Abstract
Aims: To develop methods for recovering a model virus (bacteriophage MS2) from healthcare personal protective equipment (PPE).
Methods and Results: Nine eluents were evaluated for recovery of infectious MS2 from PPE: 1·5% beef extract (BE) pH 7·5 with and without 0·1% Tween 80, 1·5% BE pH 9·0 with and without 0·1% Tween 80, 3% BE pH 7·5 with and without 0·1% Tween 80, 3% BE pH 9·0 with and without 0·1% Tween 80 and PBS with 0·1% Tween 80. Methods were applied to experimentally contaminated PPE. Elution followed by two‐step enrichment assay could recover virus inputs as low as 1·5 log10, and could recover >90% of inoculated virus from used items of experimentally contaminated PPE worn by human volunteers.
Conclusions: BE was effective for recovering infectious viruses from a range of PPE materials.
Significance and Impact of the Study: PPE plays a crucial role in interrupting transmission of infectious agents from patients to healthcare workers (HCWs). The fate of micro‐organisms when PPE is removed and disposed of has important consequences for infection control. Methods described here can be used to conduct rigorous studies of viral survival and transfer on PPE for risk assessments in infection control and HCW protection.
Keywords: healthcare worker, PPE, SARS, virus
Introduction
Although nosocomial infections are a well‐recognized risk for patients in many healthcare settings, healthcare workers (HCWs) are also affected. Caring for patients with communicable diseases places HCWs at risk for exposure to pathogens during patient care activities, and requires protective measures such as patient isolation, HCW vaccination and the use of personal protective equipment (PPE). PPE plays a crucial role in interrupting transmission of infectious bacterial and viral agents from patients to HCWs. PPE includes use of barriers (gowns, gloves and eye shields) and respiratory protection (masks and respirators) alone or in combination to protect mucous membranes, airways, skin and clothing from contact with infectious agents during patient care (Siegel et al. 2007).
The importance of PPE in preventing HCW infection was brought to the forefront of infection control by the worldwide outbreak of severe acute respiratory syndrome (SARS). This outbreak included a number of cases acquired by HCWs in the course of caring for SARS patients (Chen et al. 2004; Lau et al. 2004; Loeb et al. 2004; McDonald et al. 2004; Wong et al. 2004), and studies of the spread of SARS in healthcare environments established a crucial role for PPE in preventing the spread of SARS from patients to HCWs. Nosocomial SARS also brought renewed attention to the question of whether viruses can spread from person to person via fomites in the healthcare environment, as SARS coronavirus (SARS‐CoV) nucleic acids were recovered from hospital surfaces, including bedrails, doorknobs, telephones and ventilator controls, in outbreak settings (Dowell et al. 2004).
Furniture and healthcare equipment are not the only fomites in healthcare environments that have the potential to spread viruses, PPE itself could also play a role in viral spread. During the performance of healthcare tasks, items of PPE may become contaminated by viable pathogenic micro‐organisms spread by contact, droplets or aerosols from patients’ respiratory secretions, urine, faeces and other body fluids. Bacteria and viruses can survive for extended periods on the types of materials PPE is made from (Bean et al. 1982; Brady et al. 1990; Wang 1999). Thus, PPE items are potential fomites, and may play a role in the transmission of disease if they become contaminated with infectious micro‐organisms. The fate of contaminating micro‐organisms when PPE is removed and disposed of has important consequences for infection control, and the possibility that PPE itself may be a fomite that contributes to the spread of viruses remains a poorly understood area in need of research. In order to determine the dynamics of virus survival and transmission via contaminated PPE and the attendant health risks, levels of viral contamination on PPE need to be quantified. This requires effective and reproducible methods to recover infectious viruses from items of PPE including contact isolation gowns, N95 respirators, gloves and eye protection.
We describe methods for recovering a model virus, bacteriophage MS2, from PPE. Many of the viruses that have been identified as important causes of nosocomial viral infection or have high potential for nosocomial transmission, such as rotaviruses, rhinoviruses, parvoviruses and noroviruses, are nonenveloped viruses. MS2 is a nonpathogenic, nonenveloped virus, and has been used extensively as a surrogate for nonenveloped pathogenic viruses of humans (Allwood et al. 2003; Meschke and Sobsey 2003; Dawson et al. 2005; Sickbert‐Bennett et al. 2005; Bae and Schwab 2008). MS2 has also been shown to have a higher binding potential for PPE materials than other viruses traditionally used as viral surrogates, such as ΦX174 (Lytle et al. 1991; Lytle and Routson 1995), making it a conservative surrogate for studies of disruption of viral binding to PPE. These qualities suggest that MS2 may be a promising surrogate for evaluating methods for recovery of viruses from healthcare PPE.
