ABSTRACT
Neurovascular pathologies of the central nervous system (CNS), which are associated with barrier dysfunction, are leading causes of death and disability. The roles that neuronal and glial progenitors and mature cells play in CNS angiogenesis and neurovascular barrier maturation have been elucidated in recent years. Yet how neuronal activity influences these processes remains largely unexplored. Here, we discuss our current understanding of how neuronal and glial development affects CNS angiogenesis and barriergenesis, and outline future directions to elucidate how neuronal activity might influence these processes. An understanding of these mechanisms is crucial for developing new interventions to treat neurovascular pathologies.
KEY WORDS: Angiogenesis, Blood-brain barrier, Blood-retina barrier, Neurovascular unit, Basement membrane, Neuroglial progenitors, Neuronal activity, Radial glia, Astrocytes, Müller glia, Retinal waves, Light response
Summary: This Review discusses how neuronal and glial development affects CNS angiogenesis and barriergenesis, and how this might lead to new interventions to treat neurovascular pathologies.
Introduction
Vascular diseases of the central nervous system (CNS) are leading causes of long-term disability in adults worldwide and are among the top ten causes of death in children. Stroke is the fifth-most frequent cause of death and the greatest contributor to adult disability in the USA (Kochanek et al., 2017). CNS autoimmune diseases (e.g. multiple sclerosis; MS) and neurodegenerative diseases (e.g. Alzheimer's disease), which affect approximately 1 million (Wallin et al., 2019) and 5.8 million (Collaborators: GBD, 2016: Dementia, 2019) Americans, respectively, are also associated with vascular pathologies that contribute significantly to disease pathogenesis and progression (reviewed by Spencer et al., 2018; Sweeney et al., 2018). In the eye, diabetic retinopathy is the main cause of adult blindness within the working-age population of industrialized nations, and retinopathy of prematurity is a significant cause of blindness in preterm infants (see also Liegl et al., 2016; Prokofyeva and Zrenner, 2012). These statistics highlight the importance of correct vascularization in the CNS and indicate that neuronal/glial and vascular compartments functionally depend on each other in both normal and pathological states. Understanding how neurons and glia affect neurovascular development and maturation in the healthy CNS is therefore crucial to developing therapies aimed at restoring this delicate relationship in disease.
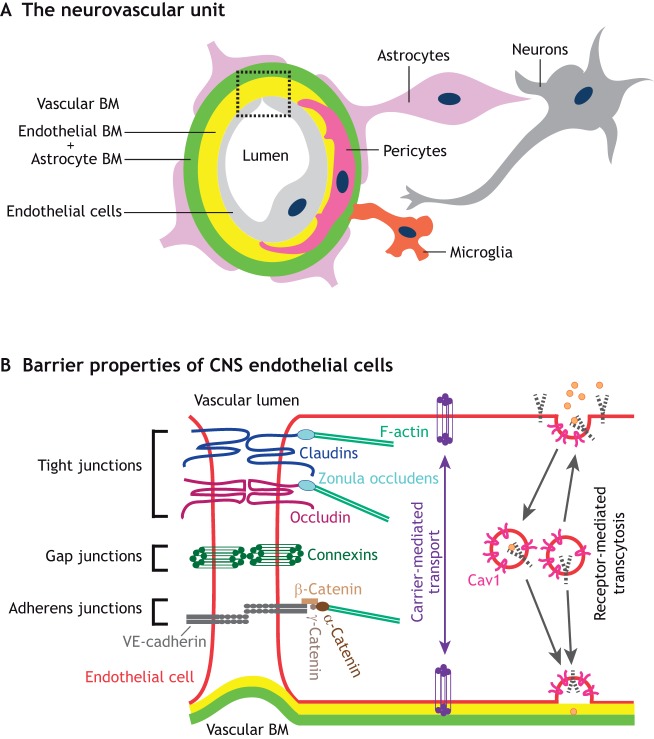
The CNS is vascularized primarily by angiogenesis, a process in which new blood vessels sprout from the existing perineural plexus that surrounds the neural tube. As they develop, CNS blood vessels are in close contact with both neuronal and glial cells and form a neurovascular unit (NVU; Fig. 1A). Reciprocal interactions between vascular components (endothelial cells, endothelial-derived basement membrane and mural cells, such as pericytes and vascular smooth muscle cells) and neuroglial components (neurons, oligodendrocytes, microglia, astrocytes and astrocyte-derived basement membrane) of the NVU not only regulate proper angiogenesis but also confer unique barrier properties to the neurovasculature, namely the blood-brain barrier (BBB) in the brain and the blood-retinal barrier (BRB) in the eye. In this Review, we summarize our current understanding of how neuronal and glial progenitors and mature cells in the brain and retina influence angiogenesis as well as BBB and BRB maturation, respectively. We then compare and contrast the development of neuronal and glial cell types with that of the neurovasculature (angiogenesis and barrier maturation) in these two distinct CNS regions. Finally, we discuss the timing of neural circuit formation and the establishment of synaptic activity in relation to the maturation of the neurovascular barrier properties in the brain and the retina, and we propose avenues for future studies to address how neuronal activity influences CNS angiogenesis and the formation of neurovascular barriers.
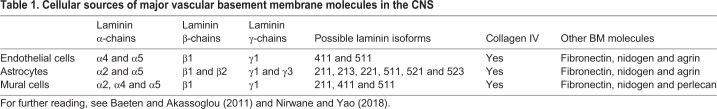
Fig. 1.
Interactions between various cell types in the neurovascular unit are crucial for the formation of neurovascular barriers. (A) In the CNS, vascular components, including endothelial cells (light gray), endothelial cell-derived basement membrane (endothelial BM; yellow) and mural cells [such as pericytes (pink)], together with astrocytes (purple), astrocyte-derived BM (astrocyte BM; green), neurons (gray) and microglia (orange) interact anatomically to form the neurovascular unit (NVU). The boxed area is presented in greater detail in B. (B) A schematic of the cell biological mechanisms that contribute to the neurovascular barrier properties of CNS endothelial cells. Tight junctions, formed by claudins (dark blue), occludin (maroon) and zonula occludens (light blue), limit the movement of small molecules between endothelial cells, thereby forming a paracellular barrier. Tight junction proteins interact with the cytoskeleton (F-actin, green) via zonula occludens proteins (light blue). Adherens junctions, which are formed by VE-cadherin (dark gray) as well as α-catenin (dark brown), β-catenin (medium brown) and γ-catenin (light brown), are crucial for cell-cell interactions and shear stress sensing. Endothelial cells communicate with each other via connexin-regulated gap junctions (dark green). Low rates of caveolin 1 (Cav1, pink)-dependent receptor (dashed black)-mediated transcytosis prevent trafficking of larger molecules and antibodies within CNS endothelial cells, establishing a transcellular barrier. Finally, CNS endothelial cells also contain specific active and passive transporters (purple) to facilitate the movement of nutrients between the blood and the brain/retina.
BBB/BRB integrity depends on proper NVU assembly and organization
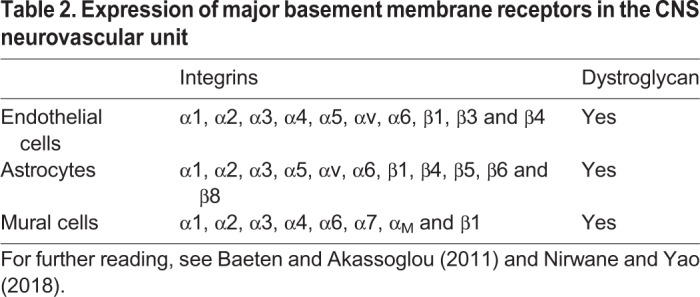
The NVU is essential for neurovascular coupling, the regulation of blood flow and the establishment of neurovascular barrier properties (reviewed by Iadecola, 2017; Liebner et al., 2018). Within the NVU, endothelial cells interact either directly (in the case of pericytes and smooth muscle cells) or indirectly (in the case of astrocytes) with other cells via the vascular basement membrane (BM, Fig. 1A). The vascular BM contains extracellular matrix (ECM) components derived from multiple cell types (see Table 1 for a summary of NVU cell sources that secrete various ECM proteins). Laminins, a class of glycoproteins made of α-, β- and γ-chains, and collagen IV are two main components of the vascular BM. Endothelial cells primarily secrete laminin α4 and α5 (Sixt et al., 2001; Vanlandewijck et al., 2018), whereas astrocytes produce laminin α2 and α5 (Sixt et al., 2001; Stenzel et al., 2011). Although endothelial cell- and astrocyte-derived laminins form a composite structure in the healthy CNS (Fig. 1A), these two components of the vascular BM can become anatomically separated during CNS inflammation, e.g. in the case of experimental autoimmune encephalomyelitis (EAE), an animal model of MS (Sixt et al., 2001). Mural cells also produce laminin α2, α4 and α5 (Menezes et al., 2014; Nirwane et al., 2019; Sixt et al., 2001; Vanlandewijck et al., 2018). The cellular sources of laminin β and γ chains are not fully understood. Laminin β1 and γ1 are produced by all three NVU cell types, whereas laminin β2 and γ3 are deposited primarily by astrocytes with some contribution from mural (β2) and endothelial cells (γ3), respectively (Biswas et al., 2017, 2018; Chen et al., 2013; Gautam et al., 2016; Gnanaguru et al., 2013; Sixt et al., 2001; Vanlandewijck et al., 2018; Yao et al., 2014). In contrast to the complexity of laminin chain secretion, collagen IV is produced by all three NVU cell types (reviewed by Baeten and Akassoglou, 2011). Endothelial cells, mural cells and astrocytes interact with the vascular BM via various cell surface receptors, such as integrins and dystroglycan (see Table 2 for a summary of integrin receptors expressed by each cell type). Microglia, the resident immune cells of the CNS, and perivascular macrophages also make contacts with the astrocyte-derived vascular BM (Fig. 1A). Neurons, notably, do not make direct contact with either endothelial cells or the vascular BM, but interact directly with pericytes and astrocytes to influence NVU function [see Iadecola (2017) for further details on neurovascular coupling].
Table 1.
Cellular sources of major vascular basement membrane molecules in the CNS
Table 2.
