Abstract
In recent years, there has been a concerted effort to improve our understanding of the quality and effectiveness of transfused blood components. The expanding use of large datasets built from electronic health records allows the investigation of potential benefits or adverse outcomes associated with transfusion therapy. Together with data collected on blood donors and components, these datasets permit an evaluation of associations between donor or blood component factors and transfusion recipient outcomes. Large linked donor-component recipient datasets provide the power to study exposures relevant to transfusion efficacy and safety, many of which would not otherwise be amenable to study for practical or sample size reasons. Analyses of these large blood banking-transfusion medicine datasets allow for characterization of the populations under study and provide an evidence base for future clinical studies. Knowledge generated from linked analyses have the potential to change the way donors are selected and how components are processed, stored and allocated. However, unrecognized confounding and biased statistical methods continue to be limitations in the study of transfusion exposures and patient outcomes. Results of observational studies of blood donor demographics, storage age, and transfusion practice have been conflicting. This review will summarize statistical and methodological challenges in the analysis of linked blood donor, component, and transfusion recipient outcomes.
In the past three decades, there has been a concerted effort to improve our understanding of the quality, safety, and effectiveness of transfused blood components.1–3 Significant research has focused on blood donors, on component manufacturing and storage, and optimal transfusion practice. Recent laboratory investigations have identified diversity in biochemical and hemolytic parameters in red blood cells (RBCs) related to blood donor sex, age, and race/ethnicity.4,5 In parallel, blood collection and manufacturing factors are known to impact immunomodulatory characteristics and rates of in vitro hemolysis of RBCs.6–8 Lastly, the findings from several randomized clinical trials (RCTs) evaluating RBC transfusions have led to the formulation of clinical practice guidelines in some, albeit not all, patient populations in need of transfusions.9,10
However, interactions between blood donor, component, and recipient factors on transfusion safety and efficacy are not well understood.11 The development of large datasets based on electronic health records has permitted expanded investigation of the potential benefits and adverse outcomes related to transfusion therapy.12–14 Incorporation of information from the blood bank and transfusion services with patient data has allowed examination of associations between blood donor or component characteristics and transfused recipient outcomes (Table 1). These linked donor-component-recipient datasets provide unique opportunities to study exposures relevant to transfusion safety, many of which would otherwise not be amenable to study for practical or sample size reasons. However, it is recognized that these analyses are complex and must account for and address a number of potential confounders that warrant the use of appropriate and often multiple statistical models.15 Given these challenges, it is important that studies be conducted across multiple linked databases to assess the reproducibility and robustness of findings.
Table 1:
Blood Donor, Component, and Recipient Characteristics
|
Donor Characteristics |
| Sex |
| Age |
| Body mass index |
| Race/Ethnicity |
| ABO/Rh-D status |
| RBC antigen phenotype |
| Donor Genetic Polymorphisms |
| History of pregnancy |
| Prior blood donation |
| Iron status |
| Hemoglobin level |
| Comorbidities |
| Tobacco use |
| Medications |
|
Component Characteristics |
| Blood collection method |
| Processing and manufacturing methods |
| Additive solution |
| RBC storage duration |
| Pathogen reduction |
| Gamma irradiation |
|
Transfusion Recipient Characteristics |
| Sex |
| Age |
| Body mass index |
| ABO/Rh-D status |
| RBC Antigen Phenotype |
| Comorbidities and severity of illness |
| Pre-transfusion laboratory values |
| History of transfusion |
| Indication for transfusion |
| Allo-immunization |
| Pulmonary edema |
| Renal failure |
| Hospital length of stay |
| Death |
In this review, we describe analyses of several ‘vein-to-vein’ databases which capture various elements of the blood donor-component-recipient continuum. These datasets encompass information on blood donor characteristics, donation activity, component processing, transfusion events, as well as various clinical and laboratory outcomes of transfused patients. These databases require significant resources to construct and maintain and thus vary in regard to the time period they include and related blood collection, manufacturing, and transfusion practices, the granularity or missingness of available data, as well as rules and regulations regarding privacy and ethics in their development. At the time of this review, databases include cohorts from Canada [Transfusion Research Utilization Surveillance and Tracking (TRUST) and from the Ottawa Hospital Research Institute], the Netherlands [Dutch Transfusion Data (DTD) warehouse], the United States [from Kaiser Permanente Northern California (KPNC) and the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III)], as well as national level data from Denmark and Sweden as part of Scandinavian Donations and Transfusions (SCANDAT).13,14,16–20
