Abstract
Differentiation of resident multipotent vascular stem cells (MVSCs) or de-differentiation of vascular smooth muscle cells (vSMCs) might be responsible for the SMC phenotype that plays a major role in vascular diseases such as arteriosclerosis and restenosis. We examined vSMCs from three different species (rat, murine and bovine) to establish whether they exhibit neural stem cell characteristics typical of MVSCs. We determined their SMC differentiation, neural stem cell marker expression and multipotency following induction in vitro by using immunocytochemistry, confocal microscopy, fluorescence-activated cell sorting analysis and quantitative real-time polymerase chain reaction. MVSCs isolated from rat aortic explants, enzymatically dispersed rat SMCs and rat bone-marrow-derived mesenchymal stem cells served as controls. Murine carotid artery lysates and primary rat aortic vSMCs were both myosin-heavy-chain-positive but weakly expressed the neural crest stem cell marker, Sox10. Each vSMC line examined expressed SMC differentiation markers (smooth muscle α–actin, myosin heavy chain and calponin), neural crest stem cell markers (Sox10+, Sox17+) and a glia marker (S100β+). Serum deprivation significantly increased calponin and myosin heavy chain expression and decreased stem cell marker expression, when compared with serum-rich conditions. vSMCs did not differentiate to adipocytes or osteoblasts following adipogenic or osteogenic inductive stimulation, respectively, or respond to transforming growth factor-β1 or Notch following γ-secretase inhibition. Thus, vascular SMCs in culture express neural stem cell markers typical of MVSCs, concomitant with SMC differentiation markers, but do not retain their multipotency. The ultimate origin of these cells might have important implications for their use in investigations of vascular proliferative disease in vitro.
Keywords: Vascular smooth muscle, Multipotent vascular stem cells, Aorta, Carotid artery, Arteriosclerosis, Vascular biology, Marker expression
Introduction
Vascular smooth muscle cell (vSMC) biology has been at the forefront of cardiovascular disease research for the last 50 years. Vascular SMCs contribute to vascular hyperplasia by rapidly expanding in injured vessels and differentiating into mature SMCs (Owens et al. 2004; Gomez and Owens 2012; Tang et al. 2013). Multipotent vascular stem cells (MVSCs) are the most recent resident vascular stem cell population isolated that might contribute in part to SMC accumulation and subsequent vascular disease progression. They were originally derived from medial tissue explants of rat carotid artery, although they are also present within the adventitia of both arterial and venous vessels from both animals and humans (Gallagher et al. 2000; Owens et al. 2004; Tang et al. 2012, 2013; Gomez and Owens 2012).
MVSCs are derived from the neural crest and so express the astrocyte marker S100β, the neural stem cell marker Sox10 and the endoderm marker Sox17 (Tang et al. 2013). S100β is a Ca2+-binding protein that promotes cell proliferation, survival and differentiation through interactions with proteins such as Ndr and p53 (Sorci 2013). Sox10 and Sox17 are both transcription factors that contain high-mobility group domains that mediate their binding to DNA. These transcription factors are involved in embryogenesis and organogenesis, and mutations within the genes that encode these proteins underlie serious developmental abnormalities (Wilson and Koopman 2002). MVSCs in culture poorly express mature vSMC contractile markers, such as smooth muscle myosin heavy chain (SM-MHC, MYH11 or Sm-2). However, during the transition to SMCs, they become mesenchymal stem cell (MSC)-like and eventually express SMC differentiation markers calponin1 (CNN1) and SM-MHC (Sm-2) in non-maintenance media or following transforming growth factor-β1 (TGF-β1) treatment or Notch activation (Tang et al. 2012). Importantly, in response to vascular injury, MVSCs differentiate to MSC-like cells, become proliferative and subsequently differentiate into SMCs thereby contributing, in part, to vascular remodelling and neointimal hyperplasia (Tang et al. 2012, 2013).
We therefore examined three commonly used, commercially available, vascular SMC lines from various species to establish whether these vSMCs in culture exhibit neural stem cell characteristics typical of MVSCs. We determined their SMC differentiation, neural stem cell marker expression and multipotency following lineage induction in vitro.
Materials and methods
Materials
All materials were of the highest purity commercially available. Primary antibodies included: anti-α actin (monoclonal mouse anti-actin antibody, Sigma Cat. No. A5228), anti-SM-MHC (monoclonal mouse anti-myosin antibody, Sigma Cat. No. clone hSM-V, M7786), anti-MHC [1G12] (Abcam Cat. No. Ab683) and goat polyclonal MYH11 antibody (N-16, Santa Cruz Cat. No. SC79079), all of which react with Sm-1 and Sm-2; anti-CNN1 (monoclonal mouse anti-calponin1 antibody, Sigma Cat. No. C2687), antiSox10 (monoclonal rabbit anti-Sox10 antibody, Abcam Cat. No. ab155279), anti-Sox17 (monoclonal rabbit anti-Sox17 antibody, Millipore Cat. No. 09–038), anti-S100β (monoclonal rabbit anti-S100β antibody, Millipore Cat. No. 04–1054), anti-CD44 (polyclonal rabbit anti-CD44, Abcam Cat. No. Ab24504), anti-CD29 (monoclonal rabbit anti-CD29, Millipore Cat. No. 04–1109) and anti-CD146 (monoclonal rabbit anti-CD146, Millipore Cat. No. 04–1147). Secondary antibodies used were Alexa Fluor 546 goat anti-rabbit, rabbit anti-mouse and goat anti-mouse (Invitrogen) and Alexa Fluor 488 goat anti-rabbit (Invitrogen). TGF-β1 was purchased from Sigma Aldrich and PeproTech UK. The γ-secretase inhibitor of Notch, DAPT, was purchased from Sigma Aldrich.
Cell culture
Murine aortic SMCs (mSMCs) were obtained from ATCC (Rockville, Md., USA; MOVAS [ATCC CRL-2797]). These cells were isolated enzymatically by collagenase–elastase digestions of murine aorta and transduced with retrovirus encoding the SV40 large T antigen and neomycin resistance, before clones were selected in the presence of 0.4 mg/ml G418 and subcloned by limiting dilution. Rat aortic SMCs (rSMCs, R354–05a) were obtained from Cell Applications (Calif., USA) that were isolated enzymatically from the tunica intima and media of healthy adult rat aorta. Bovine aortic SMCs (BASMCs) were obtained from the Coriell Cell Repositories (Coriell AG08504, N.J., USA) by explant culture from strips of the medial layer. Rat (rSMC) and bovine (BASMC) vSMCs were routinely cultured between passages 8–14 and 11–22, respectively, in Roswell Park Memorial Institute (RPMI-1640) media, supplemented with 10 % fetal bovine serum (FBS; v/v) and 1 % penicillin/streptomycin (P/S, v/v), i.e. complete RPMI medium. Murine vSMCs (mSMCs) were routinely cultured between passages 20–28 in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10 % FBS (v/v) and 1 % P/S (v/v), i.e. complete DMEM. Gibco rat mesenchymal stem cells (MSCs) were obtained from Life Technologies (Calif., USA). MSC cells were maintained in growth media made up of 50:50 minimal essential medium (α-MEM) and Ham’s F12 supplemented with 10 % MSC-defined FBS, 150 unit/ml penicillin and 150 μg/ml streptomycin. MSCs were characterised by differentiation along adipogenic and osteogenic lineages and the expression of cell surface markers indicative of MSC (i.e. CD29+, CD44+, CD90+, CD106+) using antibodies against these antigens.
