Abstract
Aims: To isolate and characterize an antifungal peptide from the seeds of Brassica parachinensis L.H.Bailey.
Methods and Results: An antifungal peptide designated as brassiparin was isolated. It exhibited a molecular mass of 5716 Da. It potently inhibited mycelial growth in a number of fungal species including Fusarium oxysporum, Helminthosporium maydis, Mycosphaerella arachidicola and Valsa mali. The antifungal activity of brassiparin toward M. arachidicola exhibited pronounced thermostability and pH stability. It inhibited proliferation of hepatoma (HepG2) and breast cancer (MCF7) cells and the activity of HIV‐1 reverse transcriptase. Its N‐terminal sequence differed from those of antifungal proteins which have been reported to date.
Conclusions: Brassiparin can be purified by using a protocol involving ion exchange chromatography, affinity chromatography and gel filtration. It manifests potent, thermostable and pH‐stable antifungal activity. It demonstrates antiproliferative activity toward tumour cells, and inhibitory activity toward HIV‐1 reverse transcriptase. Thus, brassiparin is a defense protein.
Significance and Impact of the Study: Brassiparin represents one of the few antifungal proteins reported to date from Brassica species. Its antifungal activity has pronounced pH stability and thermostability. Brassiparin exhibits other exploitable activities such as antiproliferative activity toward hepatoma and breast cancer cells and inhibitory activity toward HIV‐reverse transcriptase.
Keywords: Brassica parachinensis, Brassicaceae, brassiparin protein, protein isolation and characterization
Introduction
Antifungal proteins and peptides have been isolated from a variety of organisms including plants (Wilson et al. 2000; Wang and Ng 2001, 2003; Fujimura et al. 2003), animals (Bulet et al. 1999; Vasilevskii et al. 2007), and fungi (Wang and Ng 2004a, 2004b). Various plant tissues including seeds (1992, 1989; Cammue et al. 1995; Caruso et al. 1996; Joshi et al. 1998; Wang and Ng 2000, 2001, 2003), bulbs (Wang and Ng 2002a, 2002b;Chu and Ng 2004), leaves (Huang et al. 2000; Lam et al. 2000), tubers (Gozia et al. 1993), fruits (Pressey 1997; Wang and Ng 2002a, 2002b), and roots (Lam and Ng 2001) produce antifungal proteins and peptides.
Antifungal proteins and peptides of plant origin are divided into various types (Selitrennikoff 2001), including chitinases and chitinase‐like proteins (Vogelsang and Barz 1993; Lam et al. 2000; Lam and Ng 2001), chitin‐binding proteins (1992, 1989; Huang et al. 2000), lipid transfer proteins (Lin et al. 2007), protease inhibitors (Joshi et al. 1998), ribosome inactivating proteins (Leah et al. 1991; Pressey 1997; Parkash et al. 2002), thaumatin‐like proteins (Pressey 1997; Wang and Ng 2002a, 2002b), glucanases (Vogelsang and Barz 1993), embryo abundant protein‐like proteins (Wang and Ng 2000), and defensin‐like peptides (Wong and Ng 2003). However, only one antifungal peptide has been reported from Brassica campestris seeds (Lin et al. 2007) despite the existence of many Brassica species. The seeds of Brassica campestris and B. parachinensis have medicinal use in traditional Chinese medicine. The aim of the present study was to purify an antifungal peptide from the seeds of B. parachinensis, to compare it with the antifungal peptide previously isolated from B. campestris seeds (Lin et al. 2007), and to ascertain other potentially exploitable activities of the peptide.
Materials and methods
Materials
Seeds of Brassica parachinensis L.H.Bailey were purchased from a local seed shop that sells seeds to farmers. They were authenticated by Professor Shiuying Hu, honorary Professor of Chinese Medicine, The Chinese University of Hong Kong. The seeds were sown in soil and grew into B. parachinensis plants, testifying that they were indeed B. parachinensis seeds. The fungi were provided by Department of Microbiology, China Agricultural University, China. SP‐Sepharose, Mono S and Superdex Peptide were from GE Healthcare (Uppsala, Sweden), Affi‐gel blue gel was from Bio‐Rad (USA). All chemicals were of the highest purity available.
Isolation of antifungal peptides
The crude extract of Brassica parachinensis seeds was chromatographed on a 5 × 20 cm column of Affi‐gel blue gel in 10 mmol l−1 Tris–HCl buffer (pH 7·8). Unadsorbed proteins (fraction BG1) were eluted with the same buffer while adsorbed proteins (fraction BG2) were eluted with 10 mmol l−1 Tris–HCl buffer (pH 7·8) containing 1 mol l−1 NaCl. Fraction BG2 was subjected to ion exchange chromatography on a 2·5 × 20 cm column of SP‐Sepharose which had been equilibrated with and was then eluted with 10 mmol l−1 NH4OAc buffer (pH 4·5). After unadsorbed proteins had come off the column, the column was eluted stepwise with 10 mmol l−1 NH4OAc buffer (pH 4·5) containing 0·2, 0·5 and 1 mol l−1 NaCl to yield fractions SP1, SP2 and SP3, respectively. Fraction SP2 was further purified on a Mono S column in 10 mmol l−1 NH4OAc buffer (pH 4·5). After elution of unadsorbed proteins, the column was eluted sequentially with three linear NaCl concentration gradients (0–0·2, 0·2–0·6, and 0·6–1 mol l−1) in the starting buffer to yield fractions S1, S2, and S3, respectively. Fraction S2 was subjected to final purification on a Superdex Peptide 10/300 GL column. The main peak constituted purified antifungal peptide designated as brassiparin.
Protein determination
Protein concentration was determined with the dye‐binding method (Bio‐Rad) using bovine serum albumin as a standard.
Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis
It was conducted according to the method of Nielsen and Reynolds (1978). After electrophoresis, the gel was stained with Coomassie Brilliant Blue. The molecular mass of the isolated antifungal peptide was determined by comparison of its electrophoretic mobility with those of molecular mass marker proteins from GE Healthcare.
Mass spectrometry
Mass spectrometric analysis of the antifungal peptide was performed on a Finnigan LCQ‐MS (Applied Biosystems), an instrument that essentially consists of an atmospheric pressure electrospray positive‐ion source attached to a triple‐quadrupole mass analyzer. The purified peptide (100 pmol) was dissolved in water/methanol (50 : 50, v/v) containing 1% (v/v) acetic acid at a protein concentration of 5 μmol l−1, and then applied on the MS instrument.
N‐terminal amino acid sequence analysis
The N‐terminal amino acid sequence of the purified peptide was performed by Edman degradation using an amino acid sequencer.
Assay of antifungal activity
The assay for antifungal activity was executed using 100 × 15 mm Petri plates containing 10 ml of potato dextrose agar. After the mycelial colony had developed, sterile blank paper discs (0·625 cm in diameter) were placed at a distance of 1 cm away from the rim of the mycelial colony. An aliquot (8 μl containing 60 or 300 μg) of the purified peptide in 20 mmol l−1 PBS buffer (pH 6·0) was introduced to a disc. The plates were incubated at 23°C for 72 h until mycelial growth had enveloped peripheral discs containing the control (buffer) and had produced crescents of inhibition around discs containing samples with antifungal activity. The fungal species tested included Fusarium oxysporum, Helminthosporium maydis, Mycosphaerella arachidicola and Valsa mali (Lin et al. 2007).
To determine the IC50 value for the antifungal activity of the isolated antifungal peptide, three doses of the peptide were added separately to three aliquots each containing 4 ml potato dextrose agar at 45°C, mixed rapidly, and poured into three separate small Petri dishes. After the agar had cooled down, a small amount of mycelia, the same amount to each plate, was added. Buffer only, without containing antifungal peptide, served as a control. After incubation at 23°C for 72 h, the area of the mycelial colony was measured, and the inhibition of fungal growth determined. The concentration of the isolated antifungal peptide that brought about 50% reduction in the area of mycelial colony is the IC50 (Wang and Ng 2000). The IC50 could be read off from a graph plotting % reduction in mycelial colony area against concentration of antifungal peptide. Percentage of reduction of mycelial colony area is calculated as (Mycelial colony area in absence of antifungal peptide – mycelial colony area in presence of antifungal peptide) ÷ mycelial colony area in absence of antifungal peptide × 100%. Nystatin and a defensin‐like peptide (Wong and Ng 2003) were used as positive controls. French bean hemagglutinin (Leung et al. 2008) was used as a negative control. This assay has been checked for validity by Professor Hexiang Wang at Department of Microbiology of China Agricultural University. It has been used for monitoring the isolation of antifungal proteins (Bormann et al. 1999;Silici and Koc 2006; Park et al. 2007).
To investigate the thermal [0–100°C] stability, and pH [0–3 and 10–14] stability, the isolated antifungal peptide was pretreated accordingly and the antifungal assay was then conducted as mentioned above.
A solution of the isolated antifungal peptide [1 mg ml−1] was incubated with an equal volume of trypsin [1 mg ml−1] at 37°C for 1 h. At the end of the incubation, the reaction mixture was examined for antifungal activity.
Assay of antiproliferative activity on tumour cell lines
This assay was performed in view of reports that some antifungal proteins have this activity. Breast cancer MCF‐7 cells and hepatoma HepG2 cells were suspended in RPMI medium and adjusted to a cell density of 2 × 104 cells ml−1. A 100‐μl aliquot of this cell suspension was seeded to a well of a 96‐well plate, followed by incubation for 24 h. Different concentrations of the antifungal peptide in 100 μl complete RPMI medium were then added to the wells and incubated for 72 h. After 72 h, 20 μl of a 5 mg ml−1 solution of [3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide] [MTT] in phosphate buffered saline was spiked into each well, and the plates were incubated for 4 h. The plates were then centrifuged at 324 g for 5 min. The supernatant was carefully removed, and 150 μl of dimethyl sulfoxide was added to each well to dissolve the MTT‐formazan at the bottom of the well. After 10 min, the absorbance at 590 nm was measured by using a microplate reader (Wang and Ng 2003). Kale napin‐like polypeptide (Ngai and Ng 2004a) was used as positive control.
Assay for HIV‐1 reverse transcriptase inhibitory activity
The assay for HIV reverse transcriptase inhibitory activity was carried out in accordance with instructions supplied with the assay kit from Boehringer Mannheim (Germany) since some antifungal proteins possess this activity. The assay takes advantage of the ability of reverse transcriptase to synthesize DNA, starting from the template/primer hybrid poly (A) oligo (dT) 15. The digoxigenin‐ and biotin‐labelled nucleotides in an optimized ratio are incorporated into one of the same DNA molecule, which is freshly synthesized by the reverse transcriptase (RT). The detection and quantification of synthesized DNA as a parameter for RT activity follows a sandwich ELISA protocol. Biotin‐labelled DNA binds to the surface of microtitre plate modules that have been precoated with streptavidin. In the next step, an antibody to digoxigenin, conjugated to peroxidase, binds to the digoxigenin‐labelled DNA. In the final step, the peroxidase substrate is added. The peroxidase enzyme catalyzes the cleavage of the substrate, producing a coloured reaction product. The absorbance of the sample at 405 nm can be determined using a microtitre plate (ELISA) reader and is directly correlated to the level of RT activity. A fixed amount (4–6 ng) of recombinant HIV‐1 reverse transcriptase was used. The inhibitory activity of the antifungal peptide was calculated as percent inhibition as compared to a control without the antifungal peptide (Wang and Ng 2002a, 2002b). Ascalin (Wang and Ng 2001) was used as a positive control.
