dose 60 Gy).
RT-28 = 5.5 weeks [28 fractions] of RT (total dose 50.4 Gy).
RT-15 = 3 weeks [15 fractions] of RT (total dose 40.05 Gy).
RT-10 = 2 weeks [10 fractions] RT (total dose 34 Gy).
N/A = Not applicable.
IPLD Reconstruction
IPLD were extracted from published Kaplan–Meier curves for overall survival (OS) for total cohort and O6-methylguanine-DNA methyltransferase (MGMT) methylation status subgroups, as previously described.18–20 Outcome measures and Kaplan–Meier figures from reconstructed datasets were compared with original publications for quality assurance (Supplementary Figure 1A–E).
Incorporation of COVID-19 Mortality
We simulated COVID-19 mortality based on 2 parameters, the risk of SARS-CoV-2 infection per fraction of radiation received (i), and the risk of death from COVID-19 for patients who are infected (d).i was assumed to be a constant, per-fraction risk, independent of prior fractions. Our model did not incorporate infection risk for other treatment courses, beyond the risk per radiation fraction.
For each patient in a replicate dataset, we simulated the radiation fraction at which that patient was infected as a geometric random variable parametrized by i. Those patients for whom this value was less than the total number of fractions they received were considered to have been infected by SARS-CoV-2 during treatment (c). We then simulated the number of deaths caused by COVID-19. We first simulated Nd in this group by drawing from a binomial distribution with n = c and death rate p = d, and selected Nd individuals randomly from the pool of infected patients. These Nd individuals had their survival time adjusted as follows: We assumed a period of ~4 weeks between diagnosis and start of radiation, and added the number of fractions on treatment until infection (based on the simulations above). We added a 5-day incubation period, and simulated time from symptom onset to death as a gamma random variable with mean 18 days and shape parameter 1.0. These estimates are based on published reports of a 5-day incubation period21 and mean time of 18 days from symptom onset to death.22 The date of death in the reconstructed dataset acts as censoring time of death for COVID-19.
Parameter Values
Risk of COVID-19–associated mortality appears to depend on both age and comorbidity profile, with a range of mortality rates reported in the literature. The median age in all 5 trials was between 70 and 80 years (Table 1). Available estimates suggest an infection mortality rate of ~4.3% in this age group in the general population.22 Infection mortality rate is distinct from case fatality rate in that it includes mild and asymptomatic infections that are not tested or diagnosed. Cancer patients, however, have a higher risk of COVID-19–associated mortality compared with the general population. The rate of a composite endpoint of admission to the intensive care unit, mechanical ventilation, or death was ~2.5-fold higher in patients with comorbidities, including cancer (15% vs 6%) in a publication by Guan et al,23 and the case fatality rate in cancer patients was similarly ~2.4-fold higher (5.6% vs 2.3%) in a report by Wu et al.9 Furthermore, Liang et al reported an increase of ~5-fold in rate of severe events among cancer patients (39% vs 7.9%),3 and Zhang et al reported a COVID-19 mortality rate of ~30% in a cohort of 28 cancer patients.24
We simulated scenarios with COVID-19 fatality rates reflecting this range and felt to be representative of the 5 trials included in this analysis, each with a median age in the 70–80 years old age range. Specifically, for our low risk scenarios we assumed a fatality risk of 10%, which is approximately 2.3-fold higher than the estimated infection fatality rate of 4.3% in the general population. For medium and high risk scenarios, we increased this to 20% and 30%, respectively, which may better reflect other reports published in the literature. This parameter can be modified when applying our model to a different trial or to a specific patient to reflect the expected risks associated with a different age group.
We modeled low, medium, and high infection risk scenarios as those with risk of 1%, 5%, and 10%, respectively, of COVID-19 infection per fraction. Data are currently lacking to estimate this risk, but with development of “hotbeds” of infection, the daily risk is likely dynamic, varying widely based upon location and temporal trends of pandemic spread. It is possible that during the peak of an uncontrolled pandemic at a hospital overwhelmed with patients positive for COVID-19, this risk may exceed 10% per visit to the hospital.
