Summary
Burn lesions are one of the most devastating forms of trauma. Taking care of this type of lesion generates high costs and may lead to irreversible consequences that limit the daily activities of these patients. This is a two-year descriptive epidemiological study of burned patients treated in a specialized hospital in Mexico City. Data from 38 patients admitted to this hospital were analyzed. Population mean age was 41 years: a higher frequency of men aged 27 to 55 years were affected. The most common mechanism was direct flame, and upper and lower extremities were most frequently affected. Mean affected body surface area was 32.5%. A total of 63% required cutaneous grafting: it was observed that those grafted early (10.5 versus 41.8 days) spent fewer days in the ICU and a lower percentage of them contracted sepsis (62% versus 78%). Patients with Pseudomona aeruginosa had a longer hospital stay and underwent a greater number of surgeries for skin grafting. No difference was observed between graft integration percentages. Early grafting (<7 days) resulted in a shorter stay in the ICU and fewer overall total days in the hospital, as well as a lower rate of sepsis and admission to the ICU. The Pseudomona infection group had a longer hospital stay, greater number of surgical procedures for skin grafting and there was no difference in graft integration percentages.
Keywords: burn injury, hospital stay, skin graft, pseudomona
Abstract
Les brûlures font partie des traumatismes les plus dévastateurs, aux conséquences irréversibles pouvant laisser des handicaps définitifs. Le coût de leur prise en charge est élevé. Nous présentons une étude épidémiologique basée sur 38 patients hospitalisés en 2 ans dans un CTB de Mexico. La population la plus touchée est l’homme jeune (27 à 55 ans, moyenne 41). La brûlure est due à une flamme, touche 32,5% de la SCT, atteint préférentiellement les extrémités supérieure et inférieure. Une greffe est nécessaire dans 63% des cas. Les patients greffés précocement, aux alentours de J10 (J41,8 ailleurs), restent moins longtemps en réanimation comme dans le CTB et ont moins d’accidents septiques (62% VS 78%). La survenue d’une infection à Pseudomonas æruginosa entraîne un allongement de la durée de séjour et la nécessité de plus de séances de greffes, sans différence quant à leur intégration.
Introduction
Burn injuries have affected humanity since ancient times and represent one of the most devastating forms of trauma. Records and data about burns and their treatment go back 3500 years. However, the advances that revolutionized their treatment developed in the United States in the 18th century, when Allen et al. observed an infection incidence decrease in burned patients when applying silver sulfadiazine cream.1 These injuries are relatively frequent and have a higher incidence in third world and developing countries. In the United States 500,000 burn patients are treated each year: of these, 46% are caused by direct fire, and they represent about 3,500 deaths per year.2 In Mexico in 2011, burn lesions occupied seventeenth place in the injury incidence table, affecting 129,779 patients, predominantly males, and most frequently in the 24 to 40 years age group. The real impact lies in the complex response chain they trigger and the costs generated by their attention and care. In our country it is estimated that moderate burns generate an expense of between 30,000 and 500,000 pesos, and serious burns can reach costs of up to 40 million pesos.3 Burn-induced alterations derive mostly from the lack of skin cover. These appear when the affected body surface is greater than 10%, and they seriously alter homeostasis due to the release of insulin-regulating hormones that induce hypercatabolic states, humoral and cellular immunodeficiency, as well as water balance, temperature, hemodynamic and nutrient absorption disorders.4
Burned patient care is divided into two phases. The primary phase focuses on airway protection and establishing adequate water resuscitation. Secondary care treats the burn sequelae and provides definitive treatment through skin grafting.5
Nowadays special attention is given to post-burn recovery, since severe burn consequences or burns affecting special areas can limit work and alter interpersonal and self-care activities.
Materials and methods
This is a transversal descriptive retrospective study that included patients who met the burn reference criteria for admission to a specialized hospital over a 2-year period from January 1st, 2017 to January 1st, 2019. The following variables were measured in the general population and also by groups, considering: age, sex, body surface burned with the Wallace rule of nines, affected body segment, type of burn, trauma or associated airway injury, burn depth, germ reported in cultures, days of hospital stay, antibiotics, type of graft placed, time from burn to graft, mortality rate and graft integration percentage. We excluded burned patients with less than 10% total body surface area affected, chemical burns and those who did not have all the study variables in their electronic clinical record.
