As hospitals worldwide continue to admit an influx of coronavirus disease 2019 (COVID-19) patients, the puzzling pathogenesis behind the witnessed mortality rates is progressively being pieced together. Aside from the established respiratory involvement, the cardiac system has recently been implicated, albeit with controversial mechanisms.1 New data from cohort studies and autopsies suggest a potential role for coagulopathy in COVID-19. Although the exact mechanism may likewise remain controversial, several clinical implications are now imperative for discussion.
In a retrospective multicentre cohort study from China, charts of 191 adult patients with laboratory-confirmed COVID-19 were analysed. Of the 137 surviving patients, only one reported cardiac injury, in contrast to 32 of 54 (59%) of non-surviving patients. Aside from old age and sequential organ failure assessment scores, multivariable regression showed increasing odds of in-hospital death associated with D-dimer levels >1 μg/mL (P = 0.0033) on admission. Additionally, coagulopathy was common in 27 (50%) of 54 non-surviving patients, compared with 10 of 137 (7%) surviving patients (P < 0.0001). Since sepsis is known to be complicated by coagulopathy and is a potential controversial mechanism behind the high rates reported in COVID-19 patients, it should be mentioned that 38 (70%) of non-surviving patients had sepsis, compared with none of the surviving patients. Therefore, as per this cohort, sepsis in COVID-19 patients was not a necessary preceding condition for the development of coagulopathy.1
Additionally, although COVID-19 patients seem to have coagulative abnormalities, they are not typical of disseminated intravascular coagulopathy (DIC) of the kind seen in septicaemia. In DIC, thrombocytopenia (platelet count <150 × 109/L) is a key finding along with elevated clotting time. However, in cohorts such as the one above, non-surviving patients had an average platelet count of 165.5 × 109/L (107.0–229.0), and a prothrombin time of 11.4 s (10.4–12.6) which falls within the normal range (11– 13.5 s). It thus may be more likely that local rather than disseminated thrombin generation is at play in COVID-19 patients. In addition to platelet counts, fibrinogen can be used to differentiate COVID-19-related coagulopathy from typical acute DIC, where fibrinogen levels would normally go down. We have also seen COVID-19-confirmed patients in our practice not presenting with typical acute DIC, but rather significantly elevated D-dimers and fibrinogen levels, while platelet count did not change. A recent study that reported a 27% cumulative incidence of CT pulmonary angiogram and/or ultrasonography confirmed venous thromboembolism in 184 ICU patients with proven COVID-19 pneumonia, none of which actually developed DIC.2 Lung microthrombi formation has also been confirmed in autopsy reports.3
The mechanisms of thrombus formation are variable. Generally with viruses, evidence suggests that inflammation of immune and non-immune cells may lead to an imbalance of pro- and anticoagulant states during infection. Since the endothelium plays an important role in homeostasis regulation, and since it is disrupted in viral infections, risk of haematopathology is imminent. Additionally, viral infection-induced elevation of von Willebrand factor, Toll-like receptor activation, and tissue factor pathway activation may play a role in the coagulant cascade that follows, leading to formation of cross-linked fibrin clots. The breakdown of these clots as per the physiological response to excessive activation of the coagulation cascade is responsible for the procoagulant D-dimer markers. Upon antigen recognition, platelets are additionally activated, coordinating with white blood cells for pathogen clearance and clot formation. Immune cells, platelets, and endothelial cells thus all play a role in the coagulant state of viral infections.4 Additionally, considering the long durations of bed rest for COVID-19 patients, venous thrombo-embolisms cannot be excluded as a possible source of coagulation.
In view of the current limited evidence, and the urgency of the topic, the decision to anticoagulate or not is contentious. In a retrospective study of 449 patients with severe COVID-19 in China, 99 of which received heparin [mainly low-molecular weight heparin (LMWH)] for at least 7 days, it was found that prothrombin time was positively correlated with 28-day mortality, and platelet count negatively correlated. Generally, no significant difference in 28-day mortality was found between heparin users and non-users (30.3% vs. 29.7%, P = 0.910). However, 28-day mortality of heparin users was lower than that of non-users in patients with D-dimer levels more than six-fold the upper limit of normal (32.8% vs. 52.4%, P = 0.017), and those with sepsis-induced coagulopathy scores of >4 (40.0% vs. 64.2%, P = 0.029).5 It is important to note that the authors did not report specific doses here but rather stated that most patients were on prophylactic doses of heparin. Thus, it is important to investigate whether such data are applicable in centres where all admitted intensive care unit (ICU) patients generally receive deep venous thrombosis prophylaxis. The American Society of Hematology has also noted this fact in response to frequently asked questions.6 Despite this, the findings still highlight the potential role of anticoagulation in specific COVID-19 patients for improved mortality, and the need for a tailored approach.