Differences in results of RCTs and observational studies hint at the complexity of conducting linked blood donor-component-recipient outcomes analyses. To date, observational studies have focused on the possible deleterious health effects of blood transfusion and have provided the basis for the conduct of RCTs. While RCTs have the advantage of minimizing the effects of confounding by virtue of randomization, they often are inadequately powered to detect small but clinically meaningful differences in outcomes or subgroups and are time consuming and resource intensive to conduct. Inclusion and exclusion criteria may also limit their generalizability by restricting enrolment to a specific group of patients that differ from those more commonly seen in clinical practice. In contrast, observational studies are frequently conducted retrospectively and thus may have limitations in relation to the missingness or accuracy of relevant donor and electronic health record data. Irrespectively, observational studies have repeatedly been used to both generate hypotheses for study in RCTs and to test hypotheses, albeit with weaker evidence given limitations in causal inference. While observational studies usually provide increased statistical power and representation of a wider range of patients, they are often limited by inadequate control of indication bias and other clinical confounding factors. Consequently, for studies of transfusion therapy, association strengths are often overestimated given that transfused patients tend to be sicker than comparable patients who do not receive or require transfusions. Moreover, despite complex and ambitious efforts to control for differences between transfused and non-transfused patients, such comparisons are often fraught with residual confounding as the differences cannot be fully quantified. Lastly, neither observational studies nor RCTs have accounted for the variation in both blood donor demographic and component manufacturing characteristics when assessing the role of blood transfusion on patient outcomes.
Divergent findings between observational studies and RCTs for transfusion are perhaps best exemplified by studies conducted to evaluate the effect of RBC storage duration. A long series of retrospective observational studies found prolonged storage duration to be associated with increased morbidity and mortality in transfused patients.21 In contrast, a number of RCTs did not identify differences in outcomes in pediatric and adult populations.22–26 It is likely that some of the discrepant findings in observational studies and RCTs are due to unmeasured confounding related to the number of transfusions received by a patient, or from inadequate methodological approaches (Figure 1).27,28 In such cases, the association between the transfusion and the outcome is confounded by patient factors that were associated with both RBC storage and the clinical outcome of choice, but where investigators did not adequately control for these variables. It is also recognized that RCTs of RBC storage duration are limited in power to study the effect of red cell units stored between 35 and 42 days given the infrequency of these transfusion events.29 This limitation in power becomes further compounded when one focuses on specific patient populations, such as trauma or chronically transfused patients with hemoglobinopathies. One proposed solution would be to merge data across clinical trials to allow for relevant subgroup analyses which could otherwise not occur due to small sample size.30 Pending such analyses, large, well-conducted observational studies or mechanistic clinical investigations provide us with the best evidence regarding the effectiveness and safety profile of 35- to 42- day old RBC products.31,32
Figure 1:
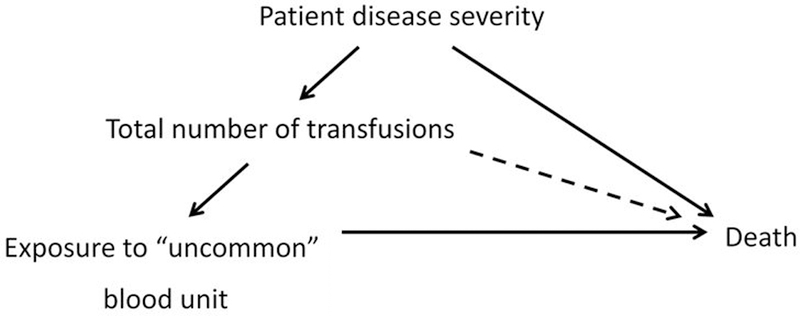
Relationship between patient disease severity, total number of transfusions, blood unit exposures, and death
“Uncommon” blood unit exposures may include RBCs from older or younger donors, previously pregnant donors, or units with prolonged storage duration
In parallel with the study of storage duration, unrecognized confounding may explain some of the discrepancies between observational analyses of blood donor demographics and recipient outcomes.15 It was initially believed that observational analyses of blood donor or component factors and their effect on recipient outcomes would not be prone to confounding. Except for blood group, these factors are not routinely taken into consideration—directly or indirectly—when blood units are allocated. Therefore, exposure to blood from particular donors or component factors, such as the type of additive solution used, is likely to be randomly distributed among recipients. However, analyses of associations between donor or component characteristics and patient outcomes can still be confounded. For example, because RBCs from younger or older blood donors are less abundant than units from middle-age donors, patients who received units from these less common donor age groups will on average have received more transfusions than patients who have not (Figure 1).33 Given that the number of transfusions is a strong risk factor for adverse outcomes such as mortality, appropriately accounting for this is critical analytically. As an example, analysis of donor age, with adjustment for the number of transfusions using a simple log-linear term, may indicate an association between exposure to a rare donor unit and increased recipient mortality (Table 2). However, after appropriate adjustment for the total number of transfusions using more flexible methods with splines, allowing departure from a log-linear trend, the association between donor age and mortality was absent.34 Similar findings were evident when analyzing exposure to RBCs from female donors. These results highlight the critical importance of statistical adjustment for recipient confounding in analyses of blood donor and component factors.
