Abstract
Objectives Radix Bupleuri represents one of the most successful and widely used herbal drugs in Asia for treatment of many diseases over the past 2000 years. Thorough studies have been carried out on many species of this genus and have generated immense data about the chemical composition and corresponding biological activity of extracts and isolated secondary metabolites. In this work, we review the chemistry and pharmacology of the genus Bupleurum and explore the relationships between the pharmacological effects and the chemical composition of these drugs.
Key findings Early studies on the genus Bupleurum had focused only on the traditional uses of the plants in the treatment of inflammatory disorders and infectious diseases. After chemical profiling, several groups of secondary metabolites were characterized with relevant biological activity: triterpene saponins (saikosaponins), lignans, essential oils and polysaccharides. As a result, present interest is now focused on the bioactivity of the isolated triterpene saponins acting as immunomodulatory, anti‐inflammatory and antiviral agents, as well as on the observed ant‐iulcer activity of the polysaccharides and anti‐proliferative activity of different lignans. Many saikosaponins exhibited very potent anti‐inflammatory, hepatoprotective and immunomodulatory activities both in vivo and in vitro.
Conclusions Further investigations and screenings are required to explore other Bupleurum species, to evaluate the clinical safety and possible interactions with other drugs or herbs. Standardization of Bupleuri extracts is crucial for them being integrated into conventional medicine due to large chemical and biological variations between different species and varieties.
Keywords: biological activity, Bupleurum, mode of action, secondary metabolites
Introduction
The family Apiaceae, or carrot family, is one of the most widely studied families of flowering plants comprising about 450 genera with 3540 species.[ 1 , 2 ] Members of this family are herbs, less often shrubs or trees, with global distribution, especially in temperate regions. [1] Many of the genera provide us with economically important food items, herbs and spices, such as carrot, anise, fennel and caraway. Many medicinally important apiaceous plants (almost 250 species) have been used for centuries in folk medicine in the treatment of various ailments. [3] These plants provide us with many potentially active compounds from all classes of secondary metabolites, including essential oils, phenolics (flavonoids, coumarins and lignans), triterpene saponins, alkaloids and polyacetylenes. These bioactive metabolites can serve as lead compounds in the treatment of many serious diseases. [4]
The name of the genus Bupleurum originates from the Latin word boupleuron (bous = ox and pleura/on = rib/s) describing the shape of the roots, which are the commonly used part of the plant. [5] Most Bupleurum species are perennial herbs, up to 150 cm in height with compound umbels. The flowers are bisexual, yellowish or rarely purplish with five stamens and the fruits occur mostly as cremocarps. Leaves are simple, long, slender and alternate with entire margin. The genus is represented by 180–190 species, which are widely distributed in the Northern Hemisphere and commonly used in Eurasia and North Africa for their medicinal properties. [2] Embedded in Asian traditional medicine, several Bupleurum species have been used either alone or in combination with other ingredients for the treatment of common cold, inflammatory disorders, hepatitis, cancer and fever in the form of over‐the‐counter herbal teas or in different pharmaceutical preparations.[ 6 , 7 ]Bupleurum species are officially listed in the Chinese and Japanese Pharmacopoeias in addition to the WHO monographs of the commonly used medicinal plants of China and Korea.[ 8 , 9 ] However, none of the Bupleurum plants were selected by the German Commission E, the British Pharmacopoeia 2009 or the British National Formulary 57. [10]
Historical and traditional uses of Bupleurum
The genus Bupleurum is a major component of Oriental folk medicine. Preparations containing the roots of Bupleurum species have been prescribed for more than 2000 years in China where the first record about their use appeared in Shen‐Nong's Herbal. [11] Inspired by the role in regulating metabolism and controlling Yin/Yang as mentioned in the old Chinese literature, Bupleurum was widely known in Korea and Japan for the treatment of fever, pain and inflammation associated with influenza and the common cold.[ 4 , 10 ] In addition, Bupleurum species were also used as analgesics in the treatment of distending pain in the hypochondriac region of the chest and against amenorrhoea. Many Bupleuri extracts have been used for improvement and protection against chronic hepatitis, nephrotic syndrome and autoimmune diseases. [12] Other uses include improvement of cholecystitis and wound healing and treatment of deafness, dizziness, vomiting, dry throat and diabetes. However, these effects are not supported by experimental or clinical data. Moreover, combinations of Bupleurum with ginseng and Astragalus are used against haemorrhoids, anal or uterine complications and diarrhoea.[ 8 , 9 , 12 ]
Chemical diversity of the secondary metabolites in the genus Bupleurum
Thorough phytochemical investigations of approximately 50 Bupleurum species led to the isolation and identification of almost 250 natural compounds from all major phytochemical classes. Nevertheless, the genus still holds other therapeutically relevant species probably containing other bioactive substances that have not yet been explored. In general, the chemical combinations and the ratio between components vary from one species to another but most of the secondary metabolites isolated belong to the classes of phenolics, lignans, terpenoids (triterpenoids and sterols), mono‐ and sesquiterpenes (essential oils) and polyacetylenes. In addition, minor components, including phenylpropanoids, polysaccharides and a few alkaloids, have also been reported. In the following sections we will summarize the current knowledge on the chemical structures of compounds isolated from the genus Bupleurum. A compilation of all isolated compounds with their original sources will be provided in supplementary materials.
Secondary metabolites present in essential oils
Like many members of the family Apiaceae, all representatives of the genus Bupleurum produce essential oils. About 200 components of essential oils from 20 species have been documented. Li and coworkers[ 13 , 14 ] examined the compositions of the essential oils obtained from the roots of 10 different species from China and they were found to consist mainly of a series of aliphatic aldehydes and acids, such as hexanal, heptanal (E)‐2‐nonenal (E,E)‐2,4‐decadienal, hexanoic acid, heptanoic acid, octanoic acid and hexadecanoic acid. These aldehydes are characteristic of the Chinese species. This was confirmed by our work on B. marginatum in which β‐caryophyllene, β‐caryophyllene oxide and spathulenol, in addition to the aforementioned aldehydes, represent the major components of the oil. [15] In contrast, essential oils from European species are characterized by the presence of a high abundance of α/β‐pinene, limonene and 1,8‐cineole rather than aliphatic aldehydes. This difference can be used to distinguish between oils from Italy or Spain and those from China.[ 16 , 17 , 18 , 19 ]
Triterpene saponins
Saikosaponin triterpenes generally constitute the main class of secondary metabolites in the genus Bupleurum amounting to up to 7% of the total dry weight in roots. To date, more than 120 glycosylated oleanane‐type and ursane‐type saponins have been isolated from Bupleurum species.[ 20 , 21 , 22 , 23 ] The aglycones of these saikosaponins are closely related oxygenated pentacyclic triterpenoidal structures that can be distinguished only by the positions and numbers of the double bonds in rings C and D and oxygenation patterns in positions 16, 23, 28 and 30 (Figure 1). These saponins generally bear one (monodesmosidic) or, less often, two (bidesmosidic) carbohydrate chains that are directly attached to the hydroxyl groups in position 3 for monodesmosidic saponins and to positions 3 and 28 or 30 in the case of the bidesmosidic saponins. The according carbohydrate chains are composed mainly of fucose, rhamnose, xylose, galacatose and glucose moieties. Generally, the bidesmosides are stored in plant vacuoles and are considered prodrugs. They are hydrolysed by the metabolizing enzymes when the plant is wounded to give the monodesmosides, which are the pharmacologically active forms. [24] Saikosaponins with the aglycone structure I represent the most abundant triterpene saponins found in Bupleurum species. [25] Of these, saikosaponins A, C and D, which had been isolated for the first time from B. falcatum roots, constitute the most common saponins in such species as B. kunmingense, B. marginatum and B. wenchuanense.[ 10 , 26 ] Variations in component abundance, as well as total saponin content, are commonly encountered between different plants and especially from different localities. [27] In addition, other saikosaponins with different aglycones or sugar chains have been also isolated from other Bupleurum species, as shown in the supplementary materials.[ 28 , 29 , 30 , 31 , 32 ] Most of these saponins are potentially active compounds and exhibit a wide range of pharmacological actions, which are discussed later.
Figure 1.


Representatives from different classes of compounds isolated from Bupleurum spp.
Sterols
This class of secondary metabolites was apparently of least interest for many phytochemists. Only 14 compounds have been identified from few species. For example, the phytosterol composition of B. falcatum was identified to be β‐sitosterol, stigmasterol, Δ7‐stigmastenol, Δ22‐stigmastenol and α‐spinasterol, [33] while aerial parts of B. flavum contain mainly betulin, betulinic acid, epibetulin and jasminol. [34] B. marginatum roots have been shown to contain β‐sitosterol and α‐spinasterol; lupeol, cycloeucalenol and erythrodiol have been isolated from B. fruticosum.[ 35 , 36 ] In general, betulin and α‐spinasterol are the most commonly occurring sterol components in Bupleurum spp.
Lignans
Lignans are the second most common class of secondary metabolite within this genus with almost 50 isolated compounds. Four major subclasses of lignans can be distinguished depending on the pattern of additional bridging between the two β‐linked phenylpropanoid units. The dibenzylbutyrolactone derivatives (Figure 1) are the most common compounds but arylnaphthalenes, aryltetralinelactones and tetrahydrofurofuranes are also present. This class of secondary metabolite has been extensively studied in B. salicifolium and about 32 compounds were isolated from different plant parts.[ 37 , 38 , 39 , 40 , 41 , 42 ] Arylnaphthalene derivatives (i.e. chinensin and isodiphyllin) are common in B. fruticescens, B. handiense and B. marginatum,[ 43 , 44 , 45 ] while tetrahydrofurofurane derivatives are only found in B. salicifolium and B. wenchuanense.[ 38 , 46 ] The majority of isolated lignans occur as aglycones and only two glycosides (phillyrin and wenchuanensin) have been isolated from B. wenchuanense. [46]
Flavonoids and related chromones
Formerly it was believed that most flavonoids in the genus are derivatives of the flavonol aglycones kaempferol, isorhamnetin or quercetin. Recently, however, some other aglycones like apigenin, acacetin, chrysin, luteolin and tamarixetin have been characterized.[ 34 , 47 , 48 , 49 ] To date about 30 flavonoids have been isolated with the diglycoside rutin being the most common. In addition, narcissin, the other diglycoside form of isorhamnetin, is present in B. flavum and B. fruticosum.[ 10 , 34 ] Flavonoids, including the minor compounds, are widely used as chemotaxonomical markers to distinguish between different Bupleurum species and different geographical sources. [47] The chromone derivatives eugenin, [50] saikochromone A [51] and saikochromoside A [52] have been isolated from B. scorzonerifolium, B. falcatum and B. chinense, respectively, and the isoflavonoid derivative saikoisoflavonoside A [53] from B. scorzonerifolium.
