Abstract
In the selection of oviposition sites female mosquitoes use various cues to assess site quality to optimize survival of progeny. The presence of conspecific larvae influences this process. Interactive effects of oviposition site selection were studied in the malaria mosquito Anopheles coluzzii Coetzee & Wilkerson in dual- and no-choice assays, by exposing single gravid mosquitoes to oviposition cups containing 1) larvae of different developmental stages, 2) larvae-conditioned water (LCW), and 3) cups where visual cues of conspecific larvae were absent. Early-stage conspecific larvae had a positive effect on the oviposition response. By contrast, late stages of conspecific larvae had a negative effect. Oviposition choice was dependent on larval density. Moreover, in oviposition cups where larvae were hidden from view, late-stage larvae had a significant negative effect on oviposition suggesting the involvement of olfactory cues. LCW had no effect on oviposition response, indicating involvement of chemicals produced by larvae in vivo. It is concluded that the presence of larvae in a breeding site affects the oviposition response depending on the development stage of the larvae. These responses appear to be mediated by olfactory cues emitted by the larval habitat containing live larvae, resulting in the enhanced reproductive fitness of the females.
Keywords: Anopheles coluzzii, olfactory cues, larval pheromones, oviposition, aggregation
Physical and chemical cues allow mosquitoes to assess the suitability of potential larval habitats and hence influence the acceptance of oviposition sites (Blackwell and Johnson 2000, McCall 2002, Abrell et al. 2005, Herrera-Varela et al. 2014). Physical cues originate from vegetation, moisture, optical density, color/contrast, temperature, and texture of the substrate (McCrae 1984; Savage et al. 1990; Clements 1999; Koenraadt et al. 2003; Huang et al. 2006b, 2007; Reiskind and Zarrabi 2012). Chemical cues are produced by microorganisms (Trexler et al. 2003, Huang et al. 2006a, Lindh et al. 2008), conspecific eggs (Laurence and Pickett 1982, 1985; Ganesan et al. 2006), conspecific larvae (Mendki et al. 2000, Mokany and Shine 2002, Seenivasagan et al. 2009), water conditioned by eggs and/or larvae (Zahiri et al. 1997, Allan and Kline 1998, Zahiri and Rau 1998), plant infusions (Olagbemiro et al. 2004; Burkett-Cadena and Mullen 2007; Ponnusamy et al. 2010a, b; Tennyson et al. 2012), odors from pollen (Wondwosen et al. 2017), and predators (Mokany and Shine 2002, 2003; Silberbush et al. 2015; Why et al. 2016).
Gravid mosquitoes may be attracted to or deterred by habitats containing conspecific larvae, because the presence of such larvae may indicate the suitability of habitats, an important factor in maximizing the fitness of their offspring (Blaustein and Kotler 1993; Allan and Kline 1998). Studies on the influence of conspecific eggs/larvae on site selection suggest that culicines are generally attracted to conspecifics (Laurence and Pickett 1982, 1985; Mendki et al. 2000). So far, studies on anophelines have given conflicting results regarding the influence of conspecifics on oviposition behavior. Some authors have reported oviposition deterrence in the presence of conspecific larvae (Bentley and Day 1989; Munga et al. 2006), whereas others have reported that several females may oviposit at the same site (Chen et al. 2006, 2008), which suggests attraction. Olfactory mediation of oviposition behavior in Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) by conspecific larva was reported, and a density-dependent factor has been suggested to regulate this behavior (Sumba et al. 2008). In these studies, no difference was made between the effects of larval stages which may determine the decision taken by the ovipositing female.
The co-existence of various developmental stages of mosquito larvae (instars) in a breeding site may lead to resource competition and cannibalism (Koenraadt and Takken 2003). First instars of the malaria vector An. gambiae s.s. have been reported to be consumed by fourth instars of the same species. It is therefore plausible for a gravid mosquito to avoid oviposition in sites where late instars are present. Therefore, mosquitoes must make a careful assessment of breeding sites before selecting one for their offspring. It has been shown, however, that mosquitoes tend to avoid oviposition in habitats with predators and competitors (Kiflawi et al. 2003, Mokany and Shine 2003). Therefore, mosquitoes are faced with the challenge of choosing a suitable habitat while minimizing the costs of intra- and/or interspecific competition (Blaustein et al. 2004). However, it is not clear whether gravid females can express an optimal oviposition strategy by using only olfactory cues or in combination with other cues emitted from the larval habitat.
The ability of a female mosquito to assess breeding site suitability relies on her ability to detect and estimate the presence and density of conspecifics, in a single breeding site as well as within and among surrounding potential sites. The contribution of breeding sites with high or low larval density to the adult population and to the epidemiology of vector-borne diseases is vital as density-dependent effects may mediate the fitness of mosquitoes emerging from the site. The mechanism, with which a gravid female recognizes various densities of larvae in the breeding site, is not well understood. Only few studies have investigated oviposition site-selection behavior, despite its large consequences for individual fitness, population dynamics, and community structure (Blaustein et al. 2004).
In insects, infochemicals can originate from specialized secretory glands, body orifices, organs involved in digestion and reproduction (e.g., mouth, anus, aedeagus) (Wertheim et al. 2005). Also infochemicals can be emitted by microorganisms living symbiotically with larvae, and attract or repel conspecific adults. Odors from bacteria are known to mediate oviposition behavior in mosquitoes (Ponnusamy et al. 2008, 2010a).
Studies with the mosquito Culiseta longiareolata Macquart (Diptera: Culicidae) (Silberbush et al. 2010) and An. gambiae (Warburg et al. 2011) reveal that gravid females avoid larval habitats containing the cues of the predatory backswimmer, Notonecta maculata Fabricus (Hemiptera: Notonectidae), as a result of detecting the hydrocarbons (n-heneicosane and n-tricosane) produced by N. maculata. On the other hand, an oviposition pheromone n-heneicosane (C21) was identified and characterized from the larval cuticle of Aedes aegypti (L.) (Diptera: Culicidae) (Mendki et al. 2000). Furthermore, it was confirmed that low doses of n-heinecosane attract gravid Ae. aegypti, whereas at higher doses they are repelled by the pheromone (Seenivasagan et al. 2012). It is not surprising that this same compound repels Culiseta. Recent studies based on electroantennographic responses (GC-EAD) and additional oviposition assays confirmed n-heneicosane to be an oviposition pheromone in Ae. aegypti and a behavior modifier of Ae. albopictus (Skuse) (Diptera: Culicidae) in larval habitats (Gonzalez et al. 2014).
Understanding mosquito oviposition behavior can provide a tool for behavioral manipulation of mosquitoes in the field and enable the development of an effective vector surveillance and control strategy. The objective of the present study was to investigate the influence of larval stages and density of conspecifics on the oviposition strategy of An. coluzzii Coetzee & Wilkerson (Diptera: Culicidae) and whether this strategy was chemically mediated. Specifically, the study aimed to explore the age at which larvae attract or deter conspecific gravid females to oviposit, and identify respective intraspecific cues involved in oviposition site selection.
