Abstract
Background
Organised inpatient (stroke unit) care is provided by multi‐disciplinary teams that manage stroke patients. This can been provided in a ward dedicated to stroke patients (stroke ward), with a peripatetic stroke team (mobile stroke team), or within a generic disability service (mixed rehabilitation ward). Team members aim to provide co‐ordinated multi‐disciplinary care using standard approaches to manage common post‐stroke problems.
Objectives
• To assess the effects of organised inpatient (stroke unit) care compared with an alternative service.
• To use a network meta‐analysis (NMA) approach to assess different types of organised inpatient (stroke unit) care for people admitted to hospital after a stroke (the standard comparator was care in a general ward).
Originally, we conducted this systematic review to clarify:
• The characteristic features of organised inpatient (stroke unit) care?
• Whether organised inpatient (stroke unit) care provide better patient outcomes than alternative forms of care?
• If benefits are apparent across a range of patient groups and across different approaches to delivering organised stroke unit care?
Within the current version, we wished to establish whether previous conclusions were altered by the inclusion of new outcome data from recent trials and further analysis via NMA.
Search methods
We searched the Cochrane Stroke Group Trials Register (2 April 2019); the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 4), in the Cochrane Library (searched 2 April 2019); MEDLINE Ovid (1946 to 1 April 2019); Embase Ovid (1974 to 1 April 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 2 April 2019). In an effort to identify further published, unpublished, and ongoing trials, we searched seven trial registries (2 April 2019). We also performed citation tracking of included studies, checked reference lists of relevant articles, and contacted trialists.
Selection criteria
Randomised controlled clinical trials comparing organised inpatient stroke unit care with an alternative service (typically contemporary conventional care), including comparing different types of organised inpatient (stroke unit) care for people with stroke who are admitted to hospital.
Data collection and analysis
Two review authors assessed eligibility and trial quality. We checked descriptive details and trial data with co‐ordinators of the original trials, assessed risk of bias, and applied GRADE. The primary outcome was poor outcome (death or dependency (Rankin score 3 to 5) or requiring institutional care) at the end of scheduled follow‐up. Secondary outcomes included death, institutional care, dependency, subjective health status, satisfaction, and length of stay. We used direct (pairwise) comparisons to compare organised inpatient (stroke unit) care with an alternative service. We used an NMA to confirm the relative effects of different approaches.
Main results
We included 29 trials (5902 participants) that compared organised inpatient (stroke unit) care with an alternative service: 20 trials (4127 participants) compared organised (stroke unit) care with a general ward, six trials (982 participants) compared different forms of organised (stroke unit) care, and three trials (793 participants) incorporated more than one comparison.
Compared with the alternative service, organised inpatient (stroke unit) care was associated with improved outcomes at the end of scheduled follow‐up (median one year): poor outcome (odds ratio (OR) 0.77, 95% confidence interval (CI) 0.69 to 0.87; moderate‐quality evidence), death (OR 0.76, 95% CI 0.66 to 0.88; moderate‐quality evidence), death or institutional care (OR 0.76, 95% CI 0.67 to 0.85; moderate‐quality evidence), and death or dependency (OR 0.75, 95% CI 0.66 to 0.85; moderate‐quality evidence). Evidence was of very low quality for subjective health status and was not available for patient satisfaction. Analysis of length of stay was complicated by variations in definition and measurement plus substantial statistical heterogeneity (I² = 85%). There was no indication that organised stroke unit care resulted in a longer hospital stay. Sensitivity analyses indicated that observed benefits remained when the analysis was restricted to securely randomised trials that used unequivocally blinded outcome assessment with a fixed period of follow‐up. Outcomes appeared to be independent of patient age, sex, initial stroke severity, stroke type, and duration of follow‐up. When calculated as the absolute risk difference for every 100 participants receiving stroke unit care, this equates to two extra survivors, six more living at home, and six more living independently.
The analysis of different types of organised (stroke unit) care used both direct pairwise comparisons and NMA.
Direct comparison of stroke ward versus general ward: 15 trials (3523 participants) compared care in a stroke ward with care in general wards. Stroke ward care showed a reduction in the odds of a poor outcome at the end of follow‐up (OR 0.78, 95% CI 0.68 to 0.91; moderate‐quality evidence).
Direct comparison of mobile stroke team versus general ward: two trials (438 participants) compared care from a mobile stroke team with care in general wards. Stroke team care may result in little difference in the odds of a poor outcome at the end of follow‐up (OR 0.80, 95% CI 0.52 to 1.22; low‐quality evidence).
Direct comparison of mixed rehabilitation ward versus general ward: six trials (630 participants) compared care in a mixed rehabilitation ward with care in general wards. Mixed rehabilitation ward care showed a reduction in the odds of a poor outcome at the end of follow‐up (OR 0.65, 95% CI 0.47 to 0.90; moderate‐quality evidence).
In a NMA using care in a general ward as the comparator, the odds of a poor outcome were as follows: stroke ward ‐ OR 0.74, 95% CI 0.62 to 0.89, moderate‐quality evidence; mobile stroke team ‐ OR 0.88, 95% CI 0.58 to 1.34, low‐quality evidence; mixed rehabilitation ward ‐ OR 0.70, 95% CI 0.52 to 0.95, low‐quality evidence.
Authors' conclusions
We found moderate‐quality evidence that stroke patients who receive organised inpatient (stroke unit) care are more likely to be alive, independent, and living at home one year after the stroke. The apparent benefits were independent of patient age, sex, initial stroke severity, or stroke type, and were most obvious in units based in a discrete stroke ward. We observed no systematic increase in the length of inpatient stay, but these findings had considerable uncertainty.
Keywords: Humans; Hospital Units; Hospitalization; Length of Stay; Outcome Assessment, Health Care; Patient Care Team; Prognosis; Randomized Controlled Trials as Topic; Stroke; Stroke/mortality; Stroke/therapy; Stroke Rehabilitation; Treatment Outcome
Plain language summary
Organised inpatient (stroke unit) care
Review question Does organised inpatient (stroke unit) care improve the recovery of people with stroke in hospital compared with conventional care in general wards?
Background Organised inpatient (stroke unit) care is a form of care provided in hospital by nurses, doctors, and therapists who specialise in looking after people with stroke. They aim to work as a co‐ordinated team to provide the most appropriate care tailored to the needs of individual people with stroke.
Study characteristics We identified 29 trials involving 5902 participants (search completed 2 April 2019). Participants who were recruited had had a recent stroke and required admission to hospital. Organised inpatient (stroke unit) care was provided in a variety of ways including stroke ward (care provided in a discrete stroke ward), mixed rehabilitation ward (setting seeking to improve care for people with stroke within a mixed rehabilitation ward), and mobile stroke team (peripatetic team looking after people with stroke across a range of wards).
Key results At an average of 12 months after their stroke, people who received organised inpatient (stroke unit) care were more likely to be alive (an extra two people surviving for every 100 receiving stroke unit care; moderate‐quality evidence) and living at home (an extra six patients for every 100 receiving stroke unit care; moderate‐quality evidence). They also were more likely to be independent in daily activities (an extra six patients for every 100 receiving stroke unit care; moderate‐quality evidence). The apparent benefits were seen in men and women, older and younger patients, and people with different types of stroke and different stroke severity. Benefits were most obvious when the stroke unit was based in a discrete stroke ward.
Quality of the evidence We downgraded the quality of evidence to 'moderate' for the main outcomes because it was impossible to hide the treating service from participants or healthcare workers. These conclusions were not dependent on trials judged to be of lower quality because of poor design or missing data. More information was missing for some of the other outcome measures and analyses, which we have downgraded to low‐quality evidence.
Conclusion People with stroke who receive organised inpatient (stroke unit) care are more likely to be alive, living at home, and independent in looking after themselves one year after their stroke. Apparent benefits were seen across a broad range of people with stroke. Various types of stroke units have been developed. The best results appear to come from stroke units based in a dedicated stroke ward.
Summary of findings
Summary of findings 1. Organised inpatient (stroke unit) care versus alternative service.
| Organised inpatient (stroke unit) care compared with alternative service | ||||||
|
Patient or population: adults with acute stroke Settings: hospital Intervention: organised inpatient (stroke unit) care Comparison: alternative service (contemporary conventional care) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alternative service | Organised inpatient (stroke unit) care | |||||
|
Poor outcome by the end of scheduled follow‐up (modified Rankin score 3 to 6 or requiring institutional care; median 12‐month follow‐up) (Analysis 1.1) |
577 per 1000 | 517 per 1000 (497 to 547) |
OR 0.77 (0.69 to 0.87) | 5336 (26) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Death by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 1.2) |
219 per 1000 | 199 per 1000 (179 to 209) |
OR 0.76 (0.66 to 0.88) |
5902 (29) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Death or institutional care by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 1.3) |
405 per 1000 | 345 per 1000 (315 to 375) |
OR 0.76 (0.67 to 0.85) |
4887 (25) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Death or dependency by the end of scheduled follow‐up (modified Rankin score 3 to 6; median 12‐month follow‐up) (Analysis 1.4) |
609 per 1000 | 549 per 1000 (519 to 567) |
OR 0.75 (0.66 to 0.85) | 4854 (24) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Subjective health status score Participant quality of life (Nottingham Health Profile; Quality of Life Scale) |
There was a pattern of improved results among stroke unit survivors, with results attaining statistical significance in 2 individual trials | N/A | 843 (3) |
⊕⊝⊝⊝ very lowa,b,c |
Data from 3 trials only High rate of missing data |
|
| Patient satisfaction or preference | We could find no systematically gathered information on patient preferences | N/A | N/A | N/A | No data available | |
| Length of stay (days) in a hospital or institution (Analysis 1.5) | Mean length of stay across the control groups ranged from 12.1 to 123 days | Mean length of stay for the intervention groups was, on average, 4.3 days less (7.9 days less to 0.7 days more) | SMD 0.16 lower (0.33 lower to 0.01 higher) |
4162 (19) |
⊕⊕⊝⊝ lowa,b | Different definitions and imprecise measures of length of stay were reported |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; OR: odds ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded for potential risk of performance bias.
bDowngraded for unexplained heterogeneity.
cDowngraded for imprecision
1.1. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 1: Poor outcome by the end of scheduled follow‐up
1.2. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 2: Death by the end of scheduled follow‐up
1.3. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 3: Death or institutional care by the end of scheduled follow‐up
1.4. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 4: Death or dependency by the end of scheduled follow‐up
1.5. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 5: Length of stay (days) in a hospital or institution or both
Summary of findings 2. Stroke ward versus general medical ward.
| Organised inpatient (stroke unit) care compared with general medical ward care for stroke | ||||||
|
Patient or population: adults with acute stroke Settings: hospital Intervention: stroke ward care Comparison: general medical ward care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| General medical ward care | Stroke ward care | |||||
|
Poor outcome by the end of scheduled follow‐up (modified Rankin score 3 to 6 or requiring institutional care; median 12‐month follow‐up) (Analysis 2.1) |
549 per 1000 | 499 per 1000 (459 to 529) |
OR 0.78 (0.68 to 0.91) | 3321 (14) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Death by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 2.2) |
242 per 1000 | 202 per 1000 (172 to 222) |
OR 0.75 (0.63 to 0.90) |
3523 (15) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Death or institutional care by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 2.3) |
383 per 1000 | 323 per 1000 (283 to 353) |
OR 0.74 (0.63 to 0.87) |
2924 (13) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Death or dependency by the end of scheduled follow‐up (modified Rankin score 3 to 6; median 12‐month follow‐up) (Analysis 2.4) |
602 per 1000 | 532 per 1000 (502 to 572) |
OR 0.75 (0.64 to 0.88) | 2839 (12) |
⊕⊕⊕⊝ moderatea | Sensitivity analysis based on trial quality suggested no alteration of conclusions |
|
Subjective health status score Participant quality of life (Nottingham Health Profile; Quality of Life Scale) |
There was a pattern of improved results among stroke unit survivors, with results attaining statistical significance in 2 individual trials | N/A | 535 (2) |
⊕⊝⊝⊝ very lowa,b,c |
Data from 3 trials only High rate of missing data |
|
| Patient satisfaction or preference | We could find no systematically gathered information on patient preferences | N/A | N/A | N/A | No data available | |
| Length of stay (days) in a hospital or institution (Analysis 2.5) | Mean length of stay across control groups ranged from 12.8 to 123 days | Mean length of stay for the intervention groups was, on average, 2.2 days less (5.2 days less to 0.8 days more) | SMD 0.13 lower (0.29 lower to 0.04 higher) |
2547 (10) |
⊕⊕⊝⊝ lowa,b | Different definitions and imprecise measures of length of stay were reported |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; OR: odds ratio; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded for potential risk of performance bias.
bDowngraded for unexplained heterogeneity.
cDowngraded for imprecision
2.1. Analysis.

Comparison 2: Stroke ward versus general medical ward, Outcome 1: Poor outcome by the end of scheduled follow‐up
2.2. Analysis.

Comparison 2: Stroke ward versus general medical ward, Outcome 2: Death by the end of scheduled follow‐up
2.3. Analysis.

Comparison 2: Stroke ward versus general medical ward, Outcome 3: Death or institutional care by the end of scheduled follow‐up
2.4. Analysis.

Comparison 2: Stroke ward versus general medical ward, Outcome 4: Death or dependency by the end of scheduled follow‐up
2.5. Analysis.

Comparison 2: Stroke ward versus general medical ward, Outcome 5: Length of stay (days) in a hospital or institution
Summary of findings 3. Mobile stroke team versus general medical ward.
| Mobile stroke team care compared with general medical ward care for stroke | ||||||
|
Patient or population: adults with acute stroke Settings: hospital Intervention: mobile stroke team care Comparison: general medical ward care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| General medical ward care | Mobile stroke team care | |||||
|
Poor outcome by the end of scheduled follow‐up (modified Rankin score 3 to 6 or requiring institutional care; median 12‐month follow‐up) (Analysis 3.1) |
712 per 1000 | 672 per 1000 (582 to 752) |
OR 0.80 (0.52 to 1.22) |
438 (2) |
⊕⊕⊝⊝ lowa,b | As dependency data were complete, these are the same data as for death or dependency |
|
Death by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 3.2) |
259 per 1000 | 279 per 1000 (189 to 359) |
OR 1.08 (0.71 to 1.65) |
438 (2) |
⊕⊕⊝⊝ lowa,b | |
|
Death or institutional care by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 3.3) |
481 per 1000 | 531 per 1000 (451 to 611) |
OR 1.27 (0.84 to 1.93) |
438 (2) |
⊕⊕⊝⊝ lowa,b | |
|
Death or dependency by the end of scheduled follow‐up (modified Rankin score 3 to 6; median 12‐month follow‐up) (Analysis 3.4) |
712 per 1000 | 672 per 1000 (582 to 752) |
OR 0.80 (0.52 to 1.22) |
438 (2) |
⊕⊕⊝⊝ lowa,b | As dependency data were complete, these are the same data as for poor outcome |
|
Subjective health status score Participant quality of life (EuroQol) |
No apparent differences between groups | N/A | 308 (1) |
⊕⊝⊝⊝ very lowa,b |
Data from 1 trial only | |
| Patient satisfaction or preference | We could find no systematically gathered information on patient preferences | N/A | N/A | N/A | No data available | |
|
Length of stay (days) in a hospital or institution (Analysis 3.5) |
No data available | N/A | N/A | N/A | No data available | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded for potential risk of performance bias.
bDowngraded for imprecision.
3.1. Analysis.

Comparison 3: Mobile stroke team versus general medical ward, Outcome 1: Poor outcome by the end of scheduled follow‐up
3.2. Analysis.

Comparison 3: Mobile stroke team versus general medical ward, Outcome 2: Death by the end of scheduled follow‐up
3.3. Analysis.

Comparison 3: Mobile stroke team versus general medical ward, Outcome 3: Death or institutional care by the end of scheduled follow‐up
3.4. Analysis.

Comparison 3: Mobile stroke team versus general medical ward, Outcome 4: Death or dependency by the end of scheduled follow‐up
3.5. Analysis.

Comparison 3: Mobile stroke team versus general medical ward, Outcome 5: Length of stay (days) in a hospital or institution
Summary of findings 4. Mixed rehabilitation ward versus general medical ward.
| Mixed rehabilitation ward care compared with general medical ward care for stroke | ||||||
|
Patient or population: adults with acute stroke Settings: hospital Intervention: mixed rehabilitation ward care Comparison: general medical ward care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| General medical ward care | Mixed rehabilitation wardcare | |||||
|
Poor outcome by the end of scheduled follow‐up (modified Rankin score 3 to 6 or requiring institutional care; median 12‐month follow‐up) (Analysis 4.1) |
574 per 1000 | 474 per 1000 (404 to 554) |
OR 0.65 (0.47 to 0.90) |
630 (6) |
⊕⊕⊝⊝ lowa,b | As dependency data were complete, these are the same data as for death or dependency |
|
Death by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 4.2) |
171 per 1000 | 161 per 1000 (101 to 211) |
OR 0.91 (0.58 to 1.42) |
630 (6) |
⊕⊕⊝⊝ lowa,b | |
|
Death or institutional care by the end of scheduled follow‐up (median 12‐month follow‐up) (Analysis 4.3) |
451 per 1000 | 371 per 1000 (291 to 451) |
OR 0.71 (0.51 to 0.99) |
578 (5) |
⊕⊕⊝⊝ lowa,b | |
|
Death or dependency by the end of scheduled follow‐up (modified Rankin score 3 to 6; median 12‐month follow‐up) (Analysis 4.4) |
574 per 1000 | 474 per 1000 (404 to 554) |
OR 0.65 (0.47 to 0.90) |
630 (6) |
⊕⊕⊝⊝ low a,b | As dependency data were complete, these are the same data as for poor outcome |
| Subjective health Status score | No data available | N/A | N/A | N/A | No data available | |
| Patient satisfaction or preference | We could find no systematically gathered information on patient preferences | N/A | N/A | N/A | No data available | |
| Length of stay (days) in a hospital or institution (Analysis 4.5) | Mean length of stay across control groups ranged from 30.5 to 129.5 days | Mean length of stay for the intervention groups was, on average, 3.9 days more (13.5 days less to 21.5 days more) | MD 0.08 more (0.21 lower to 0.37 higher) |
387 (3) |
⊕⊝⊝⊝ very lowa,b,c | Different definitions and imprecise measures of length of stay were reported |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; N/A: not applicable; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded for potential risk of performance bias.
bDowngraded for imprecision.
cDowngraded for unexplained heterogeneity.
4.1. Analysis.

Comparison 4: Mixed rehabilitation ward versus general medical ward, Outcome 1: Poor outcome by the end of scheduled follow‐up
4.2. Analysis.

Comparison 4: Mixed rehabilitation ward versus general medical ward, Outcome 2: Death by the end of scheduled follow‐up
4.3. Analysis.

Comparison 4: Mixed rehabilitation ward versus general medical ward, Outcome 3: Death or institutional care by the end of scheduled follow‐up
4.4. Analysis.

Comparison 4: Mixed rehabilitation ward versus general medical ward, Outcome 4: Death or dependency by the end of scheduled follow‐up
4.5. Analysis.

