Abstract
Targeted functional genomics represents a powerful approach for studying gene function in vivo and in vitro. However, its application to gene expression studies in human mast cells has been hampered by low yields of human mast cell cultures and their poor transfection efficiency. We developed an imaging system in which mast cell degranulation can be visualized in single cells subjected to shRNA knockdown or CRISPR-Cas 9 gene editing. By using high resolution confocal microscopy and a fluorochrome-labeled avidin probe, one can directly assess the suppression of functional responses, i.e. degranulation, in single human mast cells. The elimination of a drug or marker selection step avoids the use of potentially toxic treatment procedures and the short hands-on time of the functional analysis step enables the high-throughput screening of shRNA or CRISPR-Cas9 constructs to identify genes that regulate human mast cell degranulation. The ability to analyse single cells significantly reduces the total number of cells required, and allows for the parallel visualization of the degranulation profile of both edited and non-edited mast cells, offering a consistent internal control not found in other protocols. Moreover, our protocol offers a flexible choice between RNA interference and CRISPR-Cas9 genome editing for perturbation of gene expression using our human mast cell single-cell imaging system. Perturbation of gene expression, acquisition of microscopy data, and image analysis can be completed within 5 days, requiring only standard laboratory equipment and expertise.
Keywords: Human mast cell, mast cell, mast cell degranulation, degranulation, primary cell culture, CRISPR-Cas9, single-cell imaging, degranulation regulator, functional genomics, confocal microscopy, high-resolution confocal microscopy, single-cell imaging, single cell imaging
EDITORIAL SUMMARY
This protocol presents an an imaging system in which mast cell degranulation can be visualized in single cells subjected to shRNA knockdown or CRISPR-Cas 9 gene editing using high resolution confocal microscopy with a fluorochrome-labeled avidin probe.
INTRODUCTION
Mast cells are major effector cells of the immune system mediating IgE-dependent allergic and hypersensitivity reactions in settings such as asthma, hay fever, atopic dermatitis and anaphylaxis1. High affinity IgE receptors (FcεRI) are expressed on the surface of mast cells, and crosslinking of these receptors by cell-bound allergen-specific IgE in combination with allergen leads to mast cell degranulation1. Degranulation triggers the immediate release of preformed granular mediators (e.g., histamine and proteases), which is followed by the de novo synthesis and release of lipid mediators (e.g., leukotrienes and prostaglandins) and cytokines like TNF and IL-81. Recent studies have further expanded the roles of mast cells from allergic disorders to many additional types of inflammatory reactions involving innate and adaptive immune responses2–4. Hence, a tightly regulated spatiotemporal control of mast cell activation is important for maintaining immune homeostasis and an understanding of its mechanisms can suggest avenues for preventing allergic diseases. Experimental approaches for the identification of regulatory factors that modulate human mast cell activation and degranulation can therefore importantly contribute to the design of therapeutic agents for the treatment and/or the prevention of allergies and other mast cell-associated diseases.
Over the past decades, many laboratories have focused on the identification of protein regulators that modulate mast cell degranulation. As listed in recent entries in the Gene Ontology Annotations on “mast cell degranulation” in the Mouse Genome Informatics (MGI) website, these regulators of mast cell degranulation include cell-surface receptors, signal transduction intermediates, and effector molecules that contribute to the processes and pathways mediating mast cell degranulation (http://www.informatics.jax.org/go/term/GO:0043303). Various technological approaches (such as protein purification, differential gene expression analysis, signal transduction pathway analysis, and molecular cloning) have been employed to identify such protein regulators using rodent (primarily, mouse) mast cells, and gene-targeted knockout mice have been developed to elucidate the in vivo phenotype and functional roles of these protein regulators (http://www.informatics.jax.org/go/term/GO:0043303).
Although the human orthologues of such mouse proteins have been fully identified and sequenced, the precise functional roles of these proteins in regulating the degranulation of human mast cells have not been fully validated. The follow-up validation of the exact roles of these regulators in mediating degranulation in human mast cells has been hampered by the limited availability of primary human mast cells and the lack of suitable methodology to functionally interrogate the putative roles of such regulators of degranulation using small numbers of primary cells. To circumvent these issues, we have developed a method that couples the generation of blood-derived primary human mast cells with functional genomics and a single cell imaging protocol to assess the regulatory mechanisms of degranulation. Using this method, we have demonstrated that both single human primary mast cells in vitro and mouse dermal mast cells in vivo can respond to distinct stimuli of activation by finely regulating the dynamics and features of mast cell granule secretion5.
Development of the protocol
We developed an imaging system to probe the complex and rapidly evolving process of mast cell degranulation by high resolution confocal microscopy in single cells (Figs. 1 and 2). This method is based on the application of avidin-sulforhodamine 101 (Av.SRho), a highly cationic glycoprotein coupled to a fluorochrome, that selectively binds to rodent and human mast cell granules6. Av.SRho was initially used to stain fixed and permeabilized human and rodent mast cells in various tissues7, but we found that it could also be used to directly monitor and analyze degranulation in activated mast cells8. While parts of the externalized granule structures were released into the culture medium, a substantial amount of them were retained on the mast cell surface (as seen in Figure 1 and Supplementary Movie 1 of ref.8).
Figure 1 |. Overview of human mast cell culture, functional genomics, and high-resolution confocal microscopy procedures.
Primary human mast cells are cultured following selection enrichment of CD34+ peripheral blood hematopoietic progenitors and are then assessed for their phenotype and functional maturity after 12 weeks in culture. Perturbation of the gene-of-interest is induced using transfection of either shRNA knockdown or the CRISPR-Cas9 gene editing system. Subsequently, mast cell degranulation is visualized in single cells using high resolution confocal microscopy and a fluorochrome-labeled avidin probe. Semi-automated image analysis is performed to determine the degranulation profiles of both gene edited (degranulation suppressed) and non-edited (degranulation unaffected) mast cells. This allows the rapid identification of regulators of human mast cell degranulation. (NMD means nonsense-mediated decay.)
Figure 2 |. Flowchart illustrating three major components of our protocol: human mast cell culture, functional genomics, and high-resolution confocal microscopy.
Essential steps are shown as rounded rectangles. Time needed to complete these steps is depicted on the left. On the right, pause points are indicated, together with the timing of the different quality control checkpoints: I, purity of CD34+ selection (Step 32); II, assessment of phenotypic and functional maturity (Step 38); III, GFP expression (Step 40).
Further utilization of high resolution confocal and two-photon microscopy, in real-time and at the single-cell level, has allowed us to show that human mast cells in vitro or mouse mast cells in vivo differentially regulate their degranulation strategy in response to activation via Fc receptors (e.g., FcεRI) or G protein-coupled receptors (e.g., the receptor for cationic molecules MRGPRX2)5. Finally, confirming our interest in the validation of protein functions in human mast cells, we showed that shRNA-mediated knockdown of MRGPRX2 impaired mast cell degranulation induced by Substance P (a cationic neuropeptide that binds to and activates MRGPRX2), but not anti-IgE–induced degranulation5. This combination of RNA interference, fluorochrome-labeled Av.SRho visualization strategy, and single-cell confocal microscopy offers a highly useful approach to study the function of genes in primary human mast cells without the need to obtain large homogenous populations of fully transfected cells, which is difficult given the lack of methods the methods and protocols thatthat are currently available to study primary human mast cells.
Although shRNA-based gene silencing was useful in our previous study5, shRNA knockdown has several known drawbacks, such as (i) the difficulty to assess the level of protein knock-down in single cells, (ii) many off-target effects, and (iii) the difficulty in predicting how much protein depletion is required for measurable phenotypic effects. To this end, we have recently replaced the shRNA-mediated knockdown by the CRISPR-Cas9-mediated gene editing technique. This approach has two distinct advantages: (i) it involves specific gene deletion and has fewer off-target effects9–13. Therefore, it offers an unambiguous interpretation of functional outcomes14,15. (ii) It is possible to confirm gene deletion in sorted (or single) cells by genomic PCR16.
Accordingly, the protocol presented here can be used for the rapid identification of regulators of human mast cell degranulation and requires only standard laboratory equipment. In addition, we describe a streamlined culturing method to efficiently generate primary human mast cells from human CD34+ peripheral blood hematopoietic progenitors, yielding at least 20 × 106 functionally active human mast cells from a single buffy coat that can be stably maintained over several months (Fig. 3)5.
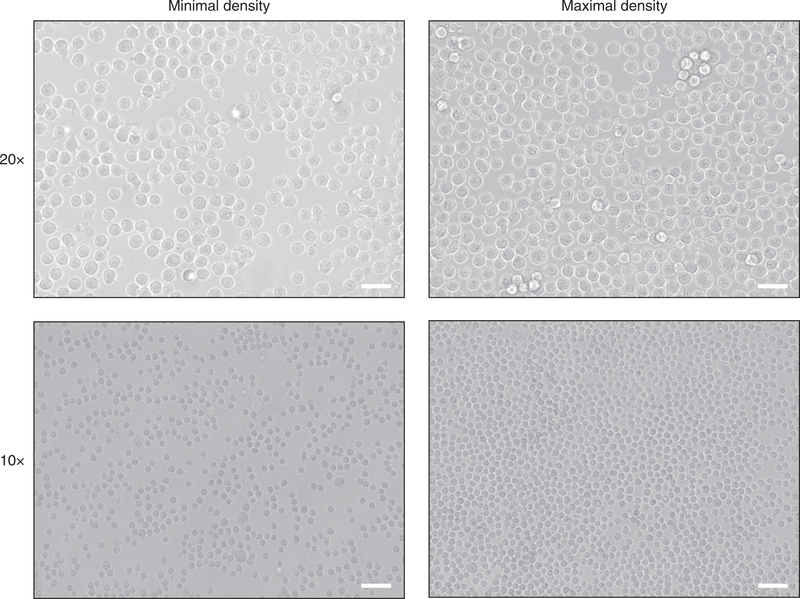
Figure 3 |. Primary human mast cell cultures generated with optimal culture conditions.