Isolation of rat MVSCs
MVSCs were isolated from rat aortic explants as described previously (Tang et al. 2012). Briefly, male Sprague Dawley rats were first anesthetized with pentobarbital sodium (0.1 mg/g) and then perfused with 10 ml phosphate-buffered saline (PBS). Arterial tissues were harvested as quickly as possible and further dissected in DMEM supplemented with 1 % FBS. The aorta was then isolated from the upper thoracic aorta to the abdominal aorta. The endothelium was removed by scraping off the cell layer on the luminal surface with a sterile scalpel blade before the adventitia was carefully removed from the media following brief enzymatic digestion with 2.5 mg/ml collagenase for 15 min at 37 °C by using forceps under a dissection microscope. The remaining media was cut into 1-mm pieces, placed onto the surface coated with 1 % CellStart (Invitrogen) in 6-well plates and grown in MVSC culture medium containing DMEM with 2 % chick embryo extract (MP Biomedical), 1 % FBS, 1 % N2 (Invitrogen), 2 % B27 (Invitrogen), 100 nM retinoic acid (Sigma-Aldrich), 50 nM 2-mercaptoethanol (Sigma-Aldrich), 1 % P/S and 20 ng ml−1 basic fibroblast growth factor (R&D Systems), i.e.maintenance medium. In parallel experiments, the remaining media were also cut into 1-mm pieces and the medial SMCs were enzymatically dispersed by using collagenase (10 mg; Sigma type I) and elastase (2.5 mg; Sigma type III) for 60–90 min, before the resulting cell suspension was centrifuged at 400g for 5 min and grown in DMEM supplemented with 10 % FCS, as previously described (Cahill and Hassid 1993). These cells served as a control differentiated SMC population.
Western blot analysis
Protein extracts (15–40 μg) were fractionated by SDS-polyacrylamide gel electrophoresis on 7–15 % (w/v) polyacrylamide resolving gels, as previously described (Gao et al. 2007). Proteins were detected by using the ProteoQwest Colorimetric Western Blotting TMB Substrate with horseradish-peroxidase-conjugated secondary antibodies as described by the manufacturer (Sigma). Minor differences in protein loading and transfer were normalised by using a Ponceau S stain and by measuring constitutive β-actin or D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein levels.
Preparation of RNA
Cells were seeded onto 6-well plates at a density of 100,000 cells/well and allowed to recover for 2 days in complete medium. After recovery, cells were cultured in their appropriate media supplemented with 0.5 % FBS and 1 % P/S for 2 days to induce quiescence. Cells were then maintained in their respective media supplemented with 0.5 % FBS or grown in media with 5 % FBS for a further 3 days. Following treatment, the cells were trypsinised and pelleted by centrifugation at 300g for 5 min at room temperature. RNA was prepared by using the 16 Cell LEV Total RNA Purification Kit from Maxwell in the automated Maxwell 16 System according to the manufacturer’s instructions. RNA samples were stored at −80 °C until they were analysed. RNA samples were quantified and their purity was validated by using the nanodrop 2000 spectrophotometer (Thermo Scientific).
Real-time quantitative polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed by using the Rotor Gene (RG-3000, Corvett Research) and the SYBR green PCR kit (Qiagen) according to the manufacturers’ instructions. All RNA samples were prepared by using the Promega Maxwell 16 Cell LEV Total RNA Purification Kit before qRT-PCR was used to analyse gene expression ratios and relative gene expression. Quantitect Primer Assays (Qiagen) were used for amplification of specific targets for SMC differentiation [smooth muscle α-actin (SMA), smooth muscle myosin heavy chain (SM-MHC), calponin 1 (CNN1) and smoothelin-B (SMO)], MVSCs [Sox10, Sox17 and S100β] and a Notch target gene (Hey1). Briefly, total RNA (1–10 ng) was then reverse-transcribed and PCR-amplified in a one-step reaction containing reverse transcription mix, SYBR green mix and RNase-free water at 55 °C for 10 min, 95 °C for 5 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 15 s and subsequent melt-curve analysis. Samples were run in triplicate with a no reverse transcriptase control. GAPDH was used as a reference gene to normalise and to compare the results of qRT-PCR between samples for all reactions. The Ct values were obtained by using the Relative Expression Software Tool (ReST; http://rest.gene-quantification.info). The mathematical model used is based on the correction for PCR efficiencies and the mean crossing point deviation between sample group(s) and control group(s).
Flow cytometry
Cells were cultured until confluency in T75 flasks and then trypsinised in 2× trypsin/0.53 mM EDTA at 37 °C. Cells were resuspended in media, counted and then fixed in BD Cytofix/Cytoperm solution (BD Bioscience) for 20 min at 4 °C. Cells were subsequently washed in 1× BD Perm/Wash solution (BD Bioscience) by centrifugation at 500g for 3 min. Following a washing step, cells were resuspended in 1× BD Perm/Wash solution containing 1 μg appropriate primary antibody and incubated at 4 °C for 30 min. Following another washing step, cells were resuspended in 1× BD Perm/Wash solution containing 1 μg appropriate enzyme-linked secondary antibody and incubated at 4 °C for 30 min. Cells were then washed in BD Perm/Wash solution and resuspended in a final volume of 500 μl BD Perm/Wash solution. The cells were then analysed by fluorescence-activated cell sorting (FACS) in the FACSCalibur System (BD Bioscience). Isotype controls, autofluorescence and nonspecific binding of cells was appropriately gated for as given in the manufacturer’s instructions. Cells were also stained with a viability dye such as propidium iodide or 7-aminoactinomycin D in order to discriminate between living and dead cells.
Immunocytochemistry
Cells were seeded onto glass slides in 6-well plates at a density of 5000 cells/well. Following treatment, the cells were washed twice with PBS and fixed at room temperature in 3.7 % formaldehyde (v/v) for 10 min. Cell permeabilisation and blocking was carried out by using 5 % bovine serum albumin (BSA), 0.1 % Triton-X in PBS at room temperature for 1 h. Primary antibodies were diluted in 5 % BSA in PBS according to the manufacturer’s instructions and cells were incubated with the primary antibodies at 4 °C overnight. The cells were washed twice with 1 ml PBS before the secondary antibodies were diluted in 5 % BSA in PBS according to the manufacturer’s instruction sand added to each well for 1 h at room temperature in the dark. An Olympus BX51 microscope was used to capture fluorescent imagery. Secondary-antibody-only controls were run in parallel to ensure no non-specific staining (data not shown). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) at a concentration of 2 μg/ml in PBS at room temperature for 10 min. Fluorescent images were collected by an Olympus DP-50 fluorescent camera with the appropriate excitation and emission spectra at 4×, 10×, 20× and 60× magnification.
Confocal microscopy
MVSCs were plated onto glass coverslips, placed in 6-well culture plates and returned to the incubator for a minimum of 24 h to allow for cell attachment and spreading. Cells were fixed and permeabilised by the addition of ice-cold acetone for 1 min. The cells were then washed three times with PBS containing 0.5 % TWEEN-20 (PBS-T), pH 7.5, and incubated for 10 min in blocking solution (5 % non-fat dry milk in PBS-T). Cells were stained for 30 min at room temperature with specific antibodies followed by incubation with an Alexa-488-labelled secondary antibody (Molecular Probes, Eugene, Ore., USA). The cells on coverslips were mounted on slides with antifade medium (Dako). Slide preparations were observed by using a Zeiss Axio Observer.Z1 equipped with a Zeiss 710 and ConfoCor3 laser scanning confocal head (Carl Zeiss). Images were analysed by using Zen 2008 software as we have previously described (McEntee et al. 2011).
Adipocyte differentiation
Cells were seeded onto 6-well plates at a density of 50,000 cells/well and allowed to recover from trypsinisation for 2 days in maintenance medium. After recovery, cells were cultured in adipocyte differentiation media for 14 days (StemPro Adipogenesis Differentiation Kit, Life Technologies) according to the manufacturer’s instructions. Adipocyte differentiation was evaluated by Oil Red O and HCS LipidTOX Green neutral lipid staining (Invitrogen).