Expression of major basement membrane receptors in the CNS neurovascular unit
The barrier properties of CNS blood vessels are mediated by two primary cell biological mechanisms that control transport from the blood into the CNS and vice versa. First, CNS endothelial cells form several types of cell-cell junctions (Fig. 1B). These include impermeable tight junctions, which prevent the movement of small molecules between endothelial cells, establishing a paracellular barrier. The major tight junction proteins at the BBB/BRB are the transmembrane proteins claudin 5, claudin 12 and occludin, which interact with similar proteins in the adjacent cell to form tight junctions, and zonula occludens (ZO) 1, ZO2 and ZO3 proteins, which anchor junctional proteins to the cytoskeleton (Fig. 1B) (reviewed in detail by Liebner et al., 2018; Zhao et al., 2015). CNS endothelial cells also form adherens and gap junctions (Fig. 1B), which play crucial roles in cell adhesion, cell communication, response to shear stress and regulation of neurovascular coupling (see Baeyens et al., 2016; Iadecola, 2017; Wallez and Huber, 2008 for reviews). Although tight junction proteins predominantly regulate paracellular permeability between CNS endothelial cells by establishing a very tight seal, adherens junction proteins (e.g. VE-Cadherin, and α-, β- and γ-catenins) are also crucial to orchestrate the assembly of tight junctions (Taddei et al., 2008) and mediate endothelial cell-pericyte interactions (Li et al., 2011). Second, CNS endothelial cells have negligible transcellular permeability due to a low rate of receptor-mediated transcytosis, which is regulated by a low number of caveolae combined with the absence of fenestrae (Fig. 1B). These properties limit the movement of larger proteins, antibodies and immune cells across endothelial cells. To overcome these two physical barriers, CNS endothelial cells express a large number of passive and active transporters that shuttle nutrients and ions across blood vessels into the CNS and regulate the efflux of toxins and drugs out of the CNS (Fig. 1B). These essential barrier properties are shared among endothelial cells in the brain and the retina (Runkle and Antonetti, 2011). However, as we discuss below, there are some differences between these two CNS regions with regard to how neurovascular barrier properties arise and are maintained.
The role of the ECM in NVU assembly and barrier formation in the brain
As the vascular BM plays a crucial role in NVU assembly and barrier maintenance in the brain and the retina, genetic and pathological conditions associated with changes in vascular BM composition lead to perturbation of neurovascular barrier properties. For example, loss of the pro-collagen type IV alpha 1 gene (Col4a1) in mice causes vascular BM disorganization and cerebral hemorrhage (Gould et al., 2005). Similarly, deletion of the laminin α2 chain gene (Lama2) (Menezes et al., 2014) or astrocyte-specific deletion of the laminin γ1 chain (Chen et al., 2013) causes BBB leakage, hemorrhagic stroke, deficits in pericyte differentiation and loss of astrocyte endfeet polarization around blood vessels, and is associated with disorganization of tight junctions at the BBB (Yao et al., 2014). Consistent with the phenotypes observed in laminin-deficient mice, neuronal- and glial-specific deletion of dystroglycan, a major receptor for laminins, increases BBB permeability (Menezes et al., 2014). In addition, loss of integrin αvβ8, an ECM receptor responsible for transforming growth factor β1 (TGFβ1) activation, has been shown to be important for CNS angiogenesis and barriergenesis. Global deletion of integrin αv causes increased CNS angiogenesis as well as intracerebral hemorrhage (Bader et al., 1998). Similarly, global deletion of integrin β8 also increases CNS angiogenesis and perivascular astrogliosis, presumably owing to diminished integrin-mediated TGFβ1 activation. However, endothelial cell tight junction integrity remains unaffected in β8-deficient strains (Mobley et al., 2009). In addition, neuroepithelium-specific deletion of integrin β8 leads to increased vascular sprouting and brain hemorrhage owing to hypo-activation of TGFβ1 signaling (Arnold et al., 2014), a phenotype similar to that of endothelial cell-specific TGFβ receptor 2 (Tgfbr2) mutant mice (Nguyen et al., 2011). However, as TGFβ signaling also plays a crucial role in CNS angiogenesis, the hemorrhagic phenotype of these mice may be secondary to abnormal vascular growth rather than due to BBB dysfunction (Arnold et al., 2014). A recent study has further elucidated the mechanisms by which integrin αvβ8 facilitates TGF-β1 activation in the CNS, showing that it acts via LRRC33, a ‘milieu’ molecule localized on the surface of microglia. Mice deficient for Lrrc33 exhibit neurological abnormalities; however, their CNS vasculature is normal, suggesting that the CNS neuroglial and vascular milieus activate TGFβ1 signaling in different manners (Qin et al., 2018). On the other hand, neuropilin 1 (Nrp1), which is expressed by brain endothelial cells, suppresses TGFβ1 activation by forming an intercellular complex with integrin β8 (Hirota et al., 2015). In summary, these genetic loss-of-function studies of ECM proteins and their cognate receptors underlie the importance of the proper NVU assembly for neurovascular development and BBB function.
In addition to proper NVU cell assembly, the correct polarization of glial endfeet around the vasculature is crucial for maintaining vascular barrier properties. In the brain, astrocytes are the only glial cells that express the water channel protein aquaporin4 (Aqp4) at their endfeet (Hubbard et al., 2015). In EAE, astrocyte endfeet lack polarized expression of Aqp4 and dystroglycan, leading to increased BBB permeability (Wolburg-Buchholz et al., 2009). However, the role of Aqp4 in BBB function is actively under debate, as some studies have shown that glial-specific deletion of Aqp4 reduces brain water content without affecting BBB permeability (Haj-Yasein et al., 2011). Loss of pericytes also causes BBB impairment (Armulik et al., 2010; Daneman et al., 2010). However, the phenotypes observed in pericyte-deficient mice are likely due to multiple factors, including changes in endothelial cell-specific gene expression, alterations in laminin deposition in the vascular BM and abnormal glial endfeet polarization around CNS blood vessels (Armulik et al., 2010; Daneman et al., 2010).
The role of the ECM in NVU assembly and barrier formation in the retina
Within the retina, vascular BM proteins are also essential for NVU assembly and BRB function. Global deletion of laminin β2 chain or β2 and γ3 chains together causes BRB leakage in the retina (Gnanaguru et al., 2013). Similarly, endothelial cell-specific deletion of integrin β1 perturbs the formation of adherens junctions, leading to aberrant polarization and lumen defects in retinal endothelial cells (Zovein et al., 2010) as well as vascular leakage (Yamamoto et al., 2015). Loss of pericytes also leads to BRB disruption (Park et al., 2017). Similar to what has been described in the brain, retinal astrocytes express integrin αvβ8, and Nestin-Cre-mediated deletion of either αv or β8 chains leads to retinal vascular abnormalities, including vascular tufts as well as intraretinal hemorrhage (Hirota et al., 2011). Moreover, Müller glia- and neuron-specific deletion of integrin β8 reduces TGF-β1 signaling and leads to increased vascular growth in the superficial plexus as well as hemorrhage within the retina, which could be due to either a secondary effect of abnormal vascular growth or to impaired BRB formation (Arnold et al., 2012).
Aqp4 is also implicated in BRB formation in the retina. However, in contrast to its expression in the brain, Aqp4 is expressed at the endfeet of two retinal glial cell types: astrocytes that surround the superficial vascular plexus; and Müller glia that contact the deeper vascular plexus (Nicchia et al., 2016). Interestingly, Aqp4−/− mice exhibit vascular leakage only in the deep retinal plexus (Nicchia et al., 2016), suggesting that Aqp4 is crucial in Müller glia, but not in astrocytes, for BRB integrity. These contrasting results between the brain and the retina suggest a complex role for Aqp4 in maintaining the vascular barrier properties of these two CNS regions, which may depend on either cell-intrinsic properties of the vascular bed or its surrounding microenvironment.
Developmental timeline of angiogenesis and BBB/BRB maturation in relation to neuronal and glial cell fate specification and neuronal activity in the developing CNS
In order to obtain a comprehensive understanding of how neurons and glia together regulate CNS angiogenesis and BBB/BRB maturation, it is important to compare the developmental timing of angiogenesis and BBB/BRB maturation with that of neurogenesis and gliogenesis and the establishment of neuronal activity in both the brain and the retina (Figs 2 and 3).
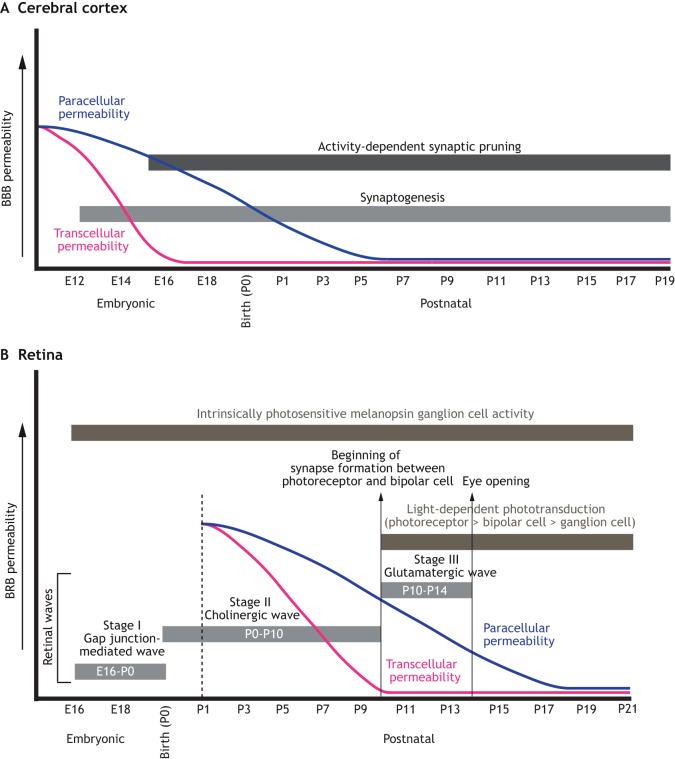
Fig. 2.
Developmental timelines for neurogenesis, gliogenesis, angiogenesis and barriergenesis in the brain and retina. (A,B) Developmental timelines (i) and schematic illustrations (ii) of the development of neurons, glial cells and blood vessels in the mouse cerebral cortex (A) and retina (B). (Ai) Neurogenesis in the cerebral cortex begins at E11, whereas gliogenesis starts at E13 (oligodendrocyte formation) and E18.5 (astrocyte formation). Neurogenesis is completed by P8-P10, whereas gliogenesis persists for prolonged periods in postnatal development corresponding to the expansion and maturation of astrocytes. Cortical angiogenesis also spans both the embryonic and postnatal stages of development, and is completed by P25. The initiation of BBB maturation [establishment of both paracellular (blue) and transcellular (pink) properties] starts soon after angiogenesis. However, by birth, both the paracellular and transcellular barrier properties of brain endothelial cells are mature. Dotted lines indicate the initiation and maturation of the endothelial barrier properties or the start of differentiation and appearance of mature astrocytes and oligodendrocytes. (Aii) Neurogenesis in the cortex occurs in an inside-out fashion (gray arrow), whereas angiogenesis occurs in an outside-in fashion (red arrow). Mature astrocytes ensheath blood vessels to ensure the maintenance of the BBB. (Bi) Amacrine cells, cones, ganglion cells and horizontal cells are born during the embryonic phase in the retina, whereas bipolar cells and Müller glia are born during the postnatal phase. Rod development occurs throughout both phases. Astrocytes migrate into the retina from E18.5 until P6. Growth of the superficial plexus (P1-P8) follows an astrocyte template, over the inner limiting membrane. Sprouts from the superficial vessels, guided by Müller glia, then form the deep (P8-P12) and intermediate (P12-P17) plexuses. The transcellular BRB matures by P10, whereas the paracellular barrier does not mature until P18. Upward arrows indicate the peak of development for each process. Dotted lines indicate the initiation and maturation of BRB properties in retinal endothelial cells. (Bii) Schematic diagram shows the relationship between distinct neuronal cell types and distinct vascular plexuses in the retina. Ganglion cell bodies reside in the ganglion cell layer, which is vascularized by the superficial plexus. Amacrine, bipolar, horizontal and Müller cell bodies reside in the inner nuclear layer, which is vascularized by the intermediate and deep vascular plexuses. Photoreceptors occupy the outer nuclear layer, which makes contacts with the deep vascular plexus.