A typical mast cell culture after 10 weeks of culture should have a minimal cell density, as illustrated in the images on the left (0.5 ×106 mast cells per mL, objective lens 20x and 10x) and a maximal cell density as illustrated in the images on the right (1–1.5 ×106 mast cells per mL, objective lens 20x and 10x). Scale bars, 50 μm (20x); 100 μm (10x). Pictures were taken 0.5 cm next to the center of the wells.
Comparison with other methods
Tissue mast cells are, at least in part, derived from CD34+ multi-potent progenitor cells in the bone marrow that migrate via the circulation to tissues, where they differentiate and mature under the influence of local environmental signals. Assessment of the functional characteristics of human primary mast cells in vitro is a complex procedure that requires the extraction and purification of mast cells from human tissues, with the substantial limitations of (i) obtaining enough mast cells, and (ii) rendering the mast cells highly homogenous. Although several human mast cell lines exist, such as HMC-1, LAD2, and more recently, LUVA, they do not appear to exhibit all of the characteristic functional and growth properties of mature human tissue mast cells. The identification of stem cell factor (SCF), the c-kit ligand17 as the key human mast cell survival and growth factor18–20 represents a critical development that has helped to advance methods for generating human mast cells in vitro. Such human mast cells have been derived from CD34+ pluripotent stem cells isolated from bone narrow21 or fetal liver22, as well as from cord blood23,24 or peripheral blood23,25.
Methods to culture primary human mast cells (often derived from peripheral- or cord-blood CD34+ hematopoietic precursors) have recently been improved, enabling the generation of higher cell yields while reducing costs and labor intensity. These methods permit the generation of functional human mast cells that exhibit morphology and functions similar to those found in human connective tissues-resident mast cells (e.g., the skin)23,25–28. An important feature of the culturing protocol detailed below, that one may consider a disadvantage, is that it takes ~ 10–12 weeks before phenotypically and functionally active human mast cells are obtained, as compared to 6 weeks in some other protocols. The high purity of our mast cell cultures is comparable with that of cultures generated using other primary human mast cell culturing methods23,25–28.
One of many current in vitro functional genomics approaches for validating the functions of mouse genes in human cells involves the transfection of suitable human cells with constructs that are designed either to suppress protein expression (such as shRNAs) or to delete the potential human orthologue gene (such as CRISPR-Cas9). However, the use of primary human mast cell cultures for such transfection studies poses several technical difficulties. The in vitro-derived cultured human mast cells, like most primary cells, exhibit low transfection efficiency. Moreover, these human mast cells typically do not survive well under selection conditions using antibiotic treatment. Therefore, the numbers of functionally and genetically-modified cultured human mast cells that can be obtained after both transfection and selection processes are usually not sufficient for in vitro functional cell-based assays, including those for quantifying degranulation by measuring the amounts of release of histamine or beta-hexosaminidase from mast cells after cell stimulation.
In addition, in the absence of selection, the CRISPR-Cas9 approach may potentially result in a polyclonal population of transfected cells. These would possibly include unedited, heterozygous knockouts, as well as homozygous knockout cells with heterogeneous sequences at the target locus due to the imperfect repair of double-strand breaks generated after cutting. Considering what has been published in the current literature, the CRISPR-Cas9 approach has so far not been applied to the study of (primary) human mast cells. Instead, gene function is usually validated using currently available RNAi systems, that require a high number of cells, thus limiting the study design to human mast cell lines, such as the LAD2 cell line29, or mouse mast cells30. Therefore, there has been a need for the development of additional technical approaches, based on single-cell readouts, to assess both editing efficacy and resulting phenotype among a heterogeneous population of transfected primary human mast cells.
In our experience, primary human mast cells display low transfection efficiencies, ranging between 20 and 40% depending on the donor, similar to other cultured cells that are derived in vitro from blood or primary tissues31. However, because we can analyse single cells in our protocol, this eliminates the need for selection and enrichment of the fully transfected cells. This approach thus provides a distinct advantage over bulk methods that rely on potentially toxic drug selection strategies. Moreover, our ability to analyse single cells significantly reduces the total numbers of cells required, thus increasing the numbers of cells available for other studies. By eliminating the selection step, this also helps to reduce labor and time requirements for the experimental studies. Moreover, our ability to visualize the degranulation profile of both gene edited and non-edited mast cells simultaneously in the same experimental condition offers a highly consistent internal control not found in other protocols (Fig. 4). Finally, our protocol offers a flexible choice between RNA interference and CRISPR-Cas9 genome editing for the perturbation of gene expression.
Figure 4 |. Representative data of selected CRISPR-Cas9-mediated gene knockout studies in primary human mast cells.
(a,b) After the focus is arranged correctly (top left panels) gene edited mast cells were identified based on GFP expression (top right panels, indicated in cyan), whereas mast cell degranulation was visualized using Av.SRho (lower left panels, indicated in red). Mast cells with a gene knockout for FcεRI-alpha chain did not respond to anti-IgE crosslinking (a, lower right panel). Mast cells with a gene knockout for MRGPRX2 do not respond to codeine (an opioid derivative and MRGPRX2 agonist) stimulation (b, lower right panel). (c-f) Semi-automated image analysis (e.g., of lower right panels from a and b; note: mast cells with GFP expression twice as high as the basal MFI level were considered GPF-positive), which separates single cells from each other and assigns a number to each individual cell as seen on panels c and d, calculates the GFP-derived MFI of each individual cell (e), and calculates Av.SRho-derived MFI of each individual cell (f). Cells number 3 and 5 (in panel d) are dead cells which stain high for Av.SRho (as seen in panel f) and should be excluded from further analysis. Scale bars, 20 μm. * indicates a high Av.SRho signal originating from dead cells. Data are representative of those obtained in multiple (>3) independent experiments, all of which gave similar results.
Applications of the method
The presented methodology allows researchers to interrogate the functional roles of specific proteins as regulators of mast cell degranulation. Although the human mast cell culturing procedures are provided here for generating primary cultured human mast cells, other types of cultured mast cells derived by other in vitro methods may be used as well. Since Av.SRho stains both rodent and human mast cell granules6, our combined functional genomics and high-resolution confocal microscopy protocol can also be applied to rodent mast cells. Although this protocol offers a tool to assess protein function using the CRISPR-Cas9-mediated gene knockout approach, it can also be adapted to other genomics approaches, such as the method of modulating transcription without modifying the genomic sequence by fusing catalytically inactive Cas9 (dCas9) to transcriptional activator and repressor domains32. CRISPR activation and CRISPR inhibition can be achieved by direct recruitment or fusion of activation and repression domains, such as VP64 and KRAB, respectively33,34. Moreover, given the relatively straightforward character of this protocol, together with the sufficient quantities of functionally active human mast cells generated by our method, the procedures described here may permit the image-based profiling of single-cell phenotypes using arrayed guide RNA libraries35–37. Therefore, by combining our protocol with arrayed CRISPR genome-editing, e.g. targeting on different pathway elements, one may be able to perform multivariate profiling of single-cell phenotypes in rodent or human mast cells. Alternatively, genes of interest resulting from prior pooled or arrayed genomic screenings can be phenotypically/functionally validated using this protocol. Finally, our previous studies have indicated that this method can also be used for in vivo studies of the genetically modified mouse mast cells5.
Limitations
Perturbation of key degranulation regulators such as the FcεRI-alpha chain or MRGPRX2 significantly reduces mast cell degranulation when such cells are stimulated with the relevant ligands, demonstrating the validity and utility of our approach. However, if protein depletion is only moderately efficient or the gene of interest has a moderate effect on degranulation, effects that are detected can be small. Consequently, that may pose a challenging task to quantify the degranulation effect. This limitation may be overcome by increasing the numbers of measured cell samples to allow appropriate statistical analysis of the acquired data sets and detection of quantitative changes in the average degranulation profile (as shown in Supplemental Figure 3 of ref. 5). We recommend that at least 25–30 single GFP positive and 25–30 single GFP negative mast cells be assessed in such situations, orresortto. For regulator proteins that may have rather subtle effects on degranulation, one may not be able to detect such effects readily using our single-cell imaging system due to its inherent sensitivity. In that case, one can attempt to employ more sensitive techniques (such as single-cell RNA-Seq or CyTOF) to assess changes in expression of genomic and proteomic biomarkers that are associated with the degranulation process.
CRISPR-mediated gene editing offers many opportunities and advantages, yet one of its main limitations is the need for a protospacer adjacent motif (PAM) sequence within the gene of interest. Because the 5′-NGG-′3 PAM sequence for the most widely used Cas9 variant, Streptococcus pyogenes Cas9 (SpCas9), occurs on average every 8–12 bp in the human genome, this usually would not hinder application38. However, if PAM sequence availability does pose a problem, one should consider switching to an engineered CRISPR-Cas9 variant with altered PAM specificity39,40.
Experimental design
The experimental workflow of the procedures is shown in Fig. 2, which depicts the different stages of the Procedure (Steps 1–68). In broad terms, the Procedure consists of four main sections, which are detailed below.
type In vitro-derived human cultured mast cells
Pragmatic selection of the mast cell model to be used in the studies can be pivotal to the outcome of the experiments. Considerations for the selection of the model should include expression of protein of interest, cell number yields and purity, costs, and labor intensity. Although the widely used mouse bone marrow-derived cultured mast cells and rat basophilic leukemia (RBL) cell line are easy to obtain and culture in large numbers and high purity, significant functional and phenotypical differences exist between rodent and human mast cells41. To obtain large numbers of functional human mast cells, human mast cell lines such as HMC-1, LAD, and LUVA may offer a solution. Although HMC-1 cells grow rapidly, their use may be limited by the absence of the expression of FcεRI and reduced quantities of granules42,43. By contrast, LAD cell lines do express FcεRI and degranulate upon IgE-crosslinking, but they exhibit a variable and long doubling time, thus making them less suitable for screening assays44,45. Finally, the relatively new LUVA cell line may bypass classical human cell line limitations, yet it can lose expression of FcεRI upon long-term culturing46. Accordingly, relevant cell line- and/or species-specific molecular abnormalities should be carefully considered before the application of our protocol.