Osteoblast differentiation
Cells were seeded onto 6-well plates at a density of 50,000 cells/well and allowed to recover from trypsinisation for 2 days in maintenance medium. After recovery, cells were cultured in the StemPro Osteogenesis Differentiation Media Kit (Life Technologies) according to the manufacturer’s instructions. Osteoblast differentiation was evaluated by using 2 % Alizarin Red S stain (Sigma).
Cell treatments with TGF-β1 and DAPT
Cells were seeded onto 6-well plates at a density of 5000 cells/well. Cells were allowed to recover from trypsinisation for 2 days in complete medium. After recovery, cells were cultured in media containing 0.5 % FBS (v/v) 1 % P/S (v/v) for 2 days to allow transition into a quiescent state. Cells were then treated with either 1 ng/ml TGF-β1 or 10 μM DAPT (γ-secretase inhibitor) in complete medium for 3 days. Control cells received HCL and DMSO vehicle controls, respectively. Following treatment, the cells were washed twice with 1 ml PBS and fixed by incubating them at room temperature in 500 μl 3.7 % formaldehyde (v/v) for 10 min before the cells were processed for immunocytochemistry.
Statistics
Results are expressed as means±SEM. Experimental points were performed in triplicate. A t-test was used for the comparison of two groups. A value of P≤0.05 was considered significant.
Results
Cultured rSMCs, mSMCs and BASMCs express SMC differentiation markers
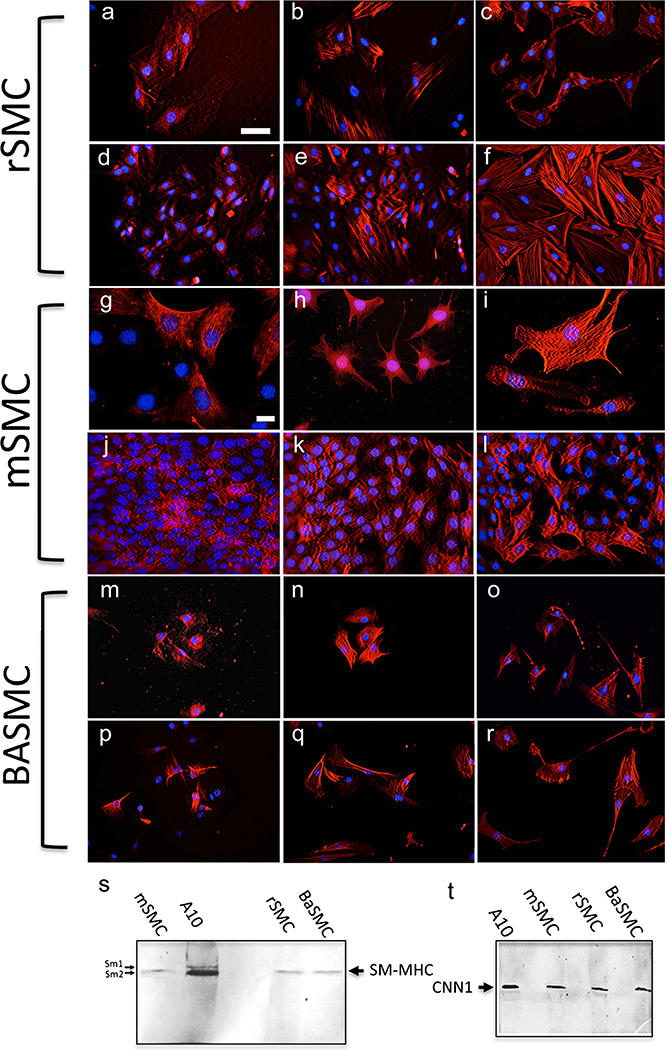
Before investigating for the presence of MVSC markers, we first confirmed the expression of SMC differentiation markers in each of the aortic vSMC lines. Commercially available BASMCs, rSMCs and mSMCs were cultured in their respective media supplemented with either 5 % FBS or 0.5 % FBS before they were analysed for SMC-specific differentiation marker expression (SM-MHC Sm-1/Sm-2, SMA and CNN1). Immunocytochemical analysis revealed extensive expression of the SMC-specific markers in each of the cell lines under both conditions (Fig. 1). In addition, consistent with previous studies of high passage de-differentiated “modulated” SMCs in culture (Chamley et al. 1974; Chamley-Campbell et al. 1979; Worth et al. 2001), the contractile and cytoskeletal proteins were re-organised, in particular SM-MHC, when compared with primary cells. Whereas each SMC line exhibited well-defined α-actin myofilaments (Fig. 1c, i, o) and, to a similar extent, CNN1 fibres (Fig. 1b, h, n) under serum-deprived conditions, the expression of SM-MHC was more diffuse within the cytoplasm with less well-defined myofilaments (Fig. 1a, g, p). Parallel immunoblot analysis confirmed that the aortic SMC lines from three different species predominantly express SM-MHC isoform, Sm-2, and CNN1 to variable degrees (Fig. 1s, t) in addition to SMA, as previously described (Zanellato et al. 1990; Babij et al. 1992; Kuang et al. 2012).
Fig. 1.
a–r Representative immunocytochemical staining of smooth muscle cell (SMC) differentiation markers. Rat (rSMC), murine (mSMC) and bovine (BASMC) cells were cultured in 0.5 % (a–c, g–i, m–o) and 5 % (d–f, j–l, p–r) fetal bovine serum (FBS)-supplemented medium for 3 days before immunocytochemistry was performed. Cells stained positively (red) for smooth muscle myosin heavy chain (SM-MHC), calponin1 (CNN1) and smooth muscle α-actin (SMA). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI, blue). Scale Bars are 50 μm (a–f, m–r) and 10 μm (g–l). Data are representative of three independent wells. s, t Representative immunoblot analysis of SMC differentiation markers, i.e. smooth muscle myosin heavy chain (SM-MHC in s) and calponin1 (CNN1 in t). Equal loading was confirmed by Ponceau S staining. Data are representative of two independent experiments
Cultured rSMCs, mSMCs and BASMCs express neural stem cell MVSC markers
MVSCs isolated from rat aortic explants and enzymatically digested primary rat vSMCs served as our control MVSC and differentiated SMC populations, respectively, as described previously (Cahill and Hassid 1993; Cappadona 1999; Tang et al. 2012). Fluorescence microscopy confirmed that the MVSCs were immunocytochemically negative for SM-MHC (Sm-2) but positive for neural stem cell markers Sox10, Sox17 and S100β, as previously described (Tang et al. 2012, 2013; Fig. 2a–l). Immunofluorescence microscopy of MVSCs also demonstrated that these cells were positive for MSC-like phenotypic markers CD44 and CD29, but were negative for CD146 (Fig. 2m–u). Immunoblot analysis of protein lysates from these cells in maintenance media confirmed that MVSCs express some SM-MHC Sm-1 (with no Sm-2 present), SMA and CNN1, while concomitantly expressing Sox10, Sox17 and S100β (Fig. 2v).