Coumarins
Coumarins are characteristic constituents of the family Apiacae and 14 compounds have so far been reported in the genus Bupleurum, especially simple alpha‐keto benzopyranes (Figure 1) from B. fruticescens and B. fruticosum. Herniarin, scopoletin, isoscopoletin, scoparone and limettin have been isolated from the aerial parts of B. fruticescens[ 45 , 54 ] and virgatenol, capensin, fraxetin, prenyletin and aesculetin from B. fruticosum.[ 55 , 56 ] Furthermore, complex pyranocoumarin derivatives, such as anomalin and praeruptorin A, have been isolated from B. falcatum and B. marginatum roots.[ 35 , 57 ]
Polyacetylenes
About 25 bioactive polyacetylenes (polyines) have been reported from seven different species. Barrero and coworkers[ 49 , 58 ] have identified bupleurynol, 2(E),9(Z)‐octadecadiene‐4,6‐diyne‐1,18‐diol with its mono‐ and diacetate derivatives and oenanthetol with its aldehyde and acetate forms, from the aerial part of B. acutifolium, and 5(E),7(E)‐pentadecadiene‐9,11,13‐triyn‐2‐one and 8(Z)‐decene‐4,6‐diyn‐1‐ol from the aerial part of B. spinosum. Saikodiyne A, B and C were isolated for the first time from the roots of B. falcatum (the prefix ‘saiko’ is derived from the Japanese name of the plant [59] ), falcarinol and diynene were isolated from the acetone extract of B. rigidum roots. [20]
Other compounds
Other minor compounds are present in the genus, such as phenylpropanoid derivatives and polysaccharides. The phenylpropanoid derivatives represent the fourth class of phenolics in this genus of which 14 different compounds have been described from aerial parts of B. fruticosum. Morinins D, G and L, in addition to ferulic acid and its derivatives, were the main compounds.[ 60 , 61 ] No data could be found regarding the other parts of B. fruticosum or other plants from the same genus.
Rare monosaccharides, like ribitol, xylose and arabinose, and pectic polysaccharides, like bupleuran 2IIb and bupleuran 2IIC, have been isolated from B. falcatum and many other species; these compounds exert anti‐ulcer, anti‐inflammatory, anti‐infective and immunomodulatory effects in autoimmune diseases as discussed later.[ 62 , 63 , 64 ]
Free organic and fatty acids, like pinellic acid, angelic acid, petroselic acid and lignoceric acid, have also been identified in many species. [10]
Other secondary metabolites, such as tannins, anthocyanidins and alkaloids, are absent in the genus, although a recent report claimed the presence of an indole‐type alkaloidal glycoside (chaihuxinoside B) in the aerial parts of B. chinense. [65]
Pharmacological properties of Bupleurum
Additive or synergistic effects may be expected from the wide range of combinations of secondary metabolites in many plants of the genus Bupleurum. In‐vitro and in‐vivo studies on Bupleurum extracts or isolated components (mainly saikosaponins) revealed significant anti‐inflammatory, anti‐ulcer and immunomodulatory activity. Other activity includes hepatoprotective, antitussive, antispasmodic, diaphoretic, antioxidant and antimicrobial effects. Some lignans of Bupleurum species may be useful as anti‐mitotic agents in the treatment of cancer by their inhibition of microtubule formation due to their high structural similarity to podophyllotoxin. Although the biological activity of certain Bupleurum essential oils and saponins had been compiled early in 2006, [10] a vast body of scientific literature on the pharmacological activity of other species and classes of secondary metabolites have since emerged.[ 63 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 ] A summary of some of the relevant literature is given in Table 1 and discussed in the following section.
Table 1.
Pharmacological activity of different species of the genus Bupleurum and their isolated compounds
| Plant species | Extract/substances | Main pharmacological activities | Source |
|---|---|---|---|
| B. chinense | Saikosaponin mixture | (−) Hepatitis B replication | 75, 76 |
| Alcoholic extract | (−) Helicobacter pylori activity | [77] | |
| Saikosaponin C | (−) Hepatitis B replication | [75] | |
| B. falcatum | Acidic polysaccharide fraction | (−) Gastric lesions | [78] |
| Saikosaponin mixture | (−) Acute and chronic inflammation | [79] | |
| Bupleuran 2IIc | (−) Gastric lesions | 80, 81, 82 | |
| (+) Gastric mucosa regeneration | |||
| Saikosaponin A | (+) B cells | 83, 84, 85, 86, 87, 88 | |
| (−) T cells | |||
| (−) PGE2 | |||
| (−) sGOT and sGPT levels | |||
| (−) Lipid peroxidation | |||
| (−) Asthmatic bronchoconstriction | |||
| Saikosaponin D | (+) Macrophage phagocytosis | 75, 84, 85, 86, 87, 92, 93, 94 | |
| (−) T cells | |||
| (+) Acid phosphatase | |||
| (+) IL‐2/IL‐4 production | |||
| (−) NF‐κB | |||
| (−) IL‐6, TNF‐α, IFN‐γ | |||
| (+) PGE2 production | |||
| (−) sGOT, sGPT and TGF‐β1 levels | |||
| (−) Lipid peroxidation | |||
| Cytotoxic against HepG2 cell line | |||
| (−) Measles and herpes viruses | |||
| Saikosaponin B1 | (+) PGE2 production | 84, 92 | |
| Saikosaponin B2 | (+) PGE2 production | 73, 84, 92 | |
| (−) Human coronavirus (HCoV) replication | |||
| Saikogenin D | (−) PGE2 production | [95] | |
| B. fruticescens | Butanol extract | (−) Acute inflammation | [96] |
| Methanol extract | (−) 5‐LOX, COX‐1 | [97] | |
| Essential oil | (−) Acute inflammation | [17] | |
| Fruticesaponin B | (−) Acute inflammation | [96] | |
| B. fruticosum | Petroleum ether extract | (−) IL‐1β, IL‐6, TNF‐α, PGE2 | [98] |
| Essential oil | (−) Acute and chronic inflammation | 18, 99 | |
| Oxytocin antagonism | |||
| Antifungal | |||
| Saikosaponin fraction | Hepatoprotective against DMN | [100] | |
| Morinin L | (−) IL‐1, IL‐6, IL‐8, TNF‐α, NF‐κB | [101] | |
| Buddlejasaponin IV | Hepatoprotective against DMN | [100] | |
| B. gibraltaricum | Methanol extract | (−) Acute inflammation | [102] |
| Essential oil | (−) Acute and chronic inflammation | 16, 19 | |
| Oxytocin antagonism | |||
| Antibacterial, antifungal | |||
| Saikosaponin I | (−) Acute inflammation | [102] | |
| B. kaoi | Saponin‐rich fraction | Hepatoprotective against CCL4 | 103, 104, 105, 106 |
| (−) sGOT, sGPT, AST and ALT levels | |||
| (+) IFN‐γ level | |||
| Cytotoxic against A549 cell line | |||
| Water extract | (+) IFN‐γ and GSH levels | 66, 105 | |
| (−) Coxsackie B1 replication | |||
| Polysaccharide‐rich fraction | (−) sGOT and sGPT levels | [105] | |
| (+) IFN‐γ and GSH levels | |||
| B. marginatum | Essential oil | (−) 5‐LOX | [15] |
| (−) PGE2 production | |||
| Antibacterial | |||
| B. rigidum | Buddlejasaponin IV | (−) Acute inflammation | 107, 108 |
| (−) 5‐LOX, COX‐1 | |||
| (−) Vesicular stomatitis virus (VSV) | |||
| Buddlejasaponin I | (−) Acute inflammation | [107] | |
| Sandrosaponin I | (−) 5‐LOX, COX‐1 | ||
| B. rotundifolium | Butanol extract | (−) Acute inflammation | [109] |
| Rotundioside F | (−) Acute inflammation | [109] | |
| Rotundioside A, H, I, J | Cytotoxic against MK‐1, HeLa and B16F10 cell lines | 30, 110 | |
| B. salicifolium | Polyacetylenes derivatives | Antibacterial | 111, 112 |
| B. scorzonerifolium | Acetone extract | Cytotoxic against A549 cell line | 113, 114, 115 |
| Wogonin, eugenin, saikochromone A | (−) IL‐2 | [50] | |
| Saikosaponin B3, bupleurosides III, VI, IX and XIII and scorzonerosides A, B, C | Hepatoprotective against DMN, LPS | 29, 116 | |
| (−) sGOT and sGPT levels | |||
| B. smithii | Acidic polysaccharide fraction | (−) Complement system | [64] |
| B. wenchuanense | 3′‐O‐Acetyl Saikosaponin A | Cytotoxic against P‐388, KB cell lines | [32] |
| 3′‐O‐Acetyl Saikosaponin D |
(–), decrease, inhibit, reduce, down‐regulate. (+), increase, activate, up‐regulate.
