Abstract
Trichloroethene (trichloroethylene, TCE) and one of its reactive metabolites dichloroacetyl chloride (DCAC) are associated with the induction of autoimmunity in MRL+/+ mice. Although oxidative stress plays a major role in TCE-/DCAC-mediated autoimmunity, the underlying molecular mechanisms still need to be delineated. Nuclear factor (erythroid-derived 2)-like2 (Nrf2) is an oxidative stress-responsive transcription factor that binds to antioxidant responsive element (ARE) and provides protection by regulating cytoprotective and antioxidant gene expression. However, the potential of Nrf2 in the regulation of TCE-/DCAC-mediated autoimmunity is not known. This study thus focused on establishing the role of Nrf2 and consequent inflammatory responses in TCE-/DCAC-mediated autoimmunity. To achieve this, we pretreated Kupffer cells (KCs) or T cells with/without tert-butylhydroquinone (tBHQ) followed by treatment with DCAC. In both KCs and T cells, DCAC treatment significantly downregulated Nrf2 and HO-1 expression along with induction of Keap-1 and caspase-3, NF-κB (p65), TNF-α, and iNOS, whereas pretreatment of these cells with tBHQ attenuated these responses. The in vitro findings were further verified in vivo by treating female MRL+/+ mice with TCE along with/without sulforaphane. TCE exposure in mice also led to reduction in Nrf2 and HO-1 but increased phospho-NF-κB (p-p65) and iNOS along with increased anti-dsDNA antibodies. Interestingly, sulforaphane treatment led to amelioration of TCE-mediated effects, resulting in Nrf2 activation and reduction in inflammatory and autoimmune responses. Our results show that TCE/DCAC mediates an impairment in Nrf2 regulation. Attenuation of TCE-mediated autoimmunity via activation of Nrf2 supports that antioxidants sulforaphane/tBHQ could be potential therapeutic agents for autoimmune diseases.
Keywords: Nrf2, trichloroethene, dichloroacetyl chloride, autoimmunity
Trichloroethene (TCE), a nonflammable chlorinated solvent, is an occupational and ubiquitous environmental contaminant (Chiu et al., 2013; Gist and Burg, 1995; Khan and Wang, 2018). TCE exposure is known to cause several adverse health effects, but mainly affects liver, kidney, reproductive, and immune system through its metabolites (Chiu et al., 2013; Cooper et al., 2009; Lash et al., 2006; Wang et al., 2018). Exposure to TCE is also associated with the induction of autoimmune diseases (ADs) such as systematic lupus erythematosus (SLE), scleroderma, and autoimmune hepatitis (AIH) (Flindt-Hansen and Isager, 1987; Garabrant et al., 2003; Gist and Burg, 1995). Experimental studies in female MRL+/+ mice show that TCE exposure causes an early induction of autoimmune responses, evident from increased autoantibodies, including antinuclear antibodies (ANA), anti-dsDNA, and anti-ssDNA antibodies (Khan et al., 1995; