ABSTRACT
Different stem cells or progenitor cells display variable threshold requirements for functional ribosomes. This is particularly true for several human ribosomopathies in which select embryonic neural crest cells or adult bone marrow stem cells, but not others, show lethality due to failures in ribosome biogenesis or function (now known as nucleolar stress). To determine if various Drosophila neuroblasts display differential sensitivities to nucleolar stress, we used CRISPR-Cas9 to disrupt the Nopp140 gene that encodes two splice variant ribosome biogenesis factors (RBFs). Disruption of Nopp140 induced nucleolar stress that arrested larvae in the second instar stage. While the majority of larval neuroblasts arrested development, the mushroom body (MB) neuroblasts continued to proliferate as shown by their maintenance of deadpan, a neuroblast-specific transcription factor, and by their continued EdU incorporation. MB neuroblasts in wild-type larvae appeared to contain more fibrillarin and Nopp140 in their nucleoli as compared to other neuroblasts, indicating that MB neuroblasts stockpile RBFs as they proliferate in late embryogenesis while other neuroblasts normally enter quiescence. A greater abundance of Nopp140 encoded by maternal transcripts in Nopp140-/- MB neuroblasts of 1–2-day-old larvae likely rendered these cells more resilient to nucleolar stress.
KEY WORDS: Nucleolar stress, Neuroblasts, Drosophila, Nopp140, Ribosomopathy
Summary: Nucleolar stress (loss of ribosome production/function) in certain human progenitor or stem cells results in disease. In fruit flies, larval mushroom body neuroblasts are relatively resilient to nucleolar stress.
INTRODUCTION
The nucleolus is the nuclear sub-compartment responsible for ribosomal subunit biogenesis (Baßler and Hurt, 2019). Functional ribosomes in the cytoplasm of eukaryotic cells consist of the small ribosomal subunit with its 18S ribosomal RNA (rRNA) assembled with 33 ribosomal proteins and the large ribosomal subunit with its 28S, 5.8S, and 5S rRNAs assembled with 47 ribosomal proteins. Subunit assembly is a complex choreography of reactions and interactions within nucleoli beginning with pre-rRNA transcription by RNA polymerase I (RNA Pol I) on tandemly repeated ribosomal DNA (rDNA) genes. The 38S pre-rRNA in Drosophila undergoes endonuclease cleavages to generate 18S, 5.8S+2S, and 28S rRNAs (Long and Dawid, 1980). These rRNAs undergo 2′-O-methylation by box C/D small nucleolar ribonucleoprotein complexes (snoRNPs) and pseudouridylation by box H/ACA snoRNPs (Wang et al., 2002; Yang et al., 2000; Bachellerie et al., 2002). Besides endonucleases and snoRNPs, subunit biogenesis requires a myriad of other factors serving as RNP chaperones, RNA helicases, and GTPase release factors (Kressler et al., 2010). The chaperones, often referred to as ribosome biogenesis factors (RBFs), act early in ribosome assembly; they include Nopp140 (Nucleolar and Cajal body phosphoprotein of 140 kDa) and treacle. While both Nopp140 and treacle are found in vertebrates, only Nopp140 orthologues are expressed in all eukaryotes.
Ribosome biogenesis requires high-energy expenditures by the cell; approximately 60% of total cellular transcription is devoted to rRNA, with some 2000 ribosomes assembled per minute in actively growing yeast cells (Warner, 1999; Woolford and Baserga, 2013). Any perturbation in ribosome biogenesis disrupts cell homeostasis; this is now called nucleolar (or ribosome) stress (Golomb et al., 2014; Tsai and Pederson, 2014; Yang et al., 2018). In humans, nucleolar stress due to mutations in RBFs, processing snoRNPs, or the ribosomal proteins themselves results in disease states collectively called ribosomopathies, of which there are several (Narla and Ebert, 2010). While each ribosomopathy has its own distinct phenotypes, and several display tissue-specificity (McCann and Baserga, 2013), there are commonalities among them: the most prevalent dysfunctions include craniofacial abnormalities, other skeletal defects, and bone marrow failures. All ribosomopathies affect only certain stem cells or progenitors despite the mutation being systemic.
One of these ribosomopathies is the Treacher Collins Syndrome (TCS), a congenital set of craniofacial birth defects caused by haplo-insufficiency mutations in the TCOF1 gene that encodes treacle (Sakai and Trainor, 2009). In TCS individuals, a particular subset of neural crest cells that normally migrate to and populate pharyngeal arches I and II on day 24–25 of human embryogenesis is insufficient in functional ribosomes. This leads to p53-dependent apoptosis (Jones et al., 2008), and loss of these select neural crest cells causes the craniofacial defects. A TCS-like phenotype can also result from mutations in genes encoding RNA Pol I and III subunit proteins, POLR1D and POLR1C, respectively (Dauwerse et al., 2011; Noack Watt et al., 2016). The question is, why are only certain progenitor cells affected while many others remain resilient?
Like treacle, metazoan Nopp140 orthologues contain alternating acidic and basic motifs constituting a large central domain of low sequence complexity (Meier, 1996). Treacle and Nopp140 also share similar roles in chaperoning C/D-box snoRNPs to the dense fibrillar component of nucleoli where pre-rRNA is modified by site-specific 2′-O-methylation. Unlike treacle, Nopp140 locates to Cajal bodies; thus Nopp140 may also play a role in snoRNP assembly and transport to nucleoli (Gonzales et al., 2005; Hayano et al., 2003; He et al., 2015). While a length polymorphism exists for the Drosophila Nopp140 gene (Baral and DiMario, 2019), and a mutation in the orthologous Caenorhabditis elegans Nopp140 gene, dao-5, has been described (Lee et al., 2014), no mutations in the human Nopp140 gene, NOLC1, have been characterized, suggesting a de novo mutation in NOLC1 may be embryonic lethal.
With predictably critical roles in ribosome biogenesis, we used RNAi to deplete Nopp140 in Drosophila (Cui and DiMario, 2007) to generate Minute-like phenotypes (Lambertsson, 1998; Marygold et al., 2007). Resulting nucleolar stress studies showed cell death occurred either by apoptosis in imaginal disc cells or by autophagy in terminally differentiated polyploid gut cells; these stress responses were p53-independent, but JNK-dependent (James et al., 2013). We used pBac elements to knock-out the Drosophila Nopp140 gene, and showed a tremendous loss of cytoplasmic ribosomes in larval polyploid cells, a corresponding accumulation of unusual electron dense granules in the cytoplasm of these same cells, a redistribution of fibrillarin from the nucleoli to the nucleoplasm in several cell types, and a resulting overall reduction in 2′-O-methylation of pre-rRNA (He et al., 2015). We did not see a detectable loss in pre-rRNA synthesis or gross morphological changes in nucleolar structure (He et al., 2015). We are now testing the hypothesis that defective ribosomes are still assembled in the absence of Nopp140, but then quickly degraded in the cytoplasm.
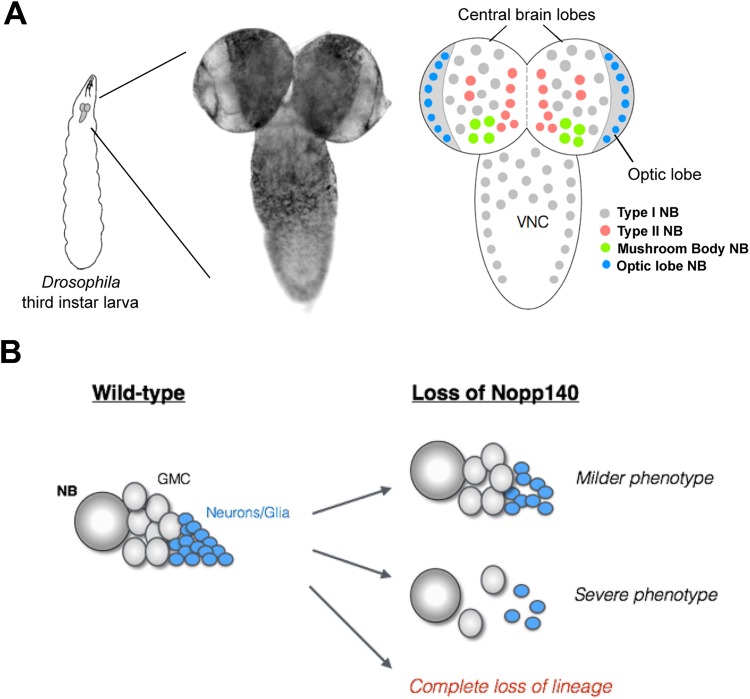
To investigate the underlying mechanisms contributing to stem cell or progenitor cell specificity as seen in the human ribosomopathies, we initiated a study of nucleolar stress in Drosophila larval neuroblasts. We wanted to determine if all neuroblast types respond similarly or differentially to nucleolar stress. The Drosophila larval brain comprises distinct neuroblast (NB) lineages generated from a fixed set of founder NBs (Homem and Knoblich, 2012; Hartenstein and Wodarz, 2013). Briefly, there are four major NB types in the Drosophila larval brain; Type I, Type II, mushroom body (MB), and optic lobe NBs (Fig. 1A). We initially hypothesized that upon nucleolar stress caused by the loss of Nopp140, different NB lineages exhibit variable phenotypes ranging from a mild loss of lineage progeny cells to substantial loss of the lineage altogether (Fig. 1B). Here we show that MB NBs are more resilient to the effects of nucleolar stress compared to the other NB types. Hence, different NB lineages respond variably to nucleolar stress, which is reminiscent of the neural crest cell-specific effects caused by the loss of treacle in TCS individuals.
Fig. 1.
Anatomy of the Drosophila larval brain and overall hypothesis. (A) Larval brains have two central brain lobes and a ventral nerve cord (VNC). There are roughly four NB types within the larval brain: Type I (grey), Type II (red), MB (green), and optic lobe (blue). These NBs are shown in their putative locations within the larval brain. MB NBs reside in the posterior of the brain lobes, which often flip forward when placing the brain on a microscope slide, thus giving a false impression of an anterior location within the brain lobe. (B) Our hypothesis is that upon nucleolar stress due to loss of Nopp140, different NB lineages exhibit variable phenotypes ranging from a mild to severe loss of lineage progeny cells, to compete loss of the lineage altogether.
RESULTS
CRISPR for homology directed repair (HDR) to disrupt the Nopp140 gene
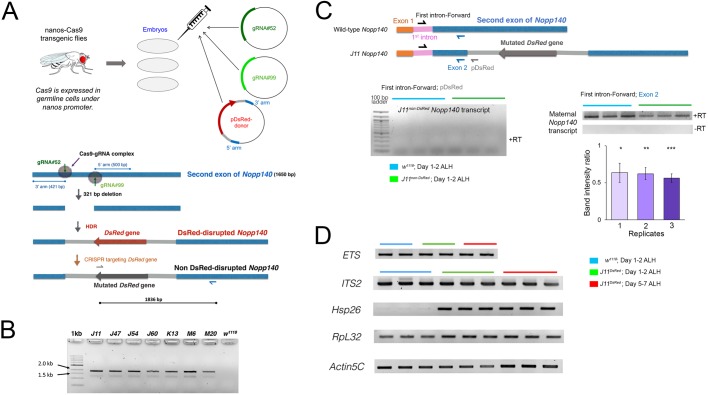
We used CRISPR-Cas9 to delete a target sequence of 321 bps from the second exon of the Nopp140 gene. A cocktail of two gRNA plasmids and the DsRed-Donor plasmid was injected into embryos homozygous for the nanos::Cas9 transgene (Fig. 2A). The gRNAs directed the Cas9-mediated deletion, and HDR inserted the DsRed gene across the deletion (Fig. 2A). DsRed then served as a selectable marker for the disrupted Nopp140 gene; it was expressed from the 3xP3 eye promoter, which is normally active in the entire embryonic and larval brain, Bolwig's organ, hind gut, anal pads and adult eyes. We recovered seven independent Nopp140 disruption lines (J11, J47, J54, J60, K13, M6 and M20) using the red fluorescence eye phenotype. Each of the seven Nopp140-disrupted chromosomes was maintained over the TM3-GFP balancer chromosome that carries a wild-type copy of Nopp140. The DsRed insertion was verified by genomic PCRs (Fig. 2B). The expected 1836 bp PCR product was amplified in all seven Nopp140 insertion alleles, with w1118 acting as a wild-type negative control (Fig. 2B). Among the seven lines initially recovered, J11DsRed/TM3-GFP was backcrossed with the Sb1/TM3-GFP fly line for at least six generations to eliminate possible off-target mutations in the J11DsRed line.
