Abstract
Background
The fungal toxin acts as effective, low-cost chemical substances for pest control worldwide and also an alternative to synthetic insecticides. This study assessed the larvicidal potential of Metarhizium anisopliae fungi derived metabolites against Aedes aegypti, Anopheles stephensi, Culex quinquefasciatus and non-targeted organisms at 24hr post treatment.
Method
Isolation of entomopathogenic fungi M. anisopliae from natural traps confirmed by using 18s rDNA biotechnological tools. Crude extracts from M. anisopliae solvent extraction and their secondary metabolites were bio-assayed following WHO standard procedures against Ae. aegypti, An. stephensi and Cx. quinquefasciatus, Artemia nauplii, Eudrilus eugeniae, and Solanum lycopersicum after 24 hr exposure. Histopathological analysis of E. eugeniae treated with fungi metabolites toxicity compared to those treated with Monocrotophos after 24hrpost-treatment. M. anisopliae metabolites were characterized using GC-MS and FT-IR analysis.
Results
The larvicidal activity was recorded in highest concentration of 75μg/ml, with 85%, 97% and 89% mortality in Ae. aegypti, An. stephensi and Cx. quinquefasciatus respectively. M. anisopliae metabolites produced LC50 values in Ae. aegypti, 59.83μg/ml, in An. stephensi, 50.16μg/ml and in Cx. quinquefasciatus, 51.15μg/ml respectively. M. anisopliae metabolites produced lower toxic effects on A. nauplii, LC50 values were, 54.96μg/ml respectively. Bio-indicator toxicity results show 18% and 58% mortality was recorded in E. eugeniae and A. nauplii and also there is no phytotoxicity that was observed on S. lycopersicum L. under semi-field condition. E. eugeniae histopathological studies shows fungal metabolites showed lower sub-lethal effects compared to synthetic chemical pesticide at 24hrs of the treatment. The GC-MS and FT-IR analysis identified five major components of active ingredients.
Conclusion
Findings of this study indicate that, M. anisopliae ethyl acetate derived secondary metabolites are effective against larvae of Ae. aegypti, An. stephensi and Cx. quinquefasciatus mosquito species, lower toxicity effects were observed on non-target organisms such as, Artemia nauplii, Eudrilus eugeniae as well as, no toxicity effect were observed on Solanum lycopersicum. Further research should be conducted in laboratory for separation of single pure molecule and be tested semifield conditions.
Introduction
In vector control programme, there is increased usage of same or different types of chemical insecticide for vector control which synthetic chemical pesticide, insect growth regulators and chemical repellent cause environmental pollution, also affect non-target organisms and mosquito species get insecticide resistance capacity [1]. For that reason, require of ecofriendly green pesticide for mosquito control. So researcher find effective molecules from biological sources such as micro-organisms and plants contain considerable amount of metabolites/essential oils which are promising alternative to synthetic insecticides [2–6].
In recent past, it has been found that, entomopathogenic fungi secondary metabolites is used for mosquito control in confined experiments [4, 5, 7]. Fungi have high toxicity to targeted insects (Arthropods), and very low toxic effect on non-target E. eugeniae. The entomopathogenic fungus contain enormous amount of secondary metabolites with high toxicity to larvae, pupae and adult mosquito species as confirmed by Vivekanandhan and others [4–6]. Many studies have reported interesting research results on the toxicity of fungi crude metabolites extracts of Beauveria bassiana, Fusarium oxysporum, Metarhizium anisopliae on larvae, pupae and adult of mosquito vector species [4–6, 8]. Until to date, many extracellular crude metabolites isolated from different fungi species such as, Metarhizium sp., Beauveria sp., Tolypocladium sp., F. oxysporum, Lagenidium giganteum have been tested for larvicidal potential on major mosquito species [4–6, 9–13]. Artemia nauplii are bisexual organisms in salt water environment. These species are adaptability in high salinity and temperatures range from 5–30°C. The A. nauplii have small lifecycle also fecundity level is high. These, Artemia organisms are perfect for the toxicological evaluations, they providing easy implementation, efficiency. Artemia sp. can be used for short and long term toxicity assay for to detect hazardous chemical and toxicant in aqueous environment, for the example, plant secondary metabolites, toxic chemicals as well as, toxic nanoparticles.
Earthworms (Eudrilus eugeniae) are important invertebrate in soil environment that are directly contact to soil environment. Leachates in soil environment may infuse their skin in addition to, alimentary tract thus causing eco-toxicological efficacy. Due to their eco-toxicological significance, high biomass and sensitivity to environmental contamination, earthworms have been reported to lookout organisms for soil monitor the eco-toxicological risks estimation of pollutant in soil ecosystems. To this effect, Eudrilus eugeniae and Artemia nauplii have been used in eco-toxicological risks monitoring and as bio-indicator of lethal and non-lethal toxicant from entomopathogenic fungi derived secondary metabolites. The present study isolated entomopathogenic fungi M. anisopliae and their secondary metabolites for evaluation of lethal and sub-lethal effects against three mosquitoes vector species and non-targeted organism for 24hr post-treatments. The Fungi secondary metabolites were characterized using GC-MS and FT-IR analysis.
Materials and methods
Source of fungi culture
Fungi infected dead A. aegypti mosquito larvae were collected from natural traps at the Sanarappatti village forest, Dharmapuri district, Tamil Nadu, India (12.0933° N, 78.2020° E). Dead mosquito larvae were washed with sterile distilled water then washed with 70% ethanol for 2-3minto remove saprophytic fungi on the surface of mosquito larvae. The dead larvae were washed with sterile distilled water then placed in sabouraud’s dextrose agar (SDA) plate and incubated for 5to 7 days at 26 ± 2°C. The grown fungi cultures were sub-cultured and pure culture was maintained at 4°C. Permission to undertake this study was granted by Periyar University. The present research does not have any evaluation/test in humans performed by any of the authors contained in this article. We followed all national and international guidelines for the use of target and non-target organisms. The Forest conservation authority in the Sanarappatti village granted permission to do sampling of the needed materials for this study.
