Abstract
The novel coronavirus disease (COVID-19) outbreak, caused by SARS-CoV-2, represents the greatest medical challenge in decades. We provide a comprehensive review of the clinical course of COVID-19, its comorbidities, and mechanistic considerations for future therapies. While COVID-19 primarily affects the lungs, causing interstitial pneumonitis and severe acute respiratory distress syndrome (ARDS), it also affects multiple organs, particularly the cardiovascular system. Risk of severe infection and mortality increase with advancing age and male sex. Mortality is increased by comorbidities: cardiovascular disease, hypertension, diabetes, chronic pulmonary disease, and cancer. The most common complications include arrhythmia (atrial fibrillation, ventricular tachyarrhythmia, and ventricular fibrillation), cardiac injury [elevated highly sensitive troponin I (hs-cTnI) and creatine kinase (CK) levels], fulminant myocarditis, heart failure, pulmonary embolism, and disseminated intravascular coagulation (DIC). Mechanistically, SARS-CoV-2, following proteolytic cleavage of its S protein by a serine protease, binds to the transmembrane angiotensin-converting enzyme 2 (ACE2) —a homologue of ACE—to enter type 2 pneumocytes, macrophages, perivascular pericytes, and cardiomyocytes. This may lead to myocardial dysfunction and damage, endothelial dysfunction, microvascular dysfunction, plaque instability, and myocardial infarction (MI). While ACE2 is essential for viral invasion, there is no evidence that ACE inhibitors or angiotensin receptor blockers (ARBs) worsen prognosis. Hence, patients should not discontinue their use. Moreover, renin–angiotensin–aldosterone system (RAAS) inhibitors might be beneficial in COVID-19. Initial immune and inflammatory responses induce a severe cytokine storm [interleukin (IL)-6, IL-7, IL-22, IL-17, etc.] during the rapid progression phase of COVID-19. Early evaluation and continued monitoring of cardiac damage (cTnI and NT-proBNP) and coagulation (D-dimer) after hospitalization may identify patients with cardiac injury and predict COVID-19 complications. Preventive measures (social distancing and social isolation) also increase cardiovascular risk. Cardiovascular considerations of therapies currently used, including remdesivir, chloroquine, hydroxychloroquine, tocilizumab, ribavirin, interferons, and lopinavir/ritonavir, as well as experimental therapies, such as human recombinant ACE2 (rhACE2), are discussed.
Keywords: COVID-19, Cardiac, Vascular, Microvascular, Endothelium, ACE2, Myocarditis, Virus, Acute coronary syndrome, Myocardial infarction
This manuscript was independently handled by Deputy Editor Professor Charalambos Antoniades
Introduction
The novel coronavirus COVID-19 outbreak, first reported on 8 December 2019 in Hubei province in China, was designated as a pandemic by the World Health Organization (WHO) on 11 March 2020. This disease, recognized as an infection with a new betacoronavirus by Dr Zhang Jixian from Hubei Provincial Hospital of Integrated Chinese and Western Medicine, has been spreading exponentially in almost all countries around the world. The epicentre shifted from China to Europe in February/March 2020 and then to the USA in March/April 2020. Current data presenting information on international case numbers and case fatality are provided by the Johns Hopkins University (JHU) Coronavirus Resource Center (https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6).1,2 There are several other web-based resources that provide informative graphics on the spread of the disease and the outcomes. The pandemic of COVID-19 has multiple medical, psychological, and socio-economic consequences. COVID-19 represents probably the greatest threat that societies will face in the 21st century. Therefore, understanding its pathophysiology and clinical implications, and development of novel preventive and therapeutic strategies are of primary importance.
Based on reviewing the available data in the public databases, the risk of infection and mortality increases with advancing age and shows sexual dimorphism. Male elderly individuals are at the highest risk of infection, as well as death.
Despite the tropism for the lungs where it causes interstitial pneumonitis, in the most severe cases multiorgan failure develops. The cardiovascular (CV) system appears to have complex interactions with COVID-19. Published reports, medRxiv, bioRxiv, and personal communications and experience of the co-authors detail evidence of myocardial injury in 20–40% of hospitalized cases manifesting as cardiac chest pain, fulminant heart failure, cardiac arrhythmias, and cardiac death. Indeed, symptoms of cardiac chest pain and palpitations are the presenting features in some patients.3–6
While COVID-19 is non-discriminatory, involving both healthy persons and those with comorbid conditions, approximately half of those admitted to hospitals in Huabei province with COVID-19 had known comorbidities. The number of patients with comorbid conditions increased to about two-thirds in those requiring intensive care unit (ICU) admission or those that did not survive. Patients with pre-existing CV conditions (hypertension in particular) had the highest morbidity (10.5%) following infection.7,8 Non-CV comorbidities, including diabetes, lung diseases, and obesity, the latter identified in current Italian and Dutch cohorts, are also major predictors of poor clinical outcomes. Similarly, in the recent analysis of 5700 Patients Hospitalized With COVID-19 in the New York City Area the most common comorbidities were hypertension (57%), obesity (42%), and diabetes (34%).167 These aspects emphasize the importance of the need for multidisciplinary assessment and treatment, including CV evaluation and therapy, during the course of COVID-19 to reduce mortality. In this rapid review, we summarize the state-of-the-art knowledge available currently, regarding COVID-19, focusing on key mechanistic and clinical aspects.
Properties of SARS-CoV-2
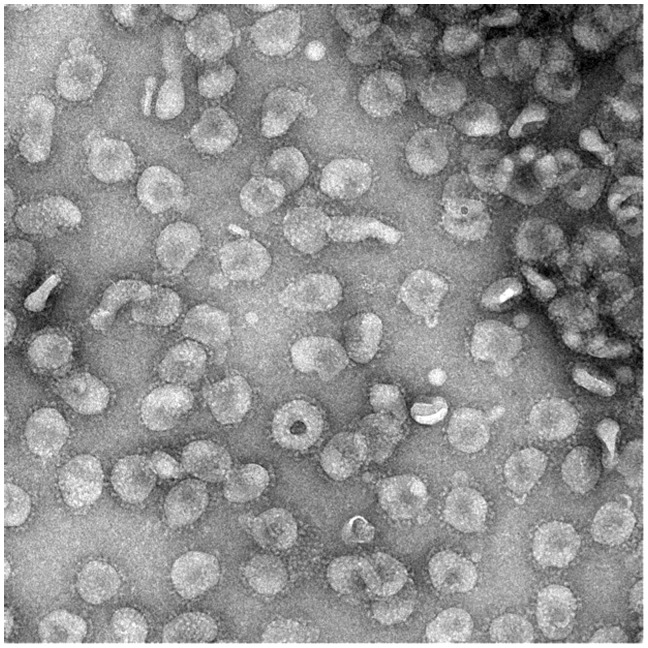
Coronaviruses are single-stranded positive-sense RNA viruses of between 26 and 32 kb in length within the family Coronaviridae. There are four genera in the subfamily Orthocoronavirinae, namely the alpha-, beta-, gamma-, and deltacoronaviruses. Of these, alpha- and betacoronaviruses infect mammals while the gamma- and deltacoronaviruses infect birds. There are seven coronaviruses that infect humans: the alphacoronaviruses HCoV-NL63 and 229E, which tend to cause a mild illness in adults; the betacoronaviruses Middle east respiratory syndrome (MERS) virus and severe acute respiratory syndrome (SARS) virus, which cause a severe respiratory illness; and OC43 and HKU1, which are associated with a mild illness. An example electron microscopy image of a betacoronavirus is shown in Figure 1. COVID-19 is caused by a novel betacoronavirus, probably originating from bats following gain-of-function mutations within the receptor-binding domain (RBD) and the acquisition of a furin-protease cleavage site. It has been named by the WHO as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).9
Figure 1.
Characteristic structure of betacoronavirus. Negative stain electron microscopy showing a betacoronavirus particles with club-shaped surface projections surrounding the periphery of the particle, a characteristic feature of coronaviruses. The photograph depicts a murine coronavirus. Kindly provided by Professor David Bhella, Scottish Centre for Macromolecular Imaging; MRC Centre for Virus Research; University of Glasgow.
Coronavirus receptor binding occurs via the spike protein (encoded by the structural S gene) which has two subunits. Subunit S1 mediates binding and a trimeric S2 stalk mediates fusion to the infected cell. The S1 subunit is divided into two domains, the N-terminal domain (S1-NTD) and the C-terminal domain (S1-CTD). These regions mediate binding to a variety of cellular receptors containing carbohydrate or protein at their binding domains. SARS-CoV and SARS-CoV-2 (and the alphacoronavirus HCoV-NL63) all bind via the S1-CTD to the angiotensin-converting enzyme 2 (ACE2) receptor (Figure 2).9 SARS-CoV-2 has a higher affinity for binding to ACE2 than SARS-CoV, and binding involves a larger number of interaction sites.10,11 A pre-requisite for binding of SARS-CoV-2 to ACE2 is cleavage of the S protein of the virus by the transmembrane serine protease TMPRSS212 (Figure 2). Replication occurs via the RNA-dependent RNA polymerase and involves discontinuous transcription of subgenomic mRNAs that encode six major open reading frames common to all coronaviruses and multiple accessory proteins.
Figure 2.

Basic pathobiology of SARS-CoV-2 infection and possible treatment strategies. Upon the viral spike protein priming by the transmembrane protease serine 2 (TMPRSS2), SARS-CoV-2 uses the host angiotensin-converting enzyme 2 (ACE2) to enter and infect the cell. Inhibiting TMPRSS2 activity (by camostat mesylate) could be used to prevent proteolytic cleavage of the SARS-CoV-2 spike protein and protect the cell against virus–cell fusion (1). Another approach could be neutralizing the virus from entering cells and keeping it in solution by activation of a disintegrin and metalloprotease 17 (ADMA17) which leads to shedding of the membrane-bound ACE2 and release of the soluble extracellular domain of ACE2 (2); with treatment with anti-ACE2 antibodies leading to blockage of the interaction between virus and receptors (3) or administration of soluble recombinant human ACE2 protein acting as a competitive interceptor for SARS-CoV-2 (4). Alternatively, purified polyclonal antibodies targeting/neutralizing the viral spike protein may offer some protection against SARS-CoV-2 (5). Interestingly, angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs), frequently used to treat hypertension, could alter ACE2 expression and intensify the SARS-CoV-2 infection.
Importantly, SARS-CoV-2 transmission occurs at a higher basic reproduction rate (R0 = 2–2.5) than SARS-CoV that caused an outbreak of severe respiratory infection in 2003 or than influenza.13 It is associated with higher viral loads in infected people (up to a billion RNA copies per milliltre of sputum) and long-term resistance on contaminated surfaces. SARS-CoV-2 is more stable on plastic and stainless steel than on copper and cardboard, and viable virus may be detected up to 72 h after application to these surfaces.14 Patients with severe COVID-19 tend to have a high viral load and a long virus-shedding period. This finding suggests that the viral load of SARS-CoV-2 might be a useful marker for assessing disease severity and prognosis.15 At the same time, pronounced nucleic acid shedding of SARS-CoV-2 was observed for 7 days in mild cases.15
To better appreciate the links between cardiovascular disease (CVD) and COVID-19, it is important to understand the underlying pathobiology of coronavirus infection. SARS-CoV-2 binds to the transmembrane ACE2 protein (a homologue of ACE) to enter type II alveolar epithelial cells, macrophages, and other cell types12 (Figure 2). The process requires priming of viral S protein by the cellular serine protease TMPRSS2.12 Thus, infection with SARS-CoV-2 requires co-expression of ACE2 and TMPRSS2 in the same cell type, as proteolytic cleavage of viral S protein is essential for binding of the virus to ACE2. Exploitation of ACE2 by coronavirus is important in predicting potential pathology as ACE2 is particularly highly expressed in pericytes, in addition to type II alveolar epithelial cells, according to the single-cell human heart atlas.16 High expression of ACE2 in pericytes could lead to development of microvascular dysfunction,17 explaining greater propensity for acute coronary syndromes (ACS).5 Moreover, ACE2 expression is up-regulated in failing human hearts, suggesting a plausible explanation for a higher infectivity of virus and a higher mortality in patients with heart failure.18 Moreover, cellular entry of coronaviruses through ACE2 has implications for vascular instability and hypotension as well as increased mortality of infected patients who have pre-existing hypertension, albeit the latter association is confounded by the older age of patients with comorbidities. In addition to pathogenicity and transmissibility of the virus, these findings also have therapeutic implications, as inhibition of the cellular serine protease TMPRSS2 and sera containing blocking antibodies against ACE2 have the potential to block viral entry and hence prevent or attenuate COVID-19 (Figure 2). In a murine model, TMPRSS2 inhibition blocked viral entry and attenuated the severity of coronavirus infection with improved survival.19,20 Two clinical trials have been started to test the efficacy of inhibition of TMPRSS2 by camostat mesilate for the treatment of patients with COVID-19 (NCT04321096 and NCT04338906).
Methodological considerations of current clinical data on COVID-19
Our understanding of COVID-19 pathomechanisms, natural clinical history, and possible therapies are evolving continuously. While in this review we have collated contemporary literature regarding this pandemic to enable a comprehensive overview, numerous methodological considerations need to be taken into account regarding study design and data collection. The sources used to generate this review are original articles published in PubMed, posted on medRxiv, bioRxiv, or ChinaXiv, or listed in clinical trial databases (ClinicalTrial.gov and EudraCT). In addition, public databases such as World Health Organization, Centers for Disease Control (CRCs), and the JHU Coronavirus Resource Center were utilized.