Table 2:
Hazard ratio of death associated with red blood cell transfusions from donors of different age groups
| Donor age | Number of RBC units (%) | Log-Linear adjustment | Restricted cubic spline |
|---|---|---|---|
| Hazard ratio (95% CI) | |||
| <20 years | 126,847 (1.9) | 1.04 (1.03–1.04) | 1.01 (1.00–1.01) |
| 20–29 years | 1,104,248 (16.3) | 1.02 (1.02–1.02) | 0.99 (0.99–1.00) |
| 30–39 years | 1,464,872 (21.6) | 1.00 (1.00–1.00) | 0.99 (0.99–1.00) |
| 40–49 years | 1,889,084 (27.9) | 1.00 (ref) | 1.00 (ref) |
| 50–59 years | 1,600,320 (23.6) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| 60–69 years | 578,194 (8.5) | 1.00 (1.00–1.01) | 1.01 (1.01–1.02) |
| ≥70 years | 3,238 (0.0) | 0.85 (0.83–0.87) | 0.96 (0.91–1.01) |
RBC=red blood cell
Included with permission from: Edgren G, Ullum H, Rostgaard K, Erikstrup C, Sartipy U, Holzmann MJ, Nyrén O, Hjalgrim H. Association of Donor Age and Sex With Survival of Patients Receiving Transfusions.
Despite the large size of many linked-donor recipient databases, they remain subject to bias not only from statistical methodologies but also due to missing data and multiple testing of subgroups. Similar to RCTs, excessive subgroup analysis increases the risk of false-positive findings (Type I error).35 Despite an overall large sample size, analyses of subgroups may produce a biased result when examining an infrequent clinical event, and caution especially needs to be taken when a significant proportion of data is missing.36 For example, in an analysis of transfused patients within the KPNC DR database between 2013 and 2016, there was no association between the number of sex-mismatched RBC transfusions (male donor-derived RBCs transfused to a female recipient OR female donor-derived RBCs transfused to a male recipient) and mortality.37 In contrast, we found an increased risk of mortality for subgroups of male and female recipients who received 3–4 or 5–6 sex-mismatched RBC units. However, this finding did not follow a dose-response pattern, as the association was not present when we examined recipient of 7 or more RBC units. Nor were similar subgroup associations apparent in the much larger REDS-III and SCANDAT cohorts which were studied using an identical analytic approach. In addition, specific patient subgroup analyses may be additionally prone to residual confounding. For example, trauma patients are predominantly young and male (<50 years of age), frequently receive more RBCs, and have higher rates of massive transfusion.38 These patients are also more likely to receive multiple types of blood components and have different mortality rates than the general transfused population. Again, merging data from multiple cohorts or inclusion of national level datasets without missing data may provide additional statistical power to conduct such subgroup analyses.37
With the development of several ‘vein-to-vein databases” we have seen an evolution in methodologic approaches to these analyses. Recent studies have accounted for blood product exposure time-dependently, allowing a recipient’s exposure status to change as they receive additional transfusions.16,17,19,34,37 These types of analyses, where patients can receive transfusion representative of more than one type of donor characteristic, do not require exclusion or separate categorization that may result in biased results. Censoring of recipients receiving more than a single component or units from multiple donor categories may seem rational, but may instead result in analysis of patient subsets who have received fewer total transfusions and thus may be biased by differences in severity of illness and mortality risk.39 Furthermore, such censoring can also influence results adversely in situations where the reason for censoring is informative of an impending outcome. This form of introduced bias is sometimes referred to as informative censoring, a common and often overlooked problem in survival analyses.