Materials and Methods
Mosquitoes
Experiments were performed using gravid An. coluzzii (Suakoko line) mosquitoes reared at the Laboratory of Entomology of Wageningen University, The Netherlands. Mosquitoes have been kept in the Laboratory of Entomology, Wageningen University since 1988 and maintained on human blood. Anopheles coluzzii were reared in a climate-controlled room at 28°C and 80% RH, with 12:12 (L:D) h photoperiod. Adults were kept in a 30-cm cubic cage, with constant access to 6% glucose solution. Larvae were reared in 2.5-liter plastic trays filled with tap water and fed Tetramin (Tetra Werke, Melle, Germany) fish food. Pupae were collected daily and placed in small trays inside the adult cage for emergence.
Bioassay Conditions
All bioassays were conducted in a standard 30 cm × 30 cm × 30 cm (length, width, height) netting cage, placed inside a climate-controlled room at 28°C and 80% RH, with 12:12 (L:D) h photoperiod. Thirty 5- to 6-d-old mosquitoes were placed in one cage, and offered a blood meal for 10 min. Unfed mosquitoes were removed from the cage and blood-fed mosquitoes were kept for 2 d while provided with 6% glucose solution on filter paper until they became fully gravid. Individual mosquitoes were randomly selected among these gravid females and used in the experiments. Selected females were placed singly in a cage with 6% glucose ad libitum and provided with two plastic oviposition cups (5 cm diameter × 4 cm height) in a two-choice bioassay. Each oviposition cup was placed diagonally in the corner of the cage at the farthest possible distance (~35 cm) from the other cup. Oviposition was checked at 24 and 48 h after the start of the experiment.
First and fourth instars (aged 2 and 7 d post-oviposition) were taken from the colony trays, using a plastic pipette. Water drops containing the larvae were placed on the bottom of an empty 2.5-liter rearing pan and the larvae counted. Rearing water was removed as much as possible by rinsing larvae through a sieve and rinsing them once with tap water. Subsequently, larvae were transferred to the oviposition cups and tap water of 28°C was added to each cup to a volume of 30 ml. Each cup contained either first instars, fourth instars, or both, depending on the experimental setup. Each experimental cage was assigned a pair of oviposition cups containing 30 ml of tap water (control) or 30 ml of tap water with larvae.
Oviposition Response in the Absence of Larvae
An oviposition response experiment in the absence of larvae was designed to investigate mosquito oviposition preference when given a dual choice of cups filled with tap water only (no treatment, negative control).
Oviposition in Response to First Instars
The influence of the density of conspecific first instar larvae on oviposition site selection of gravid An. coluzzii was investigated. Four cages were used and each was assigned two oviposition cups containing water with or without larvae. The numbers and densities (larvae per ml of water) of conspecific larvae tested against controls were 10 (0.3), 30 (1), 50 (1.7), and 100 (3.3). On each experimental day, the four treatments were tested simultaneously.
Oviposition in Response to Fourth Instars
The potential effect of fourth instars on oviposition of gravid An. coluzzii was investigated at two larval densities, each tested against a control of tap water. One cup contained 10 fourth instars and the other contained 50 fourth instars (0.3 and 1.7 larvae per ml of water, respectively). Larvae were obtained from the rearing stock and they were not fed during the experiment. On each experimental day, the two densities were tested simultaneously.
Oviposition in Response to First and Fourth Instars
Dual-choice tests were designed to investigate whether gravid An. coluzzii make a trade-off between first-stage and fourth-stage conspecific larvae of low and high densities during oviposition site selection. Two cages were used; one with 10 first instars and 10 fourth instars in cups within one cage. A second cage had 50 first and 50 fourth instars.
Oviposition Response to Larvae-Conditioned Water
This experiment investigated whether oviposition attractant/deterrent cues are present in larvae-conditioned water (LCW). Fifty first or fourth instar larvae were kept in oviposition cups for 24 h, and provided with Tetramin baby-fish food (Tetra Werke, Melle, Germany) at 0.01 g per cup. A control was prepared by adding the same amount of Tetramin to a cup of tap water and let age for 24 h. The water from these cups was filtered through Whatman filter paper no. 2 into clean cups after 24 h and used in the experiment. Two treatments tested water from first or fourth instar larvae against a negative control of water conditioned with Tetramin and against each other.
Oviposition Response to Tetramin-Conditioned Water
To investigate if the oviposition response observed with larvae of different developmental stages was caused by larval cues or by nutritional cues, gravid females were exposed to oviposition cups containing Tetramin-conditioned water (tcw) and tcw + first or fourth instars in a dual-choice assay. Tetramin (0.02 g) was added to 30 ml of tap water, shaken vigorously, and left for 12 h. Then, the water was filtered by using Whatman filter paper no. 2 into other cups and used in this experiment. Fifty first and fourth instars, respectively, were placed in oviposition cups and provided with 30 ml of tcw. In a dual-choice setup, in the standard 30 × 30 × 30 cm bioassay cage, single gravid mosquitoes were exposed to 1) tcw versus tcw + first instars, 2) tcw versus tcw + fourth instars, and 3) tcw + first instars versus tcw + fourth instars. Cups were inspected for presence of eggs after 24 and 48 h.
Oviposition Response to Cups Covered With Filter Paper
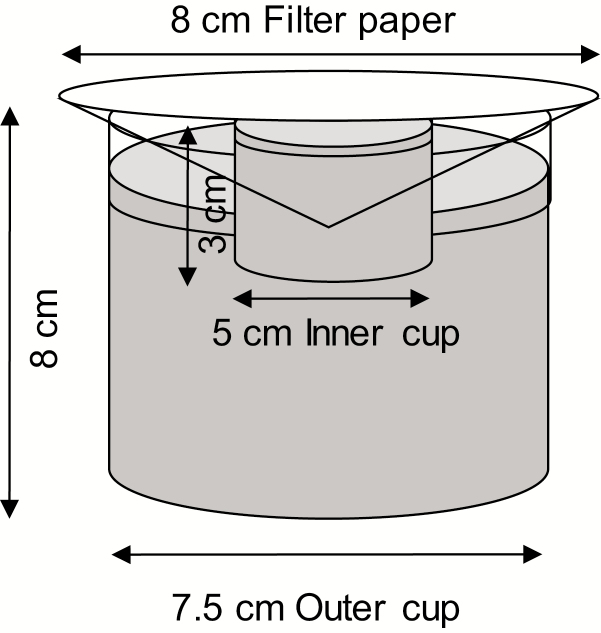
This experiment separated the influence of physical and chemical cues on oviposition site selection by gravid mosquitoes. Larger plastic cups (7.5 cm diameter) that contained 100 first or fourth stage larvae and water were prepared. A small cup (5 cm diameter) with distilled water was immersed on the large cup, serving as an oviposition cup and then, a filter paper was inserted to touch the water in the small cup but not the larval water in the large cup (Fig. 1). The entire setup was covered by a filter paper. The small cup prevented contamination of the filter paper with larval water while the wet filter paper prevented gravid females from seeing larvae in the cup, and at the same time served as an oviposition substrate. In this setup, dark color or shade due to the presence of larvae was controlled as a larva that darkens the oviposition substrate was hidden from view.
Fig. 1.
Diagram of the setup of the double cup experiment in which visual cues from larvae are hidden from the mosquito view by filter paper.
No-Choice Experiment
The aim of this experiment was to assess the response of ovipositing females to larvae of different instars (L1 and L4) in the absence of alternative oviposition sites. Individual gravid mosquitoes were exposed to a cup containing either 100 first instars or 50 fourth instars for 72 h. Oviposition was checked at 12, 24, 48, and 72 h. Any eggs remaining in the ovaries and not oviposited were counted following dissection.