The early studies in a pandemic might suffer from inclusion bias. Baseline demographics and pre-morbid status of study populations are expected to reflect the characteristics of individuals who were exposed to the disease early in the outbreak. In addition, availability and access to diagnostic testing as well as a high threshold for diagnostic testing or hospital treatment or suitability for ICU admission, because of finite resources, are expected to affect characteristics of the study populations and the clinical outcomes of the disease. For example, a large number of healthcare workers and inpatients were exposed to COVID-19 in the hospital in the early, rather than the later phase in the pandemic in China.21 The demographics of patients in the early studies from China were different from those reported later in the largest aggregate study of COVID-19 patients by Guan et al. in China22 (Table 1). Data on cardiac involvement are unfortunately not extensively presented in the study of Guan et al.22
Table 1.
| Study | Region | All patients | Severity qualification | Lower severity | High severity | P-value |
|---|---|---|---|---|---|---|
| Gender (M = 51.3%, F = 48.7% in China); n (% men) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (73%) | Non-ICU/ICU | 28 (68%) | 13 (85%) | 0.24 |
| Wang et al. | Zongnan | 138 (54%) | Non-ICU/ICU | 102 (52%) | 36 (61%) | 0.34 |
| Zhou et al. | JY-T and Wuhan | 191 (62%) | Survive/dead | 137 (59%) | 54 (70%) | 0.15 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | 0.43 |
| Liu et al. | Tongi + three others | 78 (50%) | Stable/deteriorate | 6 (48%) | 11 (64%) | 0.52 |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (58%) | Non-severe/severe | 926 (58%) | 17 3 (58%) | n/a |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (58%) | Stable/endpoint | 1032 (58%) | 67 (67%) | n/a |
| Age; n, years (IQR) | ||||||
| Huang et al. | Jin Yin-Tan | 4149 (41–58) | Non-ICU/ICU | 2849 (41–58) | 1349 (41–61) | 0.6 |
| Wang et al. | Zongnan | 138, 56 (42–68) | Non-ICU/ICU | 102, 51 (37–62) | 36, 66 (57–78) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191, 56 (46–67) | Survive/dead | 137, 52 (45–58) | 54 (63–67) | <0.001 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | <0.001 |
| Liu et al. | Tongi + three others | 78, 38 (33–57) | Stable/deteriorate | 66, 37 (32–41) | 11, 66 (51–79) | 0.001 |
| Guan et al. | 31 provinces/provincial municipalities | 1099, 47 (35–58) | Non-severe/severe | 926, 45 (34–57) | 137, 52 (40–65) | <0.001 |
| Guan et al. | 31 provinces/provincial municipalities | 1099, 47 (35–58) | Stable/endpoint | 1032, 46 (35–57) | 67, 63 (53–71) | <0.001 |
| Any comorbidity; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (32%) | Non-ICU/ICU | 28 (29%) | 13 (38%) | 0.53 |
| Wang et al. | Zongnan | 138 (46%) | Non-ICU/ICU | 102 (37%) | 36 (72%) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191 (48%) | Survive/dead | 137 (40%) | 54 (67%) | 0.001 |
| Ruan et al. | Tongji | 150 (51%) | Survive/dead | 82 (41%) | 68 (63%) | 0.0069 |
| Liu et al. | Tongi + three others | 78 | Stable/deteriorate | 66 | 11 | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (24%) | Non-severe/severe | 926 (21%) | 173 (39%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (24%) | stable/CEP | 1032 (21%) | 57 (58%) | – |
| Hypertension (prevalence 15–33% WHO data/Bundy); n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (15%) | Non-ICU/ICU | 28 (14%) | 13 (15%) | 0.93 |
| Wang et al. | Zongnan | 138 (31%) | Non-ICU/ICU | 102 (22%) | 36 (58%) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191 (30%) | Survive/dead | 137 (23%) | 54 (48%) | 0.0008 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| Liu et al. | Tongi + three others | 78 (40%) | Stable/deteriorate | 66 (9%) | 11 (18%) | 0.3 |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (15%) | Non-severe/severe | 926 (13%) | 173 (24%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 109 (15%) | Stable/endpoint | 1032 (14%) | 67 (36%) | – |
| Diabetes mellitus [general rate in China is 8.4–10% (Diabetes UK, WHO)]; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (20%) | Non-ICU/ICU | 28 (25%) | 13 (8%) | 0.16 |
| Wang et al. | Zongnan | 138 (10%) | Non-ICU/ICU | 102 (6%) | 36 (22%) | 0.009 |
| Zhou et al. | JY-T and Wuhan | 191 (19%) | Survive/dead | 137 (14%) | 45 (31%) | 0.005 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| Liu et al. | Tongi + three others | 78 (25%) | Stable/deteriorate | 66 (5%) | 11 (18%) | 0.143 |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (7%) | Non-severe/severe | 926 (5%) | 173 (16%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (7%) | Stable/endpoint | 1032 (6%) | 67 (27%) | – |
| Renal disease (CKD: 10.8% in China, Wang, Jinwei et al.); n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 | Non-ICU/ICU | 28 | 13 | – |
| Wang et al. | Zongnan | 138 (3%) | Non-ICU/ICU | 102 (2%) | 36 (6%) | 0.28 |
| Zhou et al. | JY-T and Wuhan | 191 (1%) | Survive/dead | 137 (0%) | 54 (4%) | 0.02 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| Liu et al. | Tongi + three others | 78 | Stable/deteriorate | 66 | 11 | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (8%) | Non-severe/severe | 926 (0.5%) | 173 (2%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (8%) | Stable/endpoint | 1032 (0.6%) | 67 (3%) | – |
| COPD (5.7% in 2018, Zhu B); n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (2%) | Non-ICU/ICU | 28 (0%) | 13 (8%) | 0.14 |
| Wang et al. | Zongnan | 138 (3%) | Non-ICU/ICU | 102 (1%) | 36 (8%) | 0.54 |
| Zhou et al. | JY-T and Wuhan | 191 (3%) | Survive/dead | 137 (1%) | 54 (7%) | 0.047 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| Liu et al. | Tongi + 3 others | 78 (10%) | Stable/deteriorate | 66 (1.5%) | 11 (9%) | 0.264 |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (1%) | Non-severe/severe | 926 (1%) | 173 (4%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (1%) | Stable/endpoint | 1032 (0.5%) | 67 (10%) | – |
| Cardiovascular disease/coronary heart disease (estimated 20% WHO); n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (15%) | Non-ICU/ICU | 28 (11%) | 13 (23%) | 0.32 |
| Wang et al. | Zongnan | 138 (15%) | Non-ICU/ICU | 102 (11%) | 36 (25%) | 0.04 |
| Zhou et al. | JY-T and Wuhan | 191 (8%) | Survive/dead | 137 (1%) | 54 (24%) | <0.0001 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| Liu et al. | Tongi + three others | 78 | Stable/deteriorate | 66 | 11 | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (3%) | Non-severe/severe | 926 (2%) | 173 (6%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (3%) | Stable/endpoint | 1032 (2%) | 67 (9%) | – |
| Smoking (Chinese prevalence 26.3%, WHO); n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (7%) | Non-ICU/ICU | 28 (11%) | 13 (0%) | 0.16 |
| Wang et al. | Zongnan | 138 | nonICU/ICU | 102 | 36 | – |
| Zhou et al. | JY-T and Wuhan | 191 (6%) | Survive/dead | 137 (4%) | 54 (9%) | 0.21 |
| Ruan et al. | Tongji | – | Survive/dead | – | – | – |
| Liu et al. | Tongi + three others | 78 (6%) | Stable/deteriorate | 66 (3%) | 11 (27%) | 0.018 |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (13%) | Non-severe/ severe | 926 (12%) | 173 (17%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (13%) | Stable/endpoint | 1032 (12%) | 67 (26%) | – |
| Malignancy (Chinese prevalence 0.6%, WHO); n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (2%) | Non-ICU/ICU | 28 (4%) | 13 (0%) | 0.49 |
| Wang et al. | Zongnan | 138 (7%) | Non-ICU/ICU | 102 (6%) | 36 (11%) | 0.29 |
| Zhou et al. | JY-T and Wuhan | 191 (1%) | Survive/dead | 137 (1%) | 54 (0%) | 0.037 |
| Ruan et al. | Tongji | – | Survive/dead | – | – | – |
| Liu et al. | Tongi + three others | 78 (5%) | Stable/deteriorate | 66 (10%) | 11 (18%) | 0.09 |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (1%) | Non-severe/severe | 926 (1%) | 173 (2%) | – |
| Guan et al. | 31 provinces/provincial municipalities | 1099 (1%) | Stable/endpoint | 1032 (1%) | 67 (1%) | – |
n/a, not available; ICU, intensive care unit; endpoint, composite endpoint of admission to an ICU, the use of mechanical ventilation, or death;22 CKD, chronic kidney disease.
These should be analysed in the context of recent European data which appeared after submission of this paper.27
Guan et al. present data based on disease severity at the time of assessment (using American Thoracic Society guidelines for community-acquired pneumonia) and according to composite endpoint status (EP: ICU admission, ventilation, or death).
The National Health Commission of the People's Republic of China (PRC) guidance23 recommends the use of traditional Chinese medicine alongside what is considered more conventional interventions. The published reports do not provide details of the traditional treatment regimens in patients with COVID-19. Therefore, different choices of therapy were made and any positive/negative impacts of such interventions, which may have influenced outcomes, might have introduced additional bias.
Finally, it is also difficult to assess the true prevalence, occurrence, mortality, and spectrum of the clinical course of disease since a proportion of inoculated individuals might be asymptomatic and therefore were never tested. Some in silico modelling of the infection expansion as well as in initial reports from Iceland and Italy suggest that an asymptomatic group, perhaps as high as 50% of infected individuals (DeCODE Genetics, Iceland), probably exists. This finding has considerable implications in estimating the prevalence and preventing spread of the disease. Likewise, some reports show that up to 80% of infected individuals have mild symptoms and in theory represent a group that might not seek medical care—they might not, therefore, be tested or contribute to prevalence and case fatality rate (CFR) estimates. Secondly, practically all countries experience shortage of the testing kits, therefore limiting the testing only to selected groups of individuals. Moreover, some deaths caused by SARS-CoV-2 were not attributed to COVID-19, due to the lag time when severe complications tend to develop even up to 2–3 weeks following the initial infection.8
Clinical course of COVID-19
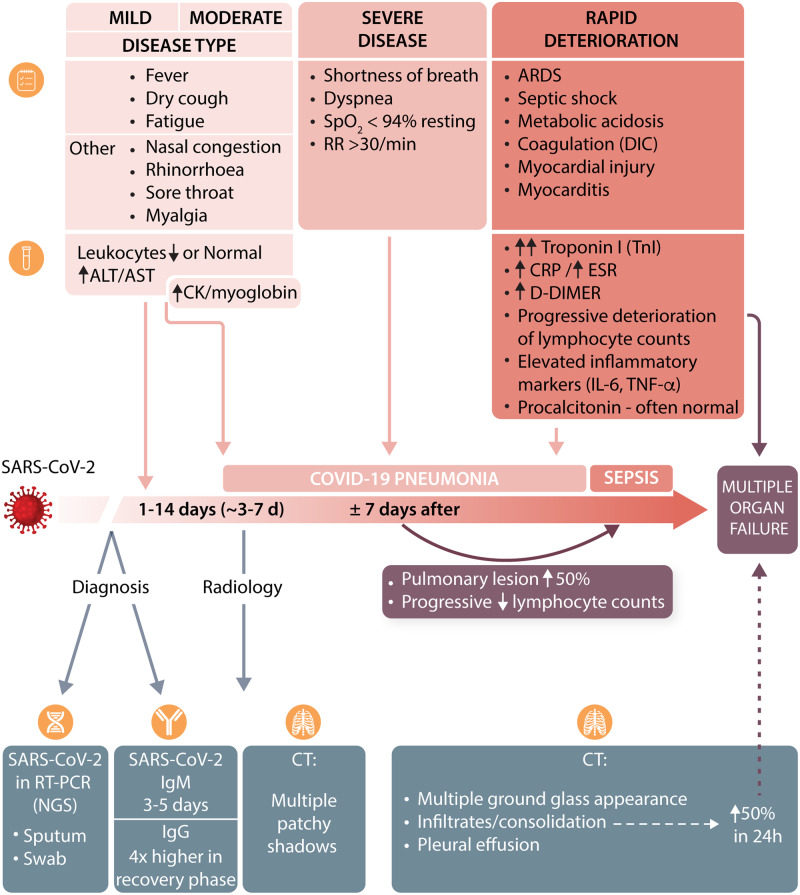
The incubation period between contact and the first set of symptoms is typically 1–14 days (but up to 24 days in individual cases).23 The median time between registered exposure and first symptoms is 5.1 days with a mean of 6.1 days.24 Duration of viral nucleic acid shedding ranges between 8 and 34 days (median 20 days) after the initial symptoms (Figure 3).
Figure 3.
Key symptoms, and biochemical and radiological features of the clinical course of COVID-19.
The main clinical symptoms develop within 11.5 days [95% confidence interval (CI) 8.2–15.6 days] and include fever, dry cough, fatigue, ageusia, anosmia, and headache.24 Other non-specific symptoms have also been reported, which included nasal congestion, rhinorrhea, sore throat, myalgia, poor appetite, and diarrhoea.21 Fever and cough typically appear concomitantly, followed by shortness of breath and severe fatigue, which appear around day 6–76 and are associated with development of severe bilateral (and occasional unilateral) pneumonia (Figure 4).
Figure 4.
Multifocal pneumonia in a patient with COVID-19. (A) A cross-sectional CT image of the lungs showing two distinct pulmonary infiltrates in the left upper lobe (arrows). (B) A large posteriorly located right lower lobe infiltrate on CT scan of the chest (arrows). Data were collected as part of a retrospective study, consent was waived, and collection of these data was approved by local ethics committee of Wuhan, China. Kindly provided by Professor Dao Wen Wang.
The most common radiological findings include multiple patchy shadows and interstitial changes in moderate disease, with consolidation, a ground glass appearance, in 56.4% of cases,22 and very occasional pleural effusions in severe cases.23 In such severe cases, pneumomediastinum and pneumothorax have been described.25,26
Pathological investigations of the lungs of deceased individuals indicate blockade of bronchi and bronchioles with large amounts of mucus plugs and bronchial epithelial cell damage.23 Lymphocyte and mononuclear cell infiltrates are present in alveolar septal spaces. Fibrinous exudate and high hyaline membranes fill alveolar cavities. Polynuclear giant cells are prominent. There is marked proliferation of type II alveolar epithelial cells. Such severe manifestations appear only in a fraction of patients. A recent study of COVID-19 cases in China reported up to 28 January 2020 indicated that severe illness may occur in 16% of cases,22 leading to an overall mortality rate estimated at 1.4% of the total reported cases22 to 4.61% in the WHO reports (accessed on 28 March 2020). In some geographical regions, due to unexplained reasons, mortality may be higher (current estimates are 11.9% in Italy, 9.0% in Spain, and 7.9% in the UK according to the JHU Coronavirus Resource Center, accessed on 2 April, 20202). It is important to note, however, that great care must be taken when calculating fatality rates based on currently available data, as these can be overestimated in relation to insufficient testing in the community or underestimated, due to long lag-time between test positivity and death or the fact that there are large differences in attributing COVID-related mortality (‘dying with’ versus ‘dying from’ as well as differences in performing post-mortem testing). Limitations of healthcare systems, abruptly overwhelmed by a surge of patients needing mechanical invasive ventilation, have also been considered a potential source of the differences. Finally, these differences may result from population structure, as Italian patients have been older than the average age reported in the Chinese patients.