Immunomodulatory activity
An early study on the effect of water‐soluble extracts and purified derivatives from the roots of B. falcatum upon the immune response of BALB/c mice used heterologous erythrocytes and bacterial lipopolysaccharide as T‐dependent or T‐independent antigens, respectively. [83] Saikosaponins A and D, but not B1, B2 or C, suppressed the response of plaque‐forming cells to heterologous erythrocytes by stimulating T and B cells in a dose‐dependent manner. The activity of saikosaponin A was higher than that of D and the optimal dose for this activity was 1 mg/kg. Further studies on the effect of other saikosaponins and saikogenins on macrophage activation in mice showed that 10 µg intraperitoneally administered saikosaponin D (ssD) potently activated peritoneal macrophages in terms of enhancement of phagocytic activity. [89] In addition, an increase in the cellular level of acid phosphatase, induction of cytostatic activity and expression of Ia antigen on the cell surface of spleen cells were observed. Moreover, saikosaponin D modulated lymphocyte activity by suppressing the T‐cell response and inducing the B‐cell response to different mitogens and up‐regulating the interleukin (IL)‐2/IL‐4 production in cell cultured thymocytes by affecting their post‐receptor signal transduction.[ 90 , 91 ]
A recent study with ssD on mouse T lymphocytes activated through the NF‐κB, nuclear factor of activated T‐cells (NF‐AT) and activator protein 1 (AP‐1) signalling pathways, cytokine secretion and IL‐2 receptor expression revealed that ssD suppressed stimulated human T‐cell proliferation and activation in vitro. The inhibitory effect of ssD on phorbol myristate acetate (PMA)‐induced T‐cell activation was associated with down‐regulation of NF‐κB signalling. In addition, ssD at 15 µ m concentration decreased the production of pro‐inflammatory cytokines IL‐6, tumour necrosis factor (TNF)‐α and interferon (IFN)‐γ. [68]
The immunomodulatory effect of some B. fruticosum extracts and isolated compounds has been investigated extensively against several markers of the immune response.[ 98 , 101 ] A petroleum ether extract (100 µg/ml) caused 80–100% release of IL‐1β and IL‐6 and prostaglandin (PG) E2 synthesis in human monocytes. However TNF‐α was inhibited using 10 µg/ml. Morinin L and one other phenylpropanoid derivative isolated from the ethyl acetate fraction were tested for their possible effect on many cytokines and immune mediators. Inhibition of the transcriptional activity of an NF‐κB‐controlled reporter gene was observed by both compounds. This inhibition took place at a post‐degradation level. Further work with the same compounds indicated that they could prevent the release of IL‐1, IL‐6, IL‐8 and TNF‐α and PGE2 synthesis. Similar results were obtained with different extracts from B. scorzonerifolium tested against the IL‐2 secretion in stimulated human peripheral blood T cells. The isolated compounds wogonin, eugenin and saikochromone A inhibited the secretion at a dose of 10 µg/ml by 99, 72 and 71%, respectively. [50]
The acidic polysaccharide fraction from B. smithii inhibited the complement activation (IC50 340 and 81 µg/ml) for both classical and alternative pathways, respectively. This inhibition was mediated through interaction with C1s, C3 and C4 complements, which are the major components of the innate immune system. [64]
Anti‐inflammatory activity
It is usually difficult to separate the inflammatory process from the immune response; drugs with immunomodulatory activity usually interfere in inflammation. A wide range of Bupleurum preparations has been shown to affect artificially induced acute and chronic inflammation in animal models and, in addition, exert a marked in‐vitro activity on inflammatory mediators resulting from the inhibition of either lipoxygenases or cyclooxygenase.
B. falcatum has been extensively investigated for its anti‐inflammatory activity. In 1975, a saikosaponin mixture (mainly saikosaponin A, C and D) isolated from the roots was tested by intramuscular and oral administration to female albino rats. Both routes of administration of the saikosaponin mixture demonstrated significant activity against acute and chronic inflammation, saikosaponin D being particularly active against chronic inflammation at a dose of 2 µg/g. [79]
Ten years later, another study measured the possible effects of isolated saponins on PGE2 production in rat peritoneal macrophages. [84] Interestingly, saikosaponins B1, B2 and D increased the production of the inflammatory mediator, while saikosaponins A and C were inhibitory. The maximal increase was observed with 10 µg/ml ssD, and the maximal inhibition after 8 h pre‐incubation with 100 µg/ml ssA. These results were confirmed in 1999 for the saikosaponins B1, B2 and D, which led to a spontaneous increase in PGE2 production in C6 rat glioma cells (IC50 15.0, 14.4 and 11.0 µ m, respectively). [92] Surprisingly, saikosaponins A and C significantly increased the amount of inflammatory mediators after 10 min of drug administration, indicating the importance of the incubation time. To clarify the possible mode of action, the aglycone of ssD, saikogenin D, was also examined for its effect on PGE2 production and intracellular free calcium ion ([Ca2+]i) concentration in C6 rat glioma cells. [95] Saikogenin D inhibited PGE2 production in a concentration‐dependent manner with an IC50 value of about 3 µ m. On the other hand, saikogenin D elevated [Ca2+]i with an EC50 value of about 35 µ m. These results suggest that saikogenin D possesses a dual effect by inhibition of PGE2 production with an elevation of [Ca2+]I, which is attributed to Ca2+ release from intracellular stores. This key finding could explain the latent inhibition of PGE2 by ssD after an 8‐h pretreatment, which might undergo a gradual cellular hydrolysis to saikogenin D being responsible for this action.
Extracts of B. fruticescens, as well as certain isolated betulinic acid derivatives (fruticesaponin A, B and C), were examined for both oral and topical anti‐inflammatory activity. The orally administered n‐butanol extract (100 mg/kg) caused an inflammatory inhibition by 43% in carrageenan‐induced acute oedema in mouse hindpaw after 5 h. Moreover, tetradecanoyl phorbol acetate (TPA)‐induced mouse ear oedema was inhibited by 74% after 4 h of topical application of the tested drug (0.5 mg/ear). Fruticesaponin B was the most active betulinic acid derivative in both oral and topical application but the maximum oral activity was observed after 3 h. [96] These results were confirmed by testing the methanol extract on different in‐vitro enzymes involved in inflammation: a significant inhibition of 5‐lipoxygenase (5‐LOX) activity was observed, which reduced both leukotriene‐B4 and 5(S)‐HETE production (IC50 112 µg/ml and 95 µg/ml, respectively). In addition, at a 200 µg/ml dose, the extract inhibited cyclooxygenase‐1 (COX‐1) and elastase activity by 90% and 54%, respectively. [97]
Another study with different Bupleurum species from Spain showed that the methanol extract of B. gibraltaricum and its isolated saponin (saikosaponin I) decreased carrageenan‐induced inflammatory oedema in a rat hindpaw model by 50% at 5 h after intraperitoneal injection of 150 and 50 mg/kg methanolic extracts and the isolated substance, respectively. [102]
Buddlejasaponin I, IV and the sulfated saikosaponin (sandrosaponin I), which were isolated from B. rigidum, exhibited a very potent in‐vivo anti‐inflammatory effect (1 mg/ear dose) on mouse ear oedema induced by PMA compared with the equivalent dose of indometacin. The effect of these compounds on other inflammatory parameters revealed that buddlejasaponins I and IV exhibited potent leukotriene‐C4 inhibition (IC50 2.4 and 2.6 µ m, respectively). The results are very close to those of the known reference inhibitor nordihydroguaiaretic acid (NDGA, IC50 = 2.0 µ m). In the PGE2 release assay, all tested saikosaponins exhibited less potency towards the inhibition of COX with IC50 values higher than 45 µ m compared with indometacin with an IC50 of 6.5 µ m. Moreover, inhibition of thromboxane B2 formation was also observed with IC50 values of 27, 4.6 and 2.4 µ m for buddlejasaponin I, IV and sandrosaponin I, respectively. These results revealed that inhibition of arachidonic acid metabolism is one of the biochemical mechanisms exerted by saikosaponins in their putative antiphlogistic activity. [107]
The n‐BuOH fraction of B. rotundifolium extract and the isolated saponins were evaluated for their possible topical effects on acute and chronic ear oedema in mice provoked by TPA. The extract caused a 73% inhibition of the acute oedema (0.5 µg/ear). The most active triterpene derivative was rotundioside F, which exerted a potent activity against acute inflammation with an IC50 value of 0.099 µ m/ear. [109]
The essential oils of Bupleurum also exhibit a positive anti‐inflammatory effect. For instance, the anti‐inflammatory activity of intraperitoneally and orally administered essential oils of B. gibraltaricum and B. fruticosum and their major components were evaluated against both acute inflammation using carrageenan‐induced oedema model and chronic proliferative inflammation by evaluating the granuloma formation.[ 16 , 18 ] The two oils showed a dose‐dependent activity in both systems but the effect was more obvious in the acute test and by the intraperitoneal route. In addition, B. gibraltaricum oil was more active than B. fruticosum oil and exhibited about one‐third of the activity shown by indometacin in the paw oedema model. Both oils had the advantage of sustaining the anti‐inflammatory activity for a longer time compared with the control drug. This activity was attributed to the main components Δ3‐carene, β‐pinene and α‐pinene, which caused a highly significant reduction of the paw oedema at the 33 mg/ml dose 3 h after oral administration. The absence of Δ3‐carene in B. fruticosum oil is apparently the reason for the reduced activity compared with B. gibraltaricum oil. [18]
Essential oils of B. gibraltaricum from different localities differed mainly in the composition of Δ3‐carene, β‐pinene and α‐pinene components. Bupleurum oils rich in Δ3‐carene and β‐pinene were active against acute inflammation while those with lower concentrations failed to exert such activity. [117]
Comparable results were also obtained with B. fruticescens; orally administered oil and its major components α‐pinene and β‐caryophyllene had a significant in‐vivo effect on both carrageenan‐ and PGE2‐induced oedema. The activity of the oil itself was much higher than that of the individual components; this was attributed to a synergistic effect of the compounds. [17]
A recent in‐vitro study was carried out by our group to evaluate the activity of B. marginatum oil against both soybean 5‐LOX and PGE2 production in MIA‐PaCa‐2 cancer cells. [15] The oil was able to inhibit 5‐LOX with an IC50 value of 63.64 µg/ml and to exert a 26% inhibition in the PGE2 production in the cancer cells treated with a 25 µg/ml dose.
Anti‐ulcer activity
Triterpene saponins like glycyrrhizin, which resemble many of the saikosaponins, exhibit potent anti‐inflammatory and anti‐ulcer effects. [118] The anti‐ulcer effects of saikosaponins have not been studied so far and in most cases the anti‐ulcer properties of Bupleurum spp. appear to be attributed only to pectic polysaccharides.