Candidate eluents for recovery of virus from PPE should be selected for their potential to disrupt viral binding to PPE materials. The attachment of viruses to surfaces is largely governed by electrostatic and Van der Waals interactions with other molecules and surfaces (Gerba 1984). PPE items are often made of synthetic polymers with both charged and hydrophobic characteristics. The protein coats of nonenveloped viruses can have pockets of hydrophobicity, and enveloped viruses have hydrophobic lipid membranes (Gerba 1984). Therefore, virus attachment to PPE may be mediated by electrostatic interactions, where charged viral surfaces encounter charged groups on PPE surfaces and hydrophobic reactions, where hydrophobic molecules on the surface of both viruses and PPE are excluded by the surrounding water molecules. Methods to elute viruses from PPE materials can be designed to disrupt these interactions. Robust and efficient virus recovery methods based on altering the adsorption and attachment behaviour of viruses in contact with surfaces have been developed for eluting viruses from charged filter media using protein solutions (Polaczyk et al. 2007). Molecules in proteinaceous eluents have a variety of surface charges, and compete with viruses for attachment and adsorption sites on surfaces (Gerba 1984). These findings suggest that protein eluents, particularly beef extract (BE), may be effective for eluting viruses from different types of surfaces. The addition of detergents that disrupt hydrophobic interactions, such as Tween 80, may also promote virus recovery from surfaces (Farrah 1982; Lukasik et al. 2000).
Currently, there are limited data on the survival, persistence and transmission of viruses on contaminated PPE. Robust methods for recovering viruses from PPE items are a necessary first step for obtaining such data. Methods using protein eluents for virus recovery, developed using a model virus, have the potential for application in studies of the recovery and survival of a range of pathogenic viruses on HCW PPE.
Materials and methods
Preparation of virus stocks
Bacteriophage MS2 was propagated in the host bacterium Escherichia. coli C3000 (ATCC no. 15597) using the soft agar coliphage propagation method. Briefly, 50 μl of virus stock was added to 30 ml of a log‐phase host bacterial culture, grown on a rotating shaker for 4 h at 37°C and purified by chloroform extraction using a 2 : 1 volume ratio of virus to chloroform, followed by centrifugation (5900 g, 30 min, 4°C). ‘Soft’ agar was prepared by adding agar to tryptic soy broth (TSB) at a final concentration of 0·7%, and bottom agar plates were prepared using full strength tryptic soy agar in 150 mm Petri dishes. Purified virus stock (0·5 ml) and log‐phase host culture (0·5 ml) were added to 30 ml of soft agar and dispensed into bottom agar plates. Plates were incubated at 37°C for 24 h. The top soft agar layer was then harvested, and soft agar from all plates was pooled, purified by chloroform extraction as described above, and stored as stock in 20% glycerol‐TSB at −80°C.
Comparison of eluents for virus elution from PPE
Nine eluents were selected for comparison of their efficiency in recovering viruses from PPE: 1·5% BE pH 7·5, 1·5% BE pH 7·5 with 0·1% Tween 80, 1·5% BE pH 9·0, 1·5% BE pH 9·0 with 0·1% Tween 80, 3% BE pH 7·5, 3% BE pH 7·5 with 0·1% Tween 80, 3% BE pH 9·0, 3% BE pH 9·0 with 0·1% Tween 80 and PBS with 0·1% Tween 80 (Table 1). BE was chosen because it has been used extensively an eluent for recovery of viruses from different surface types. Virus stocks were diluted in 0·01‐mol l−1 phosphate buffered saline, pH 7·2, to the desired concentration. Inocula for experiments were titred using the double agar layer (DAL) plaque assay on tryptic soy agar (Becton Dickinson, Franklin Lakes, NJ, USA; USEPA 2001b). Virus was applied to 4 cm2 swatches of contact isolation gown fabric in a single drop containing 10 μl. Swatches were held at room temperature in a biological safety cabinet for 15 min, then immersed 250 ml sterile eluent solution. Eluent and PPE were agitated on a reciprocal shaking platform at 120 cycles min−1 for 20 min. Swatches were removed and discarded, and eluent was diluted in TSB (Becton Dickinson) and assayed for MS2 by the DAL method.