The typical clinical course of disease is summarized in Figure 3. The heterogeneity of responses between individual patients is striking. This indicates that it is unlikely that COVID-19 can be considered from the point of view of a single disease phenotype. Rather, it seems most likely that host characteristics, which at the moment remain unknown, promote progression of the disease with a range of different presentarions, e.g. mild, severe multiorgan failure, and cytokine release storm.
While clinical symptoms of the disease are predominantly respiratory and associated with severe pneumonia, both direct and indirect involvement of other organs is common, with the CV system being particularly affected. Moreover, pre-existing conditions, largely linked to CVD, increase the risk of severe outcomes of the infection.
Cardiovascular risk factors associated with the worse outcomes of COVID-19
A number of key comorbidities are associated with worse clinical outcomes in patients with COVID-19 (Table 1). Association with age seems to dominate this relationship22 and may affect the actual importance of other factors reported in univariate analyses. Older patients (mean age 63 years old; range 53–71) are more likely to experience the composite endpoint of ICU admission, mechanical ventilation, or death compared with younger patients (mean age 46 years old, range 35–57)22 (Table 1). Males seem to be more susceptible to COVID-19-related complications, representing between 50% and 82% of the hospitalized patients in the four publications that report these data (Table 1) and the most recent report from Italy.27
Table 1 summarizes key comorbidities identified by the major studies from China showing that the presence of pre-existing morbidities increases the severity of hospital-treated COVID-19. Notably, there is a large heterogeneity of reporting, with some studies comparing death with survival and others comparing ICU with non-ICU cases (Table 1). However, regardless of the approach, pre-existing CV conditions seem to be particularly important predictors of COVID-19 severity.
The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team recently analysed all COVID-19 cases reported to China’s Infectious Disease Information System up to 11 February 2020.7 The investigators found that the fatality rate for patients with no comorbidities was ∼0.9%, whereas the CFR was much higher for patients with comorbidities. This included mortality of 10.5% for patients with CVD, 7.3% for those with diabetes, 6% for subjects with hypertension, 6.3% for those with chronic respiratory disease, and 6.0% for those with cancer.28–30 It was as high as 14.8% for patients ≥80 years of age.7,30 It is interesting that in Italian and Dutch cohorts, there are reports of higher severity in younger obese individuals as well. Severe cases accounted for 13.8%, and critical cases accounted for 4.7% of all cases. Of significance, CVD occurrence affects the mortality rate to a larger extent than the presence of pre-existing chronic obstructive pulmonary disease (COPD), which had not been the case in SARS.7
These observations are confirmed by a recent meta-analysis, based largely on these studies and an additional 44 672 patient data set reported by the China CDC.28 In this large cohort, CVD was reported in 4.2% of the total population and in 22.7% of those who died.28 By extension, it is expected that comorbidities are associated with higher rates of hospitalization in patients with COVID-19, but any effects that comorbidities may have on susceptibility to infection remain conjectural: accordingly, published frequencies of these comorbidities in China are included in Table 1. Surprisingly, a history of smoking and of chronic pulmonary disease appear to be far less powerful determinants of severity in hospitalized patients than is the history of CVD. Curiously, the prevalence of smoking in hospitalized COVID-19 patients appears far lower than might be expected from assumed population prevalence and primary respiratory infection
COVID-19 and hypertension
It is not clear if hypertension is a risk factor for susceptibility to SARS-CoV-2 infection—the available data show prevalence rates of 15–40%, largely in line with the rates of high blood pressure in the general population (∼30%).22,31 At first glance, hypertension is more prevalent in subjects with a more severe course of the disease. In a recent analysis from China,22 it was present in 13.4% of subjects with non-severe disease and in 23.7% of subjects with severe disease. This study also included a composite outcome, which was also associated with a higher prevalence of hypertension in those with a poor composite outcome (35.8% vs. 13.7%). In the cohort of 44 672 patients reported by the China CDC,28 hypertension prevalence was reported as 12.8% in the whole group of patients and as 39.7% in patients who eventually died.28 Hypertension was reported to increase the odds ratio (OR) for death by 3.05 (95% CI 1.57–5.92)32 in patients with COVID-19. These associations may, however, be largely confounded by the higher prevalence of hypertension in older people, as older individuals have significantly worse outcomes, more severe course of the disease, and a higher mortality rate than the younger patients.22 Thus, in summary, while hypertension does appear to be associated with more severe disease, a higher risk of acute respiratory distress syndrome (ARDS), and increased mortality in unadjusted analyses, there is no strong evidence to indicate increased susceptibility of patients with hypertension to COVID-19, when the association is adjusted for other risk factors.33
The mechanisms of this possible relationship and their clinical relevance have been reviewed in a recent statement of the European Society of Hypertension.33 The putative relationship between hypertension and COVID-19 may relate to the role of ACE2. ACE2 is a key element in the renin–angiotensin–aldosterone system (RAAS), which is critically involved in the pathophysiology of hypertension.34 Experimental studies demonstrated that inhibition of the RAAS with ACE inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) may result in a compensatory increase in tissue levels of ACE2,35 leading to suggestions that these drugs may be detrimental in patients exposed to SARS-CoV-2.36 It is important, however, to emphasize that there is no clear evidence that ACEIs or ARBs lead to up-regulation of ACE2 in human tissues.36 Thus, currently there is no justification for stopping ACEIs or ARBs in patients at risk of COVID-19.33 This has now been endorsed officially by many learned Societies, including the European Society of Hypertension, the International Society of Hypertension, and the European Society of Cardiology.33 It also appears that in experimental models some RAAS blockers may exert a potentially protective influence.37 Indeed, while angiotensin II promoted the internalization and intracellular degradation of ACE2, losartan reduced this effect, suggesting that ARBs may offer protection against viral entry into cells.36 The recent integrative antiviral drug repurposing analysis implicated another ARB—irbesartan—as a potential repurposable medication for COVID-19.10 In fact, the known effect of ARBs on potassium metabolism may be seen as clinically advantageous in patients infected by COVID-19 given that hypokalaemia was reported as a fairly common manifestation of COVID-19 (possibly through increased kaliuresis rather than gastrointestinal loss).38 Hypokalaemia in COVID-19 patients is difficult to manage, correlates with the severity of the disease, and has been suggested to be driven by activation of the RAAS.38 ACEIs or ARBs might offer some protection in this setting. It also needs to be emphasized that hypokalaemia has not been reported in other studies. For example, in a patient characterization by Guan et al.,22 the median value of the potassium level reported was 3.8 mmol/L with the lower margin of the interquartile range (IQR) at 3.5 mmol/L. Nevertheless, antihypertensive medications known to increase serum levels of potassium (including carvedilol and eplerenone) were implicated as potential drug repurposing opportunities for patients with COVID-19 infection.10 Moreover, observations from ICUs in Italy suggest that hypocalcaemia is a common metabolic abnormality in patients infected by COVID-19, that could be linked due to reduced albumin levels, which are commonly seen, and/or Ca2+ consumption through excessive activation of the coagulation cascade.
Another mechanism linking hypertension and COVID-19 is the immune system, which is dysregulated in hypertension and SARS-CoV-2 infection.39,40 Poor control of blood pressure may contribute to further dysregulation of the immune system. For example, it has been shown that hypertension, in humans, is associated with circulating lymphocyte counts,41 and CD8+ T cell dysfunction is observed in patients with hypertension42. Such immunosenescent CD8+ T cells are unable to efficiently combat viral infections, and contribute to pathological overproduction of cytokines—a situation providing a possible link to COVID-19. One may also postulate that ACEIs or ARBs, by providing a better control of blood pressure, may restore, at least partially, the dysregulated immune system in hypertension.
Overall it is essential to ensure that blood pressure control in hypertensive patients during viral infections is optimized, unnecessary and uncontrolled changes to therapy are discouraged, and hypertensive patients should be carefully monitored for CV and other complications during COVID-19 infection.
Cardiovascular manifestations of COVID-19
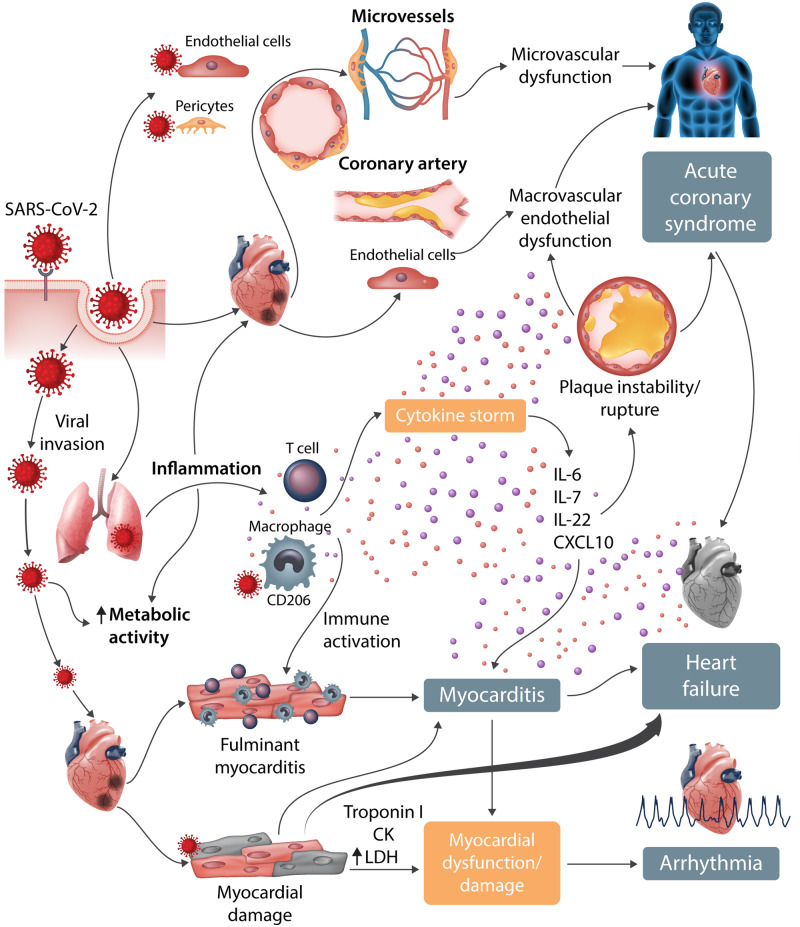
Severe COVID-19 is associated with rapidly progressing systemic inflammation, a pro-inflammatory cytokine storm, and sepsis, leading to multiorgan failure and death (Figure 5). Selected evidence and manifestations of CV injury in COVID-19 patients are summarized in Table 2. Importantly, there is a delay between initiation of symptoms and myocardial damage in studies reported so far (Table 3).
Figure 5.
Cardiovascular involvement in COVID-19—key manifestations and hypothetical mechanisms. SARS-CoV-2 anchors on transmembrane ACE2 to enter the host cells including type 2 pneumocytes, macrophages, endothelial cells, pericytes, and cardiac myocytes, leading to inflammation and multiorgan failure. In particular, the infection of endothelial cells or pericytes could lead to severe microvascular and macrovascular dysfunction. Furthermore, in conjunction with the immune over-reactivity, it can potentially destabilize atherosclerotic plaques and explain the development of the acute coronary syndromes. Infection of the respiratory tract, particularly of type 2 pneumocytes, by SARS-CoV-2 is manifested by the progression of systemic inflammation and immune cell overactivation, leading to a ‘cytokine storm’, which results in an elevated level of cytokines such as IL-6, IL-7, IL-22, and CXCL10. Subsequently, it is possible that activated T cells and macrophages may infiltrate infected myocardium, resulting in the development of fulminant myocarditis and severe cardiac damage. This process could be further intensified by the cytokine storm. Similarly, the viral invasion could cause cardiac myocyte damage directly leading to myocardial dysfunction and contribute to the development of arrhythmia.
Table 2.
Cardiac and associated outcomes in hospitalized COVID-19 disease in selected early studies3,6,18,21,22,32
| Study | Region | All patients | Severity qualification | Lower severity | High severity | P-value |
|---|---|---|---|---|---|---|
| Cardiac injury; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (12%) | Non-ICU/ICU | 28 (4%) | 13 (31%) | 0.017 |
| Wang et al. | Zongnan | 138 (7%) | Non-ICU/ICU | 102 (2%) | 36 (22%) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191 (17%) | Survive/dead | 137 (1%) | 54 (59%) | <0.001 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | |
| Heart failure; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 | Non-ICU/ICU | 28 | 13 | – |
| Wang et al. | Zongnan | 138 | Non-ICU/ICU | 102 | 36 | – |
| Zhou et al. | JY-T and Wuhan | 191 (23%) | Survive/dead | 137 (12%) | 54 (52%) | <0.001 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | |
| Arrhythmia; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 | Non-ICU/ICU | 28 | 13 | – |
| Wang et al. | Zongnan | 138 (17%) | Non-ICU/ICU | 102 (7%) | 36 (44%) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191 | Survive/dead | 137 | 54 | – |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| Shock; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (7%) | Non-ICU/ICU | 28 (0%) | 13 (23%) | 0.027 |
| Wang et al. | Zongnan | 138 (9%) | Non-ICU/ICU | 102 (1%) | 36 (31%) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191 (20%) | Survive/dead | 137 (0%) | 54 (70%) | <0.0001 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
| ARDS; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (29%) | Non-ICU/ICU | 28 (4%) | 13 (85%) | <0.001 |
| Wang et al. | Zongnan | 138 (20%) | Non-ICU/ICU | 102 (5%) | 36 (61%) | <0.001 |
| Zhou et al. | JY-T and Wuhan | 191 (31%) | Survive/dead | 137 (7%) | 54 (93%) | <0.0001 |
| Ruan et al. | Tongji | 150 | survive/dead | 82 | 68 | – |
| AKI; n (%) | ||||||
| Huang et al. | Jin Yin-Tan | 41 (7%) | Non-ICU/ICU | 28 (0%) | 13 (23%) | 0.027 |
| Wang et al. | Zongnan | 138 (4%) | Non-ICU/ICU | 102 (2%) | 36 (8%) | 0.11 |
| Zhou et al. | JY-T and Wuhan | 191 (15%) | Survive/dead | 137 (1%) | 54 (50%) | <0.0001 |
| Ruan et al. | Tongji | 150 | Survive/dead | 82 | 68 | – |
ICU, intensive care unit; ARDS, acute respiratory distress syndrome; AKI, acute kidney injury.