Fig. 3.
Developmental timeline of neuronal activity and BBB/BRB maturation in the mouse CNS. Developmental timeline of synaptogenesis, neural activity and BBB/BRB maturation in the mouse cerebral cortex (A) and retina (B). (A) Synaptogenesis (light-gray bar) in the mouse cortex begins around E13.5 (E16.5 is when activity-dependent synaptic pruning begins), whereas activity-dependent synaptic pruning (dark-gray bar) begins at E16.5 and peaks at birth. Both processes continue through P21. The transcellular barrier properties of the BBB (blue curve) in the cortex do not completely mature until E16-E17, and the paracellular BBB properties (pink curve) do not mature until birth, when the transendothelial electrical resistance (TEER) is highly increased. (B) Between E16 and P0, retinal waves (gray bars) are propagated by gap junctions (stage I). From P0-P10, these waves are cholinergic in nature (stage II), whereas between P10-P14 they switch to being glutamatergic (stage III). Most neuronal activity (dark-gray bars) in the mouse retina after P14 is light mediated (i.e. photoreceptor dependent), although some reports suggest that photoreceptor activity occurs as early as P10. Moreover, the light response by intrinsically photosensitive melanopsin ganglion cells occurs throughout this period (E16-adult). By P10, the endothelial transcellular BRB (pink curve) is mature throughout all retinal vascular beds. In contrast, the paracellular BRB (blue curve) properties of the retinal vasculature gradually arise until P18.
The relationship between neuronal and glial specification, and vascular development in the brain
Brain angiogenesis has mostly been studied in the context of the cerebral cortex; therefore, for the purpose of this Review, we focus primarily on this region. Neurogenesis in the mouse cerebral cortex occurs primarily during the embryonic phase of development. Starting from embryonic day (E) 10.0-11.5, neural progenitor cells expand in the ventricular zone and begin to differentiate into radial glia and cortical progenitors that give rise to both neurons (early) and astrocytes (later). The differentiation of radial glial cells into layer-specific neuronal subtypes begins at E11.5 and peaks around E14.5 (Martynoga et al., 2012; van den Ameele et al., 2014), although the inside-out migration of terminally differentiated neurons along radial glial processes in the cortex continues throughout the first postnatal week of development (Fig. 2A) (reviewed by Farhy-Tselnicker and Allen, 2018; Thion and Garel, 2017). The differentiation of oligodendrocytes from progenitor cells begins around E13.0-E14.5 in ventral regions of the brain and peaks between E16.5 and E18.5 in both the ventral and dorsal forebrain, including the cortex; however, a second distinct wave of oligodendrogenesis has also been reported during postnatal development (Fig. 2A) (reviewed by Rowitch and Kriegstein, 2010). Astrogenesis in the cortex begins after the wave of neurogenesis. Around E18.5, radial glial cells undergo a potency switch to generate astrocytes rather than neuronal lineages. During postnatal development, astrocyte precursors migrate to their final position and begin to acquire markers of fully mature astrocytes. However, they retain the ability to divide mitotically and can proliferate locally, which accounts for the large expansion of the astrocyte pool observed during the first postnatal month of cortical development (Fig. 2A) (reviewed by Farhy-Tselnicker and Allen, 2018; Martynoga et al., 2012). The formation of synapses in the cortex starts around E16.5; however, synapses exhibit mature morphology only around the third postnatal week of development, when the neural circuits become fully mature through activity-dependent synaptic pruning (Fig. 3A) (reviewed in detail by Farhy-Tselnicker and Allen, 2018; Thion and Garel, 2017).
Forebrain angiogenesis in the mouse was traditionally thought to begin at E10.0-E11.5, to peak around birth and then to gradually decline until postnatal day 25 (P25) when the vascular network becomes functionally mature and quiescent (reviewed by Engelhardt and Liebner, 2014). However, recent RNA sequencing studies suggest the existence of a second wave of postnatal brain angiogenesis that has not been appreciated in previous studies (Corada et al., 2019; Hupe et al., 2017). These studies have shown that endothelial cell-specific mRNA transcripts encoding: (1) angiogenic tip markers (e.g. Egfl7, Mcam, Apln and Lamb1); (2) signaling factors that regulate CNS angiogenesis, such as components of the VEGF (e.g. Flt1, Flt4 and Nrp1), Notch (e.g. Notch4, Dll4, Nrarp, Hes1 and Jag1) and Wnt (e.g. Nkd1, Tcf7, Axin2 and Ppard) pathways; (3) cell proliferation markers; or (4) cell cycle markers are decreased between E15.5 and E18.5 in the forebrain when compared with E11.5-E13.5, but exhibit a rapid increase in levels during the first week after birth (P5 and P9), indicative of a potential second wave of angiogenesis (Corada et al., 2019; Hupe et al., 2017). These molecular studies are supported by analyses of endothelial cell proliferation (e.g. via BrdU incorporation) and in vivo two-photon imaging of endothelial cell sprouts in the postnatal murine cortical vasculature, which demonstrate increased endothelial cell proliferation and sprouting from P5 to P10, followed by a gradual decline and quiescence by P25 when endothelial cell profileration ceases in the cortex (Harb et al., 2013). Moreover, endothelial cell ablation of β-catenin (Ctnnb1) or Axin overexpression (i.e. Wnt loss of function) result in hypovascularization of the postnatal forebrain (Martowicz et al., 2019), suggesting continuous postnatal angiogenesis. Therefore, forebrain angiogenesis appears to exhibit two distinct waves – an embryonic and a postnatal phase. The first rapid embryonic phase of angiogenesis in the developing cortex (E10.5-E15.5) likely corresponds to the developmental period during which progenitor cells undergo extensive proliferation to increase the size of the brain and are specified into layer-specific neuronal subtypes that migrate from deep to superficial layers of the cortex; oligodendrocyte differentiation and migration also occurs during this period (Fig. 2A,A′) (Martynoga et al., 2012; Toma and Hanashima, 2015). The decline in transcription of angiogenic markers in brain endothelial cells, which takes place between E15.5 and E18.5 (Hupe et al., 2017), occurs when astrocytes start to differentiate (Bayraktar et al., 2014). Thus, initiation of astrocyte differentiation may promote the transition from angiogenesis to vascular maturation characterized by acquisition of BBB properties (see below; Fig. 2A and A′). In contrast, the second postnatal wave of angiogenesis (P5-P10) in the developing cortex may be driven by activity-dependent mechanisms (neuronal activity), which have been shown to play a role in refining the CNS vasculature to match the metabolic demands of neurons (discussed below; Fig. 3A) (Lacoste et al., 2014).
It is important to note that the cerebellum is unique among other brain regions. The major neuronal and glial cell types in the cerebellum are specified and mature during postnatal development (Espinosa and Luo, 2008), although the territory that gives rise to the cerebellum is specified as early as E8.5 (Butts et al., 2014). Cerebellar vascular development also occurs exclusively during postnatal stages, starting at E18 when cerebellar development is initiated and continuing until P30-P45 when the cerebellum becomes functionally mature (Martinez et al., 2013; Zhou et al., 2014). Therefore, the development of the neurovasculature and the signaling pathways governing angiogenesis and barriergenesis in the cerebellum are more similar to those in the retina than the rest of the brain (see below for more details).
The development of the BBB begins immediately after the first (embryonic) wave of angiogenesis in the forebrain. Anatomical and functional studies of barrier development have suggested that BBB specification starts with the induction of a subset of barrier-specific genes, including those encoding tight junction-associated proteins (claudin 5, occludin and ZO1) and the glucose transporter (Glut1/Slc2a1), at early embryonic stages (E10.0-E11.5) in response to inductive signals (e.g. Wnts) derived from both neuronal and glial progenitor cells (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008). Recent bulk RNA sequencing data have confirmed these initial observations, i.e. that the majority of BBB-associated transcripts start to be translated by E11.5-E12.5 (Hupe et al., 2017). However, analysis of the translation of all BBB-associated transcripts during embryonic and postnatal development has revealed six important features about BBB maturation. First, distinct BBB transcripts are induced and translated at different developmental stages of CNS development. For example, transcripts and proteins for most adherens and tight junction-associated proteins (e.g. Cdh5, Cldn5, ZO1, Jam and Ocld) are highly expressed in early development, whereas transcripts for the majority of organic ion transporters (e.g. Slc28a8, Slco2b1 and Slco1a4) are induced strongly in the postnatal stages (P5-P9) (Hupe et al., 2017; Martowicz et al., 2019). Second, there is a large heterogeneity in BBB maturation even within vascular beds located in the same CNS region (Hupe et al., 2017). Third, the decrease in angiogenesis-related genes between E14.5 and E16.5 corresponds to an increase in the expression of several key transcription factors (Foxf2, Foxl2, Foxq1, Lef1 and Sox17) that play crucial roles in BBB maturation (Hupe et al., 2017; Martowicz et al., 2019; Reyahi et al., 2015). However, two additional key BBB maturation factors (Ppard and Zic3) only appear at E17.5-E18.5. Foxf2 and Zic3 induce most barrier-specific genes when tested in vitro using human umbilical cord vein cells (HUVECs), suggesting that they are master regulators of BBB specification (Hupe et al., 2017). In addition, Foxf2 and Sox17 are genetically implicated in barrier specification and maintenance (Corada et al., 2019; Reyahi et al., 2015); however, there have been no functional studies for other transcription factors implicated in barrier formation. Fourth, the expression of some of the BBB-inducing transcription factors in later gestation (E15.5-E18.5) coincides with maturation of the primitive BBB initiated earlier in development (E11.5-E13.5). For example, during late gestation, brain endothelial cells suppress caveolae-mediated transport via induction of Mfsd2a and suppression of PLVAP (PV-1; Meca32) expression to ensure a decrease in caveolae number and elimination of endothelial cell fenestrae (Ben-Zvi et al., 2014; Chow and Gu, 2015; Hallmann et al., 1995). These transcriptional changes ensure maturation of the transcellular barrier of the neurovasculature (Figs 2A and 3A). Fifth, cerebral vessels located in the pia matter significantly increase their transendothelial electrical resistance, a functional readout of the mature paracellular barrier that is regulated by tight junctions, only prior to birth (Butt et al., 1990), although these vessels express tight junction-associated proteins earlier in development. These findings suggest that the maturation of the paracellular barrier follows that of the transcellular barrier in the brain vasculature (Fig. 2A), albeit with the caveat that the Butt et al. study was focused on the pial vasculature, rather than on the vessels inside the parenchyma, due to feasibility of electrophysiological measurements. Sixth, transcripts encoding a subset of BBB-associated transporters, including ABC (e.g. Abcb1a/Mdr1a and Abcc4), amino acid (e.g. Slc1a1 and Slc1a3) and organic ion (e.g. Slco1a4, Slco2a1 and Slcob1) transporters, are highly upregulated only during postnatal development (Martowicz et al., 2019), when astrocytes mature and ensheath brain capillaries. Thus, astrocytes may play a crucial role in regulating the expression of transporters, despite the fact that they are not necessary for formation of the paracellular and transcellular barriers, as these features of the neurovasculature are developed even when astrocyte maturation in the cortex is delayed by elimination of FGF2 (Saunders et al., 2016). Overall, these analyses indicate that barrier development and maturation occur gradually, spanning both embryonic and postnatal phases of brain development.