Table 1.
Evaluation of nine candidate eluents for their efficiency in recovering MS2 from 4‐cm2 swatches of contact isolation gown material (original inoculum on each swatch 5·8 log10 PFU)
| Eluent | Total virus recovered [PFU (SD)] | % Recovery (SD) |
|---|---|---|
| 1·5% Beef extract pH 7·5 | 5·6 (0·12) | 73·0 (37·87) |
| 1·5% Beef extract pH 9·0 | 5·5 (0·22) | 60·7 (41·81) |
| 3% Beef extract pH 7·5 | 5·0 (0·54) | 17·1 (14·22) |
| 3% Beef extract pH 9·0 | 5·0 (0·33) | 14·7 (8·67) |
| 1·5% Beef extract pH 7·5 + 0·1% Tween 80 | 5·4 (0·22) | 52·5 (36·23) |
| 1·5% Beef extract pH 9·0 + 0·1% Tween 80 | 5·5 (0·24) | 67·8 (49·21) |
| 3% Beef extract pH 7·5 + 0·1% Tween 80 | 5·2 (0·25) | 20·1 (9·65) |
| 3% Beef extract pH 9·0 + 0·1% Tween 80 | 4·8 (0·68) | 21·0 (24·11) |
| PBS + 0·1% Tween 80 | 5·5 (0·24) | 63·9 (44·37) |
Each data point represents average of four trials.
Elution of viruses from PPE
Virus stocks were diluted in 0·01‐mol l−1 phosphate buffered saline to the desired concentration. For selected experiments, viruses diluted in PBS were monodispersed after dilution by sequential passage through hydrophilic polycarbonate filters with pore sizes of 0·2 μm (Isopore®; Millipore, Billerica, MA, USA) and 0·08 μm (Nuclepore® track‐etched membranes; Whatman, Kent, UK) prerinsed with 0·01% Tween 80 and sterile distilled water. Inocula for experiments were titred by DAL. Virus was applied to the surface of PPE in a single volume of 10 μl. PPE items were held at room temperature in a biological safety cabinet for 15 min, and then immersed in 1–2‐l eluent solution (depending on size of item). Eluent and PPE were agitated on a reciprocal shaking platform at 120 cycles min−1 for 20 min. PPE was removed from the eluent, the additional eluent was expressed into the container by wringing, and the item was discarded. The eluent was assayed for infectious virus using a two‐step enrichment‐spot plate lysis procedure for MS2 (USEPA 2001a).
PPE worn by human volunteers
Protocols for human volunteer experiments were approved by the University of North Carolina Chapel Hill Biomedical IRB and written informed consent was obtained. Enrolled participants met the following inclusion criteria: over 18 years of age, nonpregnant, nonlatex‐allergic, no active skin disorders and medical evaluation approval for N95 respirator fit testing and use. Experiments took place in a patient care room in the UNC Hospitals’ General Clinical Research Center. Volunteers donned contact isolation gowns (MediChoice®, Arden, NC, USA), gloves (Evolution One®; Microflex, Reno, NV, USA), respirators (N95 1860 healthcare particulate respirator, 3M Co., St Paul, MN) and splash proof plastic goggles (Monogoggle™; American Allsafe, Tonawanda, NY, USA). Items of PPE were then contaminated with MS2 in a solution containing 0·01‐mol l−1 PBS and GloGerm™ (GloGerm, Moab, UT, USA), synthetic beads that fluoresce under ultraviolet light (for visual tracking of virus). Sites of contamination were: front shoulder of the gown, back shoulder of the gown, right side of the N95 respirator, upper right front of the goggles and palm of the dominant hand. Each site was contaminated with a total of 104 plaque forming units (PFU) of MS2, in five drops of 5 μl each. Participants simulated a routine healthcare task by measuring blood pressure on a mannequin, and then removed PPE. Gowns, N95 respirators, gloves and goggles were collected after removal, immersed in eluent solution and transported immediately back to the laboratory for analysis. Eluent and PPE were agitated on a reciprocal shaking platform at 120 cycles min−1 for 20 min. PPE was removed from the eluent, the additional eluent was expressed into the container by wringing and the item was discarded. Eluent was assayed for infectious virus using the two‐step enrichment‐spot plate lysis method.
