Abstract
Background
Sexual dysfunction following stroke is common but often is poorly managed. As awareness of sexual dysfunction following stroke increases as an important issue, a clearer evidence base for interventions for sexual dysfunction is needed to optimise management.
Objectives
To evaluate the effectiveness of interventions to reduce sexual dysfunction following stroke, and to assess adverse events associated with interventions for sexual dysfunction following stroke.
Search methods
We conducted the search on 27 November 2019. We searched the Cochrane Central Register of Controlled Trials (CENTRAL; from June 2014), in the Cochrane Library; MEDLINE (from 1950); Embase (from 1980); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; from 1982); the Allied and Complementary Medicine Database (AMED; from 1985); PsycINFO (from 1806); the Physiotherapy Evidence Database (PEDro; from 1999); and 10 additional bibliographic databases and ongoing trial registers.
Selection criteria
We included randomised controlled trials (RCTs) that compared pharmacological treatments, mechanical devices, or complementary medicine interventions versus placebo. We also included other non‐pharmacological interventions (such as education or therapy), which were compared against usual care or different forms of intervention (such as different intensities) for treating sexual dysfunction in stroke survivors.
Data collection and analysis
Two review authors independently selected eligible studies, extracted data, and assessed study quality. We determined the risk of bias for each study and performed a 'best evidence' synthesis using the GRADE approach.
Main results
We identified three RCTs with a total of 212 participants. We noted significant heterogeneity in interventions (one pharmacological, one physiotherapy‐based, and one psycho‐educational), and all RCTs were small and of 'low' or 'very low' quality. Based on these RCTs, data are insufficient to provide any reliable indication of benefit or risk to guide clinical practice in terms of the use of sertraline, specific pelvic floor muscle training, or individualised sexual rehabilitation.
Authors' conclusions
Use of sertraline to treat premature ejaculation needs to be tested in further RCTs. The lack of benefit with structured sexual rehabilitation and pelvic floor physiotherapy should not be interpreted as proof of ineffectiveness. Well‐designed, randomised, double‐blinded, placebo‐controlled trials of long‐term duration are needed to determine the effectiveness of various types of interventions for sexual dysfunction. It should be noted, however, that it may not be possible to double‐blind trials of complex interventions.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Young Adult; Antidepressive Agents; Antidepressive Agents/adverse effects; Antidepressive Agents/therapeutic use; Orgasm; Pelvic Floor; Premature Ejaculation; Premature Ejaculation/drug therapy; Premature Ejaculation/etiology; Quality of Life; Randomized Controlled Trials as Topic; Resistance Training; Resistance Training/methods; Sertraline; Sertraline/adverse effects; Sertraline/therapeutic use; Sex Education; Sex Education/methods; Sexual Dysfunction, Physiological; Sexual Dysfunction, Physiological/etiology; Sexual Dysfunction, Physiological/rehabilitation; Sexual Dysfunction, Physiological/therapy; Sexual Partners; Sexual Partners/psychology; Stroke; Stroke/complications; Vitamin B 12; Vitamin B 12/analogs & derivatives; Vitamin B 12/therapeutic use; Vitamin B Complex; Vitamin B Complex/therapeutic use
Plain language summary
Treatments for sexual problems following stroke
Background
One of the most common but least talked about effects of stroke is sexual dysfunction, with 50% or more of stroke survivors experiencing a degree of sexual decline post stroke. This is not always well recognised, and it is often poorly managed. Management options are very broad and can include medications, counselling, and physical therapy.
Review question
We wanted to find out whether some treatments are better or worse than alternatives.
Search date
The evidence is current to 27 November 2019.
Study characteristics
Population: we included studies in which participants were adults who had had a stroke.
Intervention: interventions included medications or other treatments, such as rehabilitation, used to manage sexual problems following stroke.
Comparison: we compared interventions such as medications against 'fake' medications, which do not contain active substances that affect sexual function. We compared interventions such as rehabilitation, education, or therapy to usual care or alternative treatment.
Outcome: we divided outcomes into primary and secondary outcomes. Primary outcomes focused on sexual function or sexual satisfaction in stroke survivors and their partners. Secondary outcomes focused on quality of life, psychological well‐being (anxiety, depression, stress), satisfaction with intervention, sexual knowledge, and marital/relationship satisfaction (including partner satisfaction) in stroke survivors and their partners. We also reported adverse events.
Time/duration: we included studies of all durations: short (≤ 6 months), medium (between 6 and 18 months), and long (≥ 18 months).
Key results
We found three trials designed to reduce sexual dysfunction after stroke. One trial compared a medication called sertraline to methylcobalamin (vitamin B12) to help with premature ejaculation. A second trial compared a structured rehabilitation programme (which had face‐to‐face counselling and written education) to written education alone and found no clear difference in terms of sexual function, mood, stress, or quality of life. A third trial compared pelvic floor training (exercises to strengthen pelvic floor muscles) to standard rehabilitation and found no clear differences in terms of erection and quality of life. We were uncertain of the results because all three trials were small and of low or very low quality. Also, each trial compared different treatments, which meant that results could not be combined.
Side effects (mostly nausea or diarrhoea) were reported for sertraline (20 of 58 participants). No harmful events were reported with pelvic floor training, and no information was provided on harmful events related to sexual rehabilitation.
Study funding sources
The study that compared the medication (sertraline) to vitamin B12 did not describe any funding sources. The study that compared pelvic floor training to standard rehabilitation was funded through grants from the Association of Danish Physiotherapists Research Foundation, the Association of Danish Physiotherapists Practise Foundation, the Foundation of 12.12.1981, Lykkefeldts Grant, the Foundation of Lundbeck (UCSF), and the Department of Physiotherapy and Occupational Therapy Glostrup Hospital, University of Copenhagen. The study that compared a structured rehabilitation programme to written education alone was funded by the Victor Hurley Medical Research Grant‐in‐Aid and by the AFRM Ipsen Open Research Fellowship.
Quality of evidence
We are uncertain of the results because all three studies were small and of poor quality. Also, each of the three studies compared different treatments, which meant that we could not combine study results.
Conclusion
All three treatments (sertraline, structured sexual rehabilitation, and pelvic floor physiotherapy) need to be tested in further studies. Further research is needed to assess the effectiveness of treatments for sexual problems after stroke.
Summary of findings
Summary of findings 1. Pharmacological interventions compared with placebo/usual care or different forms of intervention.
| Sertraline compared with placebo (methylcobalamin) for secondary premature ejaculation after stroke | ||||||
|
Patient or population: men (aged between 23 and 45) with premature ejaculation within 3 months after stroke Settings: unclear (likely inpatient, continued into outpatient) Intervention: sertraline Comparison: methylcobalamin | ||||||
| Outcomes | Illustrative comparative risks* | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | |||||
| Placebo (Methylcobalamin) | Sertraline | |||||
| Sexual function (primary outcome) | Outcome measure 1: mean intravaginal ejaculatory latent time at 4 weeks | 2.1 (SD 0.4) | 0.8 higher (SD 0.5) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | |
| Outcome measure 1: mean intravaginal ejaculatory latent time at 8 weeks (end of intervention) | 3.8 (SD 0.5) | 2 higher (SD 0.7) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
| Outcome measure 1: mean intravaginal ejaculatory latent time at 12 weeks | 4.5 (SD 0.7) | 1.6 higher (SD 0.9) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
| Outcome measure 2: mean non‐validated measure for "sexual functioning" ** at 4 weeks | 4.4 (SD 2.1) | 1.5 higher (SD 1.9) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
| Outcome measure 2: mean non‐validated measure for "sexual functioning" ** at 8 weeks (end of intervention) | 5.3 (SD 1.9) | 1.9 higher (SD 2.2) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
| Outcome measure 2: mean non‐validated measure for "sexual functioning" ** at 12 weeks | 6.5 (SD 2.7) | 1.8 higher (SD 2.2) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
|
Sexual satisfaction (primary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Quality of life (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Psychological well‐being (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Satisfaction with intervention (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
| Marital/relationship/partner satisfaction (secondary outcome) | Outcome measure: mean non‐validated measure for "partner sexual satisfaction"** at 4 weeks | 8.9 (SD 1.5) | 1.2 higher (SD 1.4) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | |
| Outcome measure: mean non‐validated measure for "partner sexual satisfaction"** at 8 weeks (end of intervention) | 10.8 (SD 1.7) | 2.5 higher (SD 1.6) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
| Outcome measure: mean non‐validated measure for "partner sexual satisfaction"** at 12 weeks | 10.8 (SD 1.7) | 2.7 higher (SD 1.7) | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
|
Adverse effects (secondary outcome) Outcome measure: adverse effects*** |
OR 0.48 | OR 1.6 higher | 114 (1 study) | ⊕⊝⊝⊝ very lowa | ||
| *The assumed risk is based on the outcome mean of the control group. The corresponding risk is based on the outcome mean of the comparison group. The relative effect of the intervention is denoted in brackets (lower/higher/no difference). **No further description of the scale has been provided by the authors of the study. ***Adverse effects were mild and most commonly were gastrointestinal. N/A: not applicable; OR: odds ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aBased on GRADE criteria, the quality of evidence has been triple‐downgraded to "very low". First, a single randomised controlled trial is contributing to the overall quality of evidence. Second, there are significant limitations in the design and implementation of the included study (high or unclear risk of bias in all risk of bias criteria) for which we have downgraded the evidence by a further two levels. There is also a likelihood of publication bias; however, the quality of evidence is already at the lowest possible level.
Summary of findings 2. Non‐pharmacological interventions compared with placebo/usual care or different forms of intervention.
| Intervention 1. Sexual rehabilitation programme compared with written education alone for sexual dysfunction after stroke | ||||||
|
Patient or population: people within 3 months after stroke Settings: inpatient Intervention: individualised structured sexual rehabilitation Comparison: written educational materials | ||||||
| Outcomes | Illustrative comparative risks* | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | |||||
| Written educational materials alone | Sexual rehabilitation programme | |||||
| Sexual function (primary outcome) | Outcome measure: Median Sexual Functioning Questionnaire Short Form at 6 weeks | 28 (IQR 16 to 40) | 2 lower (IQR 16.8 to 39) (no significant difference) | 68 (1 study) |
low ⊕⊕⊕⊝a |
|
| Outcome measure: Median Sexual Functioning Questionnaire Short Form at 6 months | 35 (IQR 18.5 to 41) | 9 lower (IQR 16.5 to 36.5) (no significant difference) | 68 (1 study) |
low ⊕⊕⊕⊝a |
||
|
Sexual satisfaction (primary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
| Quality of life (secondary outcome) | Outcome measure: Median Stroke and Aphasia Quality of Life Scale‐39 Generic at 6 weeks | 4.5 (IQR 3.7 to 4.7) | 0.4 lower (IQR 3.5 to 4.7) (no significant difference) | 68 (1 study) |
low ⊕⊕⊕⊝a |
|
| Outcome measure: Median Stroke and Aphasia Quality of Life Scale‐39 Generic at 6 months | 4.4 (IQR 3.7 to 5) | 0.6 lower (IQR 3.2 to 4.7) (no significant difference) | 68 (1 study) |
low ⊕⊕⊕⊝a |
||
| Psychological well‐being (secondary outcome) | Outcome measure: Median Depression, Anxiety, and Stress Scale at 6 weeks | Depression 2 (IQR 0 to 9), Anxiety 2 (IQR 0 to 6) Stress 0 (IQR 0 to 10) |
Depression 2 higher (IQR 0 to 14) (no significant difference) Anxiety 3 higher (IQR 0 to 9) (no significant difference) Stress 5 higher (IQR 0 to 12.5) (no significant difference) |
68 (1 study) |
low ⊕⊕⊕⊝a |
|
| Outcome measure: Median Depression, Anxiety, and Stress Scale at 6 months | Depression 2 (IQR 0 to 10) Anxiety 2 (IQR 0 to 5) Stress 4 (IQR 0 to 11) |
Depression 2 higher (IQR 0 to 14) (no significant difference) Anxiety 2 lower (IQR 0 to 9) (no significant difference) Stress 2 higher (IQR 0 to 15) (no significant difference) |
68 (1 study) |
low ⊕⊕⊕⊝a |
||
|
Satisfaction with intervention (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Marital/relationship/partner satisfaction (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Adverse effects (secondary outcome) Outcome measure: adverse effects |
No data provided | No data provided | N/A | N/A | ||
| Intervention 2: Pelvic floor muscle training compared with standard rehabilitation for erectile dysfunction after stroke | ||||||
|
Patient or population: men with lower urinary tract symptoms and erectile dysfunction more than 1 month following stroke Settings: outpatient Intervention: pelvic floor muscle training Comparison: standard general rehabilitation without specific treatment for lower urinary tract symptoms | ||||||
| Outcomes | Illustrative comparative risks* | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | |||||
| Standard general rehabilitation | Pelvic floor muscle training | |||||
| Sexual function (primary outcome) | Outcome measure: Median International Index of Erectile Function Questionnaire at 3 months (end of intervention) | 18 (IQR 5 to 25) | 2 higher (IQR 5 to 25) (no significant difference) | 31 (1 study) | ⊕⊝⊝⊝ very lowb | |
| Outcome measure: Median International Index of Erectile Function Questionnaire at 6 months | 11 (IQR 5 to 18) | 4 higher (IQR 5 to 25) (no significant difference) | 31 (1 study) | ⊕⊝⊝⊝ very lowb | ||
|
Sexual satisfaction (primary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
| Quality of life (secondary outcome) | Outcome measure: median non‐validated "erectile dysfunction‐induced bother" questionnaire** at 3 months (end of intervention) | 3 (IQR 2 to 5) | 1 higher (IQR 3 to 4) (no significant difference) | 31 (1 study) | ⊕⊝⊝⊝ very lowb | |
| Outcome measure: median non‐validated "erectile dysfunction‐induced bother" questionnaire** at 6 months | 3 (IQR 2 to 5) | 1 lower (IQR 2 to 4) (no significant difference) | 31 (1 study) | ⊕⊝⊝⊝ very lowb | ||
|
Psychological well‐being (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Satisfaction with intervention (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Marital/relationship/partner satisfaction (secondary outcome) Not addressed |
N/A | N/A | N/A | N/A | ||
|
Adverse effects (secondary outcome) Outcome measure: adverse effects |
None reported | None reported | 31 (1 study) | ⊕⊝⊝⊝ very lowb | ||
| *The assumed risk is based on the outcome median of the control group. The corresponding risk is based on the outcome median of the comparison group. The relative effect of the intervention is denoted in brackets (lower/higher/no difference). **"If you were to spend the rest of your life with your ED problems as they are now, how would you feel about that?" IQR: interquartile range; N/A: not applicable. | ||||||
| GRADE Working Group grades of evidence. High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
We have not been able to combine the results of these two interventions given the heterogeneity of the interventions themselves.