Comparison 4: Mixed rehabilitation ward versus general medical ward, Outcome 5: Length of stay (days) in a hospital or institution
Background
Description of the condition
Stroke is now the third leading cause of disability (Murray 2012), and it is the second leading cause of mortality worldwide (Lozano 2012). The global disease burden of stroke increased by 19% between 1990 and 2010 (Murray 2012), and current projections estimate the number of deaths worldwide will rise to 6.5 million in 2015 and to 7.8 million in 2030 (Strong 2007). Interventions that are applicable to a majority of people with stroke and that aim to reduce associated mortality and disability are essential.
During their initial illness, people with stroke are frequently admitted to hospital, where they can receive care in a variety of ways and in a range of settings. Traditionally, care for people with stroke was provided within departments of general (internal) medicine, neurology, or medicine for the elderly, where they would be managed alongside a range of other patient groups. A more focused approach to the treatment of people with stroke in hospital has been developed.
Description of the intervention
Organised inpatient (stroke unit) care is the term used to describe focused care for people with stroke in hospital under a multi‐disciplinary team of individuals who specialise in stroke management (SUTC 1997a). This concept is not new, and its value has been debated for more than 30 years (Ebrahim 1990; Garraway 1985; Langhorne 1993; Langhorne 1998; Langhorne 2012). In essence, the debate has concerned whether the perceived effort and cost of focusing the care of people hospitalised with stroke within specially organised units would be matched by tangible benefits for the people receiving that care. In particular, would more people survive and make a good recovery as a result of organised inpatient (stroke unit) care?
Why it is important to do this review
A systematic review of all available trials previously described the range of characteristics of stroke unit care and addressed the question of whether improving the organisation of inpatient stroke care can bring about improvements in important patient outcomes (SUTC 1997a). This review continues to be extended and updated within the Cochrane Library (SUTC 2001; SUTC 2007; SUTC 2013). Since the last update, network meta‐analysis (NMA) has become established as an approach for handling multiple comparisons. We have added NMA to our updated review.
Objectives
To assess the effects of organised inpatient (stroke unit) care compared with an alternative service (usually contemporary conventional care)
To use a network meta‐analysis (NMA) approach to assess different types of organised inpatient (stroke unit) care for people admitted to hospital after a stroke (the standard comparator was care in a general ward)
Originally, this systematic review was conducted to address four broad questions.
What are the characteristic features of organised inpatient (stroke unit) care?
Can organised inpatient (stroke unit) care provide better patient outcomes than alternative forms of care?
Are any benefits apparent across a range of patient groups?
Are any benefits apparent across different approaches to delivering organised stroke unit care? In particular, we hypothesised that organised care would be more effective than care provided in general medical wards, but that different forms of organised care would achieve similar outcomes.
Within the current version of this review, we wished to establish whether previous conclusions were altered by the inclusion of new outcome data from recent trials and further analysis via NMA.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled clinical trials that compared an organised system of inpatient (stroke unit) care with an alternative form of inpatient care. This was usually contemporary conventional care but could include an alternative model of organised inpatient (stroke unit) care (see Types of interventions). Previous versions of this review included trials with quasi‐random treatment allocation (such as bed availability or date of admission) (SUTC 1997a; SUTC 2001; SUTC 2007). However, in an effort to ensure that this ongoing systematic review focuses on data from trials with strict randomisation procedures, we excluded all quasi‐randomised trials in the previous update (SUTC 2013). We would have included cluster‐randomised trials, but we identified none. We excluded cross‐over trials because of cross‐over of effects.
Types of participants
Any person admitted to hospital who had suffered a stroke was eligible. We recorded the delay between stroke onset and hospital admission but did not use this as an exclusion criterion. We used a clinical definition of stroke: focal neurological deficit due to cerebrovascular disease, excluding subarachnoid haemorrhage and subdural haematoma.
Types of interventions
Organised inpatient (stroke unit) care can be considered a complex organisational intervention comprising multi‐disciplinary staff providing a complex package of care to people with stroke in hospital. In the original version of this review, the first question was whether organised inpatient (stroke unit) care could improve outcomes compared with contemporary conventional care (usually in general wards) (SUTC 1997a). We then had to modify the analyses in a minor way to reflect the emerging pattern of service organisation and to allow the comparison of 'more organised' versus 'less organised' services (for which the latter was usually contemporary conventional care). We did this because some recent trials have addressed new questions and included comparisons of two services, both of which met the basic definition of organised (stroke unit) care. In the original service descriptions used in this review (SUTC 1997a), service organisation was considered as a hierarchy comprising the following.
-
Stroke ward: where a multi‐disciplinary team including specialist nursing staff based in a discrete ward cares exclusively for people with stroke. This category included the following subdivisions.
-
Acute stroke units that accept patients acutely but discharge early (usually within seven days); these appear to fall into three broad subcategories.
'Intensive' model of care with continuous monitoring, high nurse staffing levels, and the potential for life support.
'Semi‐intensive' model of care with continuous monitoring, high nurse staffing, but no life support facilities.
'Non‐intensive' model of care with none of the above.
Rehabilitation stroke units that accept patients after a delay, usually of seven days or longer, and focus on rehabilitation.
Comprehensive (i.e. combined acute and rehabilitation) stroke units that accept patients acutely but also provide rehabilitation for at least several weeks if necessary. Both the rehabilitation unit model and the comprehensive unit model offer prolonged periods of rehabilitation.
-
Mixed rehabilitation ward: where a multi‐disciplinary team including specialist nursing staff in a ward provides a generic rehabilitation service but not exclusively caring for people with stroke.
Mobile stroke team: where a peripatetic multi‐disciplinary team (usually excluding specialist nursing staff) provides care in a variety of settings.
General medical ward: where care is provided in an acute medical or neurology ward without routine multi‐disciplinary input.
For the NMA eligibility assessment, we considered the transitivity (similarity) of trials included in the network (see Data synthesis), which requires all interventions to be legitimate alternatives. All of the four main categories have been used to provide care for unselected acute stroke patients and can be considered broadly comparable for the purpose of an NMA. The general medical ward group was the reference group,
Types of outcome measures
Primary outcomes
In the previous version of this review, the primary analyses examined death, dependency, and the requirement for institutional care at the end of scheduled follow‐up of the original trial (SUTC 2013). We categorised dependency into two groups, where we took 'independent' to mean that an individual did not require physical assistance for transfers, mobility, dressing, feeding, or toileting. We considered individuals who failed any of these criteria 'dependent'. The criteria for independence were approximately equivalent to a modified Rankin score of 0 to 2 or a Barthel Index greater than 18 out of 20 (Wade 1992). We took the requirement for long‐term institutional care to mean care in a residential home, a nursing home, or a hospital at the end of scheduled follow‐up.
In view of changes in reporting standards, for this update we have now provided a single composite primary outcome: poor outcome: death or dependency or requiring institutional care (if dependency data were not available). This allowed us to keep the primary focus of the review (i.e. the focus on independent survival as an outcome), while optimising the quantity of data available.
Secondary outcomes
Secondary outcome measures now include:
death;
death or institutional care;
death or dependency;
patient subjective health status (measured using tools such as the Nottingham Health Profile, EuroQol, Short Form‐36);
patient and carer satisfaction (recorded on a Likert scale or as responses to statements); and
duration of stay in hospital or institution or both.
Outcomes are reported at the end of scheduled follow‐up. Some trials subsequently provided supplementary extended follow‐up data, which are presented separately.
Search methods for identification of studies
See the methods for the Cochrane Stroke Group Specialised register. We searched for trials in all languages and arranged the translation of relevant papers published in languages other than English.
Electronic searches
We searched the trials registers of the Cochrane Stroke Group (2 April 2019). In addition, in collaboration with the Cochrane Stroke Group Information Specialist, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019; Issue 4), in the Cochrane Library (searched 2 April 2019) (Appendix 1); MEDLINE Ovid (1946 to 1 April 2019) (Appendix 2); Embase Ovid (1974 to 1 April 2019) (Appendix 3); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (1982 to 2 April 2019) (Appendix 4).
We searched the following registers of ongoing trials using the keyword 'stroke'.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 2 April 2019).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 2 April 2019).
CenterWatch Clinical Trials Listing Service (www.centerwatch.com; searched 13 August 2018).
Community Research & Development Information Service (of the European Union) (cordis.europa.eu/en/home.html; searched 13 August 2018).
South African National Clinical Trial Register (www.sanctr.gov.za; searched 13 August 2018).
The Internet Stroke Center ‐ Stroke Trials Registry (www.strokecenter.org/trials;searched 13 August 2018).
Clinical Trials Results register (www.clinicaltrialresults.org; searched 2 April 2019).
Searching other resources
In an effort to identify further published, unpublished, and ongoing trials, we:
performed citation tracking using Web of Science Cited Reference Search for all included studies;
searched the reference lists of included trials and all relevant articles;
obtained further information from individual trialists; and
contacted other researchers in the field and publicised our preliminary findings at stroke conferences in UK, Scandinavia, Germany, Netherlands, Switzerland, Spain, Canada, Brazil, Argentina, Australia, Belgium, USA, India, Sri Lanka, Singapore, Italy, and Hong Kong.
Data collection and analysis
Selection of studies
For this updated review, one review author (PL) read the titles and abstracts of records obtained through the electronic searches and excluded obviously irrelevant studies. We obtained full copies of the remaining studies, and two review authors (PL and SR) independently selected studies for inclusion based on the following eligibility criteria.
Randomised controlled trial.
Service intervention providing a form of organised inpatient (stroke unit) care.
Service aim to improve functional recovery and survival after stroke.
Trial of stroke patients.
We tried to establish the characteristics of unpublished trials through discussion with the Cochrane Stroke Group Information Specialist before analysing the results.
Data extraction and management
If possible, the principal review author (PL) obtained descriptive information about the service characteristics of organised inpatient (stroke unit) care and conventional care settings through a structured interview or correspondence conducted with the trial co‐ordinators (n = 19). We obtained additional information from published sources. We then allocated trials to service subgroups. We confirmed outcome data from published sources and supplemented them with unpublished information provided by the co‐ordinator of each individual trial. We asked trialists to provide information on the number of participants who were dead or dependent and the number requiring institutional care or missing at the end of scheduled follow‐up. For this updated review, two review authors (PL, SR) independently extracted information using a standard data extraction form.
We sought subgroup information on potential effect modifiers primarily for the combined outcome of death or requiring institutional care. We obtained unpublished aggregated data for a majority of trials, but insufficient quantities of individual patient data were available to allow a comprehensive individual patient data analysis.
We obtained subgroup data regarding the following participant groups (see SUTC 1997a for details).
Age: up to 75 years or 75 years or older.
Sex: men or women.
-
Stroke severity: dependency at the time of randomisation (usually within one week of the index stroke).
Mild stroke: equivalent to a Barthel Index of 10 to 20 out of 20 during the first week.
Moderate stroke: equivalent to a Barthel Index of 3 to 9 out of 20 during the first week.
Severe stroke: equivalent to a Barthel Index of 0 to 2 out of 20 during the first week.
Stroke type: ischaemic or haemorrhagic based on neuroimaging.
Assessment of risk of bias in included studies
We assessed risk of bias using Cochrane's 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors identified the method of random sequence generation, the method of concealment of treatment allocation, blinding of participants and personnel, the presence of blinding of outcome assessment, completeness of follow‐up, and evidence of selective reporting. We used these potentially important factors in sensitivity analyses, but we did not use them as exclusion criteria. The principal review author then used this information to inform the categorisations within the 'Summary of findings' tables and the GRADE allocations.
Measures of treatment effect
When our analyses of poor outcome, death, dependency, or institutionalisation at the end of scheduled follow‐up were reported, we analysed these using the odds ratio (OR) and the 95% confidence interval (CI) for an adverse outcome.
We aimed to record length of stay in hospital or in an institution as the mean and standard deviation (SD). When only medians were available, we assumed these were approximate to the mean. When no other data were provided with the mean value, we inferred the SD as being at least as large as those in comparable trials using the same measure. Because length of stay was reported in a variety of ways, we checked the results obtained with the mean difference (MD) using the standardised mean difference (SMD) and the 95% CI.
We anticipated that measures of subjective health status would be analysed as mean differences, and measures of satisfaction would be analysed as odds ratios for particular responses.
Unit of analysis issues
We anticipated that most trials would have a simple parallel‐group design in which each individual was randomised to one of two treatment groups. When a trial had three (or more) treatment groups, we planned to analyse each treatment arm as a separate study. We have not included cross‐over trials because of the likelihood or carryover effects.
Dealing with missing data
When data were missing for the outcome of death, dependency, or institutionalisation, we assumed the participant to be alive, independent, and living at home. We aimed to explore the implications of these assumptions in sensitivity analyses.
Assessment of heterogeneity
We planned to determine heterogeneity by visually inspecting the forest plot and by using the I² statistic. We defined significant heterogeneity as I² greater than 50%. When significant heterogeneity occurred, we explored potential sources using pre‐planned sensitivity analyses. Assessments of transitivity and consistency are discussed in the NMA section under Data synthesis.
Assessment of reporting biases
We employed a comprehensive search strategy in an effort to avoid reporting biases. To identify unpublished studies, we searched trial registers and contacted trialists and other experts in the field. We planned to inspect funnel plots if enough studies were available.
Data synthesis
Pairwise comparisons
When we did not have access to individual patient data, two review authors (PL, SR) extracted data from published reports. When individual patient data were available, we checked them for internal consistency and consistency with published reports. One review author entered data into the Review Manager software (RevMan 2014), and a second review author checked the entries. We analysed binary outcome data using OR and 95% CI. We analysed continuous outcome data using SMD and 95% CI. By default, we used a fixed‐effect model first, but we corroborated results by using a random‐effects model if heterogeneity was significant.
When data were available, we carried out subgroup analyses for age, sex, stroke severity, and stroke type. Through subgroup analyses, we considered the degree of interaction between subgroups (Higgins 2011).
Network meta‐analysis
In this version of the review, we include a newer approach to meta‐analysis in the form of a network meta‐analysis (NMA) of trial data. The original aim of this review was to compare the effects of organised inpatient (stroke unit) care versus conventional care. We expected that within this broad definition, the included trials would comprise a range of treatment comparisons (which could include stroke wards, mobile stroke teams, mixed rehabilitation wards) with conventional care in general wards. In addition, later trials have addressed newer questions comparing different forms of organised inpatient (stroke unit) care (e.g. stroke ward versus mobile stroke team).
We have retained the previous analysis, which was organised in a hierarchical manner (organised stroke care versus alternative service; organised stroke care versus general ward; different systems of organised care). However, we now include an NMA to explore, when possible, the impact of different systems of stroke care. We used MetaInsight software, which uses a frequentist approach and is designed specifically for this role ‐ to enable us to conduct our NMA (Owen 2019).
An NMA uses information from both direct and indirect estimates of treatment effect (Tonin 2017). Direct estimates are provided by a head‐to‐head comparison (e.g. treatment A versus treatment B). Indirect estimates are provided by two or more head‐to‐head comparisons that share a common comparator (e.g. when A versus B is the comparison of interest, then trials with A versus C and with B versus C are used). A trial network is then formed, using trials that allow, through direct and indirect comparisons, calculation of the relative effects of all treatments versus each other (or versus a single comparator). The results of such analyses are usually presented as comparisons against a common comparator group (e.g. general ward). It is also possible to present a rank analysis, which ranks treatment groups on the likelihood of being most/least effective.
A key assumption in NMA is that of transitivity (or similarity). This concerns the validity of making indirect comparisons and assumes that treatment effects are 'exchangeable' across the included trials, and that all treatments are 'jointly randomisable'. In other words, all treatment categories could feasibly be randomised in the same trial, and those that are not treatment arms in any given trial are 'missing at random' (Lu 2006). As this assumption cannot be formally tested statistically, it was judged through consideration of trial settings and characteristics, patient characteristics, and treatment mechanisms, and to investigate if any differences would be expected to modify relative treatment effects. Two review authors independently extracted the relevant information, and the principal review author made the final judgement.
A second important assumption (known as the consistency assumption) assumes that it is feasible to make indirect comparisons between two treatments, and that the indirect evidence is consistent with the direct evidence (Lu 2006). The consistency assumption can be evaluated statistically by comparing the difference between the direct estimate and the indirect estimate for each loop of evidence. Therefore, we examined for any important differences in numerical results between direct, indirect, and network results.
GRADE and 'Summary of findings'
We constructed 'Summary of findings' tables and used GRADE criteria to assess the quality of evidence. 'Summary of findings' tables included the new primary outcome (poor outcome) and the three main clinical outcomes included in previous reviews (death, death or requiring institutional care, death or dependency) plus subjective health status, patient satisfaction, and length of stay in a hospital or institution. All were recorded at the end of scheduled follow‐up.
This update included an NMA whereby different types of organised inpatient (stroke unit) care were compared with care provided in a general ward (see below). For the NMA, we used the approach of the GRADE group as outlined below (Brignardello 2018; Puhan 2014).
Present direct and indirect treatment estimates for each comparison of the evidence network.
Rate the quality of each direct and indirect effect estimate (downgrading for risk of bias, inconsistency, indirectness, imprecision, and publication bias).
Present the NMA estimate for each comparison of the evidence network.
Rate the quality of each NMA effect estimate (as above).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses involved a re‐analysis stratified by participant or service subgroup using tabular subgroup data provided by trialists or obtained from published sources. We used a fixed‐effect approach unless heterogeneity was statistically significant, and all subgroup analyses considered the degree of interaction between subgroups (Higgins 2011). We applied subgroup analyses only to the main (first) comparison to minimise the risk of false‐positive results.
Sensitivity analysis
We planned sensitivity analyses around key aspects of risk of bias that we identified during our assessment of risk of bias (i.e. concealment of treatment allocation, blinding of outcome assessment, completeness of follow‐up, and a fixed period of follow‐up). We applied sensitivity analyses only to the main (first) comparison.
Results
Description of studies
This is the fifth update of this Cochrane Review. The key references are described in the following relevant tables: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search strategy for previous versions of this review yielded 28 eligible trials (Included studies), seven awaiting classification (Studies awaiting classification), three ongoing studies (Ongoing studies), and 28 excluded studies (Excluded studies).
For this updated review, searches of Embase, CINHAL, MEDLINE, and CENTRAL revealed 16,562 records. Searches of Cochrane trials registers and other ongoing trials registers identified 432 new potentially eligible trials for consideration based on the four selection criteria (Figure 1). After exclusion of duplicate records and those that were obviously irrelevant, we were left with 32 abstracts for screening. Of these, one was a new record of a previously identified study (Goteborg‐Sahlgren 1994), one was not randomised, and 19 described interventions that did not match organised inpatient (stroke unit) care.
1.

