Abstract
Mosquito surveillance has been conducted across South Dakota (SD) to record and track potential West Nile virus (WNV) vectors since 2004. During this time, communities from 29 counties collected nearly 5.5 million mosquitoes, providing data from over 60,000 unique trapping nights. The nuisance mosquito, Aedes vexans (Meigen) was the most abundant species in the state (39.9%), and most abundant in most regions. The WNV vector, Culex tarsalis Coquillett (Diptera: Culicidae), was the second most abundant species (20.5%), and 26 times more abundant than the other Culex species that also transmit WNV. However, geographic variation did exist between WNV vector species, as well as relative abundance of vector and nuisance mosquitoes. The abundance of Ae. vexans decreased from east to west in South Dakota, resulting in an increase in the relative abundance of Cx. tarsalis. Other species are reported in this study, with various relative abundances throughout the different regions of South Dakota. WNV infection rates of mosquitoes showed that Cx. tarsalis had the most positive sampling pools and the highest vector index of all the species tested. This study addressed the need for an updated summary of the predominant mosquito species present in the United States Northern Great Plain and provides infection rate data for WNV among these predominant species.
Keywords: mosquito, population, West Nile Virus, South Dakota
In the United States, mosquitoes transmit pathogens such as West Nile virus (WNV), Chikungunya, St. Louis encephalitis, Western and Eastern equine encephalitis, La Crosse virus, and most recently Zika virus (ZIKV). Out of the many mosquito species populating a region, only a few species participate in pathogen transmission. As arboviral diseases change in a region, the relative importance of each species as vectors may also change. Species that are not vectoring regionally endemic diseases, including both noncompetent species and uninfected competent species, serve only as a nuisance to the people in that region. Yet, there is growing evidence that nuisance mosquito species may be important in limiting disease transmission by altering human behaviors in ways that reduce risk of exposure to vector species (Zielinski-Gutierrez and Hayden 2006, Gujral et al. 2007, Oidtman et al. 2016). Therefore, it is not only important to understand the abundance and population dynamics for current vectors in a region, it is also important to understand these dynamics for predominant species that are currently functioning only as a nuisance.
The first human case of WNV was reported in South Dakota (SD) in 2002, causing an epidemic with 1,039 confirmed human cases the following year. Since then, WNV has become endemic throughout the Northern Great Plains (NGP) with 1,320 cases reported from 2004 to 2016, resulting in the highest incidence of neuroinvasive disease of any region in the nation (Kightlinger 2017). Given the high and persistence incidence of WNV in the NGP, which includes Montana, Nebraska, North Dakota, South Dakota, and Wyoming, a detailed understanding of South Dakota’s mosquito populations and their WNV transmission potential is essential to support ongoing disease prevention and control efforts within this region.
Studies conducted in South Dakota, Nebraska, Iowa, and North Dakota all show similarities in the predominant mosquito populations present. In these areas, Aedes vexans (Meigen) (Diptera: Culicidae) was generally the most prevelant mosquito (Easton et al. 1986, Easton 1987, Gerhardt 1966, Janousek and Kramer 1999, Bell et al. 2005, DeGroote et al. 2008, Sucaet et al. 2008, Chuang et al. 2011, Anderson et al. 2015). Studies in South Dakota and Nebraska generally reported Culex tarsalis Coquillett (Diptera: Culicidae) as the second most abundant mosquito, and the most abundant Culex species (Easton et al. 1986, Easton 1987, Gerhardt 1966, Janousek and Kramer 1999, Chuang et al. 2011). Iowa studies reported Culex pipiens L./Culex restuans Theobald complex as the second most abundant behind Ae. vexans (DeGroote et al. 2008, Sucaet et al. 2008). In North Dakota, Cx. tarsalis was reported as the second or third most abundant species in a 2002–2004 study from the northeastern edge of the state (Bell et al. 2005), and the fourth most abundant species in another 2003–2006 study from the eastern and western edge of the state (Anderson et al. 2015). When evaluated, the NGP studies found that the relative abundance of Cx. tarsalis increased from east to west. Species in the genus Culex are the most important vectors for WNV, and Culex tarsalis is one of the best amplification and bridge vectors for this virus (Turell et al. 2005), and it is likely that this species is the primary WNV vector throughout the NGP (Bell et al. 2006).
Gerhardt (1966) published a review and qualitative survey that listed 43 mosquito species found in SD based on previous reports and larval and adult mosquito collections from 37 counties. In the communities surveyed, Ae. vexans, Cx. tarsalis, Aedes dorsalis (Meigen), Aedes nigromaculis (Ludlow), Aedes triseriatus (Say), and Aedes spencerii (Theobald) were the most prevalent species. In addition to Cx. tarsalis, Gerhardt (1966) lists five Culex species from SD, including: Cx. pipiens, Cx. restuans, Culex salinarius Coquillett, Cx. territans (Walker), and Culex erraticus (Dyar and Knab). Surveys conducted in the early 1980s from 15 sites in SD documented 19 species of mosquitoes (Easton et al. 1986, Easton 1987). Overall, 13.7% of the total mosquitoes were Cx. tarsalis, but the percent ranged from 6.9% in an eastern SD site to 35.0% from the western-most site. Most recently, Chuang et al. (2011) compared the effects of land cover types on spatial distribution and the effects of weather on temporal patterns for Cx. tarsalis and Ae. vexans populations in and around the city of Sioux Falls, SD during four mosquito seasons from 2005 to 2008, and Ae. vexans was five times more abundant than Cx. tarsalis in this eastern SD location (Chuang et al. 2011).
Presence and infection rates for WNV among mosquito species in the NGP has been studied to determine the relative risks of human infection from the various species that exist within the region. Three studies in North Dakota have identified Cx. tarsalis to be the primary vector in that state. A Grand Forks study conducted during 2003 detected WNV in 31 out of the 223 Cx. tarsalis pools (5,432 individuals) tested. No WNV was found among the 50 tested pools (2,500 individuals) each of Ae. dorsalis and Ae. vexans. A subsequent study from the same area, and involving some of the same mosquitoes, reported that the minimum infection rate (MIR) for Cx. tarsalis varied from 5.7 in 2003 to 0 in 2004 and finally 1.3 in 2005 (Bell et al. 2006). A larger study that examined multiple species along the eastern and western edges of North Dakota found that nine species tested positive for WNV with Cx. tarsalis infection rate ranging between 1.12 and 12.26 while Ae.vexans ranging between < 0.01 and 0.19 per thousand specimens tested (Anderson et al. 2015).
A 2003 Nebraska study that collectively tested only various Culex species found that MIR varied in the eastern, central, and western regions from 1.4, 7.7, and 4.9, respectively (Schweitzer et al. 2006). Infection rates of WNV in Cx. tarsalis were found to be 5 times greater than the more abundant Cx. pipiens in Iowa (Dunphy et al. 2019). Though not in the NGP, a more extensive study from north-central Colorado in 2003–2004 compared WNV infection rates among 289 mosquito pools involving 13 species (Bolling et al. 2007). Three of the nine positive pools came from Cx. tarsalis and three came from Cx. pipiens, but two came from Culiseta inornata (Williston) (Diptera: Culicidae) pools and one from an Ae. vexans pool. Experimentally, Ae. vexans can become infected with WNV (Turell et al. 2005, Tiawsirisup et al. 2008); however, this species primarily feeds on mammals, leading to the suggestion that it poses a far lower risk for transmitting WNV than typical bridge mosquitoes (Kilpatrick et al. 2005, Molaei and Andreadis 2006). For example, though WNV was isolated from the Colorado pool of Ae. vexans, the calculated MIRs for the two Culex species were at least 32 times higher than that of Ae. vexans. The low transmission potential of Ae. vexans is also illustrated in a more extensive study in Connecticut involving 4,600 pools of Ae. vexans and 9,037 pools of Cx. pipiens, Cx. restuans, and Cx. salinarius, which found that the MIRs for these Culex species were 5–14 times higher than that of Ae. vexans (Andreadis et al. 2004).