aWith regards to the non‐pharmacological intervention of a sexual rehabilitation programme compared with written education alone, based on GRADE criteria, the quality of the evidence has been double‐downgraded to 'low'. First, a single randomised controlled trial is contributing to the overall quality of evidence. Second, there are some limitations in the design and implementation of the included study, specifically, participants and personnel were not blinded. It is recognised however that such blinding would be challenging to institute given the nature of the study. In recognition of the possible risk of bias introduced by the lack of such blinding, we have downgraded the evidence by a further level.
bWith regards to the non‐pharmacological intervention of pelvic floor muscle training compared with standard rehabilitation for erectile dysfunction, based on GRADE criteria, the quality of evidence has been triple‐downgraded to 'very low'. First, a single randomised controlled trial is contributing to the overall quality of evidence. Second, there are significant limitations in the design and implementation of the included study (three criteria judged as introducing high risk of bias) for which we have downgraded the evidence by a further two levels.
Summary of findings 3. Complementary medicine interventions compared with placebo/usual care or different forms of intervention.
| No included studies |
Background
Stroke is a leading cause of mortality and disability (ABS 2017). It is estimated that one in six people worldwide will experience a stroke in their lifetime – in 2018, 56,000 new strokes or recurrent strokes occurred in Australia (NSF 2018), and 795,000 in the USA (CDC 2017). In the same year, over 475,000 people in Australia and over 7,000,000 people in the USA were living with the effects of stroke (CDC 2017; NSF 2018). The burden of disease and the economic impact of stroke upon stroke survivors, their caregivers (often family members), and society are substantial, with annual national costs of AUD 5 billion in Australia (NSF 2018), along with lifetime costs per patient ranging from USD 11,787 to USD 3,3035,671 in the USA (Palmer 2005).
Description of the condition
One of the most common but least talked about effects of stroke is sexual dysfunction, with 50% or more of stroke survivors experiencing a degree of sexual decline post stroke (Giaquinto 2003; Korpelainen 1999; Schmitz 2010; Stein 2013; Tamam 2008). Sexual dysfunction is often multi‐factorial in origin, and contributing causes can be divided broadly into the following categories.
-
Primary issues with sexual dysfunction as a direct result of the stroke. Examples may include decline in libido and coital frequency, decline in vaginal lubrication, or orgasm and erection and ejaculation issues (Giaquinto 2003; Monga 1986; Tamam 2008).
Related medical issues, such as medications and premorbid medical conditions (diabetes, hypertension, cardiac issues), may also contribute to these effects (Giaquinto 2003; Sjogren 1981).
Secondary causes of sexual dysfunction, whereby the stroke results in sensorimotor problems, such as hemiplegia or spasticity, pain, or bowel or bladder dysfunction, which in turn can affect sexual function due to issues such as loss of ability to position oneself during sexual activity.
-
Tertiary causes such as psychological adjustment issues, cognitive or behavioural issues, or both.
Psychological adjustment issues may include body image changes, loss of self‐esteem, anxiety, stress, depression, fear of new stroke, and marital conflict (change in roles, fear that able‐bodied partner will leave, difficulties stemming from the spouse having a dual role of lover as well as carer) (Giaquinto 2003; Korpelainen 1999).
Cognitive or behavioural issues, or both, may include poor judgement, egocentricity, emotional lability, disinhibition, low tolerance for delayed gratification, and poor memory.
Issues may relate not only to stroke survivors but also to their spouses, with up to 88% stating they would not like to have sexual activity with a 'sick person' (Giaquinto 2003).
Sexuality is a broad concept, and it may be experienced and expressed in a variety of ways, including thoughts, fantasies, desires, beliefs, attitudes, values, behaviours, practices, roles, and relationships (WHO 2006). It is closely linked with personal identity and gender. It should be noted that studies of stroke interventions typically report the gender of participants in binary terms (male or female, men or women), and that there is generally an assumption that participants are cis‐gendered (sense of personal identity and gender corresponds with their birth sex). However, this is potentially neither accurate nor inclusive. For this review, we have chosen to use the phrase 'regardless of gender' whenever possible to include people regardless of their gender identity. Similarly, 'their' is used rather than 'his or her'. We use binary terms when necessary, such as when reporting information from studies.
Description of the intervention
Types of interventions for sexual dysfunction include:
pharmacological interventions, such as phosphodiesterase‐5 inhibitors, intracavernosal injections, intraurethral suppositories, and hormonal therapy (Vecchio 2010);
-
non‐pharmacological interventions, including:
mechanical devices (such as vacuum pumps, penile implants, penile prostheses, and lubricating gels);
psycho‐educational interventions (such as counselling and psychotherapy); and
physical therapy (such as physiotherapy for bed mobility) (Miles 2007); and
complementary medicine interventions, such as gingko biloba and ginseng (Miles 2007).
Interventions are not mutually exclusive and may be used in combination. An example of a comprehensive intervention for sexual dysfunction following stroke is sexual rehabilitation. Rehabilitation is defined as "a problem‐solving educational process aimed at reducing disability and handicap (participation) experienced by someone as a result of disease or injury" (Wade 1992). The specific aims of stroke sexual rehabilitation are to assess existing sexual issues, provide information on concerns, and support safe return to sexual activity after a stroke (Byrne 2016). Sexual rehabilitation is tailored according to individual needs and is delivered in a co‐ordinated manner by medical staff, together with representatives of one or more disciplines (physiotherapy, occupational therapy, social work, psychology, nursing). Sexual rehabilitation is designed to be person‐centred, time‐based, and functionally oriented and aims to maximise activity and participation (social integration) via a biopsychosocial model. Counselling may form a large (and potentially the only) component of sexual rehabilitation and may address sexual performance concerns, issues related to medication and comorbid conditions that may affect sexual function, and specific psychological or interpersonal factors (Lue 2004). Counselling may be delivered in a one‐on‐one or group setting. In addition to counselling, sexual rehabilitation may involve other aspects of physical rehabilitation, such as mobility training by the physiotherapist to optimise bed mobility for sexual positioning and transferring into and out of bed, and management of spasticity, such as by using a bolster between the knees for adduction spasticity. It may also include prescribed medications such as phosphodiesterase‐5 inhibitors. Sexual rehabilitation may be provided by a range of appropriately trained health professionals within the multi‐disciplinary team and may involve the stroke survivor or their partner alone, or the stroke survivor together with their partner. A range of formats may used in sexual rehabilitation, including oral information, visual information, written materials, and audiovisual and practical training. Sexual rehabilitation may be provided short term (such as one‐off counselling or a medication prescription) or longer term (such as cognitive‐behavioural therapy targeting psychological and physical aspects of sex and intimacy (Song 2011), or physiotherapy to achieve mobility goals).
At present, several international guidelines recommend that sexual function should be assessed and managed following stroke (CSN 2014; NSF 2017; RCP 2016). However, these guidelines are largely based on consensus and do not address types of interventions or their relative effectiveness.
How the intervention might work
Pharmacological interventions such as phosphodiesterase‐5 inhibitors, intracavernosal injections, and intraurethral suppositories assist with erectile function by increasing blood flow to the penis to achieve and maintain erection. Mechanisms of action vary with each medication: phosphodiesterase‐5 inhibitors prevent the breakdown of cyclic guanidine monophosphate (cGMP), which results in enhancement of penile erection, and intracavernosal injections cause vasodilatation of the penis. Hormonal treatment, such as testosterone, can be provided to treat testosterone deficiency, resulting in improved libido and erectile function.
The range of non‐pharmacological interventions is broad. Mechanical devices such as vacuum pumps and penile implants/penile prostheses treat erectile dysfunction via an external pump with a band to obtain and maintain an erection and surgical implantation of a prosthesis within the corpora cavernosa of the penis, respectively. Lubricating gels reduce friction between body parts, or between body parts and other objects, during sexual activity. Psycho‐educational interventions (such as counselling and psychotherapy) may reduce anxiety related to sexual problems and provide reassurance around fears related to sexual activity precipitating another stroke, resulting in increased confidence in sexual abilities. Other therapies may work by providing practical guidance such as ideal timing (sexual activity in the morning when the person is not tired), management of bladder and bowel issues, and working around weakness (physical support with pillows) to help stroke survivors and their partners address problems that commonly affect sexuality after a stroke.
Complementary medicine interventions such as gingko biloba and ginseng may increase nitric oxide levels, leading to improved erectile function.
Why it is important to do this review
Sexual activity is an integral part of life, and the importance of addressing sexual health after stroke is well accepted (NSF 2018). Despite this, a recent Australian National Stroke Audit Rehabilitation Services Report showed that of 3613 post‐stroke adults audited across 120 Australian public and private hospitals, only 20% received information on sexuality (NSF 2018). In addition, although current guidelines recommend assessment and management of post‐stroke sexual dysfunction (NSF 2018), little is known about what types of interventions should be provided, and how effective these interventions are. Although some clinical studies and reviews (including Cochrane Reviews) have explored the role of these interventions for sexual dysfunction in conditions such as cancer (Miles 2007), chronic kidney disease (Vecchio 2010), cardiovascular disease (Byrne 2016), chronic obstructive pulmonary disease (COPD) (Levack 2015), and diabetes (Vardi 2007), the effectiveness and safety of these interventions in stroke survivors have not yet been studied thoroughly. This review therefore aims to identify existing evidence for interventions for sexual dysfunction in stroke survivors, and to identify gaps in current knowledge, with the purpose of informing health professionals, stroke survivors and their partners, and policy makers about the effectiveness of different interventions.
Objectives
To evaluate the effectiveness of interventions to reduce sexual dysfunction following stroke, and to assess adverse events associated with interventions for sexual dysfunction following stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We excluded cohort and cross‐sectional studies.
Types of participants
We included all adults aged 18 years and over, regardless of gender, with stroke as defined by the World Health Organization (WHO) (Hatano 1976). We also included the partners of adults with stroke.
We included studies in which the cohort included a mix of stroke and non‐stroke survivors if at least 75% of the study population consisted of stroke survivors. When less than 75% of the study population consisted of stroke survivors, we included these studies if data related only to the stroke population were separately reported.
Types of interventions
We included trials comparing pharmacological treatments, mechanical devices, or complementary medicine interventions with placebo and other non‐pharmacological interventions with usual care. We also included trials with different forms of non‐pharmacological interventions (such as a comprehensive individualised psycho‐educational programme versus a basic/standard education pamphlet) for treating sexual dysfunction in stroke survivors.
Interventions could include but were not limited to:
pharmacological interventions such as phosphodiesterase‐5 inhibitors, intracavernosal injections, intraurethral suppositories, and hormonal therapy;
-
non‐pharmacological interventions, including:
mechanical devices (such as vacuum pumps, penile implants, penile prostheses, and lubricating gels);
psycho‐educational interventions (such as counselling and psychotherapy). These could range from highly structured interventions provided by an appropriately trained health professional (medical practitioner, social worker, occupational therapist, sex therapist) to education alone provided through educational pamphlets from credible sources (such as by stroke associations);
established stroke consumer or professional associations as opposed to education from friends who have not undergone appropriate training to provide such education; and
physical therapy (such as physiotherapy for bed mobility) by appropriately trained personnel; and
complementary medicine interventions such as gingko biloba and ginseng.
We excluded uncontrolled and open drug intervention trials. Therefore, we would exclude a trial comparing pharmacological intervention to usual care. It is expected that the control comparator would be in the same category as the intervention. Pharmacological and complementary medicine interventions would be compared with placebo delivered via the same route as the intervention. However, other non‐pharmacological interventions, such as counselling, could be provided at different intensities or in different formats (face‐to‐face versus group, written versus verbal). The key characteristic of an intervention is that the intention was to improve sexual dysfunction. We excluded studies in which the intervention might indirectly improve sexual dysfunction but was given primarily for another reason (such as an antidepressant for depression).
Types of outcome measures
Primary outcomes
Primary outcomes focused on sexual function or sexual satisfaction or both among stroke survivors and their partners.