Simulation Procedure
We generated simulation replicates for each scenario by sampling with replacement from each arm of a given trial. One thousand replicates were generated for each scenario. For each replicate, mortality from COVID-19 was simulated per the procedure described above.
Statistical Analysis
For each replicate, we performed Cox proportional hazards modeling to obtain estimates of the HRs and 95% CIs. For each scenario, we reported the median of the HR and upper and lower 95% CI bounds across 1000 bootstrap replicates.
Results
We examined the effects of SARS-CoV-2 infection and COVID-19 mortality in a range of different scenarios, with daily infection risks of 1%, 5%, 10% and case fatality rates of 10%, 20%, and 30% (see Methods). For the purposes of illustration, we considered a scenario with 1% daily risk and 10% mortality as low risk, 5% daily risk and 20% mortality as medium risk, and 10% daily risk and 30% mortality as high risk. A low risk scenario might be one where SARS-CoV-2 is circulating in the community but where the number of cases is far from the peak and where the health care system is not overwhelmed. A medium risk scenario might be one during the upslope of the curve, with higher numbers of cases and increased hospitalizations. A high risk scenario would be during the peak of a pandemic, with an overwhelmed health care system and limited resources. Table 2 summarizes median survival and associated HRs in the scenarios of low, medium, and high risk for each trial.
Table 2.
Overall survival from randomized trials of elderly GBM patients in setting of COVID-19–associated mortality
| Study Author, Year of publication | Arms | Hazard Ratio (95% CI) | Median OS, mo (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Low Risk | Medium Risk | High Risk | Baseline | Low Risk | Medium Risk | High Risk | ||
| Malmstrom et al, 2012 | RT-30 (ref) | – | – | – | – | 6.11 (5.21–7.66) | 6.05 (5.08–7.66) | 5.21 (4.04–6.43) | 3.96 (2.90–5.73) |
| RT-10 | 0.86 (0.65–1.15) | 0.86 (0.64–1.14) | 0.81 (0.61–1.08) | 0.80 (0.60–1.06) | 7.60 (6.59–9.18) | 7.53 (6.59–9.04) | 7.28 (5.95–8.41) | 6.15 (5.14–7.66) | |
| TMZ | 0.71 (0.53–0.95) | 0.69 (0.52–0.93) | 0.61 (0.46–0.82) | 0.54 (0.40–0.73) | 8.27 (6.96–9.70) | 8.12 (6.96–9.70) | 8.27 (6.96–9.70) | 8.27 (6.96–9.70) | |
| Perry et al, 2017 | RT15 (ref) | – | – | – | – | 7.60 (7.04–8.48) | 7.56 (6.97–8.33) | 6.97 (5.96–7.65) | 5.62 (4.48–6.70) |
| RT15 + TMZ | 0.66 (0.55–0.78) | 0.66 (0.55–0.79) | 0.69 (0.58–0.82) | 0.73 (0.61–0.86) | 9.32 (8.35–10.4) | 9.23 (8.25–10.3) | 8.31 (7.56–9.65) | 7.13 (5.84–8.14) | |
| Roa et al, 2004 | RT-30 (ref) | – | – | 5.49 (4.19–8.36) | 5.17 (4.19–8.36) | 4.24 (2.95–6.80) | 3.40 (2.20–5.70) | ||
| RT-15 | 0.92 (0.59–1.41) | 0.90 (0.58–1.39) | 0.88 (0.57–1.36) | 0.91 (0.59–1.39) | 5.71 (4.68–8.36) | 5.71 (4.68–8.36) | 5.24 (3.12–8.06) | 4.26 (2.39–5.84) | |
| Wick et al, 2012 | RT-30 (ref) | – | – | – | – | 9.41 (8.16–10.7) | 9.25 (7.90–10.3) | 7.61 (6.49–9.48) | 6.10 (4.20–7.84) |
| TMZ | 1.09 (0.84–1.42) | 1.05 (0.81–1.36) | 0.85 (0.66–1.09) | 0.68 (0.53–0.87) | 8.46 (7.44–10.4) | 8.43 (7.15–10.0) | 8.46 (7.44–10.4) | 8.46 (7.44–10.0) | |
| Keime-Guibert et al, 2007 | SC (ref) | – | – | – | – | 3.61 (2.89–4.87) | 3.61 (2.89–4.87) | 3.61 (2.89–4.87) | 3.64 (2.89–4.87) |
| RT-28 | 0.45 (0.28–0.73) | 0.46 (0.29–0.74) | 0.55 (0.34–0.88) | 0.66 (0.42–1.06) | 6.50 (5.60–8.03) | 6.50 (3.90–8.03) | 6.27 (2.89–7.76) | 3.21 (2.37–7.13) |
Baseline: Estimates from reconstructed IPLD, without incorporation of COVID-19 risk.