Assay of ability to inhibit HIV‐1 integrase. Expression and purification of recombinant HiV‐1 integrase
The plasmid that expressed His‐tagged wild‐type HIV‐1 integrase, pT7‐7‐His (Y|TX)‐HIV‐1‐IN, was a generous gift from Professor S.A. Chow (School of Medicine, UCLA). To express the protein, a 1‐liter culture of E. coli BL21 (DE3) cells containing the expression plasmid was grown at 37°C until OD600 reached 0·7–0·8. Cells were induced by addition of 0·8 mmol l−1 isopropyl‐β‐d‐thiogalactopyranoside (IPTG) and harvested, after 4 h of incubation, by centrifugation at 6000 g for 10 min at 4°C. Cells were suspended at a concentration of 10 ml g−1 wet cell paste in 20 mmol l−1 Tris–HCl buffer (pH 8·0) containing 0·1 mmol l−1 EDTA, 2 mmol l−1β‐mercaptoethanol, 0·5 mol l−1 NaCl and 5 mmol l−1 imidazole. Lysozyme was added to a concentration of 0·2 mg ml−1. After incubation at 4°C for 1 h, the lysate was sonicated and centrifuged at 40 000 g at 4°C for 20 min. The pellet was homogenized in 50 ml buffer A (20 mmol l−1 Tris–HCl, pH 8·0, 2 mol l−1 NaCl, 2 mmol l−1β‐mercaptoethanol) containing 5 mmol l−1 imidazole. The suspension was rotated at 4°C for 1 h, and cleared by centrifugation at 40 000 g at 4°C for 20 min. The supernatant was loaded onto a 1‐ml chelating Sepharose (GE Healthcare) column charged with 50 mmol l−1 imidazole. The column was washed with five column volumes of buffer A containing 5 mmol l−1 imidazole, and the protein was eluted with three column volumes of buffer A containing 200 and 400 mmol l−1 imidazole, respectively. Protein‐containing fractions were pooled, and EDTA was added to a final concentration of 5 mmol l−1. The protein was dialyzed against buffer B (20 mmol l−1 HEPES, pH 7·5, 1 mmol l−1 EDTA, 1 mol l−1 NaCl, 20% glycerol) containing 2 mmol l−1β‐mercaptoethanol, and then against buffer B containing 1 mmol l−1 dithiothreitol. Aliquots of the protein were stored at –70°C.
HIV‐1 integrase assay
A non‐radioactive ELISA‐based HIV‐1 integrase assay was performed according to the DNA‐coated plate method. In this study, 1 μg of Smal‐linearized p Bluescript SK was coated onto each well in the presence of 2 mol l−1 NaCl as target DNA. The donor DNA was prepared by annealing VU5BR (5′‐biotin‐GTGTGGAAAATCTCTAGCAGT‐3′) and VU5 (5′‐ACTGCTAGAGATTTTCCACAC‐3′) in 10 mmol l−1 Tris–HC1, pH 8·0, 1 mmol l−1 EDTA and 0·1 mol l−1 NaCl at 80°C followed by 30 min at room temperature. Integrase reaction was performed in 20 mmol l−1 HEPES (pH 7·5) containing 10 mmol l−1 MnCl2, 30 mmol l−1 NaCl, 10 mmol l−1 dithiothreitol and 0·05% Nonidet‐P40 (Sigma). After the integrase reaction, the biotinylated DNA immobilized on the wells was detected by incubation with streptavidin‐conjugated alkaline phosphatase (Boehringer‐Mannheim, Mannheim, Germany), followed by colorimetric detection with 1 mg ml−1 p‐nitrophenyl phosphate in 10% diethanolamine buffer (pH 9·8) containing 0·5 mmol l−1 MgCl2. The absorbance due to the alkaline phosphatase reaction was measured at 415 nm. The ribosome inactivating protein trichosanthin was used as a positive control (Au et al. 2000).
Screening for inhibitory effect on SARS coronavirus protease
The activity of SARS coronavirus (CoV) protease was indicated by a cleavage of designed substrate which was composed of two proteins linked by a cleavage site for SARS CoV protease. The reaction was performed in a mixture containing 5 μmol l−1 SARS CoV protease, 5 μmol l−1 sample, 20 μmol l−1 substrate and buffer [20 mmol l−1 Tris–HCl (pH 7·5), 20 mmol l−1 NaCl and 10 mmol l−1 beta‐mercaptoethanol] for 40 min at 37°C. After 40 min, the reaction was stopped by heating at 100°C for 2 min. Then the reaction mixture was analysed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE). If SARS CoV protease is inhibited by the test sample, there is only one band, which is the intact substrate, shown in SDS‐PAGE (Leung et al. 2008).
Assay for trypsin‐inhibitory activity
This assay was conducted in view of the finding that some antifungal proteins have trypsin inhibitory activity. Trypsin activity was determined by using N‐α‐benzoyl‐l‐arginine ethyl ester hydrochloride (BAEE) as the substrate. Ten microliters of a trypsin solution (250 μg ml−1) in assay buffer (50 mmol l−1 Tris–HCl, pH 8, containing 20 mmol l−1 CaCl2) were added to 980 μl of assay buffer. Then 10 μl of BAEE in assay buffer was added to give a final concentration of 0·6 mmol l−1. The reaction rate was determined by monitoring the change in absorbance at 253 nm for 1 min.