P-values are provided if they were provided in the publication.
Table 3.
Delays from illness onset to complication (adapted from Zhou et al.; n = 191; survive = 137; die = 54)
| All (191) | Non-survivors (54) | |
|---|---|---|
| Sepsis | In 59%: 9 days (7–13) | In 100%: 10 days (7–14) |
| ARDS | In 31%: 12 days (8–15) | In 93%: 12 days (8–15) |
| Acute cardiac injury | In 17%: 15 days (10–17) | In 59%: 14.5 days (9.5–17) |
| Secondary Infection | In 15%: 15 days (13–19) | In 50%: 15 days (13–19) |
| Acute kidney injury | In 15%: 15 days (13–19) | In 50%: ? days (?) |
COVID-19 and cardiac arrhythmia
Viral infections are associated with metabolic dysfunction, myocardial inflammation, and activation of the sympathetic nervous system, all of which predispose to cardiac arrhythmia. In a recent report on 138 hospitalized COVID-19 patients,21 16.7% of patients developed arrhythmias, which ranked only second among serious complications after ARDS. Arrhythmia was observed in 7% of patients who did not require ICU treatment and in 44% of subjects who were admitted to an ICU.18 Further details of these manifestations remain elusive but included atrial fibrillation, conduction block, ventricular tachycardia, and ventricular fibrillation. These arrhythmias are also observed in viral myocarditis. Interestingly, the report of the National Health Commission of China estimates that during the initial outbreak, some patients reported primarily CV symptoms, such as palpitations and chest tightness, rather than respiratory symptoms.43
COVID-19 and myocardial injury and heart failure
Most reports indicate that almost all hospitalized COVID-19 patients show elevated serum creatine kinase (CK) and lactate dehydrogenase (LDH) levels.6,43,44 In addition, a number of studies indicate that cardiac complications, including fulminant myocarditis, are potential outcomes of SARS-CoV-2 infection. Heart failure has been reported as an outcome in 23% of COVID subjects in a recent report from in-hospital Chinese subjects. Approximately 52% of non-survivors had heart failure as compared with 12% of survivors.32 Evidence of myocardial injury, such as an increase in high-sensitivity cardiac troponin I (cTnI) levels (>28 pg/mL) was detected in 5 of the first 41 patients diagnosed with COVID-19 in Wuhan.6,43,44 More recent reports indicate that 7.2%21 to 17%32 of hospitalized COVID-19 patients sustain acute myocardial injury. This may be in the form of acute myocarditis (see below) or injury secondary to an oxygen supply/demand mismatch [type 2 myocardial infarction (MI)].
In an analysis of 68 fatal cases in Wuhan, 36 patients (53%) died of respiratory failure, 5 (7%) patients with myocardial damage died from circulatory failure, and 22 patients (33%) died from both.3 Similarly, analysis of 120 COVID-19 patients reported elevated levels of N-terminal pro B-type natriuretic peptide (NT-proBNP) in 27.5% of the cases, and cTnI in 10% of deceased patients, respectively, indicating that the effects of CV injury on systemic stability may be important and should not be ignored. In another report of 138 inpatients with COVID-19 in Wuhan, the levels of biomarkers of myocardial injury were significantly higher in patients treated in the ICU as compared with those not requiring ICU care (median CK-MB level 18 U/L vs. 14 U/L, P < 0.001; hs-cTnI level 11.0 pg/mL vs. 5.1 pg/mL, P = 0.004).21. In a study of 191 patients,32 cTnI levels were strongly associated with increased mortality in the univariate analysis, but the association was not tested in a multivariate model. Similar associations between cTnI elevation and disease severity are shown when analysing cohorts on the basis of the need for ICU care.6,21 Thus patient monitoring should include a number of laboratory tests, summarized in Table 4, based on current experience and studies.
Table 4.
Diagnostic tests in patients with COVID-19 and cardiovascular involvement
| Test | Diagnostic considerations in COVID-19 patients |
|---|---|
| NT-pro BNP/BNP* |
|
| Troponin* | High-sensitivity troponin assay may be helpful for risk assessment in patients requiring ICU care and to identify individuals with silent myocardial injury. |
| D-dimer | Reports from the initial outbreak in Wuhan show a key relationship with a requirement for ICU care and mortality. |
| Procalcitonin | A marker of bacterial infection; it is more likely to be raised in patients who will require ICU care. |
| Full blood count |
|
| IL-6 | Where available; high concentrations are associated with adverse outcome. |
| Ferritin | A marker of poor outcome; very significant changes reported in COVID-19 patients. |
| Cardiac CT | To be considered in uncertain cases of patients with elevated troponins with and without signs of obstructive coronary artery disease (EACVI position166) |
| ECG | In MERS-CoV, the 12-lead ECG generally shows diffuse T wave inversion where there is myocardial involvement; this can be dynamic. Changes in COVID-19 were also described. |
| Echocardiography | May show global or regional myocardial systolic dysfunction with or without a pericardial effusion and vice versa. |
The current ACC position advises against routine measurement of troponin or BNP (ACC 18.03).
Mechanisms underlying myocardial injury remain unknown and it is unclear whether they reflect systemic/local and/or ischaemic/inflammatory process. It is still not known whether acute injury is a primary infective phenomenon or secondary to lung disease. Associations between cTnI elevation and pre-existing CV conditions (and other pre-COVID features) have not yet been examined to detect evidence of causality, and no detailed analyses of patients with CV complications of COVID-19 have been published to date. As an elevated cTnI level is associated with poorer outcomes in other (non-COVID) systemic illnesses,45 the reported association could simply reflect the severity of systemic illness (e.g. hypoxia or hypotension) rather than indicating a specific cardiac pathology. In this context, a ‘cytokine storm’ triggered by immunological dysregulation43 may be a key mediator. Plasma interleukin-6 (IL-6) concentrations are elevated in COVID-19 patients with cardiac injury,46 and abnormalities in a variety of cytokines are prominent in patients with severe COVID-19 disease.
Cardiac-specific mechanisms may also be important. Since ACE2 is expressed in the CV system,47 direct cardiomyocyte infection by SARS-CoV-2 may be a possibility, as discussed below. Moreover, therapies used in treatment of severe multiorgan dysfunction in COVID-19 patients as well as antiviral drugs may result in cardiac toxicity.
Attempts to treat COVID-19 cardiac injury have included the use of steroids, i.v. immunoglobins, hydroxychloroquine, and other antivirals, and active mechanical life support.46 While it remains uncertain if these or other therapies successfully limit myocardial injury, the detection of cardiac damage in hospitalized COVID-19 patients may help identify a subset of patients at greater risk of COVID-19 complications.
COVID-19 and myocarditis
Cardiac injury and acute myocarditis are well-recognized complications of acute viral infections. Myocyte necrosis and mononuclear cell infiltrates are reported in cardiac muscle autopsy specimens in a recent report of the National Health Commission of the PRC.23 This finding, along with case reports46,48 of fulminant myocarditis, suggests that myocarditis may be an important cause of the acute cardiac injury in COVID-19 patients. However, the prevalence, clinical importance, and mechanism(s) of myocardial inflammation in COVID-19 disease remain unclear.6,49
Clinically, COVID-19 myocarditis may manifest only as mild chest discomfort and palpitations, which may be impossible to distinguish from other causes in most patients. In some, however, myocarditis results in fulminant disease (Figure 6). Transient ECG changes are common and may help detect the presence and severity of myocardial injury. Myocarditis may progress to conduction block, tachyarrhythmias, and impairment of left ventricular function.
Figure 6.
Representative transthoracic echocardiography frames (selected from cine loop images) from a patient with COVID-19. (A) Apical four-chamber view showing globally reduced left ventricular contraction, especially in the apical segment. The right ventricle is dilated and an echo-free space, indicating pericardial effusion, is present. (B) Parasternal short axis view showing markedly reduced left ventricular contraction, enlarged right ventricle, and a mural thrombosis in the right ventricle outflow tract. (C) Two-dimensional speckle tracking echocardiography based on speckle tracking imaging technology (2D STE). Left panel showing a normal 2D STE, right showing a 2D STE from a patient with COVID-19 and myocarditis, depicting reduced regional peak systolic strain rates. Data were collected as part of a retrospective study, Wuhan, China; consent was waived and collection of these data was approved by the local ethics committee. Kindly provided by Professor Dao Wen Wang.
In other clinical settings, myocarditis is often suspected when cardiac injury is detected in the absence of an ACS. The diagnosis can often be confirmed if cardiac magnetic resonance imaging (MRI) detects typical acute myocardial injury signals.50 Endomyocardial biopsy (EMB), long considered the gold standard diagnostic test, can directly demonstrate myocyte necrosis and mononuclear cell infiltrates.51 EMB will detect evidence of a viral cause in some cases, though in others an immunologically autoimmune-mediated cause of the myocarditis is suspected.51 Biopsy studies of patients with acute myocarditis in Europe indicate that viral aetiology ranges between 37.8% and 77.4%.52,53 In COVID-19, this evidence is at the moment sparse and based on individual case series, emphasizing the need for systematic assessment. While several reports emphasize that fulminant myocarditis may be an important clinical presentation of the disease,46,48 the real prevalence of this complication remains unclear. Cardiac MRI and EMBs as diagnostic tools are likely to be inappropriate during the current COVID-19 pandemic and associated healthcare crisis, but should be considered in the future (Table 5).
Table 5.
Proposed investigations in the case of suspicion of myocarditis in COVID-19 patients
|
Animal models of viral myocarditis suggest discrete pathological phases that begin with viral-mediated myocyte lysis.54 This cardiac injury leads to activation of the innate immune response with release of proinflammatory cytokines.54 Proteins released through cell lysis might display epitopes similar to the viral antigens and be presented via the major histocompatibility complex (MHC). Myosin heavy chain, a cardiac sarcomere protein, appears to be a prime example of ‘molecular mimicry’.55 At this stage, EMBs may show inflammatory changes but no detectable viral particles because of clearance of the virus by the innate immune response. An acquired immune response is the predominant feature evidenced by activation of antibodies and T lymphocytes. CD4+ T helper (Th) cells and cytotoxic CD8+ T cells mediate their responses through activation of the inflammatory cascade and cytolysis [Th1–interferon (IFN)-γ, Th2 – e.g. IL-4, Th17 – IL-17 and Th22 – IL-22]. Macrophages migrate to the site of injury.54 In the final stage, there is either recovery or low levels of chronic inflammation with concomitant development of left ventricular dysfunction.54
Interestingly, myocarditis appears in COVID-19 patients after a prolonged period (up to 10–15 days) after the onset of symptoms (Table 3). Moreover, investigators in China point to a lack of viral particle identification on EMB (personal communication). Given these observations and the experimental context above, a question central to potential therapeutic options is the extent to which myocardial injury results from viral replication (cytopathic), is immune mediated, or is due to other mechanisms. Given that acute myocardial injury is said to begin 2 weeks after the onset of symptomatic COVID-19,32 adaptive T-cell-mediated immunity or dysregulated innate effector pathways are likely to play a pivotal in the development of myocardial inflammation. In this context, it is notable that an increase of highly proinflammatory CCR6+ Th17 in CD4+ T cells, prominent inflammatory mediators of myocarditis,56 has been reported in severe cases.
Together, the data suggest that a delay in myocardial inflammation is consistent with at least two pathogenic mechanisms: first, that the ‘cytokine storm’ unleashes a subclinical autoimmune myocarditis, and secondly that myocardial damage and/or molecular mimicry initiate a de novo autoimmune reaction.
Targeted therapeutic options remain elusive; as is the case for myocarditis in other settings, a management strategy that uses a broad range of supportive therapies remains key. A case report recently described effectiveness of the early application of steroids and i.v. immunoglobins, neuraminidase inhibitors, and active mechanical life support.46
COVID-19 and ischaemic heart disease
While little is known regarding the effects of COVID-19 on ACS, several pathways associated with viral diseases may contribute to destabilize plaques in COVID-19 patients.57 Heart failure patients are at increased risk of acute events or exacerbation; viral illness can potentially destabilize atherosclerotic plaques through systemic inflammatory responses,58 cytokine storm, as well as specific changes of immune cell polarization towards more unstable phenotypes. All of these have been observed in COVID-19. In the case of SARS and MERS, acute MI59,60 has been reported in two out of the five deaths in early reports.61
It is important to consider that type 2 MI is the most common subtype in viral conditions, thus the usefulness of invasive management with a view toward coronary revascularization (especially in type 2 MI) is limited. The decision for invasive vs. non-invasive management of patients with an ACS and COVID-19 illness should be carefully considered. Moreover, a recent single-cell atlas of the human heart indicated that pericytes express particularly high levels of ACE2 in the heart.47 One of the implications of this finding is possible local microvascular inflammation during SARS-CoV-2 infection of the pericytes, leading to severe microvascular dysfunction, contributing to myocardial infarction with non-obstructive coronary arteries (MINOCA). This could explain recent reports of the clinical course of cases of MI during COVID-19. In addition, the cytokine storm can contribute to development of endothelial dysfunction through well-characterized mechanisms.62–65
COVID-19 and coagulation abnormalities
Features of disseminated intravascular coagulation (DIC) and pulmonary embolism, characterized by increased D-dimer levels and fibrin degradation products, are highly prevalent in COVID-19. DIC has been observed in 71.4% of non-survivors.66 Massive pulmonary embolism has been reported.67 This might not be surprising given the critical condition of these subjects, although early appearance of DIC features is often evident. Notably, experience from China indicates that a D-dimer increase is highly predictive of adverse outcomes in COVID-19. In a retrospective cohort study, elevated D-dimer levels (>1 g/L) were strongly associated with in-hospital mortality, and this relationship was maintained in multivariate analysis (OR 18.4, 95% CI 2.6–128.6; P = 0.003).32 Moreover, Chinese and Italian experience emphasizes that more discrete changes in D-dimer levels are observed earlier in the course of disease preceding the rapid progression stage.
COVID-19, inflammation, and the cytokine release storm
After the lungs, immune organs are the second most affected system by COVID-19. Pathological investigations in COVID-19 victims23 have demonstrated splenic atrophy, with a very significant reduction in the number of lymphocytes and neutrophils, as well as necrosis and haemorrhages. Similarly, lymphocytes are depleted in lymph nodes and the numbers of both CD4+ and CD8+ cells are decreased.23 This corresponds to lymphopenia in peripheral blood observed in severe cases. Interestingly, an increase in systemic IL-2, IL-6, IL-7, granulocyte colony-stimulating factor, C-X-C motif chemokine 10 (CXCL10), chemokine (C-C motif) ligand 2 (CCL2), and tumour necrosis factor-α (TNF-α) has been observed in subjects with COVID-19,6 which corresponds to the characteristics of a cytokine release syndrome (CRS).16,68,69 CRS development in COVID-19 is associated with COVID-19 severity. CRS has been characterized as a complication of immune targeted therapies in oncology, in particular in relation to severe chimeric antigen receptor (CAR) T-cell-induced CRS.70 It is also reminiscent of the cytokine profile noted in haemophagocytic lymphohistiocytosis (HLH) syndromes.71 Resemblance to the latter brought considerations that COVID-19 may be a cause of secondary HLH with cytopenias, significant haemophagocytosis in bone marrow, and low fibrinogen concentration. Clinical classifications have been introduced to aid recognition of secondary HHL.71 Fluorescence-activated cell sorting (FACS) analyses of COVID-19 active cases have also shown hyperactivated T lymphocytes with large fractions of HLA-DR+ and CD38+ CD8+/CD4+ T cells and CCR6+ Th17 CD4+ cells. High concentrations of cytotoxic granules in cytotoxic T (CD8) cells have been observed. Thus, uncontrolled overactivation of T cells may account for, in part, the severe immune injury,16 similarly to atherosclerosis and other CV conditions.72,73 These aspects should also be considered in the light of sexual dimorphism related to susceptibility to CV inflammation.74–76
High serum IL-6 levels are a common feature in CRS patients. Indeed, in a recent retrospective multicentre analysis of 150 patients from Wuhan, circulating IL-6 levels were a clinical predictor of mortality in COVID-19.3 IL-6 is an important biomarker and possible target for CV morbidity and mortality linked to atherosclerosis.77–79 This is important as therapeutic targeting of the IL-6 receptor (IL-6R) with tocilizumab is used in preventing and treating CRS caused by cancer therapies and HLH.70 Tocilizumab is approved in >100 countries for the treatment of rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA),80 Castleman’s diseases, and giant cell or Takayasu arteritis.81 Other IL-6R-targeting agents, e.g. sarilumab, are similarly potentially of use. Therefore, its possible use in COVID-19 may be attractive to tackle CRS. However, when considering immunomodulation, one has to bear in mind that the primary problem is an infectious disease rather than the complications of cancer therapy. Therefore, its potentially utility must be carefully considered.
During the initial outbreak in China, the use of tocilizumab to stop severe CRS-associated organ failure and death in COVID-19 patients was attempted.71 Twenty-one severe COVID-19 cases were treated with tocilizumab in an initial pilot trial. Nineteen of them were discharged from the hospital within 2 weeks, as reported by China’s National Health Commission. The drug has now been approved in China to treat patients developing severe complications from COVID-19 and showing elevated plasma levels of IL-6.82 Chinese researchers have now registered several clinical trials for tocilizumab, expected to enrol patients with COVID-19 very soon. A partial list includes: ‘A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19)’ (ChiCTR2000029765); ‘Tocilizumab vs CRRT in Management of Cytokine Release Syndrome (CRS) in COVID-19 (TACOS)’ (ClinicalTrials.gov Identifier: NCT04306705); and ‘Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019’ (ClinicalTrials.gov Identifier: NCT04310228).
Similarly, case reports originating from Italy show that in a case series of six patients treated with tocilizumab in Naples, three have shown signs of improvement. This has prompted several studies evaluating the role of IL-6 antagonism by monoclonal antibodies in COVID-19. For example, the Italian Medicines Agency (AIFA) approved the clinical study ‘Tocilizumab in COVID-19 Pneumonia (TOCIVID-19)’ (ClinicalTrials.gov Identifier: NCT04317092). This multicentre, single-arm, open-label, phase II study will assess mortality at 1 month in 330 patients affected by COVID-19 pneumonia. The inclusion criteria comprise patients showing signs of respiratory distress syndrome or who had been subject to tracheal intubation in the preceding 24 h. The study will be led by the Instituto Nazionale Tumori IRCCS – Fondazione Pascale in Naples. Similarly, 30 participants will be enrolled in the Marche region, in the interventional clinical trial ‘Tocilizumab (RoActemra) as Early Treatment of Patients Affected by SARS-CoV-2 Infection With Severe Multifocal Interstitial Pneumonia’ (ClinicalTrials.gov Identifier: NCT04315480). In the USA, the ‘Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19’ (ClinicalTrials.gov Identifier: NCT04315298) has just started, aiming to recruit 400 patients, and will be shortly followed by the ‘Tocilizumab to Prevent Clinical Decompensation in Hospitalized, Non-critically Ill Patients With COVID-19 Pneumonitis (COVIDOSE)’ (ClinicalTrials.gov Identifier: NCT04331795) trial, which is expected to start very soon. Finally, the most recently registered trial recruiting 330 patients: A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA) (ClinicalTrials.gov Identifier: NCT04320615) is being initiated. Similar trials have been registered in France, Belgium, and Denmark. It should be noted, however, that there are currently no published clinical trial data on IL-6 targeting safety or efficacy against the virus. Moreover, tocilizumab has not received approval from China’s National Medical Product Administration to be sold for COVID-19 treatment.
The cytokine storm and increase in IL-6 signalling observed in some COVID-19 patients could have profound CV consequences causing tachycardia, hypotension, and left ventricular dysfunction. CRS-related cardiotoxicity has also been reported, mainly in the form of conduction abnormalities, atrial fibrillation, and elevation in BNP and cTnIs.83
In COVID-19 patients, medium- to long-term CV consequences may be caused by increased IL-6 signalling. Experimental evidence supports an atherogenic role for IL-6 and CRS-related cytokines,59,60,84–86 as well as its effects on cardiac fibrosis and failure.87 The cytokine increases adhesion molecule expression in human endothelial cells in vitro;88 at the same time, stimulation of human macrophages with oxidized LDLs (oxLDLs) leads to increased release of IL-6.89 In experimental atherosclerosis, IL-6 mRNA is detectable in the aorta of hyperlipidaemic mice,90 and administration of recombinant IL-6 increased plaque formation.91 Similarly, reduced pathology has been observed in LDLr–/– mice treated with a fusion protein of the IL-6 trans-signalling inhibitor soluble glycoprotein 130 (sgp130).92 Plasma IL-6 levels also have been associated with development and progression of abdominal aortic aneurysm,93 and IL-6 has been shown to influence lipid homeostasis in mice.94 IL-6 trans-signalling contributes to experimental cardiac fibrosis;87 while the up-regulation of membrane-bound IL-6R causes vascular remodelling in pulmonary arterial hypertension.95
Genetic variants leading to increased circulating levels of IL-6R and, therefore, reduced IL-6 cell signalling, have been shown to protect against coronary heart disease (CHD).96,97 Similarly, IL-6 trans-signalling is associated with increased CV risk.77,98 IL-6 is routinely used as an inflammatory biomarker in CV disease. The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) trial demonstrated a stronger effect of IL-1β inhibition in reducing secondary CV events in patients with higher circulating levels of IL-6 and C-reactive protein (CRP), indicative of residual inflammatory risk.98 Whether the observed cytokine storm and IL-6 increase in COVID-19 patients are transient or sustained remains unknown. Accordingly, monitoring inflammatory biomarkers in these patients in the medium to long term is of major importance. Similarly, CV risk should be closely evaluated during the acute phase response and in the following years.
There are, however, likely to be a range of additional cytokine moieties that will emerge to have pathway-specific contributions in the severe spectrum of COVID-19 syndrome. These include pathways driven by granulocyte–macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-17, IL-18, and IFN-γ. Moreover, the imminent prospect of single-cell and other immunological analyses will offer a more systematic insight into the immune dysregulation syndrome(s) that are emerging and especially the disease trajectory—in essence which pathways are directing COVID-related CRS and which are simply adding to the inflammatory tissue damage burden upon which the other comorbidities are operating. Thus, we propose that a useful way of thinking about this would be that the inflammatory burden might be considered as a direct effector (i.e. CRS-type), or a secondary amplificatory factor in terms of the contribution that pathways make to pathogenesis and clinical outcome.
Lessons from SARS-CoV infection
In 2002 a novel coronavirus, SARS-CoV, emerged from China, crossing from bats to humans, eventually leading to >8000 cases and the death of >700 people. SARS utilized ACE2 for cell attachment and infection through the viral envelope spike (S) protein99 and a subsequent interaction with a cellular protease, TMPRSS2, which primes S protein for cell entry.10 The closely related SARS-CoV-2, also thought to have originated in bats,9 encodes an S protein with ∼76% amino acid similarity to that of SARS-CoV and, importantly, SARS-CoV-2, as already discussed, has also recently been demonstrated to use the same cellular entry pathway via ACE2 and TMPRSS2,12 as discussed above. Both these novel coronaviruses are different from another recently emergent coronavirus, MERS virus, which crossed from the dromedary camel to humans and also caused acute respiratory failure, although utilizing a different cell entry mechanism via the receptor dipeptidyl peptidase 4 (DPP4).101 Overall, this highlights the potential divergence of respiratory coronavirus infections in humans, but emphasizes the close relationship between SARS-CoV and SARS-CoV-2. So, what can we learn from knowledge of SARS-CoV and associated CV risk to help in the current battle against COVID-19?
During human SARS-CoV infection of the murine lung, ACE2 is utilized and subsequently almost completely lost at the protein level.102 Importantly, delivery of the viral S protein alone also led to down-regulation of ACE2 and decreased lung function in normal mice, and worsened lung pathology in an acid challenge model of acute lung failure. Furthermore, disease pathology was reduced in the presence of the ARB losartan. Intriguingly, in acute lung disease triggered by acid respiration or sepsis, ACE2 has also been shown to be directly protective, acting in partnership with the angiotensin type 2 receptor (AT2R), and administration of recombinant ACE2 in this model is protective.103 Taking together the evidence from multiple experimental studies, beneficial effects of ACEIs or ARBs and also ACE2 supplementation in various animal models of lung injury or SARS have been shown and supported the concept that loss of ACE2 expression promotes the disease in lung injury models (reviewed in Kreutz et al., 202025). ACE2 is also directly regulated by cytokines.104 Decreased ACE2 levels could be a direct consequence of viral infection and/or the subsequent inflammatory and immune responses that occur in the infected lung. Interestingly, ACE2 is also reported to be detectable in macrophages,105 and its knockout in leucocytes promotes adipose inflammation,106 highlighting a role for ACE2 in the inflammatory response. Patients suffering from SARS have overwhelming immune and inflammatory responses and high mortality rates from acute respiratory failure, and furthermore they are also associated cardiac sequelae. For example, SARS patients also suffer from systolic and diastolic dysfunction and arrythmias, leading to sudden death.107,108 In murine models, intranasal administration of human SARS-CoV results in ACE2-mediated infection of the myocardium.109 These observations support a role for SARS-CoV in direct myocardial infection and a possible causative role in cardiac disease subsequent to respiratory infection. In the murine heart, ACE2 was also almost completely down-regulated at the protein level following infection. Moreover, in autopsied cardiac tissue from SARS patients with SARS-CoV-positive lung infection, viral RNA was detected in the heart, combined with decreased cardiac ACE2 protein levels and elevated cardiac macrophage infiltration. Down-regulation of ACE2 without compensatory effects on ACE may lead to the RAAS being tipped towards the detrimental ACE–Ang II–AT1R axis and away from the protective ACE2–Ang-(1-7)–Mas axis.
ACE2 is also up-regulated after MI in rodents and humans in macrophages, endothelial cells, smooth muscle cells,110,111 and cardiomyocytes,112 and may play a role in restoring RAAS homeostasis in the heart post-MI. In fact, viral vector-mediated overexpression of ACE2 in rodents also protects the heart from adverse cardiac remodelling and dysfunction post-MI.113 Overall, these findings highlight that ACE2 has a key protective function in both the lung and the heart. Therefore, SARS-CoV infection-mediated down-regulation of ACE2, as a direct mechanistic consequence of viral infection and/or as a result of the subsequent inflammatory responses, may lead to an imbalance in RAAS signalling and consequent CV sequelae. The knowledge that systemic spread of SARS from primary lung infection to other CV tissues, including the heart, is also important. Given that ACE2 functions as a receptor for virus entry into the cell, down-regulation of ACE2 upon infectioin with SARS-CoV is expected to prevent further viral entry, serving as a negative regulatory mechanism. Clearly additional investigations are needed to increase our understanding of the pathological mechanisms of acute disease and potential increased CV risk in COVID-19 patients.
Therapeutic options for COVID-19
Managing COVID-19 is challenging as there are no specific treatments for the SARS-CoV-2 virus. Obtaining high-quality randomized clinical trial data during an outbreak is difficult. Research and clinical efforts focus in parallel on development of new drugs against coronavirus as well as repurposing already approved drugs for the treatment of the disease. ClinicalTrials.gov site lists >300 studies that are testing various interventions in COVID-19 patients. This emphasis on trials as opposed to compassionate use and case reports is a major lesson from previous pandemics and it is good to see the community moving so robustly in this direction.
