Abstract
Background/Aims
Previous study has shown a positive relationship between the hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and cholangiocarcinoma (CCA); however, their correlation with different anatomical sites of CCA (i.e. ICC and ECC) has not been revealed. This study aims to evaluate the association of HBV or HCV infection with CCA, including the intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC), and to determine the roles of α-1 fetoprotein (AFP), CA19-9, and lymph node involvement in CCA with HBV infection.
Materials and Methods
Relevant studies published between 2004 and 2016 were systematically searched and retrieved from PubMed, SpringerLink, and Science Direct using key terms such as “cholangiocarcinoma”, “bile duct cancer”, “extrahepatic cholangiocarcinoma”, and “intrahepatic cholangiocarcinoma”. The demographic, clinical, and laboratory data were extracted from the included studies, and the meta-analysis was performed using RevMan and STATA 11.0 software.
Results
A total of 13 studies with CCA matched the inclusion criteria in this meta-analysis, including 7,113 CCA patients and 24,763 controls. This meta-analysis showed that the HBV or HCV infections can significantly increase the risk of CCA, including ICC and ECC. In addition, the higher levels of AFP, lower levels of CA19-9, and lymph node involvement were detected in the CCA patients with HBV infection as compared to those without.
Conclusion
The HBV and HCV infections significantly increased the risk of CCA, as well as ICC and ECC. The involvement of AFP, CA19-9, and lymph nodes may play an important role in the diagnosis of CCA.
Keywords: Hepatitis B, hepatitis C, cholangiocarcinoma
INTRODUCTION
The latest report from the World Health Organization (WHO) has revealed that around 325 million people worldwide are living with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. Meanwhile, the HBV and HCV infections are the most common viral infections worldwide (1). The WHO proposed a program to eliminate viral hepatitis in May 2016, which aims to reduce new infections by 90% and mortality by 65% before 2030 (2). Cholangiocarcinoma (CCA) is the second most frequently occurring primitive liver malignancy and is responsible for 7–10% of the malignant hepatic tumors (3). Recent epidemiological studies have shown that the incidence of CCA is increasing worldwide (4). In the United States, the incidence of intrahepatic cholangiocarcinoma (ICC) reported to the Memorial Sloan Kettering Cancer Center increased by 14.2% annually from 1990 to 2006 (5). Between 1990 and 2008, the mortality rate of ICC in Europe has increased approximately 9%, reaching the rates of 1.1 and 0.75 per 100,000 in males and females, respectively (6).
Previous studies have shown a positive relationship between the HBV or HCV infection and CCA; however, the correlation of the HBV or HCV infection with different anatomical sites of CCA (i.e. ICC and ECC) has not been revealed (7–10). So far, no meta-analysis has investigated the prognostic values of α-1 fetoprotein (AFP), CA19-9, and lymph node involvement in CCA patients infected with HBV. Therefore, we conducted this meta-analysis to evaluate the association of HBV or HCV infection with the risks of CCA, including ICC and ECC. Moreover, we determined the contribution of AFP, CA19-9, and lymph node involvement in the development of CCA in patients infected with HBV.
MATERIALS AND METHODS
In this meta-analysis, the literature search, study selection, outcome measurement, data abstraction, and statistical analyses were performed according to the Meta-analysis of the Observational Studies in Epidemiology (MOOSE) recommendations (11).
Data Sources and Searches
Relevant studies published between 2004 and 2016 were systematically searched from the electronic databases, such as PubMed, Springer Link, and Science Direct, with no restrictions on the language or country of publication. After searching the databases, we extended our search to the related articles, as well as to the reference lists of the review articles. We also manually searched the journals, theses, and books available in the library for additional articles that were not found during the previous efforts. For similar studies published more than once, only the studies with the most complete data were included.