Mass culturing of M.anisopliae
M.anisopliae broth culture was prepared for isolation of genomic DNA (genetic material) using the method developed by Vivekanandhan and others [6]. The 100ml of Sabouraud’s Dextrose Broth culture media were prepared in 250ml of the conical flask, and then sterilized at 120°C with the help of an autoclave for 20mins. After sterilization, the media were transferred to a laminar airflow chamber. Then, 1×108 conidia/ml was transferred to the liquid medium. After conidial inoculation, 0.5 g/ml of chloramphenicol was added as antibacterial agent and mixed well with culturing medium, the culturing medium was incubated at 26°C for 10 days.
Crude extraction from M. anisopliae
After incubation the fungus biomass was removed from the culturing medium using Whatman No. 1 filtering paper then fungal biomass were washed with double distilled water for remove the unwanted materials from the medium. Fungal biomass 500g was transferred to 1000 ml glass beakers contains 750ml of ethyl acetate solvent, which was mixed with the fungal mycelial mat for cold extraction for 15 days at 28±oC. Subsequent to complete crude secondary metabolites extraction the light-yellow color portion (organic phase) was separated from fungal mat using Whatman No.1 paper. Collected acetate extracts crude extract were then concentrated using a vacuum evaporator (Superfit-R/150/11, Mumbai, India).
Genomic DNA isolation
Fungi were grown in 100ml of sabouraud’s dextrose broth (SDB) in 150ml of culturing medium which was incubated at (26±2o C)for up to 5 days. After 5 days fungi mycelia filtered using Whatman no.1 filter paper and fungal mats were used for isolation of genomic DNA as described by Vivekanandhan and others [6]. Five grams (5g) of M. anisopliae biomass was ground with liquid nitrogen using sterile mortar with pestle. Then, 1.5ml freshly prepared CTAB buffer was added to grinded fungal mycelium. The mixture was transferred to sterile centrifuge tubes and placed in water bath at 62o C for incubation for the period of 1 hr. After complete incubation time, tubes were transferred to cooling condition of 4°C for 15mins, then tubes were centrifuged at 8,000rpm for 12mins (Remi cooling centrifuge-C-24BL, India).After centrifugation DNA pellet was taken and the supernatant was discarded. The pellet was washed with 70% ethanol then air-dried. Pure genomic DNA was taken for purity confirmation.
Polymerase chain reaction (PCR)
Genomic DNA was amplified, using forward (GTAGTCATATGCTTGTCTC) and reverse (CTTCCGTCAATTCCTTTAAG). PCR amplification reactions were carried out in a 20μl of PCR master mix contained 1X PCR buffer, 0.2mM each dNTPs, 1.5 μl DNA, 0.2 μl Phire Hotstart II DNA polymerase enzyme, 0.1 mg/ml BSA and 3% DMSO, 0.5M Betaine, 0.2 μl of forward and reverse primers. Polymerase chain reaction temperatures followed, initial stage at 95°C 5mins; denaturing at 94o C for 30°C and annealing temperature was 50°C for 30 seconds; elongation temperature at 72°C for 2 mins and extension temperature at 72°C with 7mins.
Sequence analysis
Sequence quality was confirmed by using sequence scanner software version-1.0 connected with a personal computer, the sequence editing and alignment were carried out by using Geneious Professional version-5.1.
Mosquito colonies rearing
An. stephensi, Ae. Aegypti and Cx. quinquefasciatus larvae were obtained from the Institute of Vector Control Zoonoses, Hosur, Tamil Nadu, India. The larval rearing was maintained at temperature of 26±2°C, 70–80% relative humidity and 14:10 light and dark photoperiod. Larvae were fed with Eleusine coracana millet powder for proper growth and development.
Non-target species rearing
Eudrilus eugeniae stock was maintained at a temperature of 27 ±2°C in Molecular Entomology Laboratory, Periyar University, Salem, Tamil Nadu, India. Artemia nauplii larvae were maintained in 1000 ml of seawater and salinity maintained at 30 parts per trillion in culture medium with pH range of 7–8 and photoperiod 17:7 light and dark. The temperature was 27 ±2°C and oxygen was supplied by aspirator.
Larval toxicity
Larval toxicity test was carried out as per the method developed by World Health Organization, [4, 5, 14]. Five different fungal concentration (0.5, 10, 25, 50 and 75μg/ml) were tested with 25 larvae of 3rd instars larvae of mosquito species. For each concentration three replicates were tested. Control was treated with DMSO as a negative control. After 24 hr post-treatment, the dead larvae were counted. Mortality was calculated using Abbott’s formula, 1925 and Probit analysis calculated [15].
Toxicity bioassay on A. nauplii
Mature A. nauplii were collected with hand pipette and used for toxicity assays under different M. anisopliae, B. bassiana and V. lecanii metabolites concentrations (0.5, 10, 25, 50 and 75μg/ml). DMSO only solution was used as control. The mortality of the A.nauplii was recorded after 24 hr. Three replicates for each concentration were performed.
Artificial soil toxicity
Artificial soil contains 25% kaolinite clay, 15% sphagnum peat, 75% fine soil was taken for artificial soil toxicity tests following OECD guideline [16]. Few drops of CaCO3 were added to regulate the pH to 6.0 ± 0.2. Soil water content was maintained for 30–32%. The soil was prepared by adding different concentrations of fungi extract and chemical insecticide (100μg/kg and 500μg/kg) on a dry weight basis. Fifteen (15) adult E. eugeniae was transferred to 1000g filled with test substrate with desired concentration and bioassay containers were closed with a proper lid for preventing the E. eugeniae escapes. After 24days exposure time, the dead E. eugeniae were counted. Each concentration had three replicates and one replicate had ten E. eugeniae. The control set without any fungal extract and replicated thrice.
Histopathological confirmation of fungal toxicity
After 24hr post-treatment of fungi crude metabolites the exposed E. eugeniae (treatment) and unexposed (control) were separately fixed with 4% formalin for 2-6hr at 4°C. Blocks were cooled at 25°C for 3hr then slash into 3μm thickness, with 0.5 mm ribbons, using a microtome (Leica, Germany). E.eugeniae was stained with Ehrlich’s hematoxylin and eosin. The dried slides were observed under a light microscope (Olympus-CH20i/India), with 100X and 400X magnifications.