Results
The study included a total of 38 patients admitted to this hospital Burn Unit. Regarding gender, 80% were men and 20% women. The population mean age was 41 years (SD 22), the most frequent age range was 27 to 55 years, 43% of the patients presented some type of associated trauma and 40% presented airway injury, 80% of the patients had burns greater than 30%, the average burned body surface was 32.5% and the interquartile range was 20 to 50% (Figs. 1 and 2). 80% of the patients suffered burns in their workplace and the most frequent mechanism of injury was flame in 60%, followed by scalding in 30% and electrical burns in 10%.
Fig. 1. Body surface affected.
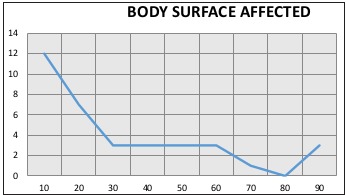
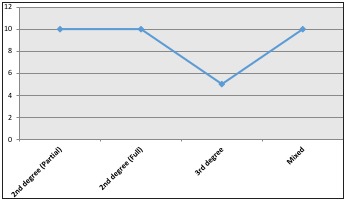
Fig. 2. Burn injury depth.
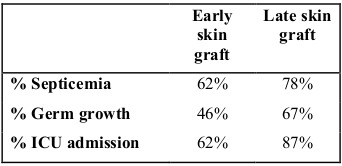
A total of 63% of the patients required tangential excision plus skin graft placement. The mean number of days from hospital admission to skin graft placement was 8.6 days, median 4.5 and interquartile range from 2 to 15 days. The total number of grafted patients were divided into two groups, an early graft group (<7 days) and a late graft group (>7 days). Days of hospital stay, sepsis development percentage, pathogen development in cultures and percentage of admission to the ICU were compared between the two groups. We observed that the early grafted group had a lower mean total hospital stay and lower stay in the ICU compared to the late grafted group, as well as a lower rate of sepsis and admission to the ICU, both statistically significant (Tables I and II).
Table I. Comparison of hospital stay according to graft group.
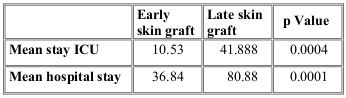
Table II. Comparison of skin graft groups according to sepsis, germs, cultures and ICU admission.
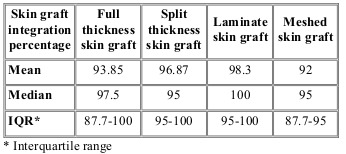
We also compared different grafting techniques and their integration percentage. We observed a similar integration percentage in both groups, without statistically significant differences between the two techniques (Table III).
Table III. Graft integration percentage according to skin harvest technique.
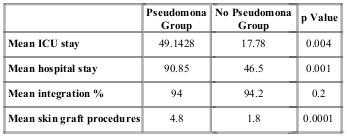
Culture was taken from all patients on admission to the unit. It was observed that 30% showed no development of pathogens and that the most frequent microorganisms were Enterobacteria, followed by the Candida family and finally Pseudomonas species (Fig. 3). Ninety-six percent of the patients received empirical antibiotic therapy and, of these, 64% received correction of the antibiotic after culture results and antibiogram matching. Third generation cephalosporins were the most frequently used antibiotics, followed by carbapenems and finally combinations of these drugs. The most frequently used combination was broad-spectrum penicillins plus an anaerobicide. Based on this, the study population was divided into two groups: patients whose culture showed development of a subtype of the pseudomonas family, and those whose culture showed other germs.We compared the two groups for mean total hospital stay and days in the ICU, graft integration percentage, and mean number of procedures for graft placement. What we observed was that the group that reported Pseudomona had longer stays in hospital and in the ICU compared to the other germ group. It was also observed that the number of procedures for skin graft placement was significantly higher in the Pseudomona group. Both comparisons showed statistical significance. Comparing the mean integration percentage of skin grafts in both groups, a discrete difference was observed in favor of the non-Pseudomona group; however, this was not statistically significant (Table IV).
Fig. 3. Incidence according to germs reported in cultures at admission.
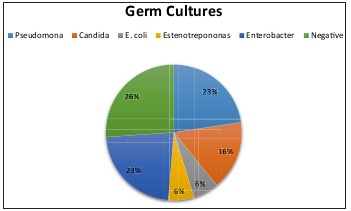
Table IV. Comparison of germ cultures.