Given the importance of WNV in the NGP, there is a clear need to better understand the various mosquito species present in this region, the population dynamics of the predominant species, and their roles in the transmission of WNV. While MIR is a common method for assessing the prevalence of viruses in mosquitoes, a more complete evaluation of the risk that each vector species provides to humans can be achieved by comparing their vector indices (VI), which includes the average abundance of each vector into its calculation (Gujral et al. 2007). Here, we report the presence and abundance of the mosquito species in South Dakota, the statewide geographic distributions, seasonal trends, and interannual variation for Cx. tarsalis and Ae. vexans, and the infection rates of mosquitoes tested for WNV.
Methods
Mosquito Collections and Identification
Mosquitoes were collected from 29 counties using CDC miniature light traps with air-actuated gates (John W. Hock Model 1012-CO2, set to deliver 0.5 l CO2/min) baited with CO2 gas in compressed tanks. Traps were suspended approximately1.5 meters from the ground and located in areas with moderate to heavy tree cover and vegetation. Trapping occurred overnight and was activated using light sensors. A second trapping method was introduced during 2016–2017 using BG-Sentinel 2 traps (Biogents, Regensbourgh, Germany) in Brookings, Lincoln, Minnehaha, and Hughes counties. These traps were baited with CO2 and the included BG-lures. BG2 traps ran for 24 h periods and mosquitoes were collected daily. Mosquitoes were brought to a local facility to be euthanized by freezing. Frozen samples were then shipped via courier in coolers with ice packs to the South Dakota Department of Health.
Yearly training and testing sessions were held to aid local municipality and Department of Health officials on the proper identification of mosquito species using morphological characteristics based upon descriptions in Darsie 2005. Additionally, computer software that contained a pictorial key to the species of South Dakota were distributed to the different municipalities to aid identifications. Differentiation of Cx. restuans and Cx. pipiens can be difficult as features used are not reliable (Harrington and Poulson 2008). Distinguishing between Cx. restuans and Cx. pipiens was based upon the presence or absence of white dots located on the scutum. Because of the difficulties in reliably identifying these two species, totals of these two species were combined for statistical analysis.
WNV Infection Rates for Mosquito Species Trapped in 2003 and 2004
During 2003, all 34,358 mosquitoes (in 1,657 pools) collected using 10 traps from 5 counties (representing 279 trapping nights) were tested for WNV. Due to resource limitations and an increased number of trap sites in 2004, some pools of Ae. vexans were not tested. During this year, 40,300 mosquitoes were tested from 1,392 pools collected using 21 traps from 10 counties representing 270 trap-nights. Among mosquitoes tested during both years, 49.2% came from counties in east-central SD, 29.7% from southeastern counties, 12.4% from central counties, and 8.7% from counties west of the Missouri River. WNV testing was performed by the South Dakota Department of Health using a standardized rRT-PCR test (Lanciotti et al. 2000). Mosquitoes were divided by day, trap and species into individual pools for testing. Traps that contained more than 50 individuals of a species for a day were divided into multiple pools containing no more than 50 individuals. Pools that tested positive were retested for confirmation, and only pools that showed positive results for both tests were recorded as ‘positive’. MIRs were calculated as the total number of infected pools divided by the total number of mosquitoes tested, and then multiplied by 1,000; this is a standard summary statistic and assumes that any positive pool contained only one infected mosquito (Gu et al. 2004). Confidence intervals with 95% coverage were calculated with the Wald approximation, and by the ‘rule of three’ whenever there were no positive pools (Louis 1981). The vector index (VI) was calculated as the product of MIR and average number of mosquitoes per trap-night density (Jones et al. 2011), and its 95% confidence interval (CI) was defined as the product of 95% CI of the MIR with the same trap-night density.
Endemic Mosquito Surveillance 2004–2017
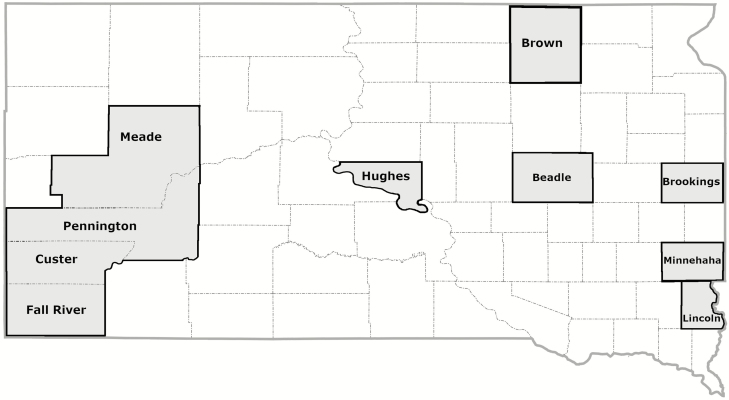
The mosquito surveillance portion of this study began after 2003, and over the next 14 yr, communities from 29 of the 66 SD counties contributed mosquitoes for varying numbers of years (Table 1). Trap locations were most commonly found in populated communities. Over half of the trap-nights occurred in the Sioux Falls area (Minnehaha and Lincoln counties), and almost 30% occurred in Brookings, Brown and Coddington counties. Collections in the southwest portion of the state occurred near the Black Hills area, including Fall River, Custer, Pennington, and Meade counties. Because of their proximity to the unique Black Hills ecoregion of the state, these counties were combined during regional analysis and labeled as ‘southwest SD’. Throughout the study, 5,486,692 mosquitoes were captured creating 60,317 unique samples. The number of data points collected in counties east of the Missouri River accounted for 96.4% of all data points, and these eastern sites trapped 97.7% of all mosquitoes included in this study. Trapping data was generally expressed as the mean number of mosquitoes trapped per trap-day or week; when expressed relative to weeks in the year, ‘weeks’ were defined as Sunday through Saturday, with the week containing January 1st defined as week 1. Trapping began as early as April and continued as late as October, but most collections were made from June 1 (generally week 22 or 23) through August 15 (generally week 33 or 34). All sites identified Cx. tarsalis, but the specificity of mosquito identification for the other species varied depending on trap location and year. As designated in Table 1, some sites (Brookings, Minnehaha/Lincoln, Hughes, Butte/Harding) identified mosquitoes to species level based upon morphological characteristics (Darsie 2005). A category called ‘non-Culex tarsalis’ was used for mosquitoes not identified to species. In some areas, such as Brown County and counties located in southwestern SD, the non-Culex tarsalis category became inflated with highly abundant mosquitoes, especially Ae. vexans. Therefore, when evaluating regional population dynamics for Ae. vexans and Cx. tarsalis, both weekly Ae. vexans and non-Cx. tarsalis were calculated because some regions did not identify most of their non-Cx. tarsalis mosquitoes to the species level.