To assay for trypsin‐inhibitory activity, 10 μl test sample in assay buffer was added to trypsin, and incubated at 25°C for 15 min before addition of substrate (BAEE) to initiate the reaction. Trypsin‐inhibitory activity was calculated as follows:
 |
where Abs control is absorbance change in absence of sample, Abs sample is absorbance change in presence of sample, trypsin (mg) is the amount of trypsin in the assay mixture. One unit of trypsin‐inhibitory activity refers to the activity capable of inhibiting 1 mg trypsin. A similar assay was conducted using casein as substrate instead of BAEE (Ye et al. 2001). Soybean trypsin inhibitor was used as a positive control.
Assay of mitogenic activity
This assay was performed in view of reports that some antifungal proteins have this activity. Four C57BL/6 mice (20–25 g) were killed by cervical dislocation, and the spleens were aseptically removed. Spleen cells were isolated by pressing the tissue through a sterilized 100‐mesh stainless steel sieve, and resuspended to 5 × 106 cells ml−1 in RPMI 1640 culture medium supplemented with 10% fetal bovine serum, 100 units penicillin ml−1, and 100 μg streptomycin/ml. The cells (7 × 105 cells/100 μl/well) were seeded into a 96‐well culture plate and serial dilutions of a solution of the isolated antifungal peptide in 100 μl medium were added. After incubation of the cells at 37°C in a humidified atmosphere of 5% CO2 for 24 h, 10 μl (methyl‐3H)‐thymidine (0·25 μCi, GE Healthcare) was added, and the cells were incubated for another 6 hr under the same conditions. The cells were then harvested with an automated cell harvester onto a glass fibre filter, and the radioactivity was measured with a Beckman model LS 6000SC scintillation counter. All reported values are the means of triplicate samples. Con A was used as a positive control and bovine serum albumin as a negative control (Wong and Ng 2003).
Results
The crude extract was fractionated on Affi‐gel blue gel into a larger unadsorbed fraction (BG1) and a smaller adsorbed fraction (BG2) (Fig. 1a). Antifungal activity resided only in fraction BG2, which was then resolved on SP‐Sepharose into an unadsorbed fraction and three adsorbed fractions (SP1, SP2 and SP3) of similar size which were desorbed with 0·2 mol l−1 NaCl, 0·5 mol l−1 NaCl, and 1 mol l−1 NaCl, respectively (Fig. 1b). Antifungal activity was concentrated in fraction SP2. Fraction SP2 was separated on Mono S into a tiny unadsorbed fraction (S1) and two adsorbed fractions (S2 and S3) (Fig. 1c). Antifungal activity was detected only in the smaller adsorbed fraction S2. Final purification of S2 on Superdex Peptide resulted in a tiny peak and a major absorbance peak, the latter representing purified antifungal peptide (Fig. 1d) which showed a molecular mass of 5·7 kDa in SDS‐PAGE (Fig. 2a). Its molecular mass as determined by mass spectrometry was 5716 Da (Fig. 2b). The yields of the chromatographic fractions with antifungal activity are presented in Table 1. It did not resemble previously reported Brassica peptides in N‐terminal sequence (Table 2). It lacked trypsin inhibitory activity toward BAEE when tested at an equimolar ratio to this substrate (Fig. 3) and was inactive on casein when tested up to 10 μmol l−1. The peptide exerted antifungal activity against various fungal species including Fusarium oxysporum (A), Helminthosporium maydis (B), Mycosphaerella arachidicola (C), and Valsa mali (D), with the ranking of potency being D>C>B>A. The IC50 values were respectively 3·9 μmol l−1 (A), 4·7 μmol l−1 (B), 2·6 μmol l−1 (C), and 0·22 μmol l−1 (D) (Fig. 4). The antifungal activity of the peptide was retained after exposure to various temperatures from 40°C to 100 °C (Fig. 5a), to the pH ranges 1–3 and 10–13 (Fig. 5b) and also after incubation with trypsin (not shown). It inhibited proliferation of HepG2 cells (Fig. 6a) and MCF7 cells (Fig. 6b), with an IC50 of 19·2 and 4·8 μmol l−1, respectively. It reduced the activity of HIV‐1 reverse transcriptase with an IC50 of 17·8 μmol l−1 (Fig. 6c). It was devoid of mitogenic activity (Fig. 6d).
Figure 1.
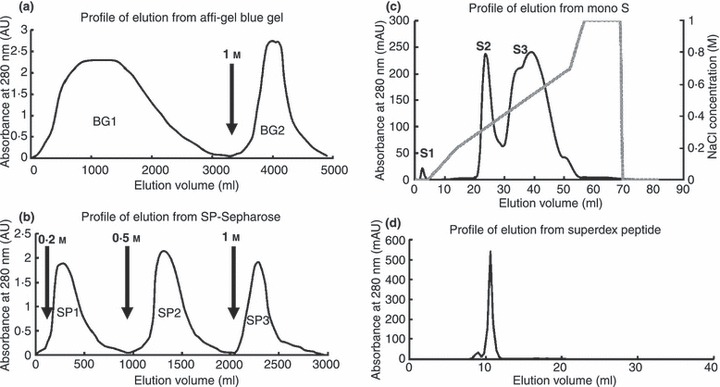
Purification of Brassica parachinensis antifungal peptide by chromatography on (a) Affi‐gel Blue gel, (b) SP‐Sepharose, (c) Mono S and (d) Superdex Peptide. Arrows indicate elution with NaCl in starting buffer.
Figure 2.