In order to provide a more comprehensive view of the specific risk factors of CCA, the combining terms of (((“cholangiocarcinoma”[MeSH]) OR “Cholangiocarcinomas” OR “Cholangiocellular Carcinoma” OR “Carcinoma, Cholangiocellular” OR “Carcinomas, Cholangiocellular” OR “Cholangiocellular Carcinomas” OR “Extrahepatic Cholangiocarcinoma” OR “Cholangiocarcinoma, Extrahepatic” OR “Cholangiocarcinomas, Extrahepatic” OR “Extrahepatic Cholangiocarcinomas” OR “Intrahepatic Cholangiocarcinoma” OR “Cholangiocarcinoma, Intrahepatic” OR “Cholangiocarcinomas, Intrahepatic” OR “Intrahepatic Cholangiocarcinomas”)) OR ((“Klatskin Tumor”[ Mesh]) OR “Tumor, Klatskin” OR “Klatskin’s Tumor” OR “Tumor, Klatskin’s” OR “Hilar Cholangiocarcinoma” OR “Cholangiocarcinoma, Hilar” OR “Cholangiocarcinomas, Hilar” OR “Hilar Cholangiocarcinomas” OR “Perihilar Cholangiocarcinoma” OR “Cholangiocarcinoma, Perihilar” OR “Cholangiocarcinomas, Perihilar” OR “Perihilar Cholangiocarcinomas”)) OR ((“Bile Duct Neoplasms”[Mesh]) OR “Bile Duct Neoplasm” OR “Neoplasm, Bile Duct” OR “Neoplasms, Bile Duct” OR “Bile Duct Cancer” OR “Bile Duct Cancers” OR “Cancer, Bile Duct” OR “Cancers, Bile Duct” OR “Cancer of the Bile Duct” OR “Cancer of Bile Duct”) were searched in PubMed. Likewise, the combining terms (“hepatitis B” OR “HBV” OR “hepatitis C” OR “HCV” OR “hepatitis B virus” OR “hepatitis C virus”) AND (“bile duct neoplasms” OR “cholangiocarcinoma”) were searched in SCIENCE DIRECT and SPRINGER.
Study Selection
After the initial search, all the articles that have been reported on the association between CCA and HBV or HCV were retrieved. This study was focused on ICC and ECC since the treatment of periampullary carcinoma and tumors of the lower parts of the choledochus are obviously different from that of ICC and ECC. In addition, the HBV infection has been defined as the presence of hepatitis B surface antigen.
The studies were included if they met the following criteria: (i) involved the HBV or HCV infection; (ii) reported the clinical outcomes of CCA, ICC, or ECC; (iii) provided the risk estimates with 95% confidence interval (CI) or the information needed to calculate these; (iv) published in full-text in English language journals; (v) included at least 30 patients. Meanwhile, the exclusion criteria were non-English language articles, non-human studies, meta-analysis, review articles, editorials, case reports, and articles not reporting the primary data of interest.
Outcome Measurement
The primary outcomes assessed were the epidemiological rates of CCA (including both ICC and ECC) in patients with HBV or HCV infection as compared to those without the HBV or HCV infection. The secondary outcomes assessed were the levels of laboratory markers (including AFP and CA-199) and lymph node involvement in the CCA patients with HBV or HCV infection as compared to those without, including ICC and ECC.
Data Extraction and Quality Assessment
Following a careful screening process, two reviewers independently judged the potential eligibility of the articles by screening their titles and abstracts. The discrepancies were resolved through discussion and consensus by a third investigator. To determine the accuracy of the assessment of eligibility, the studies with missing information were evaluated and discussed together. Then, the data were extracted independently by two other reviewers. For the studies included in the meta-analysis, the baseline characteristics and justifications of the inclusion/exclusion criteria were carefully reviewed.
The quality of each included study was assessed using the Newcastle-Ottawa quality assessment tool since this is a representative tool developed for the meta-analysis of the observational studies (12). We also evaluated the methods used to handle the missing outcome data and loss to follow-up by excluding these studies from the sensitivity analysis.
Statistical analysis
The odds ratio (OR) with 95% confidence interval (CI) were used to estimate the association of HBV or HCV infection with the risks of developing CCA, ICC, and ECC. The differences were considered statistically significant when the calculated p was less than 0.05. In the forest plots, OR > 1 was considered a risk factor while OR < 1 was considered a protective factor. To test the statistical heterogeneity, the Chi-Square based Q test and I2 statistic were analyzed using RevMan 5.3 software. When a significant Q-test (p>0.10) and I2 < 50% indicated no heterogeneity across the studies, the fixed-effects model was used to estimate the pooled OR and 95% CI; otherwise the random-effects model was used (13).
The sensitivity analysis was conducted to confirm whether the modification of the inclusion criteria would affect the results of the meta-analysis. The pooled proportion of the vertical transmission of toxoplasmosis with the corresponding 95% CI was calculated by STATA 11.0 software (Stata Corporation, College Station, TX, USA). The publication bias was considered significant if p<0.05, as evaluated by Begg’s funnel plots and Egger’s test.
The hazard ratio (HR) was used as the summary statistics, which allowed to estimate the effects of the interventions on the outcomes of interest over time. An HR of less than 1 represented a survival benefit favoring the simultaneous group and P values less than 0.05 indicated the statistical significance. Medians were converted to means using the technique described. The fixed-effect model was first used to pool the results, which assumed that all the studies shared a common effect size. In this meta-analysis, the studies were weighted by the inverse of the estimated variance of the effect size. The heterogeneity among the studies was considered statistically significant if the Cochrane Q test P value was less than 0.1.