Although peripheral blood-derived human mast cells (PBMCs) are more labor intensive to generate, and usually each culture yields no more than 20 ×106 mast cells, they are mature mast cells with a condensed non-segmented nucleus and numerous granules, express FcεRI, and are tryptase and chymase positive23,25. In contrast, cord blood-derived human mast cells (CBMCs) are relatively immature with a segmented nucleus and less abundant granules. They are only tryptase positive and do not express significant amounts of FcεRI (but this can be overcome by incubation with IgE and rhIL-4 for 5 days)23,24,48. Given the different advantages and disadvantages of various established in vitro human mast cell culturing methods, we recommend using PBMCs as the human cultured mast cell system of choice to validate the function of genes and proteins in human mast cells. The moderate cell yields and low transfection efficiency of such primary human mast cells can be compensated for in our protocol by coupling functional genomics to single cell high-resolution confocal microscopy.
Primary human mast cells can also be isolated and purified from human tissues such as the skin and intestines. However, since these cells do not need to undergo long-term in vitro culturing conditions, they may represent heterogeneous cell populations and therefore may not be suitable for single-cell imaging analysis, which is an essential feature of our protocol.
Gene expression perturbation strategies
Considering the distinct advantages of using the CRISPR-Cas9 approach over shRNA knockdown in the perturbation of gene expression, as we have outlined above, we recommend using the CRISPR-Cas9 system as the “technique of choice” to validate gene function during degranulation in human mast cells. The specificity of the Cas9 nuclease is determined by the 20-nt guide sequence within the sgRNA. For the commonly used S. pyogenes system, the target sequence must immediately precede a 5′-NGG PAM, and the 20-nt guide sequence base pairs with the opposite strand to mediate Cas9 cleavage at ~3 bp upstream of the PAM.
Therefore, two considerations must be kept in mind when designing the sgRNA: (i) the 20-nt guide sequence must contain a 5′-NGG PAM, and (ii) this should be shown to have minimal off-target activity. We recommend using the online CRISPR Design Tool (http://tools.genome-engineering.org) that reads a genomic sequence of interest and identifies suitable target sites38, and then computationally assessing the likelihood that a given guide sequence will have off-target sites. Alternatively, ready-to-use CRISPR-Cas knockout kits for specific guide sequences are available from several suppliers. We have had positive experiences with the HDR-mediated and KN2.0 non-homology-mediated (Box 1) CRISPR/Cas9 Genome Knockout Kits from Origene. These kits come with a donor/repair template, which can be used to insert reporter genes such as fluorescent proteins or antibiotic resistance markers. Depending on the study design, it is always useful to generate more plasmid DNA using DNA subcloning and purification methods for future experiments within the specific project.
Box 1. Homology directed repair and non-homologous end joining approaches in CRISPR-Cas9-mediated gene editing knockout kits.
There are currently two types of CRISPR knockout kits available: Homology Directed Repair and Non-Homology Mediated Repair (the latter also called non-homologous end joining, NHEJ). The difference between the two approaches is the repair mechanism that is used to achieve the gene knockout. In both systems, the introduction of the pCas-Guide vector, including a target sequence, causes a site-specific double stranded break in the genome. In homology-directed repair-mediated CRISPR knockouts, the donor plasmid contains a left homologous arm and a right homologous arm flanking the donor cassette. As a result, the donor cassette will be ligated into the genome via the homology-directed repair mechanism. Although homology directed repair is considered the dominant mechanism for precise double stranded break repair (since there are fewer errors or chances of mutations if the DNA template used during repair is identical to the original undamaged DNA sequence), it suffers from low efficiency as it requires higher sequence similarity between the severed and intact donor strands of DNA. As an alternative, NHEJ-break ends can be ligated without a homologous template. NHEJ is a very efficient repair mechanism that is most active in the cell. Since it is susceptible to frequent mutation errors due to nucleotide insertions and deletions (indels), the majority of gene knockouts are bi-allelic (unlike the homology-directed repair mechanism), as one allele has donor integration and the other allele has indels (insertion and deletion).
Imaging and data analysis
The choice of imaging strategy is not limited to the specific microscopes and settings presented in this protocol. However, for optimal configuration, a confocal microscope should be equipped with at least two laser lines, two photomultiplier tubes (PMT), and climate control (temperature, humidity and CO2). Furthermore, it should be considered whether measuring a time-lapse sequence is desired, which has the advantage of exposing the spatiotemporal pattern of degranulation. If the microscope is not equipped with a focus auto-adjustment control module, the focus plane needs to be readjusted manually during the imaging process. Choice of image analysis software is similarly flexible, although Image J and Fiji (an image processing package) are most commonly used among many laboratories. The macros we provide here allow for time-saving semi-automated image analysis in Fiji. Alternatively, mean fluorescence intensity (MFI) can be quantified using the measurement function of Image J software. Although laser-scanning confocal microscopes enable the control of the intensity of laser exposure and the reduction in phototoxicity, while providing better resolution, a classical wide-field fluorescent microscope may also be needed eventually for assessing mast cell degranulation.
Controls
Before alterations in the mast cell degranulation profile can be attributed to the perturbation of expression a specific gene, the reduced expression of the targeted RNA/protein has to be verified. There are a variety of techniques that can be used to achieve this goal, each with its own advantages and limitations. Specific conjugated antibodies can be used to assess the reduction in protein levels, in combination with either microscopy or FACS analysis. A major advantage of imaging-based analysis of protein levels is that they can be assessed on single cells, due to GFP expression in genome-edited cells (as we show in Fig. 5). On the other hand, the advantage of the FACS-based analysis of protein levels is the utilization of multiple laser channels to measure a set of phenotypic (e.g., FcεRI, CD117) and activation (e.g., CD63, Alexa488-coupled avidin) markers. Isolating GFP positive cells using a cell sorter allows the detection of a genomic DNA (micro) deletion or inversion and indel mutations using the SURVEYOR nuclease assay47 or genomic PCR.
Figure 5 |. Data analysis and control experiments for validation of regulators of human mast cell degranulation.
(a) A representative set of data of combined MFI values from GFP-negative (FcεRI alpha chain-sufficient, depicted in orange) and GFP-positive (FcεRI alpha chain-deficient, depicted in blue) mast cells generated from multiple images. Mast cells with GFP expression levels twice as high as the basal MFI level are considered GPF-positive. (b) Identification of regulators of human mast cell degranulation is determined by the degranulation profile of GFP-positive cells (depicted in blue) compared to that of GFP-negative cells (depicted in orange). GFP-positive mast cells lacking the FcεRI-alpha chain are significantly less responsive to anti-IgE crosslinking than are GFP-negative (wild-type) mast cells. (c) As a control for viability and functional responsiveness, GFP-positive mast cells (indicated by a high intracellular GFP signal, depicted in cyan) could still respond to non-IgE-mediated stimuli, such as Substance P. (d) CRISPR-Cas9-mediated reduction of FcεRI-alpha chain protein expression on the surface of mast cells was visualized (where GFP is depicted in cyan and FcεRI-alpha chain protein is depicted in dark blue) using specific antibodies. (e) Quantitative degranulation profile of GFP negative mast cells compared to GFP-positive mast cells stimulated with Substance P for 30 minutes. Similar numbers of mast cells degranulated in response to Substance P (14 GFP negative mast cells out of 69 total cells vs. 17 GFP-positive mast cells out of 69 total cells). No differences in degranulation profile were observed between GFP-negative and GFP-positive mast cells. Control experiments described in (c) and (e) were performed on the same day(s) and same donors were used in results shown in (a) and (b). Mean ± SEM; 2-tailed, unpaired t test. Panel (b) has n value of >30 per condition, panel (e) has n value of >14 per condition. Scale bars, 20 μm (20x); 10 μm (100x). SP = Substance P. Images displayed in panel c and d are representative of those obtained in multiple (>3) independent experiments, all of which gave similar results.
It should be noted that donor variation may be a significant concern when using primary human mast cells derived from different donors. The first approach in addressing this problem is to test multiple donors, especially when the degranulation response caused by gene perturbation is rather modest. The second approach is to employ donor cultures that exhibit an average degranulation response (~25–30% corrected histamine release) and avoid using donor cultures that represent the two extreme ends of the degranulation response spectrum. Furthermore, it should be noted that Av.SRho stains permeable dead mast cells, which should be excluded from future analysis manually (Fig. 4d, cells #3 and #5). Dead mast cells can be identified by: (i) an intracellular Av.SRho signal before degranulation is observed, (ii) an abnormally high intracellular Av.SRho signal, and/or (iii) no extracellular Av.SRho signal is observed in viable degranulating mast cells by confocal microscopy. Typically, 1 in 20 mast cells can be identified as dead during the first few days post transfection, and this number slightly increases in the next few days to 2 to 3 dead mast cells per 20 cells. Finally, the post-transfection cell viability and functionality should be addressed using stimuli that are independent of the knocked-out degranulation regulator (as we show in Fig. 5c). Since calcium ionophores can stimulate mast cell activation via the downstream signaling components of the mast cell signal transduction pathways, such compounds can represent useful reagents for a functional control of responsiveness to mast cell activation.
MATERIALS
Biological materials
Buffy coat(s) of healthy blood donor(s) obtained from the hospital blood center (we used the Stanford Blood Center and Sanquin Rotterdam).
! CAUTION All human blood samples must be handled according to the approved biosafety protocols and regulations set by each individual lab and institution. Improper handling of samples may lead to exposure to blood-borne pathogens.
! CAUTION All human blood samples, and data obtained from them, must be handled according to approved biosecurity protocols for the protection of patient confidentiality and health information in accordance with relevant institutional and national regulations.