Statistical analysis
After 24hr treatment mortality of target mosquito and non-target species, earthworm was calculated by using Abbott’s formula, 1925 [15]. Lethal concentration of mosquito larvae, E. eugeniae were calculated and their LC50 values and chi-square tests were done using SPSS (Inc., Chicago, USA) version 25.0 for analyses the derived results and the significance (P < 0.05) levels.
Results
Microscopic identification of M. anisopliae
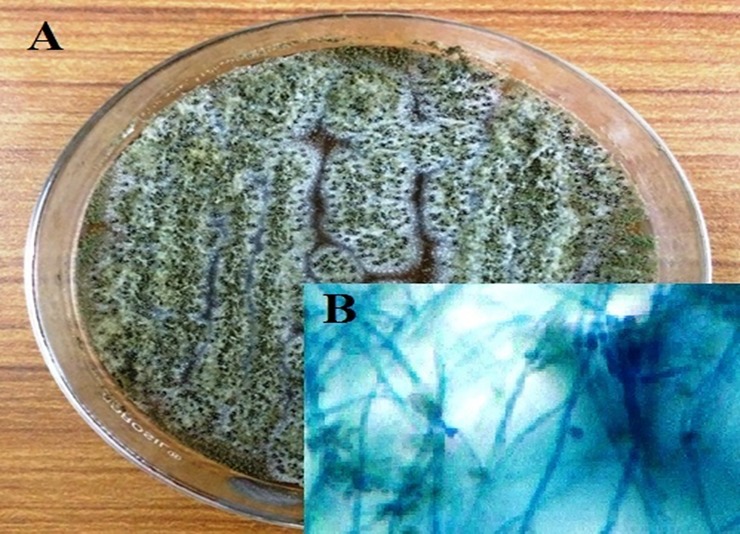
M.anisopliae fungi and their morphological were characterized. The results clearly show that the conidial color is dark green in color and their conidial structure is slender in shape (Fig 1). Based on morphological verification the fungus culture was conformed as Metarhizium genus.
Fig 1. Pure culture of M. anisopliae fungi isolated from dead A. aegypti larvae.
A). M. anisopliae; B). Fungi conidia stained with lacto phenol cotton blue stain under light microscope at 40X magnification.
Molecular identification and phylogenetic construction
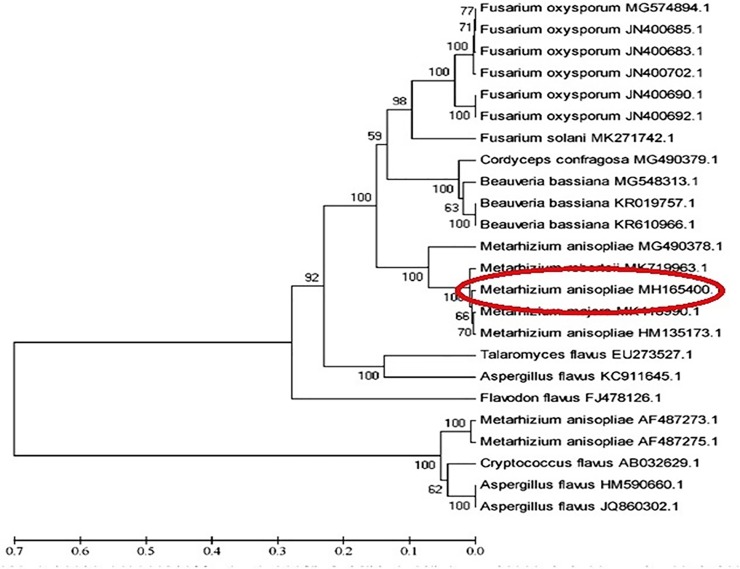
M. anisopliae genomic DNA was amplified using the polymerase chain reaction (PCR) with 18s rDNA universal primer. The M. anisopliae fungi sequence was deposited in the National Center of Biotechnology Information (NCBI) database, and their accession number is NCBI (MH165400.1). M.anisopliae18s rDNA sequence was 100% match with M. anisopliae species. Based on these results, isolated fungi culture was conformed as M. anisopliae. Neighbor-joining tree method was used for identification of M. anisopliae their taxonomy and evolution (Fig 2).
Fig 2. Phylogenetic tree construction for evolutionary steps of isolated entomopathogens was inferred using the neighbor-joining tree method.