Table 1.
Counties participating in mosquito surveillance
| County | Region | # of years collected | # mosquitoes | # of data points |
|---|---|---|---|---|
| Beadle | East Central (ER) | 12 | 170,965 | 591 |
| Brookings* | East Central (ER) | 14 | 1,207,741 | 7,060 |
| Brown | Northeastern (ER) | 14 | 2,031,810 | 8,641 |
| Butte/Harding* | Northwestern (WR) | 3 | 14,255 | 554 |
| Clay | Southeastern (ER) | 1 | 1,073 | 67 |
| Codington | East Central (ER) | 13 | 111,677 | 2,020 |
| Custer | Southwestern (WR) | 4 | 4,485 | 104 |
| Davison | Southeastern (ER) | 12 | 64,773 | 316 |
| Dewey | North Central (WR) | 2 | 6,175 | 72 |
| Edmunds | North Central (ER) | 4 | 15,943 | 155 |
| Fall River | Southwestern (WR) | 4 | 3,725 | 297 |
| Grant | Northeastern (ER) | 4 | 21,607 | 85 |
| Hand | Central (ER) | 4 | 9,692 | 68 |
| Hughes* | Central (ER) | 14 | 159,732 | 959 |
| Lake | East Central (ER) | 11 | 48,088 | 968 |
| Lincoln/Minnehaha* | Southeastern (ER) | 14 | 1,470,899 | 36,464 |
| Marshal | Northeastern (ER) | 3 | 5,652 | 52 |
| Meade | West Central (WR) | 10 | 24,969 | 764 |
| Moody | East Central (ER) | 6 | 28,731 | 519 |
| Pennington | West Central (WR) | 9 | 68,001 | 352 |
| Perkins | Northwestern (WR) | 2 | 419 | 20 |
| Sanborn | East Central (ER) | 1 | 53 | 7 |
| Spink | Northeastern (ER) | 2 | 177 | 25 |
| Turner | Southeastern (ER) | 1 | 28 | 7 |
| Union | Southeastern (ER) | 3 | 12,637 | 95 |
| Yankton | Southeastern (ER) | 2 | 1,119 | 43 |
| Ziebach | Northwestern (WR) | 1 | 2,266 | 12 |
*Denotes region that identified most mosquitoes to species.
ER denotes counties located east of Missouri River.
WR denotes counties located West of Missouri River.
Results
Infection Rate
From 2003 to 2004, 17 different species of mosquitoes were tested for WNV, but 8 of the species involved less than 205 specimens, and while WNV was not found in any of these species, their MIR upper confidence intervals were still very high (Table 2) because of low sample size. WNV was also not found in any Aedes trivittatus (Coquillett) pools, but its upper confidence interval was much lower because of the high number (2,715) of specimens tested. The 74 WNV positive mosquito pools were distributed among the remaining 8 species with sample numbers ranging from 239 to 37,931 mosquitoes. All eight species showed at least one positive pool in 2003, but WNV was only found in three species in 2004 (Cx. pipiens/restuans, Cx. tarsalis, and Ae. vexans). Pools trapped in 2003 accounted for 62.2% of all positive pools, even though there were about three times more Cx. tarsalis tested in 2004. Only 238 Aedes fitchii (Felt & Young, 1904) specimens were tested, yet two positive pools were identified, resulting in the highest mean MIR among all species. With so few samples tested, there is a very wide confidence interval for the MIR for this species. Aedes cinereus Meigen, 1818 and Cs. inornata had one positive WNV pool with sample numbers below 1,000 mosquitoes that made their mean MIRs below 2, but their confidence intervals were very wide (Table 2). Two pools out of almost 2,500 Ae. dorsalis mosquitoes were positive for WNV. Almost 38,000 Ae. vexans specimens were tested, but only four pools tested positive for WNV, and therefore, the MIR and its upper confidence interval were very low for both 2003 and 2004. The upper confidence intervals for Ae. vexans for both years were the lowest of any collected species because the number of positive pools were low while the number of tested samples were very high. Three species in the genus Culex all had MIR values above 2. More than 28,000 Cx. tarsalis specimens yielded 59 positive pools, which was 77.6% of all positive pools for the 2 yr. However, the 2-yr MIR for this species was not statistically different from the other two Culex species. Relatively few specimens were collected for Cx. pipiens/restuans (n = 900) and Cx. salinarius (n = 358), and so while their calculated MIRs are higher, their confidence intervals were within that of Cx. tarsalis. MIR for Cx. tarsalis decreased from 5.0 in 2003 to 1.1 in 2004, whereas, Cx. pipiens/restuans increased from 1.9 to 7.9 during that same time period. In the 2 yr tested, MIRs of Cx. tarsalis were 10–50 times higher than Ae. vexans.
Table 2.
Results of statewide WNV mosquito testing 2003 to 2004
| Species | Years | Pools tested | Mosquitoes tested | Positive pools | MIR (95% CI) | Vector index |
|---|---|---|---|---|---|---|
| Cx. territans | 2003–2004 | 8 | 8 | 0 | 0 (0–375) | 0 (0–5.5) |
| Ur. sapphirina | 2003–2004 | 9 | 13 | 0 | 0 (0–230.8) | 0 (0–5.5) |
| Ae. sollicitans | 2003–2004 | 11 | 21 | 0 | 0 (0–142.9) | 0 (0–5.5) |
| An. quadrimaculatus | 2003–2004 | 17 | 22 | 0 | 0 (0–136.4) | 0 (0–5.5) |
| An. punctipennis | 2003–2004 | 24 | 44 | 0 | 0 (0–68.2) | 0 (0–5.5) |
| An. walkeri | 2003–2004 | 24 | 101 | 0 | 0 (0–29.7) | 0 (0–5.5) |
| Ae. triseriatus | 2003–2004 | 64 | 157 | 0 | 0 (0–19.1) | 0 (0–5.5) |
| Cq. perturbans | 2003–2004 | 42 | 203 | 0 | 0 (0–14.8) | 0 (0–5.5) |
| Ae. trivittatus | 2003–2004 | 204 | 2,716 | 0 | 0 (0–1.1) | 0 (0–5.5) |
| Ae. fitchii | 2003 | 25 | 82 | 2 | 24.4 (<0.1–57.6) | 7.2 (0–17.1) |
| 2004 | 12 | 156 | 0 | 0 (0–19.2) | 0 (0–11.1) | |
| Cx. salinarius | 2003 | 98 | 336 | 1 | 3 (<0.1–8.8) | 3.6 (0–10.7) |
| 2004 | 22 | 22 | 0 | 0 (0–136.4) | 0 (0–11.1) | |
| Ae. cinereus | 2003 | 49 | 346 | 1 | 2.9 (<0.1–8.5) | 3.6 (0–10.6) |
| 2004 | 19 | 223 | 0 | 0 (0–13.5) | 0 (0–11.2) | |
| Cs. inornata | 2003 | 80 | 255 | 1 | 3.9 (<0.1–11.5) | 3.6 (0–10.6) |
| 2004 | 82 | 373 | 0 | 0 (0–8) | 0 (0–11.1) | |
| Cx. pipiens/restuans | 2003 | 184 | 538 | 1 | 1.9 (<0.1–5.6) | 3.7 (0–10.9) |
| 2004 | 115 | 382 | 3 | 7.9 (<0.1–16.7) | 11.2 (0–23.6) | |
| Ae. dorsalis | 2003 | 103 | 724 | 2 | 2.8 (<0.1–6.6) | 7.3 (0–17.3) |
| 2004 | 128 | 1,735 | 0 | 0 (0–1.7) | 0 (0–10.9) | |
| Cx. tarsalis | 2003 | 316 | 7,108 | 35 | 4.9 (3.3–6.5) | 125.7 (84.7–166.8) |
| 2004 | 574 | 21,095 | 24 | 1.1 (0.7–1.5) | 85.9 (54.7–117.2) | |
| Ae. vexans | 2003 | 606 | 24,018 | 3 | 0.1 (<0.1–0.2) | 8.7 (0–17.3) |
| 2004 | 352 | 13,913* | 1 | 0.1 (<0.1–0.3) | 37.2 (0–111.5)* |
* Only a portion of the Ae. vexans captured in 2004 were tested for WNV.