Reagents
Generation of peripheral blood-derived cultured human mast cells
EasySep Human CD34 Positive Selection Kit II (StemCell Technologies, cat. no. 17856)
DPBS, no calcium, no magnesium (Thermo Fisher Scientific, cat. no. 14190144)
Ficoll®-Paque Premium (Sigma-Aldrich, cat. no. GE17–5442-02)
StemSpan SFEM (StemCell technologies, cat. no. 09650)
Ciprofloxacin (Sigma-Aldrich, cat. no. 17850)
human IL-6 (Peprotech, cat. no. 200–06)
human IL-3 (Peprotech, cat. no. 200–03)
SConditioned medium from Chinese hamster ovary (CHO) cell transfectants secreting mouse stem cell factor (SCF). The CHO cells were a gift from Dr. P. Dubreuil, Marseille, France.
Iscove’s modified Dulbeccos medium with GlutaMAX-I (Thermo Fisher Scientific, cat. no. 31980030–500 ml),
2-mercaptoethanol (Sigma-Aldrich, cat. no. M6250),
Bovine Serum Albumin solution, 30% in DPBS, sterile-filtered (Sigma-Aldrich, cat. no. A9576–50ML)
Insulin-Transferrin-Selenium (ITS-G) (100X) (Thermo Fisher Scientific, cat. no. 41400045)
Ethylenediaminetetraacetic acid solution (Sigma-Aldrich, cat. no. 03690–100 mL)
Trypan Blue Solution 0.4% (Sigma-Aldrich, cat. no. T8154–100 mL)
Transfection
-
MRGPRX2 SureSilencing shRNA plasmids (Qiagen, cat. no. KM59132G)
CRITICAL: The SureSilencing shRNA vectors are shipped on cold ice packs, but should be kept at −20 °C for long-term storage. If properly stored, vectors are guaranteed to be stable for 6 months.
CRITICAL: Gene knockdown may also be achieved using kits from other manufacturers, but optimal conditions and amounts should be determined accordingly.
CRITICAL: Although SureSilencing shRNA Plasmids are not generally recommended for knocking down gene products in primary cells, we have found that they work well with this protocol, using the conditions detailed below.
Attractene Transfection Reagent (Qiagen, cat. no. 301005)
-
MRGPRX2 Human Gene Knockout Kit (CRISPR) (Origene, cat. no. KN409033)
CRITICAL: The cDNA clone is shipped at room temperature, but should be kept at −20 °C for long-term storage. If properly stored, clones are guaranteed to be stable for 12 months. Linear donor DNA can be shipped in lyophilized form; reconstitute on the day of transfection at 1 μg/μL with TE buffer.
-
FCER1A Human Gene Knockout Kit (CRISPR) (Origene, cat. no. KN203321)
CRITICAL: The cDNA clone is shipped at room temperature, but should be kept at −20 °C for long-term storage. If properly stored, clones are guaranteed to be stable for 12 months. Linear donor DNA can be shipped in lyophilized form; reconstitute on the day of transfection at 1 μg/μL with TE buffer.
TurboFectin Transfection Reagent (1 mL in 1 vial) (Origene, cat. no. TF81001)
Confocal
Avidin−Sulforhodamine 101 (Sigma-Aldrich, cat. no. A2348, RRID:AB_2810963)
Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (Thermo Fisher Scientific, cat. no. A-21245, RRID:AB_2535813)
Tyrode’s Salts (Sigma-Aldrich, cat. no. T2397–500ML)
Substance P acetate salt hydrate (Sigma-Aldrich, cat. no. S6883–1MG)
Codeine (prepared by hospital pharmacy in Rotterdam)
Human IgE, Myeloma (Merck, cat. no. 401152, RRID:AB_2810964)
Rabbit anti-Human IgE Antibody (Bethyl, cat. no. A80–109A, RRID: AB_67480)
Poly-D-lysine hydrobromide (Sigma-Aldrich, cat. no. P6407–5MG)
H2O (Sigma-Aldrich, cat. no. W3513–100ML)
Ice: for keeping reagents and buffers cold until use
Reagent setup
TE Buffer
For 10 mL of 1× TE buffer, mix 0.1 mL of tris-HCl (1 M, pH 8.0), 20 uL of EDTA (0.5 M, pH 8.0), and 9.88 mL of MilliQ water together. Autoclave and store at room temperature (18–22 °C) for up to 3 years.
MACS Buffer
For Human CD34 Positive Selection from PBMCs, prepare fresh MACS buffer, by supplementing PBS (500 mL) with 0.5% BSA and 2 mM EDTA.
Complete Medium I
For hematopoietic cell expansion, prepare complete medium I, by supplementing StemSpan medium (500 mL) with ciprofloxacin (10 μg/mL). Freeze at −20 °C until needed. Before use, warm up in a water bath (37 °C) and supplement with human IL-6 (50 ng/mL), human IL-3 (10 ng/mL) and 3% supernatant of CHO transfectants secreting mouse SCF (a gift from Pr. P. Dubreuil, Marseille University, France). In brief, approximately 1×106 CHO cells were cultured at 5×105 cells/mL in DMEM enriched with 10% FBS. Confluent cultures were split 1:4 to 1:8 every 4–7 days with 0.25% trypsin/0.53mM EDTA, until approximately 3 weeks of culture. Next, mouse SCF-containing supernatant was combined, filtered with a 0.22 μ syringe filter, aliquoted, and stored at −20 °C until further use. Complete medium I, without cytokines, can be stored at −20 °C for up to 12 months. Complete medium I, with cytokines, can be stored at 4 °C for up to 1 week.
CAUTION! Ciprofloxacin is an antibiotic, the use of personal protective equipment: Eyeshields, Gloves, type N95 (US), and type P1 (EN143) respirator filter, is recommended.
Complete Medium II
For mast cell (progenitor) expansion, prepare complete medium II, by supplementing Iscove’s modified Dulbeccos medium with GlutaMAX-I (500 ml), 50 uM 2-mercaptoethanol, 0.5% BSA, 1% Insulin-Transferrin-Selenium, ciprofloxacin (10 ug/mL). Freeze at −20 °C until needed. Before use, warm up in a water bath (37 °C) and supplement with human IL-6 (50 ng/mL), and 3% supernatant of Chinese hamster ovary transfectants secreting mouse SCF. Complete medium II, without cytokines, can be stored at −20 °C for up to 12 months. Complete medium II, with cytokines, can be stored at 4 °C for about to 1 week.
Equipment
Culture
Fume hood
Inverted contrast microscope for routine examinations of cell cultures (we use a Leica DM IL).
Water bath at 37 °C to warm cell-culture media
Incubator at 37 °C with 5% CO2
Centrifuges for 15 ml, 50 ml and Eppendorf tubes
Greiner Bio 15 mL CELLSTAR Centrifuge Tube, Sterile, DNAse/RNase Free (Greiner Bio, cat. no. 188271)
Greiner Bio 50 mL CELLSTAR Centrifuge Tube, Sterile (Greiner Bio, cat. no. 227261)
Filtered sterile pipette tips
Corning Costar TC-Treated Multiple Well Plates (Sigma-Aldrich, cat. no. CLS3516–50EA)
50 mL LeucoSep tubes, sterile (Greiner Bio-One, cat. no. 227290)
LS Columns (Milteny Biotec, cat. no. 130–042-401)
MidiMACS Separator (Milteny Biotec, cat. no. 130–042-302)
EasySep™ Magnet (StemCell Technologies, cat. no. 18000)
Pipet boy
10, 25 and 50 mL pipets
Nunc Lab-Tek Chambered Coverglass preparation
Nunc Lab-Tek 1.0 borosilicate cover glass system 8 chambers (Thermo Fisher Scientific, cat. no. 155411) (stronger than lime stone glass and more resistant to heat shock)
Microscopy
! CAUTION Most laboratories have access to confocal laser-scanning microscopes, however, appropriate training of respective microscopes should be completed BEFORE following this protocol.
-
Inverted confocal laser-scanning microscope. Many variations of hardware and acquisition software exist, and different combinations can be assembled to work well. We use a Leica SP5 AOBS with GalvoZ stage and Adaptive Focus Control (AFC):
- Objectives: HCX PL APO CS 10×/0.40 for locating GFP positive mast cells and HCX PL APO CS 20×/ 0.70 for imaging.
- Lasers used: Argon 488nM and 594HeNe.
- Detectors: 2x spectral PMT (Photo Multiplier Tube) 1x spectral HyD (Hybrid detector) 1x transmission PMT for brightfield, DIC and polarization.
- Metal-Halide lamp for EPI fluorescence GalvoZ stage with inserts for slides, 35 mm dishes, 10 cm dishes, and multi-well plates, adaptive Focus Control, large incubator with heating and CO2 control.
- Microscope enclosure with temperature, humidity and CO2 control.
CRITICAL: Alternatively, one may use any confocal microscope equipped with at least two laser lines, two PMT detectors and a microscope enclosure with temperature, humidity and CO2 control.
Software
Equipment Setup
Confocal imaging system
See Box 2 for an example of specific image acquisitions and the appropriate settings used in our system.
Box 2. Leica SP5 Settings.
In configuration, turn on the Argon (488nm) and the HeNe594.
- In Acquire – Acquisition
- Open the “Show Sequential Scan Panel”
- Add 1 additional sequential scan (keep setting to “between lines” – “Sequential scanning is an advantageous tool when detecting multiple fluorophores in one sample. It will enhance the image quality [e.g. avoiding crosstalk] by recording in a sequential order instead of a simultaneous acquisition.”).
- In sequential scan 1:
- Activate PMT 1, tick “visible”, set laser power to 15% and spectral range to 500–550 nM, click “PMT 1” and set gain to half of Max (change this later depending on signal strength) ? TROUBLESHOOTING.
- Activate PMT Trans, tick “visible”, click “PMT Trans” and set gain to half of Max (change this later depending on signal strength).
- In sequential scan 2:
- Activate PMT 2, tick “visible”, set laser power to 20% and spectral range to 605–670 nM, click “PMT 2” and set gain to half of Max (change this later depending on signal strength) ? TROUBLESHOOTING.
- In Acquire – Acquisition
- Acquisition mode: XYT
- Format: 512×512
- Line Average: 8
- Time interval: Minimize
- Duration: 30 min
Turn on regulation of temperature, humidity and CO2 control within microscope enclosure (37 °C and 5% humidified CO2).