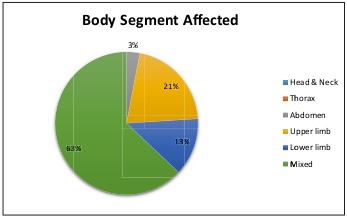
When analyzing the body segment affected, it was observed that 63% of the patients in this series showed multiple body segment involvement, while 21% had burns on the upper extremities only, followed by 13% on the lower extremities (Fig. 4). Of the burns that affected multiple body segments, 27% corresponded to the upper extremities followed by thorax injuries in 19% and lower extremities in 18% (Fig. 5). Finally, mortality rate over these 2 years of study was evaluated, yielding a total mortality rate of 11%, with the predominant causes of death being septic shock (patients who grew Pseudomona in cultures) followed by heart failure. The predominant age group was men of productive age.
Fig. 4. Body segment affected.
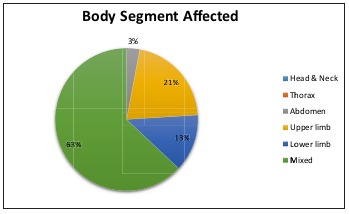
Fig. 5. Mixed burn injuries and their distribution.
Discussion
Our Hospital’s experience showed that the majority of affected patients were men of productive age, similar to that reported by Dongo et al., who reported a series of 72 patients in which the most affected age range was 20 to 29 years old. The difference in our study is that the majority of injuries occurred at home.6 Since our Hospital is a burn reference center, our patients have mostly full-thickness burns, special area burns (genitalia, face and hands) or greater than 10% of body surface burned. Likewise, a significant percentage of patients have had concomitant trauma and airway injury, hence a worse prognosis and greater complexity as regards management. In 2013, Theodorou et al. published their experience with more than 1600 patients treated in a burn unit, observing a total burn percentage of 18 to 23%, a hospital stay in the ICU of 18.8 to 19.1 days and a mortality rate of 15 to 18%.7 This correlates with the results obtained in our study, where the mortality rate was 11%, more than 70% of the patients presented burns greater than 30% total body surface and more than 60% required tangential excision with skin graft, which demonstrates a deep burn condition. Tangential excision and early skin grafting is the treatment of choice in deep burns because it minimizes fluid loss, reduces metabolic demand, protects against infections, and decreases inflammation and multi-organ failure.8 Ong et al. reported that in burned patients treated with skin graft the mortality rate was 27% compared to 36% in non-grafted patients. This difference was obvious in patients who presented airway injury, where mortality was 22% among grafted patients and 62% among non-grafted.9
Although several factors are involved in graft integration, such as nutritional status,10 surgical technique and recipient bed characteristics, early excision is essential for management.11 In our series, it was found that early graft excision significantly decreased the days of stay in the ICU and total hospital stay. Another 2005 series reported a body surface area of less than 10% in 70% of the subjects studied. 12 Tibery Queiroz in 2015 reported a mean of 26.6% burned body surface area and the most frequent cause to be flame, most often affecting men between 28 and 52 years old on the upper and lower extremities, which limits and incapacitates patients more severely.13 These results are at variance with our own. Finally, other series published in 201014 and 201515 demonstrate an epidemiology similar to what we reported in our study.
Another valuable fact in this series showed that the patients who developed Pseudomona in cultures presented a longer ICU and total hospital stay, significantly greater than those who developed other germs, and they also required a greater number of surgeries for taking and applying the skin graft. However, the skin graft integration percentage did not show a significant difference between the groups. This correlates with the study published by Dou et al., where a worse clinical course was demonstrated in patients infected with post-pseudomonas.16 Other studies also have shown that Pseudomona infection correlates with greater antibiotic resistance, prolonged hospital stay and a higher probability of death.17,18,19
Overall, there was no significant difference between different grafting techniques, but we observed a higher percentage of sepsis in patients who had a graft applied late: due to the serious condition of each of these patients we always tried as far as possible to apply the skin graft by the seventh day from burn. Also, being a reference unit, the patients treated presented greater severity and a higher number of organic systems were involved. The majority of patients received empirical antibiotics, and more than 50% underwent modifications of this treatment after culture results were obtained. We believe that this maneuver improved the severely affected patients’ prognosis.
Another factor that we believe favored recovery and resulted in a mortality rate similar to first world countries is that during the entire hospital stay there was close 24-hour surveillance by a whole group of plastic surgeons, plastic surgery residents, intensive care doctors, nurses, nutritionists and rehabilitators.