Instruments that measure sexual function may include:
International Index of Erectile Function (IIEF) (Rosen 1997);
Derogatis Interview for Sexual Functioning (DISF) and Derogatis Interview for Sexual Functioning Self‐Report (DISF‐SR) (Derogatis 1997);
Changes in Sexual Functioning Short‐Form (CSFQ‐14) (Keller 2006);
Sexual Function Questionnaire (SFQ) (Quirk 2002); and
Arizona Sexual Experience Scale (ASEX) (McGahuey 2000).
Instruments that measure sexual satisfaction may include:
Sexual Self‐Perception and Adjustment Questionnaire (SSPAQ) (Steinke 2013); and
Sexual Satisfaction Scale for Women (SSS‐W) (Meston 2005).
The primary outcomes of sexual function and sexual satisfaction were measured through the use of validated and non‐validated instruments.
Secondary outcomes
Secondary outcomes focused on quality of life, psychological well‐being (anxiety, depression, stress), satisfaction with intervention, sexual knowledge, and marital/relationship satisfaction (including partner satisfaction) among stroke survivors or their partners, or both. We also reported adverse events.
Instruments that measure quality of life may include:
36‐item Short Form Health Survey (SF‐36) or 12‐item Short Form Health Survey (SF‐12) (Ware 1992; Ware 1995); and
Stroke and Aphasia Quality of Life Scale‐39 Generic (SAQOL‐39g) (Hilari 2003).
Instruments that measure psychological well‐being may include:
Depression, Anxiety, Stress Scale (DASS) (Lovibond 1995); and
Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983).
Instruments that measure marital/relationship satisfaction include:
Evaluation and Nurturing Relationship Issues, Communication and Happiness (ENRICH) Marital Satisfaction (EMS) Scale (Olsen 1993).
We considered adverse events and side effects that may have resulted from the intervention. We defined serious adverse events as events that were life‐threatening (including death) or required prolonged hospitalisation.
Search methods for identification of studies
See the 'Specialised register' information available at the Cochrane Stroke Group website. We searched for trials in all languages and arranged for translation of relevant articles when necessary.
Electronic searches
We conducted searches on 27 November 2019. We searched the Cochrane Stroke Group trials register and the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 11), in the Cochrane Library (Appendix 1).
MEDLINE Ovid (from 1950) (Appendix 2).
Embase Ovid (from 1980) (Appendix 3).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (from 1982) (Appendix 4).
Allied and Complementary Medicine (AMED) Ovid (from 1985) (Appendix 5).
PsycINFO Ovid (from 1806) (Appendix 6).
Physiotherapy Evidence Database (PEDro) (from 1999) (http://www.pedro.org.au/) (Appendix 7).
Center for International Rehabilitation Research Information and Exchange (CIRRIE) Database of International Rehabilitation Research (http://cirrie.buffalo.edu/search/index.php) (Appendix 8).
Database of Abstracts of Reviews of Effects (DARE), in the Cochrane Library (latest issue) (Appendix 9). Please note that DARE is no longer updated as of March 2018.
ProQuest Dissertations & Theses Database (Appendix 10).
OT Search by the American Occupational Foundation and the American Occupational Therapy Association (www1.aota.org/otsearch/) (Appendix 11).
Occupational Therapy Systematic Evaluation of Evidence (OTseeker) (www.otseeker.com/) (Appendix 12).
National Rehabilitation Information Center REHABDATA Database (www.naric.com/research/rehab/) (Appendix 13).
SPORTDiscus EBSCO (Appendix 14).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases (Appendix 2). All search strategies deployed were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019)).
Searching other electronic resources
To identify further published, unpublished, and ongoing trials, we searched the following trials and research registers.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 15).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (Appendix 16).
ISRCTN Registry (www.isrctn.com) (previously known as Current Controlled Trials) (www.controlled-trials.com/) (Appendix 17).
Trials Central (www.trialscentral.org/).
Internet Stroke Center Stroke Trials Registry (www.strokecenter.org/trials).
UK Clinical Research Network Portfolio database (public.ukcrn.org.uk/search/).
Searching other resources
To identify further published, unpublished, and ongoing trials, we:
handsearched the reference lists of included trials and review articles about sexual rehabilitation after stroke;
tracked citations using Web of Science Cited Reference Search for all included studies; and
contacted experts active in this field (including authors of included trials and excluded studies identified as possible preliminary or pilot work).
Data collection and analysis
Selection of studies
Two review authors (LN, HS) independently screened titles and abstracts of references obtained as a result of our searching activities and excluded obviously irrelevant reports. We retrieved full‐text articles for the remaining references and screened full‐text articles, identified studies for inclusion, and recorded reasons for exclusion of ineligible studies. We resolved any disagreements through discussion. If required, we would have consulted a third review author (AB). We collated multiple reports of the same study so that each study ‐ not each reference ‐ would be the unit of interest in the review. We recorded the selection process and completed a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram when appropriate.
Data extraction and management
Two review authors (LN, HS) independently extracted data from included studies. We grouped studies by intervention type (pharmacological, non‐pharmacological, and complementary medicine) when possible.
We used a pre‐designed data extraction form to extract the following data from the included studies.
Participants: number of participants, age, gender, baseline functional status, or level of impairment.
Methods: inclusion criteria, time since stroke, and type, nature, and location of lesion.
-
Interventions: description of interventions given to each treatment group including duration, type, dose, route of delivery, and frequency. For interventions provided by therapists,
we documented the discipline of the person providing the intervention (e.g. physician, occupational therapist, physiotherapist, psychologist, social worker).
Outcomes: we documented primary and secondary outcomes relevant to this review. If a study used different methods for measuring the same outcome, we noted the outcome to be used for any subsequent analysis. We noted any important confounding variables. If more than two intervention groups were included in the study, we noted the method of including these groups in any subsequent analysis. The two review authors resolved any data extraction discrepancies through discussion. If disagreement persisted, we would have consulted a third review author (AB). We extracted data on whether adverse events were explicitly reported.
Assessment of risk of bias in included studies
Two review authors (LN, HS) independently assessed risk of bias for each study using the 'Risk of bias' tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved all disagreements by discussion. If required, we would have involved another review author (AB). We assessed the risk of bias according to the following seven domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded the risk of bias for each domain as high, low, or unclear and provided information from the study report together with a justification for our judgement in the ’Risk of bias’ tables.
Measures of treatment effect
We grouped studies by intervention type (pharmacological, non‐pharmacological, and complementary medicine). Given the heterogeneity of interventions, we were not able to perform a meta‐analysis. If it had been possible, we would have used the Cochrane Review Manager software to carry out statistical analyses to determine treatment effect (Review Manager 2014). For dichotomous variables, we would have calculated the treatment effect using a fixed‐effect or random‐effects model and reported it as odds ratios (ORs) with 95% confidence intervals (CIs). For continuous data, we would have calculated the treatment effect using standardised mean differences (SMDs) and 95% CIs when different studies used different scales to assess the same outcome, and we would have calculated mean differences (MDs) and 95% CIs when studies used the same method of measuring outcomes. We would have done this for all outcomes.
Unit of analysis issues
Unit of analysis issues did not arise with the included studies. If such issues had arisen, we would have addressed these as follows: if studies reported change values and the baseline value was not available, we would have used these data in meta‐analyses but planned sensitivity analyses to investigate the effect of including these data. We would have analysed adverse events as dichotomous variables. We would have done this for all outcomes.
Dealing with missing data
When an included study did not report a particular outcome but it had been included in the battery of measures administered, we contacted the study authors to ask for the original data. If we were unsuccessful in obtaining the data, we did not include that study in the analyses of that outcome.
When an included study had missing data (e.g. reports means but not standard deviations for follow‐up data), we contacted study authors to ask for the missing data. If we were unsuccessful, we took logical steps to enter an assumed value. Such steps included estimating a standard deviation based on a reported standard error, estimating a follow‐up standard deviation based on a baseline value, using the median as a proxy for the mean, and using a multiple of 0.75 times the interquartile range or 0.25 times the range as a proxy for the standard deviation values (Hozo 2005). We conducted sensitivity analyses to investigate the effect of entering assumed values.
Assessment of heterogeneity
If relevant, we would have used the I² statistic to measure heterogeneity among the trials in each analysis. When there was substantial heterogeneity (as defined by I² > 50%), we would have performed a meta‐analysis using a random‐effects model. If I² was less than or equal to 50%, we would have performed fixed‐effect meta‐analysis.
Assessment of reporting biases
We attempted to avoid reporting biases by using a comprehensive search strategy that included searching for unpublished studies and searching trials registers. We also assessed the completeness of outcome data.
Data synthesis
When we considered studies to be sufficiently similar, we would have conducted a meta‐analysis by pooling the appropriate data using RevMan 5.3 (Review Manager 2014).
GRADE and 'Summary of findings'
It was not possible to perform quantitative meta‐analysis due to the heterogeneity of studies; we used the GRADE approach instead to assess the quality of evidence (Higgins 2019). The GRADE approach defines quality of studies as the extent to which one can be certain that an estimate of effect is close to the quantity of interest. It specifies four levels of quality for a body of evidence for a given outcome: high, moderate, low, and very low. GRADE assessments of certainty are determined through consideration of the following five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Factors that may decrease the quality level of the included studies therefore include limitations in methods suggesting high likelihood of bias, indirectness of evidence such as with indirect populations, unexplained inconsistency of results, imprecision of results (e.g. with wide confidence intervals), and high probability of publication bias.
The main findings of the review, together with key information concerning the quality of evidence, are presented in the 'Summary of findings' tables. These findings are listed by intervention comparison in three categories (pharmacological interventions, non‐pharmacological interventions, and complementary medicine interventions), with outcomes divided into primary outcomes (sexual function or sexual satisfaction, or both) and secondary outcomes (quality of life, psychological well‐being, satisfaction with intervention, sexual knowledge, marital/relationship/partner satisfaction, and serious adverse events/death). When outcomes have not been addressed, we have reported this in the 'Summary of findings' table as "not addressed".
Subgroup analysis and investigation of heterogeneity
We would have explored heterogeneity by conducting additional subgroup analyses to investigate the effects of:
time since stroke;
type of intervention;
level of impairment at baseline; and
adherence with additional intervention.
Sensitivity analysis
We would have carried out a sensitivity analysis (when necessary) to explore the effects of the following methodological features.
Allocation concealment: we would have re‐analysed data, excluding trials with inadequate or unclear allocation concealment.
Masking of outcome assessor: we would have re‐analysed data, excluding trials without or with unclear masking of outcome assessor.
Missing outcome data: we would have re‐analysed data, excluding trials with inadequate or unclear methods of dealing with missing outcome data.
Results
Description of studies
Results of the search
See Figure 1 ‐ Study flow diagram.
1.
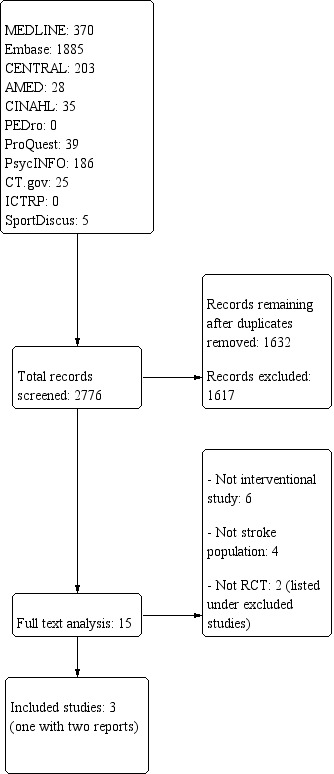
Study flow diagram.
Through searches, we identified 2776 citations (MEDLINE 370; Embase 1885; AMED 28; CINAHL Plus 35; PEDro 0; PROQUEST 39; PsycINFO 186; CT.GOV 25; ICTRP 0; SPORTDiscus 5; and CENTRAL 203). Two review authors (HS, LN) independently screened all citations. We selected 16 of these for full‐text analysis, and both review authors assessed them for inclusion with no disagreement. Three studies met the inclusion criteria (Lu 2012; Ng 2017; Tibaek 2015). We excluded two as they were not RCTs (Chae 2006; Song 2011).
Included studies
See Characteristics of included studies.
The three included studies were undertaken in three countries (Australia, China, and Denmark); all were written in English except one (Lu 2012), which was presented as an abstract written in English, but the rest of the publication was written in Chinese and required translation. The studies involved a total of 212 participants. Most participants had had a stroke within the last three months (Lu 2012; Ng 2017). Participants in Tibaek 2015 had had their stroke more than one month before the study, but duration post stroke was not otherwise reported. Ng 2017 reported that participants in the study were of "broad cultural background"; however, no further information regarding this was provided, other than the proportion of participants who did not speak English as their first language. Lu 2012 and Tibaek 2015 did not describe the cultural background of their participants. Lu 2012 and Tibaek 2015 had male participants only, whereas Ng 2017 included participants of both sexes. The age range was very broad: in Ng 2017, age ranged from 19 to 95 years, whereas participants in Tibaek 2015 ranged from 57 to 75 years of age, and participants in Lu 2012 were young (aged 23 to 45 years).