The relationship between neuronal and glial specification and vascular development in the retina
Neuronal development in the murine retina occurs during two major phases, beginning at E10 and continuing until P11 (Fig. 2B) (Rapaport et al., 2004). Cone photoreceptors, ganglion, horizontal and amacrine cells are all born during the embryonic phase, whereas bipolar cells and Müller glia are born postnatally (Fig. 2B). Rod photoreceptors appear throughout both phases, peaking at birth (Varshney et al., 2015). In contrast to the brain, astrocytes are not born in the retina but they enter via the optic nerve, beginning at E17.5-E18.5, and migrate radially towards the periphery (Chan-Ling et al., 2009). Various forms of neuronal activity occur in distinct phases of postnatal retina development, mirroring the specification of distinct neuronal subtypes (Fig. 3B). From E16.5 to P0, retinal activity (in the form of waves) is propagated by gap junctions and adenosine signaling (Fig. 3B) (Torborg and Feller, 2005). From birth to P10, there is a major switch to cholinergic retinal waves that are propagated mostly by nicotinic acetylcholine receptors (nAChRs) and inhibited by toxins directed against nAChR subunits (Fig. 3B) (Bansal et al., 2000). Finally, as the eyes open (P10-P14), excitatory waves of neural activity in the retina switch from cholinergic to glutamatergic, and are blocked by glutamate receptor antagonists (Fig. 3B) (Bansal et al., 2000). Mice lacking the β2 nAChR subunit exhibit premature onset of the glutamatergic retinal wave by P8 (Bansal et al., 2000). In contrast, mice lacking vesicular glutamate transporter 1 [vGluT1−/− (Slc17a7−/−) mice] exhibit persistent cholinergic retinal waves (Blankenship et al., 2009), indicating that glutamatergic activity is required to suppress cholinergic waves in the retina. The majority of neuronal activity in the mouse retina from P14 onwards is light mediated (i.e. photoreceptor dependent) (Fig. 3B), although a recent study reported light-dependent photoreceptor activity even prior to eye opening (P8-P10) (Tiriac et al., 2018). In contrast, light-responsive activity of intrinsically photosensitive melanopsin ganglion cells occurs from embryonic development (E16.5) into adulthood (Fig. 3B) (Rao et al., 2013; Sekaran et al., 2005). Thus, the specification of neuronal and glial cell fates, and the establishment of neural activity in the retina overlap significantly with vascular development and BRB maturation (Figs 2B and 3B).
In contrast to the brain, angiogenesis in the mouse retina occurs entirely during postnatal development. After birth, embryonic hyaloid vessels gradually regress as retinal vessels grow into the superficial, intermediate and deep vasculature (Fig. 2B,B′). The superficial vasculature expands radially from the optic nerve head (ONH) towards the retinal periphery over the ganglion cell layer (P0-P8), following a template established by astrocytes. Vascular sprouts from the superficial vessels then dive into the retina guided by Müller glial processes (Stone et al., 1995) and form the deep vascular plexus within the outer plexiform layer (P8-P12). Finally, the intermediate vascular plexus arises in the inner plexiform layer (P12 onwards), completing vascularization by P21 (Fig. 2B,B′) (see Stahl et al., 2010 for a detailed review of retinal angiogenesis).
Structurally, there are two types of BRBs in the mammalian retina. The outer BRB is composed of the retinal pigment epithelium and its underlying BM, separating photoreceptors from choroidal circulation. The inner BRB separates neurons and glia from retinal circulation by means of a structural barrier formed by retinal endothelial cells that possess the same cell biological mechanisms as those described above for brain endothelial cells forming the BBB (Fig. 1B) (Runkle and Antonetti, 2011). A recent study has suggested that the inner BRB is established as early as P3-P5 in the more mature part of the superficial vascular plexus of the central retina, whereas immature vessels around the growing vascular front remain leaky. However, BRB maturation (establishment of both paracellular and transcellular barrier properties) is completed throughout the superficial and deep vascular beds by P10; the intermediate plexus possesses an intact BRB from its emergence (Chow and Gu, 2017). Moreover, it has been suggested that retinal endothelial cells possess functional tight junctions (i.e. a mature paracellular barrier) at P1, and that establishment of the BRB along the expanding superficial vascular tree is achieved only by gradual suppression of transcellular permeability from P1 to P10 (Chow and Gu, 2017), a feature that seems unique to the retina and is not observed in the brain vasculature (see above). In contrast, using a small-molecule biocytin-tetramethylrhodamine tracer (∼890 Da), which crosses the vascular barrier via the paracellular pathway (Knowland et al., 2014; Lengfeld et al., 2017; Lutz et al., 2017), we demonstrated that the paracellular barrier of the superficial plexus is immature at P1 but gradually matures between P10 and P18 (Fig. 3B) (Mazzoni et al., 2017). Our finding suggests that the transcellular BRB matures before the paracellular BRB in the retina, similar to the developmental timeline for BBB maturation in the brain (Fig. 3A,B). Therefore, in our view, the BBB and BRB mature in a similar manner (compare Fig. 3A with B). Because the specification of neuronal and glial cell fates and the establishment of neural activity overlap significantly with vascular development and BRB maturation in the retina, it is likely that both mature neuronal and glial cells as well as neural activity play a major regulatory role in angiogenesis and vascular barrier formation (Figs 2B and 3B).
Glial- and neuronal-derived signals that promote CNS angiogenesis and barriergenesis
Glial-mediated regulation of angiogenesis and barriergenesis in the brain
Glial cells regulate several aspects of forebrain angiogenesis by secreting both pro- and anti-angiogenic factors (Fig. 4A). Three major types of glial cells are found in the cerebral cortex: radial glia, which give rise to both neuronal and glial progenitor cells; and two mature glial cells – oligodendrocytes and astrocytes. Several Wnt ligands (Wnt7a and Wnt7b) are expressed by these cells in the embryonic mouse cortex (Daneman et al., 2009; Stenman et al., 2008) (Fig. 4A). Genetic ablation of Wnt7a and Wnt7b or blockade of neuroepithelium-derived Wnt activity in endothelial cells perturbs both brain angiogenesis and BBB integrity (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008; Zhou and Nathans, 2014; Zhou et al., 2014). Although Wnt signaling is required in the initial phases of angiogenesis (E10. 5-E12.0), it is unclear whether it is necessary for later embryonic and postnatal stages of brain angiogenesis. Genetic ablation of β-catenin (Ctnnb1) in CNS endothelial cells after E11.5 does not suppress expression of angiogenesis-associated mRNA transcripts, although it does abolish induction of BBB-associated transcripts (Hupe et al., 2017). Although the direct effects of β-catenin (Ctnnb1) ablation in CNS angiogenesis (e.g. effects on cell profileration or angiogenic sprouting) were not tested, this finding suggests that Wnt/β-catenin signaling may play a more prominent role in the induction of BBB properties at late gestation, rather than in CNS angiogenesis. However, a recent study showed that endothelial cell ablation of β-catenin (Ctnnb1) or Axin overexpression results in mild hypovascularization of the postnatal brain (Martowicz et al., 2019). This effect was mediated by Sox17 (a Wnt/β-catenin transcriptional target), which induces VEFGR2 expression in endothelial cells rendering them responsive to VEGFA (Martowicz et al., 2019). Thus, Wnt/β-catenin signaling seems to be required for both embryonic and postnatal angiogenesis in the cortex, in addition to its undisputable role in BBB development. Consistent with the ability of Wnt signaling to induce BBB properties are recent in vivo studies showing that activation of the Wnt/β-catenin pathway in leaky vessels of the circumventricular organs partially induces BBB properties (Benz et al., 2019; Wang et al., 2019).
Fig. 4.
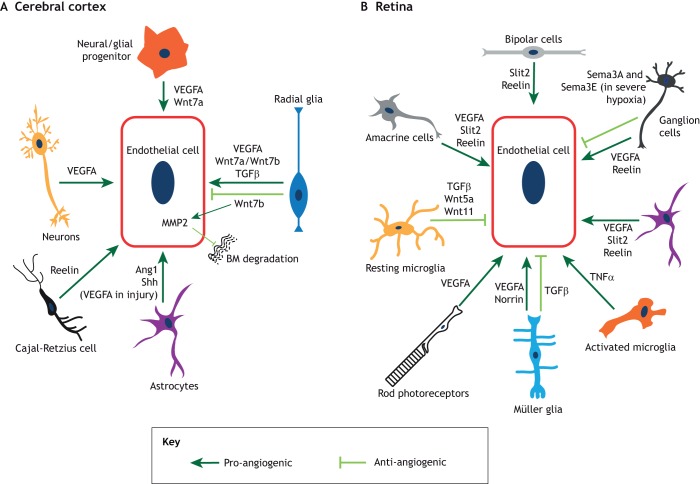
The main neuronal and glial-derived signals that influence angiogenesis and barriergenesis. Schematic representation of the signaling interactions between endothelial cells and other cell types in the cerebral cortex (A) and the retina (B). (A) In the cerebral cortex, radial glia, neuroglial progenitors and neurons secrete VEGFA. Radial glia and neuroglial progenitors also secrete several Wnt ligands; radial glia also secrete TGFβ. Astrocytes produce Ang1 and sonic hedgehog (Shh), and these same cells in the adult cortex secrete VEGFA during injury. Finally, Cajal-Retzius cells express reelin. Pro-angiogenic processes are depicted in dark green, whereas anti-angiogenic processes are depicted in light green. (B) In the retina, neuronal cell types and Müller glia secrete VEGFA, which facilitates vascular growth in distinct layers. Ganglion cells, amacrine cells and bipolar cells secrete reelin, whereas amacrine cells and bipolar cells produce Slit2, both of which promote angiogenesis. Depending on tissue physiology (such as severe ischemia), ganglion cells also secrete semaphorin 3A and semaphorin 3E (Sema3A and Sema3E), which inhibit retinal vascular growth. Müller glia secrete norrin, which promotes retinal angiogenesis and BRB formation. In contrast, Müller glia-derived TGFβ inhibits endothelial cell proliferation and vascular branching. TGFβ1 derived from resting microglia inhibits endothelial cell proliferation, while Wnt5a and Wnt11 produced by these cells negatively regulate vascular branching in the deep plexus. In contrast, activated microglia induce endothelial cell proliferation, likely by secreting tumor necrosis factor α (TNFα). Pro-angiogenic factors are depicted in dark green, whereas anti-angiogenic factors are depicted in light green.