A flow diagram of the experimental procedures is presented in Supp Fig. 1 (online only).
Statistical Analysis
The nature of a dual-choice assay with a single gravid female per cage yields discrete data as a female may lay all eggs in one oviposition cup only. Therefore, we used nonparametric statistical procedures to determine the difference in number of eggs laid on the paired oviposition substrates. Wilcoxon signed rank tests for paired samples were conducted using IBM SPSS statistics 20 for Windows. The response variable reported here is the total number of eggs collected after 48 h and each experimental setup was replicated at least eight times on different days. Data from the no-choice experiment were analyzed by a Kruskal–Wallis test.
Results
Oviposition Response in the Absence of Larvae
There was no significant difference in the mean number of eggs that mosquitoes deposited in either cup. In 20 replicates, females laid eggs randomly in one of the two cups only, with no preference for any cup (mean number of eggs per cup: 23.7 ± 5.7 and 22.5 ± 5.6, N = 20, z = −0.28, P = 0.779).
Oviposition in Response to First Instars
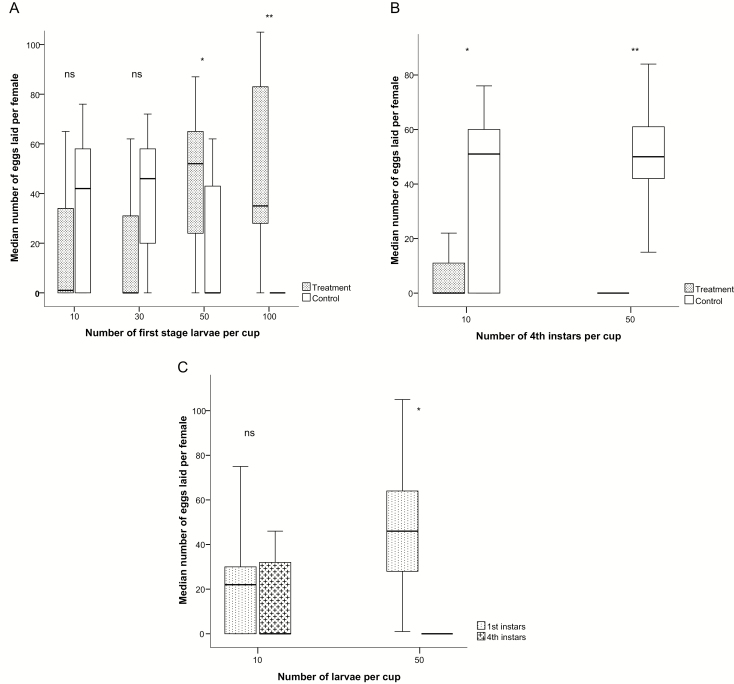
Gravid An. coluzzii deposited significantly more eggs in cups containing 50 (N = 17, z = −1.965, P = 0.049) or 100 first instars (N = 17, z = −3.432, P < 0.001) than in negative control cups. Mosquitoes laid three times as many eggs in cups with 50 first instars and 28 times as many eggs in cups containing 100 first instars compared to control cups. The difference between eggs laid in cups with 10 or 30 first instars and their controls was not significant (N = 17, z = −1.538, P = 0.124) and (N = 17, z = −1.7, P = 0.088), respectively (Fig. 2A).
Fig. 2.
(A) Number of eggs laid by single female Anopheles coluzzii per cup in a dual-choice essay, using cups with conspecific first instars in ascending densities and cups containing water only (controls). Median and quartiles are given; asterisks indicate statistical differences between treatment and control for a given density (*P < 0.05, **P < 0.001, Wilcoxon signed rank tests). The number of replicates was 17. (B) Number of eggs laid by single female An. coluzzii per cup in a dual-choice essay, using cups with conspecific fourth instars in densities of 10 and 50 per cup, respectively, against cups containing water only (controls). Median and quartiles are given; asterisks indicate statistical differences between treatment and control for a given density (*P < 0.01, **P < 0.001, Wilcoxon signed rank tests). The number of replicates was 17. (C) The number of eggs laid by An. coluzzii in a dual-choice test between cups treated with low and high densities of conspecific first and fourth instars. Median and quartiles are given; asterisks indicate statistical differences between the larval stages at a given density (*P < 0.001, Wilcoxon signed rank tests). The number of replicates was 17.
Oviposition in Response to Fourth Instars
Gravid An. coluzzii deposited significantly more eggs in control cups than in the cups containing 10 fourth instars (N = 17, z = −2.627, P = 0.009) or in cups with 50 fourth instars (N = 17, z = −3.338, P < 0.001) (Fig. 2B).
Oviposition in Response to Larvae in Two Different Development Stages
Gravid mosquitoes did not discriminate between cups with 10 first instars and cups with 10 fourth instars (N = 17, z = −0.545, P = 0.586). However, when cups with 50 first instars were tested against cups with 50 fourth instars, no eggs were deposited in the cups containing fourth instars (N = 17, z = −3.621, P < 0.001). The cup containing 50 first instars received on average 46 ± 7.1 eggs (Fig. 2C).
Oviposition Response to LCW
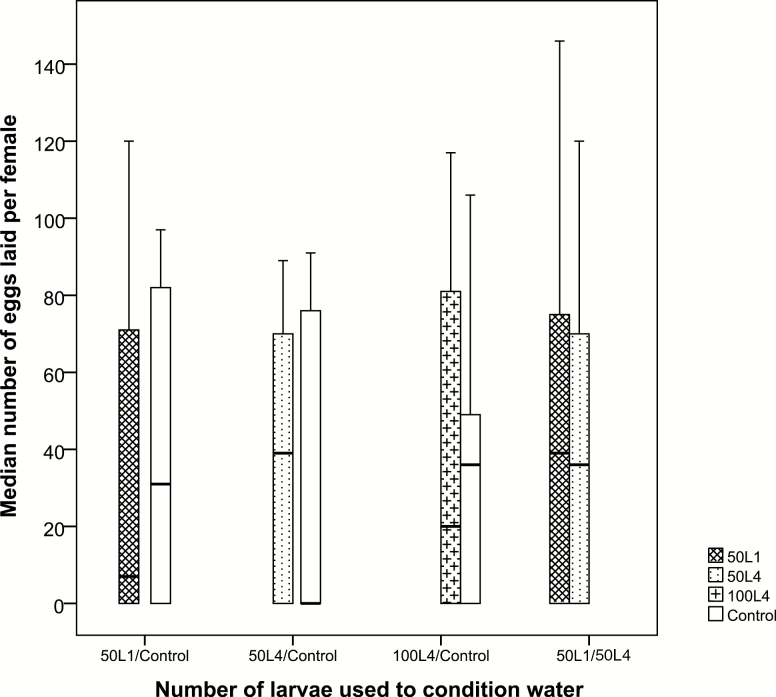
There was no significant difference in the number of eggs that females deposited in the control cup and the cup with water conditioned by first instars (N = 17, z = −0.24, P = 0.981) or by fourth instars (N = 17, z = −0.118, P = 0.906) (Fig. 3). Also, in a dual-choice test, there was no significant difference between eggs laid on water conditioned by first or fourth instars (N = 17, z = −0.379, P = 0.705) (Fig. 3).