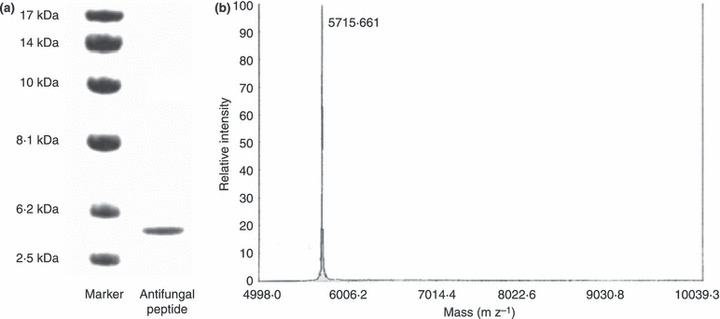
(a) SDS‐PAGE of Brassica parachinensisantifungal peptide. (b) Mass spectrometric analysis of Brassica parachinensis antifungal peptide.
Table 1.
Summary of purification of Brassica parachinensis antifungal peptide
| Column | Chromatographic fraction | Yield (mg) from 500 g seeds |
|---|---|---|
| Crude extract | 16 878 | |
| After Affi Gel Blue Gel | BG2 | 9322 |
| After SP Sepharose | SP2 | 984 |
| After Mono S | S2 | 306 |
| After Superdex Peptide | Purified peptide | 40 |
Table 2.
N‐terminal sequence of Brassica parachinensis antifungal peptide in comparison with other Brassica proteins
| N‐terminal sequence* | Mol wt (kDa) | |
|---|---|---|
| Brassica parachinensis antifungal peptide | †1DQFPQEYPGDVQFSFNALHIYPSPQVVVI29 | 5·7 |
| Brassica campestris antifungal peptide | 1ALSCGTVSGNLAACAGYV18 | 9·4 |
| Brassica parachinensis napin 8·8 kDa subunit | 1PQGPQQRPPLLQQQTNEEHE20 | 8·8 |
| Brasscia parachinensis napin 5 kDa subunit | 1PAGPFRIPKKRKKEE15 | 5 |
| Sinapis arvensis trypsin inhibitor | 1PQGPQQRPPLLQQ13 | 11 |
*The above sequences except Brassica parachinensis antifungal peptide were derived from reference (Lin et al. 2007) and (Ngai and Ng 2003).
†1D refers to D being the first N‐terminal amino acid residue.
Figure 3.
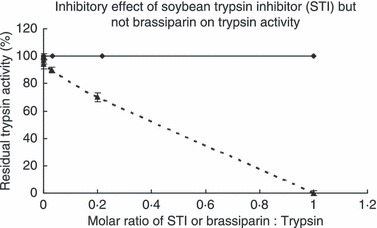
Lack of inhibitory effect of B. parachinensis antifungal peptide on the activity of trypsin. Soybean trypsin inhibitor (STI) was used as a positive control. Results are presented as mean ± SD (n = 3).( , Brassiparin on Trypsin;
, Brassiparin on Trypsin;  STI on Trypsin).
STI on Trypsin).
Figure 4.
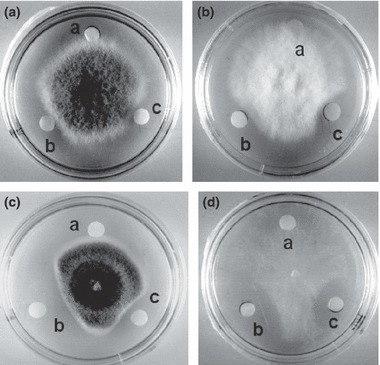
Antifungal activity of B. parachinensis antifungal peptide. The fungi tested were: plate (a) Fusarium oxysporum; plate (b) Helminthosporium maydis; plate (c) Mycosphaerella arachidicola, and plate (d) Valsa mali. The samples applied to the paper discs were as follows: (a) 10 μmol l−1 Tris–HCl buffer (pH 7·3) as control; (b) 10 μg of chestnut thaumatin‐like protein as positive control; (c) 5 μg of antifungal peptide.
Figure 5.
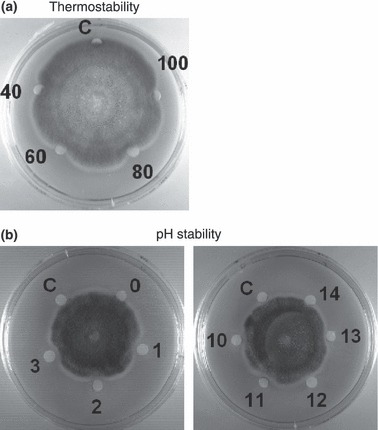
(a) Thermostability and (b) pH stability of antifungal activity of B. parachinensis antifungal peptide toward Mycosphaerella arachidicola. The same amount (2 μg) of peptide was added to each paper disc (except the control disc labelled as (c). The numbers in panel (a) (40–100) near the paper discs represent the various temperatures at which the antifungal peptide had been pretreated for 10 min before assay for antifungal activity. The numbers in panel (b), (0–3) on left plate and (10–14) on right plate represent the various pH values to which the antifungal peptide had been exposed for 30 min before assay for antifungal activity.
Figure 6.
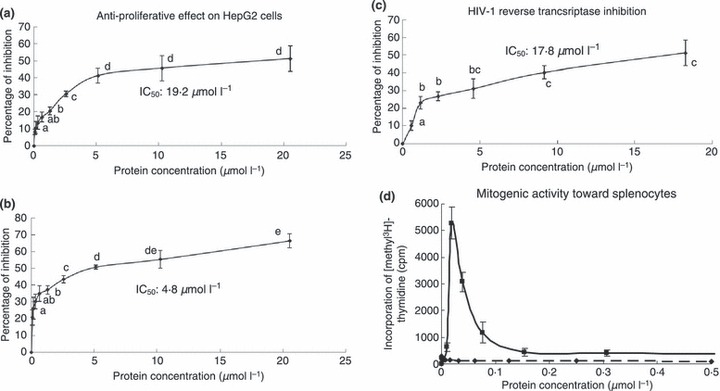
Brassica parachinensis antifungal peptide demonstrated antiproliferative activity (a, b), and HIV‐1 reverse transcriptase inhibitory activity (c), but lacked mitogenic activity (d). Data bearing different alphabets are statistically significant (P < 0·05) when tested by analysis of variance followed by Duncan’s multiple range test. ( , Antifungal peptide;
, Antifungal peptide;  , ConA).