Meanwhile, public health measures rely mostly on social measures intended to prevent viral/disease spread, in order to avoid a massive surge of patients with healthcare facilities overload, and on supportive treatment for the patients, which can be considered the mainstay of management. Available treatments once clinically evident can be classified as supportive, immune-suppressive, antiretroviral, and potential novel therapies. Supportive treatment should be the mainstay of management coordinated by the relevant specialist–multidisciplinary team. The approaches have been provided by numerous scientific and clinical societies during the early stages of the European outbreak and are continuously being updated. This includes a concise but comprehensive guidelines of the Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva.114
When disease progresses to severe phenotype, supportive treatment includes use of oxygen therapy if SpO2 is <92% on room air,23 as well as haemodynamic support. Early intubation and invasive mechanical ventilation are essential in those with progressive symptoms and increasing oxygen requirement. High flow nasal cannulae and non-invasive positive pressure ventilation (NIPPV) may play a role in some patients, especially where resources for mechanical ventilation are likely to be stretched. A lung-protective ventilation strategy is recommended by the WHO. Conservative use of i.v. fluids aiming to maintain tissue perfusion but a negative fluid balance aids lung recovery.23 Extracorporeal membrane oxygenation (ECMO) may be required in severe cases as per standard indications but should be considered early (veno-venous mode and could be initiated prior to intubation).
As cardiac damage is highly prevalent, heart failure therapies should be initiated where appropriate. Similarly, broad-spectrum antibiotics/antifungal treatments and treatment of arrhythmias are needed. Finally, due to the growing evidence of DIC as a cause of organ injury, anticoagulation should be considered.23
Approximately 75% of patients in the early Chinese cohort received antiviral therapy.6,32,43,115 The Italian recommendation is to commence treatment with antiviral therapy when COVID-19 is confirmed in patients with mild symptoms but not in a high mortality risk category or with moderate/severe signs of infection. Numerous antiviral therapies have been used to try and limit viral replication. These include protease inhibitors such as liponovir/ritonavir (used for the treatment of HIV). However, a recent rapid randomized non-placebo-controlled trial including 100 patients in each arm showed no difference in the outcome.116 Remdesivir is a nucleotide analogue and polymerase inhibitor that was previously used for the experimental treatment of Ebola in a large phase III study.117 While it had an acceptable safety profile, the remdesivir (GS-5734) arm was halted due to a higher antiviral efficacy of monoclonal antibodies in the trial. Finally chloroquine or hydroxychloroquine have been suggested as having antiviral activity against many RNA viruses including SARS and SARS-CoV-2, through an increase of the endosomal pH and interference with the glycosylation process.118 However, it has never been shown conclusively to have an antiviral effect in vivo. In alphavirus infection, while demonstrating an antiviral effect in vitro, it was found not to be associated with clinical effects in a randomized clinical trial and may even be associated with prolonged viraemia in vivo.119 While these observations cannot be directly translated to COVID-19, large phase III trials are underway with hydroxychloroquine, that will inform about the possible therapeutic value of this approach. This includes the recently initiated ‘Hydroxychloroquine Chemoprophylaxis in Healthcare Personnel in Contact With COVID-19 Patients (PHYDRA Trial)’ (ClinicalTrials.gov Identifier: NCT04318015).
As the cytokine storm appears to be a key pathogenetic process in patients exhibiting rapid deterioration, immune suppression and immune modulation approaches have been tried. This includes glucocorticoids, which are recommended by Chinese guidelines, but not Italian guidelines. Patients with evidence of lung fibrosis or severe cardiac involvement in the ICU may benefit from this approach. Methylprednisolone was used in combination with i.v. immunoglobulins in the treatment of subjects with fulminant myocarditis.118 Immunomodulatory therapies used include monoclonal antibodies against IL-6R, discussed above. IFN-β, registered for treatment of multiple sclerosis, enhances suppressor T cell activity, reducing proinflammatory cytokine production. It may be also helpful in patients with myocarditis who develop left ventricular systolic dysfunction; however, current experience is limited to enteroviruses.120 It is also being tried as an inhaled preparation. Finally, 27% of patients in the early Chinese cohort received i.v. immunoglobulins. This approach was based on the evidence of their beneficial effects in cases of myocarditis-induced dilated cardiomyopathy and is recommended in cases of viral myocarditis that are refractory to standard heart failure therapies.121
A list of planned, ongoing, and completed clinical trials can be found at: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=&cntry=&state=&city=&dist=
In addition to the many ongoing clinical trials, a new trial in Europe will investigate effects of APN01, the recombinant form of human ACE2 (rhACE2) (clinicaltrialsarena.com). HrACE2 has a dual mode of action. First, it has the potential to block infection of host cells by SARS-CoV-2, and secondly it may reduce lung injury through the protective actions of endogenous ACE2. The phase II clinical trial will be conducted in Germany, Austria, and Denmark.
Cardiovascular effects of potential therapies for COVID-19
The potential therapies for COVID-19 discussed above have important CV side effects and toxicities as well as comorbid conditions that require caution or avoidance of these drugs, as listed in Table 6. It should be noted that data for these side effects and toxicities come from patients that use these drugs chronically for the treatment of autoimmune diseases (chloroquine/hydroxychloroquine, rocilizumab), hepatitis (ribavarin, IFN-α), or HIV infection lopinivir/ritonivir). Thus, the effect of short-term use of these medications for patients without these underlying conditions is not clear. Remdesivir is an experimental drug used in the treatment of Ebola.117 Thus, its CV effects and toxicities are unknown. The antimalarial drugs chloroquine and hydroxychloroquine have recently received considerable attention and interest for the treatment and possible prophylaxis of COVID-19. However, the data to date in support of these drugs are weak and cardiac toxicities are considerable. A systematic review of the literature performed on patients treated with these drugs, albeit for an extended period of time (median 7 years) and with a high cumulative dose, demonstrated conduction disorders as the main side effect (85%).122 Other adverse cardiac events included ventricular hypertrophy (22%), hypokinesia (9.4%), heart failure (26.8%), pulmonary arterial hypertension (3.9%), and valvular dysfunction (7.1%). Cardiac function normalizes in a significant number of patients (44.9%) upon withdrawal of chloroquine and hydroxychloroquine, while others continue to show irreversible damage (12.9%) or death (30.8%).122 Thus, careful consideration should be given to the use of these drugs, particularly without stronger data regarding their efficacy. Of note, tocilizumab treatment has been shown to influence lipid metabolism in RA patients. Following tocilizumab, total-, LDL-, and HDL-cholesterol were increased, while CV risk biomarkers such as HDL-SAA, secretory phospholipase A2 IIA, and lipoprotein(a) were significantly reduced.123 Very recently, the ENTRACE clinical trial supported the CV safety of tocilizumab in RA patients;124 however, to date, IL-6 targeting has not been tested for secondary prevention in CVD.
Table 6.
Potential COVID-19 therapies and their cardiovascular effects
| CV side effects | CV warnings/toxicities | Use with caution or avoid in presence of | |
|---|---|---|---|
| Antimalarials | |||
| Chloroquine/ hydroxychloroquine |
|
|
|
| Antivirals | |||
| Ribavarin |
|
|
|
| Lopinivir/ritonivir |
|
|
|
| Remdesivir |
|
|
|
| Biologics | |||
| Tocilizumab |
|
|
|
| Interferon alpha 2B |
|
|
|
References: www.medscape.com; CAD, coronary artery disease; MI, myocardial infarction.
Follow-up of patients with cardiovascular involvement in COVID-19
While there are currently no evidence-based recommendations, considering clinical presentation, it is reasonable to propose that patients who have had cardiac involvement initially should be seen every 1–3 months. Periodic evaluation, in addition to detailed history taking and physical examination, should include a 12-lead ECG and 2D/Doppler echocardiography125 or, preferably, cardiac MRI with late gadolinium enhancement. Appropriate heart failure therapy should be initiated and maintained when required, and plans put in place to optimize doses. Patients should be given standard advice regarding physical activity. As regards unknown long-term consequences of COVID-19, regular CV risk assessment should be considered in all patients who survive COVID-19.
Ethical dilemmas brought by COVID-19
COVID-19 brings unprecedented ethical problems and situations facing the medical profession around the world. In the light of the huge imbalance between therapeutic needs and resource availability of an unprecedented scale in our generation, the Italian Society of Anesthesiology and Intensive Care (SIAARTI),126 along with other National Societies provided an ethical statement aimed to guarantee the correct psychological framework to physicians massively exposed to the need to apply hard triage rules while facing huge ethical dilemmas.126 These are derived from the fact that the need for intensive care must be integrated with other elements of ‘clinical suitability’, thus including: the type and severity of the disease, the presence of comorbidities, the impairment of other organs and systems, and their reversibility.126 Clinicians are not, either deontologically or by training, accustomed to reasoning with criteria of maxi-emergency triage, as in the current exceptional situation.126
Impact of COVID-19 on routine and emergency cardiovascular care
In preparation for the COVID-19 pandemic, many healthcare providers have had to scale down outpatient services and also defer elective cardiac procedures and surgeries. This in some instances has led to the positive integration of technology and development of virtual clinics.127 However, uptake of virtual clinics has not been universal and has also been compromised by re-deployment of the workforce to help manage the pandemic. The long-term clinical impact of scaling down outpatient activity, reduced access to diagnostics, and deferral of routine procedures is likely to be significant and extend beyond the pandemic. Similarly, the perceived risk of being exposed to COVID-19 has led to a decline or a delay in presentation of acute cardiac emergencies which is likely to contribute to cardiac mortality and morbidity.
Cardiovascular implications of social distancing
COVID-19 implications are wider than the effects of the disease on individual patients. Practically all countries affected by the disease developed mitigation and containment strategies based on social distancing. CV consequences of social distancing may be profound. Both experimental and clinical research has shown the effects of social isolation and loneliness on cognition and memory,128–132 metabolic disorders,133–136 cancer,137–139 and immune disorders.139–141 In the context of CVDs, the absence of positive relationships and the reduced chance of interaction with other people (social distancing) have been identified as major risk factors for CV mortality.142–151 A recent meta-analysis including a total of 181 006 participants152 demonstrated that the risk for ischaemic heart disease and stroke increased by 29% and 32%, respectively, in lonely and socially isolated people. Similar results were reported from a UK Biobank analysis.153
The mechanisms of detrimental effects of social isolation are multiple and are related to the activation of the hypothalamic–pituitary–adrenocortical (HPA) axis,154–157 changes in the sympathetic vascular tone,148,158,159 elevated levels of cortisol,156,160,161 and a reduced responsivity of the glucocorticoid receptor.162–165 The social distancing strategies used in COVID-19 should consider these effects and aim to mitigate them using available technological advances.
Key unanswered questions
In this comprehensive review, we aimed to highlight the current state of the art information regarding COVID-19 and CVD (Table 7). Our understanding of CV risk and consequences of COVID-19 is developing continuously. However, there are many knowledge gaps and there are many unanswered questions. Below we point out a few burning unknowns at the moment.
Table 7.
Summary of current key considerations in COVID-19 diagnosis and treatment
| Key take-home messages: |
|
| Cardiovascular comorbidities |
|
What are the factors, genetic or otherwise, that influence interindividual variability in susceptibility to COVID-19, its severity, or clinical outcomes? The mechanisms through which CVDs worsen the prognosis in COVID-19 are unknown. It remains to be addressed to what extent individual CVDs are exacerbated by COVID-19. Do pre-existing hypertension and CVDs increase infection risk and/or worsen the course of disease progression? Is the severity of CVDs related to high expression levels of ACE2, the SARS-CoV-2 receptor, in the heart and blood vessels? What influence, if any, do inhibitors of the RAAS have on susceptibility to COVID-19 and its clinical outcomes? What are the factors or therapies for CVDs that may confer protective effects against COVID-19 and its clinical outcomes? How does pre-existing CVD worsen cardiac involvement specifically? What transferable knowledge can be learned about this pathogen that would advance our understanding of CV risk for SARS-CoV-2, influenza, and other virus infections in the future? Finally, probably the most important question remains, what are the determinants of heterogeneous host responses to SARS-CoV-2 infection? The answers will be found in integrated approaches by CV immunological ID and other expertise coming together. The use of systems based on hypothesis-free in silico methodologies will be essential. This pandemic is unlike any other in arriving at the same time as humankind being in possession of remarkable molecular data science and informatic tools. This is a major test of our ability to harness such capacity for the greater good.
These questions need to be answered with the highest quality science and clinical research since the current pandemic of coronavirus might not be the last.
Acknowledgments
This work was supported by the European Research Council (TJG-InflammaTENSION: ERC-CoG-726318) and the British Heart Foundation (Guzik-FS/14/49/30838, RE/18/6/34217 to R.T., T.G. and C.B.; BHF RE/18/634217 to F.M.B. and PG/19/84/34771 to P.M and T.J.G.), and the Medical Research Council (MC_UU_12014/1 to E.T. and MC_UU_12014/7 to D.B.).
Conflicts of interest: none declared.
References
- 1. Dong E, Du H, Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John Hopkins University. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (28 March 2020).
- 3. Ruan Q, Yang K, Wang W, Jiang L, Song J.. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS.. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145–151.32064853 [Google Scholar]
- 8. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, Brown TS, Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA.. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol 2020;doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL.. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F.. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 2020;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS.. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367:1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N,, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Lou Y, Gao D, Yang L, He D, Wang MH.. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis 2020;92:214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ.. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, Peiris M, Poon LLM, Zhang W.. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020;doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E, Piacentini M, Ippolito G, Melino G.. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020;doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C.. Treatment of coronary microvascular dysfunction. Cardiovasc Res 2020;116:856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG.. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020; doi: 0.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N.. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J Virol 2019;93:e01815–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R Jr, Nunneley JW, Barnard D, Pohlmann S, McKerrow JH, Renslo AR, Simmons G.. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res 2015;116:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY,, Xiang J,, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J,, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Health Commission of the People’s Republic of China. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition).http://kjfy.meetingchina.org/msite/news/show/cn/3337.html 2020.