Characteristics of the Included Studies
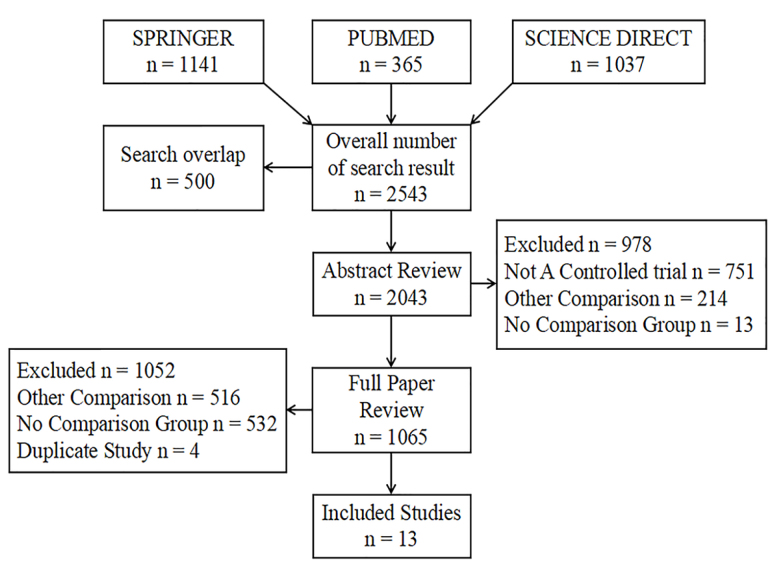
Table 1 shows the number of patients enrolled and the demographic characteristics of the included studies. A flow diagram of the selection of the studies is shown in Figure 1. A total of 13 eligible studies (14–26) were identified in this meta-analysis, including 7,405 CCA patients and 25,263 controls (Table 1). Of which, these studies were investigated on the association of the HBV infection with CCA risk (n=11) or ICC risk (n=6) or ECC risk (n=6), as well as the association of the HCV infection with CCA risk (n=5) or ICC risk (n=4). The number of studies for the association between the HCV infection and ECC risk was less than 3, and therefore, not included in the final meta-analysis model.
Table 1.
Number of patients enrolled in the demographic characteristics of the included studies.
| Authors | Year | Country | Non-CCA Controls (n) | CCA cases (n) | ICC cases (n) | ECC cases (n) | HBV infection cases in CCA patients (n) |
|---|---|---|---|---|---|---|---|
| Chul-Soo Ahn et al. | 2016 | South Korea | NA | 292 | 292 | NA | 37 |
| Lian-Yuan Tao et al. | 2016 | China | 438 | 312 | 312 | NA | 151 |
| Yan-Ming Zhou et al. | 2015 | China | 504 | 126 | NA | NA | 88 |
| Ban Seok Lee et al. | 2015 | South Korea | 552 | 276 | 83 | 193 | 28 |
| Kazuya Matsumoto et al. | 2014 | Japan | 100 | 103 | 50 | 53 | 7 |
| Yu Zhou et al. | 2013 | China | 478 | 222 | NA | 222 | 27 |
| Jeffrey S. Chang et al. | 2013 | China | 20628 | 5157 | 2978 | 2179 | 376 |
| Rui-qing Liu et al. | 2013 | China | NA | NA | NA | NA | 37 |
| Qiao Wu et al. | 2012 | China | 835 | 188 | 102 | 86 | 34 |
| Zhenliang QU et al. | 2012 | China | 317 | 212 | 98 | 212 | 19 |
| Ning-fu Peng et al. | 2011 | China | 196 | 98 | NA | NA | 31 |
| Wen-Ke Cai et al. | 2011 | China | 609 | 313 | NA | NA | 23 |
| Petcharin Srivatanakul et al. | 2010 | China | 606 | 106 | NA | NA | 11 |
| Total | 25263 | 7405 | 3915 | 2945 | 869 |
NA: not available; CCA: cholangiocarcinoma; ICC: intrahepatic cholangiocarcinoma; ECC: extrahepatic cholangiocarcinoma.
Figure 1.
A flow diagram of the selection of the studies.
All the included studies were conducted in Asian countries, including Japan, Korea, and China. Furthermore, we analyzed the influence of different risk factors on the CCA patients with HBV infection.
Quality Assessment of the Included Studies
The overall agreement between the two systematic reviewers was 0.94 for the study selection and 0.89 for the quality assessment. The quality level of the 13 included studies was evaluated using the Newcastle-Ottawa scale (Table 2). The detailed descriptions of the study design were available for most studies; however, majority of the included studies did not adequately describe the methods for handling the missing data. Moreover, the findings might have been influenced by a selection bias with respect to the patient’s distribution, including their age, sex ratio, ethnicity, and the total number between the cases and control group. Furthermore, the publication bias was detected through the visual inspection of the funnel plot, which can be interpreted as asymmetric or symmetric inverted funnel shape.