▲CRITICAL Before you begin, you will need to determine the appropriate settings for some key image acquisition parameters. It is recommended to do this right after Step 38, and after confirming that your mast cells are mature, functional and responsive to your stimuli of interest. Optimal settings should be saved and be easily accessible when needed in Step 49.
PROCEDURE
Isolation of peripheral blood-derived human mast cell progenitors – Timing 4 h
▲CRITICAL STEP The standard PBMC and CD34+ isolation from one donor typically takes between 3 to 4 h. Make sure sufficient time is reserved for the use of the appropriate biosafety hood on the day of isolation.
-
1
Order buffy coats in advance from local blood bank/supplier.
-
2
On the day of arrival, clean and organize the fume hood with alcohol and UV (optional).
-
3
Prepare the following items for processing a single buffy coat: Two 50-mL LeucoSep tubes per donor - fill each tube with 15 mL Ficoll and centrifuge at 300 g for 1 min at room temperature; Two 50-mL Falcon tubes; Disinfected clamp and scissors; Two bottles of PBS (500 mL) at room temperature; and a Pipet-Aid with 25 mL pipets.
-
4
Once the donor samples have arrived, post any required biosafety notices around your workspace, record any donor information in your logs, and equip yourself with any required personal protective equipment, including appropriate gloves, safety glasses, and lab coat.
-
5
Disinfect the buffy-coat collection bags with alcohol, dry with a clean tissue.
-
6
Tighten the clamp at the tube below the buffy coat and cut the upper tube with the scissors, while keeping the buffy coat upright.
-
7
Cut with the scissors below the clamp and hold the buffy coat above the first 50-mL Falcon tube.
-
8
Release the clamp slightly and fill the Falcon tube until 17.5 mL, tighten when done and fill the other Falcon tube until 17.5 mL.
-
9
Discard all remaining parts of the buffy coat into an appropriate waste bin and clean the scissors (and clamp if necessary) with alcohol.
-
10
Add 17.5 mL PBS to each 50-mL Falcon tube to a total volume of 35 mL, mix gently. TROUBLESHOOTING.
-
11
Repeat Steps 5 to 10 until all buffy coats are mixed 1:1 with PBS.
-
12
Transfer the 35 mL of diluted blood drop-by-drop carefully onto the LeucoSep tubes. The LeucoSep tubes help to prevent mixing of the blood with Ficoll, however, it is recommended to slowly load the blood onto the Ficoll.
▲CRITICAL STEP Mixing of blood with Ficoll is the most frequent problem leading to insufficient PBMC separation, do this very carefully.
-
13
Centrifuge for 30 min at 300 g at room temperature (without brake).
-
14
Blood should be separated into 4 layers, remove and discard the top layer and collect the interphase containing the PBMC into a new 50 ml Falcon tube.
-
15
Dilute the PBMC by adding 45 mL PBS and mix well.
-
16
Centrifuge the tubes at 200 g for 10 min at room temperature. Properties of CD34+ cells seem to be sensitive to higher g levels in centrifugation.
▲CRITICAL STEP Slow centrifugation positively influences subsequent cell culture quality and can remove debris, dead cells and red blood cells (to a certain extent).
-
17
Prepare MACS buffer (see REAGENT SETUP) and keep at RT.
-
18
Wash the cells by discarding the supernatant from Step 16, resuspend the cell pellet with 25 mL MACS buffer and centrifuge the tubes at 200 g for 10 min at room temperature. Wash the cells one more time following this procedure. The EDTA disbands cell pellets upon resuspension.
-
19
Before the last wash step, take a 20 μL sample aliquot and count the cells (dilute 10x in PBS, then dilute 1:1 with Trypan Blue to exclude Trypan Blue-positive dead cells).
-
20
Resuspend, per donor, 2 – 5 × 108 PBMCs in 1.5 mL MACS buffer.
-
21
Put the remaining MACS buffer on ice.
-
22
Transfer each donor’s sample into a new 15 mL Falcon tube.
-
23
Add CD34+ selection cocktail (100 μL/mL) per tube and keep at RT for 15 min.
-
24
Mix magnetic particles well, by pipetting up and down and add 100 μL/mL magnetic particles per tube by pipetting up and down.
-
25
Keep at room temperature for 10 min.
-
26
Wash the LS column one time with ice-cold MACS buffer.
? TROUBLESHOOTING.
-
27
Transfer the cells to the LS column and let it run though. Use an empty 15-mL Falcon tube to collect and discard the CD34-negative fraction flow-through.
-
28
Wash 3x with ice-cold 3 mL MACS buffer. Use an empty 15-mL Falcon tube to collect and discard the CD34-negative fraction flow-through.
-
29
Add 5 mL cold MACS buffer to the column, remove from the magnet and plunge into a new 15-mL tube to collect the desired CD34-positive fraction.
-
30
Take a 10 μL sample and count cell numbers.
-
31
Wash cells once (centrifuge at 200 g for 7 min at room temperature) discard supernatant and resuspend pellet in 4 mL Complete Medium I (at least 0.4 ×106 per mL), divide the cells into 2 wells (in a 6-well plate).
-
32
Optionally, perform a quality check by assessing live/dead, using a trypan blue staining, as well as CD34+ purity. You can expect 3–6 million CD34+ cells after isolation.
Cell culture – Timing: 3 months
-
33
During the first week, cells cultured in Complete Medium I will divide rapidly, maintain a cell concentration between 0.4 – 1 ×106 per mL. Once a well gets too crowded, transfer half of it into a new well. In a 6-well plate, cells will come together in the center of the well and form a dense cluster of cells visible to the naked eye. Transfer half of the cells to a new well if a well contains more than 1.5 ×106 cells, or if the patch reaches a diameter of 1 cm. If a well contains less than 2 mL after such a transfer, bring up to 2 mL by adding fresh medium.
CRITICAL STEP: It is likely that some cell debris and red blood cells will be found in the culture during the first days, this should disappear slowly over time. Debris and red blood cells can be removed by slow centrifugation (200 g for 7 min at room temperature). CRITICAL STEP: If other cells are abundantly present in the culture, such as adherent fibroblasts or macrophages, transfer all suspension cells to a new well.
-
34
During the first week, give some fresh Complete Medium I (between 25–50% of total volume) to the culture every other day. From week 2 to 4, add fresh Complete Medium I to the wells twice a week.
-
35
Keep cells in 6-well plates at least until they are 4 weeks of age.
-
36
In the 5th week, gradually switch to Complete Medium II by adding/changing medium twice a week (you should add medium if the cell population grows and is transferred to a new well; or medium can be changed [by centrifuging at 200 g for 7 min at room temperature and removing supernatant] if the cell population number is stable and no transfers are made to new wells).
-
37
Add cytokines, using fresh medium, at least once a week, at 50 ng/mL for human IL-6, and 3% supernatant of Chinese hamster ovary transfectants secreting mouse SCF. CRITICAL STEP: Around this time, some cell death can be expected: slow centrifugation (200 g for 7 min at room temperature) can be performed to prevent the accumulation of dead cells in the culture by removing the supernatant containing dead cells.
TROUBLESHOOTING.
CRITICAL STEP: Although some cell death is expected between weeks 5 to 8, cell numbers will greatly increase in this time interval (about 2.5x cell divisions per month). Between week 8 and 12, cell numbers will become stable, mainly due to slower division of the cells (about 1.5x cell divisions per month).
-
38
Confirm functional and phenotypical maturity of cultured human mast cells5, as follows:
Check that mast cells express FcεRIα and c-Kit (assessed by FACS, a mature population of human mast cells should contain at least 90% FcεRIα and c-Kit positive cells); Check that mast cells respond to IgE crosslinking (we usually sensitize the mast cells with 2 μg/mL human IgE overnight and then stimulate with 2 μg/mL anti-IgE for 1 h), or other mast cell stimuli (degranulation measured by % release of granule-associated mediators [e.g., β-hexosaminidase] and by cytokine production upon mast cell activation); Importantly, confirm that the cells express detectable levels of the protein/receptor of interest for subsequent functional genomics transfection studies, as culturing cells for weeks might potentially induce variations in the expression levels of a given receptor/protein; Assess the native promotor strength of the gene of interest. Note that certain targeted functional genomics tools (shRNA, CRISPR-Cas9 editing) use the native promotor of the knocked out/down gene. Weaker promotors may result in lower expression of GFP; Consider adding any additional validation methods and controls that may be significant to your particular study setup.
Modification of gene expression – Timing 1 h hands on, 48 h incubation
-
39
Modify expression of gene of interest using option A for CRISPR-Cas9-mediated genome editing, or option B for shRNA-mediated gene knockdown (in most settings, CRISPR-Cas9-mediated genome editing will be preferred because it is generally more precise in targeting and more versatile in its applications than shRNA-mediated gene knockdown):
A. CRISPR-Cas9 Mediated Genome Editing
▲CRITICAL Depending on the experiment size and research objective, consider making a large plasmid preparation of gRNA vectors and donor DNA (a CRISPR KO KIT from Origine provides enough plasmid and donor DNA to perform approximately 6 transfections; if the investigator wishes to perform more transfections, they should do a MIDI Prep to generate more plasmids and donor DNA).
-
I.
Define how many donors and conditions (gRNA1, gRNA2 and scramble control are provided with the kit.) should be included in the transfection and functional assays. 1×106 mast cells are needed per condition.
▲CRITICAL STEP, CRISPR-Cas9 gene editing kits often come with two gRNA vectors, we recommend running the transfection with at least two different gRNAs targeting the same gene as well as one other control (either blank or scrambled RNA). Furthermore, we recommend using primary human mast cells derived from at least two different donors for the transfection studies.
-
II.
One day before transfection, count mast cells and check viability (should be above 95%).
-
iii.
After a slow spin (200 g for 7 min at room temperature) remove 50% of old medium and reconstitute at 0.5×106 mast cells per mL with 50% fresh complete medium II.
-
iv.