Low Risk: Case fatality rate = 10%, infection risk per fraction = 1%.
Medium Risk: Case fatality rate = 20%, infection risk per fraction = 5%.
High Risk: Case fatality rate = 30%, infection risk per fraction = 10%.
ref: Reference arm.
SC: Supportive Care.
The Nordic Trial
The Nordic Clinical Brain Tumour Study Group trial (International Standard Randomised Controlled Trial #ISRCTN81470623) was designed to evaluate the optimum treatment in GBM patients 60 years or older, and it compared standard radiation (RT-30, 60 Gy in 30 fractions), hypofractionated radiation (RT-10, 34 Gy in 10 fractions), and temozolomide (TMZ) (200 mg/m2 on days 1–5 of every 28 days up to 6 cycles).11 In our reconstruction, we obtained an HR of 0.86 (ref: RT-30, 95% CI: 0.65–1.15) for RT-10 and HR of 0.71 (ref: RT-30, 95% CI: 0.53–0.95) for TMZ, which were nearly identical to published values (Table 1, Supplementary Figure 1).
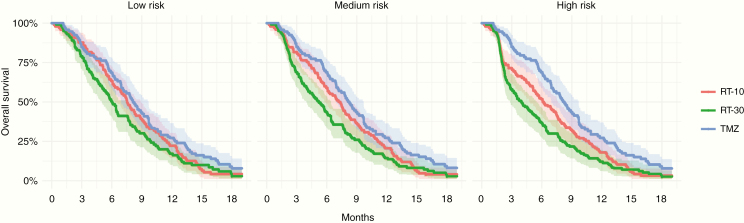
Fig. 1 demonstrates the effects on survival in low risk, medium risk, and high risk COVID-19 pandemic scenarios. We furthermore evaluated a 1%, 5%, or 10% daily risk of infection and COVID-19 fatality rates of 10%, 20%, or 30% (Supplementary Figure 2). In a low-risk scenario with 10% COVID-19–associated mortality and 1% infection risk per fraction, results remain similar, with no significant difference between RT-10 and RT-30 regimens (HR, 0.86; 95% CI: 0.64–1.14), while TMZ remains superior to RT-30 (HR, 0.69; 95% CI: 0.52–0.93). In the medium risk scenario (20% fatality rate, 5% infection risk), a hypofractionated regimen (RT-10) trends toward a benefit over a standard radiation course (HR, 0.81; 95% CI: 0.61–1.08). In the high risk scenario, TMZ alone has a greater magnitude of treatment effect over standard radiation (HR, 0.54; 95% CI: 0.40–0.73) and hypofractionated radiation (HR, 0.68; 95% CI: 0.51–0.92).
Fig. 1.
Survival curves from simulations of the Nordic trial with low risk, medium risk, and high risk COVID-19 pandemic scenarios. One thousand replicates were generated for each scenario, and the shaded bands represent the upper and lower 95% CI bounds for Kaplan–Meier estimates across the replicates.
CCTG/EORTC Hypofractionated Chemoradiation versus Hypofractionated Radiation Trial
The CCTG/EORTC trial (ClinicalTrials.gov identifier NCT00482677) compared hypofractionated radiation with concurrent and adjuvant TMZ (RT-15-TMZ) with hypofractionated radiation (RT-15) among elderly GBM patients 65 years or older.12 Reconstructed IPLD in our analysis estimated a HR of 0.66 (95% CI: 0.55–0.78) (Supplementary Figure 3), nearly equal to published values. Both arms of this trial incorporated 15 fractions of radiation therapy, and the overall results did not significantly change with varying COVID-19 risk, but the median survival with RT-15-TMZ decreased from 9.3 months (95% CI: 8.4–10.4) to 7.1 months (95% CI: 5.8–8.1) in the high risk scenario.