Two‐step enrichment‐spot plate lysis method
Eluent samples were diluted as needed using additional sterile eluent. For enrichment, 4‐mol l−1 MgCl2 (12·5 ml l−1) and 10 × TSB [1 : 20 (V/V) broth to sample] were added to the final sample dilutions. Appropriate dilutions were split into 10 replicate volumes per dilution, and 0·5 ml of log‐phase bacterial host was added to each replicate volume. Enrichment samples were incubated for 24 h at 37°C. For spot plates, 20 ml log‐phase bacterial host was added to 1 l molten half‐strength tryptic soy agar (30‐g TSB, 7·5‐g Bacto agar (Becton Dickinson) per litre) at 45°C and dispensed into 150‐mm petri dishes. After incubation of enrichment samples, 10 μl from each dilution replicate was placed on the surface of a spot plate and allowed to dry. Aliquots of bacterial host cultures were also placed on spot plates to check for viral contamination of host. Spot plates were incubated at 37°C for 24 h. After incubation, dilution replicates were scored as positive or negative based on the presence or absence of lysis zones, within and/or around the spots. Results of positive and negative enrichment‐spot plate volumes were expressed as most probable number (MPN) of viruses per unit volume of sample.
Data analysis
MPN calculations were done using the FDA Bacteriological Analytical Manual calculator (FDA, College Park, MD, USA). Data on virus recoveries were statistically analysed using excel 2003 (Microsoft, Redmond, WA, USA) and graph pad prism 5 (Graph Pad, San Diego, CA, USA).
Results
In the first phase, nine candidate eluent solutions were compared for their efficiency in eluting 5·8 log10 PFU of MS2 from 4‐cm2 swatches of contact isolation gown material. BE was chosen based on its efficacy in recovering viruses from surfaces in other applications, such as ionically charged filters (Polaczyk et al. 2007). The effects of BE concentration (1·5%vs 3%), pH (7·5 vs 9·0) and the addition of 0·1% Tween 80, a nonionic detergent, were evaluated. PBS with 0·1% Tween 80 was also used to evaluate the effect of detergent alone. Virus stocks used in these initial experiments were not monodispersed. Eluent samples were assayed by DAL. Virus recoveries using each eluent are shown in Table 1.
No significant difference was found in recoveries from any of the nine eluents using one‐way analysis of variance (anova) (P = 0·14). Four eluents with the highest mean recovery (1·5% BE pH 7·5, 73%; 1·5% BE pH 9·0, 61%; 1·5% BE pH 9·0 + 0·1% Tween 80, 68%; and PBS + 0·1% Tween 80, 64%) were then tested for their efficiency in eluting low numbers of virus (1·8 log10 PFU) from contact isolation gown swatches. To maximize recovery of low numbers of virus, the entire volume of eluent was examined using the two‐step enrichment‐spot plate lysis method. The results are shown in Fig. 1.
Figure 1.
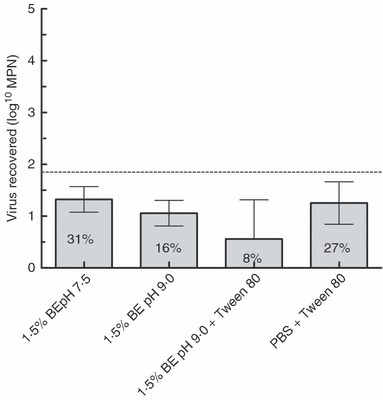
Evaluation of beef extract and PBS+Tween 80 eluents for recovery of MS2 from swatches of contact isolation gown material (average of four trials). (Original inoculum on each swatch (indicated by dashed line) = 1·8 log10 PFU; error bars represent 95% CI.).
The two eluents with the highest mean recovery, 1·5% BE pH 7·5 (mean 1·3 log10 MPN or 31% of inoculated viruses) and PBS + 0·1% Tween 80 (mean 1·25 log10 MPN or 16% of inoculated viruses), did not differ significantly (unpaired t‐test, P = 0·59). The solution with the simplest composition, 1·5% BE at pH 7·5, was chosen for subsequent experiments. This eluent was evaluated for its efficiency in eluting low numbers of MS2 (1·5 log10 PFU) from multiple PPE items using two‐step enrichment‐spot plate lysis assays of recovered eluent. PPE items tested were 4‐cm2 swatches of contact isolation gown fabric, whole contact isolation gowns, whole N95 respirators, splash proof goggles and whole latex gloves. Gown swatches were immersed in 250‐ml eluent. PPE items were immersed in 1–2 l of eluent, depending on the size of the item. Results are shown in Fig. 2.