- 24. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J.. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou C, Gao C, Xie Y, Xu M.. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020;20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun R, Liu H, Wang X.. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;doi: 10.3348/kjr.2020.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epidemiology Working Group for NCIP Epidemic Response. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol 2020;41:145–151. [Google Scholar]
- 29.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly 2020;2:x. [PMC free article] [PubMed] [Google Scholar]
- 30. Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis 2020;doi: 10.1016/S1473-3099(20)30257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beaney T, Burrell LM, Castillo RR, Charchar FJ, Cro S, Damasceno A, Kruger R, Nilsson PM,, Prabhakaran D, Ramirez AJ, Schlaich MP, Schutte AE, Tomaszewski M, Touyz R, Wang JG, Weber MA, Poulter NR, May Measurement Month Investigators. May Measurement Month 2018: a pragmatic global screening campaign to raise awareness of blood pressure by the International Society of Hypertension. Eur Heart J 2019;40:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kreutz R, Algharably E, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer T, Wang J, Burnier M.. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. European Society of Hypertension COVID-19 Task Force Review of Evidence. Cardiovasc Res 2020;116:1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD.. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE.. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 36. Danser AHJ, Epstein M, Batlle D.. Renin–angiotensin system blockers and the COVID-19 oandemic: at present there is no evidence to abandon renin–angiotensin system blockers. Hypertension 2020:HYPERTENSIONAHA12015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun ML, Yang JM, Sun YP, Su GH.. [Inhibitors of RAS might be a good choice for the therapy of COVID-19 pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:219–222. [DOI] [PubMed] [Google Scholar]
- 38. Chen DJ, Li X, Song PS, Hu CJ, Su F, Dai J.. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). medRxiv 2020;doi: https://doi.org/10.1101/2020.02.27.20028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drummond G, Vinh A, Guzik T, Sobey CG.. Immune mechanisms of hypertension. Nat Rev Immunol 2019;19:517–532. [DOI] [PubMed] [Google Scholar]
- 40. Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, Elijovich F, Laffer CL, Gnecco JS, Noonan J, Maffia P, Jasiewicz-Honkisz B, Czesnikiewicz-Guzik M, Mikolajczyk T, Sliwa T, Dikalov S, Weyand CM, Guzik TJ, Harrison DG.. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res 2018;114:1547–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siedlinski M,, Jozefczuk E, Xu X, Teumer A, Evangelou E, Schnabel RB, Welsh P, Maffia P, Erdmann J, Tomaszewski M, Caulfield MJ, Sattar N, Holmes MV, Guzik TJ.. White blood cells and blood pressure: a Mendelian randomization study. Circulation 2020;doi: 10.1161/CIRCULATIONAHA.119.045102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J,, Chang DY, Choi YS, Lee SH, Kang SM,, Jang Y, Yoo OJ, Shin EC, Park S.. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013;62:126–133. [DOI] [PubMed] [Google Scholar]
- 43. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020;doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gualandro DM, Puelacher C, LuratiBuse G, Lampart A, Strunz C, Cardozo FA, Yu PC, Jaffe AS, Barac S, Bock L, Badertscher P, du Fay de Lavallaz J, Marbot S, Sazgary L, Bolliger D, Rentsch K, Twerenbold R, Hammerer-Lercher A, Melo ES, Calderaro D, Duarte AJ, de Luccia N, Caramelli B, Mueller C, TropoVasc and BASEL-PMI Investigators. Comparison of high-sensitivity cardiac troponin I and T for the prediction of cardiac complications after non-cardiac surgery. Am Heart J 2018;203:67–73. [DOI] [PubMed] [Google Scholar]
- 46. Chen C, Zhou Y, Wang DW.. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020;doi: 10.1007/s00059-020-04909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen L, Li X, Chen M, Feng Y, Xiong C.. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M.. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallagher S, Jones DA, Anand V, Mohiddin S.. Diagnosis and management of patients with acute cardiac symptoms, troponin elevation and culprit-free angiograms. Heart 2012;98:974–981. [DOI] [PubMed] [Google Scholar]
- 51. Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Bohm M.. Update on myocarditis. J Am Coll Cardiol 2012;59:779–792. [DOI] [PubMed] [Google Scholar]
- 52. Pankuweit S, Moll R, Baandrup U, Portig I, Hufnagel G, Maisch B.. Prevalence of the parvovirus B19 genome in endomyocardial biopsy specimens. Hum Pathol 2003;34:497–503. [DOI] [PubMed] [Google Scholar]
- 53. Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP.. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005;112:1965–1970. [DOI] [PubMed] [Google Scholar]
- 54. Blyszczuk P. Myocarditis in humans and in experimental animal models. Front Cardiovasc Med 2019;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gangaplara A, Massilamany C, Brown DM, Delhon G, Pattnaik AK, Chapman N, Rose N, Steffen D, Reddy J.. Coxsackievirus B3 infection leads to the generation of cardiac myosin heavy chain-alpha-reactive CD4 T cells in A/J mice. Clin Immunol 2012;144:237–249. [DOI] [PubMed] [Google Scholar]
- 56. Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW.. Cardiac myosin–Th17 responses promote heart failure in human myocarditis. JCI Insight 2016;1:doi: 10.1172/jci.insight.85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Musher DM, Abers MS, Corrales-Medina VF.. Acute infection and myocardial infarction. N Engl J Med 2019;380:171–176. [DOI] [PubMed] [Google Scholar]
- 58. Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, Green P, Maffia P, Monaco C.. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res 2018;114:1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steven S, Dib M, Hausding M, Kashani F, Oelze M, Kroller-Schon S, Hanf A, Daub S, Roohani S, Gramlich Y, Lutgens E, Schulz E, Becker C, Lackner KJ, Kleinert H, Knosalla C, Niesler B, Wild PS, Munzel T, Daiber A.. CD40L controls obesity-associated vascular inflammation, oxidative stress, and endothelial dysfunction in high fat diet-treated and db/db mice. Cardiovasc Res 2018;114:312–323. [DOI] [PubMed] [Google Scholar]
- 60. Kusters PJH, Lutgens E, Seijkens TTP.. Exploring immune checkpoints as potential therapeutic targets in atherosclerosis. Cardiovasc Res 2018;114:368–377. [DOI] [PubMed] [Google Scholar]
- 61. Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY, HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Levy BI, Heusch G, Camici PG.. The many faces of myocardial ischaemia and angina. Cardiovasc Res 2019;115:1460–1470. [DOI] [PubMed] [Google Scholar]
- 63. Carnevale D, Wenzel P.. Mechanical stretch on endothelial cells interconnects innate and adaptive immune response in hypertension. Cardiovasc Res 2018;114:1432–1434. [DOI] [PubMed] [Google Scholar]
- 64. Petrie JR, Guzik TJ, Touyz RM.. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol 2018;34:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilk G, Osmenda G, Matusik P, Nowakowski D, Jasiewicz-Honkisz B, Ignacak A, Czesnikiewicz-Guzik M, Guzik TJ.. Endothelial function assessment in atherosclerosis: comparison of brachial artery flowmediated vasodilation and peripheral arterial tonometry. Pol Arch Med Wewn 2013;123:443–452. [DOI] [PubMed] [Google Scholar]
- 66. Tang N, Li D, Wang X, Sun Z.. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Danzi GB, Loffi M, Galeazzi G, Gherbesi E.. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020;doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ketelhuth DFJ, Lutgens E, Back M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O’Neill L, Weber C, Evans PC.. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res 2019;115:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ketelhuth DFJ. The immunometabolic role of indoleamine 2,3-dioxygenase in atherosclerotic cardiovascular disease: immune homeostatic mechanisms in the artery wall. Cardiovasc Res 2019;115:1408–1415. [DOI] [PubMed] [Google Scholar]
- 70. Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, Przepiorka D, Farrell AT, Pazdur R.. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018;23:943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gast M, Rauch BH, Nakagawa S, Haghikia A, Jasina A, Haas J, Nath N, Jensen L, Stroux A, Bohm A, Friebel J, Rauch U, Skurk C, Blankenberg S, Zeller T, Prasanth KV, Meder B, Kuss A, Landmesser U, Poller W.. Immune system-mediated atherosclerosis caused by deficiency of long non-coding RNA MALAT1 in ApoE–/– mice. Cardiovasc Res 2019;115:302–314. [DOI] [PubMed] [Google Scholar]
- 73. Gast M, Rauch BH, Haghikia A, Nakagawa S, Haas J, Stroux A, Schmidt D, Schumann P, Weiss S, Jensen L, Kratzer A, Kraenkel N, Muller C, Bornigen D, Hirose T, Blankenberg S, Escher F, Kuhl AA, Kuss AW, Meder B, Landmesser U, Zeller T, Poller W.. Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovasc Res 2019;115:1886–1906. [DOI] [PubMed] [Google Scholar]
- 74. van Koeverden ID, de Bakker M, Haitjema S, van der Laan SW, de Vries JPM, Hoefer IE, de Borst GJ, Pasterkamp G, den Ruijter HM.. Testosterone to oestradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res 2019;115:453–462. [DOI] [PubMed] [Google Scholar]
- 75. Penson P, Long DL, Howard G, Howard VJ, Jones SR, Martin SS, Mikhailidis DP, Muntner P, Rizzo M, Rader DJ, Safford MM, Sahebkar A, Toth PP, Banach M.. Associations between cardiovascular disease, cancer, and very low high-density lipoprotein cholesterol in the REasons for Geographical and Racial Differences in Stroke (REGARDS) study. Cardiovasc Res 2019;115:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crnko S, Ernens I, Van Laake LW.. New dimensions in circadian clock function: the role of biological sex. Cardiovasc Res 2018;114:203–204. [DOI] [PubMed] [Google Scholar]
- 77. Ziegler L, Gajulapuri A, Frumento P, Bonomi A, Wallen H, de Faire U, Rose-John S, Gigante B.. Interleukin 6 trans-signalling and risk of future cardiovascular events. Cardiovasc Res 2019;115:213–221. [DOI] [PubMed] [Google Scholar]
- 78. Hofmann P, Sommer J, Theodorou K, Kirchhof L, Fischer A, Li Y, Perisic L, Hedin U, Maegdefessel L, Dimmeler S, Boon RA.. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc Res 2019;115:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ferrante G, Condorelli G.. Interleukin-6 trans-signalling and risk of future cardiovascular events: a new avenue for atheroprotection? Cardiovasc Res 2019;115:8–9. [DOI] [PubMed] [Google Scholar]
- 80. Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poor G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D.. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–977. [DOI] [PubMed] [Google Scholar]
- 81. Kishimoto T. Discovery of IL-6 and development of anti-IL-6R antibody. Keio J Med 2019;68:96. [DOI] [PubMed] [Google Scholar]
- 82.The Chinese National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment–American College of Cardiology. 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/11/22/chinese-clinical-guidance-for-covid-19-pneumonia-diagnosis-and-treatment
- 83. Jamal FA, Khaled SK.. The cardiovascular complications of chimeric antigen receptor T cell therapy. Curr Hematol Malig Rep 2020;doi: 10.1007/s11899-020-00567-4. [DOI] [PubMed] [Google Scholar]
- 84. Wang HX, Li WJ, Hou CL, Lai S, Zhang YL, Tian C, Yang H, Du J, Li HH.. CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodeling via IL-10 signaling in mice. Cardiovasc Res 2018;115:83–93. [DOI] [PubMed] [Google Scholar]
- 85. van der Heijden C, Deinum J, Joosten LAB, Netea MG, Riksen NP.. The mineralocorticoid receptor as a modulator of innate immunity and atherosclerosis. Cardiovasc Res 2018;114:944–953. [DOI] [PubMed] [Google Scholar]
- 86. Brauner S, Jiang X, Thorlacius GE, Lundberg AM, Ostberg T, Yan ZQ, Kuchroo VK, Hansson GK, Wahren-Herlenius M.. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc Res 2018;114:158–167. [DOI] [PubMed] [Google Scholar]
- 87. Chou CH, Hung CS, Liao CW, Wei LH, Chen CW, Shun CT, Wen WF, Wan CH, Wu XM, Chang YY, Wu VC, Wu KD, Lin YH, TAIPAI Study Group. IL-6 trans-signalling contributes to aldosterone-induced cardiac fibrosis. Cardiovasc Res 2018;114:690–702. [DOI] [PubMed] [Google Scholar]
- 88. Watson C, Whittaker S, Smith N, Vora AJ, Dumonde DC, Brown KA.. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin Exp Immunol 1996;105:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF.. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis 2011;214:345–349. [DOI] [PubMed] [Google Scholar]
- 90. Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM.. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol 1998;18:1498–1505. [DOI] [PubMed] [Google Scholar]
- 91. Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R.. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1999;19:2364–2367. [DOI] [PubMed] [Google Scholar]
- 92. Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, Bavendiek U, von Felden J, Divchev D, Kempf T, Wollert KC, Seegert D, Rose-John S, Tietge UJ, Schieffer B, Grote K.. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2012;32:281–290. [DOI] [PubMed] [Google Scholar]
- 93. Nishihara M, Aoki H, Ohno S, Furusho A, Hirakata S, Nishida N, Ito S, Hayashi M, Imaizumi T, Fukumoto Y.. The role of IL-6 in pathogenesis of abdominal aortic aneurysm in mice. PLoS One 2017;12:e0185923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, Daemen MJ, Drexler H.. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004;110:3493–3500. [DOI] [PubMed] [Google Scholar]
- 95. Tamura Y, Phan C, Tu L, Le Hiress M, Thuillet R, Jutant EM, Fadel E, Savale L, Huertas A, Humbert M, Guignabert C.. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J Clin Invest 2018;128:1956–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium, Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, Pfister R, Mooijaart SP, Ireland HA, Leusink M, Langenberg C,, Li KW, Palmen J, Howard P, Cooper JA, Drenos F, Hardy J, Nalls MA, Li YR, Lowe G, Stewart M, Bielinski SJ, Peto J, Timpson NJ, Gallacher J, Dunlop M, Houlston R,, Tomlinson I, Tzoulaki I, Luan J, Boer JM, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Ferrucci L, Bandenelli S, Tanaka T,, Meschia JF, Singleton A, Navis G, Mateo Leach I, Bakker SJ, Gansevoort RT, Ford I, Epstein SE, Burnett MS, Devaney JM, Jukema JW, Westendorp RG, Jan de Borst G, van der Graaf Y, de Jong PA, Mailand-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Stephens JW, Eaton CB, Robinson JG, Manson JE, Fowkes FG, Frayling TM, Price JF,, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y,, Redline S, Lange LA, Kumari M, Wareham NJ, Verschuren WM, Benjamin EJ,, Whittaker JC, Hamsten A, Dudbridge F, Delaney JA, Wong A, Kuh D, Hardy R, Castillo BA, Connolly JJ, van der Harst P, Brunner EJ,, Marmot MG, Wassel CL, Humphries SE, Talmud PJ, Kivimaki M, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Hakonarson H, Reiner AP, Keating BJ, Sattar N, Hingorani AD, Casas JP.. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P,, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ,, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J,, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P,, Woodward M, Adamovic S, Rimm EB,, Meade TW, Gillum RF, Shaffer JA,, Hofman A, Onat A,, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J,, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT,, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O’Donnell CJ, Wilson JF,, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J,, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J.. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Maffia P, Guzik TJ.. When, where, and how to target vascular inflammation in the post-CANTOS era? Eur Heart J 2019;40:2492–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M.. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F.. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol 2010;84:12658–12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Raj VS, Mou H, Smits SL, Dekkers DHW, Müller MA, Dijkman R, Muth D, Demmers JAA, Zaki A, Fouchier RAM, Thiel V, Drosten C, Rottier PJM, Osterhaus ADME, Bosch BJ, Haagmans BL.. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013;495:251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM.. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM.. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. de Lang A, Osterhaus AD, Haagmans BL.. Interferon-gamma and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology 2006;353:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zulli A, Burrell LM, Widdop RE, Black MJ, Buxton BF, Hare DL.. Immunolocalization of ACE2 and AT2 receptors in rabbit atherosclerotic plaques. J Histochem Cytochem 2006;54:147–150. [DOI] [PubMed] [Google Scholar]
- 106. Thatcher SE, Gupte M, Hatch N, Cassis LA.. Deficiency of ACE2 in bone-marrow-derived cells increases expression of TNF-alpha in adipose stromal cells and augments glucose intolerance in obese C57BL/6 mice. Int J Hypertens 2012;2012:762094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JW, Yiu SF.. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 2003;108:1798–1803. [DOI] [PubMed] [Google Scholar]
- 108. Yu CM, Wong RS, Wu EB, Kong SL, Wong J, Yip GW, Soo YO, Chiu ML, Chan YS, Hui D, Lee N, Wu A, Leung CB, Sung JJ.. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 2006;82:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Oudit GY, Kassiri Z, Jiang C, Liu PP,, Poutanen SM, Penninger JM, Butany J.. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Touyz RM, Alves-Lopes R, Rios FJ, Camargo LL, Anagnostopoulou A, Arner A, Montezano AC.. Vascular smooth muscle contraction in hypertension. Cardiovasc Res 2018;114:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lacolley P, Regnault V, Avolio AP.. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res 2018;114:513–528. [DOI] [PubMed] [Google Scholar]
- 112. Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS,, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI.. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 2005;26:369–375. [DOI] [PubMed] [Google Scholar]
- 113. Zhao YX, Yin HQ, Yu QT, Qiao Y, Dai HY, Zhang MX, Zhang L, Liu YF, Wang LC, Liu DS, Deng BP, Zhang YH, Pan CM, Song HD, Qu X, Jiang H, Liu CX, Lu XT, Liu B, Gao F, Dong B.. ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum Gene Ther 2010;21:1545–1554. [DOI] [PubMed] [Google Scholar]
- 114.SIAARTI, Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva. Percorso assistenziale per il paziente affetto da COVID-19. http://www.siaarti.it/SiteAssets/News/COVID19%20-%20documenti%20SIAARTI/Percorso%20COVID-19%20-%20Sezione%201%20-%20Procedura%20Area%20Critica%20-%20Rev%202.0.pdf2020. (4 April 2020).