The effects of the acidic polysaccharide fraction from the roots of B. falcatum on induced gastric lesions in mice using different routes of administration have been evaluated. A protective effect was observed after administration by all the routes using a concentration range of 25–100 mg/kg. [78] The major acidic polysaccharide with anti‐ulcer activity was identified as bupleuran 2IIc after a bioassay‐guided fractionation of the sugar fraction. This polysaccharide, which is composed of galacturonic acid units, reduced the HCl/ethanol‐induced gastric lesions in mice more potently than the known anti‐ulcer drug sucralfate through scavenging the free oxygen radicals at a 100 mg/kg dose.[ 80 , 81 ] Similar results were obtained using acetic acid‐induced ulcers in rats in which the repair process closely resembles that of the human peptic ulcer, where an orally administered acidic polysaccharide‐rich fraction at a dose of 200 mg/kg twice daily reduced the lesions to almost 52% and regeneration of the mucosa was clearly seen. [82]
Antioxidant and hepatoprotective activity
The high concentrations of triterpene saponins and polyphenolics significantly contribute to the observed hepatoprotective effects of Bupleurum spp. The administration of saikosaponins, especially saikosaponin A and D, showed interesting effects on liver function, such as decreasing the activity of glucose‐6‐phosphatase and NADPH‐cytochrome C reductase and significantly increasing 5′‐nucleotidase activity. Moreover, an inhibitory effect on d‐galactosamine‐induced hepatic necrosis was observed in the form of significant reduction in many hepatic injury markers such as serum GOT and GPT, total and direct bilirubin and cholesterol levels after pretreatment of animals with 5 mg/kg for four successive days. [85] Similar results were obtained in other studies in which the effects of saikosaponin D on acute and chronic hepatic injury provoked by chloroform and enhanced by phenobarbitone were evaluated in rats. In addition to the reduction of hepatic enzyme levels, a significant inhibition of lipid peroxidation in the liver and a general improvement in liver weight were observed.[ 86 , 87 ]
The components of another Chinese species, B. scorzonerifolium, were studied by Yoshikawa and coworkers, where saikosaponin B3, bupleurosides III, VI, IX and XIII and scorzonerosides A, B and C reduced the cytotoxicity of both d‐galactosamine and lipopolysaccharides in primary cultured rat hepatocytes as shown in the form of a decrease in sGOT and sGPT levels.[ 29 , 116 ]
Similar results were obtained for the saponin fraction of B. fruticosum and its isolated compound buddlejasaponin IV. Both the saponin fraction and the isolated compound were ineffective against hepatic damage caused by chloroform whereas they showed substantial hepatoprotective effects against d‐galactosamine, similar to the widely used natural hepatoprotective compound silybin. [100]
An in‐vitro study of the extract and its saponin‐rich fractions of a native Taiwanese species, B. kaoi, revealed significant protection of primary hepatocytes against chloroform damage.[ 103 , 104 ] The results demonstrated that oral pretreatment of rats with the extracts at concentrations below 0.5 mg/ml 3 days before a single dose of CCl4 significantly lowered the serum levels of hepatic enzyme markers aspartae aminotransferase (AST) and alanine aminotransferase (ALT). In addition, pathological examination showed that lesions, including ballooning degeneration, necrosis and hepatitis, were partially healed by treatment with B. kaoi extracts. The extracts were also able to suppress the elevated hepatic enzyme activity of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase, which resulted from the high oxidative stress induced by CCl4. In another hepatic injury model, the hepatoprotective effects of a water extract and both polysaccharide‐ and saponin‐enriched fractions of B. kaoi were evaluated against dimethyl nitrosamine (DMN)‐induced hepatic fibrosis in rats. [105] Administration of the plant extracts markedly counteracted the harmful effects of DMN as indicated by reduction of sGOT, sGPT collagen content and elevation of the hepatic glutathione (GSH), albumin and interferon‐γ levels in the serum and liver homogenates. Another study using a tea preparation from the leaves of B. kaoi revealed a significant in‐vitro scavenging activity against both DPPH and superoxide anion radicals with IC50 values of 0.36 and 4.35 mg/ml, respectively, in addition to the inhibition of lipid peroxidation and the subsequent malondialdehyde (MDA) formation. [119] Furthermore, a leaf infusion decreased the hepatotoxicity of paracetamol and CCl4 in rat liver cells, as indicated by increased viability of intoxicated primary hepatocytes. The results are in agreement with a study carried out in 2009 with saikosaponin A. [120]
In another recent study, the therapeutic effect of saikosaponin D on liver fibrosis and cirrhosis was evaluated. [93] The results clearly demonstrated that saikosaponin D significantly reduced collagen I deposition in the liver. Moreover, it reduced the concentration of transforming growth factor β1 (TGF‐β1) in the liver, which is used as a key marker for liver fibrosis induced by DMN in addition to its role in reducing the elevated level of oxidative stress enzymes.
Cytotoxicity and anti‐tumour activity
Many Bupleurum‐containing herbal drugs have been traditionally used in the treatment of tumours and cancer. In this review we will focus on the anti‐tumour and antiproliferative effects of Bupleurum extracts and their isolated components in cell culture systems.
The cytotoxicity of different extracts from B. scorzonerifolium was assessed against A549 human lung cancer cells. [113] The acetone extract showed a dose‐dependent antiproliferative effect with an IC50 value of 59 µg/ml after a 24‐h treatment. The mechanism of this anti‐proliferative activity was assumed to be mediated through cell cycle arrest in the G2/M phase, induction of tubulin polymerization, induction of apoptosis via kinase 1/2 (ERK 1/2) activation in addition to activation of caspase‐3/9 and suppression of telomerase activity in the A549 cell line.[ 114 , 115 ] Saponin‐enriched fractions from B. kaoi exhibited similar properties on the same cell line and the IC50 value was 196 µg/ml. [106]
The cytotoxic effects of many Bupleurum saponins, especially those with an epoxy bridge, were very potent against many cancer cells. For example, saikosaponin D exerted very potent activity against the HepG2 cell line with an IC50 value of 12.5 µg/ml. The mechanism of cytotoxicity was attributed to the induction of apoptosis via activation of caspases 3 and 7 and the subsequent activation of poly‐ADP‐ribose polymerase (PARP), which leads to DNA fragmentation. [75]
The cytotoxic activity of both oleanane‐type and ursane‐type triterpene saponins isolated from B. rotundifolium by Fujioka and coworkers were examined in human gastric adenocarcinoma (MK‐1), human cervical carcinoma (HeLa) and murine melanoma (B16F10) cell lines.[ 30 , 110 ] Among the monodesmosidic ursane‐type saponins, rotundifoliosides A, H, I and J exhibited very potent cytotoxic activity with IC50 values in the range 11–71 µ m. The highest activity was observed with rotundifolioside J, which had IC50 values of 16, 21 and 11 µ m against the aforementioned cell lines, respectively. [110] The monodesmosidic oleanane saikosaponins also demonstrated different degrees of cytotoxicity but those that contained the epoxy bridge in their skeleton were highly active, with IC50 values in the range 6.6–37.3 µ m. [30] This high reactivity is attributed to the presence of the epoxy group which can covalently bond with the SH or amino groups in proteins leading to conformational changes and loss of functionality. This will be discussed in detail later. [24]
Some of the saikosaponins isolated from B. wenchuanense exhibited a potent activity against leukaemia P‐388 cells and nasopharynx carcinoma KB cells. The most active were the 3′‐O acetyl derivatives of saikosaponin A and D with IC50 values of 0.5 and 6.3 µg/ml for the former and 1.2 and 6.3 µg/ml for the latter. [32] The corresponding deacetylated forms had lower cytotoxic activity against HepG2 with IC50 values of 50 and 20 µg/ml, respectively. [121] This reduction in the cytotoxicity is due to the increase in the polarity of deacetylated forms and therefore the molecules cannot penetrate the biomembrane to exert their action. [24]
Antiviral activity
Extracts from Bupleurum have been evaluated in vitro for their possible activity against such viruses as herpes simplex, measles, hepatitis B and C viruses and many others. In general, the saikosaponins isolated from Bupleurum spp. exhibited a more potent antiviral activity than the total extracts and the individual saikosaponins showed a certain degree of selectivity towards different types of viruses.
A total aqueous extract of B. kaoi was tested against Coxsackie B virus type 1 (CVB1) and the results indicate that the extract has both direct and indirect effects on CVB1 infection. The extract was able to neutralize CVB1‐induced cytopathic effects in a human neonatal foreskin fibroblast cell line (CCFS‐1/KMC) with an IC50 value of 12.38 µg/ml. In addition, viral replication was completely inhibited at 200 µg/ml after 48 h treatment through induction of type I interferon expression. [66]
Chang and coworkers showed that a crude saikosaponin mixture obtained from B. chinense inhibited DNA replication of hepatitis B virus (HBV) and significantly reduced the HBV antigen level in transfected HepG2 cells.[ 75 , 76 ] The main active saikosaponin responsible for the activity was saikosaponin C; it showed a potent inhibition of both HBeAg secretion (IC50 of 11 µg/ml) and HBV‐DNA expression (IC50 of 13.1 µg/ml). This activity was much higher than that obtained by the well‐known antiviral drug lamivudine, which is the most widely accepted drug for treatment of chronic HBV. [75] In a recent study, other members of the saikosaponin group were identified from a B. chinense extract but only saikosaponin D was able to inhibit HBV‐DNA replication. [122]
The antiviral effects of saikosaponin D isolated from the roots of B. falcatum against herpes simplex, poliovirus and measles were investigated. [94] Saikosaponin D at a 5 mm concentration directly inactivated both measles virus and herpes simplex virus while a 500 mm concentration of the drug resulted in complete loss of viral infectivity. However, saikosaponin D was ineffective against the replication of measles virus, herpes virus, and polio virus.