Figure 2.
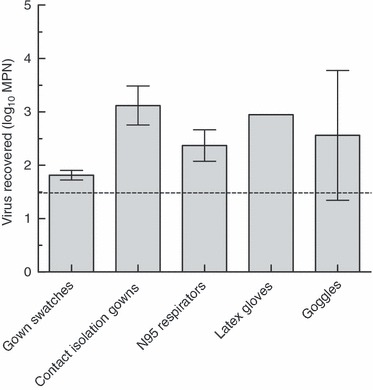
Recovery of MS2 from PPE items using 1·5% beef extract pH 7·5 (Original inoculum on each swatch (indicated by dashed line) = 1·5 log10 PFU; error bars represent 95% CI.).
Recovery from multiple PPE types using this eluent was variable, and was significantly greater than the inoculum titre, by up to fourfold. It was hypothesized that this was a result of viruses in the inoculum existing as aggregates. The titre of viruses existing in an aggregated state can be underestimated by plaque count methods because of single plaques being formed by an aggregate consisting of multiple viruses (Teunis et al. 2005). Protein solutions such as BE can disrupt aggregates formed by viruses (Gerba 1984). If aggregated viruses in the inoculum were subsequently dispersed by the BE eluent, the titre of virus recovered from the eluent would be higher than that of the inoculum. In an effort to address this problem, viruses in subsequent experiments were monodispersed by sequential filtration before being inoculated onto PPE. To evaluate the effect of monodispersion on virus recovery, monodispersed MS2 was inoculated onto multiple PPE types in amounts of 1·5, 2·5 or 4·6 log10 PFU and eluted as described above. As seen in Fig. 3, when virus is dispersed, the titre of virus recovered from gowns and other PPE types does not differ significantly (using unpaired t‐test) from the inoculum titre, with mean recoveries of 95% of initial viruses inoculated. These results indicate that monodispersion of viral inocula prior to application is necessary to accurately measure viral recovery from experimentally contaminated PPE materials.
Figure 3.
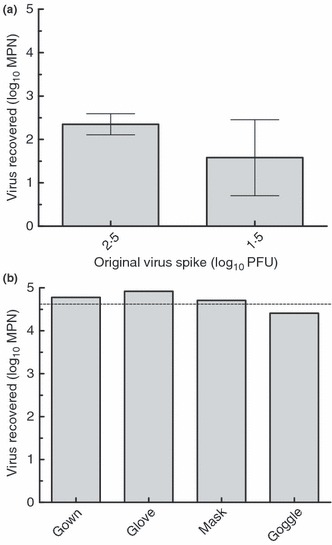
(a) Recovery of monodispersed MS2 from contact isolation gowns using 1·5% beef extract pH 7·5 (three trials). (b) Recovery of monodispersed MS2 from multiple PPE types using 1·5% beef extract pH 7·5 (two trials). (a) error bars represent 95% CI. (b) Original inoculum on each swatch (indicated by dashed line) 4·6 log10 PFU.
Based on the results of these experiments, two‐step enrichment‐spot plate lysis assay using BE eluent was then applied to items of PPE that underwent simulated viral contamination while being worn by human volunteers during a routine healthcare task. Ten subjects put on gowns, N95 respirators, gloves and goggles, which were then contaminated with 4·3 log10 PFU of monodispersed MS2 (see section ‘Materials and methods’). After a volunteer took a blood pressure on a mannequin to simulate a routine task that might be performed while wearing PPE, their PPE was removed and analysed for recovery of MS2.
As shown in Fig. 4, elution with 1·5% BE pH 7·5 followed by two‐step enrichment assay can efficiently recover >90% of infectious MS2 from contaminated PPE that has been worn during the performance of a healthcare task. Recovery did not differ significantly among PPE types (one‐way anova, P = 0·98).
Figure 4.
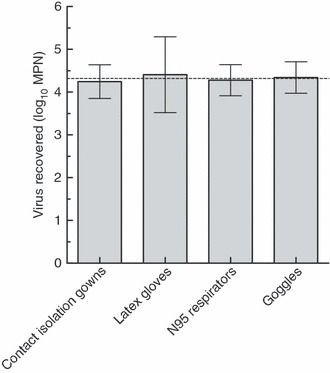
Recovery of MS2 from PPE worn by human volunteers during healthcare tasks (10 trials). (Original inoculum (indicated by dashed line) 4·3 log10 PFU; error bars represent 95% CI.).