Fig. 3.
Anopheles coluzzii oviposition (measured as the number of eggs laid per female) in cups treated with LCW and respective controls. Set 1 consists of LCW from 50 first instars and a control, set 2 consists of LCW from 50 fourth instars and a control, set 3 consists of LCW from 100 fourth instars and a control, and set 4 consists of LCW from 50 first instar larvae and LCW from 50 fourth instar larvae. Median and quartiles are given; there were no significant differences in any of the four treatments (Wilcoxon signed rank tests). The number of replicates was 20.
Oviposition Response to tcw
In dual-choice tests, there were no differences in the mean number of eggs laid per female in cups with tcw than in first instars + tcw or fourth instars + tcw (Table 1). In a choice between tcw and larvae + tcw, however, females more often selected the tcw-only cups. When first instars + tcw were tested against fourth instars + tcw, females selected more often the cups with first instars, but there was no difference in the mean number of eggs laid between treatments.
Table 1.
Oviposition response of Anopheles coluzzii in tcw and larval instars to which tcw has been added
| Treatments | ||||||
|---|---|---|---|---|---|---|
| L1 + tcw | tcw | L4 + tcw | tcw | L1 + tcw | L4 + tcw | |
| No. of females ovipositing in either cup | 4 | 9 | 4 | 9 | 9 | 5 |
| Total eggs | 342 | 781 | 368 | 736 | 924 | 401 |
| Mean no. of eggs per female | 85.5 | 86.8 | 92.0 | 81.8 | 102.7 | 80.2 |
| SE | 12.53 | 14.77 | 13.91 | 15.63 | 18.28 | 11.66 |
Number of replicates per treatment: n = 13.
Oviposition Response to Cups Covered With Filter Papers
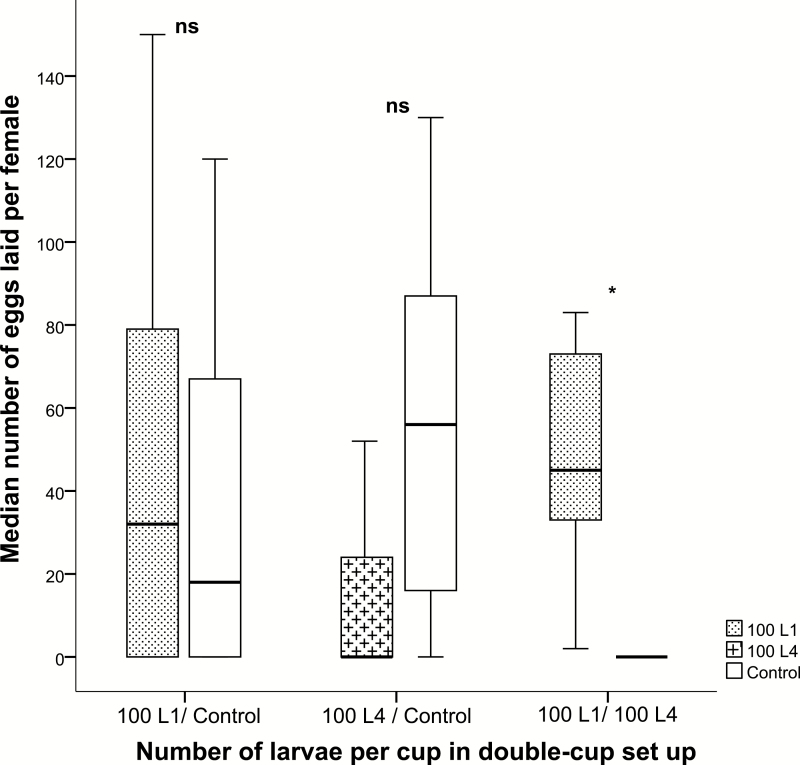
When larvae were hidden from view by covering the oviposition cups with filter paper using the double cup technique, there was no significant difference between eggs laid in cups with 100 first instars and the control cups (N = 17, z = −0.308, P = 0.758), but in the test with fourth instars, significantly more eggs were laid in the control cups than in cups with 100 fourth instars (N = 17, z = −2.225, P = 0.26) (Fig. 4). When cups with 100 first instars were tested against cups with 100 fourth instars, An. coluzzii laid significantly more eggs in cups with first instars than in those with fourth instars (N = 17, z = −3.432, P = 0.001) (Fig. 4).
Fig. 4.
Anopheles coluzzii oviposition (measured as the number of eggs laid per female) test between cups treated with 1) 100 first instars and a control, 2) 100 fourth instars and a control, and 3) between 100 first instars and 100 fourth instars using a double cup setup. Median and quartiles are given (*P < 0.05, Wilcoxon signed rank tests). The number of replicates was 17.
Oviposition Response in Single Cup (No-Choice Experiment)
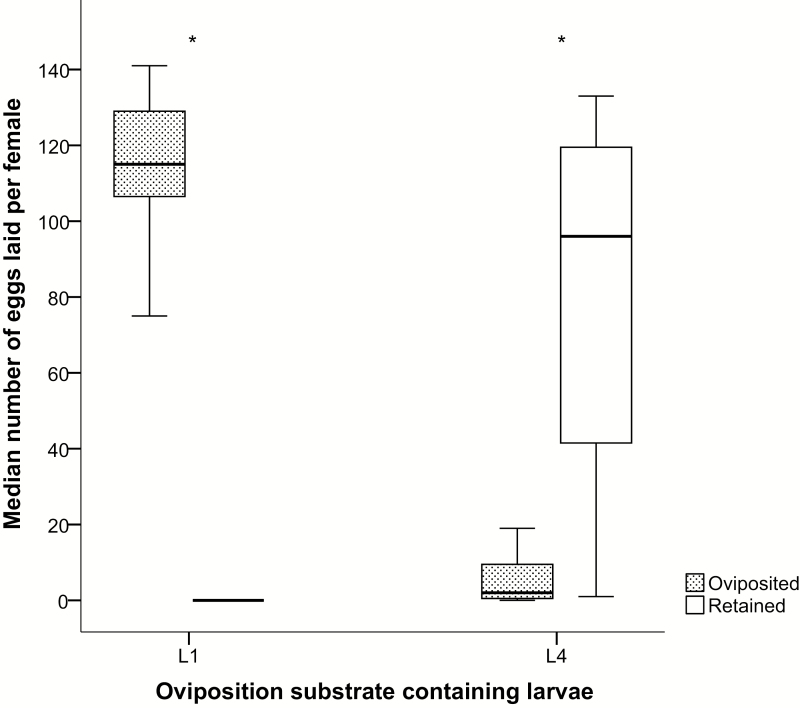
In 15 replicates, 100% of females exposed to cups with first instars laid eggs. By contrast, of the females exposed to fourth instars, only 73.3% laid eggs. Cups with first instars received significantly more eggs 103.4 ± 10.7 (Kruskal–Wallis test, P < 0.001) than cups with fourth instars, which received an average number of 7.6 ± 3.5 eggs. Moreover, all eggs in cups containing first instars were laid within the first 12 h after exposing gravid mosquitoes to the developing larvae, while females exposed to fourth instars spread their eggs between 12 and 48 h. After 72 h, all mosquitoes were removed and dissected. The mean number of eggs retained in mosquitoes exposed to fourth instars (77.5 ± 12.3) was significantly greater (P < 0.001) than the mean number of eggs retained in mosquitoes exposed to first instars (0.5 ± 0.35) (Fig. 5).