Flow diagram illustrating the results of updated searches.
Of the remaining 13 articles retrieved, we excluded 10: seven were not randomised (Akhtar 2015; Al‐Qahtany 2014; Fu 2006; He 2014; Inoue 2013; Rai 2016; Raiborirug 2017), and three did not meet the definition of stroke unit (Felix 2016; Janssen 2014; Middleton 2018). Two are ongoing studies (China (Wang) 2015; Russia 2017), and we included one new trial (New South Wales 2014).
Therefore, this updated review incorporates 29 randomised controlled trials with 5902 participants.
Included studies
Service characteristics within organised (stroke unit) care and conventional care settings
Descriptive information was available for all trials: for eight trials, we had access to published information (Birmingham 1972; Guangdong 2008; Guangdong 2009; Huaihua 2004; Hunan 2007; Illinois 1966; New South Wales 2014; New York 1962); for two trials, we had detailed unpublished information (Beijing 2004; Joinville 2003); and for the remaining 19 trials, we carried out a structured interview with the trial co‐ordinator to determine the service characteristics.
Our original publication outlined the features of stroke unit trials (SUTC 1997a). In summary, organised inpatient (stroke unit) care was characterised by:
co‐ordinated multi‐disciplinary rehabilitation;
staff with a specialist interest in stroke or rehabilitation, or both;
routine involvement of carers in the rehabilitation process; and
regular programmes of education and training.
Several factors indicating more intensive or more comprehensive input of care were also associated with the stroke unit setting. Various service models of care exist (Table 5), but core characteristics that were invariably included in the stroke unit setting were (1) multi‐disciplinary staffing, that is, medical, nursing, and therapy staff (usually including physiotherapy, occupational therapy, speech therapy, social work); and (2) co‐ordinated multi‐disciplinary team care incorporating meetings at least once per week (SUTC 1997a). When both of the compared services could satisfy the description of stroke unit care, the local conventional system of care was taken as the control service.
1. Typical characteristics of different models of organised stroke care.
| Name | Structure | Patients | Service type | Admission | Discharge | Features |
| Stroke ward | Ward | Stroke | Various (see below) | Various (see below) | Various (see below) | Various (see below) |
| Acute stroke ward | Ward | Stroke | Acute | Acute (hours) | Days | Close physiological monitoring, often followed by care in separate rehabilitation ward if required |
| Comprehensive stroke warda | Ward | Stroke | Acute, rehabilitation | Acute (hours) | Days to weeks | Acute care and rehabilitation; conventional staffing levels |
| Rehabilitation stroke ward | Ward | Stroke | Rehabilitation | Delayed (days) | Weeks | Focus on rehabilitation |
| Mobile stroke team | Mobile team | Stroke | Mobile stroke team | Variable | Days to weeks | Peripatetic care to patients in general wards; medical and rehabilitation advice |
| Mixed rehabilitation ward | Ward | Mixed | Rehabilitation | Variable | Weeks | Mixed patient group; focus on rehabilitation |
| Intensive care | Ward | Mixed | Acute, intensive | Acute (hours) | Days | High nurse staffing; life support facilities |
aTwo trials tested a comprehensive stroke ward incorporating traditional Chinese medicine (TCM) such as acupuncture.
Service comparisons within the 29 trials with outcome data are detailed in Table 6. The total number of comparisons is greater than the number of trials because in three trials, participants could be randomised to one of two alternatives to stroke unit care; two of these trials used a stratified randomisation procedure (Nottingham 1996; Orpington 1993), and one did not (Dover 1984). In two small trials, the conventional care (general medical) group also received input from a specialist nurse (Illinois 1966; New York 1962). Although this was not strictly general medical ward care, we have included this information because relatively little novel nursing input appears to be available. Exclusion of these trials would not substantially alter the conclusions of the systematic review. For one trial, some participants appear to have been treated outside the rehabilitation wards (i.e. by peripatetic team care), but the number is unclear (New York 1962). This trial is currently classified as a mixed rehabilitation ward.
2. Service comparisons in standard analyses.
| Trials | Participants | Index (stroke unit) care | Conventional care | Reference |
| 15 | 3521 | Stroke ward | General medical ward | Athens 1995, Beijing 2004, Dover 1984 (GMW), Edinburgh 1980, Goteborg‐Ostra 1988, Goteborg‐Sahlgren 1994, Guangdong 2009, Huaihua 2004, Joinville 2003, Nottingham 1996 (GMW), Orpington 1993 (GMW), Orpington 1995, Perth 1997, Svendborg 1995, Trondheim 1991 |
| 6 | 630 | Mixed rehabilitation ward | General medical ward | Birmingham 1972, Helsinki 1995, Illinois 1966, Kuopio 1985, New York 1962; Newcastle 1993 |
| 2 | 438 | Mobile stroke team (peripatetic care) | General medical ward | Manchester 2003, Montreal 1985 |
| 4 | 542 | Stroke ward | Mixed rehabilitation ward | Dover 1984 (MRW), Nottingham 1996 (MRW), Orpington 1993 (MRW), Tampere 1993 |
| 1 | 304 | Stroke ward (comprehensive) | Mobile stroke team | Orpington 2000 |
| 1 | 54 | Stroke ward (acute) | Stroke ward (comprehensive unit) | Groningen 2003 |
| 1 | 47 | Stroke ward (comprehensive) | Stroke wards (acute+rehabilitation) | New South Wales 2014 |
| 2 | 366 | Stroke ward (plus TCM) | Stroke ward (without TCM) | Guangdong 2008, Hunan 2007 |
TCM: traditional Chinese medicine.
Four trials compared a model of stroke unit care using integrated traditional Chinese medicine (TCM) (e.g. acupuncture, herbal remedies) versus standard 'Western medicine' stroke unit care (Guangdong 2008; Hunan 2007), or a general medical ward (Guangdong 2009). One trial compared a comprehensive stroke ward within a neurology unit with a general medical ward (Huaihua 2004). The duration of rehabilitation provided in all four trials was unclear, and only two trials reported the timing of randomisation (Guangdong 2009; Huaihua 2004).
Of the 29 included trials, 23 incorporated rehabilitation lasting several weeks if required: 17 of these units admitted participants acutely, and eight after a delay of one or two weeks. Two trials compared an acute stroke unit with early transfer to conventional rehabilitation if required (Groningen 2003; New South Wales 2014). One trial proved difficult to categorise as it contained elements of an acute unit but offered some rehabilitation (Athens 1995). It is classified here as a comprehensive stroke unit trial. The duration of rehabilitation was unclear for two Chinese trials (Guangdong 2008; Hunan 2007). No trials evaluated an 'intensive care' model of a stroke unit.
The classification of trials is outlined in Table 5, and the numbers in each comparison are shown in Table 6.
Excluded studies
See Characteristics of excluded studies.
Of the 38 excluded studies, 21 were not strictly randomised, six were evaluations of care pathways, four had no available outcome data, four evaluated an intervention that did not fit our description of organised inpatient (stroke unit) care, two treated intervention and control participants within the same unit, and one reported retrospective data from a previous study.
Risk of bias in included studies
See the 'Risk of bias' graph (Figure 2), the 'Risk of bias' summary (Figure 3), and the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sixteen trials used a secure and clearly concealed randomisation procedure (both random sequence generation and allocation concealment), and we judged these to be at low risk of bias (Athens 1995; Dover 1984; Edinburgh 1980; Goteborg‐Ostra 1988; Goteborg‐Sahlgren 1994; Groningen 2003; Helsinki 1995; Kuopio 1985; Manchester 2003; Montreal 1985; New South Wales 2014; Orpington 1993; Orpington 2000; Svendborg 1995; Tampere 1993; Trondheim 1991). The remaining trials were at unclear risk of bias.
Blinding
It is very challenging to blind participants or treating staff to treatment allocation, and only one trial reported any attempts to do so (New South Wales 2014). The remaining trials had unclear risk of bias. However, it is worth noting that most studies completed long‐term follow‐up at a time when participants and families often could not recall any details of their acute treatment.
Twelve trials used an unequivocally blinded final assessment for all participants (Goteborg‐Sahlgren 1994; Groningen 2003; Helsinki 1995; Hunan 2007; Joinville 2003; Kuopio 1985; Manchester 2003; Montreal 1985; New South Wales 2014; Nottingham 1996; Orpington 2000; Perth 1997). Eight trials had unclear risk of bias (Beijing 2004; Goteborg‐Ostra 1988; Guangdong 2008; Guangdong 2009; Huaihua 2004; Orpington 1995; Svendborg 1995; Trondheim 1991), and we judged eight trials to be at high risk of bias (Athens 1995; Birmingham 1972; Dover 1984; Edinburgh 1980; Illinois 1966; Newcastle 1993; Orpington 1993; Tampere 1993).
Incomplete outcome data
We judged 19 trials to be at low risk of bias (Athens 1995; Beijing 2004; Dover 1984; Goteborg‐Sahlgren 1994; Groningen 2003; Guangdong 2009; Helsinki 1995; Illinois 1966; Joinville 2003; Kuopio 1985; Manchester 2003; Montreal 1985; New South Wales 2014; Newcastle 1993; Orpington 1995; Perth 1997; Svendborg 1995; Tampere 1993; Trondheim 1991). The remaining nine trials were at unclear risk (Birmingham 1972; Edinburgh 1980; Goteborg‐Ostra 1988; Guangdong 2008; Huaihua 2004; Hunan 2007; Nottingham 1996; Orpington 1993; Orpington 2000).
Ten trials had minor omissions of death and place of residence data (26 stroke unit participants and 35 controls in total) (Birmingham 1972; Dover 1984; Edinburgh 1980; Manchester 2003; Montreal 1985; New South Wales 2014; Nottingham 1996; Orpington 1993; Orpington 2000; Tampere 1993). For the purpose of our analysis, we assumed these participants were alive and living at home, which may have introduced a minor bias in favour of the control group.
Selective reporting
We judged selective reporting bias to be low risk in 15 trials, largely because we obtained unpublished data from the trialists (Athens 1995;Beijing 2004;Birmingham 1972;Edinburgh 1980;Goteborg‐Sahlgren 1994;Groningen 2003;Joinville 2003;Manchester 2003;Nottingham 1996;Orpington 1993;Orpington 1995;Orpington 2000;Perth 1997;Tampere 1993;Trondheim 1991). We classified the remaining 13 trials as having unclear risk of reporting bias (Dover 1984; Goteborg‐Ostra 1988; Guangdong 2008; Guangdong 2009; Helsinki 1995; Huaihua 2004; Hunan 2007; Illinois 1966; Kuopio 1985; Montreal 1985; New South Wales 2014; Newcastle 1993; Svendborg 1995).
Other potential sources of bias
Most of the Stroke Unit Trialists Collaboration members carried out trials that are included in the review. However, trialists were not involved in selection or assessment of their own trials.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Results of the systematic review are presented in five sections as pairwise comparisons followed by NMA.
Pairwise comparisons
These comparisons are listed in five sections as follows.
-
Section 1. Organised inpatient (stroke unit) care versus alternative care (Comparison 1).
First, we have outlined the main outcomes for the comparison of organised inpatient (stroke unit) care with an alternative service. Therefore, this section examines the impact of all types of organised inpatient (stroke unit) care on patient outcomes. For trials where both services compared could satisfy the definition of stroke unit care (Table 5), we have presented the system of care that we considered to be conventional care in the trial as the control service.
This section includes analyses of different subgroups of participants and sensitivity analyses by trial quality.
We have then described the results for the most common comparisons of different forms of organised stroke unit care versus a general medical ward: stroke ward, mobile stroke team, and mixed rehabilitation ward.
Section 2. Stroke ward versus general medical ward (Comparison 2).
Section 3. Mobile stroke team versus general medical ward (Comparison 3).
Section 4. Mixed rehabilitation ward versus general medical ward (Comparison 4).
Finally, we have presented the results for any direct comparisons of organised care in a stroke ward versus a different form of organised stroke unit care.
Section 5. Different systems of organised care: stroke ward versus alternative organised care (Comparison 5).
Section 1. Organised inpatient (stroke unit) care versus alternative service (Comparison 1)
Outcome 1.1. Poor outcome by the end of scheduled follow‐up
Outcome data were available for 26 trials (5336 participants) (Analysis 1.1). The summary result indicated a significant reduction in the odds of a poor outcome (odds ratio (OR) 0.77, 95% confidence interval (CI) 0.69 to 0.87; moderate‐quality evidence) recorded at the end of scheduled follow‐up (median follow‐up 12 months; range 6 weeks to 12 months) with no significant heterogeneity.The main methodological difficulties when dependency was used as an outcome were the degree of blinding at final assessment and the potential for bias if the assessor was aware of the treatment allocation. The results were unchanged when restricted to those trials in which an unequivocally blinded final assessment for all participants was undertaken (OR 0.75, 95% CI 0.62 to 0.91) (Goteborg‐Sahlgren 1994; Groningen 2003; Helsinki 1995; Joinville 2003; Kuopio 1985; Manchester 2003; Montreal 1985; New South Wales 2014; Nottingham 1996; Orpington 2000).
Outcome 1.2. Death by the end of scheduled follow‐up
Outcome data were available for all 29 trials (5902 participants) in which an organised inpatient (stroke unit) intervention was compared with an alternative service (Analysis 1.2). Case fatality recorded at the end of scheduled follow‐up (median follow‐up 12 months; range 6 weeks to 12 months) was lower in the organised (stroke unit) care group in 22 of 29 trials. The overall summary estimate included an OR of 0.76 (95% CI 0.66 to 0.88; moderate‐quality evidence). A borderline significant subgroup interaction was reported (P = 0.04), with more positive effects seen in subgroups based on trials of stroke wards.
Outcome 1.3. Death or institutional care by the end of scheduled follow‐up
Outcome data were available for 24 trials (4887 participants) (Analysis 1.3). The median duration of follow‐up was 12 months (range 6 weeks to 12 months). The summary result indicated a significant reduction in the odds of a patient dying or requiring long‐term institutional care (OR 0.76, 95% CI 0.67 to 0.85; moderate‐quality evidence). A subgroup interaction was noted (P = 0.01), with more positive effects usually seen in subgroups based on trials of stroke wards. When we excluded trials that had a very short or variable period of follow‐up, we found that the overall estimate of apparent benefit was unaffected (OR 0.75, 95% CI 0.65 to 0.86) (Beijing 2004; Goteborg‐Ostra 1988; Groningen 2003; Illinois 1966; Montreal 1985; New York 1962; Orpington 1993; Orpington 1995).
Outcome 1.4. Death or dependency by the end of scheduled follow‐up
Outcome data were available for 24 trials (4854 participants) (Analysis 1.4). The summary result indicated a significant reduction in the odds of the combined adverse outcomes of death or dependency (OR 0.75, 95% CI 0.66 to 0.85; moderate‐quality evidence) with no significant heterogeneity. The main methodological difficulties when dependency was used as an outcome were the degree of blinding at final assessment and the potential for bias if the assessor was aware of the treatment allocation. The results were unchanged (OR 0.75, 95% CI 0.62 to 0.91) when restricted to those trials in which an unequivocally blinded final assessment for all participants was undertaken (Goteborg‐Sahlgren 1994; Groningen 2003; Helsinki 1995; Joinville 2003; Kuopio 1985; Manchester 2003; Montreal 1985; New South Wales 2014; Nottingham 1996; Orpington 2000).
Outcomes 1.5 and 1.6. Length of stay (days) in a hospital or institution or both
Length of stay data were available for 19 individual trials (4162 participants) (Analysis 1.5; Analysis 1.6). Mean (or median) length of stay ranged from 11 to 162 days in stroke unit groups and from 12 to 129 days in control groups. Thirteen trials reported a shorter length of stay in the organised inpatient (stroke unit) group, and six a more prolonged stay. The calculation of a summary result for length of stay was subject to major methodological limitations: length of stay was calculated in different ways (e.g. acute hospital stay, total stay in hospital or institution), two trials recorded median rather than mean length of stay, and in two trials the SD had to be inferred from the P value or from the results of similar trials. Overall, use of a random‐effects model revealed no significant reduction in length of stay in the stroke unit group. The summary estimate was complicated by considerable heterogeneity that limits the extent to which more general conclusions can be inferred.
1.6. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 6: Length of stay (days) in a hospital or hospital plus institution
We re‐analysed results according to whether length of stay was defined as stay in acute hospital only or total length of stay in a hospital or institution in the first year after stroke (Analysis 1.6). We found no significant difference between the two groups and no reduction in heterogeneity.
Participant satisfaction and subjective health status
Only three trials recorded outcome measures related to participant subjective health status (Nottingham Health Profile; EuroQol Quality of Life Scale) (Manchester 2003; Nottingham 1996; Trondheim 1991). In Nottingham 1996 and Trondheim 1991, there was a pattern of improved results among stroke unit survivors with results attaining statistical significance in the two trials. However, for Manchester 2003, there was no statistically significant difference between study groups. We could find no systematically gathered information on participant preferences.
Outcomes 1.7 to 1.10. Poor outcome, death, death or institutional care, and death or dependency at five‐year follow‐up
Three trials (1139 participants) carried out supplementary studies extending participant follow‐up to five years post stroke for the outcome of death (Athens 1995; Nottingham 1996; Trondheim 1991), and two trials (535 participants) carried out supplementary studies extending participant follow‐up to five years post stroke for the outcomes of death or institutionalisation and death or dependency (Nottingham 1996; Trondheim 1991). The OR for adverse outcomes continued to favour stroke unit care but with some heterogeneity: poor outcome 0.54 (95% CI 0.22 to 1.34), death 0.74 (95% CI 0.59 to 0.94), death or institutional care 0.59 (95% CI 0.33 to 1.05), and death or dependency 0.54 (95% CI 0.22 to 1.34).
Outcomes 1.11 to 1.14. Poor outcome, death, death or institutional care, and death or dependency at 10‐year follow‐up
Three trials (1139 participants) extended follow‐up to 10 years post stroke for the outcome of death (Athens 1995; Nottingham 1996; Trondheim 1991), and two trials (535 participants) extended follow‐up to 10 years post stroke for the outcomes of death or institutionalisation and death or dependency (Nottingham 1996; Trondheim 1991). Again, the summary results continued to favour stroke unit care but with increased heterogeneity and loss of statistical significance: poor outcome OR 0.70 (95% CI 0.27 to 1.80), death OR 0.66 (95% CI 0.43 to 1.03), death or institutional care OR 0.57 (95% CI 0.37 to 0.88), and death or dependency OR 0.70 (95% CI 0.27 to 1.80).
Sensitivity analyses by trial characteristics
Sensitivity analyses were applied only to the main (first) comparison to test confidence in the main hypothesis. In view of the variety of trial methods described, we carried out a sensitivity analysis based only on those trials with low risk of bias based on (1) secure concealment of allocation procedures, (2) unequivocally blinded outcome assessment, and (3) a fixed period of near complete follow‐up.
Secure concealment of allocation procedures: restricting analyses to the 16 trials with clearly reported random sequence generation and concealment of allocation did not substantially alter the odds of a poor outcome (0.79, 95% CI 0.69 to 0.91) (Figure 2).
Unequivocally blinded outcome assessment: restricting analyses to the 12 trials with clearly blinded outcome assessment did not substantially alter the odds of a poor outcome (OR 0.73, 95% CI 0.62 to 0.89) (Figure 2).
A fixed period of near complete follow‐up: restricting analyses to the 15 trials that clearly reported a fixed period of follow‐up (with > 90% completeness of follow‐up) did not substantially alter the odds of a poor outcome (OR 0.79, 95% CI 0.64 to 0.91) (Included studies).
Eight trials met all of these quality criteria (Goteborg‐Sahlgren 1994; Groningen 2003; Helsinki 1995; Kuopio 1985; Manchester 2003; New South Wales 2014; Nottingham 1996; Orpington 2000). Within this group of trials, stroke unit care was associated with similar reductions in the odds of a poor outcome (OR 0.75, 95% CI 0.62 to 0.91).
Subgroup analyses by patient characteristics
Pre‐defined subgroup analyses were based on previous versions of this review, and each subgroup analysis included data from at least nine trials (at least 1111 participants) (SUTC 1997a). These were based on participants' age, sex, and initial stroke severity. For this updated version of the review, we have incorporated additional data based on pathological stroke type (ischaemic or haemorrhagic stroke). See Figure 4.
4.
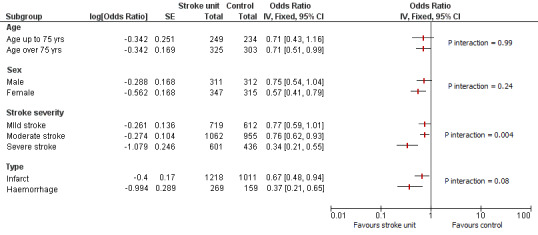
Subgroup analysis by patient characteristics: poor outcome at the end of scheduled follow‐up. Analyses used the generic inverse variance approach. P values relate to the subgroup interaction.
Caution is needed when interpreting these subgroup analyses, particularly as a relatively small number of outcome events were observed, which limits statistical power. Therefore, subgroup analyses were applied only to the main (first) comparison. Furthermore, the results may change depending on the outcome chosen. These results indicate that in general, the magnitude of benefit seemed greater for participants with more severe stroke (the only significant subgroup interactions were for stroke severity: P = 0.004). However, stroke unit benefits are apparent across a range of participant subgroups (i.e. age, sex, initial stroke severity, and stroke type).
Section 2. Stroke ward versus general medical ward (Comparison 2)
Analyses comparing a stroke ward with a general medical ward comprised two subgroups of stroke ward: comprehensive and rehabilitation (see Table 5).
Outcome 2.1. Poor outcome by the end of scheduled follow‐up
Fourteen trials (3321 participants) compared care in a stroke ward with care in a general ward (Analysis 2.1). Stroke ward care showed a reduction in the odds of a poor outcome by the end of scheduled follow‐up (OR 0.78, 95% CI 0.68 to 0.91; moderate‐quality evidence) with no subgroup interaction between the different types of stroke ward. Some minor heterogeneity (59%) was noted. Re‐analysis with a random‐effects model did not alter the conclusions.
Outcome 2.2. Death by the end of scheduled follow‐up
Fifteen trials (3523 participants) compared care in a stroke ward with care in a general ward (Analysis 2.2). Stroke ward care showed a reduction in the odds of death by the end of scheduled follow‐up (OR 0.75, 95% CI 0.63 to 0.90; moderate‐quality evidence) with no subgroup interaction between the different types of stroke ward and no significant heterogeneity.
Outcome 2.3. Death or institutional care by the end of scheduled follow‐up
Thirteen trials (2924 participants) compared care in a stroke ward with care in a general ward (Analysis 2.3). Stroke ward care showed a reduction in the odds of death or institutional care by the end of scheduled follow‐up (OR 0.74, 95% CI 0.63 to 0.87; moderate‐quality evidence) with no subgroup interaction between the different types of stroke ward and no significant heterogeneity.
Outcome 2.4. Death or dependency by the end of scheduled follow‐up
Twelve trials (2839 participants) compared care in a stroke ward with care in a general ward (Analysis 2.4). Stroke ward care showed a reduction in the odds of death or dependency by the end of scheduled follow‐up (OR 0.75, 95% CI 0.64 to 0.88; moderate‐quality evidence) with no subgroup interaction between the different types of stroke ward. Some heterogeneity (63%) was noted. Re‐analysis with a random‐effects model did not alter the conclusions.
Outcome 2.5. Length of stay (days) in a hospital or institution
Ten trials (2547 participants) compared care in a stroke ward with care in a general ward (Analysis 2.4). Overall, stroke ward care showed no reduction in length of stay (weighted mean difference (WMD) ‐2.19, 95% CI ‐5.19 to 0.82; low‐quality evidence). We found substantial heterogeneity (78%) and a significant subgroup interaction between the different types of stroke ward, which limits confidence in the results.
Participant satisfaction and subjective health status
Only two trials recorded outcome measures related to participant subjective health status (Nottingham Health Profile; EuroQol Quality of Life Scale) (Nottingham 1996; Trondheim 1991). We found a pattern of improved results among stroke unit survivors with results attaining statistical significance in the two trials. We could find no systematically gathered information on participant preferences.
Section 3. Mobile stroke team versus general medical ward (Comparison 3)
Outcome 3.1. Poor outcome by the end of scheduled follow‐up
Two trials (438 participants) compared care from a mobile stroke team with care in a general ward (Analysis 3.1). Stroke team care did not show a reduction in the odds of a poor outcome at the end of scheduled follow‐up (OR 0.80, 95% CI 0.52 to 1.22; low‐quality evidence) with no significant heterogeneity. Please note that as data were complete for dependency, this result is the same (as shown in Analysis 3.4).
Outcome 3.2. Death by the end of scheduled follow‐up
Two trials (438 participants) compared care from a mobile stroke team with care in a general ward (Analysis 3.2). Stroke team care did not show a reduction in the odds of death by the end of scheduled follow‐up (OR 1.08, 95% CI 0.71 to 1.63; low‐quality evidence) with no significant heterogeneity.
Outcome 3.3. Death or institutional care by the end of scheduled follow‐up
Two trials (438 participants) compared care from a mobile stroke team with care in a general ward (Analysis 3.3). Stroke team care did not show a reduction in the odds of death or institutional care by the end of scheduled follow‐up (OR 1.27, 95% CI 0.84 to 1.93; low‐quality evidence) with no significant heterogeneity.
Outcome 3.4. Death or dependency by the end of scheduled follow‐up
Two trials (438 participants) compared care from a mobile stroke team with care in a general ward (Analysis 3.4). Stroke team care did not show a reduction in the odds of a poor outcome at the end of scheduled follow‐up (OR 0.80, 95% CI 0.52 to 1.22; low‐quality evidence) with no significant heterogeneity.
Outcome 3.5. Length of stay (days) in a hospital or institution
No data were available for this outcome.
Participant satisfaction and subjective health status
Only Manchester 2003 recorded outcome measures related to participant subjective health status (EuroQol Quality of Life Scale). We could find no systematically gathered information on participant preferences.
Section 4. Mixed rehabilitation ward versus general medical ward (Comparison 4)
Outcome 4.1. Poor outcome by the end of scheduled follow‐up
Six trials (630 participants) compared care in a mixed rehabilitation ward with care in a general ward (Analysis 4.1). Mixed rehabilitation ward care showed a reduction in the odds of a poor outcome at the end of scheduled follow‐up (OR 0.65, 95% CI 0.47 to 0.90; moderate‐quality evidence) with no significant heterogeneity. Please note that as data were complete for dependency, this result is the same (as shown in Analysis 4.4).
Outcome 4.2. Death by the end of scheduled follow‐up
Six trials (630 participants) compared care in a mixed rehabilitation ward with care in a general ward (Analysis 4.2). Mixed rehabilitation ward care showed a reduction in the odds of death by the end of scheduled follow‐up (OR 0.91, 95% CI 0.58 to 1.42; low‐quality evidence) with no significant heterogeneity.
Outcome 4.3. Death or institutional care by the end of scheduled follow‐up
Six trials (630 participants) compared care in a mixed rehabilitation ward with care in a general ward (Analysis 4.3). Mixed rehabilitation ward care showed a reduction in the odds of death or institutional care by the end of scheduled follow‐up (OR 0.71, 95% CI 0.51 to 0.99; low‐quality evidence) with no significant heterogeneity.
Outcome 4.4. Death or dependency by the end of scheduled follow‐up
Six trials (630 participants) compared care in a mixed rehabilitation ward with care in a general ward (Analysis 4.4). Mixed rehabilitation ward care showed a reduction in the odds of death or dependency by the end of scheduled follow‐up (OR 0.65, 95% CI 0.47 to 0.90; moderate‐quality evidence) with no significant heterogeneity.
Outcome 4.5. Length of stay (days) in a hospital or institution
Three trials (387 participants) compared care in a stroke ward with care in a general ward (Analysis 4.5). Overall, stroke ward care showed no reduction in length of stay (WMD 3.85, 95% CI ‐13.49 to 21.18; very low‐quality evidence) with no significant heterogeneity.
Participant satisfaction and subjective health status
No data were available for these outcomes.
Section 5. Different systems of organised care: stroke ward versus alternative organised care (Comparison 5)
The analyses in Section 2 above indicate that organised inpatient (stroke unit) care in a stroke ward is an effective model of care. Several recent trials have compared different ways of providing care in a stroke ward. We therefore analysed those trials that directly compared a stroke ward with another form of organised inpatient (stroke unit) care that met the basic inclusion criteria (Table 5).
Of the nine trials identified for which outcome data were available, one compared an acute stroke ward with a mixed rehabilitation ward (Tampere 1993). Two small trials compared care in an acute stroke ward with care in a comprehensive stroke ward (Groningen 2003; New South Wales 2014). The Dutch trial compared care in an acute unit where there was an emphasis on close management of physiological variables (Groningen 2003). The Australian trial compared two systems of care: acute stroke ward (with transfer to a rehabilitation ward if required) with a stroke ward that combined acute care and rehabilitation (comprehensive stroke ward) (New South Wales 2014). One trial compared a stroke ward that combined acute care and rehabilitation (comprehensive stroke ward) with a general medical ward where care was co‐ordinated by a multi‐disciplinary team (mobile team care) (Orpington 2000). Two compared a stroke ward with integrated traditional Chinese medicine (TCM) with a 'Western medicine' stroke ward (Guangdong 2008; Hunan 2007). Three trials incorporated designs in which participants could be randomised to a stroke rehabilitation ward or to conventional care in a general medical ward or a mixed rehabilitation ward within a Department of Geriatric Medicine (Dover 1984; Nottingham 1996; Orpington 1993). Data were available for both of these participant subgroups.
Acute stroke ward versus mixed rehabilitation ward
Outcomes 5.1, 5.2, 5.3, 5.4, and 5.5. Poor outcome, death, death or institutional care, death or dependency by the end of scheduled follow‐up, and length of stay in hospital or institution
The trial comparing an acute unit with a mixed rehabilitation unit did not show any statistically significant difference in the odds of a poor outcome nor in death, death or requiring institutional care, death or dependency, or length of stay data (Tampere 1993).
Acute stroke ward versus comprehensive stroke ward
Outcomes 5.1, 5.2, 5.3, 5.4 and 5.5. Poor outcome, death, death or institutional care, death or dependency by the end of scheduled follow‐up, and length of stay in hospital or institution
We found no consistent evidence that an acute stroke ward was superior to a comprehensive stroke ward. The Dutch trial suggested improved outcomes within the acute ward where there was an emphasis on close management of physiological variables (Groningen 2003). However, this pilot study was underpowered to demonstrate major differences. The recent Australian trial compared a conventional model of care (acute stroke ward with transfer to rehabilitation if required) with all care provided in one (comprehensive) ward (New South Wales 2014). Study authors suggested that care in the comprehensive ward may be more efficient (with more rapid recovery), but the key outcomes reported here were not statistically different.
Comprehensive stroke ward versus mobile stroke team
Outcomes 5.1, 5.2, 5.3, 5.4, and 5.5. Poor outcome, death, death or institutional care, death or dependency by the end of scheduled follow‐up, and length of stay in hospital or institution
One trial compared a comprehensive stroke ward (providing acute care and rehabilitation) with admission to a general ward where care was provided by a mobile stroke team (Orpington 2000). Study authors found statistically significant (P < 0.001) reductions in death and the combined outcome of death or institutional care among the comprehensive stroke ward group. Fewer comprehensive stroke ward participants had a poor outcome (were dead or dependent) at the end of follow‐up, but this result did not achieve statistical significance. However, Orpington 2000 is the only trial in this analysis comparing comprehensive stroke wards with an alternative service, so these results require confirmation. Results show no significant difference in length of stay.
Rehabilitation stroke ward versus alternative service
Outcomes 5.1, 5.2, 5.3, 5.4 and 5.5. Poor outcome, death, death or institutional care, death or dependency by the end of scheduled follow‐up, and length of stay in hospital or institution
We noted a pattern of improved outcomes in the stroke rehabilitation ward with fewer deaths (OR 0.50, 95% CI 0.28 to 0.90) but no statistically significant reduction in the composite end points of poor outcome, death or requiring institutional care, and death or dependency. However, the numbers were small and no definitive conclusions could be drawn. Interpretation of length of stay data was complicated by substantial heterogeneity. There was no evidence of a systematic increase in length of stay.
Stroke ward plus TCM versus alternative service
Outcome 5.2. Death at the end of scheduled follow‐up
There was no significant difference in the odds of death in a stroke ward with integrated TCM when compared with a standard 'Western medicine' stroke ward (Guangdong 2008; Hunan 2007). The type of care provided in a stroke unit with integrated TCM has not been well described. The overall estimate is based on the results of a single trial, and no definitive conclusions can be drawn.
Analyses by service characteristics (including network meta‐analysis (NMA))
In planning our analyses, we specified in advance that an important question for service planning would be whether the benefits of organised (stroke unit) care depended upon the establishment of a ward dedicated only to stroke care (stroke ward) or could be achieved through a mobile stroke team or a generic disability service (mixed rehabilitation unit) that specialises in the management of disabling illness including stroke. We explored these questions in Sections 2, 3, and 4 above, but we also performed an NMA to explore the impact of different systems of stroke care. We used MetaInsight software, designed specifically for this role, to conduct our NMA (https://crsu.shinyapps.io/metainsightb/).
Table 5 shows the categories of organised inpatient (stroke unit) care grouped according to our service classification. In view of the difficulty of defining precisely different forms of stroke wards and the small number of comparisons of different forms of stroke wards (Table 6), we have analysed the 'stroke ward' group as a single entity. Further comparisons within this group are shown in Analysis 5.1, Analysis 5.2, Analysis 5.3, Analysis 5.4, and Analysis 5.5.
5.1. Analysis.

Comparison 5: Different systems of organised care: stroke ward versus alternative organised care, Outcome 1: Poor outcome by the end of scheduled follow‐up
5.2. Analysis.

Comparison 5: Different systems of organised care: stroke ward versus alternative organised care, Outcome 2: Death by the end of scheduled follow‐up
5.3. Analysis.

Comparison 5: Different systems of organised care: stroke ward versus alternative organised care, Outcome 3: Death or institutional care by the end of scheduled follow‐up
5.4. Analysis.

Comparison 5: Different systems of organised care: stroke ward versus alternative organised care, Outcome 4: Death or dependency by the end of scheduled follow‐up
5.5. Analysis.

Comparison 5: Different systems of organised care: stroke ward versus alternative organised care, Outcome 5: Length of stay (days) in a hospital or institution
Table 7 and Figure 5 show trial comparisons within the NMA. We believe that the transitivity (or similarity) assumption was met, as all included trials recruited people with acute or subacute stroke for treatment within a hospital system of care (Characteristics of included studies). Subgroup analyses by patient characteristics indicate that there is no substantial treatment modification by patient age, sex, or type, but possibly by stroke severity (Figure 4). Although a range of stroke severities was evident in the recruited participants, they were usually well distributed within trials.
3. Service comparisons in network meta‐analyses (NMAs)a.
| Stroke ward | |||
| 15 trials (3521 participants) |
General medical ward | ||
| 4 trials (542 participants) |
6 trials (630 participants) | Mixed rehabilitation ward | |
| 1 trial (304 participants) |
2 trials (438 participants) | ‐‐ | Mobile stroke team |
aThe table shows the numbers of trials and participants for each service comparison (e.g. two trials (438 participants) featured a comparison of a mobile stroke team with a general medical ward).
5.