Fig. 2.
CRISPR-mediated disruption of the Nopp140 gene and RT-PCR analyses. (A) Three plasmids encoding guide RNAs, gRNA#52 and gRNA#99, or the DsRed protein were injected into embryos from the nanos-Cas9 fly line. The guide RNAs directed Cas9 cleavage at two specific sites located 321 base pairs apart in the second exon of the Nopp140 gene (blue bar; 1650 bp total). The DsRed gene (red arrow) with flanking plasmid sequences (light grey) and 3′ and 5′ Nopp140 homology arms were inserted into the deletion by HDR. Seven heterozygous Nopp140 disruption lines were identified by DsRed expression in adult eyes. The DsRed gene was subsequently mutated (dark grey arrow) by CRISPR-mediated mutagenesis. (B) Genomic PCRs using a DsRed-specific forward primer (grey half arrow in panel A) and a downstream Nopp140-specific reverse primer (blue half arrow in panel A) verified the DsRed insertion within the second exon of Nopp140 gene in all seven Nopp140 heterozygous disruption lines (J11, J47, J54, J60, K13, M6 and M20) with the expected 1836 bp product. The w1118 fly line served as negative control. (C) RT-PCR analyses of Nopp140 transcript levels in control w1118 and homozygous J11non-DsRed larvae day 1–2 ALH using the Exon2 reverse primer (blue half arrow) for first strand cDNA synthesis. Subsequent PCRs used the same Exon2 reverse primer and a forward primer specific for the first intron of the Nopp140 gene. A representative gel is provided. No PCR products appeared in the minus-RT controls. Band intensity ratios (J11/w1118) were determined for three biological replicates with overall mean±s.e.m. of 0.61±0.022. Student's t-test: two-tailed with equal variance for three biological replicates (1, 2, 3) with three PCR technical replicates each, P-values=0.037*, 0.00081**, 0.0064***, respectively. No RT-PCR product was detected using the pDsRed reverse primer (grey half arrow) in the J11non-DsRed disruption line. (D) RT-PCR analyses of ETS, ITS2, Hsp26, RpL32 and Actin5C transcript levels were carried out in control w1118 larvae at day 1–2 ALH and in homozygous J11DsRed larvae at two time points, day 1–2 and 5–7 ALH using gene-specific reverse primers. Three biological replicates were performed. For each replicate, total RNA was extracted from ∼300 w1118 and 150–300 homozygous J11 larvae. PCR reactions were carried out in triplicate for each first strand cDNA. Representative gels are shown.
The GFP reporter gene on the TM3 balancer chromosome is expressed in a small cluster of larval midgut cells that are easily identifiable. Therefore, with inter se crosses of J11DsRed/TM3-GFP stock flies, we hand-selected larvae that were homozygous for J11DsRed versus larvae that were heterozygous for J11DsRed/TM3-GFP with prominent GFP signals in their midgut.
To conduct multi-channel immuno-fluorescence of the Drosophila brain, we again used CRISPR-Cas9, but now with non-homologous end joining (NHEJ) to disrupt the DsRed gene inserted in the J11DsRed allele. A cocktail of two gRNA plasmids and the pBS-Hsp70-Cas9 vector injected into J11DsRed/TM3 embryos produced several independent fly lines with mutations in DsRed (Fig. S1). We sequenced the second exon region in two of these lines, A5 and A7, and verified that each had a short deletion at the gRNA#3 target site within the DsRed gene (Fig. S1). The A7-J11non-DsRed fly line was again backcrossed six times to deplete any possible off-site targets. Either the original J11DsRed fly line or its derived A7-J11non-DsRed line was used for the experiments described below.
We next performed RT-PCR analyses to test if the disrupted Nopp140 gene was transcribed in homozygous A7-J11non-DsRed larvae (day 1–2 ALH) (Fig. 2C). The reverse primer referred to as pDsRed in Fig. 2C annealed to the pDsRed-attP plasmid sequence a few base pairs downstream of the junction between the Nopp140 second exon and the DsRed donor sequence. No transcripts containing DsRed-att sequences were detected in the total RNA samples prepared from homozygous A7-J11non-DsRed larvae 1–2 day ALH, similar to the w1118 sample that served as a negative control (Fig. 2C, but see below for a positive control). Nonsense-mediated decay (NMD) would likely degrade Nopp140 pre-mRNAs transcribed from the disrupted gene as they would likely contain premature stop codons within the pDsRed-att sequences, or these pre-mRNAs may be improperly/incompletely spliced (Garneau et al., 2007). This lack of RT-PCR products eliminates the likelihood of a dominant-negative effect due to the production of truncated Nopp140 proteins encoded by the disrupted Nopp140 gene. In summary, hand-selected larvae homozygous for the Nopp140 J11non-DsRed allele provide a null genotype (Nopp140−/−) systemic throughout the larvae.
We then determined if there were maternal wild-type Nopp140 transcripts present in the RNA preparations isolated from the same homozygous A7-J11non-DsRed 1–2 day ALH larvae used for the same RT-PCRs described in the preceding section. We performed these second RT reactions using a reverse primer (Exon2, blue in Fig. 2C) that anneals to the Nopp140 second exon a few base pairs upstream of the junction between the Nopp140 second exon and the DsRed donor sequence. Since Nopp140 transcripts harboring DsRed sequences were undetectable in these same larvae, first strand cDNAs primed with Exon2 should indicate the presence of maternal Nopp140 transcripts in homozygous J11DsRed larvae, and thus serve as a positive control for the initial RT-PCRs that showed an absence of DsRed-att-containing transcripts. These second RT-PCRs shown on the right in Fig. 2C indicated that maternal Nopp140 transcripts were indeed present in the Nopp140−/− larvae at day 1–2 ALH. The bar graph in Fig. 2C indicates that the abundance of maternal Nopp140 transcripts in the Nopp140−/− larvae was about half that seen in wild-type larvae, suggesting that both maternal and zygotic Nopp140 transcript pools exist in early wild-type larvae (Fig. 2C).
As additional controls (Fig. 2D), RT-PCRs of the external transcribed spacer (ETS) and the internal transcribed spacer 2 (ITS2) sequences within pre-rRNA showed that their levels were unaffected in homozygous J11DsRed larvae at day 1–2 ALH and at day 5–7 ALH. This indicates that loss of Nopp140 had no effect on rDNA transcription, which agrees with our earlier observations on the pBac-generated Nopp140KO121 deletion (He et al., 2015). Furthermore, Hsp26 transcript levels were upregulated in homozygous J11DsRed larvae at both day 1–2 and day 5–7 ALH, whereas the wild-type larvae had almost undetectable levels of Hsp26 transcript (Fig. 2D). Overexpression of Hsp26 in homozygous J11DsRed larvae as early as day 1 ALH indicated a cellular stress response due to the effects of Nopp140 loss (e.g. Wang et al., 2004). As final controls, we accessed RpL32 and Actin5C transcript levels: while RpL32 transcript levels remained unchanged between the wild-type and homozygous J11DsRed samples and between biological replicates, Actin5C transcript levels fluctuated slightly within the samples and between biological replicates for reasons that remain uncertain.
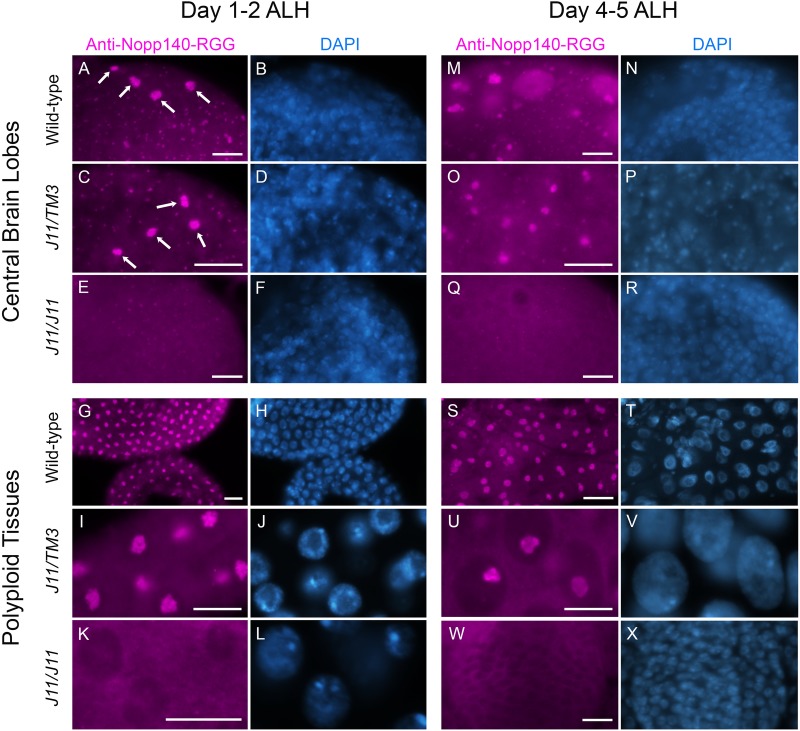
Maternal Nopp140 protein is reduced in early J11 Nopp140−/− larval brains
Since the RT-PCR analyses showed that maternal Nopp140 transcripts persisted in the homozygous J11DsRed larvae at day 1–2 ALH, we wanted to test if the Nopp140 protein could be detected in their brain and gut tissues as well. To do this, we immuno-stained homozygous A7-J11non-DsRed larvae and wild-type larvae with an antibody directed against Nopp140-RGG, one of the two Nopp140 isoforms in Drosophila. This antibody was raised against a synthetic peptide, the sequence of which is unique to the carboxyl tail region of Nopp140-RGG (see Cui and DiMario, 2007). At day 1–2 ALH, the anti-Nopp140-RGG antibody labeled nucleoli in homozygous J11non-DsRed larval brain and midgut (Fig. 3E,K), but at much lower levels compared to the same tissues in wild-type and J11/TM3 larvae (Fig. 3, panels A and C for brain cells, G and I for midgut cells). Four large nucleoli per brain lobe were routinely detected in the posterior of wild-type and J11/TM3 larval brain lobes 1–2 day ALH, and we speculated these were the MB NBs that do not undergo quiescence as do other NBs, but continue to divide throughout the embryo-to-larva transition (arrows in Fig. 3A,C). We did not observe this preferential labeling in homozygous A7-J11non-DsRed brains (Fig. 3E). By day 4–5 ALH, nucleolar labeling by anti-Nopp140-RGG was substantially reduced in homozygous A7-J11non-DsRed larval brain and midgut cells as compared to the wild-type and J11/TM3 heterozygous tissues (Fig. 3, compare panel Q with panels M and O for brain cells, and panel W with panels S and U for midgut cells). The results indicated that at least the Nopp140-RGG isoform encoded presumably by maternal transcripts persisted in the first 2 days of homozygous A7-J11non-DsRed larval development, but then diminished in most cells as these Nopp140−/− larvae aged.
Fig. 3.