Fig. 5.
Egg-laying responses of Anopheles coluzzii when exposed to oviposition substrates containing either first instar larvae (L1) or fourth instar larvae (L4) under no-choice conditions (*P < 0.001). The number of replicates was 15.
Discussion
Our study indicated that gravid females of An. coluzzii were affected in their oviposition behavior by volatiles produced by conspecific larvae, in which first instars produced attractive chemicals, whereas fourth instars produced chemicals that deterred females. This is likely the reason why females select sites with a high density of first instars, while avoiding sites with low densities of such larvae. Studies on An. gambiae oviposition behavior showed that members of this group of anophelines tend to lay eggs in groups and several females may oviposit in the same site (Chen et al. 2006, 2008). Therefore, the choice for laying eggs in sites with high densities of conspecifics can be beneficial, unless these sites confer unfavorable traits such as competition, cannibalism, or predation. When these conspecifics, however, are in an advanced age of development, they may be dangerous for young siblings, and females avoid such sites to reduce the risk of cannibalism (Koenraadt and Takken 2003). Therefore, the avoidance of oviposition in sites where late-stage larvae are present is of great advantage to mosquitoes and reveals how mosquitoes might achieve an optimal oviposition strategy by exploiting sites that confer the best chance for offspring development and survival. In nature, the presence of immature stages could indicate the suitability of that site in terms of food, site persistence, lack of predation, and appropriate abiotic conditions (Wong et al. 2011).
The findings of our experiment suggest that in the absence of conspecific larvae, mosquitoes oviposit in water bodies selected at random. The mean number of eggs laid in cups with water only was less than the mean number of eggs laid in cups containing larvae with densities starting from 1.7 larvae per ml of water. This indicates that females retain some of their eggs when there is no clear evidence of site suitability. Moreover, in the presence of first instars, oviposition responses increased with increasing larval density starting from 1.7 larvae per ml of water. Likewise, the strength of deterrence caused by fourth instars increased with increasing larval density. We can therefore conclude that the oviposition response in the presence of conspecific larvae is density-dependent. This was also found by Ogbunugafor and Sumba (2008), who reported similar olfaction-guided oviposition behavior of An. gambiae with density dependence. In Ogbunugafor and Sumba’s study, however, no distinction was made between young and older instars. In fact, their study reported deterrence of oviposition by all larval densities in distilled water. As we learned from our study, this is possibly caused by volatiles produced by fourth instars, which under all circumstances, at densities of 0.3–3.3 larvae per ml, caused deterrence. Our findings also supported an earlier hypothesis that breeding sites with low larval density are perceived as unsuitable by gravid mosquitoes (Munga et al. 2006).
Higher larval densities are common, especially in small breeding sites such as hoof/foot prints, tire prints, and road banks. In a nutrient-enriched site, a single mosquito may lay 50–500 eggs at one time (Clements 1992). As conspecific sharing of breeding sites is common among mosquitoes (Chen et al. 2008) and egg-hatching rate is high (Phasomkusolsil et al. 2013), small sites are often harboring high larval densities. Therefore, the existence of breeding sites with low larval density suggests unfavorable conditions. Anopheles gambiae s.l. tends to breed in such small, shallow, and temporary sites due to absence of predation (Service 1993, Mala and Irungu 2011, Muriu et al. 2013).
In natural conditions some habitats contain high densities of anopheline larvae, whereas many others have none despite high densities of adult mosquitoes in the immediate environment (Minakawa et al. 2002). These observations are in line with the findings from our study and observations by other authors that some aquatic habitats are more attractive or suitable for oviposition and larval development than others (Minakawa et al. 1999), and as a result, mosquitoes perform selective oviposition behavior (Davis et al. 2015). Randomly amplified polymorphic DNA used to estimate the number of full-sibling family size revealed that the average family size of Ae. aegypti mosquito larvae in a container is 11 and the family size distribution among containers is skewed toward containers with one or two families (Apostol et al. 1994). Studies on oviposition behavior of An. gambiae using a pairwise genetic relatedness method substantiate that average genetic relatedness tends to be low for breeding sites with high larval populations (Chen et al. 2006). This means that An. gambiae prefers to oviposit where others have oviposited. Our study suggests that the presence of an optimum density of first instar stage larvae in selected breeding sites may induce other conspecific mosquitoes to oviposit in the same breeding sites.
Results from the no-choice experiments suggest that substrates containing first instars attract egg-laying behavior of conspecific gravid females, whereas substrates containing fourth instars deter females intending to oviposit and induce egg-retention behavior. Egg retention is common in skip oviposition where mosquitoes do not lay all eggs in one site but retain some (Williams et al. 2008) and lay in multiple sites (Colton et al. 2003, Reiter 2007, Snell et al. 2010).
In our study we found that egg retention is common when fourth instars are present in oviposition cups, and oviposition in more than one container occurred when nutrient-poor substrates were used in both oviposition choices. This suggests that skip oviposition occurs when there is no clear indication of site suitability, as mosquitoes do not display a clear preference. Moreover, our results suggest that in natural settings, where the only available water body contains predators (e.g., conspecific fourth instars), An. coluzzii will lay few eggs in that risky environment on the first day. On the second day, An. coluzzii will lay even fewer eggs as compared to the first day while retaining the rest of the eggs. These observations are akin to skip oviposition behavior, which is displayed by other mosquitoes. For example, in Ae. aegypti, significantly higher oviposition occurred in one site and residual eggs were distributed in groups of 1–30 eggs (Oliva et al. 2014).
Our data from the double cup experiment, where visual stimuli from water and/or larvae were excluded, indicate that olfactory compounds are involved in oviposition behavior. Chemical communication has been demonstrated to operate in mosquito oviposition behavior in several mosquito species (Bentley and Day 1989, McCall and Cameron 1995) including An. gambiae s.s. (Blackwell and Johnson 2000), a sibling of An. coluzzii. However, our results demonstrate that only alive, conspecific larvae produce these cues, as LCW did not cause a behavioral response. These effects are clearly dose-dependent, as the magnitude of the influence increases with increasing larval density and at low larval densities the influence was not observed. Ogbunugafor and Sumba (2008) also reported the presence of oviposition-mediating olfactory cues derived from immature larvae of An. gambiae. In their study, however, larval water from different instars was mixed, and hence the chemical cues from one instar may have overridden the deterrent effects from fourth instars.
The effects of the larval cues appeared to be overruled by cues produced by Tetramin. Nutritional substrates are well known to affect An. gambiae oviposition behavior (Lindh et al. 2008) and an oviposition attractant cedrol was identified and associated with the presence of microbes in the breeding habitat (Lindh et al. 2015). Therefore, it is likely that chemical cues produced from nutritional constituents such as Tetramin impact mosquito oviposition behavior, masking the effects of the larval cues. Significant differences in cues from first and fourth instars, however, are evident as gravid females laid more eggs in larval substrates with first instars than with fourth instars.