Fig. 2.
a–u Representative immunocytochemical staining of SMC differentiation markers, neural stem cell markers and mesenchymal stem cell (MSC)-like markers (green). Rat aortic multipotent vascular stem cells (MVSCs) were isolated by explant and cultured in MVSC maintenance media before being analysed by immunofluorescence microscopy for SMC markers (a–c SM-MHC), neural stem cell markers (d–f Sox10, g–i Sox17, j–l S100β) and MSC (Mesenchymal) markers (m–o CD44, p–r CD29, s–u CD146). Nuclei were stained with DAPI (blue). Scale Bars are 50 μm. v Representative immunoblot analysis of SMC differentiation markers (SMA, CNN1, SM-MHC) and neural stem cell markers (Sox10, Sox17 and S100β) in MVSCs. Equal loading was confirmed by Ponceau S staining. Data are representative of two independent experiments
Whereas MVSCs expressed SMA and CNN1 in both maintenance media and DMEM supplemented with 10 % FBS (Fig. 3), the expression of SMC differentiation markers SM-MHC (Sm-2) and CNN1 was more robust following the culture of these cells for 10 days in DMEM supplemented with 10 % FBS than in maintenance media over the same time period (Fig. 3a–i). Quantitative FACS analysis of MVSCs further confirmed that these cells were Sox10-, Sox17- and S100β-positive (Fig. 3j–l). Parallel confocal immunofluorescence microscopy revealed that the MVSCs predominantly expressed S100β within the cytoplasm and Sox10 and Sox17 within the nucleus (Fig. 3m–o).
Fig. 3.
a–i Representative immunocytochemical staining (green) of SMC differentiation markers and neural stem cell markers during MVSC transition to vascular smooth muscle cells (vSMCs). MVSCs were cultured in maintenance media or differentiation media (DMEM supplemented with 10 % FBS) for 10 days before being examined for SMC differentiation markers (a, b SMA, d, e SM-MHC, g, h CNN1). Confocal immunofluorescence images for SM-MHC (c), SMA (f) and CNN1 (i). Scale Bars are 20 μm and 50 μm. j–l Representative flow cytometry analysis of Sox10 (j), Sox17 (k) and S100β (l) in MVSCs cultured in DMEM supplemented with 10 % FBS for 10 days with antibodies against Sox10, Sox17 and S100β (open curves negative IgG control cells, red filled curves cells stained with antibodies against Sox10, Sox17 and S100β). m–o Representative confocal immunofluorescence images of Sox10 (m), Sox17 (n) and S100β (o). Nuclei were stained with DAPI (blue). Bar 100 nm. Data are representative of three individual slides
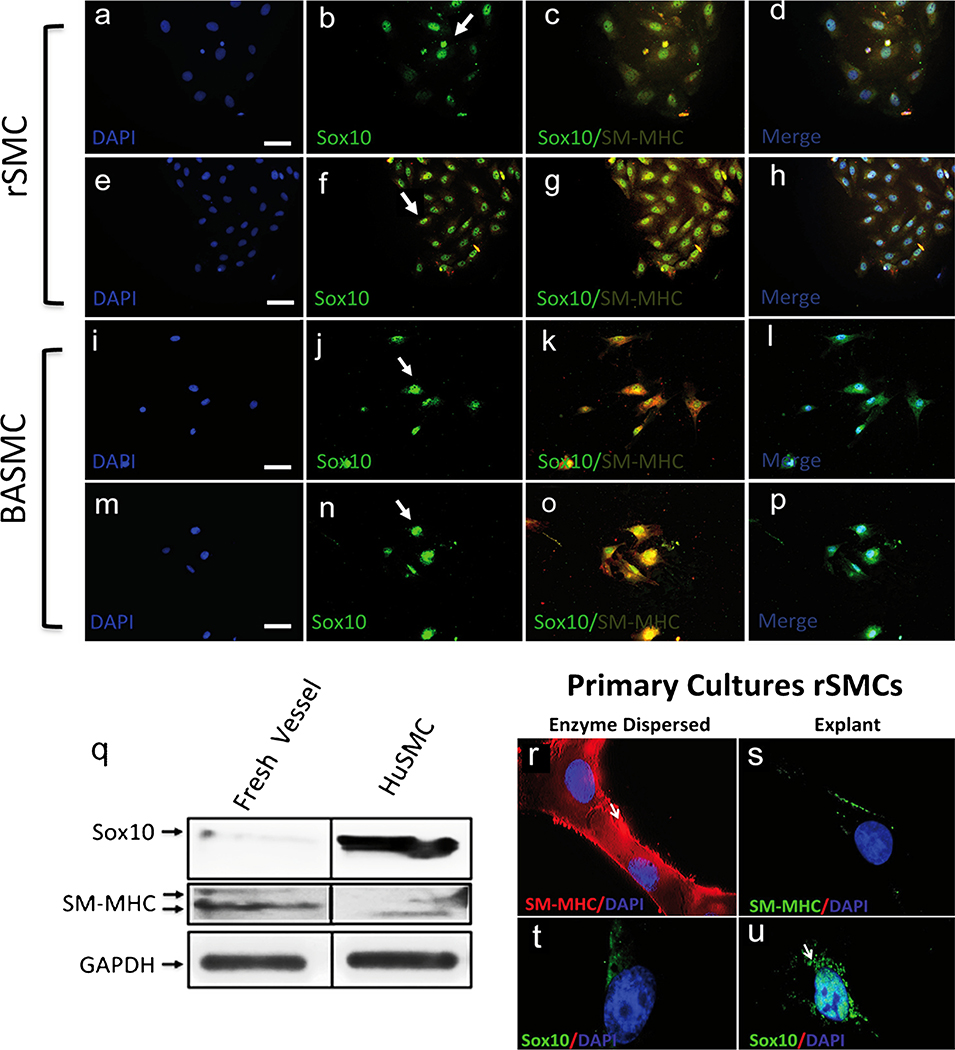
Once the aortic vSMC lines were characterised for SMC differentiation markers, we measured Sox10 and SM-MHC (Sm-2) expression to probe for the presence of “MVSC-like”-positive cells in each of the aortic vSMC lines. Using immunocytochemistry and immunoblotting, we found no evidence for SM-MHC-negative cells, (Sm-2−) present in rSMCs, mSMCs and BASMCs (Figs. 1, 4, 5). Incontrast, all of the cells examined were both SM-MHC+ and Sox10+ under both serum-rich and serum-deprived conditions (Figs. 4a–h, i–p, 5a–h).
Fig. 4.
a–p Representative immunocytochemical staining (green) of SMC differentiation markers and neural stem cell markers in rSMCs and BASMCs. Rat rSMCs and BASMCs were grown in normal media supplemented with 5 % FBS (a–d, i–l) or 0.5 % FBS (e–h, m–p) for 3 days before being analysed for Sox10 and SM-MHC (arrows localisation of Sox10 primarily to the nucleus). Nuclei were stained with DAPI (blue). Scale Bars are 25 μm. Data are representative of at least three individual slides. q–u Representative immunoblot analysis (q) and immunocytochemical staining (r–u) of Sox10 and SM-MHC in fresh isolates from murine carotid artery vessels and primary rat vascular aortic smooth muscle cells (rSMCs) in culture, generated by either enzymatic dispersal or explant Magnification x 100. Human carotid artery vascular smooth muscle cells served as an SMC control. Equal loading was confirmed by Ponceau S staining of membrane and by D-glyceralde-hyde-3-phosphate dehydrogenase (GAPDH) levels. Data are representative of three independent experiments
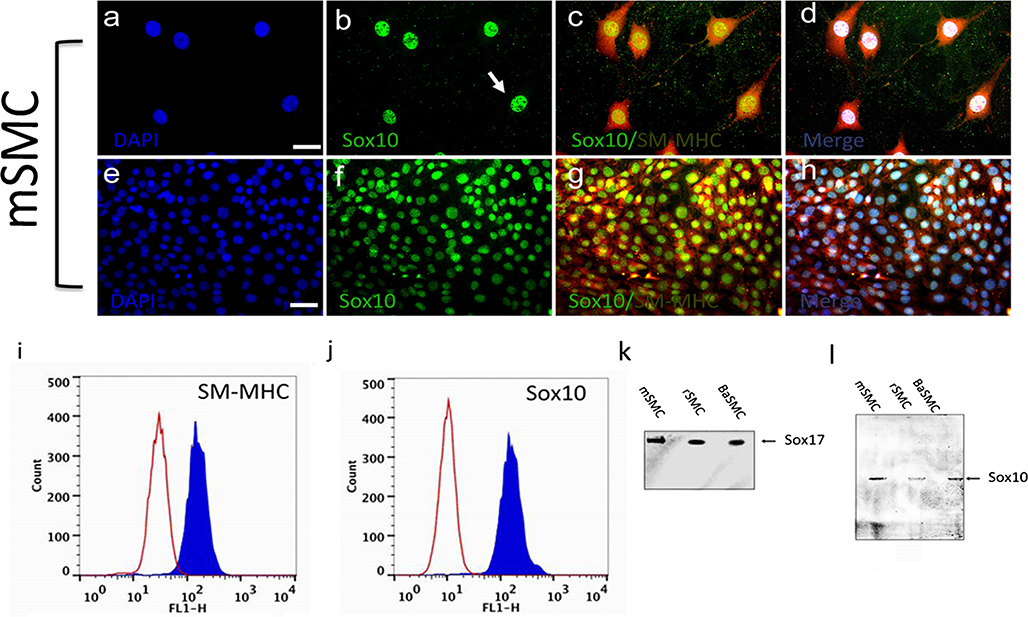
Fig. 5.