Saikosaponins isolated from B. rigidum were also evaluated for their in‐vitro antiviral activity against herpes simplex type 1 (HSV‐1), vesicular stomatitis virus (VSV) and poliovirus type 1. Buddlejasaponin IV exhibited a potent virucidal activity against VSV only at concentrations in the range 20–25 µg/ml without being toxic at this range to the cell line used and even more potent than the reference substance dextran sulfate. [108]
The possible effects of saikosaponins and their mode of action against human coronavirus (HCoV), which is the main cause of severe acute respiratory syndrome (SARS), were also examined. All the tested saikosaponins exhibited antiviral activity at concentrations of 0.25–25 mm, and the strongest activity was observed for saikosaponin B2 with an IC50 of 1.7 mm. This activity was attributed to the inhibition of viral replication at early stages of cell infection. [73]
Antibacterial and antifungal activity
Extracts, essential oils and some of the other isolated compounds from the Bupleurum spp. exhibit substantial antimicrobial activity against Gram‐positive bacteria whereas they are almost inactive towards Gram‐negative bacteria or yeasts. Different essential oils obtained from aerial parts of B. gibraltaricum were tested against different bacteria and the minimum inhibitory concentration (MIC) values were determined. Micrococcus luteus was the most sensitive bacterium with a MIC value as low as 3 µg/ml, while the Gram‐negative Escherichia coli, Pseudomonas fluorescens and Candida albicans were less sensitive. [54] The antifungal activity of the same essential oil was also evaluated against Plasmopara halstedii; the oil could reduce the sporulation frequency of the fungus after an 11‐day treatment at all tested concentrations and a complete inhibition was obtained at a concentration of 5.0 ml/l. [19]
Comparable antifungal and antibacterial activity was observed with the essential oil of B. fruticosum collected in Italy, which showed a significant effect against the Gram‐positive pathogens Streptococcus faecalis, Staphylococcus albus and, to a lesser extent, Staphylococcus aureus. [99]
We have examined the antimicrobial and antifungal activity of the essential oil and hexane–ether extract of B. marginatum on 12 different microorganisms including three methicillin‐resistant Staphylococcus aureus (MRSA) strains. Both oil and extract showed a significant antimicrobial activity against Gram‐positive pathogens (Streptococcus pyogenes and Streptococcus agalactiae) with MIC values in the range 0.125–0.50 mg/ml. In addition, a measurable inhibition of MRSA strains was recorded with an MIC value of 4 mg/ml. In contrast, the tested Gram‐negative microorganisms and yeasts were not susceptible to any of the tested samples. [15]
Another study was conducted to evaluate the possible action of many plant extracts, including both ethanol and aqueous extracts of the aerial parts of B. chinense, on the growth of the Gram‐negative microorganism Helicobacter pylori, which is the major causative agent of peptic ulcers. The alcoholic extract was more active than the aqueous extract with an MIC value of 60 µg/ml. [77]
Some of the commercially available Bupleurum preparations, like ‘Chaihu injection’, have been tested in vitro for possible antimicrobial activity. Slight inhibition of Staphylococcus aureus was observed but no effects were observed against Staphylococcus albus, Neisseria gonorrhoeae, Diplococcus pneumoniae, haemolytic Streptococcus or Pseudomonas aeruginosa. [123]
Also, other isolated secondary metabolites from B. salicifolium have been assessed for their anti‐infective activity: one of the isolated polyacetylenes (8S‐heptadeca‐2(Z)‐9(Z)‐diene‐4,6‐diyne‐1,8‐diol) exhibited significant inhibitory activity against three Gram‐positive bacteria, Staphylococcus aureus, Bacillus subtilis and Micrococcus luteus, with MIC values of 10, 5–10 and 10–15 µg/ml, respectively. However, it was inactive against Gram‐negative bacteria (E. coli, Salmonella spp., Pseudomonas aeruginosa) and the yeast Candida albicans.[ 111 , 112 ]
Kumazawa and coworkers examined in vivo the protective effect of many individual saikosaponins isolated from Bupleuri Radix against Pseudomonas aeruginosa and Listeria monocytogenes infections in mice. The protective effect was attributed to the immunomodulatory action of different saikosaponins on macrophages which enhances the resistance of the mice to the bacterial infection. [124]
In general, Bupleurum has not been used in traditional medicine to treat infections. Research on antimicrobial properties and modes of action are limited but we can anticipate a detergent effect of saikosaponins on the bacterial and fungal biomembrane which would make bacterial or fungal cells leaky and could lead to their death. [24]
Miscellaneous biological activity
Ocete and coworkers examined the antispasmodic activity of essential oils obtained from both B. gibraltaricum and B. fruticosum on the rat uterus model. Both oils were able to antagonize uterine contractions induced by oxytocin and acetylcholine to different degrees. B. gibraltaricum essential oil was more active due to its higher content of Δ3‐carene (33% of the total oil) and was able at low doses (1.1 µg/ml and 2.2 µg/ml) to competitively and noncompetitively antagonize the oxytocin‐induced contraction.[ 16 , 18 ] Saikosaponin A inhibited the passive cutaneous anaphylaxis reaction in rats and suppressed asthmatic bronchoconstriction in sensitized guinea‐pigs at a dose of 1 mg/kg. In addition, weak inhibitory activity on histamine‐induced tracheal contraction in guinea‐pigs and on histamine release induced by A‐23187 in rat mast cells were observed. These results indicate that some saikosaponins might be useful in treatment of allergic asthma. [88]
Mode of action
Generally, it is very difficult to attribute the pharmacological activity of a multi‐component mixture, as in plant extracts consisting of a diversity of secondary metabolites, to only a single compound of an extract. [125] Secondary metabolites are able to interfere with many molecular targets in the cells and in the following section we will try to distinguish the main molecular targets of secondary metabolites isolated from the genus Bupleurum in order to understand their modes of action.
The major types of molecular target in eukaryotic cells that are relevant in this context include the biomembrane, proteins and nucleic acids (DNA, RNA) as summarized in Figure 2.
Figure 2.

The main molecular targets of many secondary metabolites in the mammalian cell. ER, endoplasmic reticulum.
Some of the isolated secondary metabolites from Bupleurum act directly on the biomembrane, such as the most common triterpene saponins (saikosaponins), the sterol fraction, the polyacetylenes and the small lipophilic molecules from essential oils. Both saikosaponins and sterol glycosides are amphiphilic molecules that function as detergents and exist mostly in their monodesmosidic forms. The monodesmosides are anchored with their lipophilic moiety in the lipophilic membrane bilayer after complexing with cholesterol, while the hydrophilic sugar part remains outside the cell and can interact with other glycoproteins or glycolipids (Figure 3). As a result, loss of the membrane integrity and fluidity occurs with the subsequent leakage of many polar molecules out of the cells or the entry of unwanted molecules into a cell. Therefore, many saponins are cytotoxic against a wide range of cells (cancer, bacteria and fungi).[ 24 , 126 , 127 ]
Figure 3.
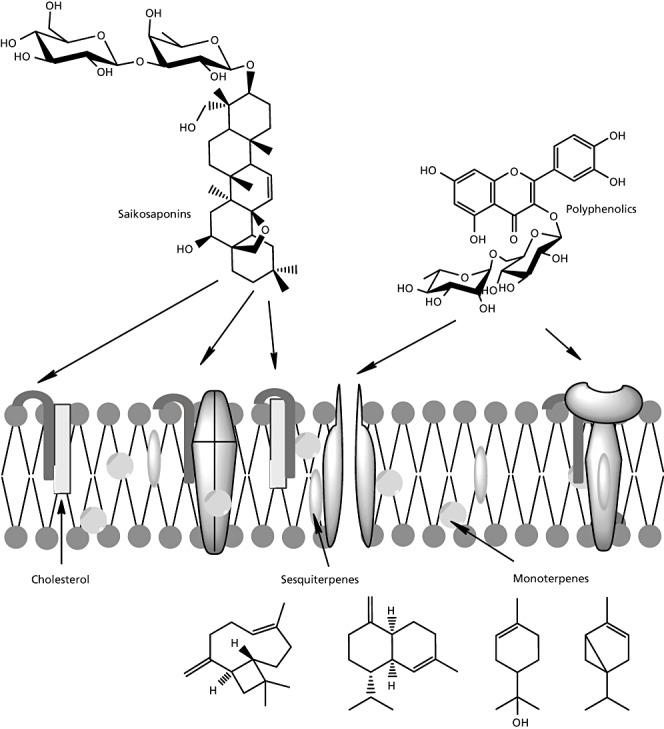
Interactions of some representatives compounds from Bupleurum with the cell membrane and membrane proteins.
In addition, other lipophilic secondary metabolites such as mono‐ and sesquiterpenes, which are the main essential oil components, can dissolve in biomembranes resulting in disturbance of the close interaction between membrane lipids and proteins thus changing the conformation of membrane proteins. These membrane proteins include ion channels, transporters for nutrients and intermediates, receptors, and proteins of signal transduction, and the cytoskeleton. [126] A change of protein conformation usually leads to a loss of function. Moreover, at higher concentrations, these secondary metabolites interact with the lipophilic inner core of biomembranes represented by fatty acids and cholesterol leading to disturbance of the membrane fluidity as shown in Figure 3. This type of interaction between the secondary metabolites and the biomembranes could explain the antimicrobial, antiviral and spasmolytic effects of many Bupleurum preparations.
Proteins represent the most important molecular targets of many secondary metabolites. Proteins have multiple functions in a cell, ranging from enzymes, transporters, ion channels, receptors, microtubules, histones to regulatory proteins (signal molecules, transcription factors etc.). Many secondary metabolites (such as coumarins, lignans, phenylpropanoids etc.) interact with proteins unselectively through formation of either noncovalent or covalent bonds that, in turn, interfere with the protein conformation leading to loss of activity in most cases.[ 125 , 126 , 128 ]
Phenolics are a major class of secondary metabolite present in all species of the genus Bupleurum in the form of phenylpropanoids, flavonoids, coumarins and lignans. These compounds are characterized by possessing one or more phenolic hydroxyl groups. The phenolic hydroxyl groups can partly dissociate under physiological conditions resulting in O– ions. The polyphenols interact with proteins by forming hydrogen bonds with electronegative atoms of the peptide or ionic bonds with positively charged side chains of basic amino acids, respectively.[ 125 , 126 ] A single of these noncovalent bonds would be quite weak but because several of them are formed concomitantly the effect is much stronger and a change in protein conformation is likely to occur, which then may lead to protein inactivation. However, the formation of covalent bonds can also occur as shown in Figure 4.
Figure 4.

Possible protein conformational changes due to formation of covalent bonds with plant secondary metabolites.
Several types of secondary metabolite carry reactive substituents like epoxide, aldehyde, triple bonds or exocyclic methylene groups that can covalently bind to amino and sulphydryl groups of proteins. [129] This alkylation also leads to a conformational change and thus loss of activity. Secondary metabolites of Bupleurum with such properties are polyacetylenes with their reactive triple bonds and aldehyde‐containing monoterpenes. This type of interaction can explain the inhibition of many enzymes such as lipoxygenases and cyclooxygenases and hence, the anti‐inflammatory, immunomodulatory and hepatoprotective activity of many Bupleurum extracts can be rationalized. Furthermore, the presence of flavonoids and other phenolics with their radical scavenging properties may assist in reducing the oxidative stress inside the cell by direct quenching of the unwanted free radicals with their damaging role in many serious diseases like cancer, atherosclerosis and inflammation.