, ConA).
Discussion
An antifungal peptide, with a molecular mass of 9 kDa and resembling nonspecific lipid transfer proteins in N‐terminal sequence, has previously been isolated from Brassica campestris seeds (Lin et al. 2007). It constitutes the first report on the isolation of an antifungal peptide from the seeds of a Brassica species. The present findings furnish evidence for the presence of an antifungal peptide in another Brassica species. However, the N‐terminal sequences of the two Brassica antifungal peptides were different although both manifested remarkable thermostability and pH stability, HIV‐1 reverse transcriptase inhibitory activity, and antiproliferative activity toward tumour cells. Both exerted antifungal activity toward F. oxysporum, M. arachidicola and a Helminthosporium species. This is noteworthy in view of the observation that some antifungal proteins exhibited antifungal activity toward only one fungus out of the several fungal species tested (Wang and Ng 2001, 2002a). Brassica parachinensis napin showed sequence similarity to Sinapis arvensis trypsin inhibitor and exhibited trypsin inhibitory activity. However, brassiparin did not resemble its homologous napin in N‐terminal sequence and exhibited no trypsin inhibitory activity. This is noteworthy in view of the fact that some of the trypsin inhibitors demonstrated antifungal activity (Ye et al. 2001). Both Brassica antifungal peptides were also isolated by using similar protocols. Ion exchange chromatography on Q‐Sepharose, affinity chromatography on Affi‐gel blue gel, ion exchange chromatography on Mono S, and gel filtration on Superdex Peptide were used for isolation of B. campestris antifungal peptide. The present protocol for brassiparin differed only in the replacement of Q‐Sepharose by SP‐Sepharose.
The yield of brassiparin (80 mg/kg) was lower than that of B. campestris antifungal peptide (175 mg kg−1). Its HIV‐1 reverse transcriptase inhibitory activity (IC50 = 17·8 μmol l−1) was also lower than that of B. campestris antifungal peptide (IC50 = 4 μmol l−1), but more potent than many anti‐HIV‐1 natural products (Ng et al. 1997). Similarly, its antiproliferative activity toward HepG2 cells and MCF‐7 cells (IC50 = 19·2 and 4·8 μmol l−1, respectively) was lower than that of B. campestris antifungal peptide (IC50 = 5·8 and 1·6 μmol l−1, respectively). Antifungal peptides from both B. parachinensis peptide and B. campestris were devoid of mitogenic activity toward mouse splenocytes. It has previously been shown that some (Ye et al. 2001; Wang and Ng 2003; Chu and Ng 2004) but not other (Lin et al. 2007) antifungal proteins/peptides exhibited mitogenic activity. Both brassiparin and B. campestris antifungal peptide had no inhibitory effect on HIV‐1 integrase, unlike some other antifungal proteins (Ng et al. 2003). They were also inactive toward SARS coronavirus proteinase. Frech bean defensin‐like antifungal peptide resembled the two Brassica antifungal peptides in that it did not have any suppressive effect on HIV‐1 integrase and SARS proteinase (Leung et al. 2008).
It is noteworthy that napin‐like polypeptides with trypsin‐inhibitory activity but devoid of antifungal activity have been isolated from seeds of various Brassica species (Ngai and Ng 2003, 2004a, 2004b; Ng and Ngai 2004). A trypsin‐inhibitor has also been isolated from Brassica napus seeds (Ceciliani et al. 1994). These proteins demonstrate N‐terminal amino acid sequences different from those of brassiparin. The isolation of this antifungal peptide adds to the literature on Brassica seeds.
When brassiparin is compared with turtle bean chitinase‐like antifungal protein (Chu and Ng, 2005) which is an example of a leguminous and non‐brassicaceous antifungal protein, it is seen that both are adsorbed on Affi‐gel blue gel and a cationic exchanger (SP‐Sepharose/CM‐cellulose). Both are devoid of mitogenic activity toward mouse splenocytes. The antifungal activity of brassiparin is more thermostable (fully preserved in brassiparin vs completely abrogated in the other after heating at 100°C for 10 min) and approximately eight‐fold more potent (IC50 = 3·9 μmol l−1 in brassiparin vs 35·1 μmol l−1 in the other toward F. oxysporum, and 3·6 μmol l−1 in brassiparin vs 18·3 μmol l−1 in the other toward M. arachidicola).
In summary, the antifungal peptide isolated from B. parachinensis L.H.Bailey seeds has potentially exploitable activities such as (i) potent antifungal activity with remarkable thermostability and pH stability, (ii) HIV‐1 reverse transcriptase inhibitory activity, and (iii) antiproliferative activity toward tumour cells. In contrast, some antifungal proteins like mungbean chitinase lack the last two activities (Lin et al. 2007).
Acknowledgements
We thank Miss Kathy Lau and Miss Grace Chan for excellent secretarial assistance.