The definition of sexuality varied between studies. Ng 2017 used a broad definition and included not just sexual intercourse but also masturbation, sexual thoughts, enjoying films with sexual content, etc., whereas Lu 2012 included only married participants who had had "regular sexual intercourse" pre‐stroke. None of the studies described the sexual orientation and/or sexual identity of participants. As for involvement of sexual partners in the study, all studies recorded the proportion of participants who were married, although it should be noted that being married and having sexual partners were not necessarily linked. This ranged from 100% (n = 114) for Lu 2012, to 70% (n = 21 of 30) for Tibaek 2015, to 42.6% (n = 29 of 68) for Ng 2017. Only one study invited sexual partners to participate in the intervention (Ng 2017); however, study authors noted that none chose to do so. In Lu 2012, sexual partners were invited to participate as part of outcome measurement (sexual satisfaction of partner). Tibaek 2015 did not report involving sexual partners in intervention nor in outcome measurement.
With regards to setting, one study was conducted in the outpatient setting (Tibaek 2015), and one was conducted in an inpatient rehabilitation unit (Ng 2017). Lu 2012 appeared to have recruited participants from neurology inpatients, but it is assumed that a portion of the study occurred in the outpatient phase given the duration of the study (12 weeks), although this was not clearly stated in the study report.
In addition to the heterogeneity of participants, we noted the heterogeneity of interventions, which could be divided into pharmacological (sertraline) and non‐pharmacological (psycho‐educational and physical therapy) treatments. The trials varied in duration. In Ng 2017 and Tibaek 2015, the last assessment time point was six months post intervention, whereas in Lu 2012, it was four weeks post intervention. Tibaek 2015 and Lu 2012 collected end‐of‐intervention data, whereas Ng 2017 did not. The earliest post‐intervention data were collected by Ng 2017 at six weeks following completion of the intervention.
Pharmacological interventions
Lu 2012 was the only study that assessed a pharmacological intervention. This study assessed the effectiveness of sertraline for secondary premature ejaculation after stroke in young men between 23 and 45 years of age. Lu 2012 recruited 114 male participants shortly after their stroke during their stay in a neurology inpatient unit. All participants were provided with "psychological and behavioural advice" by a neurologist and a urologist and were encouraged to engage in sexual activity frequently (one to two times a week). Study authors do not state the content, format, frequency, nor delivery method (verbal versus written) of this advice. In addition, the intervention group received 50 mg oral sertraline and the control group received 0.5 mg methylcobalamin. These medications were taken daily, four to six hours either before bed or before sexual activity, over the course of eight weeks. Assessment time points included baseline and four, eight, and 12 weeks from baseline. The eight‐week assessment time point was therefore the end‐of‐intervention time point. Outcomes were measured with a mix of validated (intravaginal ejaculation latency time) and non‐validated tools (sexual functioning, sexual satisfaction of participants' spouses). In addition, adverse effects of the medications were reported.
Non‐pharmacological interventions
Psycho‐educational intervention
One RCT compared the effectiveness of a structured sexual rehabilitation programme versus written education materials only (Ng 2017). The pilot study included 10 participants, and the larger subsequent study included data from those 10 and from an additional 58 participants, totalling 68 participants (Ng 2017). The mean age of participants was 63.3 years (range 19 to 95 years), and 57% were men. All participants had had a recent stroke and were undergoing inpatient rehabilitation. Both control and interventional groups received the National Stroke Foundation fact sheet "Sex and relationships after stroke" (NSF 2013), at the start of their inpatient rehabilitation, and the intervention group received a 30‐minute individualised sexual rehabilitation programme from a rehabilitation physician later during their inpatient stay. Assessment time points were baseline, six weeks, and six months following the intervention. There was no "end‐of‐intervention" assessment time point. The following validated outcome measures were used: Sexual Functioning Questionnaire Short Form; Depression, Anxiety, Stress Scale; and Stroke and Aphasia Quality of Life Scale‐39 Generic.
Physical therapy intervention
One study assessed the effectiveness of 12 consecutive weeks of pelvic floor muscle training by physiotherapists for lower urinary tract symptoms and erectile dysfunction in male stroke survivors (Tibaek 2015). This was compared to "normal standard general rehabilitation without specific treatment for lower urinary tract symptoms". This study recruited 31 men (median age 68 years). Assessment time points were baseline, three months from baseline, and six months after completion of the intervention. The three‐month assessment time point was therefore the end‐of‐intervention time point. A mix of validated (International Index of Erectile Function Questionnaire) and non‐validated (quality of life questions, termed "erectile dysfunction‐induced bother" by the study author) measures were used.
Complementary medicine intervention
No studies were included.
Excluded studies
See Characteristics of excluded studies.
We excluded two trials as they were not RCTs and therefore did not fulfil inclusion criteria (Chae 2006; Song 2011). One was a clinical controlled trial conducted in Korea (n = 46), which compared a sexual rehabilitation programme involving both verbal and written information to standard care (Song 2011). The other was a case report of a trial of quetiapine for post‐stroke hypersexuality and delusional jealousy in a 63‐year‐old South Korean man (Chae 2006).
Risk of bias in included studies
In assessing risk of bias, we attempted to contact the corresponding author for Lu 2012 for clarification because a large amount of information was not reported; however these attempts were unsuccessful. We have, therefore, based risk of bias assessments solely on published reports.
See Figure 2 ‐ 'Risk of bias' summary.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included trials were described as randomised. In Lu 2012, randomisation was stated only in the abstract ‐ not within the methods ‐ nor was the method of randomisation described, so we judged this trial to be at unclear risk of bias. In Ng 2017, randomisation was performed via a computer‐generated sequence by an independent statistician, and Tibaek 2015 randomised based on a mathematical table; therefore we judged Ng 2017 and Tibaek 2015 to be at low risk of bias. Allocation concealment through use of sealed envelopes was clearly stated in Ng 2017 and Tibaek 2015; therefore we judged Ng 2017 and Tibaek 2015 to be at low risk of bias. It is unclear through the publications whether allocation concealment occurred in Lu 2012, so we judged this trial to be at unclear risk of bias.
Blinding
It is unclear if participants, treating team, or outcome assessors were blinded in Lu 2012. No mention of blinding was made in the study, and there did not appear to be any attempt to ensure that sertraline and methylcobalamin tablets or their containers were matched in any way. We therefore judged this trial to be at high risk of bias. In Ng 2017 and Tibaek 2015, care providers and participants were not blinded but outcome assessors were blinded. We therefore judged these trials to be at high risk of bias with regards to blinding of participants and personnel but at low risk of bias with regards to blinding of outcome assessors. It is noted that blinding of participants and care providers in these two studies would not have been possible given the nature of the intervention.
Incomplete outcome data
All studies had dropouts; however, it is not clear from any of the studies how this was dealt with. Lu 2012 had a relatively low dropout rate in which only five of the total 114 participants (intervention group: 2, control group: 3) were lost to follow‐up, which was less likely to have affected the overall findings. Tibaek 2015 also had a low dropout rate, with only one participant from the control group lost to lost‐up. However, Tibaek 2015 had large quantities of missing data (13% of participants in the intervention group and 60% of participants in the control group did not complete their questionnaires). Poor self‐reporting was postulated to occur because participants were male. Participant dropout in Ng 2017 (total participants n = 68) was significant at six months (n = 8, 11.7% at six weeks and n = 17, 25% at six months). The incomplete data for Tibaek 2015 and for Ng 2017 at six months would have significantly increased the risk of bias. We therefore judged Lu 2012 to be at unclear risk of bias and Tibaek 2015 and Ng 2017 to be at high risk of bias.
Selective reporting
There was no selective reporting in Ng 2017 and Tibaek 2015. However, Lu 2012 did not report measures related to clinical characteristics of participants (ECG findings; blood panel including "liver function tests, renal function tests, cholesterol, creatine kinase, hormone levels"; and blood pressure). We therefore judged Ng 2017 and Tibaek 2015 to be at low risk of bias and Lu 2012 to be at high risk of bias.
Other potential sources of bias
Tibaek 2015 had a small cohort (n = 30) and failed to reach the required sample size of 120 participants. Ng 2017 recruited 68 participants, which was above the pre‐calculated sample size of 60 participants, and had 60 participants at six‐week follow‐up but only 51 (loss of 25%) at six‐month follow‐up. We judged Ng 2017 and Tibaek 2015 to be at low risk of bias. Lu 2012 had the largest cohort among the included studies (n = 114); however, as sample size calculations were not reported, it is unclear if this study was adequately powered.
Additionally, Lu 2012 provided very limited information on reasoning and few details for the intervention/control. For example, it is unclear as to why methylcobalamin was chosen as placebo (at an active dose at which side effects could be experienced), nor is information provided on the appearance of the medication and whether participants would have been able to determine what they received. It is also unclear as to what "psychological and behavioural advice" to the participants consisted of. The inclusion criteria for participants was highly subjective and poorly defined – for example, they had to have a "good relationship" with their wife. Finally, some of the outcome measures used in Lu 2012 were not validated instruments, but no information was provided with regards to these measures. Therefore we judged Lu 2012 to be at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
It was not possible to perform a meta‐analysis of the results, given the heterogeneity of interventions and of outcome measures. The effect of interventions has been presented, grouped by intervention and then by outcomes.
Effects of interventions by intervention type
Pharmacological interventions
Lu 2012 (n = 114) compared the effectiveness of sertraline versus placebo (methylcobalamin) for secondary premature ejaculation after stroke in young Chinese men between 23 and 45 years of age. Based on the GRADE approach, this was a 'very low‐quality' study given the high risk of bias.
The quality of evidence is 'very low' and suggests that use of sertraline to treat secondary premature ejaculation after stroke in young men may improve sexual function as measured by Intravaginal Ejaculatory Latency Time and by non‐validated questionnaires on sexual function and sexual partner satisfaction in the short term (three months).
A large number of 'mild' adverse effects were reported in both intervention (20 of 58 participants) and control (11 of 56 participants) groups, with gastrointestinal side effects the most common (n = 9 intervention group, n = 6 control group).
Non‐pharmacological interventions
Psycho‐educational intervention
Ng 2017 (n = 68) compared the effectiveness of a structured sexual rehabilitation programme versus written education materials only in Australian stroke survivors with a mean age of 63.3 years. Based on the GRADE approach, this was a 'low‐quality' study with high risk of bias.
The quality of evidence is therefore 'low', and evidence suggests that an individualised sexual rehabilitation programme compared to written educational materials alone does not further improve sexual functioning (as measured by Sexual Functioning Questionnaire Short Form), anxiety and depression (as measured by Depression, Anxiety, and Stress Score), quality of life (as measured by Stroke and Aphasia Quality of Life Scale‐39 Generic), and functional independence (as measured by Functional Independence Measure) in the short term (six months).
Adverse events data were not provided.
Physical therapy intervention
Tibaek 2015 (n = 31) compared the effectiveness of 12 weeks of pelvic floor muscle training versus standard rehabilitation in male stroke survivors (median age 68 years) with lower urinary tract symptoms. Based on the GRADE approach, this was a 'very low‐quality' study given the high risk of bias.
The quality of evidence is therefore 'very low', and evidence suggests that pelvic floor muscle training compared to standard rehabilitation does not further improve sexual function (as measured by International Index of Erectile Function Questionnaire) or quality of life (as measured by non‐validated questionnaires termed "erectile dysfunction‐induced bother" by the study author) in the short term (six months).
No adverse events were reported.
Complementary medicine interventions
No studies are included.
Effects of interventions by outcomes
Primary outcomes
Primary outcomes of sexual function and sexual satisfaction were measured through the use of validated and non‐validated instruments.
Sexual function
Sexual Functioning Questionnaire Short Form (CSFQ‐14)
Ng 2017 (n = 68) compared sexual rehabilitation to written education only and used the CSFQ‐14 as the primary outcome. The CSFQ‐14 includes questions measuring pleasure, frequency, interest, arousal, orgasm, and sexual functioning. Time points consisted of baseline, six weeks, and six months. There were no statistical differences in CSFQ‐14 between groups at any of the time points. At six weeks, change score (z‐score) was ‐0.31 (P = 0.758) (intervention median 26, interquartile ratio (IQR) 16.8 to 39; control median 28, IQR 16 to 40). At six months, change score (z‐score) was ‐1.11 (P = 0.266) (intervention median 26, IQR 16.5 to 36.5); control median 35, IQR 18.5 to 41).
International Index of Erectile Function Questionnaire (IIEF‐5)
Tibaek 2015 (n = 31) compared the effectiveness of pelvic floor muscle training to standard rehabilitation and used IIEF‐5 as the primary outcome. The IIEF‐5 is a five‐question assessment tool (scores from 1 to 25) that measures the prevalence and severity of erectile dysfunction (Rosen 1997). Time points consisted of baseline, three months, and six months. Although there was intra‐group improvement within the intervention group from baseline (median 18, IQR 5 to 24) to three months (median 20, IQR 5 to 25) (P = 0.04), there was no statistical significance for IIEF‐5 between groups at any time point. At three months, median IIEF‐5 score for the treatment group was 20 (IQR 5 to 25) and for the control group was 18 (IQR 5 to 25) (P = 0.84). At six months, median IIEF‐5 score for the treatment group was 15 (IQR 5 to 25) and for the control group was 11 (IQR 5 to 18) (P = 0.08).