a–h Representative immunocytochemical staining of expression of Sox10 and SM-MHC in mSMCs. Murine SMCs were grown in normal media containing 0.5 % FBS (a–d) or 5 % FBS (e–h) for 3 days before being analysed for Sox10 and SM-MHC (arrows localisation of Sox10 primarily to the nucleus). Nuclei were stained with DAPI (blue). Scale bars are 25 μm. i, j Representative flow cytometry analysis of mSMCs cultured for 3 days in normal media before analysis with specific antibodies against Sox10 and SM-MHC, respectively (open curves negative IgG control cells, blue curves cells stained with specific antibodies). Data are representative of three individual experiments. k, l Representative immunoblot analysis of neural stem cell markers Sox17 and Sox10, respectively, in SMCs. Equal loading was confirmed by Ponceau S staining. Data are representative of two independent experiments
We next examined for the presence of Sox10 in murine carotid artery lysates by Western blot analysis to determine whether differentiated SMCs within the vessel wall were Sox10+. Vascular lysates were prepared following the removal of adventitial and endothelial cells. In a parallel analysis, primary rat aortic vSMCs in culture (following enzymatic digestion and dispersal) were generated and examined for SM-MHC and Sox10 by immunocytochemistry. Both murine carotid artery isolates and primary rSMCs in culture expressed low levels of Sox10 (Fig. 4q). In contrast, primary explants from paired medial segments containing MVSCs were SM-MHC-negative but Sox10+ (Fig. 4r–u).
Subsequent FACS analysis of all three commercial SMC lines confirmed quantitatively that these cells were SM-MHC+ and Sox10+ (data not shown). The representative FACS analysis for mSMC is presented and confirmed that the cells co-expressed SM-MHC and Sox10 (Fig. 5i, j). Parallel immunoblot analysis of protein lysates from these cells confirmed that mSMCs expressed SMA, SM-MHC and CNN1 (Fig. 1d), while concomitantly expressing Sox10 and Sox17 (Fig. 5k, l).
Serum deprivation promotes differentiated SMC phenotype
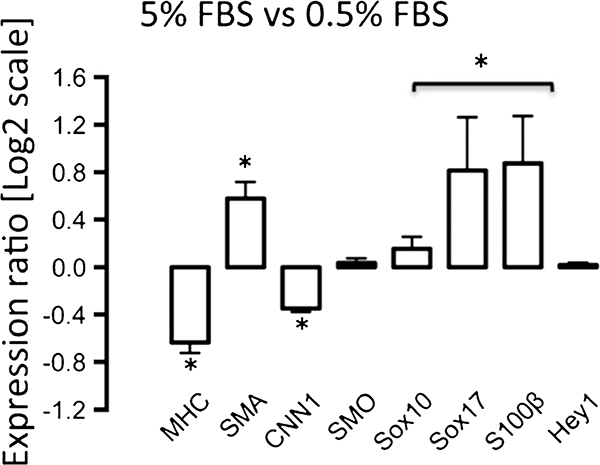
Serum deprivation is known to affect SMC marker expression differentially in both SMC and stem cells (Kato etal.1996; Tang et al. 2012). We therefore determined the relative expression levels of SMC differentiation markers and neural stem cell markers Sox10, Sox17 and S100β in mSMCs under serum-rich (5 % FBS) and serum-deprived (0.5 % FBS) conditions. Hey1 expression, a target gene of Notch signalling (Morrow et al. 2008) and an important arbiter of SMC differentiation and stem cell fate (Morrow et al. 2005; Doi et al. 2009), was also used to determine Notch activity in these cells. We observed a significant increase in MVSC marker levels and a decrease in SMC differentiation marker expression in mSMCs cultured under serum-rich conditions when compared with serum-deprived conditions (Fig. 6). In contrast, Hey1 and SMO expression was not significantly affected (Fig. 6).
Fig. 6.
Quantitative real-time polymerase chain reaction (qRT-PCR) of neural stem cell marker and SMC differentiation marker mRNA levels in a representative vSMC line. Murine SMCs were cultured in DMEM that was serum-rich (5 % FBS) or serum-deprived (0.5 % FBS) for 3 days before mRNA levels of SMC differentiation markers SMA, MHC, CNN1 and smoothelin (SMO), of neural stem cell markers Sox10, Sox17 and S100β and of the Notch target gene Hey1 were measured by qRT-PCR. GAPDH was used to normalize gene expression. The Ct values were obtained and analysed by using the Relative Expression Software Tool (ReST) to determine the relative levels of transcripts. Data are means± SEM and are representative of three independent wells. *P<0.05 when compared with 0.5 % FCS
Cultured SMC are not multipotent
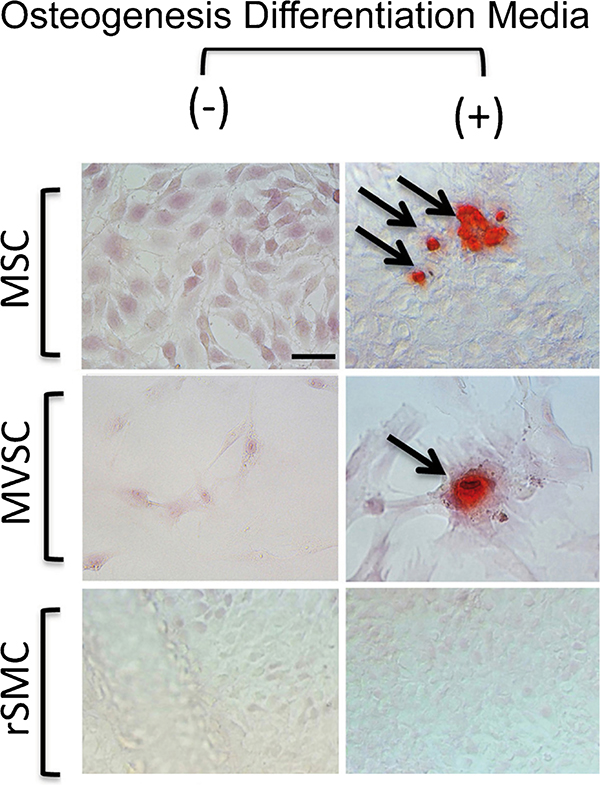
The expression of neural stem cell MVSC markers suggests that these SMC lines retain some stem cell properties. In order to assess whether SMC lines were multipotent, we determined whether rSMCs and mSMCs were capable of differentiating into discrete lineages following treatment with inductive media for adipogenic and osteogenic differentiation. Adipocyte differentiation was determined by both oil red staining and lipidTOX fluorescent staining after a 14-day treatment with the differentiation media. Rat MSCs and rat aorta-derived MVSCs were also treated with the same inductive media and served as positive controls. Bovine aortic endothelial cells served as a negative control (data not shown). Both rat MSCs and MVSCs were capable of differentiation to adipocytes after 14 days of treatment, since the number of Oil-Red-O-positive and LipidTOX-positive cells was significantly increased. In contrast, no discernible Oil Red O or LipidTOX staining was seen in rSMCs and mSMCs as these cells failed to differentiate to adipocytes under the same conditions (Fig. 7). In parallel studies, MVSCs and MSCs were both capable of differentiation to osteoblasts after a 21-day treatment with inductive stimuli, an effect that was not apparent for a representative rat SMC line (Fig. 8). Similar findings were evident for mSMCs and BASMCs (data not shown).