Despite its very high MIR in 2003, the low abundance of Ae. fitchii causes its VI to be more similar to the low levels seen in the other non-Culex minor species infected with WNV (Table 2). Because of the significant MIR increase from 2003 to 2004, the VI for Cx. pipiens/restuans also increased to above 10 in 2004. The important role of Cx. tarsalis as the primary vector for WNV is clearly demonstrated by its high VI for both years tested, which was 35 times higher than Cx. pipiens/restuans in 2003 and eight times higher in 2004. In spite of Ae. vexans very low MIR for both years, its extremely high abundance increased the VI to levels equal or higher than that of Cx. pipiens/restuans.
State-Wide Mosquito Survey
Twenty-two species were identified during the 15-yr study period and eight species were collected in every year surveyed (Table 3). Using data from counties identifying mosquitoes to species level for all years, Ae. vexans and Cx. tarsalis were the two most abundant species, accounting for 67.8% and 21.0%, respectively. Among these counties, Ae. vexans populations ranged from 31.67% to 72.10%, whereas, Cx. tarsalis ranged from 15.95% to 62.27%. Brown County reported Ae. vexans as only 2.78% of their total catch; however, this county only sporadically identified non-Culex tarsalis mosquitoes, yet Ae. vexans almost certainly contributed to a bulk of the species reported in the nonidentified category. The six other species present in each year included: Ae. trivittatus, Ae. dorsalis, Cs. inornata, Cx. restuans, Cx. salinarius, and Anopheles punctipennis Say (Diptera: Culicidae). These six species only accounted for 5.9% of the total mosquitoes in counties where all mosquitoes were identified to species (Table 3). Except for Cx. salinarius in Beadle County, these mosquitoes were recorded in all regions, but not all sites within some counties. Culex restuans and Cx. salinarius are minor vectors for WNV, accounting for less than 1% of all mosquitoes collected.
Table 3.
Mosquitoes (expressed as a percentage of the total) present annually within South Dakota areas from 2004 to 2017
| Species | Lincoln/Minnehaha | Brookings | Brown | Beadle | Hughes | Southwest SD |
|---|---|---|---|---|---|---|
| Other/unidentified | 1.49% | 6.19% | 80.56% | 4.24% | 0.63% | 43.44% |
| Ae. vexans | 72.10% | 67.23% | 2.78% | 70.01% | 31.67% | 9.43% |
| Cx. tarsalis | 18.29% | 18.84% | 15.95% | 22.40% | 62.27% | 43.42% |
| Ae. trivittatus | 3.05% | 0.89% | 0.06% | 0.69% | 0.62% | 2.13% |
| Ae. dorsalis | 1.93% | 0.85% | 0.08% | 1.10% | 2.45% | 0.58% |
| Cs. inornata | 1.40% | 0.55% | 0.07% | 0.45% | 1.16% | 0.37% |
| Cx. restuans | 0.25% | 1.23% | 0.06% | 0.88% | 0.60% | 0.23% |
| Cx. salinarius | 0.04% | 0.29% | 0.12% | 0.00% | 0.31% | 0.02% |
| An. punctipennis | 0.17% | 0.02% | 0.01% | 0.14% | 0.04% | 0.04% |
Zero values do not necessarily indicate the absence of species.
*Did not identify non-Cx. tarsalis mosquitoes during most years.
Other species were not collected every year and tended to be present in only certain areas of the state, or in certain habitat under specific conditions, and could occasionally become a dominant species within a specific area (Table 4). These species included: Coquillettidia perturbans (Walker, 1856) (Diptera: Culicidae), Ae. triseriatus, Ae. fitchii, Psorophora cyanescens (Coquillett, 1902) (Diptera: Culicidae), Aedes sollicitans (Walker), Anopheles walkeri Theobald, Uranotaenia sapphirina (Osten Sacken) (Diptera: Culicidae), Cx. territans, Anopheles quadrimaculatus Say, Aedes japonicus (Theobald), Aedes canadensis (Theobald), Ae. cinereus, and Cx. erraticus. Though these minor species only accounted for 1.25% of the mosquitoes identified statewide and during all years, occasionally their abundance became important in certain locations. For example, while Cx. pipiens does not play a major role in vectoring WNV throughout South Dakota, it could become a significant vector in Lincoln and Minnehaha counties during some years (Table 4 and Fig. 3). Overall, Cx. pipiens, Cx. restuans, Cx. salinarius, Cx. territans, and Cx. erraticus accounted for only 1.24% of the species collected, and Cx. tarsalis accounted for 17 times more individuals than all other Culex species combined. Other species could serve as vectors for diseases not currently endemic to the Northern Plains, but currently, they only function as nuisance mosquitoes in this region. For example, Cq. perturbans was an important nuisance in some sites within Brookings County (Table 3). Aedes japonicus has been sporadically detected within South Dakota. So far, nine specimens have been captured, four during 2009 in CDC miniature light traps and five during 2016 in BG-Sentinel 2 traps. All specimens were trapped in Lincoln and Minnehaha counties located on the eastern edge of the state.
Table 4.
Mosquitoes (expressed as a percentage of the total) present within South Dakota areas, but not found every year from 2004 to 2017
| Species | Lincoln/ Minnehaha | Brookings | Brown* | Beadle | Hughes | Southwest SD |
|---|---|---|---|---|---|---|
| Cx. Pipiens | 0.63% | 0.15% | 0.02% | 0.00% | 0.06% | 0.00% |
| Cq. Perturbans | 0.00% | 3.67% | 0.04% | 0.00% | 0.06% | 0.00% |
| Ae. Triseriatus | 0.23% | 0.05% | 0.01% | 0.01% | 0.04% | 0.07% |
| Ae. fitchii | 0.13% | 0.01% | 0.01% | 0.00% | 0.01% | 0.24% |
| Ps. cyanescens | 0.00% | 0.00% | 0.09% | 0.00% | 0.00% | 0.00% |
| Ae. sollicitans | 0.00% | 0.00% | 0.04% | 0.00% | 0.00% | 0.01% |
| An. walkeri | 0.01% | 0.01% | 0.01% | 0.06% | 0.07% | 0.00% |
| Ur. sapphirina | 0.00% | 0.01% | 0.00% | 0.00% | 0.00% | 0.00% |
| Cx. territans | 0.01% | 0.02% | 0.00% | 0.00% | 0.00% | 0.00% |
| An. quadrimaculatus | 0.01% | 0.00% | 0.00% | 0.00% | 0.01% | 0.00% |
| Ae. japonicus | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Ae. canadensis | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Ae. cinereus | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| Cx. erraticus | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
Zero values do not indicate absence of species.
*Did not identify non-Cx. tarsalis mosquitoes most years
Fig. 3.
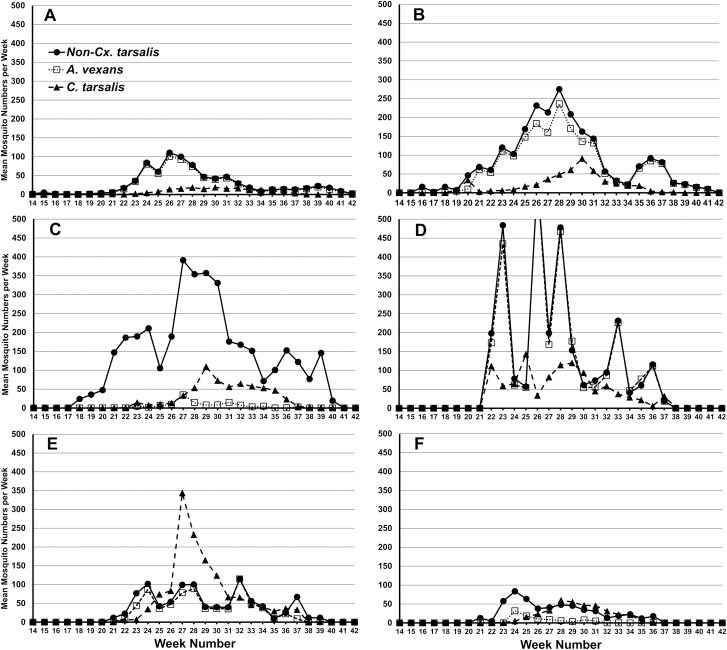
Mean number of mosquitoes collections per trap overall years within six South Dakota regions: (A) Lincoln/Minnehaha counties, (B) Brookings county, (C) Brown county, (D) Beadle County, (E) Hughes County, (F) Fall River/Custer/Pennington/Meade counties. Data are expressed relative to each numbered week (Sunday through Saturday) in the year, with the week that contained January 1st designated as week 1.
Year-to-Year Statewide Variations
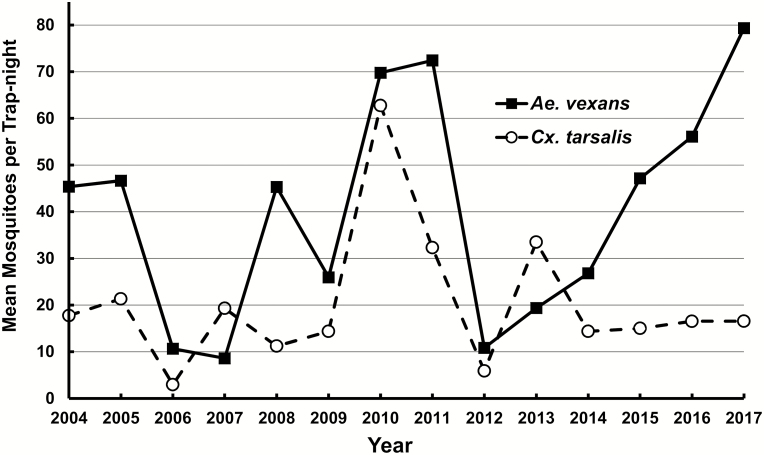
On a statewide basis, mean total number of mosquitoes per trap night for both Ae. vexans and Cx. tarsalis varied considerably among the various years (Fig. 1). Season-long mosquito abundance for Ae. vexans exceeded 40 mosquitoes/trap-night during 8 of the 14 yr, but Cx. tarsalis exceeded this threshold only during 2010, when both species exceeded 60 mosquitoes/trap-night. The mean Cx. tarsalis population for each season generally remained fairly stable between 10 and 20 mosquitoes/trap-night, whereas, Ae. vexans populations fluctuated more extensively. Aedes vexans was the most abundant species during every year except 2007 and 2013, when Cx. tarsalis became slightly more abundant (Fig. 1). During 2006 and 2012, the total populations of both Ae. vexans and Cx. tarsalis were particularly low, and these 2 yr both preceded the 2 yr when Cx. tarsalis exceeded Ae. vexans.
Fig. 1.
Yearly, season-long mean mosquito numbers per trap-night for the two most abundant mosquito species in South Dakota.
Even during years when Ae. vexans and Cx. tarsalis populations were very low, the yearly proportion for the other mosquito species never exceeded 9% of the total population. The three most common minor species each reached or exceeded 5% of the total population during at least one of the 14 yr. Two population spikes occurred for Ae. trivittatus in 2004 and 2006. Culiseta inornata and Cq. perturbans each exceeded 5% once during the study, occurring in 2012 and 2017, respectively. These spikes were not only created through an increase in abundance for these three species, but also by the low abundance of the two major species. The proportions for the less common species never exceeded 2.5%. Among the Culex species other than Cx. tarsalis, Cx. pipiens only exceeded 1% twice (i.e., 2016 and 2017), but Cx. restuans exceeded 1% during 4 yr (i.e., 2006, 2007, 2009 and 2013), and exceeded 2% during 2013.
Seasonal Population Dynamics of Cx. tarasalis and Ae. vexans
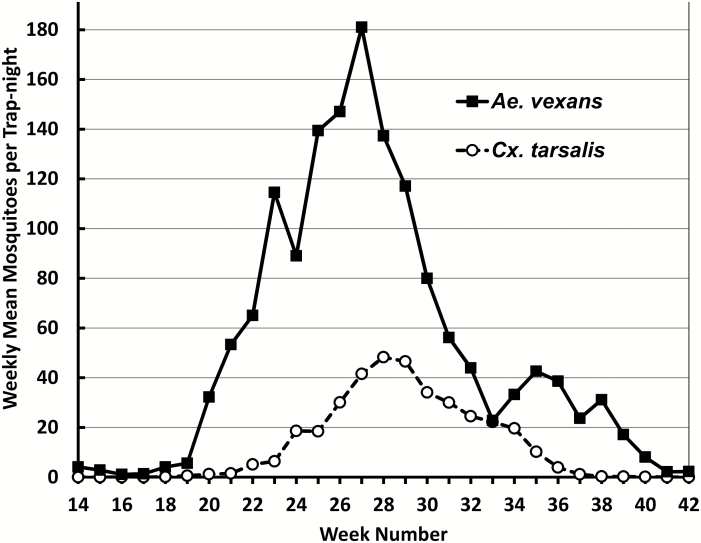
Within each mosquito season (Fig. 2), the 14-yr mean statewide trap counts for Ae. vexans tended to increase rapidly at around week 20 (generally mid-May), peaking at 180 mosquitoes per trap-night at week 27. In contrast, Cx. tarsalis populations did not begin increasing until week 22 (generally the last week in May) and reached a peak of 50 mosquito/trap-night at week 28 (generally the first week in July). Collections for half the annual Cx. tarsalis samples occurred between weeks 28 and 30 in all locations, whereas, half the annual state-wide collections for Ae. vexans occurs between weeks 27 and 28. The abundance decrease for Cx. tarsalis was slow and linear from week 28 to week 37 (Fig. 2). In contrast, the Ae. vexans population decreased rapidly until week 33 (generally the second week in August), and then increased again to week 35 until decreasing again to zero at week 42 (mid-October). It should be noted that during weeks 31–42, the abundance of Ae. vexans had typically decreased enough that they were similar to the trapped number of Cx. tarsalis (Fig. 2).