German NOA-08
The German NOA-08 study (NCT01502241) evaluated the use of standard radiation (RT-30) versus TMZ in patients 65 years or older with anaplastic astrocytoma or GBM, and reported no significant difference with respect to OS or event-free survival.13 Our reconstructed IPLD matched the published HR estimate of 1.09 (95% CI: 0.84–1.42) for OS. With increasing COVID-19–associated mortality, TMZ alone appears more favorable relative to standard radiation therapy, with HR, 0.68 (0.53–0.87) in the high risk wscenario (Table 2, Supplementary Figure 4). This is similar to findings from our analysis of the Nordic trial.
Canadian Hypofractionation versus Standard Radiation Trial
The Canadian hypofractionation trial established the use of hypofractionated radiation over 3 weeks as a comparable treatment for elderly GBM patients. In this trial of patients aged 60 years or older with KPS ≥50, there was no significant difference in survival among patients receiving standard radiation (RT-30) compared with hypofractionated radiation over 3 weeks (RT-15).15 Chemotherapy was not incorporated in upfront adjuvant therapy in this trial.
In our analysis, outcomes remained poor regardless of treatment or simulated COVID-19–associated risk in this small trial population of frail and elderly patients, without a significant difference across risk scenarios (ref RT-15, HR, 0.91; 95% CI: 0.59–1.39 in high risk scenario) (Supplementary Figure 5). The median survival was less than 6 months in both arms in the original publication and estimated to be 4–5 months with the incorporation of high COVID-19–associated risk.
Radiation Therapy versus Supportive Care Trial
The French randomized trial by Keime-Guibert et al (NCT00430911) evaluated the use of fractionated radiation over 5.5 weeks compared with supportive care alone.14 Reconstructed IPLD estimated an HR of 0.45, which matches the published point estimate. In scenarios with incorporation of COVID-19–associated risk (Supplementary Figure 6), radiation therapy was associated with a survival benefit compared with supportive care alone, although the magnitude of survival benefit was reduced with increasing COVID-19 risk (HR, 0.66; 95% CI: 0.42–1.06 in high risk scenario).
MGMT Methylation Status
The Nordic and CCTG/EORTC trials included results stratified by MGMT methylation status that we reconstructed. Patients receiving RT-10 and RT-30 were combined in the Nordic trial for these analyses.
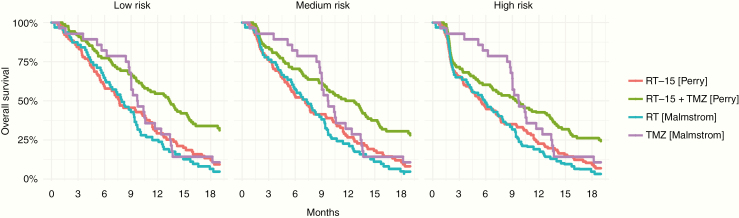
Among MGMT methylated patients, TMZ had a greater magnitude of benefit relative to radiation therapy monotherapy in the Nordic trial with increasing COVID-19–associated mortality with statistical superiority in the high risk scenario (HR, 0.57; 95% CI: 0.36–0.91) (Table 3, Fig. 2). In the CCTG/EORTC trial, median survival decreased from 13.4 to 9.5 months going from low to high risk scenarios with hypofractionated chemoradiation. Numerically, however, this remained higher than outcomes in patients with single modality treatment in the Nordic study.
Table 3.