Network meta‐analysis plot for different types of organised care. The nodes show the service groups (GMW: general medical ward; MRW: mixed rehabilitation ward; MST: mobile stroke team; SW: stroke ward), with care in a GMW as the reference.
We evaluated the consistency assumption statistically by comparing the difference between direct and indirect estimates for each loop of evidence. We examined for any inconsistency (i.e. important differences in numerical results between direct, indirect, and network results), and we presented OR estimates for each of the three comparisons. We showed the inconsistency tables from the NMA for analyses of poor outcome (Table 8), death (Table 9), death or institutional care (Table 10), and death or dependency (Table 11). These tables show the results of direct and indirect comparisons plus the NMA results. We found no statistically significant differences (P > 0.05) between any of the direct and indirect comparisons, but confidence intervals were wide.
4. Inconsistency table for the network meta‐analysis: poor outcome at the end of scheduled follow‐upa.
| Comparison | Number of studies | Log direct comparison OR | Log indirect comparison OR | Log difference (95% CI) between direct and indirect comparisons | P value of difference between direct and indirect comparisons |
| Mixed rehabilitation ward vs GMW | 6 | ‐0.413 | ‐0.266 | ‐0.147 (‐0.776 to 0.483) |
0.65 |
| Mobile stroke team vs GMW | 2 | ‐0.238 | 0.032 | ‐0.270 (‐1.111 to 0.571) |
0.53 |
| Stroke ward vs GMW | 13 | ‐0.273 | ‐0.478 | 0.205 (‐0.317 to 0.728) |
0.44 |
| Mixed rehabilitation ward vs mobile stroke team | 0 | N/A | ‐0.237 | N/A | N/A |
| Mixed rehabilitation ward vs stroke ward | 4 | 0.022 | ‐0.124 | 0.146 (‐0.483 to 0.776) |
0.65 |
| Mobile stroke team vs stroke ward | 1 | 0.321 | 0.051 | 0.270 (‐0.571 to 1.111) |
0.53 |
aThe table shows the following for each service comparison: number of trials, log direct and indirect comparisons, difference between the two estimates, and P value of that difference. There was no significant inconsistency between direct and indirect estimates.
CI: confidence interval. GMW: general medical ward. N/A: not applicable. OR: odds ratio.
5. Inconsistency table for the network meta‐analysis: death at the end of scheduled follow‐upa.
| Comparison | Number of studies | Log Direct comparison OR | Log Indirect comparison OR | Log difference (95% CI) between direct and indirect comparisons | P value of difference between direct and indirect comparisons |
| Mixed rehabilitation ward vs GMW | 6 | ‐0.101 | 0.617 | ‐0.719 (‐1.741 to 0.303) |
0.17 |
| Mobile stroke team vs GMW | 2 | 0.003 | 0.678 | ‐0.675 (‐1.997 to 0.646) |
0.32 |
| Stroke ward vs GMW | 15 | ‐0.381 | ‐1.132 | 0.750 (‐0.084 to 1.586) |
0.08 |
| Mixed rehabilitation ward vs mobile stroke team | 0 | N/A | ‐0.023 | N/A | N/A |
| Mixed rehabilitation ward vs stroke ward | 4 | 1.037 | 0.318 | 0.719 (‐0.303 to 1.741) |
0.17 |
| Mobile stroke team vs stroke ward | 1 | 1.125 | 0.449 | 0.675 (‐0.646 to 1.997) |
0.32 |
aThe table shows the following for each service comparison: number of trials, log direct and indirect comparisons, difference between the two estimates, and P value of that difference. There was no significant inconsistency between direct and indirect estimates.
CI: confidence interval. GMW: general medical ward. N/A: not applicable. OR: odds ratio.
6. Inconsistency table for the network meta‐analysis: death or institutional care at the end of scheduled follow‐upa.
| Comparison | Number of studies | Log Direct comparison OR | Log Indirect comparison OR | Log difference (95% CI) between direct and indirect comparisons | P value of difference between direct and indirect comparisons |
| Mixed rehabilitation ward vs GMW | 5 | ‐0.347 | ‐0.215 | ‐0.132 (‐0.640 to 0.375) |
0.61 |
| Mobile stroke team vs GMW | 2 | 0.242 | 0.649 | ‐0.406 (‐1.136 to 0.322) |
0.27 |
| Stroke ward vs GMW | 13 | ‐0.299 | ‐0.537 | 0.237 (‐0.193 to 0.668) |
0.28 |
| Mixed rehabilitation ward vs mobile stroke team | 0 | NA | ‐0.665 | N/A | N/A |
| Mixed rehabilitation ward vs stroke ward | 4 | 0.105 | ‐0.027 | 0.132 (‐0.375 to 0.640) |
0.61 |
| Mobile stroke team vs stroke ward | 1 | 0.964 | 0.557 | 0.406 (‐0.322 to 1.136) |
0.27 |
aThe table shows the following for each service comparison: number of trials, log direct and indirect comparisons, difference between the two estimates, and P value of that difference. There was no significant inconsistency between direct and indirect estimates.
CI: confidence interval. GMW: general medical ward. N/A: not applicable. OR: odds ratio.
7. Inconsistency table for the network meta‐analysis: death or dependency at the end of scheduled follow‐upa.
| Comparison | Number of studies | Log Direct comparison OR | Log Indirect comparison OR | Log difference (95% CI) between direct and indirect comparisons | P value of difference between direct and indirect comparisons |
| Mixed rehabilitation ward vs GMW | 6 | ‐0.413 | ‐0.317 | 0.096 (‐0.731 to 0.540) |
0.77 |
| Mobile stroke team vs GMW | 2 | ‐0.238 | ‐0.016 | ‐0.220 (‐1.070 to 0.620) |
0.61 |
| Stroke ward vs GMW | 12 | ‐0.325 | ‐0.473 | 0.150 (‐0.380 to 0.680) |
0.57 |
| Mixed rehabilitation ward vs mobile stroke team | 0 | NA | ‐0.234 | N/A | N/A |
| Mixed rehabilitation ward vs stroke ward | 4 | 0.022 | ‐0.073 | 0.100 (‐0.540 to 0.735) |
0.77 |
| Mobile stroke team vs stroke ward | 1 | 0.321 | 0.099 | 0.220 (‐0.620 to 1.070) |
0.61 |
aThe table shows the following for each service comparison: number of trials, log direct and indirect comparisons, difference between the two estimates, and P value of that difference. There was no significant inconsistency between direct and indirect estimates.
CI: confidence interval. GMW: general medical ward. N/A: not applicable. OR: odds ratio.
The NMA used the general medical ward group as the comparator, as this was clinically relevant and was the most common comparator reported by trialists (Table 6). Figure 6 shows the NMA result for a poor outcome at the end of scheduled follow‐up. The lowest odds of a poor outcome was seen with care in a stroke ward. A rank analysis, which orders treatments according to their relative effectiveness (the first ranked treatment is most likely to be the most effective treatment compared with the other treatments in the network), revealed that care in a stroke ward was the optimal option, although our confidence was limited by substantial imprecision. Finally, including a sensitivity analysis featuring only the six eligible trials that met all of the quality criteria listed in the sensitivity analysis above did not alter the ranking but resulted in wider confidence intervals (Goteborg‐Sahlgren 1994; Helsinki 1995; Kuopio 1985; Manchester 2003; Nottingham 1996; Orpington 2000).
6.

Network meta‐analysis plot for different types or organised care. The outcome is poor outcome at the end of scheduled follow‐up. The treatment column shows the service groups (GMW: general medical ward; MRW: mixed rehabilitation ward; MST: mobile stroke team; SW: stroke ward). The results are the odds ratio (95% confidence interval) for the odds of a poor outcome, with care in a GMW as the reference (OR = 1.0).
Figure 7 shows the NMA result for the outcome of death at the end of scheduled follow‐up. The lowest odds of death was seen with care in a stroke ward. No statistically significant reductions were seen for mobile stroke team or mixed rehabilitation ward care. A rank analysis revealed that care in a stroke ward was the optimal option. The sensitivity analysis did not alter the ranking.
7.
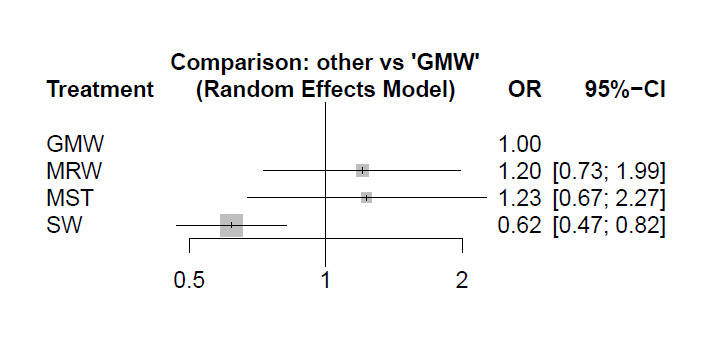
Network meta‐analysis plot for different types of organised care. The outcome is death at the end of scheduled follow‐up. The treatment column shows the service groups (GMW: general medical ward; MRW: mixed rehabilitation ward; MST: mobile stroke team; SW: stroke ward). The results are the odds ratio (95% confidence interval) for the odds of a poor outcome, with care in a GMW as the reference (OR = 1.0).
Figure 8 shows the NMA result for the outcome of death or institutional care at the end of scheduled follow‐up. The lowest odds of an adverse outcome was seen with care in a stroke ward. Reductions in poor outcome were of borderline significance for mixed rehabilitation ward, and no statistically significant reductions were seen for mobile stroke team. A rank analysis showed that care in a stroke ward was the optimal option. The sensitivity analysis did not alter the ranking.
8.