Recognizing and accounting for possible bi-directional relationships of donor and recipient variables is also critical in analyses of transfusion. While the number of transfusions could be a confounder, it could also be a mediator for a donor exposure-outcome relationship. For example, it is recognized that female donor-derived RBC units contain less hemoglobin and recipients of these units are at increased risk of requiring additional units. Therefore, recipients of female units may be more likely to receive blood from more than one donor type and consequently, more likely to be excluded from a “no-mixture” analyses.39 This scenario could further aggravate the problem of informative censoring. In principle, this case represents a complex form of time-dependent confounding, where non-standard analytic approaches may be preferable (e.g. marginal structural models).40,41
When examining patient outcomes related to changes in blood collection, component processing or manufacturing occurring over time, one needs to account for other contemporaneous changes in clinical practice. Declines in adverse pulmonary transfusion reactions have been associated with the advent of leukoreduction and improvements in patient outcomes have been correlated with reductions in blood use.42,43 However, these studies have focused on transfused populations and did not account for other secular trends in clinical practice that would affect both transfused and non-transfused patients. Recently, we examined long-term outcomes associated with implementation of patient blood management programs.44 During a period of dramatic reduction in RBC use from 2010 to 2014, risk-adjusted mortality in anemic patients declined but in parallel with that of patients without anemia. In this case, contemporaneous quality improvement initiatives to reduce the morbidity and mortality for cardiovascular disease and sepsis were likely explanations for the decline in mortality.
Beyond the acknowledgement of unmeasured confounding and statistical limitations, the a priori development and testing of hypotheses for linkage analyses is critical. Animal model studies or early mechanistic clinical trials are frequently used to develop biologically plausible hypotheses. For example, animal models suggesting improved cognitive function in older mice exposed to blood from younger mice stimulated observational studies of blood donor-recipient outcomes.45 However in studies that identified adverse outcomes related to blood donor characteristics, a lack of clear biological rationale has been an acknowledged limitation. In these studies, the authors acknowledged that their findings, at times contrary to their initial hypotheses, were tentative and would need to be corroborated by replicative studies.16 Additional analyses utilizing strict statistical techniques to control for confounding variables but also examining the role of other donor characteristics (e.g., body mass index, hemoglobin levels, smoking status) may help investigators understand the disparate results from studies of donor gender and age conducted in different countries. Additionally, examination of the cause of death in mortality studies or morbidity outcomes may provide some insight into the validity and mechanism of study findings. However, the choice of morbidity outcomes, such as hospital length of stay, need to be considered carefully as non-clinical factors may impact such measures.46 While resource intensive, future studies of donor and component characteristics could incorporate validated measures of severity of illness as a morbidity outcome as has been done for RCTs of RBC storage duration.22,25
An organized approach to incorporating more granular clinical data as part of linked analyses will help us understand interactions of relevant co-variates as well as their importance in accounting for indication bias and recipient confounding. Early studies of transfusion outcomes used claims data to account for transfusion events. However, without assessment of the timing and number of transfusions, associations of transfusion and outcomes were limited.47 Data from electronic medical records and clinical decision support systems have been used to better understand the timing as well as indications for transfusion.48 In addition, prospective development of the REDS-III vein-to-vein database allowed for enrichment of donor survey data with relevant details including donor history of pregnancy, blood transfusion, and tobacco use prior to donation.37,49 Studies have also utilized daily hemoglobin and creatinine levels as time-varying covariates to better account for patient severity, and these measures could also serve as outcomes for donor effects.17,50,51
As an example, recent publications have examined the role of blood donor and component factors on changes in hemoglobin levels following red blood cell transfusion.52–54 Among component factors, prolonged storage of RBCs has been correlated with increased levels of extravascular hemolysis and reduced hemoglobin increments following transfusion in healthy volunteers and surgical patients.55,56 As in studies of mortality, it is clear that recipient factors need to be accounted for when examining hemoglobin increments related to transfusion. For example, in a linear regression analysis of single RBC-unit transfusions within the KPNC database, mean estimated hemoglobin increments after transfusion were actually found to be increased with prolonged storage, without and following adjustment for factors associated with circulating blood volumes (Table 3). However, after additionally accounting for pre-transfusion hemoglobin levels, smaller hemoglobin increments were found after transfusion of red cell units stored for more than 35 days – a finding which may be due to increased hemolysis or clearance of these RBCs.54 Another novel approach is to examine the effects of storage duration of RBCs in a group of chronically transfused patients. In a cohort of patients with myelodysplastic syndrome, analysis of storage duration and hemoglobin increments after transfusion was feasible as patient characteristics related to circulating blood volumes, including sex and body mass index, remained constant with repeated transfusion of RBCs with varying storage age.52 Lastly, it is recognized that changes in hemoglobin levels with red blood cell transfusion may vary with recipient factors based upon on the transfusion indication, recipient comorbidities, or patient severity of illness (e.g. surgery, intensive care, hemorrhage).