References
- Au, T.K. , Collins, R.A. , Lam, T.L. , Ng, T.B. , Fong, W.P. and Wan, D.C. (2000) The plant ribosome inactivating proteins luffin and saporin are potent inhibitors of HIV‐1 integrase. FEBS Lett 471, 169–172. [DOI] [PubMed] [Google Scholar]
- Bormann, C. , Baier, D. , Hörr, I. , Raps, C. , Berger, J. , Jung, G. and Schwarz, H. (1999) Characterization of a novel, antifungal, chitin‐binding protein from Streptomyces tendae Tü901 that interferes with growth polarity. J Bacteriol 181, 7421–7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert, W.F. , Marien, W. , Terras, F.R. , De Bolle, M.F. , Proost, P. , Van Damme, J. , Dillen, L. , Claeys, M. , et al. (1992) Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine‐rich domain of chitin‐binding proteins. Biochemistry 31, 4308–4314. [DOI] [PubMed] [Google Scholar]
- Broekaert, W.F. , Van Parijs, J. , Leyns, F. , Joos, H. and Peumans, W.J. (1989) A chitin‐binding lectin from stinging nettle rhizomes with antifungal properties. Science 245, 1100–1102. [DOI] [PubMed] [Google Scholar]
- Bulet, P. , Hetru, C. , Dimarcq, J.L. and Hoffmann, D. (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23, 329–344. [DOI] [PubMed] [Google Scholar]
- Cammue, B.P.A. , Thevissen, K. , Hendriks, M. , Eggermont, K. , Goderis, I.J. , Proost, P. , Van Damme, J. , Osborn, R.W. , et al. (1995) A potent antimicrobial protein from onion seeds showing sequence homology to plant lipid transfer protein. Plant Physiol 109, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceciliani, F. , Bortolotti, F. , Menegatti, E. , Ronchi, S. , Ascenzi, P. and Palmieri, S. (1994) Purification, inhibitory properties, amino acid sequence and identification of the reactive site of a new serine proteinase inhibitor from oil‐rape (Brassica napus) seed. FEBS Lett 342, 221–224. [DOI] [PubMed] [Google Scholar]
- Caruso, C. , Caporale, C. , Chilosi, G. , Vacca, F. , Bertini, L. , Magro, P. , Poerio, E. and Buonocore, V. (1996) Structural and antifungal properties of a pathogenesis‐related protein from wheat kernel. J Protein Chem 15, 35–44. [DOI] [PubMed] [Google Scholar]
- Chu, K.T. and Ng, T.B. (2004) First report of a glutamine‐rich antifungal peptide with immunodulatory and antiproliferative activities from family Amaryllidaceae. Biochem Biophys Res Commun 325, 167–173. [DOI] [PubMed] [Google Scholar]
- Chu, K.T. and Ng, T.B. (2005) Purification and characterization of a chitinase‐like antifungal protein from black turtle bean with stimulatory effect on nitric oxide production by macrophages. Biol chem 386, 19–24. [DOI] [PubMed] [Google Scholar]
- Fujimura, M. , Minami, Y. , Watanabe, K. and Tadera, K. (2003) Isolation, characterization and sequencing of a novel type of antimicrobical peptides, Fα‐AMP1 and Fα‐AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench). Biosci Biotechnol Biochem 67, 636–642. [DOI] [PubMed] [Google Scholar]
- Gozia, O. , Ciopraga, J. , Bentia, T. , Lungu, M. , Zamfirescu, I. , Tudor, R. , Roseanu, A. and Nitu, F. (1993) Antifungal properties of lectin and new chitinases from potato tuber. C R Acad Sci III 316, 245–249. [PubMed] [Google Scholar]
- Huang, X. , Xie, W. and Gong, Z. (2000) Characteristics and antifungal activity of a chitin binding protein from Ginkgo biloba . FEBS Lett 478, 123–126. [DOI] [PubMed] [Google Scholar]
- Joshi, B.N. , Sainani, M.N. , Bastawade, K.B. , Gupta, V.S. and Ranjekar, P.K. (1998) Cysteine protease inhibitor from pearl millet: a new class of antifungal protein. Biochem Biophys Res Commun 246, 382–387. [DOI] [PubMed] [Google Scholar]
- Lam, S.K. and Ng, T.B. (2001) Isolation of a small chitinase‐like antifungal protein from Panax notoginseng (sanchi ginseng) roots. Int J Biochem Cell Biol 33, 287–292. [DOI] [PubMed] [Google Scholar]
- Lam, Y.W. , Wang, H.X. and Ng, T.B. (2000) A robust cysteine‐deficient chitinase‐like antifungal protein from inner shoots of the edible chive Allium tuberosum . Biochem Biophys Res Commun 279, 74–80. [DOI] [PubMed] [Google Scholar]
- Leah, R. , Tommerup, H. , Svendsen, I. and Mundy, J. (1991) Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem 246, 1564–1573. [PubMed] [Google Scholar]
- Leung, E.H.W. , Wong, T.H. and Ng, T.B. (2008) Concurrent purification of two defense proteins from French bean seeds: a defensin‐like antifungal peptide and a hemagglutinin. J Peptide Sci 14, 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, P. , Xia, L. , Wong, J.H. , Ng, T.B. , Ye, X.Y. , Wang, S.Y. and Shi, X.Z. (2007) Lipid transfer proteins from Brassica campestris and mung bean surpass mung bean chitinase in exploitability. J Peptide Sci 13, 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, T.B. , Au, T.K. , Lam, T.L. , Ye, X.Y. and Wan, D.C.C. (2003) Inhibitory effects of antifungal proteins on human immunodeficiency virus type 1 reverse transcriptase, protease and integrase. Life Sci 70, 927–936. [DOI] [PubMed] [Google Scholar]
