Dear Editor:
Potentially relevant to the recent appearance of COVID-19 in China is the fact that there is a belt of selenium deficiency running from northeast to southwest in the country and, indeed, China has populations that have both the lowest and the highest selenium status in the world (1). A set of interesting studies published by the Beck laboratory in the 1990s showed that host selenium deficiency increased the virulence of RNA viruses such as coxsackievirus B3 and influenza A (2, 3). Passage through a selenium-deficient animal that was unable to produce sufficient antioxidant selenoproteins for its own protection resulted in the virus mutating to a virulent form that caused more severe pathology (2, 3). Those findings shed light on a human selenium-deficiency disease, a cardiomyopathy known as Keshan disease, named after the area in northeast China where it was endemic. The disease showed a seasonal variation, suggesting a viral cofactor that was later identified as coxsackievirus B3 (2). When the population was supplemented with selenium, the incidence of Keshan disease decreased dramatically (1, 2).
Significant clinical benefits of selenium supplementation have also been demonstrated in other viral infections, as reviewed previously (4, 5), including HIV-1 [where a negative correlation between selenium status and mortality has been established (1, 6)]; in liver cancer linked to hepatitis B; and in patients with “epidemic hemorrhagic fever” that was successfully treated with oral sodium selenite, giving an overall 80% reduction in mortality (4, 7). As such, selenium appears relevant to a number of evolutionarily distinct viruses, via potential immunomodulatory effects that are fully consistent with the many essential roles of selenium in the immune system (2) and its ability (especially in deficiency) to influence viral mutation and evolution (3). These and other studies prompted us to hypothesize that selenium status was associated with COVID-19 disease outcome in China.
In this population-based, retrospective analysis, we collected real-time data from the Baidu website, a nongovernmental website that provides daily updates of the reports of the health commissions of each province, municipality, or city on numbers of COVID-19 confirmed cases, numbers cured, and numbers who died (8). [According to the National Health Commission of China, cured patients are those in whom temperature has returned to normal for >3 d, respiratory symptoms are significantly improved, lung imaging shows significant reduction of inflammation, and there is a negative nucleic acid test of respiratory pathogen on 2 consecutive occasions with a sampling interval of at least 1 d (9).] Cure rate and death rate were defined as percentage of patients cured or who died, respectively, from infection with SARS-CoV-2. We tracked the course of the outbreak from 14 February and chose data from 18 February as a “snapshot” of the progress of the outbreak to that date. We included provinces or municipalities with >200 cases and cities with >40 cases (Supplemental Table 1).
The largest data sets available on selenium status in China are on hair selenium concentration (Supplemental Table 2), which, in a previous study, was found to be highly correlated with selenium intake in different Chinese districts (R 2 = 0.74) (10). Data on hair selenium are generally more available for cities. Seventeen cities outside Hubei Province included in the study had documented hair selenium data (Supplemental Table 2).
We compared cure rate and death rate using the Stata prtest to compare 2 proportions (StataCorp 2019 Stata Statistical Software: Release 16). The prtest of the difference of 2 proportions uses an asymptotically normally distributed test statistic derived from the proportions and the SE of the difference. Associations between cure rates and mean regional or city hair selenium concentration were analyzed by fitting weighted linear regression models, weighted by the number of cases. P values (2-sided tests) from the F test of overall significance are presented.
The cure rate inside Hubei Province, of which Wuhan is the capital, was significantly lower than that in all other provinces combined (designated outside-Hubei): 13.2% compared with 40.6%, respectively (P < 0.0001; Supplemental Table 1). Correspondingly, the death rate inside Hubei Province was significantly higher than the death rate in provinces outside-Hubei: 3.0% compared with 0.6%, respectively (P < 0.0001; Supplemental Table 1). These analyses show that the outcome data for Hubei and outside-Hubei are statistically distinct, necessitating the separate treatment of Hubei (where mortality was much higher) and outside-Hubei in our subsequent analyses.