Table 3:
Effect of storage duration on hemoglobin increments after RBC transfusion
| Storage Duration |
Number of RBC units |
No Adjustment | Adjustment for age, sex, and BMI |
Adjustment for pre-TX Hb* |
|---|---|---|---|---|
| Estimated mean hemoglobin increment (95% CI) | ||||
| <21 days | 9,363 | 0.79 (0.77, 0.81) | 0.88 (0.86, 0.90) | 1.05 (1.02, 1.07) |
| 22–28 | 11,245 | 0.81 (0.79, 0.83) | 0.89 (0.87, 0.91) | 1.04 (1.01, 1.05) |
| 29–35 | 7,876 | 0.86 (0.84, 0.88) | 0.92 (0.90, 0.94) | 1.04 (1.01, 1.06) |
| 36–42 | 7,063 | 0.91 (0.89, 0.94) | 0.94 (0.92, 0.96) | 1.00 (0.98, 1.02) |
RBC=red blood cell; BMI=body mass index; TX=transfusion; Hb=hemoglobin
p-value <0.02 for mean estimated hemoglobin increment of >35-day stored RBCs vs. all other storage periods
The use of more granular patient data may allow the assessment of associations with less severe outcomes and provide an opportunity to disentangle complex causal pathways where exposure to units of different donor and component characteristics affect transfusion need and vice versa. For example, gamma irradiation has been shown to increase free hemoglobin in the supernatant of RBC units, likely through disruption of membrane integrity with increased in vitro hemolysis.57,58 However, gamma irradiated units are most frequently transfused to patients with hematologic malignancies who have been shown to have smaller hemoglobin increments with RBC transfusion.59 Therefore, it is necessary to account for recipient comorbidities and diagnoses when assessing the impact of gamma irradiation. These relationships become more complex when attempting to correlate in vitro differences of hemolysis in units from female donors compared to male-derived RBCs or changes in post-transfusion recovery of RBCs not from overall storage duration but from timing post-irradiation.60
The linking of ‘vein-to-vein databases” with relevant genomic or in vitro data from blood donors and components provides another opportunity to study the impact of donor demographic and manufacturing characteristics on recipient outcomes. Genotyping of blood donors represents the next important step in understanding the interplay between donor genetics, component storage, and transfusion efficacy. To this end, the REDS-III RBC-Omics study recently published results of analyses examining rates of post-storage osmotic and oxidative hemolysis of leukocyte-reduced RBC samples from more than 13,000 African American, Asian, Caucasian, and Hispanic blood donors. The study found that donor characteristics (age, sex, race/ethnicity, and donation frequency) were associated with in vitro measures of hemolysis at the end of storage (42 days).5,61 In parallel, metabolomic studies conducted in a subset of donors with extreme levels of hemolysis showed associations between donor demographics, storage solutions, and specific metabolic pathways.62 The investigators hypothesized that heritable variations among donor groups could modulate in vitro survival and function but also in vivo efficacy after transfusion. The data collected in this study provides an opportunity to analyze the role of donor genetic polymorphisms on patient outcomes by linking to recipients of the studied RBC units as part of the REDS-III ‘vein-to-vein’ database. By doing so, this linked analysis will be able to correlate hemolysis, metabolomic, and GWAS findings in donors with component factors (additive solutions, irradiation, storage duration) and recipient outcomes, including patient populations or conditions associated with oxidative stress (e.g. neonates, sickle cell disease). The concept of “precision transfusion medicine” was developed to move toward a future where blood donor selection, component processing and storage are optimized for transfusion recipients with the aim of improving blood safety and efficacy.63–65