Intravaginal Ejaculatory Latency Time (IELT)
Lu 2012 (n = 114) compared the effectiveness of sertraline versus placebo (methylcobalamin) for secondary premature ejaculation and used IELT as a primary outcome measure. Intravaginal ejaculation latency time is measured time between the start of vaginal intercourse and the start of intravaginal ejaculation and is used to quantify premature ejaculation, although it should be noted that there is no uniform cut‐off defining 'premature'. Expert consensus indicates one minute after penetration, and the International Classification of Diseases applies a cut‐off of 15 seconds from the beginning of sexual intercourse (Serefoglu 2014). Time points consisted of baseline, four weeks, eight weeks, and 12 weeks. There was a significant increase in the intervention group compared to the control group at every time point. At four weeks, the intervention mean was 2.9 (standard deviation (SD) 0.5) compared to 2.1 for the control (SD 0.4) (P < 0.01). At eight weeks, the intervention mean was 5.8 (SD 0.7) compared to 3.8 for the control (SD 0.5) (P < 0.01). At 12 weeks, the intervention mean was 6.1 (SD 0.9) compared to 4.5 for the control (SD 0.7) (P < 0.01) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Sertraline compared with placebo (methylcobalamin) for secondary premature ejaculation after stroke ‐ end of intervention, Outcome 1: Sexual function ‐ intravaginal ejaculatory latent time
Non‐validated measures ‐ sexual functioning
In addition to IELT, Lu 2012 used a non‐validated measure for 'sexual functioning'; however, no information (such as questions or scoring) was provided with regards to this measure. Time points consisted of baseline, four weeks, eight weeks, and 12 weeks. There was a significant increase in the intervention group compared to the control group at every time point. At four weeks, the intervention mean was 5.9 (SD 1.9) compared to 4.4 for the control (SD 2.1) (P < 0.01). At eight weeks, the intervention mean was 7.2 (SD 2.2) compared to 5.3 for the control (SD 1.9) (P < 0.01). At 12 weeks, the intervention mean was 8.3 (SD 2.2) compared to 6.5 for the control (SD 2.7) (P < 0.01) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Sertraline compared with placebo (methylcobalamin) for secondary premature ejaculation after stroke ‐ end of intervention, Outcome 2: Sexual function ‐ non‐validated measure
Sexual satisfaction
This was not measured.
Secondary outcomes
Secondary outcomes were quality of life, psychological functioning, satisfaction with sexual rehabilitation services, sexual knowledge, marital/relationship/partner sexual satisfaction, and adverse events.
Quality of life
Stroke and Aphasia Quality of Life Scale‐39 Generic (SAQOL‐39g)
Ng 2017 (n = 68) compared sexual rehabilitation to written education only and used the SAQOL‐39g to assess the quality of life at baseline, at six weeks, and at six months. The SAQOL‐39g is a stroke‐specific assessment tool that explores the physical, psychosocial, and communication domains of quality of life. There were no significant differences at any time point. At six weeks, the change score (z‐score) was ‐0.41 (P = 0.682) (intervention median 4.1, IQR 3.5 to 4.7; control median 4.5, IQR 3.7 to 4.7). At six months, the change score (z‐score) was ‐0.04 (P = 0.969) (intervention median 3.8, IQR 3.2 to 4.7; control median 4.4, IQR 3.7 to 5).
Non‐validated measures: the effect of erectile dysfunction on quality of life (termed "erectile dysfunction‐induced bother" by the study author)
Tibaek 2015 (n = 31) compared the effectiveness of pelvic floor muscle training versus standard rehabilitation and used a non‐validated questionnaire to measure the effect of erectile dysfunction on quality of life. This questionnaire contained two questions: "(1) If you were to spend the rest of your life with your ED problems as they are now, how would you feel about that?" and "(2) If you use medicine or other aids/appliances to optimise erection, is that reflected in your answer in Question 1?" There were no statistically significant differences between intervention and control groups at any time point. For Question 1, at three months the intervention group median score was 4 (IQR 3 to 4) and the control group median score was 3 (IQR 2 to 5) (P = 0.69). At six months, the treatment group median score was 2 (IQR 2 to 4) and the control group median score was 3 (IQR 2 to 5) (P = 0.88). The results of Question 2 showed a higher rate in the intervention group without the influence of medication, aids, or appliances compared to the control group but no significant differences between groups (3 months: P = 0.69; 6 months: P = 0.33).
Psychological functioning
Depression, Anxiety, Stress Scale (DASS)
Ng 2017 (n = 68) compared sexual rehabilitation to written education only and used the DASS to assess the psychological functioning of all participants at baseline, six weeks, and six months. The DASS is a self‐reported tool used to assess the negative emotional states of depression, anxiety, and stress. There were no statistical differences in DASS between groups at any time point. At six weeks, the change score (z‐score) for depression was ‐1.14 (P = 0.255) (intervention median 4, IQR 0 to 14; control median 2, IQR 0 to 9); the change score (z‐score) for anxiety was ‐0.68 (P = 0.497) (intervention median 5, IQR 0 to 9; control median 2, IQR 0 to 6); and the change score (z‐score) for stress was ‐1.18 (P = 0.240) (intervention median 5, IQR 0 to 12.5; control median 2, IQR 0 to 10). At six months, the change score (z‐score) for depression was ‐0.49 (P = 0.626) (intervention median 4, IQR 0 to 14; control median 2, IQR 0 to 10); the change score (z‐score) for anxiety was ‐0.86 (P = 0.390) (intervention median 0, IQR 0 to 9; control median 2, IQR 0 to 5); and the change score (z‐score) for stress was ‐0.34 (P = 0.738) (intervention median 6, IQR 0 to 15; control median 4, IQR 0 to 11).
Satisfaction with intervention
This was not measured.
Sexual knowledge
This was not measured.
Marital/relationship/partner sexual satisfaction
Non‐validated measures ‐ Partner Sexual Satisfaction
Lu 2012 (n = 114) compared the effectiveness of sertraline versus placebo (methylcobalamin) for secondary premature ejaculation and used a non‐validated measure of partner sexual satisfaction. No further information was provided with regards to the measure itself. There was a statistically significant increase in sexual satisfaction score in the intervention group as compared to the control group at all time points. At four weeks, the intervention mean was 10.1 (SD 1.4) and the control mean was 8.9 (SD 1.5) (P < 0.01); at eight weeks, the intervention mean was 13.3 (SD 1.6) and the control mean was 10.8 (SD 1.7) (P < 0.01); and at 12 weeks, the intervention mean was 13.5 (SD 1.7) and the control mean was 10.8 (SD1.7) (P < 0.01) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Sertraline compared with placebo (methylcobalamin) for secondary premature ejaculation after stroke ‐ end of intervention, Outcome 3: Partner sexual satisfaction ‐ non‐validated measure
Side effects/adverse events
As Lu 2012 was the only study that involved the use of medication, it is the only study that reported side effects. A large number of 'mild' adverse effects were reported in both intervention and control groups. Of the 58 participants in the treatment group, 20 experienced adverse effects (nine gastrointestinal events, five dizziness, two excessive sweating, two dry mouth, two lowered libido). Of the 56 participants in the control group, 11 experienced adverse effects (six gastrointestinal events, two headache, one excessive sweating, one lowered libido, one feeling feverish) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Sertraline compared with placebo (methylcobalamin) for secondary premature ejaculation after stroke ‐ end of intervention, Outcome 4: Adverse effects
Tibaek 2015 reported no adverse events.
Adverse events data were not provided for Ng 2017.
The 'Summary of findings' tables present the main findings of this review, together with key information concerning the quality of evidence (Table 1; Table 2; Table 3).
Discussion
Summary of main results
Sexual dysfunction is a common problem among stroke survivors. It is often multi‐factorial; this is reflected in the broad range of available pharmacological and non‐pharmacological interventions. The aims of this review were to determine existing evidence for the effectiveness and safety of these interventions in stroke survivors, to identify gaps in current knowledge, and to recommend future directions. We identified three randomised controlled trials (RCTs) with a total of 212 participants that addressed the effectiveness of interventions for sexual dysfunction following stroke. All trials had short‐term outcomes (up to six months). Due to the heterogeneity of the interventions, we were unable to perform any mathematical or statistical direct or indirect comparisons across RCTs. Instead, we used the GRADE approach to provide a best‐evidence synthesis based on the quality of evidence. Results are as follows.
Based on one 'very low‐quality' small RCT (Lu 2012), data are insufficient to provide any reliable indication of benefit or risk with the use of sertraline for improving secondary premature ejaculation and sexual satisfaction in young male stroke survivors.
Based on one 'low‐quality' small RCT (Ng 2017), data are insufficient to provide any reliable indication of benefit or risk of sexual rehabilitation programmes in an inpatient rehabilitation setting for improving sexual dysfunction.
Based on one 'very low‐quality' small RCT (Tibaek 2015), data are insufficient to provide any reliable indication of benefit or risk of physical therapy targeted towards pelvic floor muscles for improving erectile function following stroke.
No study participants reported any adverse events from pelvic floor therapy (Tibaek 2015). No adverse event data were provided by the authors of the Ng 2017 trial. A large number of 'mild' adverse effects (predominantly gastrointestinal) were reported with the use of sertraline (Lu 2012).
Overall completeness and applicability of evidence
Only RCTs were included in this review, and there was a paucity of literature in general. Many pharmacological interventions are used in clinical practice, but only one included RCT examined a pharmacological intervention (Lu 2012), and the medication studied (sertraline) is not one that is commonly used in clinical practice to treat sexual dysfunction. On the contrary, one of the well‐recognised side effects of sertraline is sexual dysfunction (reduced libido) itself. There was no commonality in the two non‐pharmacological RCTs (one described sexual rehabilitation consisting predominantly of education and counselling, and the other described pelvic floor physiotherapy), and no results could be pooled (Ng 2017). Further, no studies addressed the numerous other interventions (including devices and complementary medicine) used to manage sexual dysfunction. A limited number of outcomes were addressed, for example, sexual satisfaction, satisfaction with sexual rehabilitation services, and sexual knowledge were not addressed by any of the included studies. The impact of sexual dysfunction on sexual partners is also significant, but there was little involvement of partners, and only one of the three RCTs included an outcome measure for sexual partners (Lu 2012). There was no reporting of gender identification nor sexual orientation, and particular populations such as those who identify as lesbian, gay, bisexual, transgender, intersex, and queer (LGBTIQ+) may be particularly vulnerable, at higher risk of stigma, and less well supported. According to the Australian Department of Health (AIHW 2018), up to 11% of the Australian population identify as LBGTIQ+, representing a significant yet under‐studied population.
No recommendations can be made regarding any specific treatment for sexual dysfunction following stroke based on current evidence. Evidence is limited by the small number of low‐ to very low‐quality trials. Additionally, the RCT with the largest number of participants (N = 114) had the most restrictive participant characteristics (young, married, heterosexual Chinese males), making it impossible to generalise the results (Lu 2012).
Sexuality is an area in which the attitudes of many ‐ patients and clinicians included ‐ make management and research into this area challenging (Steinke 2013). Older patients, patients who do not identify as heterosexual, and patients without partners may be particularly wary of the social stigma reflected in society at large (Steinke 2013). All included trials however have been published in the past decade, which is encouraging, as this suggests that researchers have identified lack of evidence to support clinical practice in this area and are beginning to address it. As there is a cultural shift towards more open discussion about sexuality, this may also facilitate the willingness of participants to be involved in research. An Australia‐based research group led by McGrath et al recently published findings from a survey done with 102 participants (stroke survivors and their partners, rehabilitation clinicians, and researchers in the field) using modified Delphi methods (McGrath 2019). A high degree of consensus was evident amongst the participants that 18 core content areas should be prioritised for inclusion in sexual rehabilitation following stroke, and that sexual rehabilitation should be offered in the subacute and chronic phases of stroke recovery. Participants also expressed a preference for health professionals to deliver the intervention in a face‐to‐face format. It is likely that these findings will be used to prioritise the content of, and approaches to, sexual rehabilitation, thus paving the way for further interventional research.
Quality of the evidence
We identified three RCTs. All were at high risk of bias. It is recognised, however, that it would be difficult to blind participants when interventions such as counselling or therapy are provided. All studies included small sample sizes and poor reporting, and lack of clarification from study authors made it challenging to fully determine the risk of bias. This was particularly challenging with Lu 2012, in which a large amount of information, including participant characteristics, method of randomisation, allocation concealment, and blinding, was unclear. Reporting of the intervention/control itself was also unclear and was not consistent with CONSORT guidelines (Schulz 2011).
Potential biases in the review process
We applied a comprehensive search strategy and included searches of clinical trial registers and grey literature. We also contacted experts in the field. It is possible however that we may have missed relevant studies. Two of the three included RCTs were negative studies (Ng 2017; Tibaek 2015), which was a positive given the tendency for negative studies not to be published, resulting in publication bias.
Agreements and disagreements with other studies or reviews
The findings of this review are consistent with those of previous systematic and literature reviews on sexuality following stroke. In addition to determining the effectiveness of interventions for sexual dysfunction, Dusenbury 2017 and Grenier‐Genest 2017 described the determinants of sexual dysfunction. Dusenbury 2017 reported that most studies included male participants (90%) with moderate erectile dysfunction (ED) and mild depression. Changes in sexual activity, sexual dissatisfaction, and sexual dysfunction were common and were much worse post stroke (Dusenbury 2017). Specific changes included decreased libido, problems with orgasm, and erectile dysfunction (Dusenbury 2017). A further literature review focused on the anatomy and physiology of sexual dysfunction post stroke (Park 2015). No reviews included Lu 2012, likely because this study was listed only on the CENTRAL database and therefore could be easily missed.