Discussion
There are still knowledge gaps in assessing the risk of viral disease transmission posed by handling contaminated PPE. To accurately assess these risks, we need data on levels of viral contamination and the extent of viral survival on PPE items. The methods described in this work can help fill data gaps that currently exist in both these areas. The existing literature on viruses on PPE encompasses only some materials, and different studies have used a variety of methods to recover viruses from test materials, making comparisons between them difficult. Different methods for viral recovery may have different recovery efficiencies, especially if the method was not specifically developed for the recovery of viruses. This study demonstrated that a protein‐based eluent, BE, is effective for eluting nonenveloped viruses from a range of PPE materials, possibly because of the disruption of both charged and hydrophobic interactions between the virus and the PPE surface. Greater than 90% of input virus was recovered from PPE experimentally contaminated with MS2 after it was worn by human volunteers, showing that immersion in neutral pH BE with agitation can efficiently recover a nonenveloped virus from PPE.
Methods used for measuring viral contamination on PPE need to be able to recover low numbers of virus. The methods described here can recover as few as 30 PFU (1·5 log10) from contaminated PPE, making them applicable in studies of PPE used in healthcare practice, where levels of contamination may be very low. BE may also be efficacious for the elution of other types of pathogenic viruses from PPE, including enveloped viruses such as SARS‐CoV, influenza, parainfluenza, mumps, measles and respiratory syncytial virus, but these methods need to be evaluated using these or other enveloped viruses to determine their recovery efficacy.
The results of this study indicate that BE elution can recover nonenveloped viruses without inactivating them, making them applicable to studies of virus survival. To date, there have been few studies assessing the survival of pathogens on materials used to make PPE (Yassi et al. 2005), but they suggest that viruses have the potential to survive on PPE materials for longer than single‐use PPE is usually worn, creating the potential for viral transfer when PPE is handled after wearing. Some investigations have found that viruses can survive on materials used to make other types of PPE. When deposited in high numbers (106 TCID50), SARS‐CoV has been found to survive on gowns for up to 2 days (Lai et al. 2005). Enveloped ssRNA viruses have been shown to survive on latex glove material; human CoV 229E can survive for up to 2 h, although it loses up to 85% of its infectious titre (Sizun et al. 2000), and avian influenza virus can survive for up to 6 days without loss of infectious titre (Tiwari et al. 2006). Nonenveloped RNA viruses, such as human rotavirus and hepatitis A virus, can survive for several days on latex under ambient conditions, with only 1 log10 loss in infectious titre (Abad et al. 1994). These findings suggest that further studies are needed to determine how long pathogenic viruses can survive on PPE and what risk this might pose to HCWs. Comparative studies of survival of a range of pathogenic viruses on different PPE types can be greatly strengthened by the use of efficient elution methods designed for viruses, applied consistently across studies. The use of BE elution appears to be a candidate method for these types of investigations.
These methods are also applicable to studies of viral contamination on PPE during actual patient care activities. If patients shed viruses onto HCWs’ PPE in the course of patient care, these viruses can remain infectious when PPE is removed. Transfer of viruses from experimentally contaminated fabrics (Rusin et al. 2002), plastic surfaces (Gwaltney and Hendley 1982) and gloves (Hall et al. 1980) to hands has been demonstrated, suggesting that viruses can transfer from PPE to hands when contaminated items are handled in the course of removal and disposal. In addition, contamination can be present on skin after exposure to pathogens even when PPE is worn (Zamora et al. 2006), and may be transferred to used items of PPE, if they are handled after removal. Virus transfer between hands and PPE items can encourage both accidental autoinoculation by the HCW and subsequent transmission of viruses to other patients, staff or family members, especially when inadequate hand hygiene is practised (Pittet et al. 2006). Although this risk has been recognized, the magnitude of risk is difficult to assess because there are few data available on levels of viral contamination on PPE after use in patient care. The methods we have developed, which can recover low numbers of viruses, can be used to recover viruses from PPE items used in patient care, providing data that will aid in the assessment of risks posed by handling of contaminated PPE after patient care activities.