Fig. 2.
Statewide 14-yr mean for the number of Ae. vexans and Cx. tarsalis females captured per trap-night during each numbered week in the year (Sunday through Saturday) with the week that contained January 1st designated as week 1.
Regional Variations in Cx. tarsalis and Ae. vexans Populations
For each of the six SD regions shown in Figs. 3 and 4, weekly population dynamics for Cx. tarsalis and Ae. vexans are expressed as the mean over all 14 yr. The number of trap sites for each region is reported in Table 1. Non-Cx. tarsalis values were also added to Fig. 3 because not all mosquitoes were identified to species in some regions. Therefore, in regions where nearly all mosquitoes were identified to species, such as Minnehaha/Lincoln, Brookings, Beadle, and Hughes counties, non-Cx. tarsalis and Ae. vexans are similar; whereas in areas that did not identify all mosquitoes to species, such as Brown county and southwest South Dakota (Fall River/Custer/Pennington/Mead counties), Ae. vexans reported abundance are below their actual values, and the non-Cx. tarsalis values would more closely represent the Ae. vexans population.
Fig. 4.
South Dakota state map showing the six regions used to compare variations in Cx. tarsalis and Ae. vexans populations.
With the exception of Lincoln/Minnehaha counties, all eastern SD regions showed relatively high numbers of Ae. vexans (or non-Cx. tarsalis species) compared to western regions. These eastern counties also showed evidence of a later resurgence of Ae. vexans populations after week 33. Culex tarsalis populations were low in Lincoln/Minnehaha counties, and in southwest South Dakota. They were highest in Hughes county, located in the center of the state. Therefore, overall average abundance for Cx. tarsalis is lower than the non-Cx. tarsalis and Ae. vexans population for all regions except Hughes county.
Over 10% of the Cx. tarsalis captured for the season occurred before week 26 in Lincoln/Minnehaha counties. This occurred slightly earlier for Brookings county at week 23, for Hughes County at week 23, and Beadle County at week 22. Culex tarsalis reached this mark at week 27 in Brown County and in southwest South Dakota at week 26. At weeks 28 to 30, we consistently saw over half the annual average mosquito collections occur. Over 95% of the annual collections occurred by weeks 34 to 36 in all regions studied.
Over 10% of the Ae. vexans (or non-Cx. tarsalis mosquitoes) annual collections were captured before weeks 23 to 25 for all regions studied, and 50% were consistently captured before weeks 27 to 28, with the exception of the southwest region in which this occurred at week 25. Collections of 95% of the average annual collections varied between areas studied. In Lincoln/Minnehaha, and Brookings counties this threshold occurred at the latest between weeks 37 and 39. In Brown, Hughes, and Beadle couties this threshold occurred by weeks 34 to 35. In the southwest region of South Dakota, 95% of the average annual collections occurred at week 31. Abundance of Ae. vexans (or non-Cx. tarsalis mosquitoes) in the central and western portions of the state reached zero at or before week 40, while in the eastern portion of the state, the presence of these mosquitoes could remain until as late as week 42.
From the peak of both Ae. vexans and Cx. tarsalis, both species declined in average abundance until around week 40; however, the rate of decline was slower for Cx. tarsalis. This caused periods in which the average Cx. tarsalis met or exceeded Ae. vexans for certain weeks. These periods occurred between weeks 32 and 34 in Lincoln/Minnehaha, and Brookings counties, week 34 in Brown County, weeks 24, 25, 30, and 31 in Beadle County, and nearly all weeks for Hughes and southwest South Dakota.
Discussion
Our 2003 and 2004 findings show that almost 78% of all positive mosquito pools collected among various locations in SD were from Cx. tarsalis, confirming that this species is the primary vector of WNV in this region. WNV was found in other Culex species, and their mean MIRs were not statistically different from that of Cx. tarsalis. However, their low statewide abundance resulted in the VI of Cx. pipiens/restuans and Cx. salinarious being 14 and 57 times lower than Cx. tarsalis, respectively. However, in regions where multiple WNV vectors are more common, less-abundant vectors could be important contributors to viral amplification during years when Cx. tarsalis populations are low. The South Dakota MIRs for Cx. tarsalis were comparable with that reported during 2003 and 2004 in both North Dakota studies (Bell et al. 2006, Anderson et al. 2015). With the exceptions of Cx. tarsalis, Ae. vexans, Ae. trivittatus, and Ae. dorsalis, the MIR confidence intervals for the other species were extremely divergent due to their small sample sizes and little can be concluded about their infection levels for WNV. Our data for Ae. vexans infections rates were similar to a North Dakota study during 2003–2004 (Anderson et al. 2015). The MIR for Ae. vexans was low and had the lowest upper confidence level for MIR of any mosquito species tested; yet, this mosquito was also the most abundant, resulting in an increased relative position for VI. The VI for Cx. tarsalis was 2–14 times higher than Ae.vexans which was similar to Culex pipiens/restuans. However, Ae. vexans importance as a vector could become greater in years when WNV amplification is high or if new pathogens are introduced. Anderson et al. (2015) found that the WNV risk level for Ae. vexans was particularly high during the epidemic year of 2003. Aedes vexans is mostly considered a nuisance mosquito for this region, its high abundance may be important in diminishing human behaviors associated with WNV transmission risk (Gujral et al. 2007). The VI for Ae. dorsalis and Ae. fitchii were about half that of Ae. vexans and Cx. pipiens/restuans, but still significantly above the other species.
Previous studies from this region suggest that Cx. tarsalis and Ae. vexans are the two most common species present, and these findings are supported by the present study where both species accounted for 88.8% of all species identified. All species reported in this study have been identified in other studies from SD or neighboring states (Easton et al. 1986, Janousek and Kramer 1999, Bell et al. 2005, DeGroote et al. 2007, Friesen and Johnson 2014, Anderson et al. 2015). Small numbers of Aedes japonicus have been found in Iowa and Minnesota, but had not been identified in states within the NGP (Kaufman and Fonseca 2014), and our report of a few specimens captured on two separate years in the Sioux Falls area constitutes a new record for this region. Aedes albopictus (Skuse) has become a mosquito of concern for its potential to transmit the Zika virus. This mosquito has been found in the midwestern states of Minnesota, Iowa, Nebraska, Kansas, and Missouri, over the past 10 yr (Moore and Mitchell 1997). Some of these areas containing Ae. albopictus are within ecoregions found in SD, raising concerns that this invasive mosquito could also become established in SD (Bailey et al. 1994). Though Ae. albopictus was not detected in this study, Kaufman and Fonseca 2014 point out that Ae. japonicus share similar habitat preferences to Ae. albopictus, which indicates that the Sioux Falls area is a logical location for continued surveillance for this Zika vector.