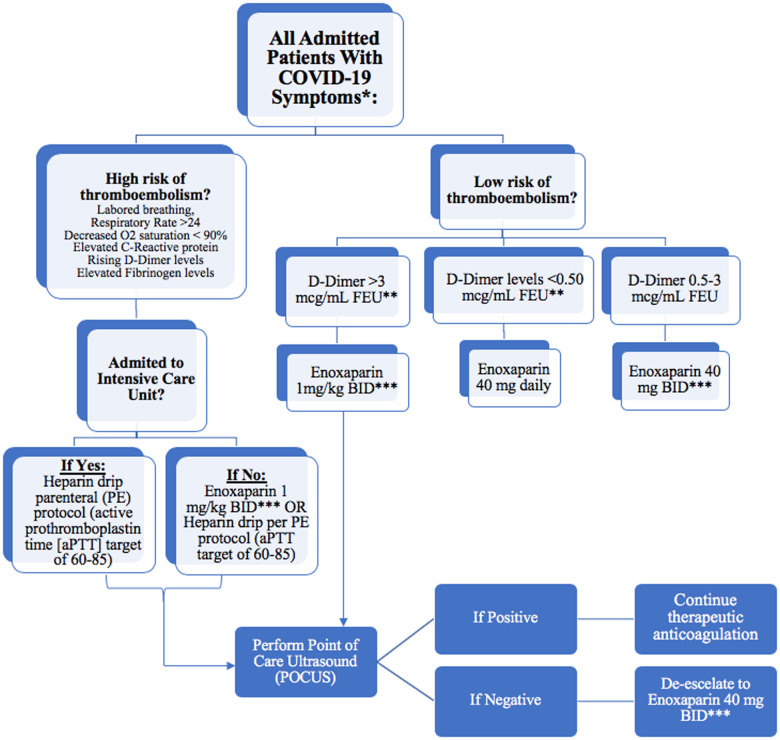
Heparin has been implicated in binding to COVID-19 spike proteins as well as down-regulating interleukin-6 (IL-6),7 which has been shown to be elevated in COVID-19 patients1, and thus unfractioned heparin or LMWH remains as the best choice of anticoagulant for admitted patients. It is possible that these patients may even require continued anticoagulation for a certain period of time following hospital discharge. We hereby recommend a patient-tailored algorithm that utilizes a specific stratification based on clinical and laboratory presentation to design a patient-specific anticoagulation regimen for admitted COVID-19 patients (Figure 1). We thus suggest implementing diagnostic tests for all admitted patients rather than for those with thrombotic complications only. It is important to note that the cut-off values for these diagnostic tests and the response to them continue to be a moving target as more data emerge on this unique pandemic.
Figure 1.
Tailored algorithm/protocol for the management of coagulopathy in COVID-19 patients. *High bleeding risk patients are excluded. Also exclude patients with platelet count <50 000; INR >2. **FEU, fibrinogen equivalent unit. ***Adjust enoxaparin dose for renal failure.
Conflict of interest: none declared.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers DA, Kant KM, Kaptein FH, van Paassen J, Stals MA, Huisman MV, Endeman H.. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T.. [A pathological report of three COVID-19 cases by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi 2020;49:E009. [DOI] [PubMed] [Google Scholar]
- 4. Giannis D, Ziogas IA, Gianni P.. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020;9:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z.. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreuziger L, Lee A, Garcia D, Cuker A, Cushman M, Connors J. COVID-19 and VTE-anticoagulation. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
- 7. Mummery RS, Rider CC.. Characterization of the heparin-binding properties of IL-6. J Immunol 2000;165:5671–569. [DOI] [PubMed] [Google Scholar]