On inspection of the Hubei data, it is notable that the cure rate in Enshi city, at 36.4%, was much higher than that of other Hubei cities, where the overall cure rate was 13.1% (Supplemental Table 1); indeed, the Enshi cure rate was significantly different from that in the rest of Hubei (P < 0.0001). Enshi is renowned for its high selenium intake and status [mean ± SD: hair selenium: 3.13 ± 1.91 mg/kg for females and 2.21 ± 1.14 mg/kg for males (11)]—compare typical levels in Hubei of 0.55 mg/kg (10)—so much so that selenium toxicity was observed there in the 1960s (11, 12). Selenium intake in Enshi was reported as 550 µg/d in 2013 (11).
Similar inspection of data from provinces outside Hubei shows that Heilongjiang Province in northeast China, a notoriously low-selenium region in which Keshan is located, had a much higher death rate, at 2.4%, than that of other provinces (0.5%; P < 0.0001). The selenium intake was recorded as only 16 µg/d in a 2018 publication (13), while hair selenium in the Songnen Plain of Heilongjiang was measured as only 0.26 mg/kg (Supplemental Table 2) (10, 13).
Finally, we found a significant association between cure rate and background selenium status in cities outside Hubei (R 2 = 0.72, F test P < 0.0001; Figure 1, Supplemental Table 2). No correlation analysis was done for cities inside Hubei because selenium status was only available for 2 cities.
FIGURE 1.
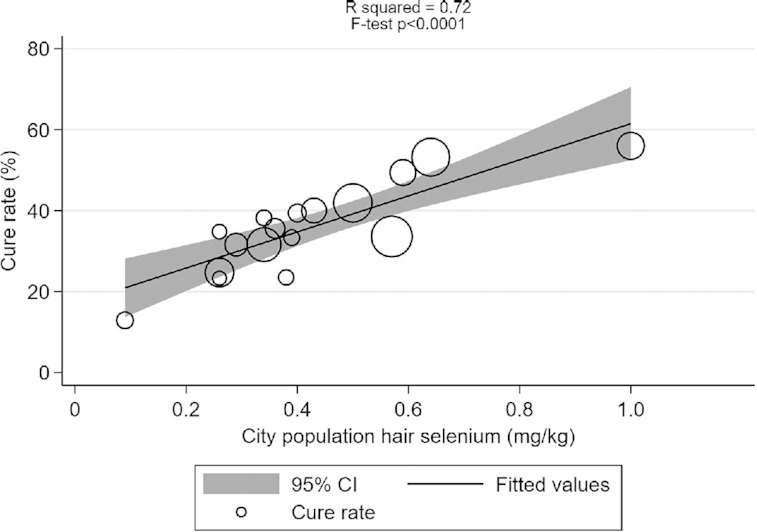
Correlation between COVID-19 cure rate in 17 cities outside Hubei, China, on 18 February, 2020 and city population selenium status (hair selenium concentration) analyzed using weighted linear regression (mean ± SD = 35.5 ± 11.1, R2 = 0.72, F test P < 0.0001). Each data point represents the cure rate, calculated as the number of cured patients divided by the number of confirmed cases, expressed as a percentage. The size of the marker is proportional to the number of cases.
Our results show an association between the reported cure rates for COVID-19 and selenium status. These data are consistent with the evidence of the antiviral effects of selenium from previous studies (2, 5, 6, 7, 14). Indeed, multiple cellular and viral mechanisms involving selenium and selenoproteins could influence viral pathogenicity, including virally encoded selenium-dependent glutathione peroxidases [reviewed in (14, 15)]. Such viral mechanisms could contribute to the well-documented oxidative stress associated with many RNA virus infections (2, 5, 6, 14, 15); increased viral replication (hence increased mutation rate); and observed higher pathogenicity or mortality under selenium deficiency, as reported here for SARS-CoV-2.