Mortality bioassay
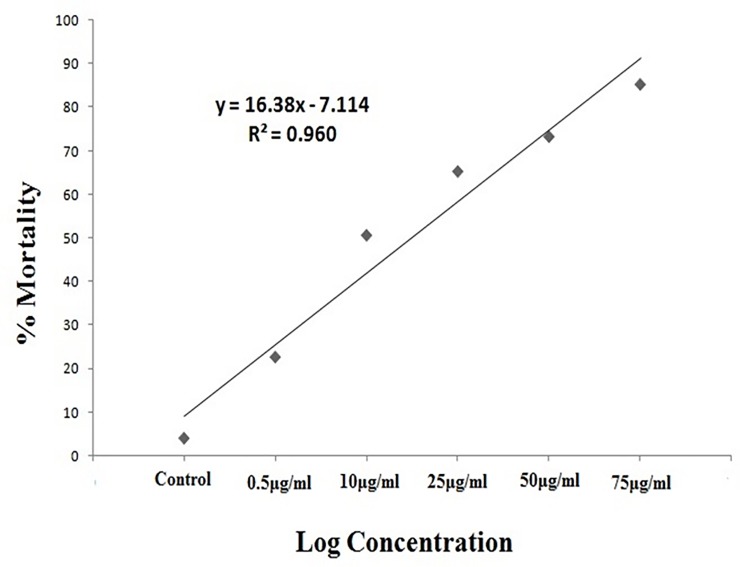
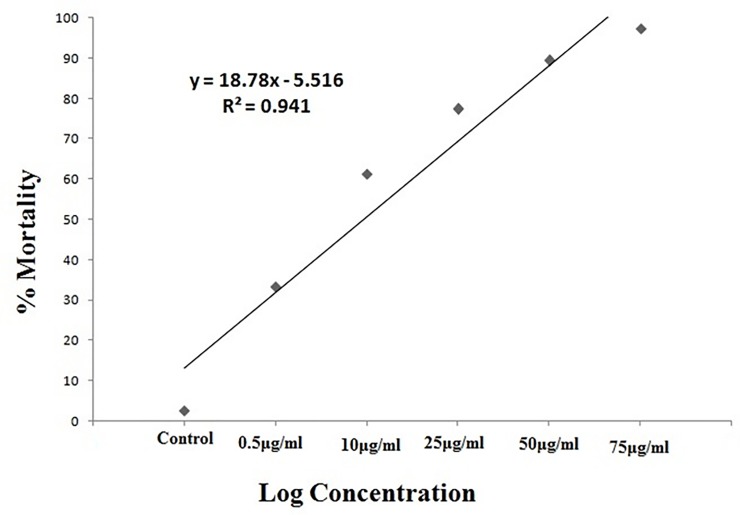
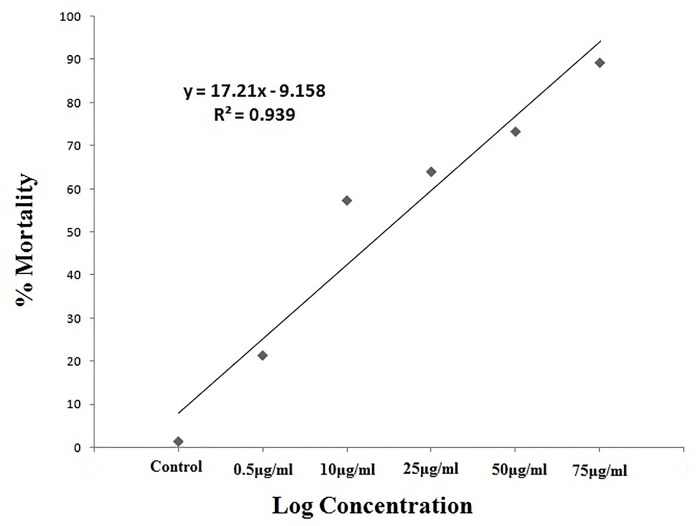
The significant differences in mortality rates were observed on larvae of Ae. Aegypti (Fig 3) An. stephensi (Fig 4) and Cx. Quinquefasciatus (Fig 5) mosquitoes in laboratory conditions at 24hr post treatment. The M. anisopliae metabolites cause mortality of larval varying from 22 to 85% (F = 660.3, P < 0.001, df = 12) in A. aegypti, for An. stephensi varied from 33 to 97% (F = 748.7, P < 0.001, df = 12) while for Cx. quinquefasciatus varied from 21 to 89% (F = 466.1, P < 0.001, df = 12) (Table 1). M.anisopliae crude metabolites produced lower LC50 values in A. aegypti (LC50, 59.83μg/ml for An. stephensi(LC50, 50.16μg/ml) and Cx. quinquefasciatus (LC50, 51.15μg/ml) (Table 1).
Fig 3. Percentage of mortality of Ae. aegypti treated with M. anisopliae derived secondary metabolites using linear regression analysis.
Fig 4. Percentage of mortality of An. stephensi treated with M. anisopliae derived secondary metabolites using linear regression analysis.
Fig 5. Percentage of mortality of Cx. quinquefasciatus treated with M. anisopliae derived secondary metabolites using linear regression analysis.
Table 1. Larvicidal activities of M. anisopliae derived secondary metabolites on larvae of Ae.aegypti, An.stephensi and Cx. quinquefasciatus mosquitoes at 24hr post treatment.
| Mosquito species (na = 450) | Concentration (μg/ml) | % Mortality (μg/ml) | LC50(LCL-UCL) (μg/ml) | χ 2 (df = 12) | F value |
|---|---|---|---|---|---|
| A. aegypti | Control | 4.00 | 59.83 (43.64–72.27) | 22.8* | 660.3 |
| 5 | 22.66 | ||||
| 10 | 50.66 | ||||
| 25 | 65.33 | ||||
| 50 | 73.33 | ||||
| 75 | 85.33 | ||||
| A n. stephensi | Control | 2.66 | 50.16 (48.163–54.97) | 32.9* | 748.7 |
| 5 | 33.33 | ||||
| 10 | 61.33 | ||||
| 25 | 77.33 | ||||
| 50 | 89.33 | ||||
| 75 | 97.33 | ||||
| Cx. quinquefasciatus | Control | 1.33 | 51.15 (43.90–60.43) | 19.2* | 466.1 |
| 5 | 21.33 | ||||
| 10 | 57.33 | ||||
| 25 | 64.00 | ||||
| 50 | 73.33 | ||||
| 75 | 89.33 |
na = total number of mosquito larvae used per each species, 25 larvae per replicate, three replicates were carried out, five concentrations were tested; LC50 = lethal concentration killing 50% of exposed organisms; LCL = 95% lower confidence limits; UCL = 95% upper confidence limits; χ2 = chi square (* s = significant (p < 0.05)); df = degrees of freedom.
Chemical characterization
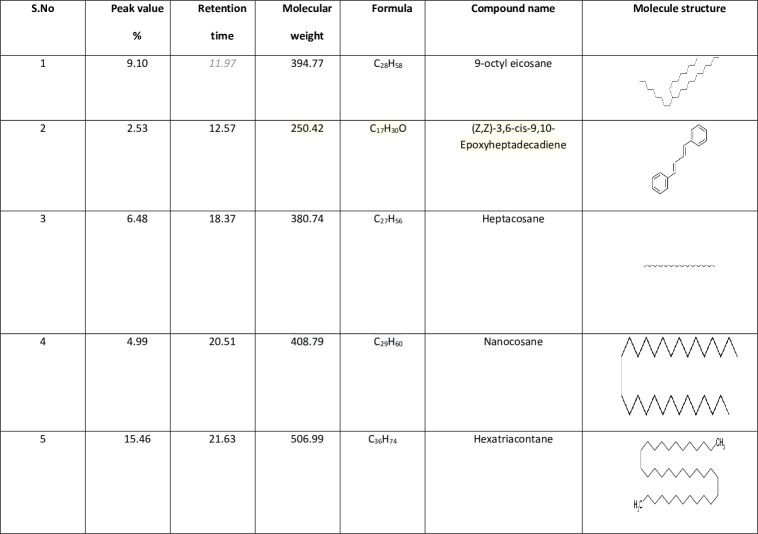
Results showed the presence of five major chemical constituents namely, Hexatriacontane (15.46%), 9-octyl eicosane (9.10%); Heptacosane (6.48%); Heptacosane (4.99%); (Z,Z)-3,6-cis-9,10-Epoxyheptadecadie (2.53%) as major and active compounds (Fig 6).