Radial glial cells can also stabilize vessels within the embryonic cerebral cortex by inhibiting Wnt signaling in endothelial cells. Ablation of radial glia decreases cortical angiogenesis and causes vascular hemorrhage (Ma et al., 2013). A recent study showed that radial glia secrete TGF-β1, which promotes both endothelial cell migration and tube formation in vitro (Siqueira et al., 2018) (Fig. 4A). In vivo, loss of TGFβ1 expression in radial glia reduces vascular growth in the cerebral cortex, whereas gain of TGF-β1 expression in the endothelium causes the opposite phenotype (Siqueira et al., 2018). Finally, neural- or glial progenitor-derived vascular endothelial growth factor A (VEGFA) is also crucially important for vascular growth in the brain (Fig. 4A). The reduction of VEGFA levels within neural progenitor cells leads to decreased vascular sprouting in the forebrain as early as E10.5, well before the differentiation of astrocytes has begun (Haigh et al., 2003; Raab et al., 2004), suggesting a crucial role for radial glia or progenitor-derived VEGF-A in CNS angiogenesis (Fig. 4A).
Immature and mature astrocytes also regulate developmental angiogenesis in the cerebral cortex. Loss of mature astrocytes reduces cortical vascular density during early postnatal development (Ma et al., 2012). Astrocytes of the adult brain have been reported to express Src-suppressed C-kinase substrate (SSeCKS), which decreases expression of astrocyte-derived VEGFA and induces expression of Ang-1, a factor involved in vessel stability (Lee et al., 2003). Similarly, loss of NG2+ oligodendrocyte or astrocyte precursor cells leads to reduced vascular density in the cerebral cortex (Minocha et al., 2015), although the precise molecular mechanisms underlying this phenotype remain unclear. Our current understanding of the role of astrocyte-derived signals in regulation of BBB physiology is very limited, despite some early studies claiming that astrocytes are crucial for BBB development. Because astrocytes differentiate relatively late, they do not play any role in barrier induction (Holash et al., 1993; Saunders et al., 2016); however, they are crucial for BBB maintenance. For example, astrocyte-derived Sonic hedgehog (Shh) is necessary for maintaining endothelial quiescence in the CNS (Alvarez et al., 2011). Finally, microglia in the brain have been shown to function as cellular chaperones that mediate anastomosis of vascular sprouts in response to a VEGFA gradient (Fantin et al., 2010). Consistent with this idea are observations that microglia-deficient brains exhibit significantly reduced vascular branching (Fantin et al., 2010).
Glial-mediated regulation of angiogenesis and barriergenesis in the retina
As in the forebrain, glial cells regulate several aspects of retinal angiogenesis by secreting both pro- and anti-angiogenic factors (Fig. 4B). Astrocytes and Müller glia have been shown to positively regulate retinal angiogenesis by secreting VEGFA (Fig. 4B) (Gerhardt et al., 2003; Stone et al., 1995). Genetic mutations that delay astrocyte migration through the malformed inner limiting membrane of the retina [e.g. global deletion of either laminin γ1 chain (Edwards et al., 2011) or β2 chain, or β2 and γ3 chains together (Gnanaguru et al., 2013)] slow down superficial vascular plexus expansion. In addition to exerting effects on vascular growth, the astrocyte-derived matrix mediates interactions with microglia in the retina. This interaction modulates microglial density and activation state, which in turn regulate vascular branching and endothelial cell proliferation, respectively. Resting microglial cells predominantly secrete TGFβ1, which inhibits CNS endothelial cell proliferation; in contrast, activated microglia produce TNFα and induce proliferation in brain endothelial cells (a similar mechanism may also operate in retinal endothelial cells) (Biswas et al., 2017; Welser et al., 2010). Resting microglia also secrete the non-canonical Wnt ligands Wnt5a and Wnt11 to restrict vascular branching in the deep vascular plexus (Stefater et al., 2011). Müller glia also regulate the formation of deeper vascular beds by secreting several factors in addition to VEGFA (Stone et al., 1995). Müller glia- and neuron-specific deletion of integrin β8 leads to hyperbranching and hyperproliferation of superficial vessels, as well as severely impaired deep vascular plexus development, most likely due to altered TGFβ1 activation (Arnold et al., 2012).
The canonical Wnt pathway also controls retinal angiogenesis and BRB development. While angiogenesis and barrier formation in most brain regions are promoted by the Wnt7a/Wnt7b-Frizzled (Fzd)-Lrp5/6 ligand-receptor complex, these functions in the retina and cerebellum are provided by the norrin-Fzd4-Lrp5/Lrp6 signaling module (Wang et al., 2012; Ye et al., 2009; Zhou et al., 2014). In the retina, norrin is secreted by Müller glia and binds to its high-affinity receptor Fzd4, which is expressed by retinal neurons as well as endothelial cells (Ye et al., 2009). Loss-of-function studies for Ndp (encoding Norrin), Fzd4, Lrp5, Lrp6 and Tspan12 (an obligatory co-receptor) in the retina show delayed growth of the superficial vascular plexus and loss of the deep vascular plexus (Chen et al., 2012; Junge et al., 2009; Luhmann et al., 2005; Xia et al., 2010; Xu et al., 2004; Ye et al., 2009; Zuercher et al., 2012). Inhibiting norrin/Fzd4/Lrp5/Lrp6 signaling, either genetically or using neutralizing antibodies, also results in loss of BRB integrity (Paes et al., 2011; Wang et al., 2012). Therefore, Muller glia-derived Norrin is crucial for both retinal angiogenesis and BRB maturation, similar to the role of Wnt7a/Wnt7b in angiogenesis and BBB maturation in the cerebral cortex (Fig. 4A,B).
Neuronal regulation of brain angiogenesis
It is generally thought that the metabolic demand of neurons drives CNS angiogenesis, although the factors mediating this process remain unclear. One factor that does appear to play an important role is VEGFA. VEGFA is primarily expressed by neurons during early postnatal development in the rat brain, whereas astrocytes begin to express VEGFA somewhat later in development (Fig. 4A) (Ogunshola et al., 2000). This scenario is probably similar in the mouse brain, although one study reported that expression levels of distinct VEGFA isoforms do not significantly change between E13 and P30 in the mouse brain (Ng et al., 2001). Furthermore, a recent study in zebrafish mechanistically explored the link between neurogenesis and angiogenesis in the brain, demonstrating that miR-9 links neurogenesis and angiogenesis via expression of VEGFA by neurons (Madelaine et al., 2017).
Neuronal regulation of retinal angiogenesis
Owing to its relatively simple architecture and postnatal vascular development, the retina has been used extensively to examine neuronal contributions to angiogenesis. Several studies have shown that multiple neuronal subtypes, including ganglion cells (Sapieha et al., 2008), photoreceptors (Joyal et al., 2016) and amacrine cells (Usui et al., 2015), positively regulate retinal angiogenesis by secreting VEGFA (Fig. 4B). VEGF receptor 2 (VEGFR2) is also expressed by both neurons and endothelial cells, and is used by retinal neurons to titrate the amount of VEGFA available for proper angiogenesis. Accordingly, neuronal-specific deletion of VEGFR2 causes misdirected vascular growth toward retinal neurons with increased perineuronal vascular density due to excess VEGFA (Okabe et al., 2014). VEGFA is also produced by ganglion cells in response to hypoxia. Hypoxic conditions in the retina lead to accumulation of succinate, a Krebs cycle intermediate, which binds to the GPR91 receptor expressed by retinal ganglion cells (RGCs), leading to VEGFA production (Sapieha et al., 2008). In line with these findings, the retinal vasculature fails to grow properly and embryonic hyaloid vessels fail to regress in RGC-deficient retinas (Edwards et al., 2012; Rao et al., 2013; Sapieha et al., 2008). As VEGFA is secreted by multiple cell types in the retina (Fig. 4B), a recent study sought to resolve the roles of astrocyte-derived versus neuronal/Müller glia-derived VEGFA in retinal vascular development. Using various cell-specific Cre lines, it was shown that deletion of VEGFA in astrocytes perturbs the growth of superficial plexus. In contrast, deletion of VEGFA in neurons and Müller glia impairs the growth of the deep plexus, leaving superficial plexus growth unaffected (Rattner et al., 2019). Therefore, VEGFA derived from distinct sources drives development of distinct vascular plexuses in the retina. Interestingly, in severe ischemia, severely hypoxic RGCs express repellent molecules, such as semaphorins 3A (Joyal et al., 2011) and 3E (Fukushima et al., 2011), rather than VEGFA, which prevent vascular growth into hypoxic zones in the retina (Fig. 4B). These observations suggest that neurons either promote or inhibit retinal angiogenesis, depending on tissue physiology.
The Robo-Slit signaling pathway has also been implicated in regulating retinal angiogenesis (Rama et al., 2015). Slit1 is expressed by retinal horizontal cells, whereas Slit2 is expressed by bipolar cells and some amacrine cells (Fig. 4B). Loss of Slit2 or both Slit1/2 leads to reduced vascular coverage and branching density in the superficial plexus (Rama et al., 2015). Robo4, and to a lesser extent Robo1 (both of which are Slit receptors), are normally expressed by retinal endothelial cells. Loss of Robo1 along with endothelial cell-specific deletion of Robo2 (as Robo1−/− endothelial cells upregulate Robo2 expression) causes a vascular phenotype similar to that observed in Slit1/2-deficient retinas (Rama et al., 2015). Another pathway that has recently emerged as a regulator of retinal angiogenesis is the reelin-ApoER2-Dab1 signaling pathway. Reelin is predominantly expressed by RGCs (Rice et al., 2001; Segarra et al., 2018) as well as amacrine and bipolar cells (Rice et al., 2001) (Fig. 4B). In contrast, ApoER2 (a reelin receptor) and Dab1 (an intracellular mediator of reelin signaling) are expressed by retinal endothelial cells. Together, these factors form a reelin-ApoER2-Dab1 signaling axis that exerts a pro-angiogenic effect in the retina via interactions between ApoER2 and VEGFR2 (Segarra et al., 2018).
The interplay between neuronal activity and CNS angiogenesis/barriergenesis
As discussed above, most studies examining the interdependence between neuronal and vascular components within the CNS have focused on the molecular crosstalk between neural progenitors or mature neurons and vascular growth and BBB/BRB specification and/or maturation. However, there is a significant overlap in the developmental timeline of synaptogenesis and the establishment of neuronal activity with neurovascular barrier formation in both the brain and retina (Fig. 3A,B), implicating a potential causal relationship between neuronal activity and vascular barrier properties. Below, we discuss some recent studies that have begun to investigate this important link.