Maternal Nopp140 protein is reduced in early J11 Nopp140−/− larval brains. Central brain lobes and mid-gut tissues from w1118 (wild-type) control, heterozygous J11non-DsRed/TM3 and homozygous J11non-DsRed larvae at day 1–2 ALH (central brain lobes A–F, polyploid gut tissues G–L) and day 4–5 ALH (central brain lobes M–R, polyploid gut tissues S–X) were immuno-stained with anti-Nopp140-RGG. Arrows in panels A and C indicate four NBs per wild-type brain lobe with large nucleoli labeled with anti-Nopp140-RGG. Nuclei were counter-stained with DAPI. n=19 (w1118); n=13 (heterozygous J11non-DsRed/TM3 larvae); n=23 (homozygous J11non-DsRed); two technical replicates. Scale bars: 10 µm.
Ultrastructural analysis
Central brain lobes from wild-type (4–5 day ALH) and J11/J11 (6–7 day ALH) larvae were examined by TEM (Fig. S2, panels A and B). Neuronal cells in the J11/J11 larvae contained ribosomes, but their density appeared reduced when compared to ribosome densities in wild-type neuronal cells. Ribonucleoprotein content within mitochondria of both wild-type and J11/J11 neuronal cells appeared comparable by lead citrate staining, indicating that the overall lighter appearance of the cytoplasm in J11/J11 cells was likely due to reduced ribosome content. Examination of midgut cells in these same J11/J11 larvae (Fig. S2D) showed a significant decline in cytoplasmic ribosomes as compared to wild-type midgut cells (Fig. S2C). In particular, there is a tremendous loss of rough endoplasmic reticulum (rER, long arrows) in the J11/J11 midgut cells. The appearance of unusual electron dense granules in the cytoplasm of J11/J11 cells was consistent with our earlier report (He et al., 2015) describing these granules upon deletion of the Nopp140 gene. Preliminary immuno-fluorescence evidence suggests these granules are related to processing (P) bodies (P. J. DiMario, unpublished observations/data).
Embryonic and larval survivability with complete or partial elimination of Nopp140
The Nopp140 disruption lines were maintained using the third chromosome balancer, TM3, which carries a wild-type copy of the Nopp140 gene. Embryos homozygous for TM3 are non-viable, hence inter se crosses within the J11DsRed/TM3 fly stock should produce 50% Nopp140DsRed/TM3 larvae and 25% homozygous J11DsRed larvae (the number of hatched larvae/total number of eggs collected). However, if the disrupted Nopp140 gene causes embryonic lethality, we would expect frequencies less than 50% and 25%, respectively. We found that only 20.8% of total eggs developed into larvae that were J11DsRed/TM3 versus the expected 50% (Fig. 4A), and only 7.1% of the total eggs developed into larvae that were homozygous for J11DsRed versus the expected 25% (Fig. 4A). These data indicated that loss of Nopp140 leads to partial embryonic lethality not only for the homozygous J11DsRed genotype as might be expected, but more interestingly for the heterozygous J11DsRed/TM3 genotype. This observation indicated for the first time that the Nopp140−/+ genotype exhibits haplo-insufficiency in Drosophila, reminiscent of the Tcof1−/+ genotype in the human TCS.
Fig. 4.
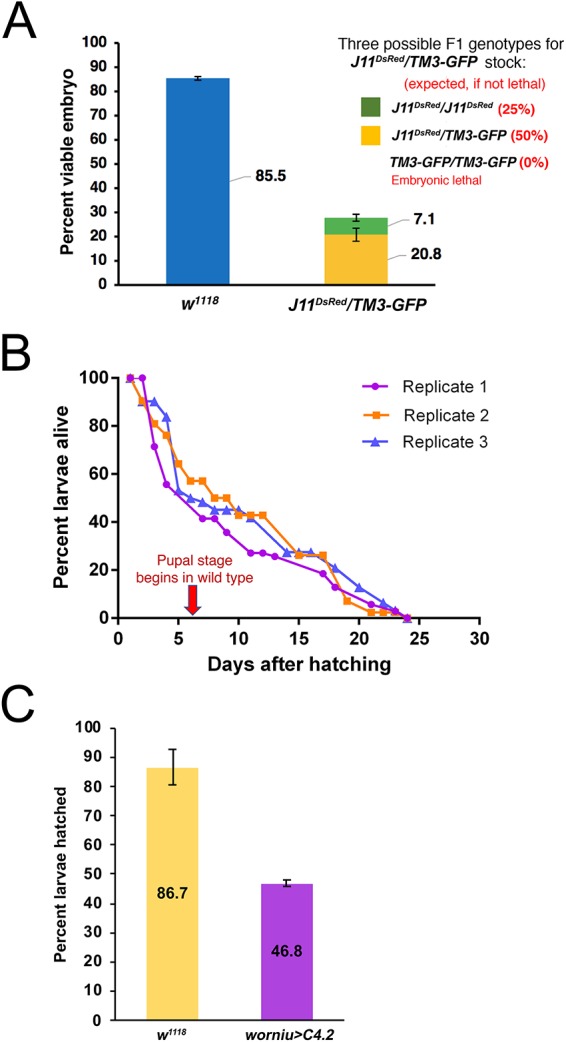
Embryonic and larval survival upon complete or partial loss of Nopp140. (A) Survival assays were performed for homozygous J11DsRed or heterozygous J11DsRed/TM3-GFP embryos, and for w1118 control embryos. Freshly laid eggs were collected from well-yeasted juice plates (n=2; total number of embryos per replicate for w1118: 200 and 299, and for J11DsRed/TM3-GFP stock: 230 and 111). The number of hatched larvae were logged for the next 2 days, and the percent viable embryos was determined. Data shows the number of larvae hatched divided by the total number of embryos collected X 100%. (B) Plot shows three replicates (number of larvae per replicate: 70, 42, 62) of survival assay for homozygous J11DsRed larvae. Newly hatched larvae were collected from a well-yeasted juice plates, and the number of living larvae were recorded in the following days until all larvae had perished. (C) Embryonic lethality and larval survivability upon Nopp140 depletion by RNAi expression using the worniu::GAL4 driver (specific for all embryonic and larval NBs) and UAS::TComC4.2 (Nopp140-RNAi line; Cui and DiMario, 2007). Compared to 86.7% of the w1118 embryos, only 46.8% of the collected embryos with Nopp140 depletion (worniu-GAL4>C4.2) hatched and developed into third instar larvae, after which all larvae developed into adults (not shown). n=3; total number of embryos collected per replicate for each genotype: 200, 265 and 330; Student's t-test: two-tailed with unequal variance, P-value=0.0069.
We earlier described growth arrest and lethality in second instar larvae that were homozygous for our original pBac-mediated Nopp140KO121 deletion (He et al., 2015). Because of the particular pBac elements available at the time, we had to delete the 3′ end of the downstream gene, P5CDh1 (He and DiMario, 2011), and this constantly forced us to control for the carboxyl truncation in P5CDh1, a mitochondrial matrix enzyme, when assessing the loss of Nopp140. Here, we assessed survivability of larvae homozygous for J11DsRed relative to wild-type larvae. Similar to our earlier findings (He et al., 2015), we found that ∼50% of the hatched homozygous J11DsRed larvae died by day 6 (which is when the pupal stage normally begins) (Fig. 4B). The remaining 50% remained as second instar larvae; they failed to grow or molt. The number of surviving homozygous J11DsRed larvae dwindled over time, but interestingly, some lingered up to day 24 (Fig. 4B).
While J11/TM3 embryos displayed partial lethality, hatched J11/TM3 larvae showed no readily apparent defects in growth or time of molting relative to wild-type larvae. In support of this observation, we depleted Nopp140 using the UAS-GAL4 system to express siRNAs. In the past, we showed that daughterless::GAL4>UAS::TComC4.2 depleted ∼70% of the Nopp140 transcripts (Cui and DiMario, 2007). Using the neuroblast-specific worniu::GAL4 driver (worniu-GAL4>UAS::TComC4.2), we found embryonic survivability was ∼46%, while the wild-type embryo survival rate was ∼86% (Fig. 4C). Similar to the surviving J11/TM3 larvae, surviving worniu::GAL4>UAS::TComC4.2 larvae developed into viable and fertile adults. While the worniu promoter is active in all embryonic and larval NBs, its peak expression is in 6–12 h embryos, perhaps explaining the survivability of nearly half the worniu::GAL4>UAS::TComC4 embryos beyond this embryonic stage.
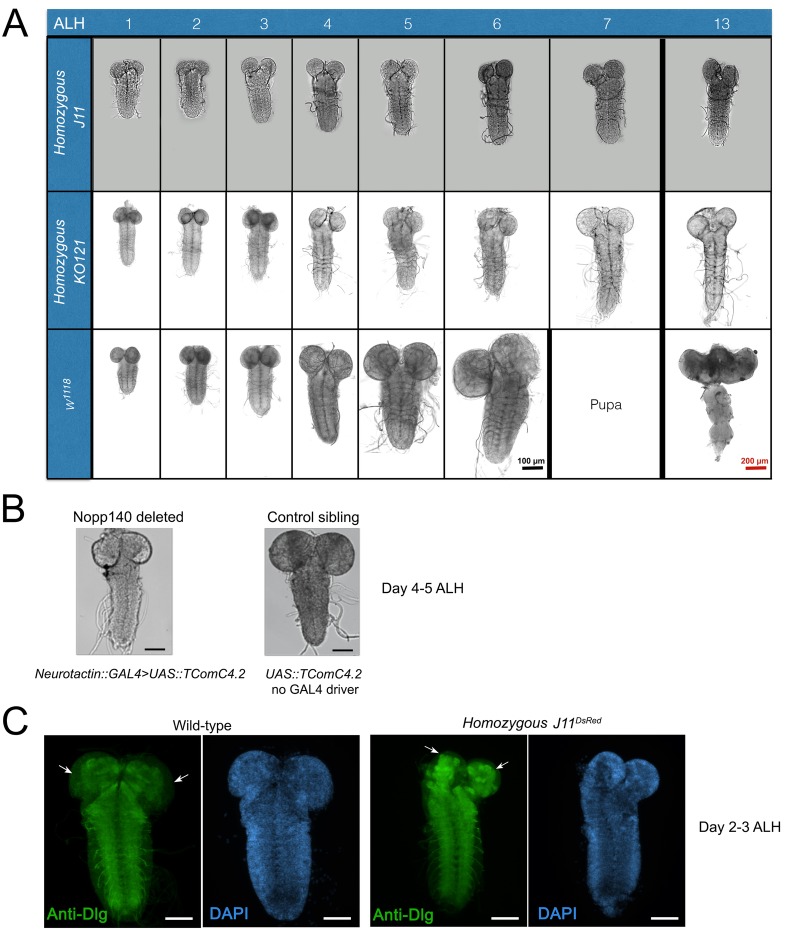
Brain hypoplasia upon nucleolar stress
We found that larval brain development was severely impaired upon loss of Nopp140 either by gene disruption (i.e. homozygous J11DsRed) or by neuron-specific RNAi depletion. During the early larval stage (day 1–2 ALH), homozygous J11DsRed brains were morphologically comparable in size to brains from newly hatched wild-type larvae. The mutant's brain continued to grow from day 3–6 ALH, but more slowly compared to wild-type larval brains (Fig. 5A). Beyond day 5–6 ALH, however, homozygous J11DsRed larval brains failed to grow. This was similar to what we saw in our original Nopp140KO121 deletion (He et al., 2015) (Fig. 5A). Likewise, brain growth was impaired in larvae upon RNAi-mediated depletion of Nopp140 using a pan-neuronal GAL4 driver (Neurotactin::GAL4>UAS::TComC4.2) (Fig. 5B).
Fig. 5.