Knowledge generated from studies of linked datasets has the potential to change the way donors are selected and how components are processed, stored and allocated. By characterizing specific donor, component, and recipient factors, these analyses also provide evidence to guide the design and conduct of future clinical trials. However, as we have outlined, it is important to recognize that the failure to adequately account for confounding or reliance on possibly biased statistical methods may introduce uncertainty into the reliability and interpretation of findings. Collaboration by investigators to uniformly capture relevant donor details or history, develop common or novel analytical approaches, and even re-analyze data using multiple statistical models will be important to resolve differences of prior studies. Such an endeavor is being planned under the auspices of the NIH-funded REDS-IV-Pediatric program. In addition to developing and carrying out some of the projects described above, this program seeks to bring together international experts to address potential methodologic challenges in their execution. With collaborative refinement of statistical approaches across multiple cohorts, it is hoped that future research projects will be able to clearly answer important questions related to blood donor and component factors on transfusion safety and efficacy.
Acknowledgements
Funding: National, Heart, Lung, and Blood Institute (NHLBI)
The content is solely the responsibility of the authors and does not represent the policy of the National Institutes of Health or the Department of Health and Human Services.
Financial Support: This work was supported by research support from the National Heart, Lung, and Blood Institute (R01HL126130)
Footnotes
Conflict of Interest Disclosures: No author reports any relevant conflicts of interest.
REFERENCES
- 1.Kleinman S, King MR, Busch MP, et al. The National Heart, Lung, and Blood Institute retrovirus epidemiology donor studies (Retrovirus Epidemiology Donor Study and Retrovirus Epidemiology Donor Study-II): twenty years of research to advance blood product safety and availability. Transfus Med Rev. 2012;26(4):281–304, 304.e281–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. 2019;133(17):1831–1839. [DOI] [PubMed] [Google Scholar]
- 3.Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood. 2019;133(17):1854–1864. [DOI] [PubMed] [Google Scholar]
- 4.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion. 2012;52(7):1388–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1(15):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almizraq RJ, Norris PJ, Inglis H, et al. Blood manufacturing methods affect red blood cell product characteristics and immunomodulatory activity. Blood Adv. 2018;2(18):2296–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acker JP, Marks DC, Sheffield WP. Quality Assessment of Established and Emerging Blood Components for Transfusion. J Blood Transfus. 2016;2016:4860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakkour S, Acker JP, Chafets DM, et al. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. 2016;111(1):22–32. [DOI] [PubMed] [Google Scholar]
- 9.Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316(19):2025–2035. [DOI] [PubMed] [Google Scholar]
- 10.Carson JL, Stanworth SJ, Roubinian N, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning S, Heddle NM, Acker JP. Exploring donor and product factors and their impact on red cell post-transfusion outcomes. Transfus Med Rev. 2018;32(1):28–35. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman S, Glynn SA. Database research in transfusion medicine: The power of large numbers. Transfusion. 2015;55(7):1591–1595. [DOI] [PubMed] [Google Scholar]
- 13.Edgren G, Rostgaard K, Vasan SK, et al. The new Scandinavian Donations and Transfusions database (SCANDAT2): a blood safety resource with added versatility. Transfusion. 2015;55(7):1600–1606. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman S, Busch MP, Murphy EL, et al. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54(3 Pt 2):942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roubinian N, Brambilla D, Murphy EL. Statistical Caution in Big Data Approaches to Transfusion Medicine Research. JAMA Intern Med. 2017;177(6):860–861. [DOI] [PubMed] [Google Scholar]
- 16.Chassé M, Tinmouth A, English SW, et al. Association of Blood Donor Age and Sex With Recipient Survival After Red Blood Cell Transfusion. JAMA Intern Med. 2016;176(9):1307–1314. [DOI] [PubMed] [Google Scholar]