Brain
Chronic sensory and behavioral stimulations have been shown to drastically reduce postnatal angiogenesis in the mouse cortex by decreasing both vascular sprouting and endothelial cell proliferation (Whiteus et al., 2014). These vascular deficits persist for a prolonged time after hyperstimulation has stopped in the cortex, suggesting the presence of a crucial period during which neural activity may affect CNS angiogenesis (Whiteus et al., 2014). However, another study in the mouse barrel cortex reported that enhanced neuronal activity increases, rather than reduces, vascular density and branching, and conversely that cortical angiogenesis is inhibited when sensory input is reduced (Lacoste et al., 2014). These apparently contradictory findings in the brain suggest that the effects of neuronal activity on postnatal cortical angiogenesis may depend on a critical developmental period. The relationship between neuronal activity and BBB development was also explored in a study focusing on insulin-like growth factor 1 (IGF1), which crosses the BBB via transcytosis (Nishijima et al., 2010). This study showed that increasing local glutamatergic neuronal activity by electrical, sensory and behavioral stimulation upregulates transcytosis of IGF1 across the BBB in stimulated regions of the brain by increasing the activity of matrix metalloprotease 9 (MMP9) (Nishijima et al., 2010).
Retina
Similar to the situation in the brain, neuronal activity in the retina – in this case, fetal light responsiveness by intrinsically photosensitive melanopsin (Opn4) ganglion cells – is a crucial regulator of angiogenesis. Both Opn4−/− mice and mice reared in the dark, in which melanopsin ganglion cells are silent from E16/17 until P8, exhibit delayed hyaloid vessel regression and overgrowth of the retinal vasculature (Rao et al., 2013). A recent study showed that transient pharmacological blockade of cholinergic waves leads to a leaky paracellular BRB, possibly by decreasing norrin/β-catenin signaling (Weiner et al., 2019). However, a thorough molecular understanding of how cholinergic and glutamatergic waves or light-dependent synaptic activity regulates BRB maturation is lacking. These limited studies nevertheless indicate that neuronal activity and neurovascular barrier development depend on each other, an important finding that remains to be further explored in the future.
Conclusions
As outlined in this Review, multiple studies have established the interdependence between neuronal and glial cell types, and vascular components of the CNS. What is lacking, however, is a detailed understanding of how neuronal activity regulates CNS vascular barrier properties. Moving forward, it will be crucial to explore the effects of sensory, behavioral and motor stimulation of neurons on CNS angiogenesis and neurovascular barrier properties. The availability of single-cell RNA sequencing approaches, coupled with both genetic and pharmacological perturbations of synaptic transmission and optogenetic manipulation of specific neural circuits, renders feasible the identification of signaling pathways that are triggered in neurons and glial cells to modulate barrier function under such conditions. Mouse models in which specific neuronal classes (e.g. rods or cones of the retina) are absent will also be valuable for dissecting the roles of specific neuronal pathways in regulating vascular barrier properties. However, in the latter case, care must be taken to distinguish between the effects of neuronal activity per se and neuronal-derived factors that are independent of activity. Such studies will not only strengthen our understanding of these complex developmental mechanisms, but also provide us with new avenues for ameliorating many neurological diseases of the brain and eye that are characterized by aberrant neuroglial and vascular function.
Acknowledgements
We thank Tyler Cutforth for scientific and editorial feedback on the Review.
Footnotes
Competing interests
The authors declare that they have no relevant affiliation or competing financial interests with any organization or entity having a financial interest or conflict with materials discussed in the manuscript.
Funding
S.B., A.C. and D.A. are supported by grants from the National Institutes of Health (R01 MH112849, R01 NS107344, RF1 AG054023), Fondation Leducq (FDNLEDQ-15CVD-02) and National Multiple Sclerosis Society (RG-1901-33218). D.A. and S.B. are partially supported by unrestricted gifts from both John. F. Castle, Newport Equities and the PANDAS Network to the Division of Stroke and Cerebrovascular Diseases, Department of Neurology at Columbia University Irving Medical Center. Deposited in PMC for release after 12 months.
References
- Alvarez J. I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P. J., Terouz S., Sabbagh M., Wosik K., Bourbonniere L., Bernard M. et al. (2011). The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727-1731. 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- Armulik A., Genové G., Mäe M., Nisancioglu M. H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K. et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557-561. 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- Arnold T. D., Ferrero G. M., Qiu H., Phan I. T., Akhurst R. J., Huang E. J. and Reichardt L. F. (2012). Defective retinal vascular endothelial cell development as a consequence of impaired integrin alphaVbeta8-mediated activation of transforming growth factor-beta. J. Neurosci. 32, 1197-1206. 10.1523/JNEUROSCI.5648-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T. D., Niaudet C., Pang M.-F., Siegenthaler J., Gaengel K., Jung B., Ferrero G. M., Mukouyama Y. S., Fuxe J., Akhurst R. et al. (2014). Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development 141, 4489-4499. 10.1242/dev.107193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader B. L., Rayburn H., Crowley D. and Hynes R. O. (1998). Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell 95, 507-519. 10.1016/S0092-8674(00)81618-9 [DOI] [PubMed] [Google Scholar]
- Baeten K. M. and Akassoglou K. (2011). Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 71, 1018-1039. 10.1002/dneu.20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N., Bandyopadhyay C., Coon B. G., Yun S. and Schwartz M. A. (2016). Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Invest. 126, 821-828. 10.1172/JCI83083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A., Singer J. H., Hwang B. J., Xu W., Beaudet A. and Feller M. B. (2000). Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 20, 7672-7681. 10.1523/JNEUROSCI.20-20-07672.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar O. A., Fuentealba L. C., Alvarez-Buylla A. and Rowitch D. H. (2014). Astrocyte development and heterogeneity. Cold Spring Harb. Perspect Biol. 7, a020362 10.1101/cshperspect.a020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A., Lacoste B., Kur E., Andreone B. J., Mayshar Y., Yan H. and Gu C. (2014). Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507-511. 10.1038/nature13324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz F., Wichitnaowarat V., Lehmann M., Germano R. F., Mihova D., Macas J., Adams R. H., Taketo M. M., Plate K. H., Guerit S. et al. (2019). Low wnt/beta-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. Elife 8, e43818 10.7554/eLife.43818.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S., Bachay G., Chu J., Hunter D. D. and Brunken W. J. (2017). Laminin-dependent interaction between astrocytes and microglia: a role in retinal angiogenesis. Am. J. Pathol. 187, 2112-2127. 10.1016/j.ajpath.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S., Watters J., Bachay G., Varshney S., Hunter D. D., Hu H. and Brunken W. J. (2018). Laminin-dystroglycan signaling regulates retinal arteriogenesis. FASEB J. 32, fj201800232R 10.1096/fj.201800232R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship A. G., Ford K. J., Johnson J., Seal R. P., Edwards R. H., Copenhagen D. R. and Feller M. B. (2009). Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron 62, 230-241. 10.1016/j.neuron.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A. M., Jones H. C. and Abbott N. J. (1990). Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J. Physiol. 429, 47-62. 10.1113/jphysiol.1990.sp018243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts T., Green M. J. and Wingate R. J. T. (2014). Development of the cerebellum: simple steps to make a ‘little brain’. Development 141, 4031-4041. 10.1242/dev.106559 [DOI] [PubMed] [Google Scholar]
- Chan-Ling T., Chu Y., Baxter L., Weible Ii M. and Hughes S. (2009). In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia 57, 39-53. 10.1002/glia.20733 [DOI] [PubMed] [Google Scholar]
- Chen J., Stahl A., Krah N. M., Seaward M. R., Joyal J.-S., Juan A. M., Hatton C. J., Aderman C. M., Dennison R. J., Willett K. L. et al. (2012). Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS ONE 7, e30203 10.1371/journal.pone.0030203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-L., Yao Y., Norris E. H., Kruyer A., Jno-Charles O., Akhmerov A. and Strickland S. (2013). Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol. 202, 381-395. 10.1083/jcb.201212032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B. W. and Gu C. (2015). The molecular constituents of the blood-brain barrier. Trends Neurosci. 38, 598-608. 10.1016/j.tins.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow B. W. and Gu C. (2017). Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron 93, 1325-1333.e1323. 10.1016/j.neuron.2017.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GBD 2016 Dementia (2019). Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 18, 88-106. 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M., Orsenigo F., Bhat G. P., Conze L. L., Breviario F., Cunha S. I., Claesson-Welsh L., Beznoussenko G. V., Mironov A. A., Bacigaluppi M. et al. (2019). Fine-tuning of Sox17 and canonical Wnt coordinates the permeability properties of the blood-brain barrier. Circ. Res. 124, 511-525. 10.1161/CIRCRESAHA.118.313316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Agalliu D., Zhou L., Kuhnert F., Kuo C. J. and Barres B. A. (2009). Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 106, 641-646. 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A. A. and Barres B. A. (2010). Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562-566. 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. M., McLeod D. S., Grebe R., Heng C., Lefebvre O. and Lutty G. A. (2011). Lama1 mutations lead to vitreoretinal blood vessel formation, persistence of fetal vasculature, and epiretinal membrane formation in mice. BMC Dev. Biol. 11, 60 10.1186/1471-213X-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. M., McLeod D. S., Li R., Grebe R., Bhutto I., Mu X. and Lutty G. A. (2012). The deletion of Math5 disrupts retinal blood vessel and glial development in mice. Exp. Eye Res. 96, 147-156. 10.1016/j.exer.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B. and Liebner S. (2014). Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res. 355, 687-699. 10.1007/s00441-014-1811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa J. S. and Luo L. (2008). Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J. Neurosci. 28, 2301-2312. 10.1523/JNEUROSCI.5157-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A., Vieira J. M., Gestri G., Denti L., Schwarz Q., Prykhozhij S., Peri F., Wilson S. W. and Ruhrberg C. (2010). Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829-840. 10.1182/blood-2009-12-257832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I. and Allen N. J. (2018). Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 13, 7 10.1186/s13064-018-0104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Okada M., Kataoka H., Hirashima M., Yoshida Y., Mann F., Gomi F., Nishida K., Nishikawa S.-I. and Uemura A. (2011). Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Invest. 121, 1974-1985. 10.1172/JCI44900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam J., Zhang X. and Yao Y. (2016). The role of pericytic laminin in blood brain barrier integrity maintenance. Sci. Rep. 6, 36450 10.1038/srep36450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D. et al. (2003). VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163-1177. 10.1083/jcb.200302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanaguru G., Bachay G., Biswas S., Pinzon-Duarte G., Hunter D. D. and Brunken W. J. (2013). Laminins containing the beta2 and gamma3 chains regulate astrocyte migration and angiogenesis in the retina. Development 140, 2050-2060. 10.1242/dev.087817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould D. B., Phalan F. C., Breedveld G. J., van Mil S. E., Smith R. S., Schimenti J. C., Aguglia U., van der Knaap M. S., Heutink P. and John S. W. (2005). Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 308, 1167-1171. 10.1126/science.1109418 [DOI] [PubMed] [Google Scholar]