Table 2.
Evaluation of the quality using Newcastle-Ottawa scale.
| Authors | HBV infection cases(n) | HCV infection cases(n) | CCA cases(n) | ICC cases(n) | ECC cases(n) | Exposure Ascertainment | Comparability of Simultaneous Group and Reference | Possible Factors Assessment in Selection Bias | |
|---|---|---|---|---|---|---|---|---|---|
| Lian-Yuan Tao et al. (2016) | 151 | 9 | 312 | 312 | NA | Hospital records | No restriction or matching | Record linkage | Unmentioned |
| Yan-Ming Zhou et al. (2015) | 88 | 1 | 126 | NA | NA | Hospital tests | 4:1 matched healthy controls for age and sex | Record linkage | Hospital-based study |
| Ban Seok Lee et al. (2015) | 28 | 11 | 276 | 83 | 193 | Hospital tests | 2:1 matched controls for age, sex, and date of diagnosis | Record linkage | Hospital-based study and diagnosis bias |
| Kazuya Matsumoto et al. (2014) | 7 | NA | 103 | 50 | 53 | Hospital tests and reviews | No restriction or matching | Record linkage | Possible bias in allocation to simultaneous group |
| Yu Zhou et al. (2013) | 27 | NA | 222 | NA | 222 | Pathological diagnosis | Controls matched for sex and age | Record linkage | Hospital-based study |
| Jeffrey S. Chang et al. (2013) | 376 | 250 | 5157 | 2978 | 2179 | The datasets of the NHIRD | 4:1 matched cancer-free controls for sex, age (birthday), and the time of case diagnosis (reference date for the controls) | Record linkage | Selection and recall biases |
| Qiao Wu et al. (2012) | 34 | NA | 188 | 102 | 86 | PUMCH Patient Information Database | 3:1 matched controls for gender and age | Record linkage | Differential referral patterns |
| Zhenliang QU et al. (2012) | 19 | 46 | 212 | NA | 212 | Hospital tests | Participants with benign biliary disease with cholelithiasis or acute cholangitis undergoing surgical intervention | Record linkage | Hospital-based study |
| Ning-fu Peng et al. (2011) | 31 | NA | 98 | 98 | NA | Medical records | 3:1 matched cancer-free controls for sex, age (birthday) | Record linkage | Mechanism confusion |
| Wen-Ke Cai et al. (2011) | 23 | NA | 313 | NA | NA | Hospital records | 2:1 matched controls for age and sex | Record linkage | Geographic regions limitation |
| Petcharin Srivatanakul et al. (2010) | 11 | NA | 106 | NA | NA | Hospital records | 1:1 matched controls for sex, age (± years) and by residence | Record linkage | Unmentioned |
| Rui-qing Liu et al. (2013) | 37(total number for study of CA19-9) | NA | NA | NA | NA | Hospital records | Non-HBV group | Record linkage | Small population |
| Chul-Soo Ahn et al. (2016) | 37(total number for study of lymphnode involvement) | NA | 292 | 292 | NA | Hospital records | Non-HBV group | Record linkage | Single-center study and limited sample size |
| Summation | 869 | 317 | 7405 | 3915 | 2945 | ||||
NA: not available; CCA: cholangiocarcinoma; ICC: intrahepatic cholangiocarcinoma; ECC: extrahepatic cholangiocarcinoma
RESULTS
HBV Infection and the Risk of CCA
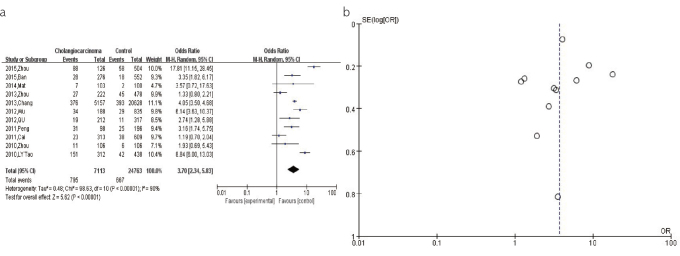
For the pooled analysis of the association between the HBV infection and CCA risk, a total of 11 studies with 24,763 participants were included. Most studies were performed in China (n=8), followed by Korea (n=2) and Japan (n=1). The result from the random-effects model (OR=3.70, 95% CI: 2.34–5.83, p<0.00001) revealed a significant association between the HBV infection and increased risk of CCA (Figure 2a). The shape of the funnel plot was asymmetrical, suggesting the presence of publication bias in this association (Figure 2b).
Figure 2. a, b.