- 115. Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, Dai J, Sun Q, Zhao F, Qu J, Yan F.. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020;80:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Geng L, Wang W, Chen Y, Cao J, Lu L, Chen Q, He R, Shen W.. Elevation of ADAM10, ADAM17, MMP-2 and MMP-9 expression with media degeneration features CaCl2-induced thoracic aortic aneurysm in a rat model. Exp Mol Pathol 2010;89:72–81. [DOI] [PubMed] [Google Scholar]
- 117. Nakkazi E. Randomised controlled trial begins for Ebola therapeutics. Lancet 2018;392:2338. [DOI] [PubMed] [Google Scholar]
- 118. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M.. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bettadapura J, Herrero LJ, Taylor A, Mahalingam S.. Approaches to the treatment of disease induced by chikungunya virus. Indian J Med Res 2013;138:762–765. [PMC free article] [PubMed] [Google Scholar]
- 120. Kuhl U, Lassner D, von Schlippenbach J, Poller W, Schultheiss HP.. Interferon-Beta improves survival in enterovirus-associated cardiomyopathy. J Am Coll Cardiol 2012;60:1295–1296. [DOI] [PubMed] [Google Scholar]
- 121. Maisch B, Alter P.. Treatment options in myocarditis and inflammatory cardiomyopathy: focus on i.v. immunoglobulins. Herz 2018;43:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM.. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf 2018;41:919–931. [DOI] [PubMed] [Google Scholar]
- 123. Gabay C, Riek M, Hetland ML, Hauge EM, Pavelka K, Tomsic M, Canhao H, Chatzidionysiou K, Lukina G, Nordstrom DC, Lie E, Ancuta I, Hernandez MV, van Riel PL, van Vollenhoven R, Kvien TK.. Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: results from a European collaborative study. Ann Rheum Dis 2016;75:1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Giles JT, Sattar N, Gabriel S, Ridker PM, Gay S, Warne C, Musselman D, Brockwell L, Shittu E, Klearman M, Fleming TR.. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol 2020;72:31–40. [DOI] [PubMed] [Google Scholar]
- 125. Zuo H, Li R, Ma F, Jiang J, Miao K, Li H, Nagel E, Tadic M, Wang H, Wang DW.. Temporal echocardiography findings in patients with fulminant myocarditis: beyond ejection fraction decline. Front Med 2019;doi: 10.1007/s11684-019-0713-9. [DOI] [PubMed] [Google Scholar]
- 126. Vergano M, Bertolini G, Giannini A, Gristina G, Livigni S, Mistraletti G, Petrini F.. Clinical Ethics Recommendations for the Allocation of Intensive Care Treatments, in Exceptional, Resource-Limited Circumstances. Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva.
- 127. Frederix I, Caiani EG, Dendale P, Anker S, Bax J, Bohm A, Cowie M, Crawford J, de Groot N, Dilaveris P, Hansen T, Koehler F, Krstacic G, Lambrinou E, Lancellotti P, Meier P, Neubeck L, Parati G, Piotrowicz E, Tubaro M, van der Velde E.. ESC e-Cardiology Working Group Position Paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol 2019;26:1166–1177. [DOI] [PubMed] [Google Scholar]
- 128. Cacioppo JT, Hawkley LC.. Perceived social isolation and cognition. Trends Cogn Sci 2009;13:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu Y, Lv L, Wang L, Zhong Y.. Social isolation induces Rac1-dependent forgetting of social memory. Cell Rep 2018;25:288–295. [DOI] [PubMed] [Google Scholar]
- 130. Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, Wildes CP, Ungless MA, Tye KM.. Dorsal Raphe dopamine neurons represent the experience of social isolation. Cell 2016;164:617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jaremka LM, Peng J, Bornstein R, Alfano CM, Andridge RR, Povoski SP, Lipari AM, Agnese DM, Farrar WB, Yee LD, Carson WE 3rd, Kiecolt-Glaser JK.. Cognitive problems among breast cancer survivors: loneliness enhances risk. Psychooncology 2014;23:1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ellwardt L, Aartsen M, Deeg D, Steverink N.. Does loneliness mediate the relation between social support and cognitive functioning in later life? Soc Sci Med 2013;98:116–124. [DOI] [PubMed] [Google Scholar]
- 133. Nonogaki K, Nozue K, Oka Y.. Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology 2007;148:4658–166. [DOI] [PubMed] [Google Scholar]
- 134. Volden PA, Wonder EL, Skor MN, Carmean CM, Patel FN, Ye H, Kocherginsky M, McClintock MK, Brady MJ, Conzen SD.. Chronic social isolation is associated with metabolic gene expression changes specific to mammary adipose tissue. Cancer Prev Res (Phila) 2013;6:634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Whisman MA. Loneliness and the metabolic syndrome in a population-based sample of middle-aged and older adults. Health Psychol 2010;29:550–554. [DOI] [PubMed] [Google Scholar]
- 136. Jaremka LM, Fagundes CP, Peng J, Belury MA, Andridge RR, Malarkey WB, Kiecolt-Glaser JK.. Loneliness predicts postprandial ghrelin and hunger in women. Horm Behav 2015;70:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Budiu RA,, Vlad AM, Nazario L, Bathula C, Cooper KL, Edmed J, Thaker PH, Urban J, Kalinski P, Lee AV, Elishaev EL, Conrads TP, Flint MS.. Restraint and social isolation stressors differentially regulate adaptive immunity and tumor angiogenesis in a breast cancer mouse model. Cancer Clin Oncol 2017;6:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, Clevenger L, Lubaroff DM, Sood AK, Cole SW.. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun 2011;25:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hawkley LC, Cacioppo JT.. Loneliness and pathways to disease. Brain Behav Immun 2003;17 Suppl 1:S98–105. [DOI] [PubMed] [Google Scholar]
- 140. Pyter LM, Yang L,, McKenzie C, da Rocha JM, Carter CS, Cheng B, Engeland CG.. Contrasting mechanisms by which social isolation and restraint impair healing in male mice. Stress 2014;17:256–265. [DOI] [PubMed] [Google Scholar]
- 141. Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK.. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology 2013;38:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Steptoe A, Kivimaki M.. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health 2013;34:337–54. [DOI] [PubMed] [Google Scholar]
- 143. Leigh-Hunt N, Bagguley D, Bash K, Turner V, Turnbull S, Valtorta N, Caan W.. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health 2017;152:157–171. [DOI] [PubMed] [Google Scholar]
- 144. Heidari Gorji MA, Fatahian A, Farsavian A.. The impact of perceived and objective social isolation on hospital readmission in patients with heart failure: a systematic review and meta-analysis of observational studies. Gen Hosp Psychiatry 2019;60:27–36. [DOI] [PubMed] [Google Scholar]
- 145. Pantell M, Rehkopf D, Jutte D, Syme SL, Balmes J, Adler N.. Social isolation: a predictor of mortality comparable to traditional clinical risk factors. Am J Public Health 2013;103:2056–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thurston RC, Kubzansky LD.. Women, loneliness, and incident coronary heart disease. Psychosom Med 2009;71:836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dennis J, Sealock J, Levinson RT, Farber-Eger E, Franco J, Fong S, Straub P, Hucks D, Song WL, Linton MF, Fontanillas P, Elson SL, Ruderfer D, Abdellaoui A, Sanchez-Roige S, Palmer AA, Boomsma DI, Cox NJ, Chen G, Mosley JD, Wells QS, Davis LK.. Genetic risk for major depressive disorder and loneliness in sex-specific associations with coronary artery disease. Mol Psychiatry 2019;doi: 10.1038/s41380-019-0614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Xia N, Li H.. Loneliness, social isolation, and cardiovascular health. Antioxid Redox Signal 2018;28:837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vigorito C, Giallauria F.. Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. Eur J Prev Cardiol 2018;25:1384–1386. [DOI] [PubMed] [Google Scholar]
- 150. Rozanski A, Blumenthal JA, Kaplan J.. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99:2192–217. [DOI] [PubMed] [Google Scholar]
- 151. Knox SS, Uvnas-Moberg K.. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology 1998;23:877–890. [DOI] [PubMed] [Google Scholar]
- 152. Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B.. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart 2016;102:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Hakulinen C, Pulkki-Raback L, Virtanen M, Jokela M, Kivimaki M, Elovainio M.. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart 2018;104:1536–1542. [DOI] [PubMed] [Google Scholar]
- 154. Koss KJ, Hostinar CE, Donzella B, Gunnar MR.. Social deprivation and the HPA axis in early development. Psychoneuroendocrinology 2014;50:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW.. The neuroendocrinology of social isolation. Annu Rev Psychol 2015;66:733–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Stafford M, Gardner M, Kumari M, Kuh D, Ben-Shlomo Y.. Social isolation and diurnal cortisol patterns in an ageing cohort. Psychoneuroendocrinology 2013;38:2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hawkley LC, Cole SW, Capitanio JP, Norman GJ, Cacioppo JT.. Effects of social isolation on glucocorticoid regulation in social mammals. Horm Behav 2012;62:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Lewis R, Wilkins B, Benjamin B, Curtis JT.. Cardiovascular control is associated with pair-bond success in male prairie voles. 2017;208:93–102. [DOI] [PubMed] [Google Scholar]
- 159. Custaud MA, Belin de Chantemele E, Larina IM, Nichiporuk IA, Grigoriev A, Duvareille M, Gharib C, Gauquelin-Koch G.. Hormonal changes during long-term isolation. Eur J Appl Physiol 2004;91:508–515. [DOI] [PubMed] [Google Scholar]
- 160. Lyons DM, Ha CM, Levine S.. Social effects and circadian rhythms in squirrel monkey pituitary–adrenal activity. Horm Behav 1995;29:177–190. [DOI] [PubMed] [Google Scholar]
- 161. Vaernes RJ, Bergan T, Warncke M, Ursin H, Aakvaag A, Hockey R.. European isolation and confinement study. Workload and stress: effects on psychosomatic and psychobiological reaction patterns. Adv Space Biol Med 1993;3:95–120. [PubMed] [Google Scholar]
- 162. Murray DR, Haselton MG, Fales M, Cole SW.. Subjective social status and inflammatory gene expression. Health Psychol 2019;38:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Mumtaz F, Khan MI, Zubair M, Dehpour AR.. Neurobiology and consequences of social isolation stress in animal model—a comprehensive review. Biomed Pharmacother 2018;105:1205–1222. [DOI] [PubMed] [Google Scholar]
- 164. Kamal A, Ramakers GM, Altinbilek B, Kas MJ.. Social isolation stress reduces hippocampal long-term potentiation: effect of animal strain and involvement of glucocorticoid receptors. Neuroscience 2014;256:262–270. [DOI] [PubMed] [Google Scholar]
- 165. Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, Tassin JP, Mombereau C, Faure P, Tronche F.. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 2013;339:332–335. [DOI] [PubMed] [Google Scholar]
- 166. Skulstad H, Cosyns B, Popescu BA,, Galderisi M, Salvo GD, Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, Almeida AG, Schulz-Menger J, Dweck MR, Pontone G, Sade LE, Gerber B, Maurovich-Horvat P, Bharucha T, Cameli M, Magne J, Westwood M, Maurer G, Edvardsen T.. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Richardson S, Hirsch JS, Narasimhan M, Crawford DM, McGinn T, Davidson KWand the Northwell COVID-19 Research Consortium. Presenting characteristics, Comorbidities, and outcomes among 5700 patients hospitalized With COVID-19 in the New York City area. JAMA 2020;doi:10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]