- Haigh J. J., Morelli P. I., Gerhardt H., Haigh K., Tsien J., Damert A., Miquerol L., Muhlner U., Klein R., Ferrara N. et al. (2003). Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 262, 225-241. 10.1016/S0012-1606(03)00356-7 [DOI] [PubMed] [Google Scholar]
- Haj-Yasein N. N., Vindedal G. F., Eilert-Olsen M., Gundersen G. A., Skare O., Laake P., Klungland A., Thoren A. E., Burkhardt J. M., Ottersen O. P. et al. (2011). Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc. Natl. Acad. Sci. USA 108, 17815-17820. 10.1073/pnas.1110655108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann R., Mayer D. N., Berg E. L., Broermann R. and Butcher E. C. (1995). Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev. Dyn. 202, 325-332. 10.1002/aja.1002020402 [DOI] [PubMed] [Google Scholar]
- Harb R., Whiteus C., Freitas C. and Grutzendler J. (2013). In vivo imaging of cerebral microvascular plasticity from birth to death. J. Cereb. Blood Flow Metab. 33, 146-156. 10.1038/jcbfm.2012.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S., Liu Q., Lee H. S., Hossain M. G., Lacy-Hulbert A. and McCarty J. H. (2011). The astrocyte-expressed integrin αvβ8 governs blood vessel sprouting in the developing retina. Development 138, 5157-5166. 10.1242/dev.069153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S., Clements T. P., Tang L. K., Morales J. E., Lee H. S., Oh S. P., Rivera G. M., Wagner D. S. and McCarty J. H. (2015). Neuropilin 1 balances β8 integrin-activated TGFbeta signaling to control sprouting angiogenesis in the brain. Development 142, 4363-4373. 10.1242/dev.113746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J. A., Noden D. M. and Stewart P. A. (1993). Re-evaluating the role of astrocytes in blood-brain barrier induction. Dev. Dyn. 197, 14-25. 10.1002/aja.1001970103 [DOI] [PubMed] [Google Scholar]
- Hubbard J. A., Hsu M. S., Seldin M. M. and Binder D. K. (2015). Expression of the astrocyte water channel aquaporin-4 in the mouse brain. ASN Neuro 7, 1759091415605486 10.1177/1759091415605486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe M., Li M. X., Kneitz S., Davydova D., Yokota C., Kele J., Hot B., Stenman J. M. and Gessler M. (2017). Gene expression profiles of brain endothelial cells during embryonic development at bulk and single-cell levels. Sci. Signal. 10, eaag2476. [DOI] [PubMed] [Google Scholar]
- Iadecola C. (2017). The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17-42. 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal J.-S., Sitaras N., Binet F., Rivera J. C., Stahl A., Zaniolo K., Shao Z., Polosa A., Zhu T., Hamel D. et al. (2011). Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 117, 6024-6035. 10.1182/blood-2010-10-311589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal J.-S., Sun Y., Gantner M. L., Shao Z., Evans L. P., Saba N., Fredrick T., Burnim S., Kim J. S., Patel G. et al. (2016). Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat. Med. 22, 439-445. 10.1038/nm.4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge H. J., Yang S., Burton J. B., Paes K., Shu X., French D. M., Costa M., Rice D. S. and Ye W. (2009). TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/β-catenin signaling. Cell 139, 299-311. 10.1016/j.cell.2009.07.048 [DOI] [PubMed] [Google Scholar]
- Knowland D., Arac A., Sekiguchi K. J., Hsu M., Lutz S. E., Perrino J., Steinberg G. K., Barres B. A., Nimmerjahn A. and Agalliu D. (2014). Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82, 603-617. 10.1016/j.neuron.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek K. D., Murphy S., Xu J. and Arias E. (2017). Mortality in the United States, 2016. NCHS Data Brief 293, 1-8. [PubMed] [Google Scholar]
- Lacoste B., Comin C. H., Ben-Zvi A., Kaeser P. S., Xu X., Costa Lda F. and Gu C. (2014). Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron 83, 1117-1130. 10.1016/j.neuron.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-W., Kim W. J., Choi Y. K., Song H. S., Son M. J., Gelman I. H., Kim Y.-J. and Kim K.-W. (2003). SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 9, 900-906. 10.1038/nm889 [DOI] [PubMed] [Google Scholar]
- Lengfeld J. E., Lutz S. E., Smith J. R., Diaconu C., Scott C., Kofman S. B., Choi C., Walsh C. M., Raine C. S., Agalliu I. et al. (2017). Endothelial Wnt/beta-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc. Natl. Acad. Sci. USA 114, E1168-E1177. 10.1073/pnas.1609905114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Lan Y., Wang Y., Wang J., Yang G., Meng F., Han H., Meng A., Wang Y. and Yang X. (2011). Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev. Cell 20, 291-302. 10.1016/j.devcel.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C. J., Reis M., Felici A., Wolburg H., Fruttiger M. et al. (2008). Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409-417. 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S., Dijkhuizen R. M., Reiss Y., Plate K. H., Agalliu D. and Constantin G. (2018). Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 135, 311-336. 10.1007/s00401-018-1815-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegl R., Hellstrom A. and Smith L. E. (2016). Retinopathy of prematurity: the need for prevention. Eye Brain 8, 91-102. 10.2147/EB.S99038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann U. F. O., Lin J., Acar N., Lammel S., Feil S., Grimm C., Seeliger M. W., Hammes H.-P. and Berger W. (2005). Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest. Ophthalmol. Vis. Sci. 46, 3372-3382. 10.1167/iovs.05-0174 [DOI] [PubMed] [Google Scholar]
- Lutz S. E., Smith J. R., Kim D. H., Olson C. V. L., Ellefsen K., Bates J. M., Gandhi S. P. and Agalliu D. (2017). Caveolin1 is required for Th1 cell infiltration, but not tight junction remodeling, at the blood-brain barrier in autoimmune neuroinflammation. Cell Rep. 21, 2104-2117. 10.1016/j.celrep.2017.10.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Kwon H. J. and Huang Z. (2012). A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS ONE 7, e48001 10.1371/journal.pone.0048001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Kwon H. J., Johng H., Zang K. and Huang Z. (2013). Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling. PLoS Biol. 11, e1001469 10.1371/journal.pbio.1001469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelaine R., Sloan S. A., Huber N., Notwell J. H., Leung L. C., Skariah G., Halluin C., Paşca S. P., Bejerano G., Krasnow M. A. et al. (2017). MicroRNA-9 couples brain neurogenesis and angiogenesis. Cell Rep. 20, 1533-1542. 10.1016/j.celrep.2017.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S., Andreu A., Mecklenburg N. and Echevarria D. (2013). Cellular and molecular basis of cerebellar development. Front. Neuroanat. 7, 18 10.3389/fnana.2013.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martowicz A., Trusohamn M., Jensen N., Wisniewska-Kruk J., Corada M., Ning F. C., Kele J., Dejana E. and Nyqvist D. (2019). Endothelial β-catenin signaling supports postnatal brain and retinal angiogenesis by promoting sprouting, tip cell formation, and VEGFR (Vascular Endothelial Growth Factor Receptor) 2 expression. Arterioscler. Thromb. Vasc. Biol. 39, 2273-2288. 10.1161/ATVBAHA.119.312749 [DOI] [PubMed] [Google Scholar]
- Martynoga B., Drechsel D. and Guillemot F. (2012). Molecular control of neurogenesis: a view from the mammalian cerebral cortex. Cold Spring Harb. Perspect Biol. 4, a008359 10.1101/cshperspect.a008359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni J., Smith J. R., Shahriar S., Cutforth T., Ceja B. and Agalliu D. (2017). The Wnt inhibitor apcdd1 coordinates vascular remodeling and barrier maturation of retinal blood vessels. Neuron 96, 1055-1069.e1056. 10.1016/j.neuron.2017.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes M. J., McClenahan F. K., Leiton C. V., Aranmolate A., Shan X. and Colognato H. (2014). The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J. Neurosci. 34, 15260-15280. 10.1523/JNEUROSCI.3678-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha S., Valloton D., Brunet I., Eichmann A., Hornung J. P. and Lebrand C. (2015). NG2 glia are required for vessel network formation during embryonic development. Elife 4, e09102 10.7554/eLife.09102.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley A. K., Tchaicha J. H., Shin J., Hossain M. G. and McCarty J. H. (2009). Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J. Cell Sci. 122, 1842-1851. 10.1242/jcs.043257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y.-S., Rohan R., Sunday M. E., Demello D. E. and D'Amore P. A. (2001). Differential expression of VEGF isoforms in mouse during development and in the adult. Dev. Dyn. 220, 112-121. [DOI] [PubMed] [Google Scholar]
- Nguyen H.-L., Lee Y. J., Shin J., Lee E., Park S. O., McCarty J. H. and Oh S. P. (2011). TGF-beta signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab. Investig. 91, 1554-1563. 10.1038/labinvest.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia G. P., Pisani F., Simone L., Cibelli A., Mola M. G., Dal Monte M., Frigeri A., Bagnoli P. and Svelto M. (2016). Glio-vascular modifications caused by Aquaporin-4 deletion in the mouse retina. Exp. Eye Res. 146, 259-268. 10.1016/j.exer.2016.03.019 [DOI] [PubMed] [Google Scholar]
- Nirwane A. and Yao Y. (2018). Laminins and their receptors in the CNS. Biol. Rev. Camb. Philos. Soc. 94, 283-306. 10.1111/brv.12454 [DOI] [PubMed] [Google Scholar]
- Nirwane A., Johnson J., Nguyen B., Miner J. H. and Yao Y. (2019). Mural cell-derived laminin-α5 plays a detrimental role in ischemic stroke. Acta. Neuropathol. Commun. 7, 23 10.1186/s40478-019-0676-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T., Piriz J., Duflot S., Fernandez A. M., Gaitan G., Gomez-Pinedo U., Verdugo J. M. G., Leroy F., Soya H., Nuñez A. et al. (2010). Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 67, 834-846. 10.1016/j.neuron.2010.08.007 [DOI] [PubMed] [Google Scholar]
- Ogunshola O. O., Stewart W. B., Mihalcik V., Solli T., Madri J. A. and Ment L. R. (2000). Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res. Dev. Brain Res. 119, 139-153. 10.1016/S0165-3806(99)00125-X [DOI] [PubMed] [Google Scholar]
- Okabe K., Kobayashi S., Yamada T., Kurihara T., Tai-Nagara I., Miyamoto T., Mukouyama Y.-S., Sato T. N., Suda T., Ema M. et al. (2014). Neurons limit angiogenesis by titrating VEGF in retina. Cell 159, 584-596. 10.1016/j.cell.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Paes K. T., Wang E., Henze K., Vogel P., Read R., Suwanichkul A., Kirkpatrick L. L., Potter D., Newhouse M. M. and Rice D. S. (2011). Frizzled 4 is required for retinal angiogenesis and maintenance of the blood-retina barrier. Invest. Ophthalmol. Vis. Sci. 52, 6452-6461. 10.1167/iovs.10-7146 [DOI] [PubMed] [Google Scholar]
- Park D. Y., Lee J., Kim J., Kim K., Hong S., Han S., Kubota Y., Augustin H. G., Ding L., Kim J. W. et al. (2017). Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 8, 15296 10.1038/ncomms15296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokofyeva E. and Zrenner E. (2012). Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 47, 171-188. 10.1159/000329603 [DOI] [PubMed] [Google Scholar]