(a). The forest plots depicting the pooled analysis of the association between the HBV infection and CCA risk. (b). The funnel plots showing the evaluation of the publication bias of the association between the HBV infection and CCA risk.
HBV Infection and the Risk of ICC
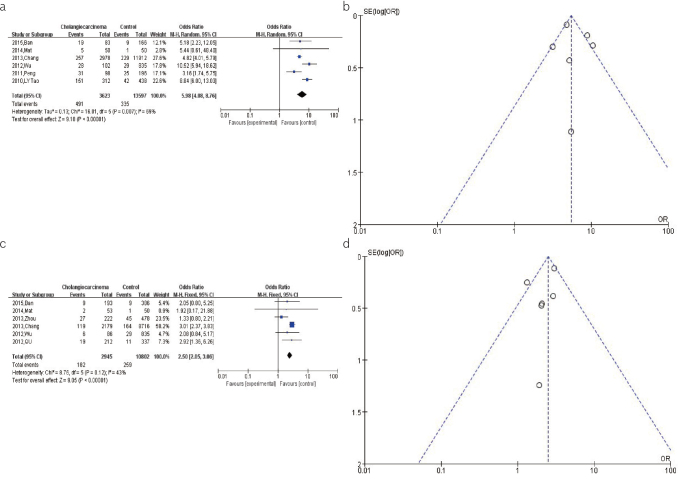
For the pooled analysis of the association between the HBV infection and ICC risk, the results from the random-effects model (OR=5.98, 95% CI: 4.08–8.76, p<0.00001) suggested that the HBV infection is significantly associated with an increased risk of ICC (Figure 3a). The shape of the funnel plot was relatively symmetrical, indicating a modest publication bias for this association (Figure 3b).
Figure 3. a–d.
(a). The forest plots depicting the pooled analysis of the association between the HBV infection and ICC risk. (b). The funnel plots showing the evaluation of the publication bias of the association between the HBV infection and ICC risk. (c). The forest plots depicting the pooled analysis of the association between the HBV infection and ECC risk. (d). The funnel plots showing the evaluation of the publication bias of the association between HBV infection and ECC risk.
HBV Infection and the Risk of ECC
For the pooled analysis of the association between the HBV infection and ECC risk, the result from the fixed-effects model (OR = 2.50, 95% CI: 2.05–3.06, p<0.00001) found a significant association between the HBV infection and increased risk of ECC (Figure 3c). The shape of the funnel plot was relatively symmetrical, indicating a modest publication bias for this association (Figure 3d).
HCV Infection and the Risk of CCA
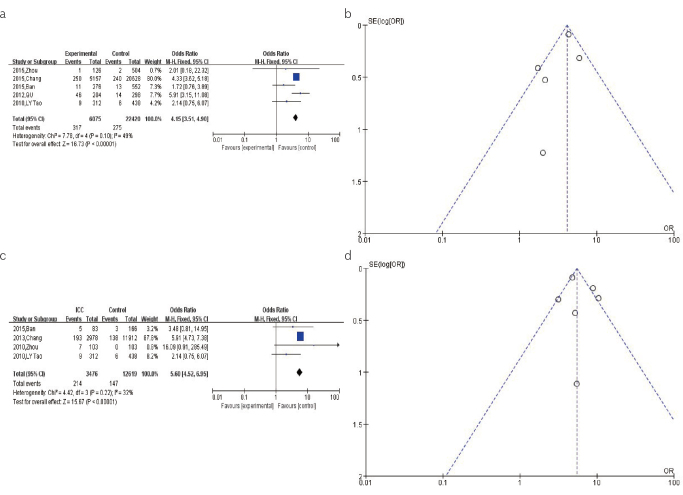
For the pooled analysis of the association between the HCV infection and CCA risk, the result from fixed-effects model (OR=4.15, 95% CI: 3.51–4.90, p<0.00001) showed a significant association between the HCV infection and increased risk of CCA (Figure 4a). The shape of the funnel plot was relatively symmetrical, indicating a modest publication bias for this association (Figure 4b).
Figure 4. a–d.
(a). The forest plots depicting the pooled analysis of the association between the HCV infection and CCA risk. (b). The funnel plots showing the evaluation of the publication bias of the association between HCV infection and CCA risk. (c). The forest plots depicting the pooled analysis of the association between the HCV infection and ICC risk. (d). The funnel plots showing the evaluation of the publication bias of the association between the HCV infection and ICC risk.
HCV Infection and the Risk of ICC
For the pooled analysis of the association between the HCV infection and ECC risk, the results from the fixed-effects model (OR=5.60, 95% CI: 4.52–6.95, p<0.00001) indicated that the HCV infection is significantly associated with an increased risk of ICC (Figure 4c). The shape of the funnel plot was relatively symmetrical, indicating a modest publication bias for this association (Figure 4d).