Some secondary metabolites probably exhibit more selective modes of action that are usually related to a particular kind of molecule rather than the whole extract. For example, many lignans with their high structural similarity to podophyllotoxin exhibit strong anti‐miotic and anti‐tumour effects by inhibiting microtubule formation in addition to inhibiting topoisomerase and thus blocking cell division in the late G2 phase.
Conclusions
The genus Bupleurum provides many efficacious herbal drugs that contain different lead compounds with a wide variety of biological effects. Substantial information on chemical and biological aspects is available but many species have not been evaluated for their biological activity and their chemical compositions remain to be investigated. Our chemical survey indicates the presence of new saponins and lignans with potential therapeutic activity, waiting to be explored. Many dated reports on biological and medicinal properties refer to Bupleuri radix, which comprises many different species. Considering the differences in the chemical compositions between different species or even the same species from different localities, we can assume the pharmacological properties to vary quite significantly. Therefore, authentication of all the drugs should be undertaken carefully by chemical fingerprinting or DNA barcoding to ensure the quality of the drug and hence the corresponding biological activity. Finally, studies on standardization of the drug and preclinical trials are required for an integration and acceptance of many Bupleurum extracts in conventional medicine.
Declarations
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
Funding
The first author would like to express his utmost gratitude to the Egyptian government for its generous scholarship.
Supporting information
Table S1 Chemical structures of the isolated triterpene saponins.
Table S2 Chemical structures of the isolated sterols.
Table S3 Chemical structures of the isolated lignans.
Table S4 Chemical structures of the isolated flavonoids and related compounds.
Table S5 Chemical structures of the isolated coumarins.
Table S6 Chemical structures of the isolated phenylpropanoids.
Table S7 Chemical structures of the isolated polyacetylenes.
Supporting info item
Acknowledgements
The authors would like to thank Mr Theodor C.T. Cole for his valuable comments on enhancement of the language of this work.
References
- 1. Judd WS et al Plant Systematics: A Phylogenetic Approach, 3rd edn. Sunderland, MA: Sinauer Associates, 2008. [Google Scholar]
- 2. Mabberley DJ. Mabberley's Plant‐Book: A Portable Dictionary of Plants, Their Classification and Uses. New York: Cambridge University Press, 2008. [Google Scholar]
- 3. Yaniv Z, Bachrach U, eds. Handbook of Medicinal Plants. New York: Haworth Medical Press, 2005. [Google Scholar]
- 4. Van Wyk B‐E, Wink M, eds. Medicinal Plants of the World: An Illustrated Scientific Guide to Important Medicinal Plants and Their Uses. Portland, OR: Timber Press, 2004. [Google Scholar]
- 5. Quattrocchi U. CRC World Dictionary of Plant Names: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology. Boca Raton, FL: CRC Press, 2000. [Google Scholar]
- 6. Wu J‐N. An Illustrated Chinese Materia Medica. New York: Oxford University Press, 2005. [Google Scholar]
- 7. Fundukian LJ, ed. The Gale Encyclopedia of Alternative Medicine, 3rd edn. Detroit, MI: Gale Cengage Learning, 2009. [Google Scholar]
- 8. World Health Organization . Medicinal Plants in the Republic of Korea: Information on 150 Commonly Used Medicinal Plants. Manila: World Health Organization, Regional Office for the Western Pacific, 1998. [Google Scholar]
- 9. World Health Organization . Medicinal Plants in China: A Selection of 150 Commonly Used Species. Manila: World Health Organization, Regional Office for the Western Pacific, 1997. [Google Scholar]
- 10. Pan S‐L, ed. Bupleurum Species: Scientific Evaluation and Clinical Applications. Boca Raton, FL: CRC/Taylor & Francis, 2006. [Google Scholar]
- 11. Xie H et al Identification of crude drugs from Chinese medicinal plants of the genus Bupleurum using ribosomal DNA ITS sequences. Planta Med 2009; 75: 89–93. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . WHO Monographs on Selected Medicinal Plants. Geneva: World Health Organization, 1999. [Google Scholar]
- 13. Li XQ et al Essential oil analyses of the root oils of 10 Bupleurum species from China. J Essent Oil Res 2007; 19: 234–238. [Google Scholar]
- 14. Li X et al Analysis of the essential oil from radix Bupleuri using capillary gas chromatography. Yakugaku Zasshi 2005; 125: 815–819. [DOI] [PubMed] [Google Scholar]
- 15. Ashour ML et al Chemical composition and biological activity of the essential oil obtained from Bupleurum marginatum (Apiaceae). J Pharm Pharmacol 2009; 61: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 16. Ocete MA et al Pharmacological activity of the essential oil of Bupleurum gibraltaricum: anti‐inflammatory activity and effects on isolated rat uteri. J Ethnopharmacol 1989; 25: 305–313. [DOI] [PubMed] [Google Scholar]
- 17. Martin S et al Anti‐inflammatory activity of the essential oil of Bupleurum fruticescens . Planta Med 1993; 59: 533–536. [DOI] [PubMed] [Google Scholar]
- 18. Lorente I et al Bioactivity of the essential oil of Bupleurum fruticosum . J Nat Prod 1989; 52: 267–272. [DOI] [PubMed] [Google Scholar]
- 19. Fernandez‐Ocana AM et al In vivo antifungal activity of the essential oil of Bupleurum gibraltarium against Plasmopara halstedii in sunflower. J Agric Food Chem 2004; 52: 6414–6417. [DOI] [PubMed] [Google Scholar]
- 20. Contreras SS et al Bioactive components of Bupleurum rigidum subsp. rigidum In: Atta‐ur‐Rahman FRS, ed. Studies in Natural Products Chemistry: Bioactive Natural Products (Part H). Amsterdam: Elsevier Science, 2002: 659–696. [Google Scholar]
- 21. Ebata N et al Saponins from the root of Bupleurum falcatum . Phytochemistry 1996; 41: 895–901. [DOI] [PubMed] [Google Scholar]
- 22. Pistelli L et al Triterpenoid saponins from Bupleurum fruticosum . J Nat Prod 1993; 56: 240–244. [DOI] [PubMed] [Google Scholar]
- 23. Ding J et al Chemical evaluation of Bupleurum species collected in Yunnan, China. Chem Pharm Bull 1986; 34: 1158–1167. [Google Scholar]
- 24. Wink M, Van Wyk B‐E. Mind‐Altering and Poisonous Plants of the World. Portland, OR: Timber Press, 2008. [Google Scholar]
- 25. Huang HQ et al Characterization and identification of saikosaponins in crude extracts from three Bupleurum species using LC‐ESI‐MS. J Sep Sci 2008; 31: 3190–3201. [DOI] [PubMed] [Google Scholar]
- 26. Kubota T, Hinoh H. The constitution of saponins isolated from Bupleurum falcatum L. Tetrahedron Lett 1968; 9: 303–306. [Google Scholar]
- 27. Huang HQ et al Fast determination of saikosaponins in Bupleurum by rapid resolution liquid chromatography with evaporative light scattering detection. J Pharm Biomed Anal 2009; 49: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 28. Barrero AF et al Saikosaponins from roots of Bupleurum gibraltaricum and Bupleurum spinosum . Phytochemistry 2000; 54: 741–745. [DOI] [PubMed] [Google Scholar]
- 29. Yoshikawa M et al Scorzonerosides A, B, and C, Novel triterpene oligoglycosides with hepatoprotective effect from Chinese Bupleuri Radix, the roots of Bupleurum scorzonerifolium WILLD. Tetrahedron Lett 1997; 38: 7395–7398. [Google Scholar]
- 30. Fujioka T et al Antiproliferative constituents from umbelliferae plants. IX. New triterpenoid glycosides from the fruits of Bupleurum rotundifolium . Chem Pharm Bull 2006; 54: 1694–1704. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Y‐Y et al Triterpenoid saponins from Bupleurum smithii var. parvifolium. Phytochemistry 1996; 42: 1673–1675. [DOI] [PubMed] [Google Scholar]
- 32. Luo S‐Q et al Saikosaponin derivatives from Bupleurum wenchuanense . Phytochemistry 1993; 33: 1197–1205. [DOI] [PubMed] [Google Scholar]
- 33. Takeda K, Kubota T. Steroidal components of domestic plants. XVIII. Components of the root of Bupleurum falcatum . Chem Pharm Bull 1958; 6536–6539. [Google Scholar]
- 34. Pistelli L et al Lupane‐triterpenes from Bupleurum flavum . Nat Prod Res 2005; 19: 783–788. [DOI] [PubMed] [Google Scholar]
- 35. Liang Z et al [Study on the constituents of the roots of Bupleurum marginatum.]. Zhongguo Yaoke Daxue Xuebao 2003; 34: 305–308. [in Chinese]. [Google Scholar]
- 36. Pistelli L. The chemistry and biological activity of the genus Bupleurum in Italy In: Pan S‐L, ed. Bupleurum Species: Scientific Evaluation and Clinical Applications. Boca Raton, FL: CRC/Taylor & Francis, 2006: 117–131. [Google Scholar]
- 37. Estevez‐Braun A et al Busaliol and busalicifol, two new tetrahydrofuran lignans from Bupleurum salicifolium . J Nat Prod 1995; 58: 887–892. [Google Scholar]
- 38. Gonzalez AG et al Salicifoliol, a new furolactone‐type lignan from Bupleurum salicifolium . J Nat Prod 1989; 52: 1139–1142. [Google Scholar]