Other Cochrane Reviews have explored the effectiveness of interventions for sexual dysfunction in various conditions. Vecchio 2010 included 15 studies (8 parallel, 7 cross‐over; 352 patients) that evaluated the effects of phosphodiesterase‐5 inhibitor (PDE5i) agents, zinc, vitamin E, vitamin D, or bromocriptine compared to placebo. Of these interventions, review authors concluded that two (PDE5i and oral zinc) were likely to confer some benefit. Review authors found that PDE5 inhibitors improved erectile function in people with chronic kidney disease; however, the quality of this evidence was limited by the small number of trials (3 trials, 142 patients). The quality of evidence supporting oral zinc supplementation for increased frequency and potency of intercourse was even weaker, with only one small trial (20 patients). Xiao 2012 found limited evidence based on two trials (420 participants in total) ‐ both judged to have high risk of attrition bias ‐ that sildenafil improved erectile function in people with multiple sclerosis. Levack 2015 included two studies involving a total of 48 participants, which evaluated the effects of interventions for sexual dysfunction in people with chronic obstructive pulmonary disease (COPD). These studies investigated different interventions ‐ one compared testosterone therapy to placebo, and the other compared one month of long‐term oxygen therapy to a single 24‐hour dose of oxygen therapy. Review authors concluded that there was low‐quality evidence suggesting that testosterone therapy for men with COPD resulted in improvements in erectile function, but that data were insufficient to provide any reliable indication of benefit or risk to guide clinical practice. Although some of these treatments are likely to have a similar effect in people with stroke, evidence to support this is required.
It is interesting that most studies have focused on erectile dysfunction, which automatically excludes the sexual experience of all women. Erectile dysfunction is only one aspect of sexuality, which, as previously mentioned, is a broad concept and may be experienced and expressed in a variety of ways (WHO 2006). It is challenging to study interventions that consider sexuality as the broad concept that it is. The broad concept of sexuality brings with it a further challenge, which is that of outcome measurement. Although outcome measures, such as the Changes in Sexual Functioning Questionnaire Short Form‐14 (CSFQ‐14) (Keller 2006), address sexuality broadly, these have not been validated in the stroke population and probably are not sensitive enough or adequately targeted for this population, for example, the effects of hemiplegia or language deficits are not considered within the questionnaire. A comprehensive, stroke‐specific tool is needed to gather accurate response data.
Authors' conclusions
Implications for practice.
A paucity of evidence is available from RCTs on the effectiveness of interventions to treat sexual dysfunction in the post‐stroke population. However, the absence of evidence should not be interpreted as proof of ineffectiveness.
Based on current evidence, data are insufficient to provide any reliable indication of benefit or risk to guide clinical practice in terms of the use of sertraline, specific pelvic floor muscle training, or individualised sexual rehabilitation.
Implications for research.
This overview has highlighted a significant gap in the current literature. There is need for:
appropriate study designs, robust methods, and longitudinal data that address the multi‐faceted and inherently sensitive nature of sexuality;
studies to assess the effectiveness of the broad range of interventions used to manage sexual dysfunction;
studies to assess the appropriate timing, content, and delivery of rehabilitation interventions;
development of stroke‐specific reliable and validated outcome measures that reflect the broad concept of sexuality;
studies specifically designed for women with stroke; and
recognition and inclusion of participants with diverse sexuality backgrounds, including those who identify as LGBTIQ+ and sexual partners in general.
History
Protocol first published: Issue 7, 2014 Review first published: Issue 5, 2020
Acknowledgements
We acknowledge the Cochrane Stroke Group, in particular Joshua Cheyne (searcher and reviewer) and Hazel Fraser, for their assistance. We thank Nina Zhang and Fary Khan for reading the draft of the protocol, and Lai Kiap Lim for assisting with translation of the study by Lu et al (2012). We thank the reviewers, Maree Hackett, Peter Langhorne, Aryelly Rodriguez, Margaret McGrath, Abhijna Vithal Yergolkar, and Nji Mbaka Fon for their thoughtful and constructive comments.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cerebrovascular Disorders] this term only #2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only #3 MeSH descriptor: [Brain Ischemia] explode all trees #4 MeSH descriptor: [Carotid Artery Diseases] explode all trees #5 MeSH descriptor: [Carotid Artery Diseases] explode all trees #6 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #7 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #8 MeSH descriptor: [Intracranial Hemorrhages] explode all trees #9 MeSH descriptor: [Stroke] explode all trees #10 MeSH descriptor: [Vasospasm, Intracranial] this term only #11 MeSH descriptor: [Vertebral Artery Dissection] this term only #12 (stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH):ti,ab,kw (Word variations have been searched) #13 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw (Word variations have been searched) #14 ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) near/5 (h?emorrhag* or h?ematoma* or bleed*)):ti,ab,kw (Word variations have been searched) #15 MeSH descriptor: [Hemiplegia] this term only #16 MeSH descriptor: [Paresis] explode all trees #17 MeSH descriptor: [Gait Disorders, Neurologic] explode all trees #18 MeSH descriptor: [Aphasia] explode all trees #19 MeSH descriptor: [Hemianopsia] this term only #20 (hemipleg* or hemipar* or paresis or paraparesis or paretic):ti,ab,kw (Word variations have been searched) #21 {or #1‐#20} #22 MeSH descriptor: [Sexual Behavior] explode all trees #23 MeSH descriptor: [Sex Counseling] this term only #24 MeSH descriptor: [Libido] this term only #25 MeSH descriptor: [Sexual Dysfunction, Physiological] explode all trees #26 MeSH descriptor: [Sexual Partners] this term only #27 ((sexual* or libido) near/5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem* or concern* or difficult*)):ti,ab,kw #28 ((sexual* near/5 (activ* or health or issue* or satisfaction or function* or experience* or adjustment or chang* or knowledge or relation* or skill* or intercourse or interest* or wish* or need* or behav* or perform* or spouse or partner or wife or husband))):ti,ab,kw #29 (sexual* near/5 (therap* or treat* or rehab* or train* or counsel* or psycholog* or psychother* or physiotherapy* or help* or advi* or inform* or guid* or intervention* or educat*)):ti,ab,kw #30 (erectile near/3 (function* or dysfunction*)):ti,ab,kw #31 (intima* near/3 (relation* or spouse or partner or wife or husband)):ti,ab,kw #32 (marital near/3 relation*):ti,ab,kw #33 {or #22‐#32} #34 #21 and #33
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. exp hemiplegia/ or exp paresis/ or gait disorders, neurologic/
6. exp aphasia/ or hemianopsia/
7. (hempar$ or hemipleg$ or paresis or paretic or aphas* or dysphas$ or hemianop$).tw.
8. or/1‐7
9. exp sexual behavior/ or sex counseling/ or libido/ or exp sexual dysfunction, physiological/ or exp sexual dysfunctions, psychological/ or sexual partners/
10. ((sexual$ or libido) adj5 (disorder$ or declin$ or dysfunct$ or impair$ or deficit$ or disabilit$ or problem$ or concern$ or difficult$)).tw.
11. (sexual$ adj5 (activ$ or health or issue$ or satisfaction or function$ or experience$ or adjustment or chang$ or knowledge or relation$ or skill$ or intercourse or interest$ or wish$ or need$ or behav$ or perform$ or spouse or partner or wife or husband)).tw.
12. (sexual$ adj5 (therap$ or treat$ or rehab$ or train$ or counsel$ or psycholog$ or psychother$ or physiotherapy$ or help$ or advi$ or inform$ or guid$ or intervention$ or educat$)).tw.
13. (erectile adj3 (function$ or dysfunction$)).tw.
14. (intima$ adj3 (relation$ or spouse or partner or wife or husband)).tw.
15. (marital adj3 relation$).tw.
16. 9 or 10 or 11 or 12 or 13 or 14 or 15
17. Randomized Controlled Trials as Topic/
18. random allocation/
19. Controlled Clinical Trials as Topic/
20. control groups/
21. clinical trials as topic/
22. double‐blind method/
23. single‐blind method/
24. Placebos/
25. placebo effect/
26. cross‐over studies/
27. Drug Evaluation/ or drug therapy.fs.
28. therapies, investigational/ or research design/
29. randomized controlled trial.pt.
30. controlled clinical trial.pt.
31. clinical trial.pt.
32. (random$ or RCT or RCTs).tw.
33. (controlled adj5 (trial$ or stud$)).tw.
34. (clinical$ adj5 trial$).tw.
35. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
36. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
37. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
38. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
39. (cross‐over or cross over or crossover).tw.
40. (placebo$ or sham).tw.
41. trial.tw
42. (assign$ or allocat$).tw.
43. (controls or groups).ab.
44. or/17‐43
45. 8 and 16 and 44
46. exp animals/ not humans.sh.
47. 45 not 46
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. exp aphasia/ or hemianopia/ 6. exp hemiplegia/ or exp paresis/ or neurologic gait disorder/ 7. (hempar$ or hemipleg$ or paresis or paretic or aphas* or dysphas$ or hemianop$).tw. 8. or/1‐7 9. sexuality/ or libido/ or exp sexual behavior/ or sexual health/ or exp sexual orientation/ or sexual counseling/ or exp sexual dysfunction/ 10. ((sexual$ or libido) adj5 (disorder$ or declin$ or dysfunct$ or impair$ or deficit$ or disabilit$ or problem$ or concern$ or difficult$)).tw. 11. (sexual$ adj5 (activ$ or health or issue$ or satisfaction or function$ or experience$ or adjustment or chang$ or knowledge or relation$ or skill$ or intercourse or interest$ or wish$ or need$ or behav$ or perform$ or spouse or partner or wife or husband)).tw. 12. (sexual$ adj5 (therap$ or treat$ or rehab$ or train$ or counsel$ or psycholog$ or psychother$ or physiotherapy$ or help$ or advi$ or inform$ or guid$ or intervention$ or educat$)).tw. 13. (erectile adj3 (function$ or dysfunction$)).tw. 14. (intima$ adj3 (relation$ or spouse or partner or wife or husband)).tw. 15. (marital adj3 relation$).tw. 16. or/9‐15 17. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 18. Randomization/ 19. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 20. control group/ or controlled study/ 21. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 22. Crossover Procedure/ 23. Double Blind Procedure/ 24. Single Blind Procedure/ or triple blind procedure/ 25. placebo/ or placebo effect/ 26. (random$ or RCT or RCTs).tw. 27. (controlled adj5 (trial$ or stud$)).tw. 28. (clinical$ adj5 trial$).tw. 29. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 30. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 31. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 32. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 33. (cross‐over or cross over or crossover).tw. 34. (placebo$ or sham).tw. 35. trial.ti. 36. (assign$ or allocat$).tw. 37. controls.tw. 38. or/17‐37 39. 8 and 16 and 38
Appendix 4. CINAHL search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units") S2 TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH) S3 TI ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) OR AB ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) S4 TI (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) OR AB (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) S5 (MH "Hemiplegia") or (MH "Gait Disorders, Neurologic+") S6 TI (hemipleg* or hemipar* or paresis or paretic) OR AB (hemipleg* or hemipar* or paresis or paretic) S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S8 (MH "Sex+") OR (MH "Sexuality+") OR (MH "Sexual Dysfunction, Male") OR (MH "Sexual Dysfunction, Female") OR (MH "Psychosexual Disorders+") OR (MH "Couples Counseling") OR (MH "Sexual Counseling") OR (MH "Sexual and Gender Disorders+") OR (MH "Intimacy") OR (MH "Intimacy Positions") S9 TI ( (sexual* or libido) n5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem* or concern* or difficult*) ) OR AB ( (sexual* or libido) n5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem* or concern* or difficult*) ) S10 TI ( (sexual* n5 (activ* or health or issue* or satisfaction or function* or experience* or adjustment or chang* or knowledge or relation* or skill* or intercourse or interest* or wish* or need* or behav* or perform* or spouse or partner or wife or husband) ) OR AB ( (sexual* n5 (activ* or health or issue* or satisfaction or function* or experience* or adjustment or chang* or knowledge or relation* or skill* or intercourse or interest* or wish* or need* or behav* or perform* or spouse or partner or wife or husband) ) S11 TI ( (sexual* n5 (therap* or treat* or rehab* or train* or counsel* or psycholog* or psychother* or physiotherapy* or help* or advi* or inform* or guid* or intervention* or educat*)) ) OR AB ( (sexual* n5 (therap* or treat* or rehab* or train* or counsel* or psycholog* or psychother* or physiotherapy* or help* or advi* or inform* or guid* or intervention* or educat*)) ) S12 TI ( (erectile n3 (function* or dysfunction*)) ) OR AB ( (erectile n3 (function* or dysfunction*)) ) S13 TI ( (intima* n3 (relation* or spouse or partner or wife or husband)) ) OR AB ( (intima* n3 (relation* or spouse or partner or wife or husband)) ) S14 TI (marital n3 relation*) OR AB (marital n3 relation*) S15 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 S16 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S17 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S18 TI random* or AB random* S19 AB "latin square" or TI "latin square" S20 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S21 MH Placebos S22 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S23 TI blind* or AB mask* or AB blind* or TI mask* S24 S22 and S23 S25 TI Placebo* or AB Placebo* or SU Placebo* S26 MH Clinical Trials S27 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S28 S16 or S17 or S18 or S19 or S20 or S21 or S24 or S25 or S26 or S27 S29 S7 AND S15 AND S28
Appendix 5. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or isch?emi$ attack$ or tia$1 or neurologic$ deficit$ or SAH or AVM).tw. 3. ((brain$ or cerebr$ or cerebell$ or cortical or vertebrobasilar or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA or anterior circulation or posterior circulation or basal ganglia) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm or obstruction or vasculopathy)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or subarachnoid or putaminal or putamen or posterior fossa) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or gait disorders/ or muscle spasticity/ 6. aphasia/ 7. (hempar$ or hemipleg$ or paresis or paretic or aphas$ or dysphas$ or hemianop$).tw. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. exp sex/ or exp sex behavior/ or exp sex counseling/ or sex disorders female/ or sex disorders male/ or "sexual and gender disorders"/ or psychosexual disorders/ or exp sexual dysfunctions/ 10. ((sexual$ or libido) adj5 (disorder$ or declin$ or dysfunct$ or impair$ or deficit$ or disabilit$ or problem$ or concern$ or difficult$)).tw. 11. (sexual$ adj5 (activ$ or health or issue$ or satisfaction or function$ or experience$ or adjustment or chang$ or knowledge or relation$ or skill$ or intercourse or interest$ or wish$ or need$ or behav$ or perform$ or spouse or partner or wife or husband)).tw. 12. (sexual$ adj5 (therap$ or treat$ or rehab$ or train$ or counsel$ or psycholog$ or psychother$ or physiotherapy$ or help$ or advi$ or inform$ or guid$ or intervention$ or educat$)).tw. 13. (erectile adj3 (function$ or dysfunction$)).tw. 14. (intima$ adj3 (relation$ or spouse or partner or wife or husband)).tw. 15. (marital adj3 relation$).tw. 16. or/9‐15 17. 8 and 16