Overall survival by MGMT status from 2 randomized trials of elderly GBM patients in setting of COVID-19–associated mortality
| MGMT Status | Study | Arm | Hazard Ratio (95% CI) | Median OS, mo (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Low Risk | Medium Risk | High Risk | Baseline | Low Risk | Medium Risk | High Risk | |||
| Methylated | Perry et al. 2017 | RT–15 (ref) | – | – | – | – | 7.68 (5.94–11.1) | 7.67 (5.94–11.1) | 7.11 (5.22–10.3) | 5.44 (3.52–7.73) |
| RT–15 + TMZ | 0.52 (0.38–0.72) | 0.52 (0.38–0.73) | 0.55 (0.39–0.76) | 0.60 (0.43–0.84) | 13.45 (10.5–15.6) | 13.40 (10.3–15.6) | 12.20 (9.34–14.6) | 9.47 (6.51–13.5) | ||
| Malmstrom et al. 2012 | RT* (ref) | – | – | – | – | 8.21 (6.67–9.58) | 7.95 (6.57–9.58) | 7.31 (5.65–9.34) | 5.91 (3.96–8.27) | |
| TMZ | 0.72 (0.45–1.14) | 0.71 (0.44–1.12) | 0.65 (0.41–1.03) | 0.57 (0.36–0.91) | 9.72 (9.00–13.4) | 9.72 (9.00–13.4) | 9.72 (9.00–13.4) | 9.72 (9.00–13.4) | ||
| Unmethylated | Perry et al. 2017 | RT–15 (ref) | – | – | – | – | 7.92 (7.16–10.2) | 7.90 (7.08–10.1) | 7.53 (6.00–9.00) | 6.05 (4.33–7.90) |
| RT–15 + TMZ | 0.77 (0.57–1.04) | 0.77 (0.57–1.03) | 0.80 (0.59–1.08) | 0.81 (0.60–1.09) | 9.95 (8.41–11.0) | 9.90 (8.24–10.9) | 8.83 (7.68–10.2) | 7.85 (6.06–9.80) | ||
| Malmstrom et al. 2012 | RT* (ref) | – | – | – | – | 6.96 (5.24–9.80) | 6.95 (5.15–9.80) | 6.16 (4.70–8.34) | 4.82 (3.42–6.98) | |
| TMZ | 1.14 (0.77–1.69) | 1.13 (0.76–1.69) | 0.99 (0.66–1.47) | 0.84 (0.57–1.25) | 6.92 (6.06–8.62) | 6.92 (6.06–8.62) | 6.92 (6.06–8.62) | 6.92 (6.17–8.62) |
Baseline: Estimates from reconstructed IPLD, without incorporation of COVID-19 risk.
Low Risk: Case fatality rate = 10%, infection risk per fraction = 1%.
Medium Risk: Case fatality rate = 20%, infection risk per fraction = 5%.
High Risk: Case fatality rate = 30%, infection risk per fraction = 10%.
ref: Reference arm.
*: 10 or 30 fractions
Fig. 2.
Survival curves from simulations for MGMT methylated patients enrolled in the CCTG/EORTC hypofractionated chemoradiation trial and the Nordic trial, under
low risk, medium risk, and high risk COVID-19 pandemic scenarios.
Among MGMT unmethylated patients in the Nordic trial, OS decreased in the radiation arm with increasing risk (median survival, 7.0 mo in lowest risk and 4.8 mo in highest risk scenario), but there was no significant difference compared with TMZ alone. These trends were reflected in the CCTG/EORTC trial as well, where in MGMT unmethylated patients, median survival ranged from 7.9 to 6.1 months with RT alone in the low and high risk scenarios, respectively (Supplementary Figure 7).
Discussion
During the COVID-19 pandemic, contact with the health care system carries a risk of infection and mortality. This is especially relevant for cancer patients receiving radiation therapy, which requires daily contact with health care facilities over a period of several weeks. Clinicians must therefore weigh COVID-19–associated risk when recommending a radiation therapy regimen for GBM patients. The expected effect of COVID-19 on clinical outcomes of patients treated with radiation therapy depends on the risk of becoming infected with SARS-CoV-2 per fraction of radiation received and the risk of death from COVID-19 for patients who are infected. The daily risk of becoming infected by SARS-CoV-2 during radiation therapy is based on a variety of factors that include the prevalence of the pandemic in a certain location, precautions and social distancing employed at local and federal levels, as well as the safety measures taken by a specific radiation oncology facility to ensure the safety of patients and staff. The context in which radiation therapy is delivered can also be a factor. The predominant model for RT delivery in Europe and North America involves daily outpatient visits to a community or academic cancer center, but there is variation globally. The risk of infection with daily treatment will depend more on risk mitigation strategies in the community and outpatient clinics for outpatient models of RT delivery, whereas it will be influenced more by hospital-specific factors for inpatient models of RT delivery. Pertinent to our analysis of elderly GBM patients, initial reports from China have also suggested that the elderly are at highest risk.9 Cancer patients also appear to be at higher risk of SARS-CoV-2 infection and at several-folds higher risk of experiencing severe events (intensive care admission or death) from COVID-19.3
Hypofractionated radiation has been widely encouraged to mitigate risk of infection associated with treatment4,5,25 but without rigorous quantification of risks and benefits of different regimens. Survival analysis and modeling of COVID-19–associated mortality can provide a means to evaluate how decisions can affect patient outcomes. Our study is the first to our knowledge to provide a quantitative assessment of the risks of COVID-19 on patient outcomes, by incorporating COVID-19 risk into the best available evidence from randomized clinical trials. We focused on elderly GBM patients in this work, but our approach could be similarly applied across neuro-oncology.