- 39. Estevez‐Braun A et al 13C‐NMR assignemts of some dibenzyl‐gamma‐butyrolactone lignans. Phytochemistry 1996; 43: 885–886. [Google Scholar]
- 40. Estevez‐Braun A et al Lignanolides from Bupleurum salicifolium . Phytochemistry 1992; 31: 2841–2845. [Google Scholar]
- 41. Estevez‐Braun A et al Structural elucidation and conformational analysis of new lignan butenolides from the leaves of Bupleurum salicifolium . Tetrahedron 1994; 50: 5203–5210. [Google Scholar]
- 42. Estevez‐Reyes R et al New lignan butenolides from Bupleurum salicifolium . J Nat Prod 1993; 56: 1177–1181. [Google Scholar]
- 43. Liu Y et al Three new arylnaphthalide lignans from the aerial parts of Bupleurum marginatum WALL. ex DC. Helv Chim Acta 2008; 91: 2316–2320. [Google Scholar]
- 44. Lopez H et al Lignans from Bupleurum handiense . J Nat Prod 1996; 59: 493–494. [Google Scholar]
- 45. Gonzalez AG et al Umbelliferous compounds. IV. Lignans from Bupleurum fruiticescens . An Quim (1968–1979). 1975;71: 109–110. [Google Scholar]
- 46. Luo S‐Q et al Lignan glucosides from Bupleurum wenchuanense . Phytochemistry 1993; 33: 193–196. [DOI] [PubMed] [Google Scholar]
- 47. Zhang T et al Flavonoids from aerial part of Bupleurum chinense DC. Biochem Syst Ecol 2007; 35: 801–804. [Google Scholar]
- 48. Zhang T‐T et al [Chemical constituents of the aerial part of Bupleurum longicaule.]. Zhongguo Tianran Yaowu 2008; 6: 430–434. [in Chinese]. [Google Scholar]
- 49. Barrero AF et al Polyacetylenes, terpenoids and flavonoids from Bupleurum spinosum . Phytochemistry 1998; 48: 1237–1240. [Google Scholar]
- 50. Chang WL et al Immunosuppressive flavones and lignans from Bupleurum scorzonerifolium . Phytochemistry 2003; 64: 1375–1379. [DOI] [PubMed] [Google Scholar]
- 51. Kobayashi M et al Studies on the constituents of Umbelliferae plants. XVIII. : Minor constituents of Bupleuri Radix: occurrence of saikogenins, polyhydroxysterols, a trihydroxy C18 fatty acid, a lignan and a new chromone. Chem Pharm Bull 1990; 38: 3169–3171. [Google Scholar]
- 52. Liang H et al A new chromone glycoside from Bupleurum chinense . Chin Chem Lett 1998; 9: 69–70. [Google Scholar]
- 53. Tan L et al New isoflavonoside from Bupleurum scorzonerifolium . Chin Chem Lett 1998; 9: 71–73. [Google Scholar]
- 54. Barrero AF et al The chemistry and biological activity of the genus Bupleurum in Spain In: Pan S‐L, ed. Bupleurum Species: Scientific Evaluation and Clinical Applications. Boca Raton, FL: CRC/Taylor & Francis, 2006: 97–116. [Google Scholar]
- 55. Pistelli L et al Minor constituents from Bupleurum fruticosum roots. Phytochemistry 1996; 41: 1579–1582. [DOI] [PubMed] [Google Scholar]
- 56. Estévez‐Braun A, González AG. Coumarins. Nat Prod Rep 1997; 14: 465–475. [DOI] [PubMed] [Google Scholar]
- 57. Banerji MJ et al Occurrence of (‐)‐anomalin in Bupleurum falcatum Linn. (Umbelliferae). Indian J Chem B 1977; 15B: 293–294. [Google Scholar]
- 58. Barrero AF et al Lignans and polyacetylenes from Bupleurum acutifolium . J Nat Prod 1999; 62: 946–948. [DOI] [PubMed] [Google Scholar]
- 59. Morita M et al Polyacetylenes from roots of Bupleurum falcatum . Phytochemistry 1991; 30: 1543–1545. [Google Scholar]
- 60. Pistelli L et al Phenylpropanoids from Bupleurum fruticosum . J Nat Prod 1995; 58: 112–116. [Google Scholar]
- 61. Massanet GM et al Phenylpropanoids from Bupleurum fruticosum . Phytochemistry 1997; 44: 173–177. [Google Scholar]
- 62. Matsumoto T et al Stimulatory effect of a pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L., on G‐CSF secretion from intestinal epithelial cells. Int Immunopharmacol 2008; 8: 581–588. [DOI] [PubMed] [Google Scholar]
- 63. Wang Z et al Beneficial effect of Bupleurum polysaccharides on autoimmune disease induced by Campylobacter jejuni in BALB/c mice. J Ethnopharmacol 2009; 124: 481–487. [DOI] [PubMed] [Google Scholar]
- 64. Xu H et al Isolation and characterization of an anti‐complementary polysaccharide D3‐S1 from the roots of Bupleurum smithii . Int Immunopharmacol 2007; 7: 175–182. [DOI] [PubMed] [Google Scholar]
- 65. Kuang H et al New megastigmane sesquiterpene and indole alkaloid glucosides from the aerial parts of Bupleurum chinense DC. Fitoterapia 2009; 80: 35–38. [DOI] [PubMed] [Google Scholar]
- 66. Cheng PW et al Bupleurum kaoi inhibits Coxsackie B virus type 1 infection of CCFS‐1 cells by induction of type I interferons expression. Food Chem Toxicol 2007; 45: 24–31. [DOI] [PubMed] [Google Scholar]
- 67. Sun Y et al Saikosaponin a inhibits the proliferation and activation of T cells through cell cycle arrest and induction of apoptosis. Int Immunopharmacol 2009; 9: 978–983. [DOI] [PubMed] [Google Scholar]
- 68. Wong VKW et al Mechanistic study of saikosaponin‐d (ssd) on suppression of murine T lymphocyte activation. J Cell Biochem 2009; 107: 303–315. [DOI] [PubMed] [Google Scholar]
- 69. Matsumoto T et al A pectic polysaccharide isolated from the roots of Bupleurum falcatum L. stimulates the tyrosine phosphorylation of lipid rafts of murine B cells. Biol Pharm Bull 2008; 31: 931–934. [DOI] [PubMed] [Google Scholar]
- 70. Chen SM et al Effects of Bupleurum scorzoneraefolium, Bupleurum falcatum, and saponins on nephrotoxic serum nephritis in mice. J Ethnopharmacol 2008; 116: 397–402. [DOI] [PubMed] [Google Scholar]
- 71. Kim SY, Yun‐Choi HS. Platelet anti‐aggregating activities of bupleurumin from the aerial parts of Bupleurum falcatum . Arch Pharm Res 2007; 30: 561–564. [DOI] [PubMed] [Google Scholar]
- 72. Cho B‐S et al Effects of Bupleurum falcatum and its combination with an angiotensin II receptor blocker on cytokine and chemokine expression in human mesangial cells. Phytother Res 2010; 24: 339–343. [DOI] [PubMed] [Google Scholar]
- 73. Cheng P‐W et al Antiviral effects of saikosaponins on human coronavirus 229E in vitro . Clin Exp Pharmacol Physiol 2006; 33: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen Y‐L et al In vitro and in vivo studies of a novel potential anticancer agent of isochaihulactone on human lung cancer A549 cells. Biochem Pharmacol 2006; 72: 308–319. [DOI] [PubMed] [Google Scholar]
- 75. Chiang LC et al Cytotoxicity and anti‐hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med 2003; 69: 705–709. [DOI] [PubMed] [Google Scholar]
- 76. Chang JS et al Sho‐saiko‐to (Xiao‐Chai‐Hu‐Tang) and crude saikosaponins inhibit hepatitis B virus in a stable HBV‐producing cell line. Am J Chin Med 2007; 35: 341–351. [DOI] [PubMed] [Google Scholar]
- 77. Li Y et al In vitro anti‐Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol 2005; 98: 329–333. [DOI] [PubMed] [Google Scholar]
- 78. Sun XB et al Effects of a polysaccharide fraction from the roots of Bupleurum falcatum L. on experimental gastric ulcer models in rats and mice. J Pharm Pharmacol 1991; 43: 699–704. [DOI] [PubMed] [Google Scholar]
- 79. Yamamoto M et al Structure and actions of saikosaponins isolated from Bupleurum falcatum L. I. Anti‐inflammatory action of saikosaponins. Arzneimittelforschung 1975; 25: 1021–1023. [PubMed] [Google Scholar]
- 80. Yamada H et al Purification of anti‐ulcer polysaccharides from the roots of Bupleurum falcatum . Planta Med 1991; 57: 555–559. [DOI] [PubMed] [Google Scholar]
- 81. Matsumoto T et al Role of polymorphonuclear leucocytes and oxygen‐derived free radicals in the formation of gastric lesions induced by HCl/ethanol, and a possible mechanism of protection by anti‐ulcer polysaccharide. J Pharm Pharmacol 1993; 45: 535–539. [DOI] [PubMed] [Google Scholar]
- 82. Matsumoto T et al Effect of the antiulcer polysaccharide fraction from Bupleurum falcatum L. on the healing of gastric ulcer induced by acetic acid in rats. Phytother Res 2002; 16: 91–93. [DOI] [PubMed] [Google Scholar]
- 83. Yamaguchi N et al Effect of saikosaponin derivatives upon the immune response against T‐dependent and T‐independent antigens in mice. Int J Immunopharmacol 1985; 7: 827–832. [DOI] [PubMed] [Google Scholar]
- 84. Ohuchi K et al Pharmacological influence of saikosaponins on prostaglandin E2 production by peritoneal macrophages. Planta Med 1985; 3: 208–212. [DOI] [PubMed] [Google Scholar]
- 85. Abe H et al Pharmacological actions of saikosaponins isolated from Bupleurum falcatum. 1. Effects of saikosaponins on liver function. Planta Med 1980; 40: 366–372. [DOI] [PubMed] [Google Scholar]
- 86. Abe H et al Protective effect of saikosaponin‐d isolated from Bupleurum falcatum L. on CCl4‐induced liver injury in the rat. Naunyn Schmiedebergs Arch Pharmacol 1982; 320: 266–271. [DOI] [PubMed] [Google Scholar]
- 87. Abe H et al Effects of saikosaponin‐d on enhanced CCl4‐hepatotoxicity by phenobarbitone. J Pharm Pharmacol 1985; 37: 555–559. [DOI] [PubMed] [Google Scholar]
- 88. Park KH et al Effect of saikosaponin‐A, a triterpenoid glycoside, isolated from Bupleurum falcatum on experimental allergic asthma. Phytother Res 2002; 16: 359–363. [DOI] [PubMed] [Google Scholar]