In a 6-well plate, plate 2 mL (1×106 cells) per condition, clearly mark intended treatment per well and put at 37 oC overnight.
-
v.
On the day of transfection, per condition, dilute 2 μg (40 μL of 50 ng/uL stock solution) of one of the gRNA vectors (or control) in 250 uL of Iscove’s modified Dulbeccos medium with GlutaMAX-I (Thermo Fisher Scientific), vortex gently. Then add 2 μg (2 μL of 1 μg/μL stock solution) of the donor DNA into the same 250 μL of Iscove’s modified Dulbeccos medium. Vortex gently.
-
vi.
Repeat for each condition before moving on to the next step.
-
vii.
Add 12 μL of Turbofectin 8.0 to the diluted DNA from the previous Step and pipet gently to mix completely.
? TROUBLESHOOTING.
-
viii.
Incubate the mixture for 15 min at room temperature.
-
ix.
Add the mixture drop-wise to the appropriate cell containing wells plated in Step 39Aiv (no need to change the media). Gently rock the plate back-and-forth and side-to-side to distribute the mixture evenly. CRITICAL STEP: Note that directly after transfection, the mast cells can take on strange elongated shapes. Cell morphology is known to change in response to transfection-induced stress. This effect is transient and apparently not harmful to the mast cells).
-
x.
Incubate transfected mast cells in a 5% CO2 incubator for at least 48 h.
B. shRNA Mediated Gene Knockdown
-
I.
Repeat Steps 39Ai-iv
-
II.
On the day of transfection, per condition: dilute 1.2 μg of gene-specific shRNA plasmid or negative control shRNA plasmid into 59.6 μl aliquots of Iscove’s modified Dulbeccos medium with GlutaMAX-I (Thermo Fisher Scientific), vortex gently. Prepare separate mixtures for each replicate of mast cells to be transfected with the same plasmid.
-
iii.
Repeat for each condition before moving on to the next step.
-
iv.
For each well, add 9.0 μl Attractene Transfection Reagent into 60 μl donor DNA containing Iscove’s modified Dulbecco’s medium from Step 39BII of this paragraph (7.5 μl Attractene Transfection Reagent per μg of plasmid). Mix gently.
-
v.
Repeat Steps 39Aviii-x.
-
40
In the days after transfection, check for single cell GFP expression by measuring MFI using the imaging configuration as detailed in “BOX 2” or following your own preferential settings. Do this on a daily basis to identify the peak of expression (in our culture condition: 48 h after transfection)
? TROUBLESHOOTING.
Preparing Nunc Lab-Tek 8-well Chambered Coverglass - Timing: 10 min hands on, 30 min incubation
▲CRITICAL In determining how many wells are to be used per run, we recommend filling not more than 4 wells per experiment. Keeping the mast cells for too long in a well together with poly-D-Lysine may result in overall decreased mast cell activation. In addition, most confocal microscopes are not able to take numerous high-quality pictures per minute. We therefore recommend marking multiple positions (>4) in fewer wells, followed by a multi-position time-lapse for 30 min to gain the most out of the limited number of transfected mast cells available. Each position should contain at least 2 GFP-positive cells and 2 GFP-negative cells and each experiment should contain at least 25–30 single GFP-positive and 25–30 single GFP-negative mast cells.
-
41
Prepare a 100x dilution from the poly-D-Lysine stock (500 μL/mL), 20 uL stock in 2000 uL water is enough to coat four Nunc Lab-Tek wells. Wash wells twice with Tyrode’s buffer (400 uL). ?TROUBLESHOOTING.
-
42
Coat wells with 400 uL diluted Poly-D-Lysine.
-
43
Immediately transfer the Nunc Lab-Tek coverglass to a 37 °C incubator for 2 min.
-
44
Wash wells twice with water (400 uL) and once with Tyrode’s buffer (400 uL).
-
45
Gently resuspend transfected mast cells from Step 39 and transfer 100 μL (5 ×104) of them per well and add 300 μL Tyrode’s buffer pre-warmed at 37 °C.
-
46
Repeat Step 45 until all samples with different conditions are in the Nunc Lab-Tek coverglass.
-
47
Note down clearly the contents of each well.
▲CRITICAL STEP The Nunc Lab-Tek coverglass is too small to write on, note down clearly, in a lab journal, the contents of each well relative to the small overhang on the left side of the cover glass.
-
48
Incubate for 20 min in a 37 °C incubator.
-
49
Turn on the confocal imaging system and load the imaging configuration as detailed in “BOX 2” or following your own preferential settings.
-
50
Transfer the Nunc Lab-Tek Coverglass to the stage of the confocal microscope pre-warmed for 30 min at 37 °C and incubate for another 10 min.
High-resolution confocal microscopy imaging - Timing: 20 min hands on, 30 min incubation
-
51
In the imaging software, click “Show Mark and Find Panel”.
-
52
Use the 488 nm laser to detect GFP-positive mast cells and mark their positions.
CRITICAL STEP: It is recommended to mark positions that include both GFP-positive and GFP-negative mast cells, which can act as an intra-experiment positive control.
CRITICAL STEP: Although you can mark as many positions as you want, keep in mind the numbers of images per marking.
-
53
A. Once all positions are marked, add 0.4 μL Av.SRho (final concentration: 5 μg/ml) per well followed by the stimuli of your choice (we usually stimulate mast cells (which were IgE-sensitized overnight with 2 μg/mL IgE) with 2 μg/mL rabbit anti-human IgE, 5 μg/mL codeine or 10 μM Substance P).
B. Alternatively, validation of protein expression can be addressed in this step using appropriate antibodies. To assess FcεRI alpha chain expression, we added, after activating the mast cells using the rabbit anti-human IgE crosslinking described in Step 53. A, 2 ug/ml Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 647.
-
54
A. Start a multi-position time-lapse recording on the marked positions for 30 min and readjust the focus plane during the imaging process, if your microscope is not equipped with a module of auto-adjustment control of focus.
B. Alternatively, make before-and-after images of marked positions instead of a multi-position time-lapse. Such images can be obtained by taking images of marked positions after adding Av.SRho to the wells, but before stimulation, and again 30 minutes after stimulation.
CRITICAL STEP: Degranulation should be visible in GFP-negative or GFP–positive mast cells (depending on the chosen stimuli) within 15 min upon addition of stimuli.
-
55
End image acquisition and save files. We typically save the acquired images per experiment as .lif (complete experiments) or .lsm in case of individual images, which are accessible using a wide variety of free software.
PAUSE POINT: You can proceed to data analysis at the time of your choosing.
Semi-automated image analysis in Fiji – 2 h
CRITICAL: first time users are recommended to get familiar with Fiji and macros, using the detailed Supplementary Tutorial (Fiji/Macro) and Supplementary Data (Demo Dataset), before proceeding to Step 56.
-
56
Open the original data in Fiji, by dragging the file into the work bar of Fiji.
-
57
View stack with: Hyperstack, keep all other boxes unchecked and click OK.
-
58
Select the images you wish to analyze and click OK.
▲CRITICAL STEP For first time users, we recommend starting with a single image (such as an .lsm file, or a selected image from an .lif file named “Series 1: Image001: 512 × 512; 3 planes (3C)”). After getting acquainted with semi-automated image analysis as described in step 59A-C, one can move on to analysis of multi-position time-lapses described in step 59D.
▲CRITICAL STEP Although our provided macros make it easy to set correct colors, enhance contrast, measure fluorescence intensity, and export ready-to-use figures and data-sets, some fine-tuning of the settings may be required depending on your individual equipment setup and configuration. In the TROUBLESHOOTING section we provide some tips for optimizing the provided macros to your individual equipment setup and configuration.
-
59
With Fiji running, go to “Plugins” in the taskbar, then to “New”, then select “Macro”. A new window will open, here you can load/edit the macros detailed below. Load one of the four macro options (A-D) below.
To assign channel colors and enhance the contrast of a single image.
To assign channel colors, enhance the contrast of a single image and export images as .JPEG (individually and combined) and .tif.
To calculate and export MFI per cell, to compare GFP vs. Av.SRho (without generating images) per single image.
To calculate and export MFI per cell and compare GFP vs. Av.SRho in a time-lapse measurement.
CRITICAL: You can save (edited) macros by selecting “File” in the taskbar and select “Save As”, and create a name for the macro ending with “.ijm”.
- Assigning colors and enhancing the contrast of a single image:
- Stack.setChannel(1);
- run(“Cyan”);
- run(“Enhance Contrast”, “saturated=0.35”);
- Stack.setChannel(2);
- run(“Grays”);
- Stack.setChannel(3);
- run(“Red”);
- run(“Enhance Contrast”, “saturated=0.35”);
-
Assigning colors, enhance the contrast of a single image and export images as .JPEG.
name=getTitle();
Stack.setChannel(1);
run(“Cyan”);
run(“Enhance Contrast”, “saturated=0.35”);
saveAs(“Jpeg”, “/Users/Username/Desktop/GFP-”+name);
Stack.setChannel(2);
run(“Grays”);
saveAs(“Jpeg”, “/Users/ Username /Desktop/Grey-”+name);
Stack.setChannel(3);
run(“Red”);
run(“Enhance Contrast”, “saturated=0.35”);
saveAs(“Jpeg”, “/Users/ UserName /Desktop/Avidin-”+name);
Stack.setDisplayMode(“composite”);
roiManager(“Show All”);
saveAs(“Jpeg”, “/Users/ UserName /Desktop/Combined-”+name);
saveAs(“Tiff”, “/Users/ UserName /Desktop/Combined”+name);
-
Calculate and export MFI per cell, to compare GFP vs. Av.SRho.
name=getTitle();
run(“Duplicate...”, “title=Mask duplicate channels=2”);
selectWindow(“Mask”);
run(“Find Edges”);
run(“Threshold...”);
setAutoThreshold(“Default dark”);
waitForUser(“set treshhold manually”);
selectWindow(“Mask”);
run(“Analyze Particles...”, “size=300-Infinity pixel show=Masks exclude include”);
run(“Grays”);
run(“Watershed”);
roiManager(“reset”);
run(“Analyze Particles...”, “size=300-Infinity pixel show=Nothing display exclude clear include add”);
selectWindow(name);
Stack.setChannel(1);
run(“Clear Results”);
roiManager(“Measure”);
String.copyResults();
waitForUser(“Export Data Chanel 1”);
Stack.setChannel(3);
run(“Clear Results”);
roiManager(“Measure”);
String.copyResults();
waitForUser(“Export Data Chanel 3”);
//run(“Channels Tool...”);
Stack.setDisplayMode(“composite”);
Stack.setChannel(3);
run(“Red”);
run(“Enhance Contrast”, “saturated=0.35”);
Stack.setChannel(1);
run(“Cyan”);
run(“Enhance Contrast”, “saturated=0.35”);
Stack.setChannel(2);
run(“Grays”);
roiManager(“Show All”);
saveAs(“Tiff”, “/Users/ UserName /Desktop/”+name);
selectWindow(“Mask of Mask”);
close();
selectWindow(“Mask”);
close();
-
Calculate and export MFI per cell and compare GFP vs. Av.SRho in a time-lapse. Select the time-lapse of interest and click OK. Multi-position time-lapses are indicated slightly differently than regular images, e.g. “Series 30: Mark and Find 006/Pos001 S001: 512 × 512; 105 planes (3C x 35T)”.