GBM patients over the age of 65, often frail and with medical comorbidities, are particularly vulnerable to infection and will have very high mortality rates in the setting of a full pandemic and overwhelmed health care resources. While 6 weeks of radiation with concurrent and adjuvant TMZ is established as the standard treatment for younger patients,17 there currently is no consensus on the optimal adjuvant regimen in elderly patients with newly diagnosed GBM, who make up the majority of new GBM diagnoses.26 Of note, while randomized trials have used varying age criteria for inclusion in elderly-focused trials, most included patients 65 years or older (Table 1). For these elderly patients, several alternative regimens have emerged as options, in an effort to improve treatment compliance, reduce acute toxicity, and offer comparable rates of oncologic control.10 Our simulations demonstrate that COVID-19 mortality deleteriously affects survival in more protracted radiation schedules, with the magnitude of effect dependent on the local risk of SARS-CoV-2 infection and COVID-19–associated mortality.
The combination of TMZ with short-course radiation is superior to radiation therapy alone in elderly patients with good performance status with respect to OS without compromising quality of life.12 In our simulations of low and medium COVID-19 risk scenarios, the use of hypofractionated chemoradiation over 3 weeks demonstrates favorable results. This pattern persists when we examine outcomes stratified by MGMT methylation status in the Nordic and CCTG/EORTC trials (Table 3). Thus, with the exception of situations where the risk of COVID-19 is felt to be very high (mortality risk >20% with daily infection risk of >5%), hypofractionated radiation over 3 weeks with TMZ is an excellent option for elderly GBM patients with good performance status. The combination of TMZ with more hypofractionated radiation regimens (25 Gy in 5 fractions or 34 Gy in 10 fractions) is appealing but has not been evaluated in a prospective clinical trial.
For elderly GBM patients who are more frail with moderate or poor performance status, single modality treatment with radiation alone or TMZ alone has been established as a reasonable treatment approach.11,13,27 MGMT methylation status can guide treatment decisions in this setting, and TMZ may be an acceptable alternative to radiation in patients harboring MGMT methylation.7,19,28,29 In our simulations, the benefit of TMZ, relative to hypofractionated or standard radiation, trended favorably with increasing COVID-19–associated risks (Table 3), and is more pronounced in MGMT methylated patients. In high pandemic risk settings, our results suggest that TMZ alone may be an option for frail elderly MGMT methylated patients who are suitable for single modality treatment, but this modality requires further evaluation before widespread use, given the immunosuppressive effects of TMZ. In MGMT unmethylated frail patients, our results suggest that a hypofractionated course of radiation can be used and should be recommended. Both 10-fraction and 15-fraction radiation alone regimens have been evaluated in randomized trials, but there is no prospective comparison of these 2 regimens. In higher risk pandemic settings, 34 Gy in 10 fractions is an appealing option to minimize visits required by patients. We were not able to analyze the International Atomic Energy Agency trial that evaluated a 5-fraction regimen for newly diagnosed GBM,16 which delivers a lower biologic effective dose but could be an option in the setting of high risk and resource strain. In unmethylated MGMT patients, caution would be necessary in considering omission of RT in favor of TMZ, as TMZ can be immunosuppressive and has an unclear benefit in these patients.