Because almost 98% of mosquitoes collected in this study were from counties east of the Missouri River, population intensities described here more closely resembled distributions in Iowa and eastern Nebraska, than those found in neighboring states to the west. Further studies involving additional locations from western SD would be useful in identifying more subtle mosquito differences exist in western regions that had little sampling data in our study. There were regions in central and western SD where the abundance of Ae. vexans decreased such that Cx. tarsalis became the most abundant species. The observed east-to-west decrease in relative abundance of Ae. vexans to Cx. tarsalis is supported by a multiregional study in Nebraska that clearly showed a similar decrease in Ae. vexans abundance in sites located in the western region of the state, whereas, the abundance of Cx. tarsalis remained stable or even increased (Janousek and Kramer 1999).
These changes in relative abundance between vectors and nonvectors could impact human avoidance behaviors if important nuisance mosquitoes are not as abundant. Reductions in biting pressure generated by nonvector mosquitoes could reduce avoidance behaviors such as applying repellent or seeking shelter. Previous studies have shown that the public is more inclined to take action to prevent bites when they are aware of mosquito presence (Zielinski-Gutierrez and Hayden 2006). Control efforts in SD often target Ae. vexans and Cx. tarsalis and a recent study has shown that both Ae. vexans and Cx. tarsalis have similar susceptibility to permethrin; however, Ae. vexans has an increased level or resistance compared to members of that genus elsewhere in the world (Vincent et al. 2018).
In addition to Cx. tarsalis, the present study identified the five other Culex species reported in other studies from the region (Gerhardt 1966, Easton et al. 1986, Easton 1987, Janousek and Kramer 1999). These species (Cx. pipiens, Cx. restuans, Cx. salinarius. Cx. territans, and Cx. Erraticus) collectively accounted for less than 1.3% of the mosquitoes identified, and the relative importance of Cx. tarsalis as the primary vector for WNV in this region is illustrated by its vector index and in our finding that it accounted for about 17 times more of the Culex population than all other species combined. In Iowa, Cx. pipiens is the most abundant vector statewide, and Cx. tarsalis is found primarily in the western regions of the state (DeGroote et al. 2008). In Nebraska, the abundance of Cx. tarsalis increased from east to west while the abundance of Cx. pipiens decreased in the central and western portions of that state (Janousek and Kramer 1999). We found that the small numbers of Cx. pipiens found within South Dakota were primarily located along the eastern region, where they may become an important WNV vector in some localized areas. South Dakota appears to be part of a large longitudinal boundary in the upper Great Plains for a transitional shift between two prominent WNV vectors, Cx. tarsalis and Cx. pipiens. This geospatial shift between mosquito vectors and between vectors and nonvectors abundance may be important risk factors in the mosquito transmission of WNV to humans in this very endemic region.
Despite the growing recognition of Cx. tarsalis’s importance for WNV transmission and Ae. vexans’s importance as the primary nuisance species throughout the NGP, very few studies have evaluated year-to-year population fluctuations or weekly population changes during the mosquito season for either species. Our 14-yr study showed that Cx. tarsalis populations were generally lower than Ae. vexans and remained stable throughout the various years, whereas, Ae. vexans populations tended to be higher and fluctuated more extensively. This trend was also found in a 2005 to 2008 study from Sioux Falls, SD that also demonstrated that Ae. vexans was more positively influenced by precipitation, whereas, Cx. tarsalis was more positively influenced by higher temperatures (Chuang et al. 2011). It is also suggested that these differences are related to developmental and survival differences in the life-cycle among both species (Chuang et al. 2011). Our study showed that statewide Cx. tarsalis populations exceeded that of Ae. vexans during only two out of the 14 yr, and both times followed years when the population of both species had dropped to extremely low levels. This would suggest that Cx. tarsalis populations can recover from factors such as severe drought conditions more quickly than Ae. vexans.
The average abundance for Ae. vexans and Cx. tarsalis within a year tended to follow a similar pattern in most areas of the state. Aedes vexans tends to be collected in the traps 2 to 3 wk before Cx. tarsalis and peaks around one to 2 wk earlier. The immediate decline in Ae. vexans after its peak occurs at the same time that Cx. tarsalis peaks in its abundance. Furthermore, after the peak, Ae. vexans decreases in abundance at a greater rate than Cx. tarsalis and it is at this initial decrease in Ae. vexans abundance that human cases of WNV begin to sharply rise (Wimberly et al. 2013, Kightlinger 2017). These seasonal patterns were similar to those found in North Dakota (Anderson et al. 2015).
Recently, South Dakota has used a new WNV prediction model which has shown success over the past few years (Davis et al. 2017, 2018). This technique uses mosquito infection data along with climate variables to predict human risk of WNV, however, the model does not use mosquito abundance data. Future modeling efforts could utilize mosquito population information to build forecasting tools to assist communities in determining when and where control methods may be most effective in reducing important mosquito species. Understanding the roles and impacts of various mosquitoes have on infection rates, human behaviors, and ultimately human infection of WNV, could enhance models currently used to predict human risk of WNV.
Acknowledgments
The authors would like to extend thanks to the state of South Dakota and all of their employees for their hours collecting and identifying mosquitoes. This publication was supported by Cooperative Agreement NU50CK000380 funded by the Centers for Disease Control and Prevention. This publication was also supported by a grant from the NASA Applied Sciences Health and Air Quality Program (Grant no. NNX15AF74G) and a grant from the National Institutes of Health National Institute of Allergy and Infectious Diseases (Grant no. R01-AI079411). The authors declare that there are no conflicts of interest regarding the publication of this paper.