Network meta‐analysis plot for different types or organised care. The outcome is death or institutional care at the end of scheduled follow‐up. The treatment column shows the service groups (GMW: general medical ward; MRW: mixed rehabilitation ward; MST: mobile stroke team; SW: stroke ward). The results are the odds ratio (95% confidence interval) for the odds of a poor outcome, with care in a GMW as the reference (OR = 1.0).
Figure 9 shows the NMA result for the poor outcome (death or dependency) at the end of scheduled follow‐up. The lowest odds of poor outcome was seen with care in a stroke ward or in a mixed rehabilitation ward. No statistically significant reductions were seen for mobile stroke team. A rank analysis found that care in a mixed rehabilitation ward was the optimal option. The sensitivity analysis did not alter the ranking.
9.
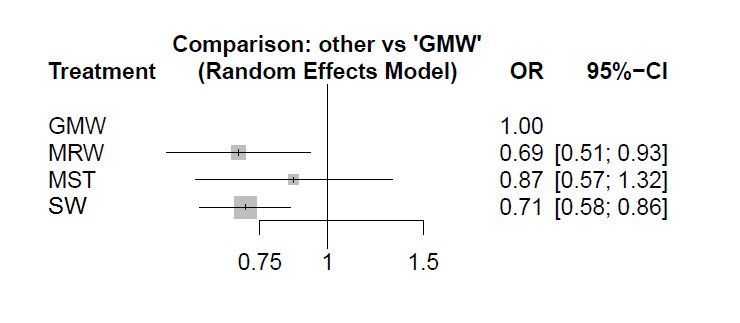
Network meta‐analysis plot for different types of organised care. The outcome is death or dependency at the end of scheduled follow‐up. The treatment column shows the service groups (GMW: general medical ward; MRW: mixed rehabilitation ward; MST: mobile stroke team; SW: stroke ward). The results are the odds ratio (95% confidence interval) for the odds of a poor outcome, with care in a GMW as the reference (OR = 1.0).
Finally, we present a 'Summary of findings' table for the NMA (Table 12).
8. Summary of findings table for the network meta‐analysis of different types of organised inpatient (stroke unit) care.
| Intervention organised inpatient (stroke unit) care | Comparison GMW | Number of studies (participants) with direct comparison evidence |
Direct comparison evidence OR (95% CI) |
Quality of the evidence (GRADE) for direct comparisons |
Direct plus indirect evidence in NMA OR (95% CI) |
Quality of the evidence (GRADE) for NMA |
| Poor outcome at end of scheduled follow‐up | ||||||
| Stroke ward | GMW | 14 (3321) | 0.78 (0.68 to 0.91) | Moderatea | 0.74 (0.62 to 0.89) | Moderatea |
| Mobile stroke team | GMW | 2 (438) | 0.80 (0.52 to 1.22) | Lowa,b | 0.88 (0.58 to 1.34) | Lowa,b |
| Mixed rehabilitation ward | GMW | 6 (630) | 0.65 (0.47 to 0.90) | Moderatea | 0.70 (0.52 to 0.95) | Lowa,b |
| Death at end of scheduled follow‐up | ||||||
| Stroke ward | GMW | 15 (3523) | 0.75 (0.63 to 0.90) | Moderatea | 0.62 (0.47 to 0.82) | Moderatea |
| Mobile stroke team | GMW | 2 (438) | 1.08 (0.71 to 1.65) | Lowa,b | 1.23 (0.67 to 2.27) | Lowa,b |
| Mixed rehabilitation ward | GMW | 6 (630) | 0.91 (0.58 to 1.42) | Lowa,b | 1.20 (0.73 to 1.99) | Lowa,b |
| Death or institutional care at end of scheduled follow‐up | ||||||
| Stroke ward | GMW | 13 (2924) | 0.74 (0.63 to 0.87) | Moderatea | 0.72 (0.62 to 0.83) | Moderatea |
| Mobile stroke team | GMW | 2 (438) | 1.27 (0.64 to 1.27) | Lowa,b | 1.46 (1.03 to 2.05) | Lowa,b |
| Mixed rehabilitation ward | GMW | 5 (578) | 0.71 (0.51 to 0.99) | Lowa,b | 0.75 (0.58 to 0.96) | Lowa,b |
| Death or dependency at end of scheduled follow‐up | ||||||
| Stroke ward | GMW | 12 (2839) | 0.75 (0.64 to 0.88) | Moderatea | 0.71 (0.58 to 0.86) | Moderatea |
| Mobile stroke team | GMW | 2 (438) | 0.80 (0.52 to 1.22) | Lowa,b | 0.87 (0.57 to 1.32) | Lowa,b |
| Mixed rehabilitation ward | GMW | 6 (630) | 0.65 (0.47 to 0.90) | Moderatea | 0.69 (0.51 to 0.93) | Lowa,b |
The characteristics of different models of care are outlined in Additional Table 1.
aDowngraded for risk of performance bias.
bDowngraded for imprecision.
CI: confidence interval. GMW: general medical ward. NMA: network meta‐analysis. OR: odds ratio.
Discussion
Summary of main results
Main analysis
The updated information in Section 1 confirms our previous observations that people receiving organised inpatient (stroke unit) care were more likely to survive, regain independence, and return home than those receiving an alternative service. These benefits were based on moderate‐quality evidence. The observed reductions in combined adverse outcomes (poor outcome, death or institutionalisation, death or dependency) are relatively robust and would be negated only by several new neutral trials. The three trials that have extended follow‐up for 5 or 10 years suggest sustained benefit among stroke unit patients.
The requirement for long‐term care is a useful surrogate for disability and is likely to be less prone to observer bias than measurement of disability (Barer 1993). The absolute rates of institutionalisation, however, will be influenced by a variety of national and cultural factors. The combined adverse outcome of death or dependency is a more direct measure of patient outcome but is subject to potential observer bias where final assessments were not carried out in a blinded manner. The sensitivity analysis based on those trials that used an unequivocally blinded assessment suggested that such bias has not seriously influenced the results.
The analysis of length of stay is complicated by different methods of reporting results, widely varying control group lengths of stay, and statistically significant heterogeneity between trials. The most reasonable conclusion appears to be that no systematic increase in length of stay is associated with organised (stroke unit) care, and there may be a modest reduction.
Subgroup analyses by patient characteristics
In any discussion of the comparison of results from different subgroups, it is worth bearing in mind that the main issue is not whether a subgroup result is statistically different from the comparator result, but whether there is statistically significant heterogeneity between estimates of effect in each of the relevant subgroups. Our analyses are limited by relatively low statistical power and so must be interpreted with great caution. The subgroup analyses indicate that observed benefits of organised stroke unit care are not limited to any one subgroup of patients. Apparent benefits were seen in people of both sexes, younger and older than 75 years, with ischaemic or haemorrhagic stroke, and across a range of stroke severities. The apparent interaction between stroke severity and outcome must be interpreted with caution. People with more severe stroke symptoms are at greater risk of death or requiring institutional care and hence stand to gain more from treatment. People with a mild stroke appeared to benefit from stroke unit care when death or dependency was the chosen outcome (Figure 4), but this effect was less certain for the outcomes of death and death or institutional care (data not shown).
Analyses by different types of service
An original aim of this review was to compare effects of organised inpatient (stroke unit) care with effects of contemporary conventional care. We expected that within this broad definition, the included trials would comprise a range of treatment comparisons (which could include stroke wards, mobile stroke teams, and mixed rehabilitation wards) with conventional care in a general ward. However, later trials have addressed newer questions by comparing different forms of organised inpatient (stroke unit) care (e.g. stroke ward versus mobile stroke team). This means that although the analysis in Section 1 tells us about the likely impact of organising inpatient care, it is much more limited in providing advice about specific service models. We have therefore retained the previous analyses but now include a network meta‐analysis (NMA) to explore, when possible, the impact of different models of organised inpatient (stroke unit) care.
Of the four approaches to organised inpatient (stroke unit) care tested in the NMA (stroke ward, mixed rehabilitation ward, mobile stroke team, general medical ward), care within a ward for stroke patients (stroke ward) was generally the most effective approach. Mixed rehabilitation units also showed some reduction in the composite outcomes (poor outcome, death or requiring institutional care, death or dependency).
In direct comparisons with care in a general medical ward, two different types of stroke wards (comprehensive stroke ward and mixed rehabilitation ward) tended to be more effective. There was a similar but less convincing pattern for rehabilitation stroke units. However, mobile stroke team care appeared to have a neutral effect. Apparent benefits were seen in units with acute admission policies, as well as in those with delayed admission policies and in units that could offer a period of rehabilitation lasting several weeks.
The final section (Section 5) focused on trials that directly compared two different forms of care, both of which met our basic definition of organised inpatient (stroke unit) care: multi‐disciplinary team care co‐ordinated through regular meetings. The results of this analysis indicate improved results from a comprehensive stroke ward over a mobile stroke team. Results also suggest better survival within the stroke rehabilitation ward setting as opposed to the mixed rehabilitation ward setting. Comparisons of a comprehensive ward with alternative pathways of care were limited by wide confidence intervals. No firm conclusions could be drawn for the comparisons of a stroke ward integrated with traditional Chinese medicine (TCM) versus a 'Western medicine' stroke ward.
In summary, subgroup analyses indicate that organised inpatient (stroke unit) care based in a dedicated stroke ward is likely to be most effective. Within the stroke ward approach, benefits were apparent for both comprehensive and rehabilitation stroke wards, indicating that organised stroke unit care is of benefit in both acute and rehabilitation phases of care. Mixed rehabilitation wards also showed some benefit in reducing dependency.
Overall completeness and applicability of evidence
This update includes one new trial (47 participants), and the overall conclusions remain unaltered from those provided in previous versions of the review. The review now summarises data from a total of 29 trials (5902 participants) from 12 countries in Asia, Australia, Europe, North America, and South America. A majority of trials have been performed in high‐income countries. Observational studies indicate that stroke units are likely to be effective in low‐ or middle‐income countries (Langhorne 2018; Seenan 2007; Urimubenshi 2017), but there is a shortage of information from randomised clinical trials (Langhorne 2012).
As discussed above, our subgroup analyses suggest that the benefits of organised inpatient (stroke unit) care are seen across a wide range of stroke patients. The current analysis does not explain how stroke units may improve patient outcomes, but this could be the result of greater staff expertise, better diagnostic procedures, better nursing care, early mobilisation, prevention of complications, or more effective rehabilitation procedures (Langhorne 1998).
Since the original publication of this review, stroke services in many developed countries have undergone substantial reorganisation in line with national strategies and clinical practice guidelines to enable improvements in access to stroke unit care. More recently, stroke services in many countries have been further re‐organised to reflect a two‐tiered (or hub‐and‐spoke) model of care in which a central 'comprehensive stroke centre' (based around a 'hyper‐acute stroke unit') is equipped with facilities for acute intravenous or intra‐arterial treatments, intensive monitoring, advanced imaging, and neurosurgery. These then serve a number of 'primary stroke centres' or stroke units within a hospital network or geographical location. Although these developments appear almost intuitive to many stroke clinicians, they have never been formally tested in randomised controlled trials with important patient outcomes. Until such trials are available, stroke services should ensure that every stroke patient receives the core service characteristics identified in randomised trials of organised inpatient (stroke unit) care.
Costs and benefits
Stroke units appear to improve outcomes, but at what cost? In cost terms, length of stay is likely to dominate any individual component of acute patient care and rehabilitation. Longer‐term costs are likely to be dominated by the need for nursing care. Studies from several developed countries have shown that fixed costs (particularly nursing staff salaries) account for over 90% of spending on people with acute stroke (Warlow 2008). Remedial therapy represents only a small proportion of the total cost of hospitalisation. In one analysis, stroke unit care was not clearly associated with an increase in total health and social care costs, but these conclusions were sensitive to some variation in cost estimates (Major 1998). More research is required to elucidate the cost implications of stroke units.
Quality of the evidence
The quality of evidence was made more uniform in the previous review update by the exclusion of several quasi‐randomised controlled clinical trials that were originally included in the data synthesis (see Description of studies) (SUTC 2013). The main goal of this change was to simplify the inclusion criteria for this and future updates. However, it is worth noting that exclusion of these trials did not affect the overall estimate of treatment effect (SUTC 2013).
The most common potential source of bias is the difficulty of concealing treatment allocation from participants (patients) and treating staff (performance bias); this is very difficult to achieve with this type of intervention. Although we have downgraded our recommendations for the risk of performance bias, it is worth noting that most studies completed long‐term follow‐up at a time when participants and families often could not recall any details of their treatment.
We judged some trials to be at high risk of bias due to poor allocation concealment and unblinded outcome assessment; in others, these important methodological aspects were not clearly reported, making a judgement of risk of bias difficult. We have not downgraded the GRADE recommendations because (1) the key outcomes (survival, return home) are unlikely to be sensitive to observer bias, and (2) sensitivity analyses restricted to the eight trials at low risk of bias did not alter effect sizes of the estimates. In particular, effect sizes for the composite adverse outcomes of death or institutionalisation and death or dependency remained largely unaltered.
We recognise that some of the included trials are relatively old, possibly applying entirely different standards of care from those used currently. Similarly, although a majority of included trials were conducted fairly recently, most would still have been undertaken in an era without routine access to re‐perfusion therapies (intravenous thrombolysis or mechanical thrombectomy) for acute ischaemic stroke. Although essentially all stroke patients would be eligible for admission to a stroke unit, only a small proportion would be eligible for treatment with re‐perfusion therapies even in the most established acute centres (Langhorne 2012). Moreover, all included trials were randomised; therefore any differences in the standard of care should not have had a confounding effect on the final conclusions.
Potential biases in the review process
Through a comprehensive search strategy and established connections with other researchers in the field, we have gone to considerable lengths to identify all potentially relevant studies. However, we did not search Chinese databases, and we were unable to classify or obtain useable outcome data for 7 of the 11 Chinese studies that we did identify (Anhui 2008; China (Hao) 2010; China (Pei) 2011; China (Wang) 2008; China (Wu) 2007; Haikou 2007; Shanghai 2006). We recognise that the absence of data from these studies in our meta‐analysis could potentially introduce bias.
Methodological limitations may also have influenced the analysis of descriptive information about service organisation (SUTC 1997a). First, our system of classifying services is an attempt to bring some structure to a complex topic, and it will lack precision (Table 5). In addition, we collated service descriptions retrospectively through discussion with the trialists who ran the organised (stroke unit) care. Our findings may therefore be biased towards the expectations of trialists and by a tendency to discuss the results with trialists who ran the organised stroke unit care more than with those who ran the conventional care unit. At best, this represents a strictly factual account of service characteristics; at worst, it represents a consensus view of the trialists about which features of stroke unit care were effective.
Agreements and disagreements with other studies or reviews
This systematic review has been updated multiple times over the last 25 years with broadly similar conclusions (SUTC 1997a; SUTC 1997b; SUTC 2001; SUTC 2007; SUTC 2013). Other versions of the review have tended to focus on the method (Sun 2013), on a subgroup of trials (Chan 2013), or on studies of implementation in routine services (Seenan 2007; Urimubenshi 2017). Although the emphasis may have varied between reviews, the overall conclusion about the effectiveness of organised inpatient (stroke unit) care has not changed.
Authors' conclusions
Implications for practice.
Moderate‐quality evidence shows that people with acute stroke are more likely to survive, return home, and regain independence if they receive organised inpatient (stroke unit) care. This is typically provided by a co‐ordinated multi‐disciplinary team operating within a discrete stroke ward that can offer a substantial period of rehabilitation if required. There are no firm grounds for restricting access according to a person's age, sex, stroke severity, or pathological stroke type (i.e. ischaemic or haemorrhagic). Stroke unit care provided in a dedicated stroke ward seems to be most effective, with some evidence for effectiveness of a mixed rehabilitation ward model.
Since the original publication of this review, many stroke services have been re‐organised to support newer treatments. Many have promoted a two‐tiered model of care in which a central 'comprehensive stroke centre' is equipped with facilities ('hyperacute' stroke units) for re‐perfusion therapies (intravenous thrombolysis and mechanical thrombectomy), advanced imaging, and neurosurgery. These in turn serve a number of 'primary stroke centres' within a hospital network or geographical location. These newer service models have not been formally tested in randomised controlled trials. Until such trials are available, clinicians and planners must ensure that every stroke patient receives the core service characteristics described in randomised trials of organised inpatient (stroke unit) care.
Implications for research.
Future trials should focus on examining the potentially important components of stroke unit care and on performing direct comparisons of different models of organised stroke unit care, particularly with regard to the hyperacute stroke unit model. In low‐income healthcare settings, appropriately powered clinical trials could help define how barriers to the establishment of stroke units could be overcome (Langhorne 2012). Outcome measures should not include only the outcomes of death, dependency, and institutionalisation; they should also include the domains of patient satisfaction, quality of life, and cost. Pre‐planned collaboration between comparable trials could alleviate some of the problems of retrospective systematic reviews by, for example, ensuring that similar variables and outcomes are recorded in any new trial.
Anyone carrying out a relevant randomised trial of a stroke service component is invited to contact Peter Langhorne regarding a future collaborative review.
Feedback
Patient subgroups,
Summary
The 95% CI includes 1.0 for patients with mild stroke. I would conclude that for this subgroup, there is no significant benefit insofar as preventing death or institutional care. I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms. Don Hess 2000‐09‐12 16:05
Criticism editor summary Regarding the outcome 'death or institutional care' for patients with mild stroke, the 95% CIs around the odds ratio suggest that stroke unit care is not beneficial in this subgroup of patients. This is not made clear in the review's abstract, results, and discussion.
Reply
Thank you for your comment. The proper test in a subgroup analysis is not whether a subgroup result is statistically different from zero, but whether there is statistically significant heterogeneity between the estimates of effect in each of the relevant subgroups. In our subgroup analysis, the mild stroke patient group does indeed have CIs that include no effect (odds ratio = 1.0). However, we do not believe we can at present conclude that this subgroup of patients have a different result from the totality of patients. First, the statistical power of this analysis is limited because the mild stroke subgroup had relatively few outcome events (death or institutional care). Second, the mild stroke subgroup result is not significantly different from that of the moderate and severe subgroups. These analyses are explored in greater detail in the Stroke Unit Trialists' Collaboration. How do stroke units improve patient outcomes? A collaborative systematic review of the randomised trials. Stroke 1997;28:2139‐44.
Contributors
Peter Langhorne 07/03/2001.
Numerical error, 30 June 2014
Summary
Possible typo? The abstract states "... odds of death recorded at final (median one year) follow‐up (odds ratio (OR) 0.87..." but text on page 15 (Comparisons 2.1, 2.2, 2.3 ...) and forest plot for 2.1 report OR = 0.81.
Reply
Dr Zekowski is correct. There appears to have been an error when updating the review. In the abstract, the correct OR for death should be (OR 0.81, 95% CI 0.69 to 0.94; P = 0.005). This has now been corrected in the text.
Contributors
Feedback: Steven Zekowski, MD.
Response: Peter Langhorne.
Long‐term health outcome, 4 December 2016
Summary
To my knowledge, there are no published randomised controlled trials directly comparing long‐term health outcomes in patients managed at defined stroke units with those of conventional care. I would much appreciate if you can comment on this and also refer me to scientific articles estimating the 'number needed to care for' (analogous to number needed to treat in RCTs) regarding various health outcomes as well as health care utilization effects in stroke patients.
Reply
Thank you for your recent feedback submission on the stroke unit review. I will address your queries in turn.
Long‐term health outcomes: to my knowledge, three randomised trials (Athens 1995; Nottingham 1996; Trondheim 1991) carried out 5‐ and 10‐year follow‐up of all participants. However, this is limited to a few fundamental outcomes (death, place of residence, disability). As you can imagine, almost all participants were dead or disabled at 10‐year follow‐up. There is very limited information on other outcomes.
Estimation of 'number needed to care for': I cannot reference a recent scientific article addressing your specific question. However, it is possible to calculate this information relatively easily from the current Cochrane Review. For instance, in Table 2.1 (Organised stroke unit care versus general medical ward), the absolute risk difference in deaths is ‐3 per 100 cared for (95% confidence interval ‐6 to ‐1). This translates into a number needed to care for of 33. The equivalent numbers needed to treat to avoid death or institutional care and death or disability are 20 and 17, respectively.
Other health outcomes (such as ADL score or quality of life) often are not reported in the trials. When they are, they tend to favour stroke unit care.
Information on health utilisation: once again, healthcare utilisation has been measured different ways in different trials. Overall, stroke unit patients tended to have a shorter length of stay in hospital, and length of stay is the main driver of costs in hospital. Several independent analyses have modelled potential healthcare utilisation effects of stroke unit care. Most have concluded that stroke unit costs are equivalent to or slightly lower than general medical costs.
I hope these comments are useful.
Contributors
Comment: Gunnar Akner, MD, PhD
Response: Peter Langhorne, Professor of Stroke Care
What's new
| Date | Event | Description |
|---|---|---|
| 2 April 2019 | New search has been performed | This update incorporated (1) a revised literature search (updated to 2 April 2019), (2) 1 new included trial, (3) a single primary outcome (poor outcome: death or dependency or requiring institutional care) in addition to the previous outcomes, (4) a revision of the data presentation, (5) a new network meta‐analysis, and (6) new 'Summary of findings' tables with GRADE quality of evidence classifications. The review now includes 29 studies involving 5902 participants |
| 2 April 2019 | New citation required but conclusions have not changed | The conclusions have not changed and remain similar to the previous version |
History
Protocol first published: Issue 1, 1995 Review first published: Issue 1, 1995
| Date | Event | Description |
|---|---|---|
| 6 January 2017 | Feedback has been incorporated | Feedback has been incorporated |
| 14 July 2014 | Feedback has been incorporated | Feedback has been incorporated and numerical error in the Abstract has been corrected with no change to the conclusions |
| 29 January 2013 | New search has been performed | This updated review identified 4 new trials (763 participants). We have excluded 7 previously included quasi‐randomised prospective controlled clinical trials. This review now incorporates an individual patient data meta‐analysis of 28 randomised controlled trials (5855 participants). More recent stroke unit trials have addressed different ways of providing organised care. This update contains data from trials comparing stroke unit care with care given in general medical wards and comparing 2 different forms of organised (stroke unit) care |
| 29 January 2013 | New citation required but conclusions have not changed | The conclusions of the review have not changed |
| 9 September 2008 | Amended | The review has been converted to new review format |
| 28 November 2006 | New search has been performed | New data on 2027 participants from 8 new trials (Athens, Beijing, Cape Town, Groningen, Joinville, Manchester, Osaka, and Pavia) have become available. More recent stroke unit trials have addressed different ways of providing organised care. This update contains new information and data from trials comparing stroke unit care with care provided on general medical wards and comparing 2 different forms of organised (stroke unit) care |
Acknowledgements
This review is dedicated to the memory of Peter Berman, Mona Britton, and Richard Stevens.
We are grateful to our two consumer reviewers (Odie Geiger and Dee Shneiderman) for their comments and input.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL search strategy (April 2019) #1 MeSH descriptor: [Cerebrovascular Disorders] this term only #2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees #3 MeSH descriptor: [Brain Ischemia] explode all trees #4 MeSH descriptor: [Carotid Artery Diseases] explode all trees #5 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #6 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #7 MeSH descriptor: [Intracranial Hemorrhages] explode all trees #8 MeSH descriptor: [Stroke] this term only #9 MeSH descriptor: [Brain Infarction] explode all trees #10 MeSH descriptor: [Vertebral Artery Dissection] explode all trees #11 ((stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex*)):ti,ab,kw #12 (((brain* or cerebr* or cerebell* or vertebrobasilar or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) NEAR/5 (isch?emi* or infarct* or thrombo* or emboli*))):ti,ab,kw #13 (((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli*) NEAR/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*))):ti,ab,kw #14 {or #1‐#13} #15 MeSH descriptor: [Hospital Units] this term only #16 MeSH descriptor: [Patient Care Team] this term only #17 ((stroke NEAR/3 (unit or units or ward or wards or hospital or hospitals or centre* or team or teams))):ti,ab,kw #18 (((organi?ed or structured) NEAR/3 care)):ti,ab,kw #19 ((rehabilitation NEAR/3 (unit or units or ward or wards or hospital or hospitals or centre* or team or teams))):ti,ab,kw #20 ((multidisciplinary NEAR/3 (team or teams or staff* or care or rehabilitation or unit or units or ward or wards))):ti,ab,kw #21 (((dedicated or discrete or comprehensive) NEAR/5 (ward or wards or unit or units or stroke care))):ti,ab,kw #22 (((specialist or specialized or specialised) NEAR/5 (nurs* or staff* or care or unit or units or ward or wards))):ti,ab,kw #23 ((organi?ed NEAR/3 (unit or units or ward or wards))):ti,ab,kw #24 (focus* care):ti,ab,kw #25 ((package* NEAR/3 care)):ti,ab,kw #26 ((intensive NEAR/3 stroke NEAR/3 care)):ti,ab,kw #27 MeSH descriptor: [Intensive Care Units] this term only #28 MeSH descriptor: [Critical Care] this term only #29 {or #15‐#28} #30 #14 and #29
Appendix 2. MEDLINE search strategy
MEDLINE (Ovid) search strategy 1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or exp vertebral artery dissection/ 2. (stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. 1 or 2 or 3 or 4 6. hospital units/ or patient care team/ 7. (stroke adj3 (unit or units or ward or wards or hospital or hospitals or centre$ or team or teams)).tw. 8. ((organi?ed or structured) adj3 care).tw. 9. (rehabilitation adj3 (unit or units or ward or wards or hospital or hospitals or centre$ or team or teams)).tw. 10. (multidisciplinary adj3 (team or teams or staff$ or care or rehabilitation or unit or units or ward or wards)).tw. 11. ((dedicated or discrete or comprehensive) adj5 (ward or wards or unit or units or stroke care)).tw. 12. ((specialist or specialized or specialised) adj5 (nurs$ or staff$ or care or unit or units or ward or wards)).tw. 13. (organi?ed adj3 (unit or units or ward or wards)).tw. 14. focus$ care.tw. 15. (package$ adj care).tw. 16. (intensive adj3 stroke adj3 care).tw. 17. Intensive Care Units/ or critical care/ or intensive care/ 18. or/6‐17 19. 5 and 18 20. Randomized Controlled Trials as Topic/ 21. random allocation/ 22. Controlled Clinical Trials as Topic/ 23. control groups/ 24. clinical trials as topic/ 25. double‐blind method/ 26. single‐blind method/ 27. Research Design/ 28. Program Evaluation/ 29. randomised controlled trial.pt. 30. controlled clinical trial.pt. 31. clinical trial.pt. 32. random$.tw. 33. (controlled adj5 (trial$ or stud$)).tw. 34. (clinical$ adj5 trial$).tw. 35. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 36. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 37. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 38. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 39. (assign$ or allocat$).tw. 40. controls.tw. 41. trial.ti. 42. or/20‐41 43. 19 and 42 44. exp animals/ not humans.sh. 45. 43 not 44
Appendix 3. Embase search strategy
Embase (Ovid) search strategy 1. cerebrovascular disease/ or basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/ 2. stroke patient/ 3. (stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$).tw. 4. ((brain$ or cerebr$ or cerebell$ or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$)).tw. 5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 6. 1 or 2 or 3 or 4 or 5 7. "hospital subdivisions and components"/ 8. ward/ or emergency ward/ or nursing unit/ 9. intensive care unit/ 10. exp intensive care/ 11. (stroke adj3 (unit or units or ward or wards or hospital or hospitals or centre$ or team or teams)).tw. 12. ((organi?ed or structured) adj3 care).tw. 13. (rehabilitation adj3 (unit or units or ward or wards or hospital or hospitals or centre$ or team or teams)).tw. 14. (multidisciplinary adj3 (team or teams or staff$ or care or rehabilitation or unit or units or ward or wards)).tw. 15. ((dedicated or discrete or comprehensive) adj5 (ward or wards or unit or units or stroke care)).tw. 16. ((specialist or specialized or specialised) adj5 (nurs$ or staff$ or care or unit or units or ward or wards)).tw. 17. (organi?ed adj3 (unit or units or ward or wards)).tw. 18. focus$ care.tw. 19. (package$ adj care).tw. 20. (intensive adj3 stroke adj3 care).tw. 21. or/7‐20 22. 6 and 21 23. stroke unit/ 24. 22 or 23 25. Randomized Controlled Trial/ 26. Randomization/ 27. Controlled Study/ 28. control group/ 29. clinical trial/ 30. Double Blind Procedure/ 31. Single Blind Procedure/ or triple blind procedure/ 32. Parallel Design/ 33. random$.tw. 34. (controlled adj5 (trial$ or stud$)).tw. 35. (clinical$ adj5 trial$).tw. 36. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 37. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 38. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 39. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 40. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 41. controls.tw. 42. trial.ti. 43. or/25‐42 44. 24 and 43 45. heart stroke volume/ or heat stroke/ or stroke volume.tw. or heat stroke.tw. 46. 44 not 45
Appendix 4. CINAHL search strategy
S44 .S28 and S43 S43 .S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S38 or S39 or S40 or S41 or S42 S42 .TI trial S41 .TI controls OR AB controls S40 .TI ( assign* or allocat* ) OR AB ( assign* or allocat* ) S39 .TI ( (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) ) OR AB ( (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) ) S38 .TI ( (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) ) OR AB ( (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) ) S37 .TI ( quasi‐random* or quasi random* or pseudo‐random* or pseudo random* ) OR AB ( quasi‐random* or quasi random* or pseudo‐random* or pseudo random* ) S36 .TI ( (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) ) OR AB ( (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) ) S35 .TI clinical* N5 trial* OR AB clinical* N5 trial* S34 .TI ( controlled N5 (trial* or stud*) ) OR AB ( controlled N5 (trial* or stud*) ) S33 .TI random* OR AB random* S32 .(MH "Program Evaluation") S31 .(MH "Random Assignment") S30 .(ZT "clinical trial") or (ZT "randomised controlled trial") S29 .(MH "Clinical Trials") OR (MH "Double‐Blind Studies") OR (MH "Intervention Trials") OR (MH "Randomized Controlled Trials") OR (MH "Single‐Blind Studies") OR (MH "Therapeutic Trials") OR (MH "Triple‐Blind Studies") S28 .S1 or S27 S27 .S11 and S26 S26 .S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 S25 .TI intensive N3 stroke N3 care OR AB intensive N3 stroke N3 care S24 .TI package* N3 care OR AB package* N3 care S23 .TI focus* care OR AB focus* care S22 .TI ( organi?ed N3 (unit or units or ward or wards) ) OR AB ( organi?ed N3 (unit or units or ward or wards) ) S21 .TI ( (specialist or specialized or specialised) N5 (nurs* or staff* or care or unit or units or ward or wards) ) OR AB ( (specialist or specialized or specialised) N5 (nurs* or staff* or care or unit or units or ward or wards) ) S20 .TI ( (dedicated or discrete or comprehensive) N5 (ward or wards or unit or units or stroke care) ) OR AB ( (dedicated or discrete or comprehensive) N5 (ward or wards or unit or units or stroke care) ) S19 .TI ( multidisciplinary N3 (team or teams or staff* or care or rehabilitation or unit or units or ward or wards) ) OR AB ( multidisciplinary N3 (team or teams or staff* or care or rehabilitation or unit or units or ward or wards) ) S18 .TI ( rehabilitation N3 (unit or units or ward or wards or hospital or hospitals or centre* or team or teams) ) OR AB ( rehabilitation N3 (unit or units or ward or wards or hospital or hospitals or centre* or team or teams) ) S17 .TI ( (organi?ed or structured) N3 care ) OR AB ( (organi?ed or structured) N3 care ) S16 .TI ( stroke N3 (unit or units or ward or wards or hospital or hospitals or centre* or team or teams) ) OR AB ( stroke N3 (unit or units or ward or wards or hospital or hospitals or centre* or team or teams) ) S15 .(MH "Critical Care Nursing") S14 .(MH "Critical Care") S13 .(MH "Multidisciplinary Care Team") S12 .(MH "Hospital Units") OR (MH "Intensive Care Units") S11 .S2 or S3 or S4 or S7 or S10 S10 .S8 and S9 S9 .TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S8 .TI ( brain brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli* ) or AB (brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli* ) S7 .S5 and S6 S6 .TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* ) S5 .TI (brain* or cerebr* or cerebell* or vertebrobasilar or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) or AB (brain* or cerebr* or cerebell* or vertebrobasilar or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia ) S4.TI (stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex*) or AB (stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* ) S3 .(MH "Stroke Patients") S2 .(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") S1 .(MH "Stroke Units")
Appendix 5. ClinicalTrials.gov search strategy
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) ( Hospital Unit OR Intensive Care OR Critical Care Or Stroke Unit ) AND INFLECT EXACT "Interventional" [STUDY‐TYPES] AND ( Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke ) [DISEASE] AND INFLECT ( "09/01/2012": "08/13/2018" ) [STUDY‐FIRST‐POSTED]
Appendix 6. World Health Organization International Clinical Trials Registry search strategy
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) Basic search/ Phases are: ALL: stroke AND inpatient OR stroke AND hospital unit OR stroke AND patient care or stroke AND "stroke unit".
Data and analyses
Comparison 1. Organised stroke care versus alternative service.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Poor outcome by the end of scheduled follow‐up | 29 | 5336 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 1.1.1 Stroke ward vs general medical ward | 14 | 3321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.68, 0.91] |
| 1.1.2 Mixed rehabilitation ward vs general medical ward | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 1.1.3 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 1.1.4 Stroke ward vs mixed rehabilitation ward | 4 | 542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.68, 1.50] |
| 1.1.5 Stroke ward vs mobile stroke team | 1 | 304 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.46, 1.14] |
| 1.1.6 Stroke ward vs stroke ward | 2 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.21, 1.04] |
| 1.2 Death by the end of scheduled follow‐up | 32 | 5902 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.66, 0.88] |
| 1.2.1 Stroke ward vs general medical ward | 15 | 3521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.63, 0.90] |
| 1.2.2 Mixed rehabilitation ward vs general medical ward | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.58, 1.42] |
| 1.2.3 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.71, 1.65] |
| 1.2.4 Stroke ward vs mixed rehabilitation ward | 4 | 542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.54, 1.24] |
| 1.2.5 Stroke ward vs mobile stroke team | 1 | 304 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.19, 0.65] |
| 1.2.6 Stroke ward vs stroke ward | 4 | 467 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.14, 0.94] |
| 1.3 Death or institutional care by the end of scheduled follow‐up | 27 | 4887 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.67, 0.85] |
| 1.3.1 Stroke ward vs general medical ward | 13 | 2924 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.63, 0.87] |
| 1.3.2 Mixed rehabilitation ward vs general medical ward | 5 | 578 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 1.3.3 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.84, 1.93] |
| 1.3.4 Stroke ward vs mixed rehabilitation ward | 4 | 542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.64, 1.27] |
| 1.3.5 Stroke ward vs mobile stroke team | 1 | 304 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.23, 0.68] |
| 1.3.6 Stroke ward vs stroke ward | 2 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.16, 0.93] |
| 1.4 Death or dependency by the end of scheduled follow‐up | 27 | 4854 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.66, 0.85] |
| 1.4.1 Stroke ward vs general medical ward | 12 | 2839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 1.4.2 Mixed rehabilitation ward vs general medical ward | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 1.4.3 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 1.4.4 Stroke ward vs mixed rehabilitation ward | 4 | 542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.68, 1.50] |
| 1.4.5 Stroke ward vs mobile stroke team | 1 | 304 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.46, 1.14] |
| 1.4.6 Stroke ward vs stroke ward | 2 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.21, 1.04] |
| 1.5 Length of stay (days) in a hospital or institution or both | 20 | 4162 | Mean Difference (IV, Random, 95% CI) | ‐4.28 [‐7.86, ‐0.71] |
| 1.5.1 Stroke ward | 17 | 3775 | Mean Difference (IV, Random, 95% CI) | ‐4.76 [‐8.46, ‐1.05] |
| 1.5.2 Mixed rehabilitation ward | 3 | 387 | Mean Difference (IV, Random, 95% CI) | 3.85 [‐13.49, 21.18] |
| 1.6 Length of stay (days) in a hospital or hospital plus institution | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Acute hospital stay only | 7 | 1817 | Mean Difference (IV, Random, 95% CI) | ‐2.78 [‐6.13, 0.56] |
| 1.6.2 Hospital and institution stay | 13 | 2345 | Mean Difference (IV, Random, 95% CI) | ‐4.84 [‐14.52, 4.84] |
| 1.7 Poor outcome at 5‐year follow‐up | 2 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.34] |
| 1.8 Death at 5‐year follow‐up | 3 | 1139 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.59, 0.94] |
| 1.9 Death or institutional care at 5‐year follow‐up | 2 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 1.10 Death or dependency at 5‐year follow‐up | 2 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.34] |
| 1.11 Poor outcome at 10‐year follow‐up | 2 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.27, 1.80] |
| 1.12 Death at 10‐year follow‐up | 3 | 1139 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.43, 1.03] |
| 1.13 Death or institutional care at 10‐year follow‐up | 2 | 535 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.37, 0.88] |
| 1.14 Death or dependency at 10‐year follow‐up | 2 | 535 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.27, 1.80] |
1.7. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 7: Poor outcome at 5‐year follow‐up
1.8. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 8: Death at 5‐year follow‐up
1.9. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 9: Death or institutional care at 5‐year follow‐up
1.10. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 10: Death or dependency at 5‐year follow‐up
1.11. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 11: Poor outcome at 10‐year follow‐up
1.12. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 12: Death at 10‐year follow‐up
1.13. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 13: Death or institutional care at 10‐year follow‐up
1.14. Analysis.