Fig. 7.
Adipogenic potential of vSMCs in vitro. The ability of MSCs, MVSCs, rSMCs and mSMCs to differentiate to adipocytes was determined following treatment with adipocyte differentiation media for 14 days. Adipocyte differentiation was determined by both Oil Red O and LipidTOX staining of lipid droplets (arrows cells positive for adipocyte characteristics). Data are representative of three independent wells Magnification x 40
Fig. 8.
Osteogenic potential of SMCs in vitro. The ability of MSCs, MVSCs and rSMCs to differentiate to osteoblasts was determined following treatment with osteogenic differentiation media for 21 days. Osteoblast differentiation was determined by measuring calcium deposition with Alizarin Red (arrows cells positive for osteoblast characteristics). Scale bars are 25 μm. Data are representative of three independent wells
TGF-β1 and DAPT do not affect stem-like properties of mSMCs in serum-containing medium
As both TGF-β1 and Notch are critical arbiters of the MSC transition to vascular lineages (Gallagher et al. 2000; Kurpinski et al. 2010) and since MVSC transition to a MSC-like intermediate occurs en route to their differentiation to vSMCs (Owens et al. 2004; Tang et al. 2012), we investigated whether TGF-β1 stimulation or Notch inhibition following treatment with a γ-secretase inhibitor (DAPT) could force mSMCs to adopt a more mature contractile phenotype and Qualitatively decrease stem cell marker expression; to achieve this aim, we monitored SM-MHC and Sox10 expression by using immunocytochemistry. Similar to rat (Sweeney et al. 2004) and human (Morrow et al. 2005) SMCs, Notch1 receptors were present on mSMCs (data not shown). Treatment of mSMCs with recombinant TGF-β1 (1 ng/ml) or DAPT in DMEM supplemented with 10 % FBS did not significantly alter the ratio of Sox10+, SM-MHC+ cells, despite a change in cell number (Fig. 9a–v).
Fig. 9.
Effects of transforming growth factor-β1 (TGF-β1) and Notch inhibition by γ-secretase inhibitor (DAPT) on Sox10 and SM-MHC levels in a representative vSMC line, namely mSMCs. The expression of Sox10 and SM-MHC was determined by immunocytochemistry. Cells were grown in media containing 10 % FBS supplemented with (a–j TGF-β1 (1 ng/ml) or (k–t) 10 xM DAPT for 3 days. Scale bars are 25 μm. Ratio of Sox10:SM-MHC (sm-2)-positive cells in untreated and in (u) TGF-β1-treated and (v) DAPT-treated cells. Data are representative of three independent wells
Discussion
The discovery of resident vascular stem cells within the vessel wall has raised the possibility that cultured vSMC lines are derived from these stem cells during the culture process and share similar phenotype characteristics to MVSCs. In this context, we have determined whether commonly used, commercially available, aortic SMC lines from three different species share any phenotypic characteristics typical of MVSCs by examining their SMC differentiation marker expression, neural stem cell marker expression and multipotency. The expression of neural stem cell markers typical of MVSCs in all three aortic cell lines is consistent with the hypothesis that these vascular cells are of neural stem origin and may well be derived from resident vascular stem cells. However, unlike bone-marrow-derived MSCs and aortic MVSCs, these Sox10+ vSMCs have lost their multipotency in response to specific inductive signals.
Notwithstanding the recent controversy as to whether neointimal “modulated” SMCs following injury are derived from resident vascular stem cells or medial differentiated SMCs, or both, no controversy surrounds the phenotype of medial SMCs in normal arterial vessels in situ before isolation and culture in vitro (Metz et al. 2012; Tang et al. 2012). The majority of medial SMCs in situ are strongly positive for SM-MHC (Sm-2; Holifield et al. 1996; Worth et al. 2001). SMCs from the medial layer of arteries of SM-MHC-Cre/LoxP-enhanced green fluorescence protein (EGFP) mice are clearly predominantly EGFP (SM-MHC)-positive and express little to no Sox10, Sox17 or S100β (Tang et al. 2012). This profile is maintained when cells are enzymatically digested, dispersed and cultured in primary culture (Tang et al. 2012). In contrast, when cells are prepared by explant culture from arterial vessels of the same SM-MHC-Cre/LoxP-EGFP mice, these SMCs are predominantly immunocytochemically EGFP-negative (and hence SM-MHC-negative) but Sox10-, Sox17- and S100β-positive and yet acquire EGFP (SM-MHC) positivity when sub-cultured or activated by a Notch ligand or TGF-β1 in vitro (Tang et al. 2012, 2013). Notably, this is in contrast to de-differentiated SMCs, which have reduced expression of SMC differentiation markers, in particular SM-MHC, when compared with cells in situ or in primary culture (Holifield et al. 1996; Kato et al. 1996).
Our data suggest that arterial SMCs of neuroectodermal origin within the murine carotid artery express SM-MHC but little Sox10 in situ. In addition, this profile for SMCs is maintained in cultured rat primary aortic vSMCs generated by enzymatic dispersal of cells from the media, in that these cells are also SM-MHC+ but Sox10−. Moreover, sub-cultured rSMCs derived by enzymatic dispersal and passaged numerous times are immunocytochemically SM-MHC-positive (albeit poorly compared with differentiated SMCs) but are also positive for Sox10, Sox17 and S100β. In contrast, primary explanted SMCs derived from rat aorta and maintained in stem cell maintenance media at early passage (1–4) are SM-MHC (Sm-2)-negative but are positive for Sox10, Sox17 and S100β. Importantly, they can transition to Sm-2-positive SMCs, adipocytes or osteoblasts following specific inductive stimulation and enhance their expression of SMC differentiation markers when grown in non-maintenance stem cell media (DMEM + 10 % FCS).
The commercial vSMC lines used in our study were obtained by either enzymatic dispersal (rat and murine) or explant (bovine) culture. Hence, irrespective of the method of isolation, we find all SMCs express neural stem cell markers typical of MVSCs in late passage. Whereas the murine cell line was an immortalized line, all three mimicked the phenotypic profile of MVSCs. Interestingly, Tang et al. (2012, 2013) have reported that the transition of MVSCs and differentiation to SMCs occurs concomitantly with the complete loss of Sox10 expression after 8 weeks in culture. In contrast, the current study clearly demonstrates the retention of neural stem cell marker expression (Sox10+) concurrent with the expression of SMC markers in all the SMC lines after multiple passages in culture. The co-expression of Sox10 and SM-MHC might be indicative of a proliferative transition between MVSCs and SM-MHC+ Sox10− SMCs, as previously reported (Tang et al. 2012). Of note, rat SMCs also express another neural stem cell marker, namely nestin, when grown in serum or stimulated with thrombin (Huang et al. 2009). Moreover, the expression of nestin is significantly enhanced following vascular injury (Oikawa et al. 2010). The percentage of nestin-positive cells in our three SMC lines was considerably lower under our culture conditions (data not shown). The reason(s) for the observed co-expression of SM-MHC and Sox10 in these cells awaits further analysis.