Appendix 6. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ 6. exp aphasia/ 7. hemianopia/ 8. (hempar$ or hemipleg$ or paresis or paretic or aphas* or dysphas$ or hemianop$).tw. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. psychosexual behavior/ or "erection (penis)"/ or exp human courtship/ or masturbation/ or exp orgasm/ or safe sex/ or exp sexual arousal/ or exp sexual function disturbances/ or exp "sexual intercourse (human)"/ or psychosexual development/ or exp sex/ or sexual attraction/ or sexual fantasy/ or sexual satisfaction/ or sexual partners/ 11. ((sexual$ or libido) adj5 (disorder$ or declin$ or dysfunct$ or impair$ or deficit$ or disabilit$ or problem$ or concern$ or difficult$)).tw. 12. (sexual$ adj5 (activ$ or health or issue$ or satisfaction or function$ or experience$ or adjustment or chang$ or knowledge or relation$ or skill$ or intercourse or interest$ or wish$ or need$ or behav$ or perform$ or spouse or partner or wife or husband)).tw. 13. (sexual$ adj5 (therap$ or treat$ or rehab$ or train$ or counsel$ or psycholog$ or psychother$ or physiotherapy$ or help$ or advi$ or inform$ or guid$ or intervention$ or educat$)).tw. 14. (erectile adj3 (function$ or dysfunction$)).tw. 15. (intima$ adj3 (relation$ or spouse or partner or wife or husband)).tw. 16. (marital adj3 relation$).tw. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. clinical trials/ or treatment effectiveness evaluation/ or placebo/ 19. (random$ or RCT or RCTs).tw. 20. (controlled adj5 (trial$ or stud$)).tw. 21. (clinical$ adj5 trial$).tw. 22. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 23. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 24. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 25. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 26. (cross‐over or cross over or crossover).tw. 27. (placebo$ or sham).tw. 28. trial.ti. 29. (assign$ or allocat$).tw. 30. controls.tw. 31. or/18‐30 32. 9 and 17 and 31
Appendix 7. PEDro search strategy
neurology in the <Subdiscipline> field clinical trial in the <Method> field (sexual OR erectile dysfunction OR arousal OR intimacy OR impotence) in the <Title & Abstract> field
Appendix 8. CIRRIE search strategy
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Appendix 9. DARE search strategy
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Appendix 10. ProQuest search strategy
(ti,ab((sexual* OR libido) NEAR/5 (disorder* OR declin* OR dysfunct* OR impair* OR deficit* OR disabilit* OR problem* OR concern* OR difficult*)) OR ti,ab(sexual* NEAR/5 (activ* OR health OR issue* OR satisfaction OR function* OR experience* OR adjustment OR chang* OR knowledge OR relation* OR skill* OR intercourse OR interest* OR wish* OR need* OR behav* OR perform* OR spouse OR partner OR wife OR husband)) OR ti,ab(sexual* NEAR/5 (therap* OR treat* OR rehab* OR train* OR counsel* OR psycholog* OR psychother* OR physiotherapy* OR help* OR advi* OR inform* OR guid* OR intervention* OR educat*)) OR ti,ab(erectile NEAR/3 (function* OR dysfunction*))) AND ti,ab(stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH)
Appendix 11. OT Search by the American Occupational Foundation and the American Occupational Therapy Association
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Appendix 12. OTSeeker search strategy
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Appendix 13. National Rehabilitation Information Center REHABDATA Database search strategy
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Appendix 14. SPORTDiscus search strategy
S1 DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis" OR DE "STROKE" OR DE "BRAIN ‐‐ Wounds & injuries" OR DE "BRAIN damage" S2 DE "CEREBROVASCULAR disease patients" S3 TI ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) or AB ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) S4 TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) S5 TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* ) S6 S4 AND S5 S7 TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) S8 TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S9 S7 AND S8 S10 DE "HEMIPLEGIA" OR DE "HEMIPLEGICS" S11 TI ( hemipleg* or hemipar* or paresis or paretic or brain injur* ) or AB ( hemipleg* or hemipar* or paresis or paretic or brain injur*) S12 S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 S13 ((DE "HUMAN sexuality") OR (DE "SEXUAL exercises")) OR (DE "GENITALIA") S14 TI ( (sexual* or libido) n5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem* or concern* or difficult*)) ) OR AB ( (sexual* or libido) n5 (disorder* or declin* or dysfunct* or impair* or deficit* or disabilit* or problem* or concern* or difficult*)) ) S15 TI ( (sexual* n5 (activ* or health or issue* or satisfaction or function* or experience* or adjustment or chang* or knowledge or relation* or skill* or intercourse or interest* or wish* or need* or behav* or perform* or spouse or partner or wife or husband)) ) OR AB ( (sexual* n5 (activ* or health or issue* or satisfaction or function* or experience* or adjustment or chang* or knowledge or relation* or skill* or intercourse or interest* or wish* or need* or behav* or perform* or spouse or partner or wife or husband)) ) S16 TI ( (sexual* n5 (therap* or treat* or rehab* or train* or counsel* or psycholog* or psychother* or physiotherapy* or help* or advi* or inform* or guid* or intervention* or educat*)) ) OR AB ( (sexual* n5 (therap* or treat* or rehab* or train* or counsel* or psycholog* or psychother* or physiotherapy* or help* or advi* or inform* or guid* or intervention* or educat*)) ) S17 TI ( (erectile n3 (function* or dysfunction*)) ) OR AB ( (erectile n3 (function* or dysfunction*)) ) S18 TI ( (intima* n3 (relation* or spouse or partner or wife or husband)) ) OR AB ( (intima* n3 (relation* or spouse or partner or wife or husband)) ) S19 TI (marital n3 relation*) OR AB (marital n3 relation*) S20 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21 S12 AND S20
Appendix 15. CT.gov search strategy
( sexual OR erectile dysfunction OR arousal OR intimacy OR impotence ) AND ( Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke ) [DISEASE]
Appendix 16. World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search strategy
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Appendix 17. ISRCTN Registry search strategy
Basic search: stroke AND sexual OR stroke AND erectile dysfunction OR stroke AND arousal OR stroke AND intimacy OR stroke AND impotence
Data and analyses
Comparison 1. Sertraline compared with placebo (methylcobalamin) for secondary premature ejaculation after stroke ‐ end of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Sexual function ‐ intravaginal ejaculatory latent time | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 2.00 [1.78, 2.22] |
| 1.2 Sexual function ‐ non‐validated measure | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.90 [0.15, 1.65] |
| 1.3 Partner sexual satisfaction ‐ non‐validated measure | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 2.50 [1.89, 3.11] |
| 1.4 Adverse effects | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.92, 5.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lu 2012.
| Study characteristics | ||
| Methods | Study design: RCT Study duration: 12 weeks Follow‐up: 4 weeks, 8 weeks, 12 weeks Country: China Setting: presumed outpatients given duration of study, although initial recruitment was sought from neurology inpatients | |
| Participants | Number (intervention/control): 58/56 Age (mean): 41 years Sex (both groups): 100% men Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group: 8 weeks of oral sertraline 50 mg daily 4 to 6 hours before bed or sexual intercourse. Participants encouraged to have sexual intercourse at least 1 to 2 times weekly Neurologist and urologist provided psychological and behavioural advice Control group: 8 weeks of oral methylcobalamin 0.5 mg daily 4 to 6 hours before bed or sexual intercourse All participants received "psychological and behavioural advice" from a neurologist and urologist and were encouraged to have sexual intercourse at least 1 to 2 times weekly | |
| Outcomes |
|
|
| Funding source | Funding is not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is mentioned in the abstract; method is not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not stated. No attempt was made to ensure sertraline and methylcobalamin tablets or their containers are matched in any way |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 dropouts from treatment group and 3 from control group. Unclear how missing data were handled |
| Selective reporting (reporting bias) | High risk | No reporting of outcome measures such as ECG findings, blood panel including "liver function tests, renal function tests, cholesterol, creatine kinase, hormone levels," and blood pressure |
| Other bias | High risk | Patient characteristics are not reported Other than IELT, other outcome measures are not validated in stroke populations nor described Screening process for recruitment of patients is unclear. Some criteria (such as 'good relationship') were not clearly defined Setting is unclear: patients were recruited as inpatients, but it is unclear if and how much of the programme was continued in an outpatient setting No information on psychological and behavioural advice given was provided Unclear as to why methylcobalamin was chosen as the control |
Ng 2017.
| Study characteristics | ||
| Methods | Single‐blinded RCT Study duration: 14 months Follow‐up: 6 weeks, 6 months Country: Australia Setting: inpatient rehabilitation unit | |
| Participants | Number (intervention/control): 35/33
Mean age (intervention/control): 62/67 years
Sex (intervention/control): 60%/54.5% men
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group: single 30‐minute individualised sexual rehabilitation programme provided by a rehabilitation physician and written educational material (National Stroke Foundation fact sheet). Partners were included when possible Rehabilitation physicians delivering the individualised component of the programme had 7 years of experience each in delivery of sexual rehabilitation. Additional input from occupational therapy, physiotherapy, and or psychology as required, for counselling or training to optimise bed mobility for sexual positioning or to address other aspects of sexuality. Programmes were individually tailored and based on the "PLISSIT" model: Permission ‐ reassurance that sexual dysfunction is common; Limited information ‐ such as safety of resuming sexual activity; Specific Suggestions ‐ such as positioning; and Intensive Therapy ‐ such as in‐depth counselling. Intensive therapy was not provided as part of the 30‐minute session, but participants could be referred when deemed appropriate by the participant and the physician. Content included (1) information regarding common changes in sexuality post stroke; (2) counselling on fears regarding post‐stroke sexuality; (3) challenging stereotypical views on sexuality and sexual satisfaction (such as sexual intercourse being the only expression of sexual activity, having sexual intercourse only at night, or believing that sexual intercourse must be spontaneous to achieve sexual satisfaction); and (4) tips and strategies to minimise post‐stroke sexual dysfunction, such as choosing a suitable time, reviewing sexual side effects from medications, finding safe and comfortable sexual positions, and managing reduced vaginal lubrication, urinary continence issues, or erectile difficulties Control group: written educational material only (National Stroke Foundation fact sheet). Could request additional information if desired |
|
| Outcomes |
|
|
| Funding source | Victor Hurley Medical Research Grant‐in‐Aid and the AFRM Ipsen Open Research Fellowship | |
| Notes | This study included data from its pilot study. A large proportion of the intervention group were not sexually active with a partner | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated: "randomized ... using a computer‐generated sequence by an independent statistician" |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, opaque, sealed envelopes were used for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and care providers were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded and had no access to previous treatments or documentation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participant dropout of 11% at 6 weeks and 25% at 6 months was reported. How this was addressed in terms of data handling was not stated |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Other bias | Low risk | Study was adequately powered |
Tibaek 2015.
| Study characteristics | ||
| Methods | Study design: RCT Study duration: 35 months Follow‐up: 3 months, 6 months Country: Denmark Setting: outpatient | |
| Participants | Number (intervention/control): 15/15
Median age (intervention/control): 68/70 years
Sex (intervention/control): 100% men
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group: PFMT programme delivered by a specialised physiotherapist consisting of:
Control group received:
|
|
| Outcomes |
|
|
| Funding source | Funding sources: grants from the Association of Danish Physiotherapists Research Foundation, the Association of Danish Physiotherapists Practise Foundation, the Foundation of 12.12.1981, Lykkefeldts Grant, the Foundation of Lundbeck, and the Department of Physiotherapy and Occupational Therapy Glostrup Hospital, University of Copenhagen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated: "randomisation was based on a mathematical table, delivered in sealed envelope" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded Physiotherapist treating participants with PFMT was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6% were lost to follow‐up, but a large quantity of data was missing due to failure to complete questionnaires (17% in treatment group and 40% to 60% in control group) It is unclear how missing data were managed |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Did not reach sample size of 120 participants |
CSFQ‐14: Changes in Sexual Functioning Questionnaire Short Form‐14. DASS 21: Depression, Anxiety, and Stress Scale. ECG: electrocardiogram. FIM: Functional Independence Measure. IELT: intravaginal ejaculatory latency time. IIEF‐5: International Index of Erectile Function Questionnaire. LUTS: lower urinary tract symptoms. MMSE: Mini‐Mental State Examination. MRI: magnetic resonance imaging. PFM: pelvic floor muscle. PFMT: pelvic floor muscle training. RCT: randomised controlled trial. SAQOL‐39g: Stroke and Aphasia Quality of Life Scale‐39 Generic. WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
CCT: clinical controlled trial. RCT: randomised controlled trial.
Differences between protocol and review
2019: The secondary outcome measure of 'satisfaction with sexual rehabilitation services' has been changed to 'satisfaction with intervention' for better coverage of all interventions ‐ not just those related to sexual rehabilitation. We have also broadened the secondary outcome of 'marital/relationship satisfaction' to include partner satisfaction.
For future updates, we will separate device interventions and psychological interventions into two categories instead of including them in the same 'non‐pharmacological' subgroup of interventions. We will also consider sex‐disaggregated analyses.
Contributions of authors
Louisa Ng led the review, identified relevant articles, assisted with data extraction, reviewed the papers, provided methodological and content expertise, revised and finalised drafts of the review, and responded to all editorial comments.
Hezekiah Stratton identified relevant articles, assisted with data extraction, reviewed the papers, and wrote the drafts of the review.
Anita Brown‐Major, Joshua Sansom, and Paul Anderson provided content expertise and comments on the final drafts of the review.
Sources of support
Internal sources
University of Melbourne, Australia
External sources
None, Other
Declarations of interest
Hezekiah Stratton: none known. Joshua Sansom: author of an included study (Ng 2017). Anita Brown‐Major: none known. Paul Anderson: none known. Louisa Ng: author of an included study (Ng 2017).
New
References
References to studies included in this review
Lu 2012 {published data only}
- Lu Z, Ye F, Shen S. Clinical study of sertraline in treatment of secondary premature ejaculation after stroke in young men [舍曲林治疗青年脑卒中后继发性早泄的临床研究]. Chinese Journal of Andrology 2012;26(3):52-4. [Google Scholar]
Ng 2017 {published data only}
- *.Ng L, Sansom J, Zhang N, Amatya B, Khan F. Effectiveness of a structured sexual rehabilitation programme following stroke: a randomized controlled trial. Journal of Rehabilitation Medicine 2017;49(4):330-40. [DOI] [PubMed] [Google Scholar]
- Sansom J, Ng L, Zhang N, Khan F. Let’s talk about sex: a pilot randomised controlled trial of a structured sexual rehabilitation programme in an Australian stroke cohort. International Journal of Therapy and Rehabilitation 2015;22(1):21-9. [Google Scholar]
Tibaek 2015 {published data only}
- Tibaek S, Gard G, Dehlendorff C, Iversen HK, Erdal J, Biering-Sorensen F, et al. The effect of pelvic floor muscle training on sexual function in men with lower urinary tract symptoms after stroke. Topics in Stroke Rehabilitation 2015;22(3):185-93. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chae 2006 {published data only}
- Chae B-J, Kang B-J. Quetiapine for hypersexuality and delusional jealousy after stroke. Journal of Clinical Psychopharmacology 2006;26(3):331-2. [DOI] [PubMed] [Google Scholar]
Song 2011 {published data only}
- Song HS, Oh HS, Kim HS, Seo WS. Effects of a sexual rehabilitation intervention program on stroke patients and their spouses. NeuroRehabilitation 2011;28(2):143-50. [DOI: ] [DOI] [PubMed] [Google Scholar]
Additional references
ABS 2017
- Australian Bureau of Statistics. 3303.0 - Causes of Death, Australia, 2017. Released 26 September 2018. https://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/47E19CA15036B04BCA2577570014668B?opendocument (accessed 20 January 2020).
AIHW 2018
- Australian Institute of Health and Welfare. Australia’s Health 2018. Australia’s Health Series https://www.aihw.gov.au/getmedia/61521da0-9892-44a5-85af-857b3eef25c1/aihw-aus-221-chapter-5-5.pdf.aspx (accessed 20 January 2020);(16):1-4.
Byrne 2016
- Byrne M, Doherty S, Fridlund BGA, Martensson J, Steinke EE, Jaarsma T, et al. Sexual counselling for sexual problems in patients with cardiovascular disease. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD010988.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2017
- Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion, Division for Heart Disease and Stroke Prevention (updated 1 September 2017). Stroke Facts. http://www.cdc.gov/stroke/facts.htm (accessed 20 January 2020).
CSN 2014
- Canadian Stroke Network and the Heart and Stroke Foundation of Canada. Canadian Best Practice Recommendations for Stroke Care 2014. http://www.strokebestpractices.ca/ (accessed 20 January 2020).
Derogatis 1997
- Derogatis LR. The Derogatis Interview for Sexual Functioning (DISF/DISF-SR): an introductory report. Journal of Sex and Marital Therapy 1997;23(4):291-304. [DOI] [PubMed] [Google Scholar]
Dusenbury 2017
- Dusenbury W, Palm Johansen P, Mosack V, Steinke EE. Determinants of sexual function and dysfunction in men and women with stroke: a systematic review. International Journal of Clinical Practice 2017;71(7):e-pub. [DOI: 10.1111/ijcp.12969] [DOI] [PubMed] [Google Scholar]
Giaquinto 2003
- Giaquinto S, Buzzelli S, Di Francesco L, Nolfe G. Evaluation of sexual changes after stroke. Journal of Clinical Psychiatry 2003;64(3):302-7. [DOI] [PubMed] [Google Scholar]
Grenier‐Genest 2017
- Grenier-Genest A, Gerard M, Courtois F. Stroke and sexual functioning: a literature review. NeuroRehabilitation 2017;41(2):293-315. [DOI: 10.3233/nre-001481] [DOI] [PubMed] [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54(5):541-53. [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). www.training.cochrane.org/handbook.
Hilari 2003
- Hilari K, Byng S, Lamping DL, Smith SC. Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke 2003;34(8):1944-50. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;1(5):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keller 2006
- Keller A, McGarvey EL, Clayton AH. Reliability and construct validity of the Changes in Sexual Functioning Questionnaire short-form (CSFQ-14). Journal of Sex and Marital Therapy 2006;32(1):43-52. [DOI] [PubMed] [Google Scholar]
Korpelainen 1999
- Korpelainen JT, Nieminen P, Myllyla VV. Sexual functioning among stroke patients and their spouses. Stroke 1999;30(4):715-9. [DOI] [PubMed] [Google Scholar]
Levack 2015
- Levack WMM, Poot B, Weatherall M, Travers J. Interventions for sexual dysfunction in people with chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011442.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovibond 1995
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd edition. Sydney: Psychology Foundation, 1995. [Google Scholar]
Lue 2004
- Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson KE, Althof S. Summary of the recommendations on sexual dysfunctions in men. Journal of Sexual Medicine 2004;1:6-23. [DOI] [PubMed] [Google Scholar]
McGahuey 2000
- McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. Journal of Sex and Marital Therapy 2000;26(1):25-40. [DOI] [PubMed] [Google Scholar]
McGrath 2019
- McGrath M, Lever S, McCluskey A, Power E. Developing interventions to address sexuality after stroke: findings from a four-panel modified Delphi study. Journal of Rehabilitation Medicine 2019;51(5):352-60. [DOI: 10.2340/16501977-2548] [DOI] [PubMed] [Google Scholar]
Meston 2005
- Meston C, Trapnell P. Development and validation of a five-factor sexual satisfaction and distress scale for women: the Sexual Satisfaction Scale for Women (SSS-W). Journal of Sexual Medicine 2005;2(1):66-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miles 2007
- Miles CL, Candy B, Jones L, Williams R, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005540.pub2] [DOI] [PubMed] [Google Scholar]
Monga 1986
- Monga TN, Lawson JS, Inglis J. Sexual dysfunction in stroke patients. Archives of Physical Medicine and Rehabilitation 1986;67(1):19-22. [PubMed] [Google Scholar]
NSF 2013
- National Stroke Foundation. Deloitte Access Economics Report. The economic impact of stroke in Australia. 2013. Melbourne, Australia. Available from http://strokefoundation.com.au/2013/03/the-economic-impact-of-stroke-in-australia/ (accessed 2 January 2014).
NSF 2017
- National Stroke Foundation. Clinical Guidelines for Stroke Management 2017. Melbourne, Australia. Available from http://strokefoundation.com.au/health-professionals/tools-and-resources/clinical-guidelines-for-stroke-prevention-and-management/ (accessed 20 January 2020).
NSF 2018
- National Stroke Foundation. National Stroke Audit – Rehabilitation Services Report 2018. Melbourne, Australia. Available from https://informme.org.au/-/media/42056DD67049480FACAE79440B440FEC.ashx?la=en (accessed 20 January 2020).
Olsen 1993
- Olsen D, Fowers BJ. Enrich Marital Satisfaction Scale: a brief research and clinical tool. Journal of Family Psychology 1993;7:176-85. [Google Scholar]
Palmer 2005
- Palmer AJ, Valentine WJ. Overview of costs of stroke. Current Medical Research and Opinion 2005;21:19-26. [DOI] [PubMed] [Google Scholar]
Park 2015
- Park JH, Ovbiagele B, Feng W. Stroke and sexual dysfunction - a narrative review. Journal of the Neurological Sciences 2015;350(1-2):7-13. [DOI: 10.1016/j.jns.2015.02.001] [DOI] [PubMed] [Google Scholar]
Quirk 2002
- Quirk FH, Heiman JR, Rosen RC, Laan E, Smith MD, Boolell M. Development of a sexual function questionnaire for clinical trials of female sexual dysfunction. Journal of Womens Health and Gender Based Medicine 2002;11(3):277-89. [DOI] [PubMed] [Google Scholar]
RCP 2016
- Intercollegiate Stroke Working Party. National Clinical Guideline for stroke. 5th edition. London: Royal College of Physicians, 2016. Available from http://www.rcplondon.ac.uk/resources/stroke-guidelines (accessed 20 January 2020).
Review Manager 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822-30. [DOI] [PubMed] [Google Scholar]
Schmitz 2010
- Schmitz MA, Finkelstein M. Perspectives on poststroke sexual issues and rehabilitation needs. Topics in Stroke Rehabilitation 2010;17(3):204-13. [DOI] [PubMed] [Google Scholar]
Schulz 2011
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2011;9(8):672-7. [DOI: 10.1016/j.ijsu.2011.09.004] [DOI] [PubMed] [Google Scholar]
Serefoglu 2014
- Serefoglu EC, McMahon CG, Waldinger MD, Althof SE, Shindel A, Adaikan G, et al. An evidence-based unified definition of lifelong and acquired premature ejaculation: report of the second International Society for Sexual Medicine ad hoc committee for the definition of premature ejaculation. Journal of Sexual Medicine 2014;2(2):41-59. [DOI: 10.1002/sm2.27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sjogren 1981
- Sjogren K, Fugl-Meyer AR. Sexual problems in hemiplegia. Internal Journal of Rehabilitation Medicine 1981;3(1):26-31. [DOI] [PubMed] [Google Scholar]
Stein 2013
- Stein J, Hillinger M, Clancy C, Bishop L. Sexuality after stroke: patient counseling preferences. Disability Rehabilitation 2013;35:1842–7. [DOI] [PubMed] [Google Scholar]
Steinke 2013
- Steinke EE, Mosack V, Hill TJ. Sexual self-perception and adjustment of cardiac patients: a psychometric analysis. Journal of Research in Nursing 2013;18(3):191-201. [Google Scholar]
Tamam 2008
- Tamam Y, Tamam L, Akil E, Yasan A, Tamam B. Post-stroke sexual functioning in first stroke patients. European Journal of Neurology 2008;15(7):660-6. [DOI] [PubMed] [Google Scholar]
Vardi 2007
- Vardi M, Nini A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002187.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vecchio 2010
- Vecchio M, Navaneethan SD, Johnson DW, Lucisano G, Graziano G, Saglimbene V, et al. Interventions for treating sexual dysfunction in patients with chronic kidney disease. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007747.pub2] [DOI] [PubMed] [Google Scholar]
Wade 1992
- Wade DT. Measurement in Neurology Rehabilitation. Oxford: Oxford University Press, 1992. [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Ware 1995
- Ware JE Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Medical Care 1995;33(4 Suppl):AS264-79. [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Defining sexual health. Department of Reproductive Health and Research 2006 https://www.who.int/reproductivehealth/topics/sexual_health/sh_definitions/en/ (accessed 14 August 2013).
Xiao 2012
- Xiao Y, Wang J, Luo H. Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009427.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ng 2014
- Ng L, Sansom J, Zhang NY, Khan F. Interventions for sexual dysfunction following stroke. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011189] [DOI] [PMC free article] [PubMed] [Google Scholar]