Comparison 1: Organised stroke care versus alternative service, Outcome 14: Death or dependency at 10‐year follow‐up
Comparison 2. Stroke ward versus general medical ward.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Poor outcome by the end of scheduled follow‐up | 14 | 3321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.68, 0.91] |
| 2.1.1 Comprehensive stroke ward vs general medical ward | 10 | 2788 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.66, 0.91] |
| 2.1.2 Rehabilitation stroke ward vs general medical ward | 4 | 533 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.57, 1.23] |
| 2.2 Death by the end of scheduled follow‐up | 15 | 3523 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.63, 0.90] |
| 2.2.1 Comprehensive stroke ward vs general medical ward | 11 | 2988 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.63, 0.93] |
| 2.2.2 Rehabilitation stroke ward vs general medical ward | 4 | 535 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.46, 1.05] |
| 2.3 Death or institutional care by the end of scheduled follow‐up | 13 | 2924 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.63, 0.87] |
| 2.3.1 Comprehensive stroke ward vs general medical ward | 9 | 2391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.62, 0.88] |
| 2.3.2 Rehabilitation stroke ward vs general medical ward | 4 | 533 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.52, 1.09] |
| 2.4 Death or dependency by the end of scheduled follow‐up | 12 | 2839 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 2.4.1 Comprehensive stroke ward vs general medical ward | 8 | 2306 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.62, 0.87] |
| 2.4.2 Rehabilitation stroke ward vs general medical ward | 4 | 533 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.57, 1.23] |
| 2.5 Length of stay (days) in a hospital or institution | 10 | 2547 | Mean Difference (IV, Random, 95% CI) | ‐2.19 [‐5.19, 0.82] |
| 2.5.1 Comprehensive stroke ward vs general medical ward | 9 | 2373 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐5.68, 0.10] |
| 2.5.2 Rehabilitation stroke ward vs general medical ward | 1 | 174 | Mean Difference (IV, Random, 95% CI) | 16.34 [2.82, 29.86] |
Comparison 3. Mobile stroke team versus general medical ward.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Poor outcome by the end of scheduled follow‐up | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 3.1.1 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 3.2 Death by the end of scheduled follow‐up | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.71, 1.65] |
| 3.2.1 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.71, 1.65] |
| 3.3 Death or institutional care by the end of scheduled follow‐up | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.84, 1.93] |
| 3.3.1 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.84, 1.93] |
| 3.4 Death or dependency by the end of scheduled follow‐up | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 3.4.1 Mobile stroke team vs general medical ward | 2 | 438 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.52, 1.22] |
| 3.5 Length of stay (days) in a hospital or institution | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.5.1 Mobile stroke team vs general medical ward | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | Not estimable |
Comparison 4. Mixed rehabilitation ward versus general medical ward.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Poor outcome by the end of scheduled follow‐up | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 4.1.1 Mixed rehabilitation ward vs general medical ward | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 4.2 Death by the end of scheduled follow‐up | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.58, 1.42] |
| 4.2.1 Mixed rehabilitation ward vs general medical ward | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.58, 1.42] |
| 4.3 Death or institutional care by the end of scheduled follow‐up | 5 | 578 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 4.3.1 Mixed rehabilitation ward vs general medical ward | 5 | 578 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 4.4 Death or dependency by the end of scheduled follow‐up | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 4.4.1 Mixed rehabilitation ward vs general medical ward | 6 | 630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.47, 0.90] |
| 4.5 Length of stay (days) in a hospital or institution | 3 | 387 | Mean Difference (IV, Random, 95% CI) | 3.85 [‐13.49, 21.18] |
| 4.5.1 Mixed rehabilitation ward vs general ward | 3 | 387 | Mean Difference (IV, Random, 95% CI) | 3.85 [‐13.49, 21.18] |
Comparison 5. Different systems of organised care: stroke ward versus alternative organised care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Poor outcome by the end of scheduled follow‐up | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1.1 Acute ward vs mixed rehabilitation ward | 1 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.72, 2.14] |
| 5.1.2 Acute stroke ward vs comprehensive stroke ward | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.18, 3.69] |
| 5.1.3 Comprehensive stroke ward vs mobile stroke team | 1 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.14] |
| 5.1.4 Rehabilitation stroke ward vs mixed rehabilitation ward | 3 | 331 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.45, 1.45] |
| 5.2 Death by the end of scheduled follow‐up | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.2.1 Acute stroke ward vs mixed rehabilitation ward | 1 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.76, 2.58] |
| 5.2.2 Acute stroke ward vs comprehensive stroke ward | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 2.90] |
| 5.2.3 Comprehensive stroke ward vs mobile stroke team | 1 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.64] |
| 5.2.4 Rehabilitation stroke ward vs mixed rehabilitation ward | 3 | 331 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.28, 0.90] |
| 5.2.5 Stroke ward (plus TCM) vs stroke ward (without TCM) | 2 | 366 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.13, 2.30] |
| 5.3 Death or institutional care by the end of scheduled follow‐up | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.3.1 Acute ward vs mixed rehabilitation ward | 1 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [0.76, 2.30] |
| 5.3.2 Acute stroke ward vs comprehensive stroke ward | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.14, 11.31] |
| 5.3.3 Comprehensive stroke ward vs mobile stroke team | 1 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.68] |
| 5.3.4 Rehabilitation stroke ward vs mixed rehabilitation ward | 3 | 331 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.45, 1.09] |
| 5.4 Death or dependency by the end of scheduled follow‐up | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.4.1 Acute ward vs mixed rehabilitation ward | 1 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.72, 2.14] |
| 5.4.2 Acute stroke ward vs comprehensive stroke ward | 2 | 101 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.18, 3.69] |
| 5.4.3 Comprehensive stroke ward vs mobile stroke team | 1 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.14] |
| 5.4.4 Rehabilitation stroke ward vs mixed rehabilitation ward | 3 | 331 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.45, 1.45] |
| 5.5 Length of stay (days) in a hospital or institution | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.5.1 Acute stroke ward vs mixed rehabilitation ward | 1 | 211 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐11.19, 7.19] |
| 5.5.2 Acute (±rehabilitation) stroke ward vs comprehensive stroke ward | 2 | 101 | Mean Difference (IV, Random, 95% CI) | ‐2.89 [‐20.10, 14.33] |
| 5.5.3 Comprehensive stroke ward vs mobile stroke team | 1 | 304 | Mean Difference (IV, Random, 95% CI) | 2.50 [‐5.42, 10.42] |
| 5.5.4 Rehabilitation stroke ward vs mixed rehabilitation ward | 3 | 331 | Mean Difference (IV, Random, 95% CI) | 15.80 [‐45.71, 77.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Athens 1995.
| Study characteristics | ||
| Methods | RCT Sealed envelopes Unblinded follow‐up | |
| Participants | People with acute stroke admitted to emergency department within 24 hours of symptoms Excluded TIA or recurrent stroke | |
| Interventions | Small (6‐bed) ward within Internal Medicine department Used the American Heart Association protocol, management of physiological abnormalities, multi‐disciplinary team approach Compared with conventional care in general medical ward | |
| Outcomes | Death, cause of death, length of stay Recorded up to 6.5 years (12‐month data used in primary analysis) | |
| Notes | Unpublished at present | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised ... using numbered opaque sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Beijing 2004.
| Study characteristics | ||
| Methods | RCT Divided randomly using SPSS software package | |
| Participants | People with stroke admitted to hospital with first or recurrent stroke Subarachnoid haemorrhage and tumour were excluded | |
| Interventions | New comprehensive stroke unit, early multi‐disciplinary rehabilitation Control participants were admitted to general medical or general neurology ward | |
| Outcomes | Death, NIHSS, Barthel Index, Oxford Handicap Scale, patient satisfaction at time of discharge | |
| Notes | Some unpublished data included No institutional care available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Divided randomly into two groups using SPSS software package" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers in treatment (n = 20) and control (n = 21) groups with missing data |
| Selective reporting (reporting bias) | Low risk | Data on all pre‐specified outcomes reported |
Birmingham 1972.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with stroke within 2 weeks of stroke onset Able to tolerate active rehabilitation | |
| Interventions | Intensive rehabilitation in rehabilitation centre (mixed rehabilitation unit) (n = 29) vs normal care in general medical ward (n = 23) Organised care provided for months if required | |
| Outcomes | Death and functional status at end of follow‐up (6 to 8 months) | |
| Notes | Timing of outcomes not clearly stated Intervention not clearly defined 3 control participants lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Evenly divided on a random basis" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 control participants (almost 10%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported |
Dover 1984.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with stroke up to 9 weeks after stroke onset (majority within 3 weeks) Fit for transfer to rehabilitation ward | |
| Interventions | Stroke rehabilitation ward (dedicated stroke unit) (n = 116) vs general medical ward (n = 89) or geriatric medical ward (mixed rehabilitation unit) (n = 28) Organised care provided for months if required | |
| Outcomes | Death, Rankin score, place of residence, length of stay in hospital up to 1 year after stroke | |
| Notes | Randomisation resulted in marginally poorer prognosis in participants in the control group Numbers differ slightly from the published report after re‐analysis of original data 2 control participants lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation ... by the secretary opening the next in a stack of serially numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data explained; broadly similar numbers between intervention and control groups |
| Selective reporting (reporting bias) | Unclear risk | Rankin score pre‐specified but not reported Disability reported with a different measure |
Dover 1984 (GMW).
| Study characteristics | ||
| Methods | RCT Subgroup of Dover 1984 (stroke unit vs general medical ward) | |
| Participants | People with stroke up to 9 weeks after stroke onset (majority within 3 weeks) Fit for transfer to rehabilitation ward | |
| Interventions | Stroke rehabilitation ward (dedicated stroke unit) (n = 98) vs general medical ward (n = 89) Organised care provided for months if required | |
| Outcomes | Death, Rankin score, place of residence, length of stay in hospital up to 1 year after stroke | |
| Notes | Stroke severity subgroup data inferred from distribution in the whole group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation ... by the secretary opening the next in a stack of serially numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data explained; broadly similar numbers between intervention and control groups |
| Selective reporting (reporting bias) | Unclear risk | Rankin score pre‐specified but not reported Disability reported in an alternate way |
Dover 1984 (MRW).
| Study characteristics | ||
| Methods | RCT Subgroup of Dover 1984 (stroke unit vs mixed rehabilitation ward) | |
| Participants | People with stroke up to 9 weeks after stroke onset (majority within 3 weeks) Fit for transfer to rehabilitation ward. | |
| Interventions | Stroke rehabilitation ward (dedicated stroke unit) (n = 18) vs geriatric medical ward (mixed rehabilitation unit) (n = 28) Organised care provided for months if required | |
| Outcomes | Death, Rankin score, place of residence, length of stay in hospital up to 1 year after stroke | |
| Notes | Stroke severity subgroup data inferred from distribution in the whole group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation ... by the secretary opening the next in a stack of serially numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data explained; broadly similar numbers between intervention and control groups |
| Selective reporting (reporting bias) | Unclear risk | Rankin score pre‐specified but not reported Disability reported in an alternate way |
Edinburgh 1980.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with acute stroke within 7 days of stroke onset Stroke of moderate severity | |
| Interventions | Comprehensive stroke ward (dedicated stroke unit) (n = 155) vs general medical ward (n = 156) Organised care provided for a maximum of 16 weeks | |
| Outcomes | Death, dependency, place of residence, length of initial hospital admission up to 1 year after stroke | |
| Notes | 6 intervention and 10 control participants lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using numbered sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Serially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants in control group 'dropped out' after randomisation; no outcome data provided |
| Selective reporting (reporting bias) | Low risk | Outcomes were not clearly pre‐specified but all expected outcomes are reported |
Goteborg‐Ostra 1988.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with acute stroke within 7 days of stroke | |
| Interventions | Comprehensive stroke ward (n = 215) within general medical service vs conventional care in general medical ward (n = 202) | |
| Outcomes | Death, Barthel Index, place of residence, length of hospital stay recorded at discharge | |
| Notes | Not yet published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in closed envelopes |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Goteborg‐Sahlgren 1994.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with acute stroke within 7 days of onset | |
| Interventions | Combined service continuum linking 2 acute and 2 rehabilitation stroke wards (n = 166) vs conventional care in general medical ward (n = 83) | |
| Outcomes | Death, dependency (Barthel Index), place of residence, satisfaction, length of hospital stay up to 1 year | |
| Notes | – | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Serially numbered sealed envelopes (randomization in blocks of 10)" |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All dichotomous outcomes reported, but proportionately more follow‐up assessments missing in control group (7/83) than in intervention group (6/166) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Groningen 2003.
| Study characteristics | ||
| Methods | RCT Blinded assessment of outcomes | |
| Participants | People with acute ischaemic stroke admitted within 24 hours (conscious, hemiparetic, no prior dependency) | |
| Interventions | Acute stroke unit with continuous physiological monitoring and intervention for 48 hours All other care as per conventional stroke unit Transfer to conventional stroke unit after 48 hours Conventional stroke unit: comprehensive stroke ward with intermittent physiological monitoring Both units had a multi‐disciplinary team meeting once per week Both units had discharge for rehabilitation at about 2 weeks | |
| Outcomes | Death or poor outcome (institutional care or Rankin score > 3 or Barthel Index < 12) recorded at 3 months Complications and interventions, length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised ... using an envelope system on a one to one basis ..." |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal but similar care routines |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome data reported |
Guangdong 2008.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People (56 men) with acute ischaemic stroke; timing of randomisation unclear Mean age intervention group: 61.4 years (SD 9.05); mean age control group: 60.9 years (SD 8.2) |
|
| Interventions | Stroke unit plus integrated traditional Chinese medicine (n = 58) vs 'Western medicine' stroke unit (n = 42) | |
| Outcomes | Death, NIHSS at 30 days, Barthel Index Length of follow‐up unclear |
|
| Notes | Limited translated data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Guangdong 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Participants (137 men) with acute ischaemic stroke, randomised on admission Average age 61.9 years in intervention group vs 63.4 years in control group |
|
| Interventions | Stroke unit with integrated traditional Chinese medicine (n = 100) vs general medical ward (n = 100) | |
| Outcomes | Death, dependency (Barthel Index, OHS), discharge NIHSS Cost‐effectiveness analysis |
|
| Notes | Limited translated data available Overall numbers in intervention and control groups differed between original publication and data in published meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Helsinki 1995.
| Study characteristics | ||
| Methods | RCT Blinded assessment of outcomes | |
| Participants | People with acute stroke within 7 days of stroke Unselected people over the age of 65 years | |
| Interventions | Mixed rehabilitation unit within neurology ward (n = 121) vs conventional care in general medical ward (n = 122) Organised care provided for several weeks if required | |
| Outcomes | Death, Barthel Index, Rankin score, length of hospital stay up to 1 year after stroke | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was carried out in blocks of 10, with numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐specified outcome data reported |
Huaihua 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People (292 men) with acute ischaemic stroke, randomised on admission Age 38 to 79 years (mean age 59.2 years) |
|
| Interventions | Comprehensive stroke unit within neurology department (n = 324) vs general medical ward (n = 73) | |
| Outcomes | Death or poor outcome at 1 year Functional ability at 1 year, but scale used not clear |
|
| Notes | Limited translated data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" Numbers in intervention group much greater than in control group |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Hunan 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People (163 men: 61.2%) with acute stroke; timing of randomisation unclear Mean age in intervention group: 62.3 years (SD 10.7); mean age in control group: 61.2 years (SD 11.8) |
|
| Interventions | Stroke unit with integrated traditional Chinese medicine (n = 139) vs Western medicine stroke unit (n = 127) | |
| Outcomes | Death and NIHSS, Barthel Index and mRS at 90 days Length of stay |
|
| Notes | Limited translated data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Illinois 1966.
| Study characteristics | ||
| Methods | RCT with 3:2 allocation to intervention:control | |
| Participants | People with stroke up to 1 year after stroke onset Appropriate for rehabilitation service | |
| Interventions | Rehabilitation service (mixed rehabilitation unit) (n = 56) vs general medical ward (which had some specialist nursing input) (n = 35) Organised care provided for months if required | |
| Outcomes | Functional status and place of residence at end of follow‐up | |
| Notes | Intervention and control services not clearly defined No deaths reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fisher's table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not clearly pre‐specified |
Joinville 2003.
| Study characteristics | ||
| Methods | RCT by means of randomised numbers in the emergency room Blinded follow‐up | |
| Participants | Clinical stroke diagnosis (confirmed on CT scan) within 7 days of onset | |
| Interventions | Comprehensive stroke unit within neurology department (n = 35) vs conventional care in general medical ward | |
| Outcomes | Death, Rankin score, length of stay up to 6 months | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "List of randomized numbers available in the emergency room" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Kuopio 1985.
| Study characteristics | ||
| Methods | RCT Blinded assessment of outcome | |
| Participants | People with stroke within 7 days of stroke onset Able to tolerate intensive rehabilitation | |
| Interventions | Intensive rehabilitation in neurological rehabilitation unit (mixed rehabilitation ward) (n = 50) vs general ward (n = 45) Organised care provided for months if required | |
| Outcomes | Death, Lehman (disability) score, place of residence, total time in hospital up to 1 year after stroke | |
| Notes | Majority of people screened failed to meet inclusion criteria for the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised using sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not clearly pre‐specified |
Manchester 2003.
| Study characteristics | ||
| Methods | RCT Telephone randomisation and blinded follow‐up | |
| Participants | People with acute stroke within 5 days of symptoms No recent myocardial infarction or fracture | |
| Interventions | Mobile stroke team (stroke physician, therapist) in 2 acute hospitals provided early assessment and advice to staff, co‐ordinated early therapy input, encouraged guideline adherence Controls received usual medical ward‐based care | |
| Outcomes | Death, institutional care, dependency, simple questions, Nottingham extended ADL score, Frenchay Aphasia Screening Test, EuroQol, Hospital Anxiety and Depression Scale Recorded up to 12 months | |
| Notes | 5 intervention and 4 control missing from final follow‐up 23 people underwent secondary randomisation in trial of early supported discharge team | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Offsite office using a computer generated schedule" |
| Allocation concealment (selection bias) | Low risk | "Allocated using a simple computer generated procedure ... initially and then in the later stages a minimisation procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportionately small; similar numbers missing from intervention and control groups at 12 months |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Montreal 1985.
| Study characteristics | ||
| Methods | RCT Blinded assessment of outcome | |
| Participants | Unselected people with stroke within 7 days of stroke onset | |
| Interventions | Mobile stroke team (dedicated stroke unit) (n = 65) vs conventional care on general medical ward (n = 65) Study ended at 6 weeks post stroke | |
| Outcomes | Death, Barthel Index, place of residence, length of initial hospital stay up to 6 weeks after stroke | |
| Notes | Short follow‐up period 1 intervention and 3 control patients lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were stratified ... "block randomization within each stratum" |
| Allocation concealment (selection bias) | Low risk | "Two series of numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants (1 intervention; 2 control) removed from study due to non‐stroke diagnosis following randomisation 1 additional participant not admitted from the emergency room |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐specified outcomes reported |
New South Wales 2014.
| Study characteristics | ||
| Methods | RCT: prospective, single‐blind, randomised controlled trial across 4 hospitals | |
| Participants | People with stroke (ischaemic and haemorrhagic) on day 1 of admission if vacant beds existed in both acute and rehabilitation hospitals and person had an acute stroke within previous 24 to 48 hours with sufficient neurological impairment and disability to require ongoing rehabilitation | |
| Interventions | The 'intervention' was the 'early' commencement of a rehabilitation process, with a significant portion of acute care to be spent in a rehabilitation setting Traditional stroke care (TSC): participants were admitted into an acute stroke unit (ASU) and were transferred to a rehabilitation unit at the end of their acute stroke phase (after completion of investigations and acute treatment, and when medically stable as per usual practice). Therefore participants were cared for in 2 different stages (acute and rehabilitation) by different nursing and allied health teams Comprehensive stroke care (CSC): participants were pressed to be transferred to a rehabilitation bed, aiming within 24 to 48 hours after arrival at ASU (or the next working day if weekend). This occurred when participants were still in acute stroke phase and might require attention to acute medical problems should they arise. Hence it was not equivalent to early transfer to a rehabilitation unit. Participants in the CSC arm were cared for by the same nursing and allied health team for a larger portion of their hospital stay All standard and best possible care was given to participants in both arms and the same treatment interventions were available to participants admitted to either arm (with the exception of rehabilitation process allowed to happen earlier after faster arrival in rehabilitation setting in the CSC arm) |
|
| Outcomes | Death FIM at discharge and at 3 months Modified Rankin score at discharge FIM efficiency (11) (change in FIM score ÷ total LOS) between participants who received CSC and those who received TSC. The FIM efficiency is an indicator of the rate of functional improvement per day of hospital stay Total hospital length of stay (acute and rehabilitation units combined) All FIM assessments were performed by the same research officer who had passed training sessions in performing the assessment tool (i.e. 1 research officer for each pair of acute rehabilitation units/hospitals) Length of stay for all participants was decided by the team caring for the participants, and information was extracted from medical records and counter‐checked with the team by research officers upon discharge of participants |
|
| Notes | Numbers independent at 3 months calculated as number FIM > 110 calculated from mean and SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was generated centrally by a biostatistician using a computer software program Stata11 (StataCorp LP, College Station, TX, USA)" |
| Allocation concealment (selection bias) | Low risk | "The generated results were concealed and stored locally. Clinicians at the rehabilitation services were not informed as to whether a patient was randomized into the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Clinicians at the rehabilitation services were not informed as to whether a patient was randomized into the study" "All standard and best possible care was given to participants in both arms, and the same treatment interventions were available to patients admitted to either arm" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The research officers who conducted baseline measures and subsequent FIM assessments at discharge and 90 days telephone follow‐up interview were also blind to group allocation" "The rehabilitation teams were blind to the group allocation of the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 4/47 (9%) participants withdrew |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
New York 1962.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with stroke up to 2 months after stroke Appropriate for rehabilitation centre | |
| Interventions | Mixed rehabilitation team working in rehabilitation centre or attending participants in other wards (n = 42) vs programme of care in general ward (n = 40) that had some specialist nursing input Organised care provided for months if required | |
| Outcomes | Functional status and place of residence at end of follow‐up (approximately 1 year) | |