- 17.Heddle NM, Cook RJ, Liu Y, et al. The association between blood donor sex and age and transfusion recipient mortality: an exploratory analysis. Transfusion. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54(10 Pt 2):2678–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caram-Deelder C, Kreuger AL, Evers D, et al. Association of Blood Transfusion From Female Donors With and Without a History of Pregnancy With Mortality Among Male and Female Transfusion Recipients. JAMA. 2017;318(15):1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasan SK, Chiesa F, Rostgaard K, et al. Lack of association between blood donor age and survival of transfused patients. Blood. 2016;127(5):658–661. [DOI] [PubMed] [Google Scholar]
- 21.Qu L, Triulzi DJ. Clinical effects of red blood cell storage. Cancer Control. 2015;22(1):26–37. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372(15):1410–1418. [DOI] [PubMed] [Google Scholar]
- 23.Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N Engl J Med. 2016;375(20):1937–1945. [DOI] [PubMed] [Google Scholar]
- 24.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308(14):1443–1451. [DOI] [PubMed] [Google Scholar]
- 25.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of Transfusion of Red Blood Cells With Longer vs Shorter Storage Duration on Elevated Blood Lactate Levels in Children With Severe Anemia: The TOTAL Randomized Clinical Trial. JAMA. 2015;314(23):2514–2523. [DOI] [PubMed] [Google Scholar]
- 27.Edgren G, Kamper-Jørgensen M, Eloranta S, et al. Duration of red blood cell storage and survival of transfused patients (CME). Transfusion. 2010;50(6):1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middelburg RA, van de Watering LM, van der Bom JG. Blood transfusions: good or bad? Confounding by indication, an underestimated problem in clinical transfusion research. Transfusion. 2010;50(6):1181–1183. [DOI] [PubMed] [Google Scholar]
- 29.Pereira A Will clinical studies elucidate the connection between the length of storage of transfused red blood cells and clinical outcomes? An analysis based on the simulation of randomized controlled trials. Transfusion. 2013;53(1):34–40. [DOI] [PubMed] [Google Scholar]
- 30.Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion. 2016;56(6):1462–1468. [DOI] [PubMed] [Google Scholar]
- 31.Halmin M, Rostgaard K, Lee BK, et al. Length of Storage of Red Blood Cells and Patient Survival After Blood Transfusion: A Binational Cohort Study. Ann Intern Med. 2017;166(4):248–256. [DOI] [PubMed] [Google Scholar]
- 32.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2017;127(1):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgren G, Hjalgrim H. Epidemiology of donors and recipients: lessons from the SCANDAT database. Transfus Med. 2019;29 Suppl 1:6–12. [DOI] [PubMed] [Google Scholar]
- 34.Edgren G, Ullum H, Rostgaard K, et al. Association of Donor Age and Sex With Survival of Patients Receiving Transfusions. JAMA Intern Med. 2017;177(6):854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fletcher J Subgroup analyses: how to avoid being misled. BMJ. 2007;335(7610):96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pocock SJ, Alonso AC. Ever-Pregnant Female Blood Donors and Mortality Risk in Male Recipients. JAMA. 2018;319(10):1048. [DOI] [PubMed] [Google Scholar]
- 37.Edgren G, Murphy E, Brambilla D, et al. Association of blood donor sex and prior pregnancy with mortality among red blood cells transfusion recipients: JAMA; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali O, Wasfi MF, Uzoigwe CE. Ever-Pregnant Female Blood Donors and Mortality Risk in Male Recipients. JAMA. 2018;319(10):1048–1049. [DOI] [PubMed] [Google Scholar]
- 39.Cable RG, Edgren G. Blood Transfusions From Previously Pregnant Women and Mortality: Interpreting the Evidence. JAMA. 2017;318(15):1445–1447. [DOI] [PubMed] [Google Scholar]
- 40.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 41.Neugebauer R, Schmittdiel JA, van der Laan MJ. Targeted learning in real-world comparative effectiveness research with time-varying interventions. Stat Med. 2014;33(14):2480–2520. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg N, Heal JM, Gettings KF, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54(10 Pt 2):2753–2759. [DOI] [PubMed] [Google Scholar]