Drosophila larval brain development is impaired under nucleolar stress induced by the loss of Nopp140. (A) Larval brain development in homozygous KO121 (Nopp140 gene deletion, He et al., 2015), homozygous J11 (CRISPR-mediated Nopp140 disruption), and wild-type (w1118) starting at day 1 after larval hatching (ALH) until day 7 and day 13 ALH. Homozygous KO121 and J11 larval brains at day 13 ALH are shown, but wild-type individuals have developed into adults by day 13 ALH, hence an adult fly brain is shown. (B) RNAi-depletion of Nopp140 using pan-neuronal GAL4 driver, Neurotactin (Nrt)::GAL4, and the UAS::TComC4.2 (Nopp140 RNAi line) resulted in impaired larval brain development similar to that seen in Nopp140 homozygous deletion background. Representative larval brains from three independent crosses at day 4–5 ALH comparing Nopp140-depleted brains with control sibling brains (not expressing RNAi) are shown. Scale bars: 100 µm (C) Conventional fluorescence images of the neuropil immuno-stained with antibody against Discs large (Dlg; green) in second instar w1118 control larvae and homozygous J11DsRed larvae at day 2–3 ALH. White arrows show unstained peripheral cell body layers, which are reduced in homozygous J11non-DsRed larvae. n=15 (wild-type); n=18 (homozygous J11non-DsRed); >3 technical replicates. Scale bars: 50 µm.
To determine where brain growth was impaired, we immuno-stained brains from homozygous J11DsRed larvae and wild-type larvae at day 2–3 ALH with an antibody against discs large (anti-Dlg). This antibody labels axon bundles (the neuropil), but not the cell body mass which we counter-stained with DAPI. Neuropils within the two central brain lodes were reduced in homozygous J11DsRed brains as compared to wild-type brains, but there were no observable physical defects in the ventral nerve cord (VNC) neuropil of homozygous J11DsRed larvae when compared to wild-type larvae. Besides the reduced central brain lobe neuropils, we found the cell body mass of the central brain lobe was also reduced in homozygous J11DsRed brains when compared to wild-type brains (Fig. 5C).
Reduced NB numbers and proliferation upon nucleolar stress
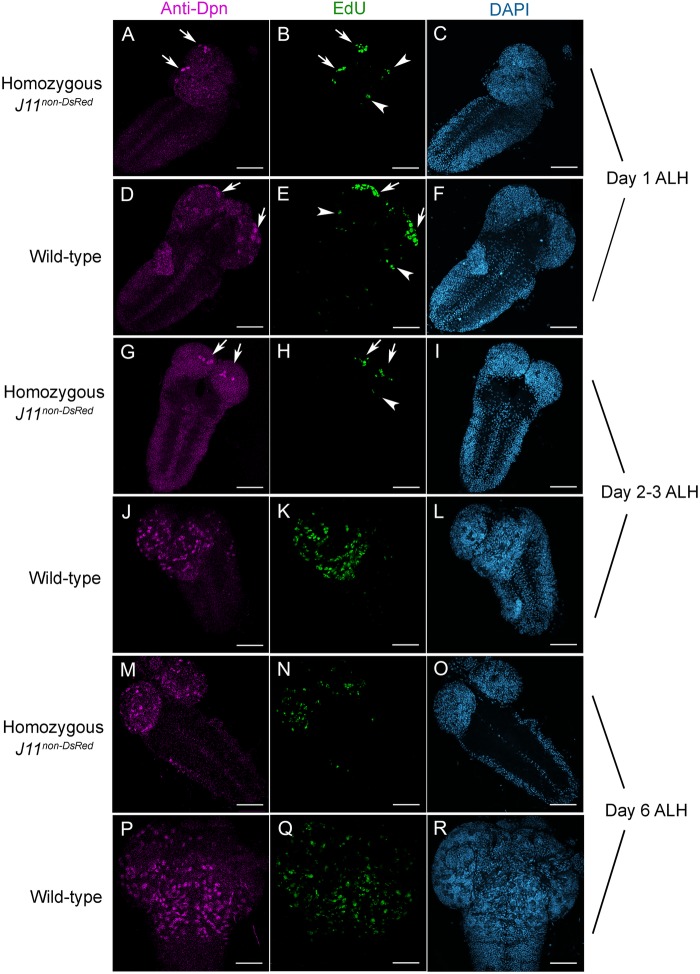
We hypothesized that the hypoplasia in Nopp140−/− larval brains was due to either a reduction in NB numbers, a reduction in the proliferative capacity of NBs, or both. To assess these possibilities, we first performed a Click-iT EdU labeling assay on living brains. EdU is a thymidine analog, which is incorporated into genomic DNA during S-phase of the cell cycle, and hence cells labeled by EdU are committed to cell division. We used 30 min EdU pulse-labeling assay in wild-type, homozygous A7-J11non-DsRed larval brains at 1, 2–3, and 6 days ALH (Fig. 6), and in heterozygous A7-J11non-DsRed/TM3 larval brains at 1–2 and 4–5 days ALH (Fig. S5). After pulse-labeling, brains were fixed with paraformaldehyde and the incorporated EdU was fluorescently labeled by Click-iT chemistry. We then immuno-stained the brains with anti-deadpan to visualize the relative numbers and distribution of NBs within the brain lobes. Deadpan (Dpn) is a NB-specific transcription factor necessary for self-renewal.
Fig. 6.
Neuroblast proliferation is reduced upon nucleolar stress. Confocal images of homozygous J11DsRed and control w1118 larval brains at day 1 ALH (A–F), days 2–3 ALH (G–L), and day 6 ALH (M–R) are shown after EdU-labeling (Click-iT Alexa Fluor 488) followed by anti-deadpan (anti-Dpn) immuno-staining. Dpn-stained cells (magenta) are NBs. After a 30 min pulse, EdU-labeled S-phase cells (green) were committed to cell division. Arrows indicate four likely MB NBs that were EdU- and Dpn-positive and clustered near presumably the posterior end of the central brain (A,B,D,E,G,H). Arrowheads indicate a few EdU-positive cells, likely arising from AL MBs, at the lateral side of the central brain (B,E,H). n=10, 15 and 10 for days 1, 2–3, and 6, respectively, for both wild-type and homozygous J11DsRed samples; three technical replicates. Scale bars: 50 µm.
Anti-Dpn labeling showed that NBs were present in homozygous J11non-DsRed larval brains from all age groups; however, their numbers were consistently reduced compared to either wild-type brains of similar age (Fig. 6; compare homozygous J11non-DsRed panels A, G, and M with wild-type panels D, J, and P), or to J11non-DsRed/TM3 brains of similar age (Fig. S5). This suggested that the observed hypoplasia in the homozygous J11non-DsRed larval brains was due in part by fewer NBs.
Strikingly, homozygous J11non-DsRed larvae at day 1 and day 2–3 ALH consistently showed four NBs in each central brain lobe that were prominently labeled by anti-Dpn as compared to the surrounding Dpn-stained NBs (Fig. 6; arrows in panels A and G; Fig. S5, panel D). These four NBs were visible in wild-type and heterozygous J11non-DsRed/TM3 brains as well, but only in brains from day 1–2 ALH larvae (Fig. 6D; Fig. S5D) as these four cells became less discernible in older larval brains among the many other NBs also present (Fig. S5G).
Subsets of EdU-positive cells in homozygous J11non-DsRed, wild-type and J11non-DsRed /TM3 brains were identified as NBs by the anti-Dpn nuclear staining. But EdU labeling also displayed the ganglion mother cells (GMCs) that were in S-phase. Overall, homozygous J11non-DsRed larval brains in all examined age groups showed fewer EdU-positive cells (both NBs and GMCs) as compared to wild-type brains (Fig. 6; homozygous J11non-DsRed panels B, H and N compared to wild-type panels E, K and Q) or to heterozygous J11non-DsRed/TM3 brains (Fig. S5, homozygous J11non-DsRed panels E and K compared to J11non-DsRed/TM3 panels B and H). This suggested a slower rate of NB proliferation in the homozygous J11non-DsRed larval brains. We did notice other EdU-positive cells in the lateral regions of the central brain lobes from both homozygous J11non-DsRed and wild-type larvae at day 1 ALH (Fig. 6, arrowheads in panels B and E). These should be the antennal lobe (AL) NBs. We occasionally detected them in central brain lobes of day 2–3 ALH homozygous J11non-DsRed (Fig. 6, arrowheads in panel H).
In larvae at day 2–3 ALH, we again observed only four NBs that co-labeled with both EdU and anti-Dpn in homozygous J11non-DsRed brains (Fig. 6, arrows in panels G and H). We predicted that these were the MB NBs based on their location and consistency in number. Wild-type larval brains, however, had far more EdU-positive and Dpn-positive cells, suggesting that the majority of wild-type NBs had now exited quiescence and started to proliferate as expected (Fig. 6, panels J and K).
Taken together, these observations indicated that upon nucleolar stress, a subset of NBs and GMCs, preferentially located within the MBs, proliferated in homozygous J11non-DsRed brains, but that these NBs gave rise to lineages that were comparatively smaller than those in wild-type brains under non-stressed conditions. Indeed, using an antibody against Prospero, a nuclear marker specific for GMCs and their descendent glia and neurons, we found significantly fewer GMC populations in the homozygous J11non-DsRed brains than in wild-type brains at day 1–2 and 6–7 ALH (Fig. S3). The apparent loss of most Type I and Type II neural lineages due to their inability to proliferate upon nucleolar stress likely contributed significantly to the brain hypoplasia. Additionally, we found reduced areas of cell nuclei in homozygous J11non-DsRed larval NBs and neurons compared to those in wild-type larval brains at day 2–3 ALH (Fig S4). This nuclear area reduction may further contribute to the brain hypoplasia in homozygous J11non-DsRed larvae.
MB NBs are resilient to nucleolar stress
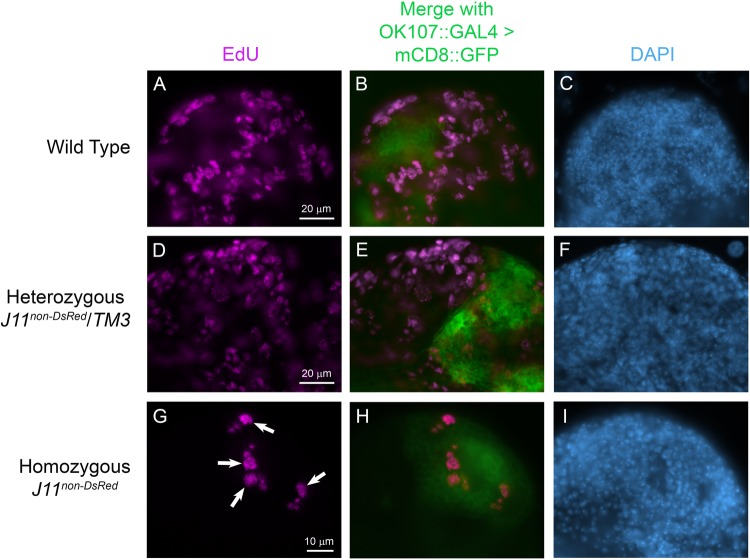
To test if the four EdU-positive NBs were in fact MB NBs, we used a MB lineage-specific GAL4 driver to express a GFP-tagged plasma membrane reporter protein, mCD8-GFP (OK107::GAL4>mCD8::GFP), and again performed co-EdU labeling in brains from homozygous J11non-DsRed and control (wild-type and J11non-DsRed/TM3) larvae at day 3 ALH. EdU labeling again showed many S-phase cells in wild-type and J11non-DsRed/TM3 larval brains; however, while a subset of these cells were typically found within the mCD8-GFP-positive MB-lineage cell cluster, it was often difficult to identify the MB NBs and descendent glia (Fig. 7, panels B and E). In homozygous J11non-DsRed larval brains at day 3 ALH, EdU-positive cells were always located within the MB lineage-cell cluster as identified by mCD8-GFP (Fig. 7, panel H). This suggested that the four Dpn-positive and EdU-positive NBs that we observed in homozygous J11non-DsRed larval brains at day 2–3 ALH (Fig. 6, panels G and H) were indeed MB NBs. The combined results of Figs 6 and 7 suggest that the MB NBs are more resilient to nucleolar stress induced by the loss of Nopp140 as compared to most other NBs within these brains. We base this MB NB resiliency on continued Deadpan labeling and co-EdU incorporation.