Conclusion
Burned patient care is one of the most complex therapeutic challenges in trauma, since it requires multidisciplinary intervention, high specialty management and it represents a high socio-economic impact. Early skin grafting is the treatment of choice that decreases hospital stay and diminishes infection percentage and admission to the ICU. It is necessary to create prevention programs at home and in the workplace that reduce the incidence and severity, and develop an effective health system that includes reference centers and specialized burn care units. This is essential to increasing survival in patients who have been affected by burns, and offers them a quick recovery so they can return to their daily activities sooner. It is essential to pay closer attention to post-burn consequences, and the sequelae and functional limitations entailed, as few studies focus on this aspect.
References
- 1.Hettiaratchy S, Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328(7453):1427–1429. doi: 10.1136/bmj.328.7453.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peck MD. Epidemiology of burns throughout theWorld. Part II: intentional burns in adults. Burns. 2012;38(5):630–637. doi: 10.1016/j.burns.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Moctezuma-Paz L, Páez-Franco I, Jimenez-González S, Miguel-James KD. Epidemiologia de las quemaduras en México. Rev Esp Méd Quir. 2015;20:78–82. [Google Scholar]
- 4.Evers LH, Bhavsar D, Mailänder P. The biology of burn injury. Exp Dermatol. 2010;19(9):777–783. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 5.Daigeler A, Kapalschinski N, Lehnhardt M. Therapy of burns. Chirurg. 2015;86(4):389–401. doi: 10.1007/s00104-014-2919-3. [DOI] [PubMed] [Google Scholar]
- 6.Dongo AE, Irekpita EE, Oseghale LO, Ogbebor CE. A fiveyear review of burn injuries in Irrua. BMC Health Serv Res. 2007;23(7):171. doi: 10.1186/1472-6963-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theodorou P, Xu W, Weinand C, Perbix W. Incidence and treatment of burns: a twenty-year experience from a single center in Germany. Burns. 2013;39(1):49–54. doi: 10.1016/j.burns.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Janzekovic Z. A new concept in the early excision and immediate grafting of burns. J Trauma. 1970;10(2):1103–1108. [PubMed] [Google Scholar]
- 9.Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360(9):893–901. doi: 10.1056/NEJMct0804451. [DOI] [PubMed] [Google Scholar]
- 10.García-Espinoza JA, Aguilar-Aragón VB, García-Méndez S. Use of the CONUT index as a predictor of integration of cutaneous grafts in burn patients. J Cutan Aesthet Surg. 2017;10(3):172–176. doi: 10.4103/JCAS.JCAS_83_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daigeler A, Kapalschinski N, Lehnhardt M. Therapy of burns. Chirurg. 2015;86(4):389–401. doi: 10.1007/s00104-014-2919-3. [DOI] [PubMed] [Google Scholar]
- 12.Song C, Chua A. Epidemiology of burn injuries in Singapore from 1997 to 2003. Burns. 2005;31 Suppl1:S18–S26. doi: 10.1016/j.burns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Queiroz LF, Anami EH, Zampar EF, Tanita MT. Epidemiology and outcome analysis of burn patients admitted to an Intensive Care Unit in a University Hospital. Burns. 2016;42(3):655–62. doi: 10.1016/j.burns.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Ben DF, Ma B, Chen XL, Zhu SH. Burn injuries caused by ship fire: a 12-year study in Shanghai. Burns. 2010;36(4):576–580. doi: 10.1016/j.burns.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Oh B, Boo S. Burns in South Korea: an analysis of nationwide data from the Health Insurance Review and Assessment Service. Burns. 2016;42(3):675–81. doi: 10.1016/j.burns.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Dou Y, Huan J, Guo F, Zhou Z, Shi Y. Pseudomonas aeruginosa prevalence, antibiotic resistance and antimicrobial use in Chinese burn wards from 2007 to 2014. J Int Med Res. 2017;45(3):1124–1137. doi: 10.1177/0300060517703573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yali G, Jing C, Chunjiang L, Cheng Z. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns. 2014;40(3):402–407. doi: 10.1016/j.burns.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Panghal M, Singh K, Kadyan S, Chaudary U, Yadav JP. The analysis of distribution of multidrug resistant Pseudomonas and Bacillus species from burn patients and burn ward environment. Burns. 2015;41(4):812–819. doi: 10.1016/j.burns.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Issler-Fisher AC, Fakin RM, Fisher OM, McKew G. Microbiological findings in burn patients treated in a general versus a designated intensive care unit: Effect on length of stay. Burns. 2016;42(8):1805–1818. doi: 10.1016/j.burns.2016.06.019. [DOI] [PubMed] [Google Scholar]