Fig 6. The M. anisopliae derived secondary metabolites chemical constituent were identified using GC-MS analysis.
Major functional groups identified using FT-IR analysis of M. anisopliae crude metabolites showed peaks range from 3952.19 to 495.02. These findings demonstrate that, M. anisopliae crude metabolites contain major functional group namely; Alcohols and Carboxylic acids. Major broad peaks were observed in 3952.19cm-1 this wave number assigned to O–H stretching, 3592.61cm-1 wave number assigned to = NOH (OH stretching) and 495.02cm-1 wave number assigned to S-S disulfide asym (Table 2).
Table 2. FT-IR analysis of M. anisopliae secondary metabolites for identification functional group present in the crude metabolites.
| S/N | Observed Wave Numbers (cm-1) | Peak Assignment | Functional Group | Visible Intensity |
|---|---|---|---|---|
| 1 | 3952.19 | O-H stretching | Alcohols | Small sharp peack |
| 2 | 3763.45 | O-H stretching | Alcohols | Small peack |
| 3 | 3699.51 | O-H stretching | Alcohols | Sharp peack |
| 4 | 3592.61 | = NOH (OH stretching) | Misc | Small sharp peack |
| 5 | 3376.04 | Dimer OH | Carboxylic acids | Small peack |
| 6 | 3459.69 | O-H stretching | Alcohols | Small peack |
| 7 | 3242.27 | Dimer OH | Carboxylic acids | Small peack |
| 8 | 495.02 | S-S disulfide asym | Misc | Small peack |
E. eugeniae toxicity assay
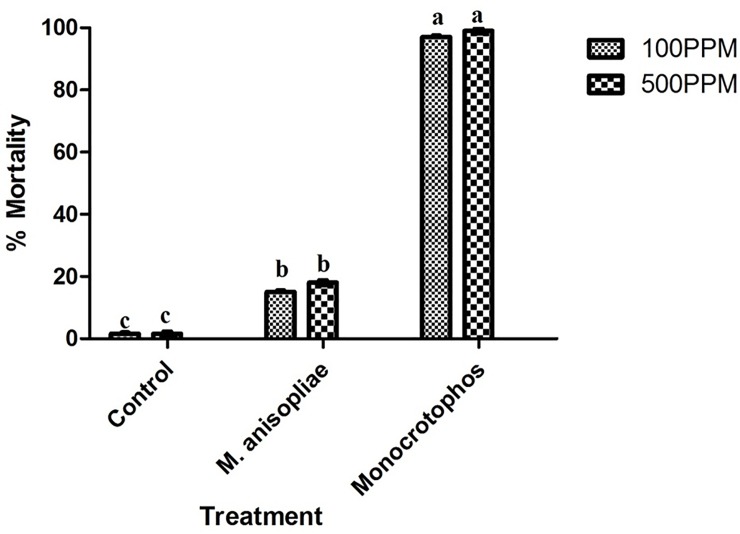
The lowest lethal toxicity was observed in E. eugeniae and limited mortality was observed in M. anisopliae compared to Monocrotophos at after 24hrpost-treatment. Eighteen percent (18%) mortality observed in M. anisopliae, 97% mortality in Monocrotophos and 4% mortality in control. The highest E. eugeniae mortality was observed in Monocrotophos pesticide treatment that recorded 97% (Fig 7).
Fig 7. Percentage mortality of E. eugeniae after treatment with M. anisopliae derived secondary metabolites and Monocrotophos at 24h post treatment.
Histopathology of E. eugeniae
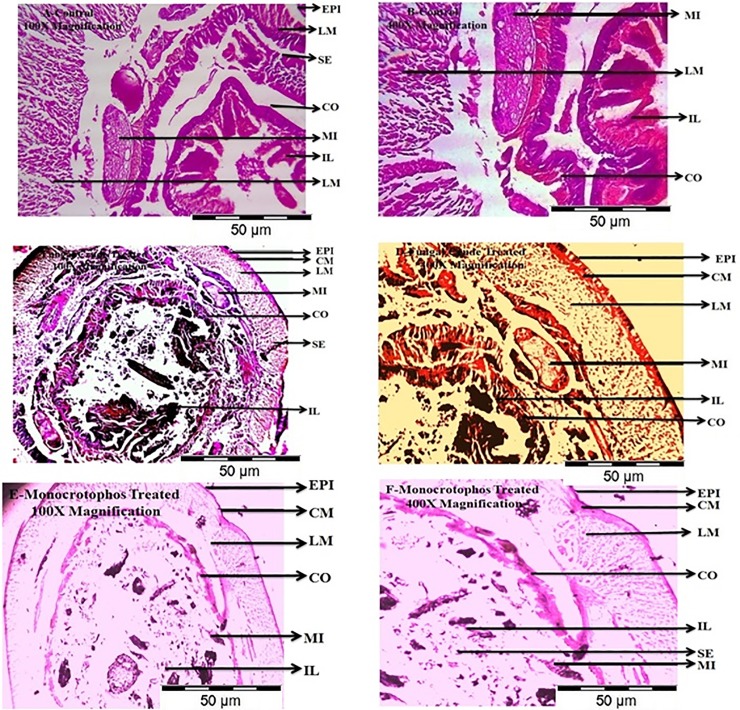
Sub-lethaleffect was observed in E. eugeniae gut tissues after 24hr post-treatment. At 100μg/ml of M. anisopliae fungi crude metabolites produced lower damage to gud tissues namely, intestinal lumen and intestine compared to Monocrotophos (Fig 8). The thickness of the epidermis, intestinal epithelium and the body wall of E. eugeniae were evaluated after treatments (Table 3).