Fig. 7.
MB NBs are resilient to nucleolar stress. Larval brains from control w1118 (wild-type), heterozygous J11non-DsRed/TM3, and homozygous J11non-DsRed larvae at day 3 ALH were used for 30 min EdU pulse labeling (Click-iT Alexa Fluor 594). Merged confocal images show EdU-labeled cells (magenta) nestled within the GFP-labeled MB lineage (green) near the central brain lobes. n=12 (control); n=10 (heterozygous J11non-DsRed/TM3 larvae); n=20 (homozygous J11non-DsRed); three technical replicates. Scale bar dimensions are provided in panels A, D and G.
MB NBs in Nopp140−/− larval brains retain fibrillarin in their nucleoli
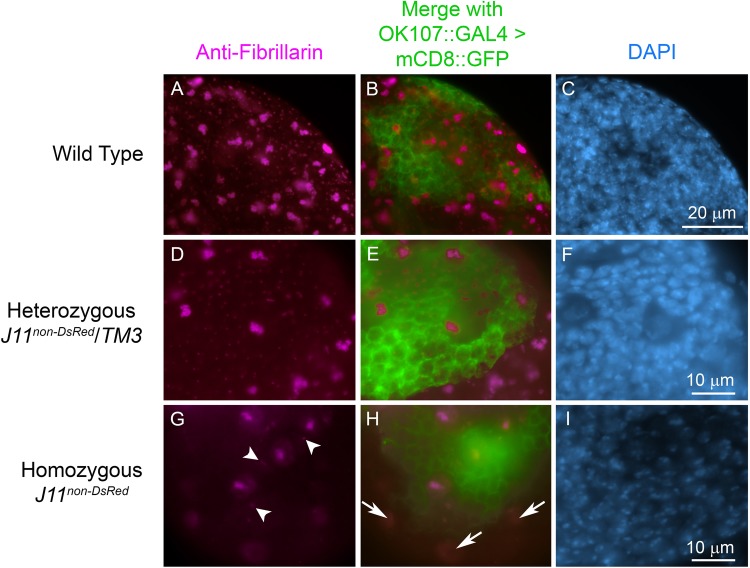
Nopp140 is a chaperone for C/D-box snoRNPs that catalyze 2′-O-methylation of pre-rRNA during ribosome biogenesis. Previous work in our lab showed that the C/D-box snoRNP methyl-transferase, fibrillarin, redistributes to the nucleoplasm upon complete loss of Nopp140 in larval tissues homozygous for the Nopp140KO121 gene deletion. Loss of fibrillarin from the nucleoli caused a corresponding reduction in 2′-O-methylation of pre-rRNA, clearly indicating nucleolar dysfunction, even though gross nucleolar morphology and rDNA transcription remained normal (He et al., 2015). Since MB NBs, but not others, continue to divide in Nopp140−/− larval brains at day 2–3 ALH, we predicted that MB NBs might retain fibrillarin within their nucleoli, while other NBs and their lineages redistribute fibrillarin to the nucleoplasm. To test this, we used anti-fibrillarin to immuno-stain brains from homozygous J11non-DsRed and control (wild-type and J11non-DsRed/TM3) larvae at day 3 ALH. All three genotypes expressed mCD8::GFP in the MB (OK107::GAL4>mCD8::GFP). Anti-fibrillarin stained large nucleoli in neuroblasts and smaller nucleoli in neurons in the wild-type and J11non-DsRed/TM3 larval brains (Fig. 8, panels A and D) with minimal nucleoplasmic labeling. Conversely, in homozygous J11non-DsRed larval brains, anti-fibrillarin failed to label the nucleoli in the majority of brain NBs outside the MBs. Instead, it labeled the nucleoplasm in the majority of these NBs. The exception was a small number of NBs, again just four and likely the MB NBs, usually located within the MB-lineage as marked by mCD8-GFP labeling (Fig. 8, panels G and H). These few cells showed clear nucleolar labeling with anti-fibrillarin, even though there was still some nucleoplasmic labeling (Fig. 8, panels G and H). Closer examination showed other smaller nucleoli in the near vicinity of the MB NBs (arrowheads in Fig. 8G). These may be nucleoli in the GMCs, but the identity of these cells awaits further analysis. Overall, this result indicates that the MB-lineage cells, and not others, are able to retain at least some nucleolar fibrillarin, indicating that their nucleoli are partially functional.
Fig. 8.
MB lineage cells retain nucleolar fibrillarin under nucleolar stress. Larval brains from control w1118 (wild-type), heterozygous J11non-DsRed/TM3, and homozygous J11non-DsRed larvae at day 3 ALH were immuno-stained with anti-fibrillarin (magenta). Image panels B, E and H show anti-fibrillarin stained central brain lobes along with mCD8::GFP (green) expressed under MB-lineage specific OK107::GAL4 driver in the same tissues. Four large nucleoli, presumably in the MB NBs, are apparent in the homozygous J11non-DsRed larval brains (G). Arrowheads in G show small nucleoli that reside in putative GMCs. Arrows in H indicate redistributed nucleolar fibrillarin in NBs residing outside of mCD8::GFP expressing MB lineage cells (green). n=10 (wild-type); n=14 (heterozygous J11non-DsRed/TM3 larvae); n=10 (homozygous J11non-DsRed); two technical replicates. Scale bar dimensions are provided in panels C, F and I.
A transcriptomics perspective
With nucleoli in MB NBs retaining fibrillarin, we used the NB-lineage specific transcriptome data set from Yang et al. (2016) to ask if MB NBs express more Nopp140 and fibrillarin relative to other NBs. We found the expression levels of four RBFs (Nopp140, fibrillarin, Nop56 and Nop60B) were generally higher in the MB NBs compared to the AL NBs and Type II NBs (Fig. S6A). As controls, we checked the data set for expression levels of Deadpan, which encodes a NB-specific transcription factor, Prospero, which encodes a NB- and GMC-specific transcription factor, and Elav, which encodes a Drosophila neuron-specific protein. As expected, Deadpan and Prospero expression levels were higher in NBs compared to neurons, and Elav expression was higher in neurons compared to NBs (Fig. S6B). Therefore, the transcriptomics data supports our experimental data, indicating further that RBFs are preferentially enriched in MB NBs.
DISCUSSION
Ontogenesis of the MB NB lineages
A wealth of knowledge exists for Drosophila neurogenesis making it possible to analyze developing brains at the level of individual NB lineages (Birkholz et al., 2015; Egger et al., 2008; Hartenstein and Wodarz, 2013; Homem and Knoblich, 2012; Urbach and Technau, 2003). This is particularly true for the MB. Insect MBs are central hubs for olfactory sensory input, learning, and memory (Thum and Gerber, 2019). Formation of the MBs begins during embryogenesis during which each MB NB differentially expresses unique combinations of the regulatory genes (Kunz et al., 2012; Yang et al., 2016). As far as we know, none of these gene products have direct links to ribosome production. During the embryo-to-larva transition, only the four MB NBs and one AL NBs continue to proliferate independently of dietary nutrients and PI3-kinase activity (Kunz et al., 2012; Prokop and Technau, 1994; Ito and Hotta, 1992; Lin et al., 2013; Sipe and Siegrist, 2017). The majority of other NBs, however, enter a period of quiescence (Kunz et al., 2012; Prokop and Technau, 1994; Ito and Hotta, 1992) and require dietary nutrients and PI3-kinase activity to exit quiescence ∼24 h after hatching (Lovick and Hartenstein, 2015; Sipe and Siegrist, 2017). During subsequent larval development, each MB NBs generates an almost identical repertoire of intrinsic Kenyon cells and continues to proliferate on into the pupal stages (∼85–90 h after pupa formation) (Ito and Hotta, 1992; Ito et al., 1997). As a result, the adult MB neuropil in each CB lobe is densely packed with around 2000–3000 Kenyon cells per lobe (Technau and Heisenberg, 1982; Aso et al., 2009).
Identifying MB NBs as resilient to nucleolar stress
We asked if Type I and II neuroblasts, MB NBs, OL NBs, and AL NBs are affected variably upon nucleolar stress, as are stem cells and precursor cells in the human ribosomopathies. To induce nucleolar stress, we used CRISPR-Cas9 to disrupt the Nopp140 gene, which encodes two isoforms that function as RBFs early in the nucleolar subunit assembly. While we could detect maternal Nopp140 transcripts in 1–2 day Nopp140−/− larvae by RT-PCR, we failed to detect zygotically expressed Nopp140 transcripts in these larvae (Fig. 2C). A polyclonal antibody directed against a unique peptide sequence within the RGG carboxyl domain of Nopp140-RGG showed reduced levels of this isoform in brain tissue of 1–2 day J11/J11 larvae, and eventually no detectable fluorescence signal in brain tissue in 4–5 day J11/J11 larvae (Fig. 3E and Q). An antibody directed against the carboxyl terminus of the other isoform, Nopp140-True, has proven much weaker in immuno-fluorescence assays, and was not used here. Neither antibody works well on western blots. Drosophila larvae homozygous for J11non-DsRed arrested in the second instar stage and showed smaller brains by day 4 ALH (Fig. 5A). While some of these homozygous larvae lingered to day 24 ALH, none of them survived (Fig. 4B). Compared to wild-type brains, homozygous J11non-DsRed larval brains at day 2–3 ALH had far fewer proliferating NBs (Fig. 6; Fig. S5). Data presented here indicate that the brain hypoplasia in homozygous J11non-DsRed larvae is due to both the loss of Type I and Type II NB divisions and to reduced sizes of the remaining NBs and neuronal cells (Fig. S4). Clonal analyses currently underway should provide detailed information on cell proliferation and sizes within the Nopp140-deficient clones versus surrounding, phenotypically normal cells.
On the other hand, anti-deadpan and EdU labeling of homozygous J11non-DsRed larvae showed that MB NBs in particular, and in some cases the AL NBs, proliferated through the embryo-to-larva transition, and they continued to proliferate at day 2–3 ALH and at day 4–5 ALH as other NB lineages remained arrested (Fig. 6). From this observation we conclude that MB NBs predominantly exhibited resilience to nucleolar stress caused by the loss of zygotic Nopp140 gene expression. Thus, the MB NBs (and AL NBs) are inherently different in their proliferative schedules compared to the rest of the NBs in the Drosophila larval brain. This may explain in part their resilience to nucleolar stress; that is, continued NB proliferation and already high synthetic rates (e.g. ribosome production) may temporarily sustain the MB NBs upon nucleolar stress, while the other NBs may not be able to rekindle high synthetic levels as they exit quiescence (Bertoli et al., 2013).
Phenotypes
For the first time, we showed that the Nopp140 gene in Drosophila is haplo-insufficient where J11DsRed/TM3 displayed embryonic lethality (Fig. 4A). This was a surprise since a previous segmental aneuploidy study indicated no haplo-insufficiency genes existed in cytological region 78F4 of the left arm of chromosome 3 (Lindsley et al., 1972). Haplo-insufficiency of the Drosophila Nopp140 gene would be analogous to haplo-insufficiency of the human Tcof1 gene, which encodes treacle, a vertebrate RBF related to Nopp140 in peptide structure and function in early subunit assembly. Loss of treacle in Tcof1+/− human embryos results in TCS, a ribosomopathy leading to apoptosis in select embryonic neural crest cells ultimately leading to the craniofacial birth defects.