The results of this study also suggest that MS2 is a promising surrogate for studying the dynamics of nonenveloped virus survival and transfer in healthcare settings. It has been used in studies of the efficacy of healthcare handwashing agents (Sickbert‐Bennett et al. 2005), virus transfer between hands and objects (Hall et al. 1980; Gwaltney and Hendley 1982; Rusin et al. 2002) and HCW contamination after removal of contaminated PPE (Casanova et al. 2008). As a nonpathogenic virus that can be efficiently recovered and assayed for infectivity, MS2 may be a useful surrogate for modelling the spread viral of contamination during PPE use and removal in healthcare environments. Robust methods to measure viral contamination on PPE items can be applied to more accurately assess these and other microbial risks of PPE removal, handling and reuse in all healthcare settings. The methods described here are the first that have been shown to give high virus recovery with multiple types of PPE. These methods can be used with model viruses or evaluated for pathogenic viruses to conduct rigorous studies of viral survival and transfer on PPE for risk assessments in infection control and HCW protection.
Acknowledgements
We thank Dr Edie Alfano‐Sobsey for assistance with human volunteer experiments. Funding for this work was provided by the Centers for Disease Control and Prevention through Cooperative Agreement number U01/CI000299 and was also supported in part by a grant (RR00046) from the General Clinical Research Centers programme of the Division of Research Resources, National Institutes of Health.
References
- Abad, F. , Pinto, R. and Bosch, A. (1994) Survival of enteric viruses on environmental fomites. Appl Environ Microbiol 60, 3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood, P. , Malik, Y. , Hedberg, C. and Goyal, S. (2003) Survival of F‐specific RNA coliphage, feline calicivirus, and Escherichia coli in Water: a comparative study. Appl Environ Microbiol 69, 5707–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, J. and Schwab, K. (2008) Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol 74, 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean, B. , Moore, B. , Sterner, B. , Peterson, L. , Gerding, D. and Balfour, H. Jr (1982) Survival of influenza viruses on environmental surfaces. J Infect Dis 146, 47–51. [DOI] [PubMed] [Google Scholar]
- Brady, M. , Evans, J. and Cuartas, J. (1990) Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control 18, 18–23. [DOI] [PubMed] [Google Scholar]
- Casanova, L. , Alfano‐Sobsey, E. , Rutala, W.A. , Weber, D.J. and Sobsey, M. (2008) Virus transfer from personal protective equipment to healthcare employees’ skin and clothing. Emerg Infect Dis [serial on the Internet]. Available from: http://www.cdc.gov/EID/contents/14/8/1291.htm; accessed March 9, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Chen, P. , Chang, S. , Kao, C. , Wang, S. and Wang, L. (2004) Infection control and SARS transmission among healthcare workers, Taiwan. Emerg Infect Dis 10, 895–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, D. , Paish, A. , Staffell, L. , Seymour, I. and Appleton, H. (2005) Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J Appl Microbiol 98, 203–209. [DOI] [PubMed] [Google Scholar]
- Dowell, S. , Simmerman, J. , Erdman, D. , Wu, J. , Chaovavanich, A. , Javadi, M. , Yang, J. , Anderson, L. et al. (2004) Severe acute respiratory syndrome coronavirus on hospital surfaces. Clin Infect Dis 39, 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah, S. (1982) Chemical factors influencing adsorption of bacteriophage MS2 to membrane filters. Appl Environ Microbiol 43, 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba, C. (1984) Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol 30, 133–168. [DOI] [PubMed] [Google Scholar]
- Gwaltney, J. and Hendley, J. (1982) Transmission of experimental rhinovirus infection by contaminated surfaces. Am J Epidemiol 116, 828–833. [DOI] [PubMed] [Google Scholar]
- Hall, C. , Douglas, R. Jr and Geiman, J. (1980) Possible transmission by fomites of respiratory syncytial virus. J Infect Dis 141, 98–102. [DOI] [PubMed] [Google Scholar]