Fig 8. Mid gut cross section of the earthworm, E. eugeniae exposed to M.anisopliae secondary metabolites for 24hr post treatment with 500μg/ml concentration.
E. eugeniae cross section was magnified at 100X and 400X under light microscope. A and B is control; C and D is Monocrotophos 500μg/ml treated; E and F is to M.anisopliae extract treated. Among the control and treated few histopathological changes was observed in intestinal lumen and intestine was totally damaged compare with control (EPI-epidermis; CM-circular muscle; LM-longitudinal muscle; SE-setae; CO-coelom; MI-mitochondrion; IL-intestinal lumen).
Table 3. Thickness of the epidermis, intestinal epithelium and body wall of E. eugeniae after the 24hr post treatment.
| Treatments | E. eugeniae | ||
|---|---|---|---|
| Epidermis (μm) | Intestinal epithelium (μm) | Body wall (μm) | |
| Control | 39.00±0.0c | 75.17±0.6c | 296.15±0.0c |
| M.anisopliae | 37.80±0.5b | 70.15±0.3b | 280.15±0.6b |
| Monocrotophos | 23.08±0.1a | 50.20±0.6a | 211.15±0.3a |
Values followed by different superscripts between treatments are significantly different according to the ANOVA-Tukey’s honestly significantly different (HSD) multiple comparison test (at P<0.05). Per replicate have ten earthworm and 45 earthworms per treatment, each treatment has three replicates. The each one replicates has 15 earth worms.
Non-target bioassay
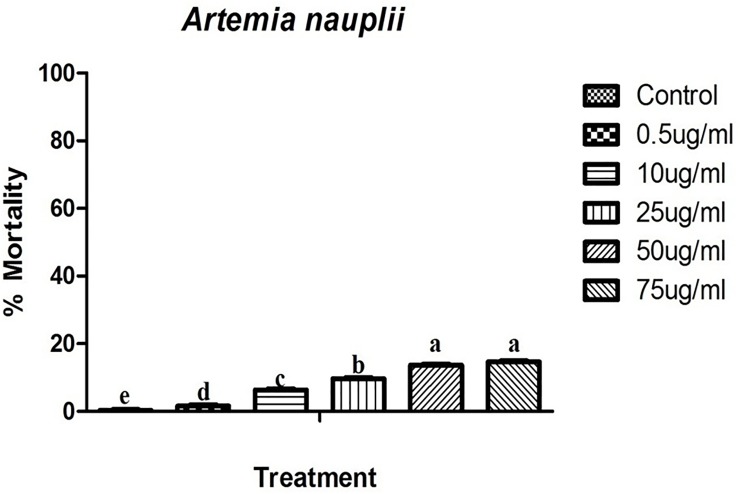
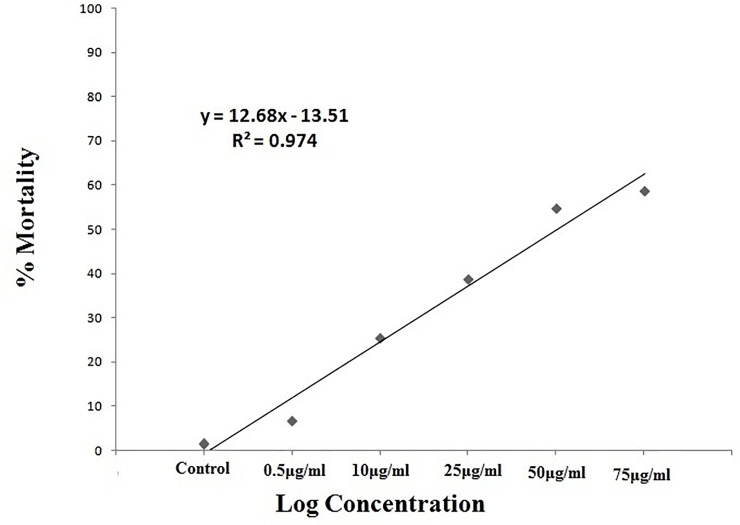
M.anisopliae fungi crude metabolites showed lower toxicity on the non-target species A. nauplii than mosquitoes. The results of M.anisopliae derived crude metabolites indicated low (less than 20%) mortality on A. nauplii at 24hr post treatment (F = 325.0, Df = 12,P = 0.001) (Fig 9). The LC50 values were, 54.96μgml for the treatment of M.anisopliae derived secondary metabolites (Fig 10). No behavioral changes were observed during the treatment.
Fig 9. Percentage mortality of A. nauplii after treatment with M. anisopliae derived secondary metabolites at 24hr post treatment.
Fig 10. Percentage of mortality of A. nauplii treated with M. anisopliae derived secondary metabolites using linear regression analysis.
Phyto-toxicity
Thirty dayspost-treatment in semi-field level application our results clearly showed that high yield of flowers, more root and shoot length and no bacterial and fungal infection was observed compared with control. Fungi crude metabolite toxicity was not observed on treated tomato plant (Solanum lycopersicum L).
Discussion
This study has shown that, in unpolluted environment several beneficial and economical species of microbial community can be found including entomopathogenic fungi which have shown to have mortality effect to mosquito vector species and less effect to non-targeted organisms. These findings are similar to previous studies which showed toxicity effect to variety of arthropods [17–19]. At present, there is a better consideration of the use of microbial-derived pesticides to reduce the use of synthetic chemical pesticides [4, 5, 20]. These findings indicates that, the selection of entomopathogenic fungi and their effective toxins are possible alternative to synthetic insecticides for control of larvae, pupae and adult mosquitoes in laboratory and field application [4, 20, 21]. Microbial derived compound show several advantages in vector control program such as the high toxicity to larvae, pupae, adults and lower toxicity to non-target species. The entomopathogenic fungi species producing stable compounds including; Aspergillus sp, Fusarium sp, Metarhizium sp, Beauveria sp and Verticillium sp, have shown mortality effect to insect pests in the laboratory and field condition [4–6, 22].