References Cited
- Andreadis T. G., Anderson J. F., Vossbrinck C. R., and Main A. J.. . 2004. Epidemiology of West Nile Virus in Connecticut: a five-year analysis of mosquito data 1999-2003. Vector Borne Zoonotic Dis. 4: 360–378. [DOI] [PubMed] [Google Scholar]
- Anderson J. F., Main A. J., Armstrong P. M., Andreadis T. G., and Ferrandino F. J.. . 2015. Arboviruses in North Dakota, 2003-2006. Am. J. Trop. Med. Hyg. 92: 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey R., Avers P., King T., and McNab W.. . 1994. Ecoregions and subregions of the United States (map). 1: 7,500,000. With supplementary table of map Unit descriptions, compiled and edited by WH McNab & RG Bailey. USDA Forest Service, Washington, DC. [Google Scholar]
- Bell J. A., Mickelson N. J., and Vaughan J. A.. . 2005. West Nile virus in host-seeking mosquitoes within a residential neighborhood in Grand Forks, North Dakota. Vector Borne Zoonotic Dis. 5: 373–382. [DOI] [PubMed] [Google Scholar]
- Bell J. A., Brewer C. M., Mickelson N. J., Garman G. W., and Vaughan J. A.. . 2006. West Nile virus epizootiology, central Red River Valley, North Dakota and Minnesota, 2002-2005. Emerg. Infect. Dis. 12: 1245–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling B. G., Moore C. G., Anderson S. L., Blair C. D., and Beaty B. J.. . 2007. Entomological studies along the Colorado Front Range during a period of intense West Nile virus activity. J. Am. Mosq. Control Assoc. 23: 37–46. [DOI] [PubMed] [Google Scholar]
- Chuang T. W., Hildreth M. B., Vanroekel D. L., and Wimberly M. C.. . 2011. Weather and land cover influences on mosquito populations in Sioux Falls, South Dakota. J. Med. Entomol. 48: 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie R. F. 2005. Identification and geographical distribution of the mosquitos of North America, north of Mexico, Gainesville. University Press of Florida, Gainesville, FL. [Google Scholar]
- Davis J. K., Vincent G., Hildreth M. B., Kightlinger L., Carlson C., and Wimberly M. C.. . 2017. Integrating environmental monitoring and mosquito surveillance to predict vector-borne disease: prospective forecasts of a West Nile virus outbreak. PLoS Curr. Outbreaks. 9. doi:10.1371/currents.outbreaks.90e80717c4e67e1a830f17feeaaf85de [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Vincent G. P., Hildreth M. B., Kightlinger L., Carlson C., and Wimberly M. C.. . 2018. Improving the prediction of arbovirus outbreaks: a comparison of climate-driven models for West Nile virus in an endemic region of the United States. Acta Trop. 185: 242–250. [DOI] [PubMed] [Google Scholar]
- DeGroote J., Mercer D. R., Fisher J., and Sugumaran R.. . 2007. Spatiotemporal investigation of adult mosquito (Diptera: Culicidae) populations in an eastern Iowa county, USA. J. Med. Entomol. 44: 1139–1150. [DOI] [PubMed] [Google Scholar]
- DeGroote J. P., Sugumaran R., Brend S. M., Tucker B. J., and Bartholomay L. C.. . 2008. Landscape, demographic, entomological, and climatic associations with human disease incidence of West Nile virus in the state of Iowa, USA. Int. J. Health Geogr. 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy B. M., Kovach K. B., Gehrke E. J., Field E. N., Rowley W. A., Bartholomay L. C., and Smith R. C.. . 2019. Long-term surveillance defines spatial and temporal patterns implicating Culex tarsalis as the primary vector of West Nile virus. Sci. Rep. 9: 6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton E. R. 1987. Mosquito surveillance employing New Jersey light traps on Indian reservations in Iowa, Nebraska and South Dakota in 1984 and 1985. J. Am. Mosq. Control Assoc. 3: 70–73. [PubMed] [Google Scholar]
- Easton E. R., Coker R. S., and Ballinger R.. . 1986. Occurrence and seasonal incidence of mosquitoes on Indian reservations in Iowa, Nebraska and South Dakota during 1983. J. Am. Mosq. Control Assoc. 2: 190–195. [PubMed] [Google Scholar]
- Friesen K. M., and Johnson G. D.. . 2014. Mosquito and West Nile virus surveillance in northeast Montana, U.S.A., 2005 and 2006. Med. Vet. Entomol. 28: 85–93. [DOI] [PubMed] [Google Scholar]
- Gerhardt R. W. 1966. South Dakota mosquitoes and their control, pp. 531 InResearch bulletins of the South Dakota Agricultural Station (1887–2011). South Dakota Experiment Station, South Dakota State University, Brookings, SD. Available from http://openprairie.sdstate.edu/agexperimentsta [Google Scholar]
- Gu W., Lampman R., and Novak R. J.. . 2004. Assessment of arbovirus vector infection rates using variable size pooling. Med. Vet. Entomol. 18: 200–204. [DOI] [PubMed] [Google Scholar]
- Gujral I. B., Zielinski-Gutierrez E. C., LeBailly A., and Nasci R.. . 2007. Behavioral risks for West Nile virus disease, northern Colorado, 2003. Emerg. Infect. Dis. 13: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L. C., and Poulson R. L.. . 2008. Considerations for accurate identification of adult Culex restuans (Diptera: Culicidae) in field studies. J. Med. Entomol. 45: 1–8. [DOI] [PubMed] [Google Scholar]
- Janousek T. E., and Kramer W. L.. . 1999. Seasonal incidence and geographical variation of Nebraska mosquitoes, 1994-95. J. Am. Mosq. Control Assoc. 15: 253–262. [PubMed] [Google Scholar]
- Jones R. C., Weaver K. N., Smith S., Blanco C., Flores C., Gibbs K., Markowski D., and Mutebi J. P.. . 2011. Use of the vector index and geographic information system to prospectively inform West Nile virus interventions. J. Am. Mosq. Control Assoc. 27: 315–319. [DOI] [PubMed] [Google Scholar]
- Kaufman M. G., and Fonseca D. M.. 2014. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu. Rev. Entomol 59: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kightlinger L. 2017. West Nile review: 15 years of human disease in South Dakota, 2002-2016. S. D. Med. 70: 346–351. [PubMed] [Google Scholar]
- Kilpatrick A. M., Kramer L. D., Campbell S. R., Alleyne E. O., Dobson A. P., and Daszak P.. . 2005. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 11: 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Kerst A. J., Nasci R. S., Godsey M. S., Mitchell C. J., Savage H. M., Komar N., Panella N. A., Allen B. C., Volpe K. E., . et al. 2000. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis T. A. 1981. Confidence intervals for a binomial parameter after observing no successes. The American Statistician 35: 154. [Google Scholar]
- Molaei G., and Andreadis T. G.. . 2006. Identification of avian- and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, U.S.A. J. Med. Entomol. 43: 1088–1093. [DOI] [PubMed] [Google Scholar]
- Moore C. G., and Mitchell C. J.. . 1997. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg. Infect. Dis. 3: 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oidtman R. J., Christofferson R. C., ten Bosch Q. A., Espana G., Kraemer M. U. G., Tatem A., Barker C. M., and Perkins T. A.. . 2016. Pokémon go and exposure to mosquito-borne diseases: how not to catch ‘em all. PLOS Currents Outbreaks. Nov 15. Edition 1. 8. doi:10.1371/currents.outbreaks.2d885b05c7e06a9f72e4656d56b043cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer B. K., Kramer W. L., Sambol A. R., Meza J. L., Hinrichs S. H., and Iwen P. C.. . 2006. Geographic factors contributing to a high seroprevalence of West Nile virus-specific antibodies in humans following an epidemic. Clin. Vaccine Immunol. 13: 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucaet Y., Van Hemert J., Tucker B., and Bartholomay L.. . 2008. A web-based relational database for monitoring and analyzing mosquito population dynamics. J. Med. Entomol. 45: 775–784. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S., Kinley J. R., Tucker B. J., Evans R. B., Rowley W. A., and Platt K. B.. . 2008. Vector competence of Aedes vexans (Diptera: Culicidae) for West Nile virus and potential as an enzootic vector. J. Med. Entomol. 45: 452–457. [DOI] [PubMed] [Google Scholar]
- Turell M. J., Dohm D. J., Sardelis M. R., Oguinn M. L., Andreadis T. G., and Blow J. A.. . 2005. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J. Med. Entomol. 42: 57–62. [DOI] [PubMed] [Google Scholar]
- Vincent G. P., Davis J. K., Wimberly M. C., Carlson C. D., and Hildreth M. B.. . 2018. Permethrin susceptibility for the vector Culex tarsalis and a nuisance mosquito Aedes vexans in an area endemic for West Nile virus. Biomed Res. Int. 2018: 2014764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly M. C., Giacomo P., Kightlinger L., and Hildreth M. B.. . 2013. Spatio-temporal epidemiology of human West Nile virus disease in South Dakota. Int. J. Environ. Res. Public Health. 10: 5584–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski-Gutierrez E. C., and Hayden M. H.. . 2006. A model for defining West Nile virus risk perception based on ecology and proximity. EcoHealth 3: 28–34. [Google Scholar]