| Notes | No deaths reported Minor anomaly in published data table Not clear how many participants were managed in a peripatetic way | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly drawn unmarked envelopes |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Newcastle 1993.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Stroke patients within 3 days of stroke onset | |
| Interventions | Mixed rehabilitation ward in geriatric medicine department (n = 34) vs general medical ward (n = 33) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, Rankin score, place of residence, length of stay in hospital up to 6 months after stroke | |
| Notes | Majority of patients screened failed to meet trial inclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Stratified based on continence and then randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Nottingham 1996.
| Study characteristics | ||
| Methods | RCT with 5:4 allocation of intervention:control Blinded assessment of outcome | |
| Participants | Patients with stroke at 2 weeks after stroke onset Able to participate actively in rehabilitation | |
| Interventions | Stroke rehabilitation ward in department of geriatric medicine (n = 176) vs conventional care in geriatric medical (mixed rehabilitation) ward (n = 63) or general medical ward (n = 76) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, Nottingham Health Profile, length of hospital stay up to 1 year after stroke | |
| Notes | Some cross‐over from general medical ward to geriatric medicine department 3 intervention and 4 control participants lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified based on admission ward ... then randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Small numbers (3 intervention; 4 control) lost to follow‐up Some secondary outcome assessments not completed or partially completed; this varied between groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Nottingham 1996 (GMW).
| Study characteristics | ||
| Methods | RCT Subgroup of Nottingham (stroke unit vs general medical ward) | |
| Participants | People with stroke at 2 weeks after stroke onset Able to participate actively in rehabilitation | |
| Interventions | Stroke rehabilitation ward in department of geriatric medicine (n = 78) vs conventional care in geriatric medical (mixed rehabilitation) ward (n = 63) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, Nottingham Health Profile, length of hospital stay up to 1 year after stroke | |
| Notes | Some cross‐over from general medical ward to geriatric medicine department | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified based on admission ward ... then randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some secondary outcome assessments not completed or partially completed; this varied between groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Nottingham 1996 (MRW).
| Study characteristics | ||
| Methods | RCT Subgroup of Nottingham (stroke unit vs mixed rehabilitation ward) | |
| Participants | People with stroke at 2 weeks after stroke onset Able to participate actively in rehabilitation | |
| Interventions | Stroke rehabilitation ward in department of geriatric medicine (n = 98) vs conventional care in general medical ward (n = 76) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, Nottingham Health Profile, length of hospital stay up to 1 year after stroke | |
| Notes | Some cross‐over from general medical ward to geriatric medicine department | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified based on admission ward... then randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some secondary outcome assessments not completed or partially completed; this varied between groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Orpington 1993.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with stroke who had survived for 2 weeks Suitable for transfer to rehabilitation ward | |
| Interventions | Stroke rehabilitation ward (n = 124) vs conventional care in geriatric (mixed rehabilitation unit) (n = 73) or general medical (n = 48) ward Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, length of initial hospital stay at end of follow‐up 2 intervention and 5 control patients lost to follow‐up |
|
| Notes | Variable duration of follow‐up (hospital discharge) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised with the use of Geigy table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was computerized" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 intervention and 5 control participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Orpington 1993 (GMW).
| Study characteristics | ||
| Methods | RCT Subgroup of Orpington 1993 (stroke unit vs general medical ward) | |
| Participants | People who survived stroke for 2 weeks Suitable for transfer to rehabilitation ward | |
| Interventions | Stroke rehabilitation ward (n = 53) vs conventional care in general medical (n = 48) ward Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, length of initial hospital stay at end of follow‐up | |
| Notes | Stroke severity subgroup data inferred from distribution in whole group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised with the use of Geigy table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was computerized" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 intervention and 5 control participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Orpington 1993 (MRW).
| Study characteristics | ||
| Methods | RCT Subgroup of Orpington 1993 (stroke unit vs mixed rehabilitation ward) | |
| Participants | People who survived stroke for 2 weeks Suitable for transfer to rehabilitation ward | |
| Interventions | Stroke rehabilitation ward (n = 71) vs conventional care in geriatric (mixed rehabilitation) ward (n = 73) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, length of initial hospital stay at end of follow‐up | |
| Notes | Stroke severity subgroup data inferred from distribution in whole group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised with the use of Geigy table of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was computerized" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 intervention and 5 control participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Orpington 1995.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People who had a poor prognosis 2 weeks after stroke Suitable for transfer to rehabilitation ward | |
| Interventions | Stroke rehabilitation ward in geriatric medicine department (n = 36) vs general medical ward (n = 37) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, length of hospital stay at end of follow‐up | |
| Notes | Variable duration of follow‐up (hospital discharge) 2 control participants lost to follow‐up; assumed to be alive and independent (ITT analysis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Were randomized" "The process of randomization was not limited by bed availability" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Orpington 2000.
| Study characteristics | ||
| Methods | RCT Blinded outcome assessment | |
| Participants | People with acute stroke (meeting WHO definition of stroke) from a community stroke register Intermediate stroke severity | |
| Interventions | 3‐arm comparison of:
|
|
| Outcomes | Death, dependency (Barthel Index), place of residence, length of stay, resource use up to 12 months 3 control participants lost to follow‐up |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Unstratified ... using the block randomization technique .... computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | "Allocation schedule prepared using computer generated random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 control participants lost to follow‐up at 12 months |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Perth 1997.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with acute stroke within 7 days of stroke onset | |
| Interventions | Comprehensive stroke ward (dedicated stroke unit) (n = 29) vs general medical ward (n = 30) Organised care provided for months if required | |
| Outcomes | Death, Barthel Index, place of residence, length of hospital stay up to 6 months after stroke | |
| Notes | Most people screened did not enter trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Outcomes not clearly pre‐specified but all expected outcomes reported |
Svendborg 1995.
| Study characteristics | ||
| Methods | RCT by means of sealed envelopes (stratified by age and side of lesion) | |
| Participants | People with acute stroke patients (within 8 days of symptoms) meeting WHO diagnostic criteria | |
| Interventions | Comprehensive stroke ward (n = 31) vs conventional care in general medical ward (n = 34) | |
| Outcomes | Death, dependency (Rankin score), place of residence, length of hospital stay at 6 months after randomisation | |
| Notes | Staffing levels were higher in the stroke unit group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Translation ‐ randomised by the envelope method (drawing lots), stratified by age and side of lesion |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No obvious missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Tampere 1993.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with acute stroke within 7 days of stroke (usually earlier) | |
| Interventions | Acute (semi‐intensive) stroke ward in neurology department (n = 98) vs conventional care in neurology department (mixed rehabilitation unit) (n = 113) Organised care provided for approximately 1 week only | |
| Outcomes | Death, Rankin score, place of residence, length of hospital stay up to 1 year after stroke 1 intervention and 1 control participant removed due to non‐stroke diagnosis |
|
| Notes | Short duration (1 week) in stroke unit before transfer to conventional service | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed with the aid of a table of random numbers" "Randomly assigned using serially numbered, sealed, envelopes" |
| Allocation concealment (selection bias) | Low risk | "Serially numbered, sealed, envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in intervention group and 1 participant in control group removed due to incorrect diagnosis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
Trondheim 1991.
| Study characteristics | ||
| Methods | RCT | |
| Participants | People with stroke within 7 days (usually within 24 hours) of stroke onset Exclusion of deeply unconscious patients and those previously residing in a nursing home | |
| Interventions | Comprehensive stroke ward (dedicated stroke unit) (n = 110) vs general medical ward (n = 110) Organised care provided for a maximum of 6 weeks | |
| Outcomes | Death, Barthel Index, place of residence, length of stay in hospital or institution up to 1 year after stroke | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned ... using serially numbered sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Serially numbered sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Difficult to conceal |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Both blinded and open assessments available for 50% of participants at 52 weeks; open assessments available for only 50% Correlation between blinded and open was high, but risk of bias remains unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
ADL: activity of daily living. CT: computerised tomography. FIM: Functional Independence Measure. GMW: general medical ward. ITT: intention‐to‐treat. LOS: length of stay. mRS: modified Rankin Scale. MRW: mixed rehabilitation ward. NIHSS: National Institutes of Health Stroke Scale. OHS: Oxford Handicap Scale. RCT: randomised controlled trial. SD: standard deviation. SPSS: Statistical Package for the Social Sciences. TIA: transient ischaemic attack. WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abissi 1995 | Trial tested a care plan protocol only No other aspect of organisation was under evaluation |
| Akhtar 2015 | Not an RCT |
| Al‐Qahtany 2014 | Not an RCT |
| Asplund 2000 | Trial of a geriatric assessment unit |
| Cavallini 2003 | Quasi‐randomised treatment allocation |
| Davis 2000 | Intervention and control arms of trial were treated within the same stroke unit |
| Di Lauro 2003 | Intervention and control arms of trial were treated within the same stroke unit |
| Diagana 2008 | Quasi‐randomised treatment allocation |
| Durastanti 2005 | Quasi‐randomised treatment allocation |
| Felix 2016 | Trial of stroke education |
| Fu 2006 | Not an RCT |
| HAMLET 2009 | Does not report outcomes for different medical treatment arms |
| Hamrin 1982 | Quasi‐randomised treatment allocation |
| He 2014 | Not a fully randomised cluster‐RCT |
| Inoue 2013 | Not an RCT |
| Janssen 2014 | Trial of enriched environment |
| Koton 2005 | Treatment allocated by selection criteria |
| Langhorne 2001 | Study tested a care plan protocol only No other aspect of organisation was under evaluation |
| Middleton 2006 | Care pathway study only |
| Middleton 2018 | Care pathway study |
| Moloney 1999 | Care pathway study only |
| Pappa 2009 | Non‐randomised |
| Patel 2000 | Quasi‐randomised treatment allocation |
| Pearson 1988 | No available outcome data |
| Rai 2016 | Not a full RCT |
| Raiborirug 2017 | Not an RCT |
| Ricauda 2004 | Trial comparing home care team vs general medical ward |
| Ronning 1998 | Quasi‐randomised treatment allocation |
| Ronning 1998a | A portion of the data was collected retrospectively All prospective data are included in the Akershus study (Ronning 1998) |
| Ronning 1998b | Comparison of stroke rehabilitation ward vs discharge to community‐based stroke rehabilitation |
| Shiraishi 2004 | Non‐randomised treatment allocation |
| Silva 2004 | Treatment allocated by the study neurologist |
| Stone 1998 | No available outcome data |
| Strand 1985 | Quasi‐randomised treatment allocation |
| Von Arbin 1980 | Quasi‐randomised treatment allocation |
| Walter 2005 | Non‐randomised treatment allocation |
| Wang 2004 | No available outcome data |
| Yagura 2005 | Quasi‐randomised treatment allocation |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Anhui 2008.
| Methods | RCT |
| Participants | People with acute stroke |
| Interventions | "Standardised tertiary rehabilitation" (n = 51) vs usual inpatient care (n = 51) |
| Outcomes | Functional outcome (unknown scale) and quality of life (WHOQOL‐BREF) at 1, 3, and 6 months Cost analysis |
| Notes | Currently no useable data |
China (Hao) 2010.
| Methods | Possible RCT |
| Participants | People with pneumonia (n = 159) after acute stroke (within 2 weeks) |
| Interventions | Management in comprehensive stroke unit versus general ward Allocated 'treatment' group depended on which ward the person was in when pneumonia developed |
| Outcomes | Death, NIHSS, Barthel Index at 21 days Length of stay Cost analysis |
| Notes | Method of randomisation unclear |
China (Pei) 2011.
| Methods | RCT |
| Participants | People with stroke (n = 236 ) |
| Interventions | Randomly assigned to organised stroke care model with integrated Chinese medicine (n = 121) vs traditional care model (n = 115) |
| Outcomes | Death, NIHSS, Barthel Index, OHS score at 21 days |
| Notes | Currently no useable data |
China (Wang) 2008.
| Methods | RCT |
| Participants | People with 'acute cerebral infarction' |
| Interventions | Randomly assigned to stroke rehabilitation unit group (n = 77) vs ordinary care group (n = 73) |
| Outcomes | NIHSS Barthel Index (duration of follow‐up unclear) Length of stay |
| Notes | ‐ |
China (Wu) 2007.
| Methods | RCT |
| Participants | 2367 people with acute stroke |
| Interventions | Randomly assigned to organised stroke ward vs general ward |
| Outcomes | Death, 'non‐recovery', and 'improvement' over 5 years |
| Notes | Currently no useable data |
Haikou 2007.
| Methods | RCT |
| Participants | People with acute ischaemic stroke randomised within 1 week |
| Interventions | Randomised to extended stroke unit vs general medical ward for 3 weeks |
| Outcomes | Discharge Barthel Index and NIHSS |
| Notes | Currently no useable data |
Shanghai 2006.
| Methods | RCT |
| Participants | Cerebral stroke from 22 hospitals |
| Interventions | "Standardised tertiary rehabilitation" vs routine care |
| Outcomes | Functional recovery (unknown scale) Cost‐effectiveness analysis |
| Notes | Currently no useable data |
NIHSS: National Institutes of Health Stroke Scale. OHS: Oxford Handicap Scale. RCT: randomised controlled trial. WHOQOL‐BREF: World Health Organization Quality of Life Project.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐OCH‐09000335.
| Study name | A study of the stroke unit of traditional Chinese and Western medicine in the treatment of ischaemic stroke |
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Starting date | ‐ |
| Contact information | Qiujuan Zhang; zqiyyy@hotmail.com |
| Notes | Yueyang Hospital, Shanghai |
China (Wang) 2015.
| Study name | Rationale and design of a cluster‐randomised multifaceted intervention trial to improve stroke care quality in China: the GOLDEN BRIDGE‐Acute Ischemic Stroke |
| Methods | Cluster‐RCT |
| Participants | 40 hospitals in China |
| Interventions | Multi‐faceted quality improvement intervention (experimental group) or routine standard of care (control group) |
| Outcomes | Measure of adherence to evidence‐based performance measures: in‐hospital death; new vascular event; disability; all‐cause death at 3, 6, and 12 months after initial symptom onset |
| Starting date | ‐ |
| Contact information | Wang Yilong, Tiantan Clinical Trial and Research Center for Stroke, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; China National Clinical Research Center for Neurological Diseases, Beijing, China |
| Notes | ClinicalTrials.gov/NCT02212912 |
NCT00544622.
| Study name | Structured stroke management improves outcomes at 6 months |
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Starting date | ‐ |
| Contact information | ‐ |
| Notes | Kantonsspital Baden |
NCT00843765.
| Study name | Efficiency study of traditional Chinese medicine (TCM) versus Western medicine (WM) on ischaemic stroke |
| Methods | ‐ |
| Participants | ‐ |
| Interventions | ‐ |
| Outcomes | ‐ |
| Starting date | ‐ |
| Contact information | ‐ |
| Notes | Dongzhimen Hospital and Beijing Tiantan Hospital |
Russia 2017.
| Study name | Development of medical rehabilitation in Russia (DOME): rehabilitation in stroke units and rehabilitation centres |
| Methods | Large clinical trial |
| Participants | Acute stroke |
| Interventions | |
| Outcomes | Recovery of functions, activity, and participation assessed with modified Rankin scale (mRS) |
| Starting date | |
| Contact information | G Ivanova, Pirogov Russian National Medical Research University, Department of Medical and Social Rehabilitation, Ministry of Health of Russia, Moscow, Russian Federation |
| Notes | ClinicalTrials.gov Identifier: NCT02793934 |
RCT: randomised controlled trial.
Differences between protocol and review
This review update incorporated four new features. First, a minor revision of the data presentation such that comparisons are made at three levels: (1) organised inpatient (stroke unit) care versus any conventional service, (2) organised inpatient (stroke unit) care in a stroke ward versus care in a general medical ward, and then (3) organised inpatient (stroke unit) care in a stroke ward versus an alternative form of organised care (e.g. mobile stroke team).
Second, changes in reporting expectations required us to define a single primary outcome. We have therefore used 'poor outcome' ‐ death or dependency or requiring institutional care (if dependency data were not available). This allowed us to keep the primary focus of the review while optimising the quantity of data available.
Third, we added an exploratory network meta‐analysis (NMA) at the first level of comparison (see Comparison 1 above) to complement the previous analysis, which used a series of individual service comparisons. Both approaches are discussed here.
Finally, we have included a 'Summary of findings' table (plus GRADE) for both conventional and network meta‐analyses.
Contributions of authors
Peter Langhorne initiated and co‐ordinated the original review project, was principal grant holder, and revised the updated report.
For this version of the review, Peter Langhorne and Samantha Ramachandra selected trials and extracted data. Peter Langhorne performed updated literature searches, re‐analysed the data, and re‐drafted the manuscript.
For the previous version of the review, Patricia Fearon performed the updated literature searches, selected trials and extracted data, assisted with data analysis, and re‐drafted the manuscript.
The following collaborators provided original data, advice, and comment, and assisted with re‐drafting of the report: C Blomstrand (Goteborg, Sweden); NL Cabral (Joinville, Brazil); A Cavallini (Pavia, Italy); P Dey (Manchester, England); E Hamrin (Uppsala, Sweden); Graeme J Hankey (Perth, Australia); B Indredavik (Trondheim, Norway); L Kalra (Orpington, England); M Kaste (Helsinki, Finland); SO Laursen (Svendborg, Denmark); RH Ma (Beijing, China); N Patel (Cape Town, South Africa); H Rodgers (Newcastle, England); MO Ronning (Akershus, Norway); J Sivenius (Kuopio, Finland); G Sulter (Groningen, Netherlands); A Svensson (Goteborg, Sweden); K Vemmos (Athens, Greece); S Wood‐Dauphinee (Montreal, Canada); and H Yagura (Osaka, Japan).
Previous versions of the review also received data, advice, and comment from K Asplund (Umea, Sweden); P Berman (Nottingham, England); M Britton (Stockholm, Sweden); J Douglas (Administrator); T Erila (Tampere, Finland); M Garraway (Edinburgh, Scotland); M Ilmavirta (Tampere, Finland); R Stevens (Dover, England); SP Stone (London, England); and B Williams (Glasgow, Scotland).
Important contributions were also made by the following individuals, who supplied useful information and comment: D Deleo (Perth, Australia); A Drummond (Nottingham, England); R Fogelholm (Jyvaskyla, Finland); N Lincoln (Nottingham, England); H Palomaki (Helsinki, Finland); J Slattery (London, England); T Strand (Umea, Sweden); CP Warlow (Edinburgh, Scotland); and L Wilhelmsen (Goteborg, Sweden).
Sources of support
Internal sources
University of Glasgow, UK
University of Edinburgh, UK
External sources
Chest, Heart and Stroke Scotland, UK
Declarations of interest
Most of the Stroke Unit Trialists Collaboration members carried out trials that are included in the review.
Peter Langhorne: none known.
Samantha Ramachandra: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Athens 1995 {published and unpublished data}
- Pappa T, Xynos K, Theodorakis M, Salliaris M, Kostopoulos K, Vemmos K. The impact of acute stroke unit treatment on long term survival. Cerebrovascular Diseases 2007;23 Suppl 2:64. [Google Scholar]
- Spengos K, Tsivgoulis G, Manios E, Papamichael C, Konstastinopoulou A, Vemmos K. Which patients benefit most from treatment in a stroke unit? Stroke 2004;35(1):294.
- *.Vemmos K, Takis K, Madelos D, Synetos A, Volotasiou V, Tzavellas H. Stroke unit treatment versus general medical wards: long term survival. Cerebrovascular Diseases 2001;11 Suppl 4:8. [Google Scholar]
Beijing 2004 {published data only}
- *.Ma RH, Wang YJ, Qu H, Yang ZH. Assessment of the early effectiveness of a stroke unit in comparison to the general ward. Chinese Medical Journal 2004;117(6):852-5. [PubMed] [Google Scholar]
- Ma RH, Wang YJ, Zhao XQ, Wang CX, Yang ZH, Qu H. The impact of stroke unit on early outcome of cerebral infarction patients. Zhonghua Nei Ke Za Zhi 2004;43(3):183-5. [PubMed] [Google Scholar]
- Wang YJ, Ma RH, Zhao XQ, Xing D, Cai J, Li L, et al. Comparison of early effects between stroke unit and general ward. Chinese Journal of Clinical Rehabilitation 2005;8(19):3716-7. [Google Scholar]
Birmingham 1972 {published data only}
- Peacock PB, Riley CHP, Lampton TD, Raffel SS, Walker JS. Trends in Epidemiology: The Birmingham Stroke, Epidemiology and Rehabilitation Study. Springfield, IL (USA): Thomas, 1972. [Google Scholar]
Dover 1984 {published and unpublished data}
- Stevens RS, Ambler NR, Warren MD. A randomised controlled trial of a stroke rehabilitation ward. Age and Ageing 1984;13:65-75. [DOI] [PubMed] [Google Scholar]
Dover 1984 (GMW) {published and unpublished data}
Dover 1984 (MRW) {published and unpublished data}
Edinburgh 1980 {published and unpublished data}
- Garraway WM, Akhtar AJ, Hockey L, Prescott RJ. Management of acute stroke in elderly: follow up of a controlled trial. British Medical Journal 1980;281:827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goteborg‐Ostra 1988 {unpublished data only}
- Svensson A, Harmsen P, Wilhelmsen L. Goteborg Ostra Stroke Unit Trial. Personal communication .
Goteborg‐Sahlgren 1994 {published and unpublished data}
- Claesson L, Gosman-Hedstrom G, Fagerberg B, Blomstrand C. Hospital re-admissions in relation to acute stroke unit care versus conventional care in elderly patients the first year after stroke: the Goteborg 70+ stroke study. Age and Ageing 2003;32:109-13. [DOI] [PubMed] [Google Scholar]
- Claesson L, Gosman-Hedstrom G, Johannesson M, Fagerberg B, Blomstrand C. Resource utilization and costs of stroke unit care integrated in a care continuum: a 1-year controlled, prospective, randomized study in elderly patients. The Goteborg 70+ stroke study. Stroke 2000;31:2569-77. [DOI] [PubMed] [Google Scholar]
- Claesson L, Gosman-Hedstrom G, Johannesson M, Fagerberg B, Blomstrand C. Stroke unit care - effects on ADL, health-related quality of life and health economy. Cerebrovascular Diseases 1999;9 Suppl 1:115. [Google Scholar]
- Fagerberg B, Blomstrand C. Do stroke units save lives? Lancet 1993;342:992. [PubMed]
- *.Fagerberg B, Claesson L, Gosman-Hedstrom G, Blomstrand C. Effect of acute stroke unit care integrated with care continuum versus conventional treatment: A randomized 1-year study of elderly patients. The Goteborg 70+ stroke study. Stroke 2000;31:2578-84. [DOI] [PubMed] [Google Scholar]
- Gosman-Hedstrom G, Claesson L, Blomstrand C. Consequences of severity at stroke onset for health-related quality of life (HRQL) and informal care: a 1-year follow-up in elderly stroke survivors. Archives of Gerontology and Geriatrics 2008;47:79-91. [DOI] [PubMed] [Google Scholar]
Groningen 2003 {published data only}
- Sulter G, Elting JW, Langedijik M, Maurits NM, De Keyser J. Admitting acute ischaemic stroke patients to a stroke care monitoring unit versus a conventional stroke unit: a randomized pilot study. Stroke 2003;34:101-4. [DOI] [PubMed] [Google Scholar]
Guangdong 2008 {published data only}
- Yang N, LI G, Zhang Z, Wang B. Clinical efficacy observing of the treatment in stroke unit of integrated TCM and western medicine on patients with ischaemic stroke in acute stage. Chinese Archives of Traditional Chinese Medicine 2008;26:2750-3. [Google Scholar]
Guangdong 2009 {published data only}
- Yang N, Zheng L. Cost benefit and effect analysis for patients with ischaemic stroke on stroke unit of integration traditional Chinese and western medicine. Chinese Archives of Traditional Chinese Medicine 2009;27:1331-3. [Google Scholar]
Helsinki 1995 {published and unpublished data}
- *.Kaste M, Palomaki H, Sarna S. Where and how should elderly stroke patients be treated? A randomised trial. Stroke 1995;26:249-53. [DOI] [PubMed] [Google Scholar]
- Kaste M, Palomaki H. By whom should elderly stroke patients be treated? Stroke 1992;23(1):163. [DOI] [PubMed] [Google Scholar]
- Kaste M, Palomaki H. Who should treat elderly stroke patients? Journal of Stroke and Cerebrovascular Diseases 1992;2 Suppl 1:S27. [Google Scholar]
Huaihua 2004 {published data only}
- Liao Y, Zeng JS, Zhou J, Xie CM, Yang DY, Liu SX, et al. Pattern and superiority of stroke unit in treating patients with stroke. Chinese Journal of Clinical Rehabilitation 2004;8:6014-5. [Google Scholar]
Hunan 2007 {published data only}
- Li X, Wu Q, Liu W. The effects of the treatment in stroke unit of integrated therapy TCM and WM on patients with stroke in acute stage. Journal of Emergency in Traditional Chinese Medicine 2007;16(4):381-3. [Google Scholar]
Illinois 1966 {published data only}
- Gordon EE, Kohn KH. Evaluation of rehabilitation methods in the hemiplegic patient. Journal of Chronic Diseases 1966;19:3-16. [DOI] [PubMed] [Google Scholar]
Joinville 2003 {published data only}
- Cabral NL, Moro C, Silva GR, Scola RH, Werneck LC. Study comparing the stroke unit outcome and conventional ward treatment: a randomised study in Joinville, Brazil. Arquivos de Neuro-Psiquatria 2003;61(2-A):188-93. [DOI] [PubMed] [Google Scholar]
Kuopio 1985 {published and unpublished data}