In the present study, isolated M. anisopliae crude metabolites showed a dose dependent larval toxic efficacy on three major mosquito species. Similarly, in the previous report F. oxysporum derived crude secondary metabolites showed high toxicity on three mosquito species namely, An. stephensi, Ae. aegypti and Cx. quinquefasciatus under laboratory condition [5]. After 24hr post-treatment with M. anisopliae crude metabolites, a remarkable larval toxic efficacy was observed on mosquito larvae. Similarly, Ragavendran and others reported that, P. dahlia derived extracellular metabolites showed remarkable toxicity on Ae. aegypti and Cx. quinquefasciatus larvae and lower toxicity effect was observed on non-target species [23]. The A. nauplii were treated with secondary metabolites of M.anisopliae and morphological changes were observed. In a other study conducted using fungal secondary metabolites from P. daleae produced changes in eye shape, eye color, and eyes fading [23]. These secondary metabolites from different fungi have varying levels of toxicity to non-target organisms.
The FT-IR analysis identifies the functional groups present in the M. anisopliae crude metabolite to be alcohols, carboxylic acids and others in minor proportions. These functional groups maybe involved in mosquito larvicidal activity. Similarly,Vivekanandhan and others reported that the B. bassiana and F. oxysporum derived crude metabolites and silver nanoparticles have similar kind of functional groups involved for mosquitocidal activity [4–6].The GC-MS analysis of M. anisopliae crude metabolites results clearly shows that five major chemical constituents namely, 9-octyl eicosane; (Z,Z)-3,6-cis-9,10-Epoxyheptadecadie; Heptacosane; Heptacosane; Hexatriacontane. Among the chemical constituents, Hexatriacontane as major chemical constituents presents in fungal crude metabolites so these chemical constituents maybe involved in mosquito larvicidal activity. Previous research found that, B. bassiana, F. oxysporum and plant secondary metabolites/essential oils contain similar kind of organic chemical constituents with larvicidal, pupicidal and adulticidal activities against mosquitoes [4, 5, 24].
This study also found that, the crude secondary metabolites had lower toxicity against E. eugeniae (non-targeted) compared to the synthetic chemicals. The M. anisopliae crude metabolites produce 18% mortality while chemical pesticide produces 97% mortality rate. E. eugeniae gut histopathological revealed that, the lower sub-lethal effect was observed in gut tissues. In the Monocrotophos treatment the E. eugeniae gut tissues were highly damage compared with fungal crude metabolites treatment. This study also found that, in 30days post-treatment bacteria and fungi infections were observed in treated plants compared with control on tomato plant using phytotoxicity bioassays method. This present study; show that the entomopathogenic fungi M. anisopliae show lower toxicity effects were observed on the non-target species A. nauplii. In previous study, Larsen and others reported swimming speed alterations were observed on Artemia species after treatment 24hrs treatment of different fungal toxins [25]. Our studies clearly show there is low sub-lethal effect observed as well as no morphological changes were observed on non-target organisms.
Zimmermann clearly reported that there is no pathogenicity/toxicity were observed on humans and other animals such as, mice, fish, guinea pigs, birds and rats after exposure of M. anisopliae conidia and their toxin [26]. Similarly, Strasser and others report the entomopathogenic fungi cause no effect to human population and green environment [27]. Previouse report, Vivekanandhan and others researchers tested entomopathogenic fungi B. bassiana and M. anisopliae derived crude metabolites against A. nauplii and E. eugeniae, their results show there is low sub-lethal effects were observed on non-target species, so they concluded that the fungal crude metabolites are effective, cheaper, lower toxicity to non-target organisms and also best alternative for synthetic chemical pesticides [4].
Conclusion
In the present study, M. anisopliae derived secondary metabolites showed strong larvicidal activity on larvae of An. stephensi, Ae. aegypti and Cx. quinquefasciatus mosquitoes. The present findings suggest that, M. anisopliaederived toxins are target specific with low toxicity to non-target species such as A. nauplii. Histopathological studies indicated that the crude metabolites produced lower damages in gut tissues while the complete gut tissue damage was observed in treatments of synthetic insecticide (Monocrotophos).
Acknowledgments
Authors thank the Institute of Vector Control and Zoonoses (IVCZ) Hosur for supplying eggs. We also express our thanks to Department of Environmental Science, Department of Botany, Department of Zoology, Periyar University, Salem, for providing infrastructural facility for us to carry out our research successfully.
Abbreviations
- BLAST
Basic Local Alignment Search Tool
- CTAB buffer
Clear To Auscultation Bilaterally
- DMSO
Dimethyl sulfoxide
- FT-IR
Fourier Transform Infrared
- GC-MS
Gas chromatography mass spectrometry
- ITS
Internal transcribed spacer
- NCBI
National Center of Biotechnology Information
- PCR
polymerase chain reaction
- rDNA
Recombinant deoxyribonucleic acid
- SDB
Sabouraud’s dextrose broth
- WHO
World Health Organization
- rDNA
Recombinant deoxyribonucleic acid
Data Availability
All relevant data are within the paper.
Funding Statement
Periyar University Research Fellowship Scheme (Ref No. PU/AD-3/URF/2016).