AFP level and the Risk of CCA
The secondary outcomes assessed were the levels of the laboratory markers (namely, AFP and CA-199) and lymph node involvement in the CCA patients with the HBV or HCV infection as compared to those without. With regard to the secondary outcome results, a total of 11 studies with 24,763 participants were included. These studies were conducted in the Asian countries, including Japan (n=1), Korea (n=2), and China (n=8).
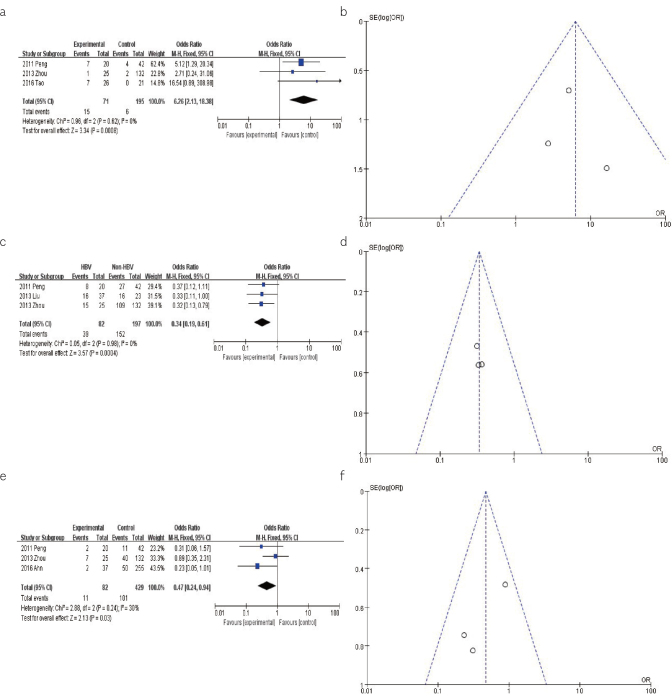
For the pooled analysis of the association between the levels of AFP and CCA with HBV or HCV infection, the results from the fixed-effects model (OR=6.26, 95% CI: 2.13–18.38, p<0.001) found a significant association between high levels of AFP and increased risk of CCA (Figure 5a). The shape of the funnel plot was symmetrical, indicating no evidence of publication bias for this association (Figure 5b).
Figure 5. a–f.
(a). The forest plots depicting the pooled analysis of the association between the AFP level and CCA with HBV or HCV infection. (b). The funnel plots showing the evaluation of the publication bias of the association between AFP level and CCA with 16HBV or HCV infection. (c). The forest plots depicting the pooled analysis of the association between the CA-199 level and CCA with HBV or HCV infection. (d). The funnel plots showing the evaluation of the publication bias of the association between the CA-199 level and CCA with HBV or HCV infection. (e). The forest plots depicting the pooled analysis of the association between the lymph node involvement and CCA with HBV or HCV infection. (f). The funnel plots showing the evaluation of the publication bias of the association between the lymph node involvement and CCA with HBV or HCV infection.
CA-199 level and the Risk of CCA
For the pooled analysis of the association between the levels of CA-199 and CCA with HBV or HCV infection, the results from the fixed-effects model (OR=0.34, 95% CI: 0.19–0.61, p<0.001) suggested a significant association between the levels of CA-199 and lower incidence of CCA (Figure 5c). The shape of the funnel plot was symmetrical, indicating no evidence of publication bias for this association (Figure 5d).
Lymph Node involvement and the Risk of CCA
For the pooled analysis of the association between the lymph node involvement and CCA with HBV or HCV infection, the results from the fixed-effects model (OR=0.47, 95% CI: 0.24–0.94, p=0.03) suggested a reduced risk of CCA associated with lymph node involvement (Figure 5e). The shape of the funnel plot was symmetrical, indicating no evidence of publication bias for this association (Figure 5f).
DISCUSSION
The current meta-analysis was performed to establish the relationship between the HBV or HCV infection and the risks of CCA, ICC, and ECC across 13 included studies. The impact of the levels of AFP and CA19-9, and the lymph node involvement on the incidence of CCA in the patients with HBV infection were analyzed in this meta-analysis. To our knowledge, few meta-analyses have been performed to comprehensively evaluate the association of the HBV or HCV infection with CCA, ICC, and ECC, and to determine the roles of AFP, CA19-9, and lymph node in the CCA with HBV infection.