When female An. coluzzii were offered a choice between oviposition cups with fourth instars and a control, they deposited more eggs in the control cups. When subjected to a choice between same high densities of early- and late-stage larvae, female mosquitoes opted for the former exclusively. These results indicate the presence of different types of larval pheromones involved in the oviposition strategy of An. coluzzii. Pheromones produced by early instars that signal to the gravid female suitability of the site and pheromones produced by late instars that signal unsuitability. The pheromone might be of the same nature, but produced at a different concentration by early- and late-stage larvae. On the other hand, the difference between eggs laid in cups with low densities of first and fourth larval instars was not significant, and confirms our earlier findings that a low density of first instars does not signal the quality of a site. It is possible that the emission of chemical cues that are likely to mediate oviposition behavior is too low when larval density is low.
Under laboratory conditions, gravid An. gambiae usually touch the oviposition substrate briefly or hover 5–10 cm above the site before depositing her eggs (Huang et al. 2006b). Such an assessment procedure may involve visual, tactile, or olfactory cues, alone or in combination. Experiments with LCW did not show a significant difference between conditioned water and controls. The disappearance of attractiveness or deterrence after the removal of larvae may suggest two things: first, vision is also involved in the previously displayed behavior. Secondly, the presence of live larvae is necessary to evoke the observed behavior. Chemicals released by mosquito larvae are highly volatile and present in low concentrations (Allan and Kline 1998). Therefore, it is possible that the removal of larvae and volatilization of chemicals have contributed to absence of attractiveness or deterrence response in the LCW experiments.
Studies by Huang et al. (2018) emphasized that cannibalization of newly hatched An. gambiae larvae by fourth instar larvae was a result of egg reduction in egg counts and not olfactory deterrent cues. However, results from the double cup experiments, in which the larvae are hidden from view by a filter paper (Fig. 1), suggest that chemical cues are involved in the oviposition behavior of An. coluzzii. Our study further suggests that oviposition-deterrent chemicals are responsible for counteracting resource competition and cannibalism among larvae of An. coluzzii in field settings. We therefore confirm recent findings that ovipositing An. coluzzii females are less inclined to lay eggs in pools that have late instars compared to those with early instars (Sumba et al. 2008). These observations can partly explain why an initial population peak of Anopheles larvae observed at a fixed point in a rice field was not immediately followed by a sustained high population peak of early instars (Mutero et al. 2004). Our study was conducted in the laboratory, using mosquitoes that originated from Liberia. When the study was repeated in Tanzania using mosquitoes that originated from Tanzania, we obtained similar results (V. S. Mwingira, unpublished data). However, further studies need to be conducted in various places with different climatic and geographic conditions to clarify the existence of a region-specific cue in An. coluzzii (Ogbunugafor and Sumba 2008).
Larval-based cues can be used for manipulation of mosquito behavior in a push-pull system by making protected resources unsuitable to mosquitoes (by repellents) while luring them toward attractive sources (by attractants) where they can be eliminated by insecticides. It appears evident that larval hydrocarbons can be detected by conspecific (Seenivasagan et al. 2009) and heterospecific mosquitoes (Gonzalez et al. 2014). Moreover, depending on the concentration, female mosquitoes were attracted to a low dose, while higher doses enforced repellency (Seenivasagan et al. 2009). We suspect that the observed pattern in our study might be explained by a similar mechanism whereby early instars emit chemical cues at low concentrations that attract, while late instars emit high concentrations which repel female mosquitoes.
Conclusion
Conspecific larvae, as demonstrated by this study, mediate the oviposition of An. coluzzii to enhance successful development of their offspring. Low densities of first instars did not attract gravid mosquitoes, while higher densities attracted gravid mosquitoes significantly. In contrast, fourth instars caused an oviposition-deterring response. These effects occurred only in vivo, in the presence of live conspecific larvae, as LCW neither stimulated nor deterred oviposition. The study further suggests that the oviposition attraction and deterring effects of larvae are masked by chemical cues from larval nutriments such as Tetramin, and therefore volatiles from other organisms present in natural breeding sites may interact with the instar-associated cues as found in the present study. The attractive and deterrent effects on ovipositing females were caused, at least in part, by nonvisual cues emitted by live larvae and suggests that the oviposition behavior of An. coluzzii is chemically mediated.
Supplementary Material
Acknowledgments
We thank Leo Koopman, Frans van Aggelen, and André Gidding for rearing mosquitoes, Niels Verhulst and Maaike Bruinsma for feeding mosquitoes and assisting in data analyses. We also thank Bart Knols for his earlier comments on this study. This work was supported by funds from Wageningen University, Project No: 55510 and the Dutch Organisation for Internationalisation in Education (Nuffic) and is published with permission from the Director General, National Institute for Medical Research, Tanzania.
References Cited
- Abrell L., Guerenstein P. G., Mechaber W. L., Stange G., Christensen T. A., Nakanishi K., and Hildebrand J. G.. 2005. Effect of elevated atmospheric CO2 on oviposition behavior in Manduca sexta moths. Glob. Chang. Biol. 11: 1272–1282. [Google Scholar]
- Allan S. A., and Kline D. L.. 1998. Larval rearing water and preexisting eggs influence oviposition by Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 35: 943–947. [DOI] [PubMed] [Google Scholar]
- Apostol B. L., Black W. C. IV, Reiter P., and Miller B. R.. 1994. Use of randomly amplified polymorphic DNA amplified by polymerase chain reaction markers to estimate the number of Aedes aegypti families at oviposition sites in San Juan, Puerto Rico. Am. J. Trop. Med. Hyg. 51: 89–97. [DOI] [PubMed] [Google Scholar]
- Bentley M. D., and Day J. F.. 1989. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 34: 401–421. [DOI] [PubMed] [Google Scholar]
- Blackwell A., and Johnson S. N.. 2000. Electrophysiological investigation of larval water and potential oviposition chemoattractants for Anopheles gambiae sensu stricto. Ann. Trop. Med. Parasitol. 94: 389–398. [DOI] [PubMed] [Google Scholar]