- Lai, M.Y. , Cheng, P.K. and Lim, W.W. (2005) Survival of severe acute respiratory syndrome coronavirus. Clin Infect Dis 41, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, J. , Fung, K. , TzeWai, W. , Kim, J. , Wong, E. , Chung, S. , Ho, D. , Chan, L. et al. (2004) SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 10, 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb, M. , McGeer, A. , Henry, B. , Ofner, M. , Rose, D. , Hlywka, T. , Levie, J. , McQueen, J. et al. (2004) SARS among critical care nurses, Toronto. Emerg Infect Dis 10, 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasik, J. , Scott, T. , Andryshak, D. and Farrah, S. (2000) Influence of salts on virus adsorption to microporous filters. Appl Environ Microbiol 66, 2914–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle, C. and Routson, L. (1995) Minimized virus binding for tests of barrier materials. Appl Environ Microbiol 61, 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle, C. , Truscott, W. , Budacz, A. , Venegas, L. , Routson, L. and Cyr, W. (1991) Important factors for testing barrier materials with surrogate viruses. Appl Environ Microbiol 57, 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, L. , Simor, A. , IhJen, S. , Maloney, S. , Ofner, M. , KowTong, C. , Lando, J. , McGeer, A. et al. (2004) SARS in healthcare facilities, Toronto and Taiwan. Emerg Infect Dis 10, 777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschke, J. and Sobsey, M. (2003) Comparative reduction of Norwalk virus, poliovirus type 1, F+ RNA coliphage MS 2 and Escherichia coli in miniature soil columns. Water Sci Technol 47, 85–90. [PubMed] [Google Scholar]
- Pittet, D. , Allegranzi, B. , Sax, H. , Dharan, S. , Pessoa‐Silva, C. , Donaldson, L. and Boyce, J. (2006) Evidence‐based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis 6, 641–652. [DOI] [PubMed] [Google Scholar]
- Polaczyk, A. , Roberts, J. and Hill, V. (2007) Evaluation of 1MDS electropositive microfilters for simultaneous recovery of multiple microbe classes from tap water. J Microbiol Methods 68, 260–266. [DOI] [PubMed] [Google Scholar]
- Rusin, P. , Maxwell, S. and Gerba, C. (2002) Comparative surface‐to‐hand and fingertip‐to‐mouth transfer efficiency of gram‐positive bacteria, gram‐negative bacteria, and phage. J Appl Microbiol 93, 585–592. [DOI] [PubMed] [Google Scholar]
- Sickbert‐Bennett, E. , Weber, D. , Gergen‐Teague, M. , Sobsey, M. , Samsa, G. and Rutala, W. (2005) Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control 33, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, J. , Rhinehart, E. , Jackson, M. , Chiarello, L. and the Healthcare Infection Control Practices Advisory Committee . (2007) Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Available from: URL: http://cdc.gov/ncidod/dhqp/pdf/guidelines/Isolation2007.pdf; accessed 12 December 2007. [Google Scholar]
- Sizun, J. , Yu, M. and Talbot, P. (2000) Survival of human coronaviruses 229E and OC43 in suspension and after drying onsurfaces: a possible source of hospital‐acquired infections. J Hosp Infect 46, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis, P. , Lodder, W. , Heisterkamp, S. and De Roda Husman, A. (2005) Mixed plaques: statistical evidence how plaque assays may underestimate virus concentrations. Water Res 39, 4240–4250. [DOI] [PubMed] [Google Scholar]
- Tiwari, A. , Patnayak, D. , Chander, Y. , Parsad, M. and Goyal, S. (2006) Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis 50, 284–287. [DOI] [PubMed] [Google Scholar]
- USEPA (2001a) EPA method 1602: Male‐specific (F+) and Somatic Coliphage in Water by Single Agar Layer (SAL) Procedure. EPA document 821‐R‐01‐029, Washington, DC: Office of Water, USEPA. [Google Scholar]
- USEPA (2001b) Method 1601: Male‐Specific (F+) and Somatic Coliphage in Water by Two‐Step Enrichment Procedure. EPA document 821‐R‐01‐030. Washington, DC: Office of Water, USEPA. [Google Scholar]
- Wang, Z. (1999) Survival of Bacteria on Respirator Filters. Aerosol Sci Technol 30, 300–308. [Google Scholar]
- Wong, T. , Lee, C. , Tam, W. , Lau, J. , Yu, T. , Lui, S. , Chan, P. , Li, Y. et al. (2004) Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis 10, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassi, A.M. , Moore, D. , FitzGerald, J.M. , Bigelow, P. , Hon, C.‐Y. , Bryce, E. and other members of The BC Interdisciplinary Respiratory Protection Study Group (2005) Research gaps in protecting healthcare workers from SARS and other respiratory pathogens: an interdisciplinary, multi‐stakeholder, evidence‐based approach. J Occup Environ Med 47, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora, J. , Murdoch, J. , Simchison, B. and Day, A. (2006) Contamination: a comparison of 2 personal protective systems. Can Med Assoc J 175, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]