- Ng, T.B. , Huang, B. , Fong, W.P. and Yeung, H.W. (1997) Anti‐HIV natural products with special emphasis on HIV reverse transcriptase inhibitors. Life Sci 61, 933–949. [DOI] [PubMed] [Google Scholar]
- Ng, T.B. and Ngai, P.H.K. (2004) The trypsin‐inhibitory, immunostimulatory and antiproliferative activities of a napin‐like polypeptide from Chinese cabbage seeds. J Peptide Sci 10, 103–108. [DOI] [PubMed] [Google Scholar]
- Ngai, P.H.K. and Ng, T.B. (2003) Isolation of a napin‐like polypeptide with potent translation‐inhibitory activity from Chinese cabbage (Brassica parachinensis cv green‐stalked) seeds. J Peptide Sci 9, 442–449. [DOI] [PubMed] [Google Scholar]
- Ngai, P.H.K. and Ng, T.B. (2004a) A napin‐like polypeptide from dwarf Chinese white cabbage seeds with translation‐inhibitory, trypsin‐inhibitory, and antibacterial activities. Peptides 25, 171–176. [DOI] [PubMed] [Google Scholar]
- Ngai, P.H.K. and Ng, T.B. (2004b) A napin‐like polypeptide with translation‐inhibitory, trypsin‐inhibitory, antiproliferative and antibacterial activities from kale seeds. J Peptide Res 64, 202–208. [DOI] [PubMed] [Google Scholar]
- Nielsen, T.B. and Reynolds, J.A. (1978) Measurements of molecular weights by gel electrophoresis. Meth Enzymol 48, 3–10. [DOI] [PubMed] [Google Scholar]
- Park, M.J. , Gwak, K.S. , Yang, I. , Choi, W.S. , Jo, H.J. , Chang, J.W. , Jeung, E.B. and Choi, I.G. (2007) Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J Microbiol 45, 460–465. [PubMed] [Google Scholar]
- Parkash, A. , Ng, T.B. and Tso, W.W. (2002) Isolation and characterization of luffacylin, a ribosome inactivating peptide with antifungal activity from sponge gourd (Luffa cylindrical) seeds. Peptides 23, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Pressey, R. (1997) Two isoforms of NP24: a thaumatin‐like protein in tomato fruit. Phytochemistry 44, 1241–1245. [DOI] [PubMed] [Google Scholar]
- Selitrennikoff, C.P. (2001) Antifungal proteins. Appl Environ Microbiol 67, 2883–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silici, S. and Koc, A.N.. (2006) Comparative study of in vitro methods to analyse the antifungal activity of propolis against yeasts isolated from patients with superficial mycoses. Lett Appl Microbiol 43, 318–324. [DOI] [PubMed] [Google Scholar]
- Vasilevskiĭ, A.A. , Kozlov, S.A. , Zhmak, M.N. , Kudelina, I.A. , Dubovskiĭ, P.V. , Shaturskiĭ, O.Ia. , Arsen’ev, A.S. and Grishin, E.V. (2007) Synthetic analogues of antimicrobial peptides from the venom of the Central Asian spider Lachesana tarabaevi . Bioorg Khim 33, 405–412. [PubMed] [Google Scholar]
- Vogelsang, R. and Barz, W. (1993) Purification, characterization and differential hormonal regulation of a β‐1, 3‐glucanase and two chitinases from chickpea (Cicer arietinum L.). Planta 189, 60–69. [DOI] [PubMed] [Google Scholar]
- Wang, H. and Ng, T.B. (2000) Ginkbilobin, a novel antifungal protein from Ginkgo biloba seeds with sequence similarity to embryo‐abundant protein. Biochem Biophys Res Commun 279, 407–411. [DOI] [PubMed] [Google Scholar]
- Wang, H. and Ng, T.B. (2001) Isolation of a novel deoxyribonuclease with antifungal activity from Asparagus officinalis seeds. Biochem Biophys Res Commun 289, 102–104. [DOI] [PubMed] [Google Scholar]
- Wang, H. and Ng, T.B. (2002a) Ascalin, a new antifungal peptide with human immunodeficiency virus type 1 reverse transcriptase inhibitory activity from shallot bulbs. Peptides 23, 1025–1029. [DOI] [PubMed] [Google Scholar]
- Wang, H. and Ng, T.B. (2002b) Isolation of an antifungal thaumatin‐like protein from kiwi fruits. Phytochemistry 61, 1–6. [DOI] [PubMed] [Google Scholar]
- Wang, H. and Ng, T.B. (2004a) Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii . Peptides 25, 1–5. [DOI] [PubMed] [Google Scholar]
- Wang, H. and Ng, T.B. (2004b) Alveolarin, a novel antifungal polypeptide from the wild mushroom, Polyorus alveolaris . Peptides 25, 693–696. [DOI] [PubMed] [Google Scholar]
- Wang, H.X. and Ng, T.B. (2003) Isolation of cucurmoschin, a novel antifungal peptide abundant in arginine, glutamate and glycine residues from black pumpkin seeds. Peptides 24, 969–972. [DOI] [PubMed] [Google Scholar]
- Wilson, S. , Mahiou, B. , Reiger, R. , Tentler, S. , Schimoler, R. , Orndorff, S. and Selitrennikoff, C.P. (2000) Pilot‐scale purification of zeamatin, an antifungal protein from maize. Biotechnol Prog 16, 38–43. [DOI] [PubMed] [Google Scholar]
- Wong, J.H. and Ng, T.B. (2003) Gymnin, a potent defensin‐like antifungal peptide from the Yunnan bean Gymnocladus chinensis Baill. Peptides 24, 963–968. [DOI] [PubMed] [Google Scholar]
- Ye, X.Y. , Ng, T.B. and Rao, P.F. (2001) A Bowman‐Birk‐type trypsin‐chymotrypsin inhibitor from broad beans. Biochem Biophys Res Commun 289, 91–96. [DOI] [PubMed] [Google Scholar]