- 44.Roubinian NH, Murphy EL, Mark DG, et al. Long-Term Outcomes Among Patients Discharged From the Hospital With Moderate Anemia: A Retrospective Cohort Study. Ann Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villeda SA, Plambeck KE, Middeldorp J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiessen J, Kambara H, Sakai T, Kato K, Yamauchi K, McMillan C. What causes international variations in length of stay: a comparative analysis for two inpatient conditions in Japanese and Canadian hospitals. Health Serv Manage Res. 2013;26(2–3):86–94. [DOI] [PubMed] [Google Scholar]
- 47.Menis M, Izurieta HS, Anderson SA, et al. Outpatient transfusions and occurrence of serious noninfectious transfusion-related complications among US elderly, 2007–2008: utility of large administrative databases in blood safety research. Transfusion. 2012;52(9):1968–1976. [DOI] [PubMed] [Google Scholar]
- 48.Triulzi D, Gottschall J, Murphy E, et al. A multicenter study of plasma use in the United States. Transfusion. 2015;55(6):1313–1319; quiz 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karafin MS, Tan S, Tormey CA, et al. Prevalence and risk factors for RBC alloantibodies in blood donors in the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Transfusion. 2019;59(1):217–225. [DOI] [PubMed] [Google Scholar]
- 50.Dupuis C, Garrouste-Orgeas M, Bailly S, et al. Effect of Transfusion on Mortality and Other Adverse Events Among Critically Ill Septic Patients: An Observational Study Using a Marginal Structural Cox Model. Crit Care Med. 2017;45(12):1972–1980. [DOI] [PubMed] [Google Scholar]
- 51.Heddle NM, Arnold DM, Acker JP, et al. Red blood cell processing methods and in-hospital mortality: a transfusion registry cohort study. Lancet Haematol. 2016;3(5):e246–254. [DOI] [PubMed] [Google Scholar]
- 52.Rydén J, Clements M, Hellström-Lindberg E, Höglund P, Edgren G. A longer duration of red blood cell storage is associated with a lower hemoglobin increase after blood transfusion: a cohort study. Transfusion. 2019. [DOI] [PubMed] [Google Scholar]
- 53.DeSimone RA, Hayden JA, Mazur CA, et al. Red blood cells donated by smokers: A pilot investigation of recipient transfusion outcomes. Transfusion. 2019. [DOI] [PubMed] [Google Scholar]
- 54.Roubinian N, Plimier C, Woo J, et al. Effect of donor, component and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood. 2019;blood.2019000773; doi: 10.1182/blood.2019000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118(25):6675–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunsicker O, Hessler K, Krannich A, et al. Duration of storage influences the hemoglobin rising effect of red blood cells in patients undergoing major abdominal surgery. Transfusion. 2018;58(8):1870–1880. [DOI] [PubMed] [Google Scholar]
- 57.Harm SK, Raval JS, Cramer J, Waters JH, Yazer MH. Haemolysis and sublethal injury of RBCs after routine blood bank manipulations. Transfus Med. 2012;22(3):181–185. [DOI] [PubMed] [Google Scholar]
- 58.Ran Q, Hao P, Xiao Y, Zhao J, Ye X, Li Z. Effect of irradiation and/or leucocyte filtration on RBC storage lesions. PLoS One. 2011;6(3):e18328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karafin M, Bruhn R, Roubinian N, et al. The impact of recipient factors on hemoglobin increment in transfused outpatients with hematologic disease. Vol. in press. Transfusion; 2019. [DOI] [PubMed] [Google Scholar]
- 60.de Korte D, Thibault L, Handke W, et al. Timing of gamma irradiation and blood donor sex influences in vitro characteristics of red blood cells. Transfusion. 2018;58(4):917–926. [DOI] [PubMed] [Google Scholar]
- 61.Kanias T, Stone M, Page GP, et al. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion. 2019;59(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klein HG, Flegel WA, Natanson C. Red Blood Cell Transfusion: Precision vs Imprecision Medicine. JAMA. 2015;314(15):1557–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spitalnik SL, Triulzi D, Devine DV, et al. 2015 proceedings of the National Heart, Lung, and Blood Institute’s State of the Science in Transfusion Medicine symposium. Transfusion. 2015;55(9):2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spitalnik SL, Devine DV. Translating red cell “omics” into new perspectives in transfusion medicine: mining the gems in the data mountains. Transfusion. 2019;59(1):2–5. [DOI] [PubMed] [Google Scholar]