This meta-analysis revealed that the risk of CCA (including ICC and ECC) in the HBV patients was 3.70-fold higher than that in the patients without HBV infection. Similarly, our meta-analysis showed 5.98-fold and 2.50-fold increase in the risk of developing ICC and ECC, respectively, among the patients infected with HBV, with a significant P-value of less than 0.00001. As compared with ECC, ICC may be more closely related to the HBV infection, suggesting that the etiology of ICC and ECC may be different. The HBV infection is a global health issue, and China has a high incidence of the HBV infection (27). It is therefore postulated that the incidence of CCA (including ICC and ECC) can be reduced by preventing the HBV infection.
This study also indicated that the risks of CCA and ICC in the patients with HCV infection were 4.15-fold and 5.60-fold higher that in the non-infected patients, respectively, with a statistical significance of p<0.00001. In addition to the HBV infection, we found that the HCV infection significantly increased the risks of CCA and ICC. As compared to the overall CCA, the incidence of ICC is more closely related to the HCV infection. However, the relationship between HCV infection and ECC risk was not analyzed due to lack of sufficient literature.
The possible reasons for these findings include: (i) HBV and HCV are hepatotropic viruses, moreover, the liver and bile duct are homologous during embryogenesis and have anatomical continuity; (ii) Bile duct is responsible for bile secretion from the liver cells, and the upper part of the bile duct that is located near the liver is more likely to suffer from HBV and HCV infections; (iii) both the liver and bile duct cells originated from the primitive cells where HBV and HCV can cause hepatocellular carcinoma and further lead to CCA; and (iv) most CCA is presented in the upper part of the bile duct (28). Through this meta-analysis, we have discovered that both HBV and HCV are the high-risk factors for CCA. Therefore, we can reduce the incidence of CCA by preventing the occurrence of these risk factors.
The results from the meta-analysis showing the levels of the laboratory markers in the CCA patients with HBV infection as compared to those without HBV infection suggested that the levels of AFP and CA19-9, and the lymph node involvement changed by 6.26-, 0.34- and 0.47-fold, respectively, among the HBV infection group. Hence, there was statistically significant increased risk of incidence of CCA with high AFP levels, whereas significantly decreased risk of CCA incidence with decreased CA19-9 levels and lymph node involvement. Meanwhile, all the funnel plots are symmetrical, which indicate that no publication bias existed for these associations. Recent studies have reported that the bile duct cells and hepatocytes differentiate from the same hepatic progenitor cells, which can produce AFP through the process of malignant transformation (29). Previous study showed that the serum CA 19-9 levels are correlated with the tumor burden and not the magnitude of cholestatic liver dysfunction (30). Lymph node invasion is considered an important index to evaluate the prognosis of the ICC patients that underwent hepatectomy (31). Our study found that the HBV-infected CCA has a better prognosis. Therefore, we can utilize the levels of AFP and CA19-9, and lymph node involvement as well as the HBV or HCV infection to assist in the diagnosis and treatment of CCA.
There are some limitations in this study. First, this meta-analysis included the studies from China, South Korea, and Japan; thus, the studies from different countries may have different effects on the results of this study. Second, only 13 studies were included in this meta-analysis which may lead to selection bias. Third, the severity of the HBV or HCV infection was not detailed in most studies, which may influence the true results since the condition of the hepatitis virus can affect the prognosis.
Our findings suggest that both the HCV and HBV infections can significantly increase the risk of developing CCA, as well as ICC and ECC; therefore, prevention, screening, and treatment of both may reduce the risk of CCA in these patients. Furthermore, higher levels of AFP and lower levels of CA19-9, and lymph node involvement were observed in the CCA patients with the HBV infection as compared to those without.
Footnotes
Ethics Committee Approval: Ethics committee approval not received for this study as there are no human or animal subjects directly recruited.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - G.W.Z., J.H.T., W.Y.Z.; Design - G.W.Z.; Supervision - L.Z., R.C.C.; Resource - G.W.Z.; Materials - J.H.T., W.Y.Z.; Data Collection and/or Processing - W.Y.Z., L.Z.; Analysis and/ or Interpretation - J.H.T., R.C.C.; Literature Search - L.Z., R.C.C.; Writing - J.H.T., W.Y.Z.; Critical Reviews - G.W.Z.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declare that this study was supported by the Scientific Research Startup Program of the Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (CX2018N012), Clinical Research Program of the Nanfang Hospital, Southern Medical University (2018CR046), and Clinical Research Startup Program of the Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (LC2016PY011).