References
- 1.Wang R, Yuan Y, Yen H, Grieneisen M, Arnold J, Wang D, et al. A review of pesticide fate and transport simulation at watershed level using SWAT: Current status and research concerns. Science of The Total Environment. 2019;669:512–26. 10.1016/j.scitotenv.2019.03.141 [DOI] [PubMed] [Google Scholar]
- 2.Mdoe FP, Cheng S-S, Lyaruu L, Nkwengulila G, Chang S-T, Kweka EJ. Larvicidal efficacy of Cryptomeria japonica leaf essential oils against Anopheles gambiae. Parasites & Vectors. 2014;7(1):426 10.1186/1756-3305-7-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mdoe FP, Cheng S-S, Msangi S, Nkwengulila G, Chang S-T, Kweka EJ. Activity of Cinnamomum osmophloeum leaf essential oil against Anopheles gambiae s.s. Parasites & Vectors. 2014;7(1):209 10.1186/1756-3305-7-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivekanandhan P, Deepa S, Kweka EJ, Shivakumar MS. Toxicity of Fusarium oxysporum-VKFO-01 Derived Silver Nanoparticles as Potential Inseciticide Against Three Mosquito Vector Species (Diptera: Culicidae). Journal of Cluster Science. 2018;29(6):1139–49. [Google Scholar]
- 5.Vivekanandhan P, Karthi S, Shivakumar MS, Benelli G. Synergistic effect of entomopathogenic fungus Fusarium oxysporum extract in combination with temephos against three major mosquito vectors. J Pathogens global health. 2018;112(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vivekanandhan P, Kavitha T, Karthi S, Senthil-Nathan S, Shivakumar M, health p. Toxicity of Beauveria bassiana-28 mycelial extracts on larvae of Culex quinquefasciatus mosquito (Diptera: Culicidae). International journal of environmental research. 2018;15(3):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivekanandhan P, Kavitha T, Karthi S, Senthil-Nathan S, Shivakumar SM. Toxicity of Beauveria bassiana-28 Mycelial Extracts on Larvae of Culex quinquefasciatus Mosquito (Diptera: Culicidae). International Journal of Environmental Research and Public Health. 2018;15(3). 10.3390/ijerph15030440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurya P, Mohan L, Sharma P, Srivastava CJJEB. Larval susceptibility of Aloe barbadensis and Cannabis sativa against Culex quinquefasciatus, the filariasis vector. 2008;29(6):941–3. [PubMed] [Google Scholar]
- 9.Vijayan VK, Balaraman K. Metabolites of fungi and actinobacteria active against mosquito larvae. Indian J Med Res. 1991;93:115 [PubMed] [Google Scholar]
- 10.Vyas N, Dua K, Prakash S. Bioassay of secondary metabolite of Lagenidium giganteum on mosquito larvae for vector control. Bull Bio Sci. 2006;4:65–9. [Google Scholar]
- 11.Vyas N, Dua K, Prakash S. Laboratory efficacy of metabolites of Lagenidium giganteum (Couch) on Anopheles stephensi (Liston) after filterations by Column Chromatography. Journal of Communicable Diseases. 2006;38(2):176 [PubMed] [Google Scholar]
- 12.Prakash S. Laboratory efficacy tests for fungal metabolites of Chrysosporium tropicum against Culex quinquefasciatus. Journal of the American Mosquito Control Association. 2003;19(4):404–7. [PubMed] [Google Scholar]
- 13.Matha V, Weiser J, Olejnicek J. The effect of tolypin in Tolypocladium niveum crude extract against mosquito and blackfly larvae in the laboratory. J Folia parasitologica. 1988;35(4):379–81. [PubMed] [Google Scholar]
- 14.WHO. Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. WHO, Geneva. Geneva: WHO/CDS/WHOPES/GCDPP/1.3, 2005.
- 15.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18(2):265–7. [Google Scholar]
- 16.OECD. OECD Guideline for Testing of Chemicals. Earthworm Acute Toxicity. Paris, France: OECD, 1984.
- 17.Chaurasia A, Lone Y, Wani O, Gupta US. Effect of certain entomopathogenic fungi on oxidative stress and mortality of Periplaneta americana. Pesticide Biochemistry and Physiology. 2016;127:28–37. 10.1016/j.pestbp.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Scholte E-J, Ng, habi K, Kihonda J, Takken W, Paaijmans K, et al. An Entomopathogenic Fungus for Control of Adult African Malaria Mosquitoes. Science. 2005;308(5728):1641 10.1126/science.1108639 [DOI] [PubMed] [Google Scholar]
- 19.Ullah MI, Altaf N, Afzal M, Arshad M, Mehmood N, Riaz M, et al. Effects of Entomopathogenic Fungi on the Biology of Spodoptera litura (Lepidoptera: Noctuidae) and its Reduviid Predator, Rhynocoris marginatus (Heteroptera: Reduviidae). International Journal of Insect Science. 2019;11:1179543319867116 10.1177/1179543319867116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butt TM, Greenfield BPJ, Greig C, Maffeis TGG, Taylor JWD, Piasecka J, et al. Metarhizium anisopliae Pathogenesis of Mosquito Larvae: A Verdict of Accidental Death. PLOS ONE. 2013;8(12):e81686 10.1371/journal.pone.0081686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logeswaran C, Vivekanandhan P, Shivakumar M. Chemical constituents of thermal stress induced Ganoderma applantum (Per.) secondary metabolites on larvae of Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus and histopathological effects in mosquito larvae. Biocatalysis Agricultural Biotechnology. 2019;20:101253. [Google Scholar]
- 22.Jeger MJ, Madden LV, Van den Bosch F. The effect of transmission route on plant virus epidemic development and disease control. Journal of theoretical biology. 2009;258(2):198–207. 10.1016/j.jtbi.2009.01.012 [DOI] [PubMed] [Google Scholar]
- 23.Ragavendran C, Dubey NK, Natarajan D. Beauveria bassiana (Clavicipitaceae): a potent fungal agent for controlling mosquito vectors of Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). RSC Advances. 2017;7(7):3838–51. [Google Scholar]
- 24.Pratheeba T, Vivekanandhan P, Faeza AN, Natarajan D. Chemical constituents and larvicidal efficacy of Naringi crenulata (Rutaceae) plant extracts and bioassay guided fractions against Culex quinquefasciatus mosquito (Diptera: Culicidae). Biocatalysis Agricultural Biotechnology. 2019;19:101137. [Google Scholar]
- 25.Larsen J, Johansen A, Larsen SE, Heckmann LH, Jakobsen I, Krogh PHJSB, et al. Population performance of collembolans feeding on soil fungi from different ecological niches. 2008;40(2):360–9. [Google Scholar]
- 26.Zimmermann GJPS. The entomopathogenic fungus Metarhizium anisopliae and its potential as a biocontrol agent. 1993;37(4):375–9. [Google Scholar]
- 27.Shahid AA, Rao QA, Bakhsh A, Husnain T. Entomopathogenic fungi as biological controllers: new insights into their virulence and pathogenicity. J Archives of Biological Sciences. 2012;64(1):21–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.