- 89. Kumazawa Y et al Activation of murine peritoneal macrophages by saikosaponin a, saikosaponin d and saikogenin d. Int J Immunopharmacol 1989; 11: 21–28. [DOI] [PubMed] [Google Scholar]
- 90. Kato M et al Cell type‐oriented differential modulatory actions of saikosaponin‐d on growth responses and DNA fragmentation of lymphocytes triggered by receptor‐mediated and receptor‐bypassed pathways. Immunopharmacology 1995; 29: 207–213. [DOI] [PubMed] [Google Scholar]
- 91. Ushio Y, Abe H. The effects of saikosaponin on macrophage functions and lymphocyte proliferation. Planta Med 1991; 57: 511–514. [DOI] [PubMed] [Google Scholar]
- 92. Kyo R et al Antagonism of saikosaponin‐induced prostaglandin E2 release by baicalein in C6 rat glioma cells. Biol Pharm Bull 1999; 22: 1385–1387. [DOI] [PubMed] [Google Scholar]
- 93. Fan J et al Saikosaponin‐d attenuates the development of liver fibrosis by preventing hepatocyte injury. Biochem Cell Biol 2007; 85: 189–195. [DOI] [PubMed] [Google Scholar]
- 94. Ushio Y, Abe H. Inactivation of measles virus and herpes simplex virus by saikosaponin d. Planta Med 1992; 58: 171–173. [DOI] [PubMed] [Google Scholar]
- 95. Kodama Y et al Dual effect of saikogenin D: in vitro inhibition of prostaglandin E2 production and elevation of intracellular free Ca2+ concentration in C6 rat glioma cells. Planta Med 2003; 69: 765–767. [DOI] [PubMed] [Google Scholar]
- 96. Just MJ et al Anti‐inflammatory activity of unusual lupane saponins from Bupleurum fruticescens . Planta Med 1998; 64: 404–407. [DOI] [PubMed] [Google Scholar]
- 97. Prieto JM et al Dual inhibition of cyclooxygenase‐1 and 5‐lipoxygenase by aerial part of Bupleurum fruticescens methanol extract. Fitoterapia 2004; 75: 179–186. [DOI] [PubMed] [Google Scholar]
- 98. Bremner P et al Assessing medicinal plants from South‐Eastern Spain for potential anti‐inflammatory effects targeting nuclear factor‐Kappa B and other pro‐inflammatory mediators. J Ethnopharmacol 2009; 124: 295–305. [DOI] [PubMed] [Google Scholar]
- 99. Manunta A et al [L’ Huile essentielle du Bupleurum fruticosum L.]. Plantes Med Phytother 1987; 21: 20–25. [in French]. [Google Scholar]
- 100. Guinea MC et al Biologically active triterpene saponins from Bupleurum fruticosum . Planta Med 1994; 60: 163–167. [DOI] [PubMed] [Google Scholar]
- 101. Bremner P et al Phenylpropanoid NF‐κB inhibitors from Bupleurum fruticosum . Planta Med 2004; 70: 914–918. [DOI] [PubMed] [Google Scholar]
- 102. Utrilla MP et al Isolation of a saikosaponin responsible for the antiinflammatory activity of Bupleurum gibraltaricum lam. root extract. Phytother Res 1991; 5: 43–45. [Google Scholar]
- 103. Lin CC et al The pharmacological and pathological studies on Taiwan folk medicine (III): The effects of Bupleurum kaoi and cultivated Bupleurum falcatum var. komarowi. Am J Chin Med 1990; 18: 105–112. [DOI] [PubMed] [Google Scholar]
- 104. Wang BJ et al Hepatoprotective and antioxidant effects of Bupleurum kaoi Liu (Chao et Chuang) extract and its fractions fractionated using supercritical CO2on CCl4‐induced liver damage. Food Chem Toxicol 2004; 42: 609–617. [DOI] [PubMed] [Google Scholar]
- 105. Yen MH et al The hepatoprotective effect of Bupleurum kaoi, an endemic plant to Taiwan, against dimethylnitrosamine‐induced hepatic fibrosis in rats. Biol Pharm Bull 2005; 28: 442–448. [DOI] [PubMed] [Google Scholar]
- 106. Hsu YL et al The antiproliferative activity of saponin‐enriched fraction from Bupleurum Kaoi is through Fas‐dependent apoptotic pathway in human non‐small cell lung cancer A549 cells. Biol Pharm Bull 2004; 27: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 107. Benito PB et al In vivo and in vitro antiinflammatory activity of saikosaponins. Life Sci 1998; 63: 1147–1156. [DOI] [PubMed] [Google Scholar]
- 108. Bermejo P et al Antiviral activity of seven iridoids, three saikosaponins and one phenylpropanoid glycoside extracted from Bupleurum rigidum and Scrophularia scorodonia . Planta Med 2002; 68: 106–110. [DOI] [PubMed] [Google Scholar]
- 109. Navarro P et al In vivo anti‐inflammatory activity of saponins from Bupleurum rotundifolium . Life Sci 2001; 68: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 110. Fujioka T et al Antiproliferative constituents from Umbelliferae plants VI. New ursane‐type saikosaponin analogs from the fruits of Bupleurum rotundifolium . Chem Pharm Bull 2003; 51: 365–372. [DOI] [PubMed] [Google Scholar]
- 111. Gonzalez JA et al Biological activity of secondary metabolites from Bupleurum salicifolium (Umbelliferae). Experientia 1995; 51: 35–39. [PubMed] [Google Scholar]
- 112. Estevez‐Braun A et al Antibiotic activity and absolute configuation of 8S‐heptadeca‐2(Z),9(Z)‐diene‐4,6‐diyne‐1,8‐diol from Bupleurum salicifolium . J Nat Prod 1994; 57: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 113. Cheng YL et al Acetone extract of Bupleurum scorzonerifolium inhibits proliferation of A549 human lung cancer cells via inducing apoptosis and suppressing telomerase activity. Life Sci 2003; 73: 2383–2394. [DOI] [PubMed] [Google Scholar]
- 114. Chen YL et al Requirement for ERK activation in acetone extract identified from Bupleurum scorzonerifolium induced A549 tumor cell apoptosis and keratin 8 phosphorylation. Life Sci 2005; 76: 2409–2420. [DOI] [PubMed] [Google Scholar]
- 115. Cheng YL et al Anti‐proliferative activity of Bupleurum scrozonerifolium in A549 human lung cancer cells in vitro and in vivo . Cancer Lett 2005; 222: 183–193. [DOI] [PubMed] [Google Scholar]
- 116. Matsuda H et al New hepatoprotective saponins, bupleurosides III, VI, IX, and XIII, from Chinese Bupleuri Radix: structure‐requirements for the cytoprotective activity in primary cultured rat hepatocytes. Bioorg Med Chem Lett 1997; 7: 2193–2198. [Google Scholar]
- 117. Gil ML et al Comparative study of different essential oils of Bupleurum gibraltaricum Lamarck. Pharmazie 1989; 44: 284–287. [PubMed] [Google Scholar]
- 118. Yano S et al Antiulcer activities of glycyrrhetinic acid derivatives in experimental gastric lesion models. Chem . Pharm Bull 1989; 37: 2500–2504. [DOI] [PubMed] [Google Scholar]
- 119. Liu CT et al Antioxidative and in vitro hepatoprotective activity of Bupleurum kaoi leaf infusion. Phytother Res 2006; 20: 1003–1008. [DOI] [PubMed] [Google Scholar]
- 120. Wu SJ et al Curcumin or saikosaponin a improves hepatic antioxidant capacity and protects against CCl4‐induced liver injury in rats. J Med Food 2008; 11: 224–229. [DOI] [PubMed] [Google Scholar]
- 121. Motoo Y, Sawabu N. Antitumor effects of saikosaponins, baicalin and baicalein on human hepatoma cell lines. Cancer Lett 1994; 86: 91–95. [DOI] [PubMed] [Google Scholar]
- 122. Yin F et al Saikosaponins from Bupleurum chinense and inhibition of HBV DNA replication activity. Nat Prod Commun 2008; 3: 155–157. [Google Scholar]
- 123. Du X‐M et al The pharmacological activity of chinese ‘Chaihu’–the root of Bupleurum species In: Pan S‐L, ed. Bupleurum Species: Scientific Evaluation and Clinical Applications. Boca Raton, FL: CRC/Taylor & Francis, 2006: 135–149. [Google Scholar]
- 124. Kumazawa Y et al Protective effect of saikosaponin A, saikosaponin D and saikogenin D against Pseudomonas aeruginosa infection in mice. Int J Immunopharmacol 1990; 12: 531–537. [DOI] [PubMed] [Google Scholar]
- 125. Wink M. Evolutionary advantage and molecular modes of action of multi‐component mixtures used in phytomedicine. Curr Drug Metab 2008; 9: 996–1009. [DOI] [PubMed] [Google Scholar]
- 126. Wink M. Importance of plant secondary metabolites for protection against insects and microbial infections In: Rai M, Carpinella MC, eds. Advances in Phytomedicine Series: Naturally Occurring Bioactive Compounds. Amsterdam: Elsevier, 2006: 251. [Google Scholar]
- 127. Kuljanabhagavad T, Wink M. Biological activities and chemistry of saponins from Chenopodium quinoa Willd. Phytochem Rev 2009; 8: 473–490. [Google Scholar]
- 128. Wink M. Molecular modes of action of cytotoxic alkaloids: from DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance In: Cordell GA, ed. The Alkaloids: Chemistry and Biology series, 64. California, USA: Academic Press, 2007: 1–47. [DOI] [PubMed] [Google Scholar]
- 129. Lobo R et al Curcuma zedoaria Rosc. (white turmeric): a review of its chemical, pharmacological and ethnomedicinal properties. J Pharm Pharmacol 2009; 61: 13–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Chemical structures of the isolated triterpene saponins.
Table S2 Chemical structures of the isolated sterols.
Table S3 Chemical structures of the isolated lignans.
Table S4 Chemical structures of the isolated flavonoids and related compounds.
Table S5 Chemical structures of the isolated coumarins.
Table S6 Chemical structures of the isolated phenylpropanoids.
Table S7 Chemical structures of the isolated polyacetylenes.
Supporting info item