- Sivenius J, Pyorala K, Heinonen OP, Salonen JT, Reikkinen P. The significance of intensity of rehabilitation after stroke - a controlled trial. Stroke 1985;16:928-31. [DOI] [PubMed] [Google Scholar]
Manchester 2003 {published and unpublished data}
- *.Dey P, Woodman M, Gibbs A, Steele R, Stocks SJ, Wagstaff S, et al. Early assessment by a mobile stroke team: a randomised controlled trial. Age and Ageing 2005;34:331-8. [DOI] [PubMed] [Google Scholar]
- Dey P, Woodman M, Gibbs A. Fast track assessment and rehabilitation for stroke patients (FASTAR). Report (Project number RD0/28/1/02) 2003.
Montreal 1985 {published and unpublished data}
- Wood-Dauphinee S, Shapiro S, Bass E, Fletcher C, Georges P, Hensby V, et al. A randomised trial of team care following stroke. Stroke 1984;5:864-72. [DOI] [PubMed] [Google Scholar]
Newcastle 1993 {published and unpublished data}
- Aitken PD, Rodgers H, French JM, Bates D, James OFW. General medical or geriatric unit care for acute stroke? A controlled trial. Age and Ageing 1993;22 Suppl 2:4-5. [Google Scholar]
New South Wales 2014 {published data only}
- Chan D, Levi C, Pollack M, Cordato D, O'Rourke F, Chen J, et al. A randomised controlled trial to evaluate a model of comprehensive stroke care. International Journal of Stroke 2014;9:13. [DOI] [PubMed] [Google Scholar]
- *.Chan DKY, Levi C, Cordato D, O’Rourke F, Chen DL, Redmond H, et al. Health service management study for stroke: a randomized controlled trial to evaluate two models of stroke care. International Journal of Stroke 2014;9:400-5. [DOI] [PubMed] [Google Scholar]
New York 1962 {published data only}
- Feldman DJ, Lee PR, Unterecker J, Lloyd K, Rusk HA, Toole A. A comparison of functionally orientated medical care and formal rehabilitation in the management of patients with hemiplegia due to cerebrovascular disease. Journal of Chronic Diseases 1962;15:297-310. [DOI] [PubMed] [Google Scholar]
Nottingham 1996 {published and unpublished data}
- Drummond A, Lincoln N, Juby L. Effects of stroke unit on knowledge of stroke and experiences in hospital. Age and Ageing 2001;30:129-33. [Google Scholar]
- Drummond A, Pearson B, Lincoln NB, Berman P. Ten year follow up of a randomised controlled trial of care in a stroke rehabilitation unit. BMJ 2005;331:491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands SL, Lincoln NB, Drummond AER, Gladman J, Trescoli C. Five-year results of a randomized controlled trial of a stroke rehabilitation unit. Clinical Rehabilitation 1999;13(6):530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Juby LC, Lincoln NB, Berman P. The effect of a stroke rehabilitation unit on functional and psychological outcome. A randomised controlled trial. Cerebrovascular Diseases 1996;6:106-10. [Google Scholar]
- Lincoln NB, Husbands S, Trescoli C, Drummond AER, Gladman JRF, Berman P. Five year follow up of a randomised controlled trial of a stroke rehabilitation unit. BMJ 2000;320:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nottingham 1996 (GMW) {published and unpublished data}
- Juby LC, Lincoln NB, Berman P. The effect of a stroke rehabilitation unit on functional and psychological outcome. A randomised controlled trial. Cerebrovascular Diseases 1996;6:106-10. [Google Scholar]
Nottingham 1996 (MRW) {published and unpublished data}
- Juby LC, Lincoln NB, Berman P. The effect of a stroke rehabilitation unit on functional and psychological outcome. A randomised controlled trial. Cerebrovascular Diseases 1996;6:106-10. [Google Scholar]
Orpington 1993 {published and unpublished data}
- *.Kalra L, Dale P, Crome P. Improving stroke rehabilitation: a controlled study. Stroke 1993;24:1462-7. [DOI] [PubMed] [Google Scholar]
- Kalra L. Inpatient rehabilitation for elderly stroke patients. Journal of the American Geriatrics Society 1994;42:1027. [DOI] [PubMed] [Google Scholar]
Orpington 1993 (GMW) {published and unpublished data}
- Kalra L, Dale P, Crome P. Improving stroke rehabilitation: a controlled study. Stroke 1993;24:1462-7. [DOI] [PubMed] [Google Scholar]
Orpington 1993 (MRW) {published and unpublished data}
- Kalra L, Dale P, Crome P. Improving stroke rehabilitation: a controlled study. Stroke 1993;24:1462-7. [DOI] [PubMed] [Google Scholar]
Orpington 1995 {published and unpublished data}
- Kalra L, Eade J. Role of stroke rehabilitation units in managing severe disability after stroke. Stroke 1995;26:2031-4. [DOI] [PubMed] [Google Scholar]
Orpington 2000 {published and unpublished data}
- Evans A, Harraf F, Donaldson N, Kalra L. Randomised controlled study of stroke unit care versus stroke team care in different stroke subtypes. Stroke 2002;33:449-55. [DOI] [PubMed] [Google Scholar]
- Evans A, Perez I, Harraf H, Melbourn A, Steadman J, Donaldson N, et al. Can differences in management processes explain different outcomes between stroke unit and stroke team care? Lancet 2001;358:1586-92. [DOI] [PubMed] [Google Scholar]
- Evans A, Perez I, Melbourn A, Steadman J, Kalra L. Alternative strategies in stroke: a randomised controlled trial of three strategies of stroke management and rehabilitation. Cerebrovascular Diseases 2000;10 Suppl 2:60. [Google Scholar]
- *.Kalra LL, Evans A, Perez I, Knapp M, Donaldson N, Swift C. Alternative strategies for stroke: a prospective randomised controlled trial. Lancet 2000;356:894-9. [DOI] [PubMed] [Google Scholar]
- Patel A, Knapp M, Perez I, Evans A, Kalra L. Alternative strategies for stroke care: cost-effectiveness analysis from a prospective randomised controlled trial. Cerebrovascular Diseases 2003;16 Suppl 4:101. [Google Scholar]
Perth 1997 {published and unpublished data}
- Hankey GJ, Deleo D, Stewart-Wynne EG. Stroke units: an Australian perspective. Australian and New Zealand Journal of Medicine 1997;27:437-8. [DOI] [PubMed] [Google Scholar]
Svendborg 1995 {published data only}
- Henriksen IO, Laursen SO. Acute stroke - treatment in a non-intensive stroke unit. Scandinavian Journal of Rehabilitation Medicine 1992;Suppl 26:153. [Google Scholar]
- *.Laursen SO, Henriksen IO, Dons U, Jacobsen B, Gundertofte L. Intensive rehabilitation following stroke: controlled pilot study. Ugeskr Laeger 1995;157:1996-9. [PubMed] [Google Scholar]
Tampere 1993 {unpublished data only}
- Ilmavirta M, Frey H, Erila T, Fogelholm R. Stroke outcome and outcome of brain infarction. A prospective randomised study comparing the outcome of patients with acute brain infarction treated in a stroke unit and in an ordinary neurological ward. In: Doctoral Thesis. Vol. 410; series A. Tampere: University of Tampere Faculty of Medicine, 1994. [Google Scholar]
Trondheim 1991 {published and unpublished data}
- Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Stroke unit treatment improves long-term quality of life: a randomized controlled trial. Stroke 1998;29:895-9. [DOI] [PubMed] [Google Scholar]
- Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Stroke unit treatment: 10 year follow-up. Stroke 1999;30:1524-7. [DOI] [PubMed] [Google Scholar]
- Indredavik B, Bakke F, Slordahl SA, Rokseth R, Haheim LL. Treatment in a combined acute and rehabilitation stroke unit. Which aspects are important? Stroke 1999;30:917-23. [DOI] [PubMed] [Google Scholar]
- *.Indredavik B, Bakke F, Solberg R, Rokseth R, Haahein LL, Home I. Benefit of stroke unit: a randomised controlled trial. Stroke 1991;22:1026-31. [DOI] [PubMed] [Google Scholar]
- Indredavik B, Fjaertoft H, Ekeberg RN, Loge A, Morch B. Benefit of an extended stroke unit service with early supported discharge. A randomised control trial. Stroke 2000;31:2989-94. [DOI] [PubMed] [Google Scholar]
- Indredavik B, Slordahl SA, Bakke F, Rokseth R, Haheim LL. Stroke unit treatment: long-term effects. Stroke 1997;28:1861-6. [DOI] [PubMed] [Google Scholar]
- Indredavik B, Slordahl SA, Bakke F. Stroke unit treatment - 10 years follow-up. Cerebrovascular Diseases 1999;9 Suppl 1:122. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abissi 1995 {published data only}
- Abissi CJ, Sepe E, Patiak C, Davis JN. Cerebral infarction: comparison of care with case-management to traditional care. Neurology 1995;45 Suppl 4:A240. [Google Scholar]
Akhtar 2015 {published data only}
- Akhtar N, Kamran S, D'Souza A, Imam Y, Bourke P, Joseph S, et al. Establishment of stroke ward results in immediate reduction of major complications. In: Neurology. Conference: 67th American Academy of Neurology Annual Meeting, AAN 2015. Vol. 84(Suppl 14). Washington, DC, USA, 2015.
Al‐Qahtany 2014 {published data only}
- Al-Qahtany AM, Ahmed A, Alamry A, Al Khathaami AM. The impact of the stroke unit on stroke outcomes in an academic medical center in Saudi Arabia. Cerebrovascular Diseases 2014;37 Suppl 1:457. [Google Scholar]
Asplund 2000 {published data only}
- Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, et al. Geriatric-based general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. Journal of the American Geriatrics Society 2000;48(11):1381-8. [DOI] [PubMed] [Google Scholar]
Cavallini 2003 {published and unpublished data}
- *.Cavallini A, Micieli G, Marcheselli S, Quaglini S. Role of monitoring in the management of acute ischaemic stroke patients. Stroke 2003;34(11):2599-603. [DOI] [PubMed] [Google Scholar]
- Cavallini A, Micieli G, Zambrelli E, Quaglini S, Rogoni C. Role of continuous physiological monitoring in the acute phase of stroke. Cerebrovascular Diseases 2001;11 Suppl 4:117. [Google Scholar]
Davis 2000 {published data only}
- Davis M, Chambers B, Birschel P. A pilot study of monitoring in acute stroke. Stroke 2000;31(11):2869. [Google Scholar]
- Davis M, Hollymann C, McGiven M, Chambers I, Egbuji J, Barer D. Physiological monitoring in acute stroke. Age and Ageing 1999;28 Suppl 1:45. [Google Scholar]
Diagana 2008 {published data only}
- Diagana M, Ould A, Salem B, N'Diaye M, LeCornet C, Quet F, et al. Impact of acute unit care improving post-stroke functionality outcomes in Noukachott, Mauritania. African Journal of Neurological Sciences 2008;27:38-46. [Google Scholar]
Di Lauro 2003 {published data only}
- Di Lauro A, Pellegrino L, Savastano G, Ferraro C, Fusco M, Balzarano F, et al. A randomised trial on the efficacy of intensive rehabilitation in the acute phase of ischemic stroke. Journal of Neurology 2003;250(10):1206-8. [DOI] [PubMed] [Google Scholar]
Durastanti 2005 {published data only}
- Durastanti L, Puca E, Angelantonio E, Di Lorenzano S, Falcou A, Gori MC, et al. Efficacy of acute ischemic stroke patient management in an emergency department stroke unit (EDSU). Cerebrovascular Diseases 2005;19 Suppl 2:57. [Google Scholar]
Felix 2016 {published data only}
- Felix C, Felix J, Harvey L, Rajan R, Iype T, Lindley R. KNIT (know stroke symptoms, use hotline number, intervene at stroke unit support system, act on time) study: evaluate running of a co-ordinated (Well-KNIT) stroke unit support system in Kerala, India. Cerebrovascular Diseases 2016;42 Suppl 1:56. [Google Scholar]
Fu 2006 {published data only}
- Fu XH, Wang H, Sun J, Sun HY, Song QY, Liu Y, Li H. Comprehensive therapeutic effect of the stroke rehabilitation unit in a medium-sized comprehensive community hospital. Neural Regeneration Research 2004;1(4):1673-74. [Google Scholar]
HAMLET 2009 {published data only}
- Hofmeijer J, den Worp HB, Amelink GJ, Algra A, Gijn J, Kapelle LJ. HAMLET hemicraniectomy after MCA infarction with life-threatening oedema trial (Abstract 11). In: Proceedings of the European Stroke Conference, 21-24 May, Valencia, Spain. 2003.
Hamrin 1982 {published and unpublished data}
- *.Hamrin E. Early activation after stroke: does it make a difference? Scandinavian Journal of Rehabilitation Medicine 1982;14:101-9. [PubMed] [Google Scholar]
- Hamrin E. One year after stroke: a follow-up of an experimental study. Scandinavian Journal of Rehabilitation Medicine 1982;14:111-6. [PubMed] [Google Scholar]
He 2014 {published data only}
- He M, Wang J, Gong L, Dong Q, Ji N, Xing H, et al. Community-based stroke system of care for Chinese rural areas. Stroke 2014;45:2385-90. [DOI] [PubMed] [Google Scholar]
Inoue 2013 {published data only}
- Inoue T, Fushimi K. Stroke care units versus general medical wards for acute management of stroke in Japan. Stroke 2013;44(11):3142-7. [DOI] [PubMed] [Google Scholar]
Janssen 2014 {published data only}
- Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, et al. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: a pilot non-randomized controlled trial. Disability Rehabilitation 2014;36(3):255-62. [DOI] [PubMed] [Google Scholar]
Koton 2005 {published data only}
- *.Koton S, Schwammenthal Y, Merzeliak O, Philips T, Tsabari R, Bruk B, et al. Effectiveness of establishing a dedicated acute stroke unit in routine clinical practice in Israel. Israel Medical Association Journal 2005;7:688-93. [PubMed] [Google Scholar]
- Koton S, Schwammenthal Y, Merzeliak O, Philips T, Tsabari R, Bruk B, et al. Management and outcome of acute stroke: differences between organised short-term acute stroke unit and general medical wards. Cerebrovacular Diseases 2005;19 Suppl 2:58. [Google Scholar]
Langhorne 2001 {published data only}
- Langhorne P, Fraser P, Wright F, Shields M, MacIntosh G, Stott D. Evaluation of an acute care protocol for stroke patients: a controlled clinical trial. Cerebrovascular Diseases 2001;11 Suppl 4:35. [Google Scholar]
Middleton 2006 {published data only}
- Middelton S. An outcomes approach to stroke care; the importance of teamwork and evidence-based nursing care. International Journal of Stroke 2012;7:224-6. [DOI] [PubMed] [Google Scholar]
- *.Middleton S, Levi C, Griffiths R, Grimshaw J, Ward J. Quality in Acute Stroke Care project: protocol for a cluster randomised control trial to evaluate the management of fever, sugar and swallowing after stroke. Journal of Internal Medicine 2006;36:A1-A14. [Google Scholar]
- Middleton S, Levi C, Ward J, Grimshaw J, Griffiths R, D'Este C, et al. Fever, hyperglycaemia and swallowing dysfunction management in acute stroke: a cluster randomised controlled trial of knowledge transfer. Implementation Science 2009;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton S, McElduff P, Ward J, Grimshaw J, Dale S, D'Este C, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia and swallowing dysfunction in acute stroke (QASC): a cluster randomised control trial. Lancet 2011;378:1699-706. [DOI] [PubMed] [Google Scholar]
Middleton 2018 {published data only}
- Middleton S, Considine J, Dale S, Cheung NW, McInnes E, Levi C, et al. Improving triage, treatment and transfer of patients with stroke in emergency departments: the T3 trial. Emergency Medicine Australasia 2018;30 Suppl 1:12. [Google Scholar]
Moloney 1999 {published data only}
- Moloney A, Critchlow B, Jones K. A multi-disciplinary care pathway in stroke - does it improve care? Age and Ageing 1999;28 Suppl 1:42-3. [Google Scholar]
Pappa 2009 {published data only}
- Pappa T, Spengos K, Skoufi M, Zafiriou I, Barbaressou Z, Peppa K, et al. Acute stroke unit improves long-term survival in very old stroke patients. Cerebrovascular Diseases 2009;27 Suppl 6:13. [Google Scholar]
Patel 2000 {unpublished data only}
- Patel N, Louw S, Zwarenstein M. Organised Care of Acute Stroke at Groot Schuur Hospital (MPhil in Epidemiology). Cape Town, South Africa: University of Cape Town, 2000. [Google Scholar]
Pearson 1988 {published data only}
- Pearson A, Durand I, Punton S. Effects of admission to a nursing unit. Australian Journal of Advanced Nursing 1988;6(1):38-42. [PubMed] [Google Scholar]
Rai 2016 {published data only}
- Rai N, Prasad K, Bhatia R, Vibha D, Singh MB, Rai VK, et al. Development and implementation of acute stroke care pathway in a tertiary care hospital in India: a cluster-randomized study. Neurology India 2016;64:S39-45. [DOI] [PubMed] [Google Scholar]
Raiborirug 2017 {published data only}
- Rajborirug K, Tumviriyakul H, Suwanno J. Effects of stroke unit care in acute ischemic stroke patient ineligible for thrombolytic treatment. Journal of the Medical Association of Thailand 2017;100(4):410-7. [PubMed] [Google Scholar]
Ricauda 2004 {published data only}
- Ricauda NA, Bo M, Molaschi M, Massaia M, Salerno D, Amati D, et al. Home hospitalization service for acute uncomplicated first ischemic stroke in elderly patients: a randomized trial. Journal of the American Geriatrics Society 2004;52:278-83. [DOI] [PubMed] [Google Scholar]
Ronning 1998 {published and unpublished data}
- Ronning OM, Guldvog B, Stavem K. The benefit of an acute stroke unit in patients with intracerebral haemorrhage: a controlled trial. Journal of Neurology Neurosurgery and Psychiatry 2001;70:631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ronning OM, Guldvog B. Stroke units versus general medical wards, II: neurological deficits and activities of daily living: a quasi-randomised controlled trial. Stroke 1998;29:586-90. [DOI] [PubMed] [Google Scholar]
- Stavem K, Ronning OM. Survival over 12 years following acute stroke: initial treatment in a stroke unit versus general medical ward. Acta Neurologica Scandinavica 2011;124(6):429-33. [DOI] [PubMed] [Google Scholar]
Ronning 1998a {published data only}
- Ronning OM, Guldvog B. Stroke units versus general medical wards, I: twelve- and eighteen-month survival. A randomized controlled trial. Stroke 1998;29:58-62. [DOI] [PubMed] [Google Scholar]
Ronning 1998b {published data only}
- Ronning OM, Guldvog B. Outcome of subacute stroke rehabilitation. A randomized controlled trial. Stroke 1998;29:779-84. [DOI] [PubMed] [Google Scholar]
Shiraishi 2004 {published data only}
- Shirashi N, Mizutani C, Menjho M, Deguchi A, Takase K, Hamaguchi H, et al. Comparison of daily living for a convalescent rehabilitation ward and general ward for stroke patients. Japanese Journal of Geriatrics 2004;41:646-52. [DOI] [PubMed] [Google Scholar]
Silva 2004 {published data only}
- *.Silva Y, Piugdemont M, Castellanos M, Serena J, Suner RM, Davalos A. Semi-intensive monitoring in acute stroke and long term outcome. Stroke 2002;33(1):411. [DOI] [PubMed]
- Silva Y, Puigdemont M, Castellanos M, Serena J, Suner RM, Garcia MM, et al. Semi-intensive monitoring in acute stroke and long term outcome. Cerebrovascular Diseases 2005;19(1):23-30. [DOI] [PubMed] [Google Scholar]
- Silva Y, Serena J, Castellanos M, Ramio L, Osuna T, Davalos A. Stroke units: the effect of continuous monitoring on clinical outcome. Cerebrovascular Diseases 2001;11 Suppl 4:122. [Google Scholar]
Stone 1998 {unpublished data only}
- Stone SP. A trial of stroke unit (SU) versus comprehensive geriatric service (CGS) management of all stroke patients. Neurorehabilitation and Neural Repair 1999;13(1):13. [Google Scholar]
Strand 1985 {published and unpublished data}
- *.Strand T, Asplund K, Eriksson S, Hagg E, Lithner F, Wester PO. A non-intensive stroke unit reduced functional disability and the need for long-term hospitalisation. Stroke 1985;16:29-34. [DOI] [PubMed] [Google Scholar]
- Strand T, Asplund K, Eriksson S, Hagg E, Lithner F, Wester PO. Stroke unit care - who benefits? Comparisons with general medical care in relation to prognostic indicators on admission. Stroke 1986;17:377-81. [DOI] [PubMed] [Google Scholar]
Von Arbin 1980 {published data only}
- Von Arbin M, Britton M, Faire U, Helmers C, Miah K, Murray V. A study of stroke patients treated in a non-intensive stroke unit or in general medical wards. Acta Medica Scandinavica 1980;208:81-5. [DOI] [PubMed] [Google Scholar]
Walter 2005 {published data only}
- Walter A, Seidel G, Thie A, Raspe HH, for the SSSH Study Group. German stroke units versus conventional care in acute ischemic stroke and TIA - a prospective study. Cerebrovascular Diseases 2005;19 Suppl 2:30. [Google Scholar]
Wang 2004 {published data only}
- Wang YJ, Gao XL, Ma RH, Wu D. Beijing Organized Stroke Care Study (BOSS). In: Proceedings of the 29th International Stroke Conference, San Diego, CA, USA. 5-7 February 2004.
Yagura 2005 {published data only}
- Yagura H, Miyai I, Suzuki T, Yanagihara T. Patients with severe stroke benefit most by interdisciplinary rehabilitation team approach. Cerebrovascular Diseases 2005;20:258-63. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Anhui 2008 {published data only}
- Ni C, Li C, Han R, Chen J, Sun H, Liu S. A randomised controlled trial of standardised tertiary rehabilitation after stroke. Journal of Rehabilitation Medicine 2008;Suppl 46:71. [Google Scholar]
China (Hao) 2010 {published data only}
- Hao JJ. Effect of comprehensive stroke unit on patients with pneumonia after acute stroke. Chinese Journal of Cerebrovascular Diseases 2010;7:120-3. [Google Scholar]
China (Pei) 2011 {published data only}
- Pei Z. Clinical research of organised stroke care model with integrated Chinese traditional and Western medicine in primary hospital. Chinese Journal of Contemporary Neurology and Neurosurgery 2011;11:221-5. [Google Scholar]
China (Wang) 2008 {published data only}
- Wang ZM, Wang P, Chen J, Luo DH, Shen WM. Application of stroke rehabilitation in municipal hospitals during the acute phase of cerebral infarction. Chinese Journal of Epidemiology 2008;29:724-5. [PubMed] [Google Scholar]
China (Wu) 2007 {published data only}
- Wu WL, Lu XL, Zheng MY, Liang W, Yao XL, Hu ZL. Impact of organised stroke unit on the therapeutic effect in stroke patients. Journal of Southern Medical University 2010;30:555-6. [PubMed] [Google Scholar]
Haikou 2007 {published data only}
- Su Q, Lin T, Wu Y, Cai M, Chen Z. Benefit of an extended stroke unit to acute cerebral infarction. Cerebrovascular Diseases 2007;24:490. [Google Scholar]
Shanghai 2006 {published data only}
- *.Hu YS. Standardized tertiary rehabilitation (STR) for stroke patients with hemiplegia in promoting the neurological function. Neurorehabilitation and Neural Repair 2006;20:163. [Google Scholar]
- Hu YS. Standarized tertiary rehabilitation (STR) for patients with cerebral strokes accompanied by hemiplegia. Neurorehabilitation and Neural Repair 2006;20:163. [Google Scholar]
- Jiang CY, Hu YS, Wang Q, Wu Y, Zhu YL. The cost-effectiveness analysis of early rehabilitation for stroke patients. Neurorehabilitation and Neural Repair 2006;20:205. [Google Scholar]
- Sun LM, Hu YS, Wu Y, Zhu YL, Fan WK. Effect of standardized tertiary rehabilitation on the motor function in patients with cerebral stroke accompanied by hemiplegia. Neurorehabilitation and Neural Repair 2006;20:163. [Google Scholar]
References to ongoing studies
ChiCTR‐OCH‐09000335 {unpublished data only}
- ChiCTR-OCH-09000335. A study of the stroke unit of traditional Chinese and western medicine in the treatment of ischemic stroke. http://www.chictr.org.cn/showprojen.aspx?proj=9196 (first posted 22 February 2009).
China (Wang) 2015 {published data only}
- Wang Y, Li Z, Xian Y, Zhao X, Li H, Shen H, GOLDEN BRIDGE-AIS investigators. Rationale and design of a cluster-randomized multifaceted intervention trial to improve stroke care quality in China: the GOLDEN BRIDGE-Acute Ischemic Stroke. American Heart Journal 2015;169(6):767-74. [DOI] [PubMed] [Google Scholar]
NCT00544622 {unpublished data only}
- NCT00544622. Structured stroke management improves outcome at 6 months. https://clinicaltrials.gov/ct2/show/NCT00544622 (first posted 16 October 2007).
NCT00843765 {unpublished data only}
- NCT00843765. Efficiency study of traditional Chinese medicine (TCM) versus Western medicine (WM) on ischemic stroke. https://clinicaltrials.gov/ct2/show/NCT00843765 (first posted 13 February 2009).
Russia 2017 {published data only}
- Ivanova G, Melnikova E, Shmonin A, Shamalov N, Khasanova D, Bodrova R, et al. Preliminary results of large clinical trial 'development of medical rehabilitation in Russia' (DOME): rehabilitation in stroke units and rehabilitation centers. European Stroke Journal 2017;2(1 Suppl 1):177-8. [Google Scholar]
- *.Ivanova G, Shamalov N, Melnikova E, Belkin A, Khasanova D, Prokopenko S, et al. Results of large clinical trial 'development of medical rehabilitation in Russia': patient-oriented, multidisciplinary and problem-focused model of rehabilitation patients with stroke or cerebral hemorrhage. European Stroke Journal 2018;3(1 Suppl 1):54. [Google Scholar]
Additional references
Barer 1993
- Barer D, Gibson OP, Ellul J, the "GUESS" Group. Outcome of hospital care for stroke in 12 centres. In: Proceedings of the British Geriatrics Society, Spring Meeting, Glasgow. 15-17 April 1993.
Brignardello 2018
- Brignardello‐Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. Advances in the GRADE approach to rate the certainty in estimates from a network meta‐analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan DKY, Cordato D, O’Rourke F, Chan DL, Pollack M, Middleton S, et al. Comprehensive stroke units: a review of comparative evidence and experience. International Journal of Stroke 2013;8(4):260-4. [DOI] [PubMed] [Google Scholar]
Ebrahim 1990
- Ebrahim S. Clinical Epidemiology of Stroke. 1st edition. Oxford: Oxford University Press (Medical Publications), 1990. [Google Scholar]
Garraway 1985
- Garraway WM. Stroke rehabilitation units: concepts, evaluation, and unresolved issues. Stroke 1985;16:178-81. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Higgins 2019
- Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 [updated July 2019]. The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Langhorne 2012
- Langhorne P, Villiers L, Pandian JD. Applicability of stroke-unit care to low-income and middle-income countries. Lancet Neurology 2012;11:341-8. [DOI] [PubMed] [Google Scholar]
Langhorne 2018
- Langhorne P, O'Donnell MJ, Chin SL, Zhang H, Xavier D, Avezum A, the INTERSTROKE Investigators. Practice patterns and outcomes after stroke across countries at different economic levels (INTERSTROKE): an international observational study. Lancet 2018;391:2019-27. [DOI] [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades A. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447-59. [Google Scholar]
Murray 2012
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. [DOI] [PubMed] [Google Scholar]
Owen 2019
- Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: an interactive web-based tool for analyzing, interrogating, and visualizing network metaanalyses using R-shiny and netmeta. Research Synthesis Methods 2019;10(4):569-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puhan 2014
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [DOI: 10.1136/bmj.g5630] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Seenan 2007
- Seenan P, Long M, Langhorne P. Stroke units in their natural habitat: a systematic review of observational studies. Stroke 2007;38:1886-92. [DOI] [PubMed] [Google Scholar]
Strong 2007
- Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurology 2007;6:182-7. [DOI] [PubMed] [Google Scholar]
Sun 2013
- Sun Y, Paulus D, Eyssen M, et al. A systematic review and meta-analysis of acute stroke unit care: what’s beyond the statistical significance? BMC Medical Research Methodology 2013;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonin 2017
- Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharmacy Practice 2017;15(1):943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Urimubenshi 2017
- Urimubenshi G, Langhorne P, Cadilhac DA, Kagwiza JN, Wu O. Association between patient outcomes and key performance indicators of stroke care quality: a systematic review and meta-analysis. European Stroke Journal 2017;2:287-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 1992
- Wade D. Measurement in Neurological Rehabilitation. 1st edition. Oxford: Oxford University Press, 1992. [Google Scholar]
Warlow 2008
- Warlow C, Van Gijn J, Dennis M, Wardlaw J, Bamford J, Hankey G, et al. Stroke: Practical Management. 3rd edition. Oxford: Blackwell Publishing, 2008. [Google Scholar]
References to other published versions of this review
Langhorne 1993
- Langhorne P, Williams BO, Gilchrist W, Howie K. Do stroke units save lives? Lancet 1993;342:395-8. [DOI] [PubMed] [Google Scholar]
Langhorne 1998
- Langhorne P, Dennis MS, on behalf of the Stroke Unit Trialists' Collaboration. Stroke Units: An Evidence Based Approach. London: BMJ Books, 1998. [Google Scholar]
Major 1998
- Major K, Walker A. Economics of stroke unit care. In: Langhorne P, Dennis MS, editors(s). Stroke Units: An Evidence Based Approach. London: BMJ Books, 1998. [Google Scholar]
SUTC 1997a
- Stroke Unit Trialists' Collaboration. Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ 1997;314:1151-9. [PMC free article] [PubMed] [Google Scholar]
SUTC 1997b
- Stroke Unit Trialists' Collaboration. How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke 1997;28:2139-44. [DOI] [PubMed] [Google Scholar]
SUTC 2001
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD000197] [DOI] [PMC free article] [PubMed] [Google Scholar]
SUTC 2007
- Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000197.pub2] [DOI] [PubMed] [Google Scholar]
SUTC 2013
- Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000197.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