- Blaustein L., and Kotler B. P.. 1993. Oviposition habitat selection by the mosquito, Culiseta longiareolata: effects of conspecifics, food and green toad tadpoles. Ecol. Entomol. 18: 104–108. [Google Scholar]
- Blaustein L., Kiflawi M., Eitam A., Mangel M., and Cohen J. E.. 2004. Oviposition habitat selection in response to risk of predation in temporary pools: mode of detection and consistency across experimental venue. Oecologia. 138: 300–305. [DOI] [PubMed] [Google Scholar]
- Burkett-Cadena N. D., and Mullen G. R.. 2007. Field comparison of Bermuda-hay infusion to infusions of emergent aquatic vegetation for collecting female mosquitoes. J. Am. Mosq. Control Assoc. 23: 117–123. [DOI] [PubMed] [Google Scholar]
- Chen H., Fillinger U., and Yan G.. 2006. Oviposition behavior of female Anopheles gambiae in western Kenya inferred from microsatellite markers. Am. J. Trop. Med. Hyg. 75: 246–250. [PubMed] [Google Scholar]
- Chen H., Minakawa N., Cui L. W., and Yan G. Y.. 2008. Conspecific sharing of breeding sites by anopheline female mosquitoes (Diptera: Culicidae) inferred from microsatellite markers. J. Insect Behav. 21: 24–33. [Google Scholar]
- Clements A. N. 1992. The biology of mosquitoes, vol. I Chapman & Hall, London, United Kingdom. [Google Scholar]
- Clements A. N. 1999. The biology of mosquitoes, vol. II CABI Publishers, Wallingford, United Kingdom. [Google Scholar]
- Colton Y. M., Chadee D. D., and Severson D. W.. 2003. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med. Vet. Entomol. 17: 195–204. [DOI] [PubMed] [Google Scholar]
- Davis T. J., Kaufman P. E., Hogsette J. A., and Kline D. L.. 2015. The effects of larval habitat quality on Aedes albopictus skip oviposition. J. Am. Mosq. Control Assoc. 31: 321–328. [DOI] [PubMed] [Google Scholar]
- Ganesan K., Mendki M. J., Suryanarayana M. V. S., Prakash S., and Malhotra R. C.. 2006. Studies of Aedes aegypti (Diptera: Culicidae) ovipositional responses to newly identified semiochemicals from conspecific eggs. Aust. Entomol. 45: 75–80. [Google Scholar]
- Gonzalez P. V., Audino P. A. G., and Masuh H. M.. 2014. Electrophysiological and behavioural response of Aedes albopictus to n-heinecosane, an ovipositional pheromone of Aedes aegypti. Entomol. Exp. Appl. 151: 191–197. [Google Scholar]
- Herrera-Varela M., Lindh J., Lindsay S. W., and Fillinger U.. 2014. Habitat discrimination by gravid Anopheles gambiae sensu lato–a push-pull system. Malar. J. 13: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Walker E. D., Otienoburu P. E., Amimo F., Vulule J., and Miller J. R.. 2006a. Laboratory tests of oviposition by the African malaria mosquito, Anopheles gambiae, on dark soil as influenced by presence or absence of vegetation. Malar. J. 5: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Miller J. R., Chen S. C., Vulule J. M., and Walker E. D.. 2006b. Anopheles gambiae (Diptera: Culicidae) oviposition in response to agarose media and cultured bacterial volatiles. J. Med. Entomol. 43: 498–504. [DOI] [PubMed] [Google Scholar]
- Huang J., Walker E. D., Vulule J., and Miller J. R.. 2007. The influence of darkness and visual contrast on oviposition by Anopheles gambiae in moist and dry substrates. Physiol. Entomol. 32: 34–40. [Google Scholar]
- Huang J., Miller J. R., and Walker E. D.. 2018. Cannibalism of egg and neonate larvae by late stage conspecifics of Anopheles gambiae (Diptera: Culicidae): implications for ovipositional studies. J. Med. Entomol. 55: 801–807. [DOI] [PubMed] [Google Scholar]
- Kiflawi M., Blaustein L., and Mangel M.. 2003. Predation-dependent oviposition habitat selection by the mosquito Culiseta longiareolata: a test of competing hypotheses. Ecol. Lett. 6: 35–40. [Google Scholar]
- Koenraadt C. J. M., and Takken W.. 2003. Cannibalism and predation among larvae of the Anopheles gambiae complex. Med. Vet. Entomol. 17: 61–66. [DOI] [PubMed] [Google Scholar]
- Koenraadt C. J., Paaijmans K. P., Githeko A. K., Knols B. G., and Takken W.. 2003. Egg hatching, larval movement and larval survival of the malaria vector Anopheles gambiae in desiccating habitats. Malar. J. 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence B. R., and Pickett J. A.. 1982. Erythro-6-acetoxy-5-hexadecanolide, the major component of a mosquito oviposition attractant pheromone. J. Chem. Soc. Chem. Commun.1: 59–60. [DOI] [PubMed] [Google Scholar]
- Laurence B. R., and Pickett J. A.. 1985. An oviposition attractant pheromone in Culex quinquefsciatus Say (Diptera: Culicidae). Bull. Entomol. Res. 75: 283–290. [Google Scholar]
- Lindh J. M., Kännaste A., Knols B. G., Faye I., and Borg-Karlson A. K.. 2008. Oviposition responses of Anopheles gambiae s.s. (Diptera: Culicidae) and identification of volatiles from bacteria-containing solutions. J. Med. Entomol. 45: 1039–1049. [DOI] [PubMed] [Google Scholar]
- Lindh J. M., Okal M. N., Herrera-Varela M., Borg-Karlson A. K., Torto B., Lindsay S. W., and Fillinger U.. 2015. Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex. Malar. J. 14: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mala A. O., and Irungu L. W.. 2011. Factors influencing differential larval habitat productivity of Anopheles gambiae complex mosquitoes in a western Kenyan village. J. Vector Borne Dis. 48: 52–57. [PubMed] [Google Scholar]
- McCall P. J. 2002. Chemoecology of oviposition in insects of medical and veterinary importance. Chemoecology of insect egg deposition, pp. 265–289. InHilker M. and Meiners T. (eds.), Chemoecology of insect eggs and egg deposition. Blackwell Verlag GmbH, Berlin, Germany. [Google Scholar]
- McCall P. J., and Cameron M. M.. 1995. Oviposition pheromones in insect vectors. Parasitol. Today. 11: 352–355. [DOI] [PubMed] [Google Scholar]
- McCrae A. W. R. 1984. Oviposition by African malaria vector mosquitoes. II. Effects of site tone, water type and conspecific immatures on target selection by freshwater Anopheles gambiae Giles, sensu lato. Ann. Trop. Med. Parasitol. 78: 307–318. [PubMed] [Google Scholar]
- Mendki M. J., Ganesan K., Prakash S., Suryanarayana M. V. S., Malhotra R. C., Rao K. M., and Vaidyanathaswamy R.. 2000. Heneicosane: an oviposition-attractant pheromone of larval origin in Aedes aegypti mosquito. Curr. Sci. 78: 1295–1296. [Google Scholar]
- Minakawa N., Mutero C. M., Githure J. I., Beier J. C., and Yan G.. 1999. Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am. J. Trop. Med. Hyg. 61: 1010–1016. [DOI] [PubMed] [Google Scholar]
- Minakawa N., Seda P., and Yan G.. 2002. Influence of host and larval habitat distribution on the abundance of African malaria vectors in western Kenya. Am. J. Trop. Med. Hyg. 67: 32–38. [DOI] [PubMed] [Google Scholar]
- Mokany A., and Shine R.. 2002. Competition between tadpoles and mosquitoes: the effects of larval density and tadpole size. Aust. J. Zool. 50: 549–563. [Google Scholar]
- Mokany A., and Shine R.. 2003. Competition between tadpoles and mosquito larvae. Oecologia. 135: 615–620. [DOI] [PubMed] [Google Scholar]