Earlier work in our lab showed that complete loss of Nopp140 in Drosophila induced nucleolar stress with the redistribution of the C/D box methyl-transferase, fibrillarin, to the nucleoplasm (He et al., 2015). Here, we showed that Nopp140 transcripts and at least the Nopp140-RGG isoform were reduced, but not completely absent in early larvae homozygous for the disrupted Nopp140 allele, J11non-DsRed (Figs 2 and 3). Interestingly, each wild-type central brain lobe showed four anterior cells that appeared to contain more Nopp140-RGG than other cells within the same lobes (Fig. 3). The observation suggested that MB NBs contain more Nopp140 than do other NBs. We then showed that mCD8::GFP-labeled MB lineages in homozygous J11non-DsRed larvae retained nucleolar fibrillarin, whereas fibrillarin was noticeably redistributed to the nucleoplasm in the majority of other NBs (Fig. 8). This latter observation indicated that nucleoli in the MB lineages preferentially retained more RBFs and perhaps maintained functional production of ribosomes longer, although to what extent requires future molecular analyses.
Differential RBF expressions
Most cells within the central brain lobes of homozygous J11non-DsRed larvae 1–2 day ALH showed reduced anti-Nopp140-RGG labeling compared to brain cells in similarly aged wild-type larvae. Interestingly, wild-type larvae clearly showed four cells, identified as MB NBs, per central brain lobe with prominent anti-Nopp140-RGG labeling (Fig. 3). The observation suggests that wild-type MB NBs contain more zygotically expressed Nopp140 than do other NBs. Recent findings supporting this notion show that various RBFs such as treacle, fibrillarin, Nop56, mbm and NS3 are overexpressed in stem cell and progenitor cell populations (Brombin et al., 2015; Watanabe-Susaki et al., 2014; Wang et al., 2013; Johnson et al., 2018; Hovhanyan et al., 2014; Hartl et al., 2013; Dixon et al., 2006), and that the quantity and spatiotemporal expression of RBFs can vary in different stem cell or progenitor cell populations (Weiner et al., 2012; Bouffard et al., 2018).
Related to differential expression of RBFs in stem cells and progenitor cells is the perplexing problem of why some larvae homozygous for the disrupted Nopp140 gene survive up to day 24 ALH (Fig. 4B). While we have yet to pursue this question rigorously, we suspect these Nopp140−/− individuals may inherent more maternal Nopp140 mRNA and/or protein, and this may be a function of the nutritional state and health of the mothers. Earlier work in our lab (McCain et al., 2006) followed maternally expressed exogenous GFP-Nopp140 protein into embryogenesis, and noted that it lingered for several days. Individual Nopp140−/− embryos that inherited extra maternal Nopp140 transcripts or protein would likely produce more ribosomes and survive longer.
The possibility of heterogeneous ribosomes
Ribosomes are not all the same even within a single cell (Xue and Barna, 2012; Guo, 2018). Werner et al. (2015) showed that the translation program of human embryonic stem cells (hESCs) differentiating into neural crest cells changed after the depletion of KBTBD8, a substrate adapter for the vertebrate-specific ubiquitin ligase, CUL3. CUL3 mono-ubiquitylates human Nopp140 (NOLC1) and treacle, and forms a Nopp140-treacle platform that connects RNA Pol I machinery with ribosome modification factors. Based on these results, the authors hypothesized that the change in translational profile was the result of differential alteration of ribosomes. Modifications such as rRNA pseudouridylation and methylation, or phosphorylation and ubiquitylation of ribosomal proteins or ribosome-associated factors may ultimately contribute to translational control of gene expression (Sloan et al., 2017). Thus, the abundance of Nopp140 in different Drosophila NBs could potentially lead to differential modifications of the ribosome pools, and thus changes in the translational profile in different NBs.
Finally, transcriptome profiles (Fig. S6) of the different Drosophila larval brain NBs support our finding that RBFs Nopp140 and fibrillarin exist in higher levels in the MB NBs. The transcriptomics suggest that Nop56 and Nop60B may also exist in higher levels in MB NBs. Are there heterogeneous pools of ribosomes within a Drosophila larval brain? The Drosophila nervous system should serve well to explore differential threshold requirements for ribosome production and the diversity of resulting ribosome pools.
MATERIALS AND METHODS
Fly stocks
Fly lines used in this study included: w1118 (used as a wild-type control, Bloomington stock #3605), the third chromosome balancer stock w*; Sb1/TM3, P{ActGFP}JMR2, Ser1 (referred to as TM3-GFP, Bloomington stock #4534), y1 M{nos-Cas9.P}ZH-2A w* (referred to as nanos-Cas9, Bloomington stock #54591 provided by Fillip Port and Simon Bullock, MRC Laboratory of Molecular Biology), w*; P{GawB}OK107 eyOK107/In(4) ciD, ciD panciD svspa-pol (referred to as OK107-GAL4, Bloomington stock #854), w*; P{wor.GAL4.A}2; Dr1/TM3, P{Ubx-lacZ.w+}TM3, Sb1 (referred to as worniu-GAL4, Bloomington stock #56553), w1118; P{GMR37H04-GAL4}attP2 [referred to as Scabrous (Sca)-GAL4, Bloomington stock #49969], w1118; P{y[+t7.7] w[+mC]=GMR38F05-GAL4}attP2 [referred to as Neurotactin (Nrt)-GAL4, Bloomington stock #49383], y1 w*; P{w+mC=UAS-mCD8::GFP.L}LL5, P{UAS-mCD8::GFP.L}2 (referred to as UAS-mCD8-GFP, Bloomington stock #5137), KO121 Nopp140 gene deletion line (He et al., 2015), and the UAS-TComC4.2 Nopp140 RNAi line (Cui and DiMario, 2007). Flies were maintained in the laboratory at room temperature (22–24°C) on standard cornmeal-molasses medium. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Homology directed insertion of DsRed into Nopp140
We used CRISPR-Cas9 and HDR to insert the DsRed gene into the second exon of the Nopp140 gene. The CRISPR optimal target finder tool (http://targetfinder.flycrispr.neuro.brown.edu/) provided 271 gRNA target sites, each 20 nt in length excluding the NGG PAM sequence. Among these, six gRNAs had zero off-targets in coding regions of the Drosophila genome. The gRNAs were additionally verified to have no off-targets by the TagScan tool (Genome-wide Tag Scanner; https://ccg.epfl.ch//tagger/tagscan.html), and the Cas-OFFinder tool (Bae et al., 2014). Two gRNA targets, gRNA#52 (5′GGGCTTTGCCGGTTCTTCCTCGG on the minus strand of Nopp140; with the PAM sequence underlined) and gRNA #99 (5′CAAGTTGGCTCCTGCTAAGAAGG on the plus strand of Nopp140), were chosen and used for CRISPR gene editing. Successful CRISPR-Cas9 cleavage at both gRNA target sites would delete 321 bps from the second exon.
To express these gRNAs, sense and anti-sense oligos that included BbsI restriction site overhangs were prepared for both gRNAs by Integrated DNA Technologies (IDT; see Table 1 for gRNA sequences). Mixtures of sense and anti-sense oligos for each gRNA were annealed (heated at 95°C for 5 min, and then cooled to room temperature over 1 h in 1× ligation buffer). The resulting double-strand DNAs were ligated separately into pCFD3-dU6:3gRNA at the BbsI site. pCFD3-dU6:3gRNA was a gift from Simon Bullock [Addgene plasmid # 49410; http://n2t.net/addgene:49410; RRID:Addgene_49410; (Port et al., 2014; Ren et al., 2013)]. The resulting plasmids are referred to as gRNA#55 and gRNA#99 (Fig. 2A).
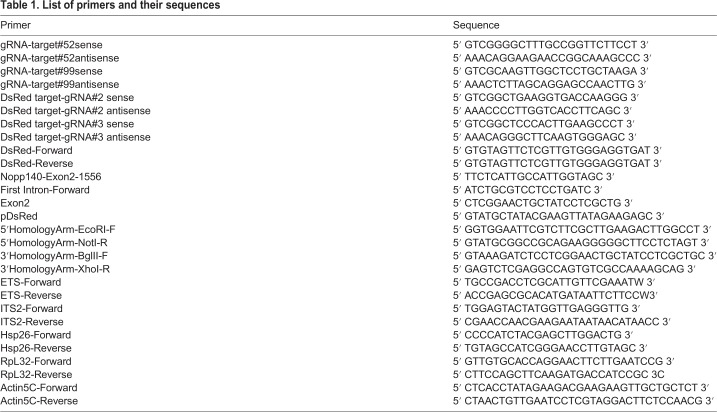
Table 1.
List of primers and their sequences
To mark the disrupted Nopp140 gene, the DsRed gene was inserted at the Cas9-mediated deletion site by HDR. We used the donor plasmid, pDsRed-attP, which was a gift from Melissa Harrison, Kate O'Connor-Giles, and Jill Wildonger (University of Wisconsin-Madison) (Addgene plasmid # 51019; http://n2t.net/addgene:51019; RRID:Addgene_51019; Gratz et al., 2014). We followed general guidelines (Gratz et al., 2014) to insert the homology arms into the multiple cloning sites available on either side of the DsRed gene in pDsRed-attP. The 5′ and 3′ homology arms from the Nopp140 second exon were prepared by PCR using forward and reverse primers listed in Table 1. These homology arms flank the 321 bp deletion region described in the preceding paragraph (Fig. 2A). We first inserted the 421 bp 3′ arm into pDsRed-attP at the BglII and XhoI sites upstream of the DsRed gene, and then inserted the 500 bp 5′ arm at NotI and EcoRI sites downstream of the DsRed gene. The orientation of the homology arms relative to DsRed should insert the DsRed sequence by HDR such that transcription of DsRed is in the opposite direction relative to transcription of the Nopp140 gene. The final plasmid is referred to as pDsRed-Donor (Fig. 2A).
NHEJ disruption of DsRed gene inserted within Nopp140 second exon
To mutate the DsRed gene within the Nopp140 gene in the J11 DsRed fly line, we used Cas9 endonuclease expressed from the pBS-Hsp70-Cas9 plasmid, a gift from Melissa Harrison, Kate O'Connor-Giles, and Jill Wildonger (Addgene plasmid # 46294; http://n2t.net/addgene:46294; RRID:Addgene_46294; Gratz et al., 2013). To find gRNA target sites within the DsRed gene, we again used the CRISPR optimal target finder tool, which yielded 38 gRNA target sites that were 18-nt in length. Twelve of the 38 gRNA targets had no matches to the Drosophila genome. Among the twelve gRNA targets, we chose gRNA#2 (5′GCTGAAGGTGACCAAGGGCGG on the plus strand of DsRed) and gRNA#3 (5′GCTCCCACTTGAAGCCCTCGG on the minus strand of DsRed). Sense and anti-sense oligos for each gRNA target site were prepared by IDT (see Table 1 for sequences). Each double stranded DNA encoding the respective gRNAs was separately ligated into the pCFD3-dU6:3gRNA plasmid at the BbsI restriction site following the same procedures described above for the preparation of gRNA#52 and gRNA#99 plasmids. The resulting plasmids for DsRed gene mutagenesis are gRNA#2 and gRNA#3.
Drosophila embryo injections
All plasmids used for embryo injections were extracted from transformed Escherichia coli cells using a plasmid Midiprep kit from Thermo Fisher Scientific. To disrupt the Nopp140 gene, the plasmid injection mixture contained 15 ng µl−1 of gRNA#52, 15 ng µl−1 of gRNA#99, and 230 ng µl−1 of pDsRed-Donor. The mixture was injected into homozygous nanos-Cas9 transgenic embryos. To disrupt the DsRed gene, the CRISPR injection mixture contained 75 ng µl−1 of gRNA#2, 75 ng µl−1 of gRNA#3, and 350 ng µl−1 of pBS-Hsp70-Cas9. This mixture was injected into J11 DsRed/TM3-GFP embryos. All injections were performed by GenetiVision Corporation (Houston, TX, USA).