In areas of very high infection risk and mortality, there could be an inclination to consider supportive care only for elderly GBM patients. Some groups have gone as far as to recommend consideration of omission of radiation therapy for patients over the age of 65.30 To address this, we examined a French randomized trial that demonstrated that radiation therapy provides a robust survival benefit over supportive care.14 In simulations of low and medium risk pandemic settings, our results support a continued survival advantage with radiation therapy, and underscores the need to treat elderly GBM patients who can tolerate treatment in all but the highest risk pandemic scenarios (Supplementary Figure 6).
In this analysis, we focused on modeling infection risks associated with daily RT, but TMZ is also associated with hematologic toxicity and immunosuppression, though the resulting effect on risk of infection or mortality due to COVID-19 is not currently well characterized. Early data do suggest an elevated risk of adverse events or death among cancer patients receiving chemotherapy compared with the general population.31 Reported toxicity data from published trials can provide estimates of risks associated with TMZ use. In the Nordic trial, a 12% rate of neutropenia and 19% rate of infection/fever was reported with TMZ versus 0% neutropenia and 7% infection/fever rate with hypofractionated RT.11 In the CCTG/EORTC trial, rate of grade 3+ lymphopenia was 27.2% with short-course chemoradiation versus 10.3% with short-course radiation alone.12 It remains unclear how these reported hematologic toxicity rates translate to infection risk during the COVID-19 pandemic. As additional data become available, TMZ-associated risk could be incorporated into our quantitative framework, and it may disproportionately affect chemoradiation regimens that include the risk of both exposure from daily radiation visits and increased susceptibility due to TMZ-associated immunosuppression.
There are several limitations to our analysis. We focused on the possible effects of COVID-19 on patient outcomes but did not address broader implications of cancer care in the setting of a pandemic such as the stress on health care resources, use of personal protective equipment by patients and staff, and the necessity of possible redeployment of health care resources to manage COVID-19 patients. Analyses were based on reconstructed data, and while these are felt to be representative of actual patient level data, known prognostic variables other than MGMT status could not be incorporated. Several assumptions were necessary to model the effect of COVID-19 on patient outcomes, and COVID-19 pathophysiology and associated risks are not yet well understood. For this reason, we focused on several scenarios to generate a general framework that could be tailored to different clinical contexts.
Conclusion
We developed a quantitative framework using published results from randomized clinical trials to incorporate the risk of COVID-19–associated mortality during radiation therapy and quantify the risks and benefits of various treatment regimens under different pandemic scenarios. In elderly GBM patients, we demonstrate that COVID-19–associated risks at the local level should be incorporated in making treatment recommendations. Use of short-course chemoradiation should be prioritized during the pandemic for elderly GBM patients. Monotherapy with hypofractionated RT or TMZ can be considered for frail patients, and MGMT methylation status can be used to guide clinical decision making.
Funding
There were no funding sources for this work.
Conflict of interest statement. B.M.A.: Employment – Foundation Medicine, Inc.; Equity – Hoffman-La Roche; Research support – Puma, Eli Lilly, Celgene. P.W.: Research Support from Agios, Astra Zeneca, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics, VBI Vaccines. Advisory Board for Agios, Astra Zeneca, Bayer, Blue Earth Diagnostics, Deciphera, Elevate Bio, Immunomic Therapeutics, Imvax, Karyopharm, Kiyatec, Puma, QED, Taiho, Vascular Biogenics, VBI Vaccines, Voyager, Tocagen. Speaker for Merck, Prime Oncology. Consultant: Integral Health. D.C.: Research Support from NH Theraguix.
Authorship statement. Study design and analysis: RR and STabrizi. Figure generation: GF. Statistical review and recommendations: LT and SV. Writing of manuscript: RR and STabrizi. Review and revision of manuscript: LT, SV, DC, STanguturi, PYW, BMA. Supervision for project: RR.
Supplementary Material
References
- 1. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19, 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed March 28, 2020.