REFERENCES
- 1.World Health Organization. Global hepatitis report. [Accessed 18 Feb. 2018]. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- 2.World Health Organization. Global Health sector strategy on viral hepatitis 2016–2021: Towards ending viral hepatitis. [Accessed 2 Feb. 2018]. http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
- 3.Cardinale V, Bragazzi MC, Carpino G. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2:272–80. doi: 10.3978/j.issn.2304-3881.2013.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Hahn T, Ciesek S, Wegener G, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroentero. 2011;46:1092–8. doi: 10.3109/00365521.2011.589472. [DOI] [PubMed] [Google Scholar]
- 5.Endo I, Gonen M, Yopp AC, Dalal KM, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 6.Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667–74. doi: 10.1093/annonc/mds652. [DOI] [PubMed] [Google Scholar]
- 7.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–7. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhu B, Zhang H, Liang J, Zeng W. HBV Infection Status and the Risk of Cholangiocarcinoma in Asia: A Meta-Analysis. Biomed Res Int. 2016;2016 doi: 10.1155/2016/3417976. 3417976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Sheng YY, Dong QZ, Qin LX. Hepatitis B virus and hepatitis C virus play different prognostic roles in intrahepatic cholangiocarcinoma: A meta-analysis. World J Gastroenterol. 2016;22:3038–51. doi: 10.3748/wjg.v22.i10.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Li J, Li P, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol. 2012;27:1561–8. doi: 10.1111/j.1440-1746.2012.07207.x. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn CS, Hwang S, Lee YJ, et al. Prognostic impact of hepatitis B virus infection in patients with intrahepatic cholangiocarcinoma. ANZ J Surg. 2018;88:212–7. doi: 10.1111/ans.13753. [DOI] [PubMed] [Google Scholar]
- 15.Tao LY, He XD, Xiu DR. Hepatitis B virus is associated with the clinical features and survival rate of patients with intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2016;40:682–7. doi: 10.1016/j.clinre.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YM, Zhang XF, Wu LP, Sui CJ, Yang JM. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-controlstudy. World J Gastroenterol. 2014;20:12615–20. doi: 10.3748/wjg.v20.i35.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol. 2015;21:502–10. doi: 10.3748/wjg.v21.i2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto K, Onoyama T, Kawata S, et al. Hepatitis B and C Virus Infection is a Risk Factor for the Development of Cholangiocarcinoma. Intern Med. 2014;53:651–4. doi: 10.2169/internalmedicine.53.1410. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Zhou Q, Lin Q, et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer. 2013;133:1867–75. doi: 10.1002/ijc.28196. [DOI] [PubMed] [Google Scholar]
- 20.Chang JS, Tsai CR, Chen LT. Medical risk factors associated with cholangiocarcinoma in Taiwan: a population-based case-control study. PLoS One. 2013;8:e69981. doi: 10.1371/journal.pone.0069981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu RQ, Shen SJ, Hu XF, Liu J, Chen LJ, Li XY. Prognosis of the intrahepatic cholangiocarcinoma after resection: hepatitis B virus infection and adjuvant chemotherapy are favorable prognosis factors. Cancer Cell International. 2013;13:99. doi: 10.1186/1475-2867-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q, He XD, Yu L, Liu W, Tao LY. The Metabolic Syndrome and Risk Factors for Biliary Tract Cancer: A Case-control Study in China. Asian Pac J Cancer Prev. 2012;13:1963–9. doi: 10.7314/APJCP.2012.13.5.1963. [DOI] [PubMed] [Google Scholar]
- 23.Qu Z, Cui N, Qin M, Wu X. Epidemiological survey of biomarkers of hepatitis virus in patients with extrahepatic cholangiocarcinomas. Asia Pac J Clin Oncol. 2012;8:83–7. doi: 10.1111/j.1743-7563.2011.01466.x. [DOI] [PubMed] [Google Scholar]
- 24.Peng NF, Li LQ, Qin X, et al. Evaluation of Risk Factors and Clinicopathologic Features for Intrahepatic Cholangiocarcinoma in Southern China: A Possible Role of Hepatitis B Virus. Ann Surg Oncol. 2011;18:1258–66. doi: 10.1245/s10434-010-1458-5. [DOI] [PubMed] [Google Scholar]
- 25.Cai WK, Sima H, Chen BD, Yang GS. Risk factors for hilar cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2011;17:249–53. doi: 10.3748/wjg.v17.i2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivatanakul P, Honjo S, Kittiwatanachot P, Jedpiyawongse A, Khuhaprema T, Miwa M. Hepatitis Viruses and Risk of Cholangiocarcinoma in Northeast Thailand. Asian Pac J Cancer Prev. 2010;11:985–8. [PubMed] [Google Scholar]
- 27.Ott JJ, Horn J, Krause G, Mikolajczyk RT. Time trends of chronic HBV infection over prior decades-A global analysis. J Hepatol. 2017;66:48–54. doi: 10.1016/j.jhep.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253–60. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 29.Zhou H, Wang H, Zhou D. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056–61. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–7. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 31.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824–9. doi: 10.1097/SLA.0b013e318236c21d. [DOI] [PubMed] [Google Scholar]