PCR verification of Homology Directed Cas9-mediated donor sequence insertion
Approximately 30 healthy well-fed adults were homogenized in 100 mM Tris-HCl (pH 7.5), 100 mM EDTA, 100 mM NaCl and 0.5% SDS, followed by 30 min incubation at 70°C. Genomic DNA was precipitated in a 1:2 ratio of 5 M KOAc:6 M LiCl on ice for 10 min, followed by phenol-chloroform purification and ethanol precipitation. PCR reactions contained 20–70 ng of genomic DNA, 0.40 µM of each primer, 0.20 mM of each dNTP, 0.50 mM of MgCl2, 1 X Phusion GC Buffer and 0.40 unit of Phusion high-fidelity DNA polymerase (M0530S, New England Biolabs). Amplification was performed in a Bio-Rad C1000 Thermal Cycler (cycling conditions: 32 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 62°C, and elongation at 72°C for 1 min 20 s). Primers used for PCR verification were DsRed-Reverse and Nopp140-Exon2-1556. Their sequences are provided in Table 1.
Sequence analyses
PCR products were extracted from agarose gels using phenol-chloroform, ethanol precipitated, and then sequenced using a BigDye Terminator Cycle Sequencing kit v.3.1 and an ABI 3130XL Genetic Analyzer (Applied Biosystems). Sequencing primers are indicated wherever the sequence reads are provided. Sequences were analyzed and aligned using CLC Sequence Viewer (Qiagen Bioinformatics).
RT-PCR analysis
Larvae at day 1–2 after larval hatching (ALH) or day 5–7 ALH were collected from well-yeasted grape juice plates, placed into an Eppendorf tube, and rinsed with distilled water to remove yeast and other debris. Total RNA was extracted from wild-type or Nopp140−/− larvae using TRIzol (Invitrogen) according to the manufacturer's recommendations. First-strand cDNA synthesis was performed using M-MuLV Reverse Transcriptase (NEB M0253S) according to the manufacturer's recommendations with either oligo(dT) primers or gene-specific reverse primers (same as the reverse primers used in PCR). Oligo(dT) primers were used to synthesize the first-strand cDNA of Hsp26, RpL32 and Actin5C. Gene-specific reverse primers were used for the ETS and ITS2 regions of pre-ribosomal RNA. Specific forward and reverse PCR primers are described in Table 1.
EdU labeling
For 5-ethynyl-2-deoxyuridine (EdU) labeling, larval brains were dissected in PBS (without detergent or azide), and within 5 min of dissection, the brains were incubated with 20 µM EdU in PBS for 30 min at room temperature. The tissues were then fixed for 30 min at room temperature in Buffer B (16.7 mM KH2PO4/K2HPO4, 75 mM KCl, 25 mM NaCl, 3.3 mM MgCl2, pH 7.0–7.2) with 2% paraformaldehyde (from a freshly prepared 10% stock) (de Cuevas and Spradling, 1998). EdU incorporated into S-phase cells was detected by a Click-iT Alexa Fluor 488 EdU imaging kit (Invitrogen) according to the manufacturer's recommendations. EdU was also detected by Alexa Fluor 594-Azide (Product No.1295, AF 594 Azide from Click Chemistry Tools) used with the reagents provided by Invitrogen Click-iT EdU imaging kit. Following EdU labeling, the larval brains were immuno-stained with antibodies followed by DAPI counterstaining.
Immuno-staining and fluorescence microscopy
Larval brains and other tissues were dissected directly into fixation Buffer B described in the previous section. Tissues were fixed for 30–35 min total starting from the point when the dissection commenced. All washings were done with PBS with 0.1% TX-100 detergent. The blocking solution was 3% BSA prepared in PBS with 0.1% TX-100 which was also used for preparing dilutions of primary and secondary antibodies. In all cases, tissues were incubated in the primary antibody overnight at 4°C on a shaker, and in the secondary antibody for 4 h at 4°C on a shaker. Primary antibodies included the polyclonal guinea pig anti-Nopp140-RGG (Cui and DiMario, 2007) used at 1:100, a rat monoclonal anti-deadpan (Abcam, 195173, stock 1 mg ml−1) used at 1:250, the mouse monoclonal anti-fibrillarin mAb 72B9 (Reimer et al., 1987; hybridoma supernatant used without dilution), the mouse monoclonal anti-prospero (deposited at the DSHB by C.Q. Doe; DSHB Hybridoma Product: Prospero MR1A) used at 1:50, and the mouse monoclonal anti-discs large (dlg) (deposited at the DSHB by C. Goodman; DSHB Hybridoma Product: 4F3 anti-discs large) used at 1:30. Secondary antibodies included the Alexa Fluor 546 conjugated goat anti-rat (A-11081, Thermo Fisher Scientific) used at 1:1000, the Alexa Fluor 594 conjugated goat anti-guinea pig (A-11073, Thermo Fisher Scientific) used at 1:500, and the DyLight 488 conjugated goat anti-mouse (35503, Thermo Fisher Scientific) used at 1:500. Tissues were counter-stained with 4′,6-diamino-2-phenylindole (DAPI, Polysciences) at 1 µg ml−1. To image the tissues, we used either a conventional fluorescence microscope, a Zeiss Axioskop equipped with a SPOT RTSE digital camera, or a Leica SP8 Confocal Microscope equipped with the White Light Laser system in the Shared Instrumentation Facility (SIF) at Louisiana State University.
TEM
Wild-type and Nopp140−/− larval tissues were prepared for transmission electron microscopy (TEM) using standard techniques as described by He et al. (2015). Images were captured using a JEOL 1400 TEM in the Shared Instrumentation Facility (SIF) at Louisiana State University.
Determination of nuclear area
The 2D confocal images of the Nopp140−/− and wild-type larval brains at day 2–3 ALH were analyzed using Fiji software. After setting scale for each image, the free hand selection tool was used to draw outlines of each nucleus, and the nuclear area was subsequently recorded. Deadpan-stained larval brains were used to determine the nuclear area of NBs. Neuronal nuclear area was obtained from the DAPI-stained larval brains. The nuclear areas were plotted into a box-scatter plot using Microsoft Excel, and a Student's t-test (one-tailed) was performed on the data.
Supplementary Material
Acknowledgements
We thank Kate O'Connor-Giles for advice on CRISPR applications in Drosophila. We thank Simon Bullock, Melissa Harrison, Jill Wildonger and Kate O'Connor-Giles for plasmids used in HDR and NHEJ. We also thank David Burk of the LSU Shared Instrumentation Facility for assistance in confocal microscopy.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.S.B., P.J.D.; Methodology: S.S.B., M.E.L., P.J.D.; Validation: S.S.B., P.J.D.; Formal analysis: S.S.B., P.J.D.; Investigation: S.S.B., M.E.L., P.J.D.; Resources: S.S.B., P.J.D.; Data curation: S.S.B., P.J.D.; Writing - original draft: S.S.B.; Writing - review & editing: S.S.B., P.J.D.; Visualization: S.S.B., M.E.L., P.J.D.; Supervision: S.S.B., P.J.D.; Project administration: P.J.D.; Funding acquisition: P.J.D.
Funding
This study was funded by the National Science Foundation [grant numbers MCB0919709 and MCB1712975].
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.046565.supplemental
References
- Aso Y., Grübel K., Busch S., Friedrich A. B., Siwanowicz I. and Tanimoto H. (2009). The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 23, 156-172. 10.1080/01677060802471718 [DOI] [PubMed] [Google Scholar]
- Bachellerie J.-P., Cavaillé J. and Hüttenhofer A. (2002). The expanding snoRNA world. Biochimie 84, 775-790. 10.1016/S0300-9084(02)01402-5 [DOI] [PubMed] [Google Scholar]
- Bae S., Park J. and Kim J.-S. (2014). Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473-1475. 10.1093/bioinformatics/btu048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral S. S. and DiMario P. J. (2019). The Nopp140 gene in Drosophila melanogaster displays length polymorphisms in its large repetitive second exon. Mol. Genet. Genomics 294, 1073-1083. 10.1007/s00438-019-01568-6 [DOI] [PubMed] [Google Scholar]
- Baßler J. and Hurt E. (2019). Eukaryotic ribosome assembly. Annu. Rev. Biochem. 88, 281-306. 10.1146/annurev-biochem-013118-110817 [DOI] [PubMed] [Google Scholar]
- Bertoli C., Skotheim J. M. and de Bruin R. A. M. (2013). Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 14, 518-528. 10.1038/nrm3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkholz O., Rickert C., Nowak J., Coban I. C. and Technau G. M. (2015). Bridging the gap between postembryonic cell lineages and identified embryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Biol. Open 4, 420-434. 10.1242/bio.201411072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard S., Dambroise E., Brombin A., Lempereur S., Hatin I., Simion M., Corre R., Bourrat F., Joly J.-S. and Jamen F. (2018). Fibrillarin is essential for S-phase progression and neuronal differentiation in zebrafish dorsal midbrain and retina. Dev. Biol. 437, 1-16. 10.1016/j.ydbio.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Brombin A., Joly J.-S. and Jamen F. (2015). New tricks for an old dog: ribosome biogenesis contributes to stem cell homeostasis. Curr. Opin. Genet. Dev. 34, 61-70. 10.1016/j.gde.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Cui Z. and DiMario P. J. (2007). RNAi knockdown of Nopp140 induces Minute-like phenotypes in Drosophila. Mol. Biol. Cell 18, 2179-2191. 10.1091/mbc.e07-01-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwerse J. G., Dixon J., Seland S., Ruivenkamp C. A. L., van Haeringen A., Hoefsloot L. H., Peters D. J. M., Boers A. C.-D., Daumer-Haas C., Maiwald R. et al. (2011). Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat. Genet. 43, 20-22. 10.1038/ng.724 [DOI] [PubMed] [Google Scholar]
- de Cuevas M. and Spradling A. C. (1998). Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development 125, 2781-2789. [DOI] [PubMed] [Google Scholar]
- Dixon J., Jones N. C., Sandell L. L., Jayasinghe S. M., Crane J., Rey J.-P., Dixon M. P. and Trainor P. A. (2006). Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl. Acad. Sci. USA 103, 13403-13408. 10.1073/pnas.0603730103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B., Chell J. M. and Brand A. H. (2008). Insights into neural stem cell biology from flies. Philos. Trans. R. Soc. B. Biol. Sci. 363, 39-56. 10.1098/rstb.2006.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau N. L., Wilusz J. and Wilusz C. J. (2007). The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 8, 113-126. 10.1038/nrm2104 [DOI] [PubMed] [Google Scholar]
- Golomb L., Volarevic S. and Oren M. (2014). p53 and ribosome biogenesis stress: the essentials. FEBS Lett. 588, 2571-2579. 10.1016/j.febslet.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Gonzales B., Henning D., So R. B., Dixon J., Dixon M. J. and Valdez B. C. (2005). The Treacher Collins syndrome (TCOF1) gene product is involved in pre-rRNA methylation. Hum Mol. Genet. 14, 2035-2043. 10.1093/hmg/ddi208 [DOI] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K., Harrison M. M., Wildonger J. and O'Connor-Giles K. M. (2013). Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194, 1029-1035. 10.1534/genetics.113.152710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Ukken F. P., Rubinstein C. D., Thiede G., Donohue L. K., Cummings A. M. and O'Connor-Giles K. M. (2014). Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961-971. 10.1534/genetics.113.160713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. (2018). Specialized ribosomes and the control of translation. Biochem. Soc. Trans. 46, 855-869. 10.1042/BST20160426 [DOI] [PubMed] [Google Scholar]