As with most ecological studies, our study has several important limitations. The association between hair selenium and COVID-19 cure rate that we note is based on city population selenium status data, mostly dating from 2011, although some data are considerably older. Furthermore, we were unable to collect city- or patient-level data for the following likely confounders: age and comorbidities such as cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and cancer (16). We also lack information on variation in medical facilities and therapy protocols (including the use of traditional Chinese medicine or anti-viral therapies). Clearly, we were not able to adjust for these possible confounders in the analysis.
We are fully aware, therefore, that the association shown is far from being robust to criticisms of confounding. At best, it points towards the need for further research, particularly when viewed in the context of associations between selenium status and disease outcome found with other viruses (3, 5, 6, 7). In due course, more individual-level data will emerge, and the association between the severity of COVID-19 and many factors, including selenium, can be explored.
Footnotes
The authors reported no funding received for this study. The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
The authors’ responsibilities were as follows—JZ and EWT: joint first authors; EWT and MPR: designed the study; JZ: researched the data on COVID-19 cases, cure rate, and death rate; KB: performed the data analysis and created the figure; RS: described a counterintuitive severity ratio requiring the recognition of an environmental factor; MPR: drafted the manuscript; and all authors: contributed to creating the data tables and read and approved the final manuscript.
SUPPORTING INFORMATION
nqaa095_Supplemental_File
References
- 1.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck MA, Handy J, Levander OA. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004;12:417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor EW, Ruzicka JA, Premadasa L, Zhao L. Cellular selenoprotein mRNA tethering via antisense interactions with Ebola and HIV-1 mRNAs may impact host selenium biochemistry. Curr Top Med Chem. 2016;16:1530–1535. doi: 10.2174/1568026615666150915121633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbrenner H, Al-Quraishy S, Dkhil MA, Wunderlich F, Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, Sauberlich H, Page JB. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hou JC. Inhibitory effect of selenite and other antioxidants on complement-mediated tissue injury in patients with epidemic hemorrhagic fever. Biol Trace Elem Res. 1997;56:125–130. doi: 10.1007/BF02778988. [DOI] [PubMed] [Google Scholar]
- 8.Baidu. Epidemic real-time big data report. [Internet] [cited 18 February, 2020]. Available from: https://voice.baidu.com/act/newpneumonia/newpneumonia/?from=osari_pc_3.
- 9.Baidu. Commission answered the questions of covered journalists: the discharge standard of patients with new coronary pneumonia is unified nationally [Internet] [cited 18 February, 2020]. Available from: https://baijiahao.baidu.com/s?id=1657688134616589632&wfr=spider&for=pc.
- 10.Li S, Banuelos GS, Wu L, Shi W. The changing selenium nutritional status of Chinese residents. Nutrients. 2014;6:1103–1114. doi: 10.3390/nu6031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Wang Q, Gao J, Lin Z, Banuelos GS, Yuan L, Yin X. Daily dietary selenium intake in a high selenium area of Enshi, China. Nutrients. 2013;5:700–710. doi: 10.3390/nu5030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang GQ, Xia YM. Studies on human dietary requirements and safe range of dietary intakes of selenium in China and their application in the prevention of related endemic diseases. Biomed Environ Sci. 1995;8:187–201. [PubMed] [Google Scholar]
- 13.Dinh QT, Cui Z, Huang J, Tran TAT, Wang D, Yang W, Zhou F, Wang M, Yu D, Liang D. Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int. 2018;112:294–309. doi: 10.1016/j.envint.2017.12.035. See also Online Supplementary Material, Table S5, Human daily dietary Se intake in China. [DOI] [PubMed] [Google Scholar]
- 14.Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Cox AG, Ruzicka JA, Bhat AA, Zhang W, Taylor EW. Molecular modeling and in vitro activity of an HIV-1-encoded glutathione peroxidase. Proc Natl Acad Sci USA. 2000;97:6356–6361. doi: 10.1073/pnas.97.12.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epidemiological Group of Emergency Response Mechanism of New Coronavirus Pneumonia, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China [J] Chinese J Epidemiol. 2020;41(2):145–151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nqaa095_Supplemental_File