-
ii.
Play the time-lapse by pressing the play button. Individual channels can be viewed by selecting them.
-
iii.
Load the following macro into Fiji:
name=getTitle();
run(“Duplicate...”, “title=Mask duplicate channels=2”);
selectWindow(“Mask”);
run(“Find Edges”);
run(“Threshold...”);
setAutoThreshold(“Default dark”);
waitForUser(“set treshhold manually”);
run(“Stack Particle Analyzer”)
selectWindow(“Mask”);
run(“Analyze Particles...”, “size=300-Infinity pixel show=Masks exclude include stack”);
run(“Grays”);
run(“Watershed”);
roiManager(“reset”);
run(“Analyze Particles...”, “size=300-Infinity pixel show=Nothing display exclude clear include add stack”);
selectWindow(name);
Stack.setChannel(1);
run(“Clear Results”);
roiManager(“Measure”);
String.copyResults();
waitForUser(“Export Data Chanel 1”);
Stack.setChannel(3);
run(“Clear Results”);
roiManager(“Measure”);
String.copyResults();
waitForUser(“Export Data Chanel 3”);
//run(“Channels Tool...”);
Stack.setDisplayMode(“composite”);
Stack.setChannel(3);
run(“Red”);
run(“Enhance Contrast”, “saturated=0.35”);
Stack.setChannel(1);
run(“Cyan”);
run(“Enhance Contrast”, “saturated=0.35”);
Stack.setChannel(2);
run(“Grays”);
roiManager(“Show All”);
saveAs(“Tiff”, “/Users/Username/Desktop/”+name);
selectWindow(“Mask of Mask”);
close();
selectWindow(“Mask”);
close();
-
ii.
-
60
With the macro loaded, select the image on which you want to execute the macro (otherwise the macro will be executed on the most forward image). Then select “Run”.
CRITICAL STEP: If you have loaded macro A or B, no further input from the user is needed and you can analyze the images as you like. If macro C or D was loaded, some additional steps are required to obtain the data, as detailed in Steps 61–65.
? TROUBLESHOOTING.
-
61
On the screen, you will see different “masks” being applied to the image, followed by two pop-ups “Action Required – set threshold manually” and “Threshold”. Reduce the threshold with the upper bar until small red dots appear in the image (these will not be included in the final data analysis, due to the minimal particle size settings). Click “OK”.
-
62
The next action required is “Export Data Chanel 1” – which contains the mean GFP MFI values per cell. Open Excel and paste the data from the clipboard to the spreadsheet, and click “OK”.
? TROUBLESHOOTING.
-
63
A new action required states “Export Data Chanel 3” – which contains the mean Av.SRho MFI values per cell. In Excel, paste the data from the clipboard to the spreadsheet, directly next to the GFP data and click “OK”.
-
64
Finally, a .tif image is created (and saved to the Desktop) which visually correlates the cell numbers in the spreadsheet to cells in the image. In the spreadsheet, above the MFI data, note down the analyzed image information (e.g. image identification, experiment name and information, date, donor number, etc.) and save the data as an excel file.
-
65
Repeat Steps 60 – 64 until all desired images have been analyzed.
PAUSE POINT: You can proceed to statistical analysis at the time of your choosing.
-
66
Load the data into a statistics software of your choosing for further analysis and statistics.
Timing
Steps 1–38, Cell Culture: Depends on specific protocol (10–12 weeks for the provided protocol)
Steps 39–40, Modification of gene expression: 1 h hands on, 48 h incubation
Steps 41–50, Preparation of Nunc Lab-Tek Coverglass: 10 min hands on, 20 min incubation
Steps 49, Setting up of Confocal Microscope: 10 min hands on, 10 m incubation
Steps 51–55, Imaging: 20 min hands on, 30 min imaging time-lapse per run
Steps 56–66, Image analysis and initial data visualization: 2 h
Troubleshooting
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 10 | Insufficient PBMC layer separation and/or too many red blood cells | Buffy coat is not diluted enough with PBS | Dilute future samples 1:3 or 1:4 with PBS |
| 26 | Working with StemCell EasySep Magnet is preferred, rather than LS columns. | StemCell EasySep Magnet is already available in-house | Both systems are tested and work well with the subsequently described techniques. However, we have experienced a higher and purer yield using LS columns from Milteny Biotec. |
| 37 | Cells form big clumps of living cells | Overactive progenitor cells | There is nothing to prevent this problem (it seems to be a donor-specific rather than a protocol-specific problem) nor is there a way to treat it. Donor cells displaying such large clumps should be discarded, as they will not grow into mature mast cells. |
| 39, 40 | Low GFP expression | Mast cells are old | Check GFP expression every day. After 5 days there should be GFP expression in older mast cells as well. |
| GFP expression is driven under a weak endogenous promotor | Assess native promotor strength of the gene of interest, consider ordering new donor DNA with a strong promotor driving GFP expression. | ||
| 39B | Low transfection efficiency after using less/more vector DNA | Turbofectin/DNA ratio needs to be adjusted | Keep Turbofectin and DNA a 3:1 ratio. |
| Low transfection efficiency | Too little DNA added | Increase the amount of DNA in the transfection. We do not recommend using another transfection reagent, as we have found that other reagents do not work as well with primary human mast cells. | |
| Equipment setup - Confocal imaging system, 40, 53 | Low signal strength | Laser power differs per optical configuration and loses power throughout time | Increase the laser power by 5%. Your core facility should check the net power of each laser from time to time. Ask them which settings to use to achieve 20 μW for the 488 laser and 36 μW for the 594 laser. |
| 41 | Diminished mast cell activation using self-made Tyrode’s buffer | pH is not correctly adjusted for the Tyrode’s buffer | Check pH often or buy the buffer as a solution; the latter is a cheap and safe solution to prevent the problem. |
| 60 | The supplied Macros (Supplementary Tutorial) do not call individual mast cells correctly | Particle size parameters need to be adjusted | Depending on the magnification of the image, adjust particle size parameters. We recommend a particle size of 300 for images shot at 20x, and a particle size of 100 for images shot at 10x. |
| Threshold is too high | Reduce threshold with every image until you see small dots appearing around the mast cells (these will not be calculated due to the particle size settings). | ||
| 62 | Information in the results box is incomplete | Measurements settings are not set correctly | Right click the Results box in Fiji and select “Set Measurements”. In case of time-lapse analysis, select “Stack Position”. |
Anticipated results
Outlined in the protocol described above, our approach is comprised of three major technical components: human mast cell culture, functional genomics, and single cell high-resolution confocal microscopy. By implementing the procedures described in our protocol, one can readily employ the approach of shRNA- or CRISPR-Cas9-mediated modulation of gene expression to functionally knockdown/knockout gene expression of regulators of human mast cell degranulation and assess visually the individual transfected cells for changes in degranulation response. We anticipate that the data that can be acquired in other laboratories that use the three components of our protocol to identify human mast cell degranulation regulators will be highly similar to those we present in this report.
In our studies designed to validate the approach outlined in our protocol, we knocked out the FcεRI alpha chain or MRGPRX2 receptors in primary human mast cells using CRISPR-Cas9 gene editing (Fig. 4a, b) and stimulated these transfected cell populations with anti-IgE or codeine (a known activator of human mast cells via MRGPRX2 receptors, and an inducer of pseudo-allergic drug reactions48), respectively. In both studies, mast cell degranulation response was observed in GFP-negative mast cells but not in GFP-positive mast cells (Fig. 4a, b). Note that although we have used a controlled substance, i.e., codeine, in our study, alternative compounds also can be used.
By quantitative measurements (the raw MFI data derived from “Semi-automated image analysis in Fiji“), the activation of mast cell degranulation by receptor stimuli was negatively correlated with GFP expression (e.g., Fig. 4c–f), but statistical significance should be calculated instead using the combined MFI values of multiple images. In our image analysis studies, we labelled those mast cells as “GPF-positive” if their GFP expression levels were twice as high as basal MFI levels (Fig. 5a). Further quantitative and statistical analysis confirmed a reduced degranulation profile detected in GFP-positive cells, compared to GFP-negative cells, in response to the cognate receptor-mediated stimuli (Fig. 5b). Absence of the FcεRI-alpha chain expression in GFP-positive cells was shown by staining an goat anti-rabbit IgG antibody (Alexa Fluor 647) (Fig. 5c) Moreover, mast cell degranulation induced by stimuli other than those associated with the deleted receptors, e.g., Substance P, was observed in both GFP-negative and GFP-positive mast cells (Fig. 5d, e). This responsiveness of GFP-positive mast cells to non-IgE-mediated stimuli, in this case, Substance P (Fig. 5d, e), constituted a control for the GFP-positive mast cells’ viability and functional responsiveness. Similarly, GFP-positive mast cells with reduced MRGPRX2 expression were still viable and fully responsive to IgE-crosslinking (Supplemental Figure 3 of ref. 5).