The exact origin of these SM-MHC+ and Sox10+ cells remains an issue. Differentiated SMCs are clearly Sox10− but SM-MHC+ (Sm-2). Whereas lineage mapping studies and single cell resolution imaging studies of the SM-MHC (Sm-2) epigenetic signature have confirmed the origin of early passage cultured SMCs, even when cells have lost a particular phenotype (and hence specific marker) or changed to multiple phenotypes (Gomez and Owens 2012; Gomez et al. 2013), the current SMC lines of later passage notably express both MVSC and SMC markers to variable degrees. Until a specific (epi)genetic signature for MVSCs has also been identified and validated, the ultimate origin of cultured SMCs awaits final determination. At present, we cannot rule out the possibility that these SM-MHC+ and Sox10+/Sox17+/ S100β+ cells are derived from differentiated SMCs, MVSCs or both.
Increasing evidence now supports the plasticity of vascular cells and so SMCs might de-differentiate and revert to a more multipotent state in response to specific cues. Such plasticity might be the ultimate driver for various vascular pathologies. Indeed, differentiated vascular cells could be re-programmed to become multipotent/pluripotent given the right stimulus. In this context, mSMCs are capable of trans-differentiation to macrophage-like cells following cholesterol treatment (Rong et al. 2003). Moreover, several studies have suggested that, under certain circumstances, cultured SMCs can be induced to osteogenic and skeletal lineages in vitro (Graves and Yablonka-Reuveni 2000; Ciceri et al. 2012; Liao et al. 2013). This further supports a plasticity and stemness associated with SMCs possibly acquiring stem cell phenotypes following the de-differentiation of contractile SMCs. Interestingly, human SMCs are capable of trans-differentiation to a neural-stem-like phenotype typical of MVSCs if treated with a combination of sex steroids (Bukovsky 2009). As SMCs appear to adopt a more neural-stem-like phenotype in serum-rich conditions, we suspected that the expression of SMC and MVSC markers could be altered following exogenous stimuli such as inductive differentiation media, TGF-β1 stimulation or Notch inhibition. Our data suggest that, whereas mSMCs do indeed retain their MVSC markers in culture, they lose their multipotent capabilities in as much as they fail to mimic either MVSCs or MSCs and differentiate into adipocytes or osteoblasts following the same inductive stimulation. In addition, TGF-β1 stimulation and DAPT treatment fail to have any qualitative effect on the ratio of SM-MHC+ to Sox10+ cells in mSMC populations. TGF-β1 is known to promote the differentiation of stem cells into SMCs (Guo et al. 2013) and has been reported to suppress Sox10 expression directly in progenitors of neural crest origin (John et al. 2011). Conversely, DAPT inhibits Notch signalling, which is a critical component during MSC to SMC transition (Kurpinski et al. 2010; Kane et al. 2011; Wang et al. 2012; Tang et al. 2012). Interestingly, Sox17 acts upstream of the Notch system and downstream of the canonical Wnt system in orchestrating arterial specification and might thus be critical for MVSC transition to SMCs in vitro (Corada et al. 2013).
In conclusion, we provide evidence that aortic SMCs used routinely in culture to examine SMC behaviour and functionality in vitro express neural stem cell markers typical of resident vascular stem cells. Moreover, as SMCs in situ and primary dispersed medial SMCs in culture exhibit low levels of MVSC markers (unlike explanted primary SMCs, which are Sox10+ but SM-MHC−), these SMC lines clearly acquire MVSC markers either by de-differentiation to Sox10+ cells during culture or are outgrown by a small percentage of Sox10+ MVSCs over time or both. Moreover, to our knowledge, this is the first study to describe a SM-MHC+, Sox10+ phenotype for aortic SMCs derived from three different species in culture. Based on previous lineage tracing studies of the SM-MHC (Sm-2) promoter in cultured SMCs in vitro and of de-differentiated SMCs following injury in vivo, uncertainty remains as to whether these Sox10+, Sox17+, S100β+ SMCs are reminiscent of SMCs derived from differentiated SMCs, MVSCs or both. Further study of stem-cell-derived SMCs in culture will provide a new perspective on vascular biology and may lead to the development of new therapies for vascular diseases that target these cells. In this context, multiple SMC phenotypes will assist in this goal and further elucidate the role of stem-cell-derived SMCs and differentiated SMCs in determining whether vascular disease is a stem cell disease, a differentiated SMC disease or both.
Acknowledgments
This study was supported in part by funds from Science Foundation Ireland (SFI-11/PI/1128 to P.A. Cahill) and the National Institutes of Health (R00HL095650 to D. Morrow and R21AA020365 to E.M. Redmond).
Contributor Information
Eimear Kennedy, Vascular Biology and Therapeutics Laboratory, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland.
Ciaran J. Mooney, Vascular Biology and Therapeutics Laboratory, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland
Roya Hakimjavadi, Vascular Biology and Therapeutics Laboratory, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland.
Emma Fitzpatrick, Vascular Biology and Therapeutics Laboratory, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland.
Shaunta Guha, Vascular Biology and Therapeutics Laboratory, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland.
Laura E. Collins, Immunomodulation Research Group, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland
Christine E. Loscher, Immunomodulation Research Group, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland
David Morrow, Department of Surgery, University of Rochester Medical Center, Rochester NY 14642, USA.