- Qin Y., Garrison B. S., Ma W., Wang R., Jiang A., Li J., Mistry M., Bronson R. T., Santoro D., Franco C. et al. (2018). A milieu molecule for TGF-beta required for microglia function in the nervous system. Cell 174, 156-171.e116. 10.1016/j.cell.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S., Beck H., Gaumann A., Yüce A., Gerber H.-P., Plate K., Hammes H.-P., Ferrara N. and Breier G. (2004). Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 91, 595-605. 10.1160/TH03-09-0582 [DOI] [PubMed] [Google Scholar]
- Rama N., Dubrac A., Mathivet T., Ní Chárthaigh R.-A., Genet G., Cristofaro B., Pibouin-Fragner L., Ma L., Eichmann A. and Chedotal A. (2015). Slit2 signaling through Robo1 and Robo2 is required for retinal neovascularization. Nat. Med. 21, 483-491. 10.1038/nm.3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Chun C., Fan J., Kofron J. M., Yang M. B., Hegde R. S., Ferrara N., Copenhagen D. R. and Lang R. A. (2013). A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature 494, 243-246. 10.1038/nature11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport D. H., Wong L. L., Wood E. D., Yasumura D. and LaVail M. M. (2004). Timing and topography of cell genesis in the rat retina. J. Comp. Neurol. 474, 304-324. 10.1002/cne.20134 [DOI] [PubMed] [Google Scholar]
- Rattner A., Williams J. and Nathans J. (2019). Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Invest. 129, 3807-3820. 10.1172/JCI126655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyahi A., Nik A. M., Ghiami M., Gritli-Linde A., Pontén F., Johansson B. R. and Carlsson P. (2015). Foxf2 is required for brain pericyte differentiation and development and maintenance of the blood-brain barrier. Dev. Cell 34, 19-32. 10.1016/j.devcel.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Rice D. S., Nusinowitz S., Azimi A. M., Martinez A., Soriano E. and Curran T. (2001). The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron 31, 929-941. 10.1016/S0896-6273(01)00436-6 [DOI] [PubMed] [Google Scholar]
- Rowitch D. H. and Kriegstein A. R. (2010). Developmental genetics of vertebrate glial-cell specification. Nature 468, 214-222. 10.1038/nature09611 [DOI] [PubMed] [Google Scholar]
- Runkle E. A. and Antonetti D. A. (2011). The blood-retinal barrier: structure and functional significance. Methods Mol. Biol. 686, 133-148. 10.1007/978-1-60761-938-3_5 [DOI] [PubMed] [Google Scholar]
- Sapieha P., Sirinyan M., Hamel D., Zaniolo K., Joyal J.-S., Cho J.-H., Honoré J.-C., Kermorvant-Duchemin E., Varma D. R., Tremblay S. et al. (2008). The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 14, 1067-1076. 10.1038/nm.1873 [DOI] [PubMed] [Google Scholar]
- Saunders N. R., Dziegielewska K. M., Unsicker K. and Ek C. J. (2016). Delayed astrocytic contact with cerebral blood vessels in FGF-2 deficient mice does not compromise permeability properties at the developing blood-brain barrier. Dev. Neurobiol. 76, 1201-1212. 10.1002/dneu.22383 [DOI] [PubMed] [Google Scholar]
- Segarra M., Aburto M. R., Cop F., Llao-Cid C., Hartl R., Damm M., Bethani I., Parrilla M., Husainie D., Schanzer A. et al. (2018). Endothelial Dab1 signaling orchestrates neuro-glia-vessel communication in the central nervous system. Science 361, eaao2861 10.1126/science.aao2861 [DOI] [PubMed] [Google Scholar]
- Sekaran S., Lupi D., Jones S. L., Sheely C. J., Hattar S., Yau K.-W., Lucas R. J., Foster R. G. and Hankins M. W. (2005). Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr. Biol. 15, 1099-1107. 10.1016/j.cub.2005.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira M., Francis D., Gisbert D., Gomes F. C. A. and Stipursky J. (2018). Radial glia cells control angiogenesis in the developing cerebral cortex through TGF-beta1 signaling. Mol. Neurobiol. 55, 3660-3675. 10.1007/s12035-017-0557-8 [DOI] [PubMed] [Google Scholar]
- Sixt M., Engelhardt B., Pausch F., Hallmann R., Wendler O. and Sorokin L. M. (2001). Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J. Cell Biol. 153, 933-946. 10.1083/jcb.153.5.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J. I., Bell J. S. and DeLuca G. C. (2018). Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 89, 42-52. 10.1136/jnnp-2017-316011 [DOI] [PubMed] [Google Scholar]
- Stahl A., Connor K. M., Sapieha P., Chen J., Dennison R. J., Krah N. M., Seaward M. R., Willett K. L., Aderman C. M., Guerin K. I. et al. (2010). The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 51, 2813-2826. 10.1167/iovs.10-5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater J. A. III, Lewkowich I., Rao S., Mariggi G., Carpenter A. C., Burr A. R., Fan J., Ajima R., Molkentin J. D., Williams B. O. et al. (2011). Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474, 511-515. 10.1038/nature10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J. M., Rajagopal J., Carroll T. J., Ishibashi M., McMahon J. and McMahon A. P. (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247-1250. 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- Stenzel D., Franco C. A., Estrach S., Mettouchi A., Sauvaget D., Rosewell I., Schertel A., Armer H., Domogatskaya A., Rodin S. et al. (2011). Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 12, 1135-1143. 10.1038/embor.2011.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., Itin A., Alon T., Pe'er J., Gnessin H., Chan-Ling T. and Keshet E. (1995). Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 15, 4738-4747. 10.1523/JNEUROSCI.15-07-04738.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. D., Sagare A. P. and Zlokovic B. V. (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133-150. 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A., Giampietro C., Conti A., Orsenigo F., Breviario F., Pirazzoli V., Potente M., Daly C., Dimmeler S. and Dejana E. (2008). Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 10, 923-934. 10.1038/ncb1752 [DOI] [PubMed] [Google Scholar]
- Thion M. S. and Garel S. (2017). On place and time: microglia in embryonic and perinatal brain development. Curr. Opin. Neurobiol. 47, 121-130. 10.1016/j.conb.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Tiriac A., Smith B. E. and Feller M. B. (2018). Light prior to eye opening promotes retinal waves and eye-specific segregation. Neuron 100, 1059-1065.e1054. 10.1016/j.neuron.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma K. and Hanashima C. (2015). Switching modes in corticogenesis: mechanisms of neuronal subtype transitions and integration in the cerebral cortex. Front. Neurosci. 9, 274 10.3389/fnins.2015.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg C. L. and Feller M. B. (2005). Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 76, 213-235. 10.1016/j.pneurobio.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Usui Y., Westenskow P. D., Kurihara T., Aguilar E., Sakimoto S., Paris L. P., Wittgrove C., Feitelberg D., Friedlander M. S., Moreno S. K. et al. (2015). Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Invest. 125, 2335-2346. 10.1172/JCI80297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ameele J., Tiberi L., Vanderhaeghen P. and Espuny-Camacho I. (2014). Thinking out of the dish: what to learn about cortical development using pluripotent stem cells. Trends Neurosci. 37, 334-342. 10.1016/j.tins.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mäe M. A., Andrae J., Ando K., Del Gaudio F., Nahar K., Lebouvier T., Lavina B., Gouveia L. et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475-480. 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- Varshney S., Hunter D. D. and Brunken W. J. (2015). Extracellular matrix components regulate cellular polarity and tissue structure in the developing and mature retina. J. Ophthalmic. Vis. Res. 10, 329-339. 10.4103/2008-322X.170354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez Y. and Huber P. (2008). Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta 1778, 794-809. 10.1016/j.bbamem.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Wallin M. T., Culpepper W. J., Campbell J. D., Nelson L. M., Langer-Gould A., Marrie R. A., Cutter G. R., Kaye W. E., Wagner L., Tremlett H. et al. (2019). The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 92, e1029-e1040. 10.1212/WNL.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Rattner A., Zhou Y., Williams J., Smallwood P. M. and Nathans J. (2012). Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332-1344. 10.1016/j.cell.2012.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Sabbagh M. F., Gu X., Rattner A., Williams J. and Nathans J. (2019). Beta-catenin signaling regulates barrier-specific gene expression in circumventricular organ and ocular vasculatures. Elife 8, e43257 10.7554/eLife.43257.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner G. A., Shah S. H., Angelopoulos C. M., Bartakova A. B., Pulido R. S., Murphy A., Nudleman E., Daneman R. and Goldberg J. L. (2019). Cholinergic neural activity directs retinal layer-specific angiogenesis and blood retinal barrier formation. Nat. Commun. 10, 2477 10.1038/s41467-019-10219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welser J. V., Li L. and Milner R. (2010). Microglial activation state exerts a biphasic influence on brain endothelial cell proliferation by regulating the balance of TNF and TGF-beta1. J. Neuroinflammation 7, 89 10.1186/1742-2094-7-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteus C., Freitas C. and Grutzendler J. (2014). Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature 505, 407-411. 10.1038/nature12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg-Buchholz K., Mack A. F., Steiner E., Pfeiffer F., Engelhardt B. and Wolburg H. (2009). Loss of astrocyte polarity marks blood-brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol. 118, 219-233. 10.1007/s00401-009-0558-4 [DOI] [PubMed] [Google Scholar]
- Xia C.-H., Yablonka-Reuveni Z. and Gong X. (2010). LRP5 is required for vascular development in deeper layers of the retina. PLoS ONE 5, e11676 10.1371/journal.pone.0011676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Wang Y., Dabdoub A., Smallwood P. M., Williams J., Woods C., Kelley M. W., Jiang L., Tasman W., Zhang K. et al. (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883-895. 10.1016/S0092-8674(04)00216-8 [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Ehling M., Kato K., Kanai K., van Lessen M., Frye M., Zeuschner D., Nakayama M., Vestweber D. and Adams R. H. (2015). Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat. Commun. 6, 6429 10.1038/ncomms7429 [DOI] [PubMed] [Google Scholar]
- Yao Y., Chen Z.-L., Norris E. H. and Strickland S. (2014). Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 5, 3413 10.1038/ncomms4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Wang Y., Cahill H., Yu M., Badea T. C., Smallwood P. M., Peachey N. S. and Nathans J. (2009). Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139, 285-298. 10.1016/j.cell.2009.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Nelson A. R., Betsholtz C. and Zlokovic B. V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064-1078. 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. and Nathans J. (2014). Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev. Cell 31, 248-256. 10.1016/j.devcel.2014.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang Y., Tischfield M., Williams J., Smallwood P. M., Rattner A., Taketo M. M. and Nathans J. (2014). Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 124, 3825-3846. 10.1172/JCI76431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein A. C., Luque A., Turlo K. A., Hofmann J. J., Yee K. M., Becker M. S., Fassler R., Mellman I., Lane T. F. and Iruela-Arispe M. L. (2010). Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev. Cell 18, 39-51. 10.1016/j.devcel.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuercher J., Fritzsche M., Feil S., Mohn L. and Berger W. (2012). Norrin stimulates cell proliferation in the superficial retinal vascular plexus and is pivotal for the recruitment of mural cells. Hum. Mol. Genet. 21, 2619-2630. 10.1093/hmg/dds087 [DOI] [PubMed] [Google Scholar]