- 2. Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10(6):589–597. [DOI] [PubMed] [Google Scholar]
- 3. Liang W, Guan W, Chen R, et al. . Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braunstein LZ, Gillespie EF, Hong L, et al. . Breast radiotherapy under COVID-19 pandemic resource constraints—approaches to defer or shorten treatment from a comprehensive cancer center in the United States. Adv Radiat Oncol. 2020;0(0). doi: 10.1016/j.adro.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaorsky NG, Yu JB, McBride SM, et al. . Prostate cancer radiotherapy recommendations in response to COVID-19. Adv Radiat Oncol. 2020;0(0). doi: 10.1016/j.adro.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Summary, COVID-19 Resources, American Society for Radiation Oncology (ASTRO), American Society for Radiation Oncology (ASTRO) ASTRO; https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Summary. Accessed April 4, 2020. [Google Scholar]
- 7. Zarnett OJ, Sahgal A, Gosio J, et al. . Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol. 2015;72(5):589–596. [DOI] [PubMed] [Google Scholar]
- 8. Kalra B, Kannan S, Gupta T. Optimal adjuvant therapy in elderly glioblastoma: results from a systematic review and network meta-analysis. J Neurooncol. 2020;146(2):311–320. [DOI] [PubMed] [Google Scholar]
- 9. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. February 2020. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 10. Nassiri F, Taslimi S, Wang JZ, et al. . Determining the optimal adjuvant therapy for improving survival in elderly patients with glioblastoma: a systematic review and meta-analysis. Clin Cancer Res. January 2020. doi: 10.1158/1078-0432.CCR-19-3359 [DOI] [PubMed] [Google Scholar]
- 11. Malmström A, Grønberg BH, Marosi C, et al. . Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. [DOI] [PubMed] [Google Scholar]
- 12. Perry JR, Laperriere N, O’Callaghan CJ, et al. . Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 2017;376(11):1027–1037. [DOI] [PubMed] [Google Scholar]
- 13. Wick W, Platten M, Meisner C, et al. ; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. [DOI] [PubMed] [Google Scholar]
- 14. Keime-Guibert F, Chinot O, Taillandier L, et al. ; Association of French-Speaking Neuro-Oncologists Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. [DOI] [PubMed] [Google Scholar]
- 15. Roa W, Brasher PM, Bauman G, et al. . Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. [DOI] [PubMed] [Google Scholar]
- 16. Roa W, Kepka L, Kumar N, et al. . International atomic energy agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2015;33(35):4145–4150. [DOI] [PubMed] [Google Scholar]
- 17. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 18. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rahman R, Ventz S, Fell G, Vanderbeek AM, Trippa L, Alexander BM. Divining responder populations from survival data. Ann Oncol. 2019;30(6):1005–1013. [DOI] [PubMed] [Google Scholar]
- 20. Rahman R. Deviation from the proportional hazards assumption in randomized phase 3 clinical trials in oncology: prevalence, associated factors and implications. Clin Cancer Res. 2019. doi: 10.1158/1078-0432.ccr-18-3999 [DOI] [PubMed] [Google Scholar]
- 21. Lauer SA, Grantz KH, Bi Q, et al. . The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. March 2020. doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Verity R, Okell LC, Dorigatti I, et al. . Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020. doi: 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guan W, Ni Z, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang L, Zhu F, Xie L, et al. . Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. March 2020. doi: 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Achard V, Tsoutsou P, Zilli T. Radiotherapy in the time of the coronavirus pandemic: when less is better. Int J Radiat Oncol Biol Phys. 2020. doi: 10.1016/j.ijrobp.2020.03.008 [DOI] [Google Scholar]
- 26. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-Oncol. 2012;14 Suppl 5:v1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wick A, Kessler T, Platten M, et al. . Superiority of temozolomide over radiotherapy for elderly patients with RTK II methylation class, MGMT promoter-methylated malignant astrocytoma. Neuro-Oncol. February 2020. doi: 10.1093/neuonc/noaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 29. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 30. Coronavirus (COVID-19) | cancer treatment documents | The Royal College of Radiologists; https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/coronavirus-covid-19-resources/coronavirus-covid-19-1. Accessed April 6, 2020. [Google Scholar]
- 31. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak [published online ahead of print April 28, 2020]. Cancer Discov. 2020;CD-20-0422. doi: 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.