- Hartenstein V. and Wodarz A. (2013). Initial neurogenesis in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 2, 823-823 10.1002/wdev.117 [DOI] [PubMed] [Google Scholar]
- Hartl T. A., Ni J., Cao J., Suyama K. L., Patchett S., Bussiere C., Gui D. Y., Tang S., Kaplan D. D., Fish M. et al. (2013). Regulation of ribosome biogenesis by nucleostemin 3 promotes local and systemic growth in Drosophila. Genetics 194, 101-115. 10.1534/genetics.112.149104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano T., Yanagida M., Yamauchi Y., Shinkawa T., Isobe T. and Takahashi N. (2003). Proteomic analysis of human Nop56p-associated pre-ribosomal ribonucleoprotein complexes. J. Biol. Chem. 278, 34309-34319. 10.1074/jbc.M304304200 [DOI] [PubMed] [Google Scholar]
- He F. and DiMario P. J. (2011). Drosophila delta-1-pyrroline-5-carboxylate dehydrogenase (P5CDh) is required for proline breakdown and mitochondrial integrity—establishing a fly model for human type II hyperprolinemia. Mitochondrion 11, 397-404. 10.1016/j.mito.2010.12.001 [DOI] [PubMed] [Google Scholar]
- He F., James A., Raje H., Ghaffari H. and DiMario P. (2015). Deletion of Drosophila Nopp140 induces subcellular ribosomopathies. Chromosoma 124, 191-208. 10.1007/s00412-014-0490-9 [DOI] [PubMed] [Google Scholar]
- Homem C. C. and Knoblich J. A. (2012). Drosophila neuroblasts: a model for stem cell biology. Development 139, 4297-4310. 10.1242/dev.080515 [DOI] [PubMed] [Google Scholar]
- Hovhanyan A., Herter E. K., Pfannstiel J., Gallant P. and Raabe T. (2014). Drosophila mbm is a nucleolar myc and casein kinase 2 target required for ribosome biogenesis and cell growth of central brain neuroblasts. Mol. Cell. Biol. 34, 1878-1891. 10.1128/MCB.00658-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. and Hotta Y. (1992). Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev. Biol. 149, 134-148. 10.1016/0012-1606(92)90270-Q [DOI] [PubMed] [Google Scholar]
- Ito K., Awano W., Suzuki K., Hiromi Y. and Yamamoto D. (1997). The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761-771. [DOI] [PubMed] [Google Scholar]
- James A., Cindass R. Jr, Mayer D., Terhoeve S., Mumphrey C. and DiMario P. (2013). Nucleolar stress in Drosophila melanogaster. Nucleus 4, 123-133. 10.4161/nucl.23944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. W., Doe C. Q. and Lai S.-L. (2018). Drosophila nucleostemin 3 is required to maintain larval neuroblast proliferation. Dev. Biol. 440, 1-12. 10.1016/j.ydbio.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Lynn M. L., Gaudenz K., Sakai D., Aoto K., Rey J.-P., Glynn E. F., Ellington L., Du C., Dixon J. et al. (2008). Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat. Med. 14, 125-133. 10.1038/nm1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressler D., Hurt E. and Baßler J. (2010). Driving ribosome assembly. Biochim. Biophys. Acta 1803, 673-683. 10.1016/j.bbamcr.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Kunz T., Kraft K. F., Technau G. M. and Urbach R. (2012). Origin of Drosophila mushroom body neuroblasts and generation of divergent embryonic lineages. Development 139, 2510-2522. 10.1242/dev.077883 [DOI] [PubMed] [Google Scholar]
- Lambertsson A. (1998). The Minute genes in Drosophila and their molecular functions. Adv. Genet. 38, 69-134. 10.1016/S0065-2660(08)60142-X [DOI] [PubMed] [Google Scholar]
- Lee C.-C., Tsai Y.-T., Kao C.-W., Lee L.-W., Lai H.-J., Ma T.-H., Chang Y.-S., Yeh N.-H. and Lo S. J. (2014). Mutation of a Nopp140 gene dao-5 alters rDNA transcription and increases germ cell apoptosis in C. elegans. Cell Death Dis 5, e1158 10.1038/cddis.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Marin E. C., Yang C.-P., Kao C.-F., Apenteng B. A., Huang Y., O'Connor M. B., Truman J. W. and Lee T. (2013). Extremes of lineage plasticity in the Drosophila brain. Curr. Biol. 23, 1908-1913. 10.1016/j.cub.2013.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T. C., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. et al. (1972). Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics 71, 157-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O. and Dawid I. B. (1980). Alternative pathways in the processing of ribosomal RNA precursor in Drosophila melanogaster. J. Mol. Biol. 138, 873-878. 10.1016/0022-2836(80)90070-4 [DOI] [PubMed] [Google Scholar]
- Lovick J. K. and Hartenstein V. (2015). Hydroxyurea-mediated neuroblast ablation establishes birth dates of secondary lineages and addresses neuronal interactions in the developing Drosophila brain. Dev. Biol. 402, 32-47. 10.1016/j.ydbio.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marygold S. J., Roote J., Reuter G., Lambertsson A., Ashburner M., Millburn G. H., Harrison P. M., Yu Z., Kenmochi N., Kaufman T. C. et al. (2007). The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8, R216 10.1186/gb-2007-8-10-r216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain J., Danzy L., Hamdi A., Dellafosse O. and DiMario P. (2006). Tracking nucleolar dynamics with GFP-Nopp140 during Drosophila oogenesis and embryogenesis. Cell Tissue Res. 323, 105-115. 10.1007/s00441-005-0044-9 [DOI] [PubMed] [Google Scholar]
- McCann K. L. and Baserga S. J. (2013). Mysterious ribosomopathies. Science 341, 849-850. 10.1126/science.1244156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U. T. (1996). Comparison of the rat nucleolar protein Nopp140 with its yeast homolog SRP40. J. Biol. Chem. 271, 19376-19384. [PubMed] [Google Scholar]
- Narla A. and Ebert B. L. (2010). Ribosomopathies: human disorders of ribosome dysfunction. Blood 115, 3196-3205. 10.1182/blood-2009-10-178129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack Watt K. E., Achilleos A., Neben C. L., Merrill A. E. and Trainor P. A. (2016). The roles of RNA polymerase I and III subunits Polr1c and Polr1d in craniofacial development and in zebrafish models of Treacher Collins Syndrome. PLoS Genet. 12, e1006187 10.1371/journal.pgen.1006187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F., Chen H.-M., Lee T. and Bullock S. L. (2014). Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl. Acad. Sci. USA 111, E2967-E2976. 10.1073/pnas.1405500111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. and Technau G. M. (1994). Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev. Biol. 161, 321-337. 10.1006/dbio.1994.1034 [DOI] [PubMed] [Google Scholar]
- Reimer G., Pollard K. M., Penning C. A., Ochs R. L., Lischwe M. A., Busch H. and Tan E. M. (1987). Monoclonal autoantibody from a (New Zealand black×New Zealand white) F1 mouse and some human scleroderma sera target an MTr 34,000 nucleolar protein of the U3 RNP particle. Arthritis. Rheum. 30, 793-800. 10.1002/art.1780300709 [DOI] [PubMed] [Google Scholar]
- Ren X., Sun J., Housden B. E., Hu Y., Roesel C., Lin S., Liu L.-P., Yang Z., Mao D., Sun L. et al. (2013). Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc. Natl. Acad. Sci. USA 110, 19012-19017. 10.1073/pnas.1318481110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. and Trainor P. A. (2009). Treacher Collins syndrome: unmasking the role of Tcof1/treacle. Int. J. Biochem. Cell Biol. 41, 1229-1232. 10.1016/j.biocel.2008.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe C. W. and Siegrist S. E. (2017). Eyeless uncouples mushroom body neuroblast proliferation from dietary amino acids in Drosophila. eLife 6, e26343 10.7554/eLife.26343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan K. E., Warda A. S., Sharma S., Entian K.-D., Lafontaine D. L. J. and Bohnsack M. T. (2017). Tuning the ribosome: the influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol. 14, 1138-1152. 10.1080/15476286.2016.1259781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau G. and Heisenberg M. (1982). Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster. Nature 295, 405-407. 10.1038/295405a0 [DOI] [PubMed] [Google Scholar]
- Thum A. S. and Gerber B. (2019). Connectomics and function of a memory network: the mushroom body of larval Drosophila. Curr. Opin. Neurobiol. 54, 146-154. 10.1016/j.conb.2018.10.007 [DOI] [PubMed] [Google Scholar]
- Tsai R. Y. L. and Pederson T. (2014). Connecting the nucleolus to the cell cycle and human disease. FASEB J. 28, 3290-3296. 10.1096/fj.14-254680 [DOI] [PubMed] [Google Scholar]
- Urbach R. and Technau G. M. (2003). Molecular markers for identified neuroblasts in the developing brain of Drosophila. Development 130, 3621-3637. 10.1242/dev.00533 [DOI] [PubMed] [Google Scholar]
- Wang C., Query C. C. and Meier U. T. (2002). Immunopurified small nucleolar ribonucleoprotein particles pseudouridylate rRNA independently of their association with phosphorylated Nopp140. Mol. Cell. Biol. 22, 8457-8466. 10.1128/MCB.22.24.8457-8466.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-D., Kazemi-Esfarjani P. and Benzer S. (2004). Multiple-stress analysis for isolation of Drosophila longevity genes. Proc. Natl. Acad. Sci. USA 101, 12610-12615. 10.1073/pnas.0404648101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen X., He T., Zhou Y. and Luo H. (2013). Evidence for tissue-specific Jak/STAT target genes in Drosophila optic lobe development. Genetics 195, 1291-1306. 10.1534/genetics.113.155945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. (1999). The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24, 437-440. 10.1016/S0968-0004(99)01460-7 [DOI] [PubMed] [Google Scholar]
- Watanabe-Susaki K., Takada H., Enomoto K., Miwata K., Ishimine H., Intoh A., Ohtaka M., Nakanishi M., Sugino H., Asashima M. et al. (2014). Biosynthesis of ribosomal RNA in nucleoli regulates pluripotency and differentiation ability of pluripotent stem cells. Stem Cells 32, 3099-3111. 10.1002/stem.1825 [DOI] [PubMed] [Google Scholar]
- Weiner A. M. J., Scampoli N. L. and Calcaterra N. B. (2012). Fishing the molecular bases of Treacher Collins syndrome. PLoS ONE 7, e29574 10.1371/journal.pone.0029574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Iwasaki S., McGourty C. A., Medina-Ruiz S., Teerikorpi N., Fedrigo I., Ingolia N. T. and Rape M. (2015). Cell-fate determination by ubiquitin-dependent regulation of translation. Nature 525, 523-527. 10.1038/nature14978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L. Jr and Baserga S. J. (2013). Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195, 643-681. 10.1534/genetics.113.153197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S. and Barna M. (2012). Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol. 13, 355-369. 10.1038/nrm3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Isaac C., Wang C., Dragon F., Pogačić V. and Meier U. T. (2000). Conserved composition of mammalian Box H/ACA and Box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol. Biol. Cell. 11, 567-577. 10.1091/mbc.11.2.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. P., Fu C. C., Sugino K., Liu Z., Ren Q., Liu L. Y., Yao X., Lee L. P. and Lee T. (2016). Transcriptomes of lineage-specific Drosophila neuroblasts profiled by genetic targeting and robotic sorting. Development 143, 411-421. 10.1242/dev.129163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Yang J. and Yi J. (2018). Nucleolar Stress: hallmarks, sensing mechanism and diseases. Cell Stress 2, 125-140. 10.15698/cst2018.06.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.