- Munga S., Minakawa N., Zhou G., Barrack O. O., Githeko A. K., and Yan G.. 2006. Effects of larval competitors and predators on oviposition site selection of Anopheles gambiae sensu stricto. J. Med. Entomol. 43: 221–224. [DOI] [PubMed] [Google Scholar]
- Muriu S. M., Coulson T., Mbogo C. M., and Godfray H. C.. 2013. Larval density dependence in Anopheles gambiae s.s., the major African vector of malaria. J. Anim. Ecol. 82: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutero C. M., Ng’ang’a P. N., Wekoyela P., Githure J., and Konradsen F.. 2004. Ammonium sulphate fertiliser increases larval populations of Anopheles arabiensis and culicine mosquitoes in rice fields. Acta Trop. 89: 187–192. [DOI] [PubMed] [Google Scholar]
- Ogbunugafor C. B., and Sumba L.. 2008. Behavioral evidence for the existence of a region-specific oviposition cue in Anopheles gambiae s.s. J. Vector Ecol. 33: 321–324. [DOI] [PubMed] [Google Scholar]
- Olagbemiro T. O., Birkett M. A., Mordue Luntz A. J., and Pickett J. A.. 2004. Laboratory and field responses of the mosquito, Culex quinquefasciatus, to plant-derived Culex spp. oviposition pheromone and the oviposition cue skatole. J. Chem. Ecol. 30: 965–976. [DOI] [PubMed] [Google Scholar]
- Oliva L. O., Correia J. C., and Albuquerque C. M. R.. 2014. How mosquito age and the type and color of oviposition sites modify skip-oviposition behavior in Aedes aegypti (Diptera: Culicidae)? J. Insect Behav. 27: 81–91. [Google Scholar]
- Phasomkusolsil S., Tawong J., Monkanna N., Pantuwatana K., Damdangdee N., Khongtak W., Kertmanee Y., Evans B. P., and Schuster A. L.. 2013. Maintenance of mosquito vectors: effects of blood source on feeding, survival, fecundity, and egg hatching rates. J. Vector Ecol. 38: 38–45. [DOI] [PubMed] [Google Scholar]
- Ponnusamy L., Xu N., Nojima S., Wesson D. M., Schal C., and Apperson C. S.. 2008. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc. Natl. Acad. Sci. USA. 105: 9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy L., Wesson D. M., Arellano C., Schal C., and Apperson C. S.. 2010a. Species composition of bacterial communities influences attraction of mosquitoes to experimental plant infusions. Microb. Ecol. 59: 158–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy L., Xu N., Böröczky K., Wesson D. M., Abu Ayyash L., Schal C., and Apperson C. S.. 2010b. Oviposition responses of the mosquitoes Aedes aegypti and Aedes albopictus to experimental plant infusions in laboratory bioassays. J. Chem. Ecol. 36: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind M. H., and Zarrabi A. A.. 2012. Water surface area and depth determine oviposition choice in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 49: 71–76. [DOI] [PubMed] [Google Scholar]
- Reiter P. 2007. Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 7: 261–273. [DOI] [PubMed] [Google Scholar]
- Savage H. M., Rejmankova E., Arredondo-Jim’enez J. I., Roberts D. R., and Rodr’iguez M. H.. 1990. Limnological and botanical characterization of larval habitats for two primary malarial vectors, Anopheles albimanus and Anopheles pseudopunctipennis, in coastal areas of Chiapas State, Mexico. J. Am. Mosq. Control Assoc. 6: 612–620. [PubMed] [Google Scholar]
- Seenivasagan T., Sharma K. R., Sekhar K., Ganesan K., Prakash S., and Vijayaraghavan R.. 2009. Electroantennogram, flight orientation, and oviposition responses of Aedes aegypti to the oviposition pheromone n-heneicosane. Parasitol. Res. 104: 827–833. [DOI] [PubMed] [Google Scholar]
- Seenivasagan T., Sharma K. R., and Prakash S.. 2012. Electroantennogram, flight orientation and oviposition responses of Anopheles stephensi and Aedes aegypti to a fatty acid ester-propyl octadecanoate. Acta Trop. 124: 54–61. [DOI] [PubMed] [Google Scholar]
- Service M. W. 1993. Mosquito ecology - field sampling methods, 2nd ed. Elsevier Applied Science, London, United Kingdom. [Google Scholar]
- Silberbush A., Markman S., Lewinsohn E., Bar E., Cohen J. E., and Blaustein L.. 2010. Predator-released hydrocarbons repel oviposition by a mosquito. Ecol. Lett. 13: 1129–1138. [DOI] [PubMed] [Google Scholar]
- Silberbush A., Abramsky Z., and Tsurim I.. 2015. Effects of fish cues on mosquito larvae development. Acta Trop. 150: 196–199. [DOI] [PubMed] [Google Scholar]
- Snell A. E., Knox R. L., and Cane R. P.. 2010. Aspects of nutrition and oviposition in the endemic rockpool mosquito Opifex fuscus Hutton (Diptera: Culicidae). N. Z. Entomol. 33: 79–83. [Google Scholar]
- Sumba L. A., Ogbunugafor C. B., Deng A. L., and Hassanali A.. 2008. Regulation of oviposition in Anopheles gambiae s.s.: role of inter- and intra-specific signals. J. Chem. Ecol. 34: 1430–1436. [DOI] [PubMed] [Google Scholar]
- Tennyson S., Ravindran K. J., Eapen A., and William S. J.. 2012. Effect of Ageratum houstonianum Mill. (Asteraceae) leaf extracts on the oviposition activity of Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 111: 2295–2299. [DOI] [PubMed] [Google Scholar]
- Trexler J. D., Apperson C. S., Zurek L., Gemeno C., Schal C., Kaufman M., Walker E., Watson D. W., and Wallace L.. 2003. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 40: 841–848. [DOI] [PubMed] [Google Scholar]
- Warburg A., Faiman R., Shtern A., Silberbush A., Markman S., Cohen J. E., and Blaustein L.. 2011. Oviposition habitat selection by Anopheles gambiae in response to chemical cues by Notonecta maculata. J. Vector Ecol. 36: 421–425. [DOI] [PubMed] [Google Scholar]
- Wertheim B., van Baalen E. J., Dicke M., and Vet L. E.. 2005. Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annu. Rev. Entomol. 50: 321–346. [DOI] [PubMed] [Google Scholar]
- Why A. M., Lara J. R., and Walton W. E.. 2016. Oviposition of Culex tarsalis (Diptera: Culicidae) differs on water conditioned by potential fish and insect predators. J Med Entomol 53: 1093–1099. [DOI] [PubMed] [Google Scholar]
- Williams C. R., Leach K. J., Wilson N. J., and Swart V. R.. 2008. The Allee effect in site choice behaviour of egg-laying dengue vector mosquitoes. Trop. Biomed. 25: 140–144. [PubMed] [Google Scholar]
- Wondwosen B., Hill S. R., Birgersson G., Seyoum E., Tekie H., and Ignell R.. 2017. A(maize)ing attraction: gravid Anopheles arabiensis are attracted and oviposit in response to maize pollen odours. Malar. J. 16: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Stoddard S. T., Astete H., Morrison A. C., and Scott T. W.. 2011. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl. Trop. Dis. 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahiri N., and Rau M. E.. 1998. Oviposition attraction and repellency of Aedes aegypti (Diptera: Culicidae) to waters from conspecific larvae subjected to crowding, confinement, starvation, or infection. J. Med. Entomol. 35: 782–787. [DOI] [PubMed] [Google Scholar]
- Zahiri N., Rau M. E., and Lewis D. J.. 1997. Oviposition responses of Aedes aegypti and Ae. atropalpus (Diptera: Culicidae) females to waters from conspecific and heterospecific normal larvae and from larvae infected with Plagiorchis elegans (Trematoda: Plagiorchiidae). J. Med. Entomol. 34: 565–568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.