Data presented are pooled: (a) from studies using two different gRNAs for the same targeted gene per donor, (b) from studies using two different donors, or (c) from high-resolution confocal microscopy imaging studies performed on 3 different days. Also, some of the figures shown (e.g., Fig. 4 and Fig. 5c and d), depict representative images from such experiments.
To validate the functional roles of proteins in specifically regulating human mast cell degranulation, our protocol offers easy-to-use human mast cell progenitor isolation and culturing procedures, providing 20–30 ×106 primary human mast cells per donor after 10–12 weeks of culture (Fig. 3). By estimation, 20–30 transfections can be performed per donor, allowing the imaging of 200–300 individual wells/condition using high-resolution confocal microscopy. We anticipate that there will be differences in total cell numbers from different donors, and further studies may reveal variations in the phenotype and function of mast cells derived from different donors.
Taken together, our protocol details a functional genomics approach coupled to high-resolution confocal microscopy that can provide a methodological platform for rapidly identifying regulators of human mast cell degranulation. Given the relatively straightforward character of the procedures, including the choice between RNA interference and CRISPR-Cas9 genome editing for perturbation of gene expression, our protocol can also be applied in other experimental settings as well, such as image-based profiling of single-cell phenotypes using arrayed guide RNA libraries for the elucidation of regulatory mechanisms mediating degranulation in human mast cells.
Supplementary Material
Supplementary Tutorial - Fiji/Macro | Tutorial for first time users to gain familiarity with Fiji and its macros in semi-automated image analysis. The tutorial outlines (i) system requirements, (ii) the installation procedure, and (iii) a step by step demo to get familiar with Fiji, the provided macros and initial data visualization. Tips and tricks on “How to run the software on your data” are provided and can be kept on hand when proceeding to Step 56.
Supplementary Data – Demo Dataset | An exemplary dataset displaying an image taken 30 min after stimulation with anti-IgE in a heterogeneous CRISPR-Cas9 mediated FcεRI-alpha chain-knockout mast cell population. This demo data set is to be used in combination with the “Supplementary Tutorial - Fiji/Macro”.
Acknowledgements
We thank C. Liu for technical support and A. van Stigt for helpful discussions regarding MRGPRX2 expression and gene knockout in human mast cell models. This research was supported by NIH grants U19AI104209, R01 AR067145 and R01 AI32494 (to S.J.G.) and the Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2016 #749629), the European Research Council (ERC-2018-STG #802041) and the INSERM ATIP-Avenir program (to N. G.) and the Lung Foundation Netherlands 4.1.18.226 (to R.W.H). J.F. is supported by a Fulbright Fellowship (financed by the Netherland-America Foundation). R.S. is supported by an NWO Veni Fellowship (grant no. 91617114) and an Erasmus MC Fellowship.
Footnotes
Competing interests
The authors declare no competing interests.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author(s) on reasonable request, and an example dataset is provided as Supplementary Data.
Code Availability Statement
The custom Fiji macros described in the main text of this study can be accessed and used by readers without restrictions.
TWEET Rapid identification of human mast cell degranulation regulators using shRNA knockdown or CRISPR-Cas 9 gene editing coupled to high-resolution confocal microscopy
Related links
Key reference using this protocol
Gaudenzio, N. et al. J. Clin. Invest. 126, 3981–3998 (2016) [DOI: 10.1172/JCI85538]
References
- 1.Galli SJ & Tsai M IgE and mast cells in allergic disease. Nat. Med 18, 693–704 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galli SJ et al. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv. Immunol 126, 45–127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnzon C-F, Rönnberg E & Pejler G The Role of Mast Cells in Bacterial Infection. Am. J. Pathol 186, 4–14 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Reber LL et al. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight 2, 6930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudenzio N et al. Different activation signals induce distinct mast cell degranulation strategies. J. Clin. Invest 126, 3981–3998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tharp MD, Seelig LL, Tigelaar RE & Bergstresser PR Conjugated avidin binds to mast cell granules. J. Histochem. Cytochem 33, 27–32 (1985). [DOI] [PubMed] [Google Scholar]
- 7.Gaudenzio N, Laurent C, Valitutti S & Espinosa E Human mast cells drive memory CD4+ T cells toward an inflammatory IL-22+ phenotype. J Allergy Clin Immunol 131, 1400–7.e11 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Joulia R et al. Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence. Nat Commun 6, 6174 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Kim D, Cho SW, Kim J & Kim J-S Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doench JG et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol 34, 184–191 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinstiver BP et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529, 490–495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slaymaker IM et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu BXH, Hansen LL, Artiles KL, Nonet ML & Fire AZ Landscape of target: Guide homology effects on Cas9-mediated cleavage. Nucleic Acids Research 42, 13778–13787 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu PD et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31, 827–832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattanayak V et al. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31, 839–843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evers B et al. CRISPR knockout screening outperforms shRNA and CRISPRi in identifying essential genes. Nat Biotechnol 34, 631–633 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Zsebo KM et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63, 213–224 (1990). [DOI] [PubMed] [Google Scholar]
- 18.Valent P et al. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood 80, 2237–2245 (1992). [PubMed] [Google Scholar]
- 19.Costa JJ et al. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med 183, 2681–2686 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlin JS et al. KIT signaling is dispensable for human mast cell progenitor development. Blood 130, 1785–1794 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu Y et al. Characterization of ‘adult-type’ mast cells derived from human bone marrow CD34(+) cells cultured in the presence of stem cell factor and interleukin-6. Interleukin-4 is not required for constitutive expression of CD54, Fc epsilon RI alpha and chymase, and CD13 expression is reduced during differentiation. Clin. Exp. Allergy 32, 872–880 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Shimizu Y, Irani AM, Brown EJ, Ashman LK & Schwartz LB Human mast cells derived from fetal liver cells cultured with stem cell factor express a functional CD51/CD61 (alpha v beta 3) integrin. Blood 86, 930–939 (1995). [PubMed] [Google Scholar]
- 23.Andersen HB et al. Comparison of short term in vitro cultured human mast cells from different progenitors - Peripheral blood-derived progenitors generate highly mature and functional mast cells. J. Immunol. Methods 336, 166–174 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Mitsui H et al. Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-kit ligand. Proceedings of the National Academy of Sciences 90, 735–739 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito H, Kato A, Matsumoto K & Okayama Y Culture of human mast cells from peripheral blood progenitors. Nat Protoc 1, 2178–2183 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Schmetzer O et al. A novel method to generate and culture human mast cells: Peripheral CD34+ stem cell-derived mast cells (PSCMCs). J. Immunol. Methods 413, 62–68 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Tam IYS, Ng CW, Tam S-Y & Lau HYA Novel six-week protocol for generating functional human connective tissue-type (MCTC) mast cells from buffy coats. Inflammation Research 1–13 (2016). doi: 10.1007/s00011-016-0989-z [DOI] [PubMed] [Google Scholar]
- 28.Yin Y, Bai Y, Olivera A, Desai A & Metcalfe DD An optimized protocol for the generation and functional analysis of human mast cells from CD34+ enriched cell populations. J. Immunol. Methods 1–23 (2017). doi: 10.1016/j.jim.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navinés-Ferrer A et al. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci. Rep 8, 11628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeil BD et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamm A, Krott N, Breibach I, Blindt R & Bosserhoff AK Efficient transfection method for primary cells. Tissue Eng. 8, 235–245 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Joung J et al. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc 12, 828–863 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert LA et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konermann S et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrotis A & Ketteler R A new age in functional genomics using CRISPR/Cas9 in arrayed library screening. Front Genet 6, 300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Groot R, Lüthi J, Lindsay H, Holtackers R & Pelkmans L Large-scale image-based profiling of single-cell phenotypes in arrayed CRISPR-Cas9 gene perturbation screens. Mol. Syst. Biol 14, e8064 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canver MC et al. Integrated design, execution, and analysis of arrayed and pooled CRISPR genome-editing experiments. Nat Protoc 13, 946–986 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ran FA et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ran FA et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleinstiver BP et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischoff SC Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nature Reviews Immunology 7, 93–104 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Nilsson G et al. Phenotypic characterization of the human mast-cell line HMC-1. Scand. J. Immunol 39, 489–498 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Xia YC et al. Human mast cell line-1 (HMC-1) cells transfected with FcεRIα are sensitive to IgE/antigen-mediated stimulation demonstrating selectivity towards cytokine production. Int. Immunopharmacol 11, 1002–1011 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Rådinger M, Jensen BM, Kuehn HS, Kirshenbaum A & Gilfillan AM Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol Chapter 7, Unit 7.37–7.37.12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guhl S, Babina M, Neou A, Zuberbier T & Artuc M Mast cell lines HMC-1 and LAD2 in comparison with mature human skin mast cells--drastically reduced levels of tryptase and chymase in mast cell lines. Exp. Dermatol 19, 845–847 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Laidlaw TM et al. Characterization of a novel human mast cell line that responds to stem cell factor and expresses functional FcεRI. J Allergy Clin Immunol 127, 815–22.e1–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guschin DY et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol. Biol 649, 247–256 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Sheen CH, Schleimer RP & Kulka M Codeine induces human mast cell chemokine and cytokine production: involvement of G-protein activation. Allergy 62, 532–538 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tutorial - Fiji/Macro | Tutorial for first time users to gain familiarity with Fiji and its macros in semi-automated image analysis. The tutorial outlines (i) system requirements, (ii) the installation procedure, and (iii) a step by step demo to get familiar with Fiji, the provided macros and initial data visualization. Tips and tricks on “How to run the software on your data” are provided and can be kept on hand when proceeding to Step 56.
Supplementary Data – Demo Dataset | An exemplary dataset displaying an image taken 30 min after stimulation with anti-IgE in a heterogeneous CRISPR-Cas9 mediated FcεRI-alpha chain-knockout mast cell population. This demo data set is to be used in combination with the “Supplementary Tutorial - Fiji/Macro”.