Eileen M. Redmond, Department of Surgery, University of Rochester Medical Center, Rochester NY 14642, USA
Paul A. Cahill, Vascular Biology and Therapeutics Laboratory, School of Biotechnology, Faculty of Science and Health, Dublin City University, Dublin 9, Ireland
References
- Babij P, Kawamoto S, White S, Adelstein RS, Periasamy M (1992) Differential expression of SM1 and SM2 myosin isoforms in cultured vascular smooth muscle. Am J Physiol 262:C607–C613 [DOI] [PubMed] [Google Scholar]
- Bukovsky A (2009) Sex steroid-mediated reprogramming of vascular smooth muscle cells to stem cells and neurons: possible utilization of sex steroid combinations for regenerative treatment without utilization of in vitro developed stem cells. Cell Cycle 8:4079–4084 [DOI] [PubMed] [Google Scholar]
- Cahill PA, Hassid A (1993) Differential antimitogenic effectiveness of atrial natriuretic peptides in primary versus subcultured rat aortic smooth muscle cells: relationship to expression of ANF-C receptors. J Cell Physiol 154:28–38. doi: 10.1002/jcp.1041540105 [DOI] [PubMed] [Google Scholar]
- Cappadona C (1999) Phenotype dictates the growth response of vascular smooth muscle cells to pulse pressure in vitro. Exp Cell Res 250: 174–186. doi: 10.1006/excr.1999.4502 [DOI] [PubMed] [Google Scholar]
- Chamley JH, Campbell GR, Burnstock G (1974) Dedifferentiation, redifferentiation and bundle formation of smooth muscle cells in tissue culture: the influence of cell number and nerve fibres. J Embryol Exp Morphol 32:297–323 [PubMed] [Google Scholar]
- Chamley-Campbell J, Campbell GR, Ross R (1979) The smooth muscle cell in culture. Physiol Rev 59:1–61 [DOI] [PubMed] [Google Scholar]
- Ciceri P, Volpi E, Brenna I, Arnaboldi L, Neri L, Brancaccio D, Cozzolino M (2012) Combined effects of ascorbic acid and phosphate on rat VSMC osteoblastic differentiation. Nephrol Dial Transplant 27: 122–127. doi: 10.1093/ndt/gfr284 [DOI] [PubMed] [Google Scholar]
- Corada M, Orsenigo F, Morini MF, Pitulescu ME, Bhat G, Nyqvist D, Breviario F, Conti V, Briot A, Iruela-Arispe ML, Adams RH, Dejana E (2013) Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun 4:2609. doi: 10.1038/ncomms3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Iso T, Shiba Y, Sato H, Yamazaki M, Oyama Y, Akiyama H, Tanaka T, Tomita T, Arai M, Takahashi M, Ikeda U, Kurabayashi M (2009) Notch signaling regulates the differentiation of bone marrow-derived cells into smooth muscle-like cells during arterial lesion formation. Biochem Biophys Res Commun 381:654–659. doi: 10.1016/j.bbrc.2009.02.116 [DOI] [PubMed] [Google Scholar]
- Gallagher PJ, Jin Y, Killough G, Blue EK, Lindner V (2000) Alterations in expression of myosin and myosin light chain kinases in response to vascular injury. Am J Physiol Cell Physiol 279:C1078–C1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Ferguson G, Connell P, Walshe T, Murphy R, Birney YA, O’Brien C, Cahill PA (2007) High glucose concentrations alter hypoxia-induced control of vascular smooth muscle cell growth via a HIF-1alpha-dependent pathway. J Mol Cell Cardiol 42:609–619. doi: 10.1016/j.yjmcc.2006.12.006 [DOI] [PubMed] [Google Scholar]
- Gomez D, Owens GK (2012) Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 95:156–164. doi: 10.1093/cvr/cvs115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Shankman LS, Nguyen AT, Owens GK (2013) Detection of histone modifications at specific gene loci in single cells in histological sections. Nat Methods 10:171–177. doi: 10.1038/nmeth.2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DC, Yablonka-Reuveni Z (2000) Vascular smooth muscle cells spontaneously adopt a skeletal muscle phenotype: a unique Myf5-/MyoD+ myogenic program. J Histochem Cytochem 48:1173–1193. doi: 10.1177/002215540004800902 [DOI] [PubMed] [Google Scholar]
- Guo X, Stice SL, Boyd NL, Chen SY (2013) A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells. Am J Physiol Cell Physiol 304:C289–C298. doi: 10.1152/ajpcell.00298.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holifield B, Helgason T, Jemelka S, Taylor A, Navran S, Allen J, Seidel C (1996) Differentiated vascular myocytes: are they involved in neointimal formation? J Clin Invest 97:814–825. doi: 10.1172/JCI118481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YL, Shi GY, Lee H, Jiang MJ, Huang BM, Wu HL, Yang HY (2009) Thrombin induces nestin expression via the transactivation of EGFR signalings in rat vascular smooth muscle cells. Cell Signal 21:954–968. doi: 10.1016/j.cellsig.2009.02.005 [DOI] [PubMed] [Google Scholar]
- John N, Cinelli P, Wegner M, Sommer L (2011) Transforming growth factor β-mediated Sox10 suppression controls mesenchymal progenitor generation in neural crest stem cells. Stem Cells 29:689–699. doi: 10.1002/stem.607 [DOI] [PubMed] [Google Scholar]
- Kane NM, Xiao Q, Baker AH, Luo Z, Xu Q, Emanueli C (2011) Pluripotent stem cell differentiation into vascular cells: a novel technology with promises for vascular re(generation). Pharmacol Ther 129:29–49. doi: 10.1016/j.pharmthera.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Kato S, Shanley JR, Fox JC (1996) Serum stimulation, cell-cell interactions, and extracellular matrix independently influence smooth muscle cell phenotype in vitro. Am J Pathol 149:687–697 [PMC free article] [PubMed] [Google Scholar]
- Kuang S-Q, Kwartler CS, Byanova KL, Pham J, Gong L, Prakash SK, Huang J, Kamm KE, Stull JT, Sweeney HL, Milewicz DM (2012) Rare, nonsynonymous variant in the smooth muscle-specific isoform of myosin heavy chain, MYH11, R247C, alters force generation in the aorta and phenotype of smooth muscle cells. Circ Res 110:1411–1422. doi: 10.1161/CIRCRESAHA.111.261743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S (2010) Transforming growth factor-β and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28:734–742. doi: 10.1002/stem.319 [DOI] [PubMed] [Google Scholar]
- Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, Hu YR, Yuan ZS, Gu L, Li SJ, Mao DA, Lu Q, Zhou XM, de Jesus Perez VA, Yuan LQ (2013) MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 154:3344–3352. doi: 10.1210/en.2012-2236 [DOI] [PubMed] [Google Scholar]
- McEntee G, Minguzzi S, O’Brien K, Ben Larbi N, Loscher C, O’Fágáin C, Parle-McDermott A (2011) The former annotated human pseudogene dihydrofolate reductase-like 1 (DHFRL1) is expressed and functional. Proc Natl Acad Sci U S A 108:15157–15162. doi: 10.1073/pnas.1103605108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz R, Patterson J, Wilson E (2012) Vascular smooth muscle cells: isolation, culture, and characterization In: Peng X, Antonyak M (eds) Methods in molecular biology. Humana, Totowa, pp 169–176 [DOI] [PubMed] [Google Scholar]
- Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA (2005) Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol 289:C1188–C1196. doi: 10.1152/ajpcell.00198.2005 [DOI] [PubMed] [Google Scholar]
- Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O’Brien C, Walls D, Redmond EM, Cahill PA (2008) Notch and vascular smooth muscle cell phenotype. Circ Res 103:1370–1382. doi: 10.1161/CIRCRESAHA.108.187534 [DOI] [PubMed] [Google Scholar]
- Oikawa H, Hayashi K, Maesawa C, Masuda T, Sobue K (2010) Expression profiles of nestin in vascular smooth muscle cells in vivo and in vitro. Exp Cell Res 316:940–950. doi: 10.1016/j.yexcr.2009.10.025 [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801. doi: 10.1152/physrev.00041.2003 [DOI] [PubMed] [Google Scholar]
- Rong JX, Shapiro M, Trogan E, Fisher EA (2003) Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A 100:13531–13536. doi: 10.1073/pnas.1735526100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G (2013) S100B protein in tissue development, repair and regeneration. World J Biol Chem 4:1–12. doi: 10.4331/wjbc.v4.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA (2004) Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. FASEB J 18:1421–1423. doi: 10.1096/fj.04-1700fje [DOI] [PubMed] [Google Scholar]
- Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S (2012) Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun 3:875. doi: 10.1038/ncomms1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Wang A, Wang D, Li S (2013) Smooth muscle cells: to be or not to be?: response to Nguyen et al. Circ Res 112:23–26. doi: 10.1161/CIRCRESAHA.112.281055 [DOI] [PubMed] [Google Scholar]
- Wang A, Tang Z, Li X, Jiang Y, Tsou DA, Li S (2012) Derivation of smooth muscle cells with neural crest origin from human induced pluripotent stem cells. Cells Tissues Organs 195:5–14. doi: 10.1159/000331412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Koopman P (2002) Matching SOX: partner proteins and cofactors of the SOX family of transcriptional regulators. Curr Opin Genet Dev 12:441–446. doi: 10.1016/S0959-437X(02)00323-4 [DOI] [PubMed] [Google Scholar]
- Worth NF, Rolfe BE, Song J, Campbell GR (2001) Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskeleton 49:130–145. doi: 10.1002/cm.1027 [DOI] [PubMed] [Google Scholar]
- Zanellato AM, Borrione AC, Giuriato L, Tonello M, Scannapieco G, Pauletto P, Sartore S (1990) Myosin isoforms and cell heterogeneity in vascular smooth muscle. I. Developing and adult bovine aorta. Dev Biol 141:431–446. doi: 10.1016/0012-1606(90)90398-3 [DOI] [PubMed] [Google Scholar]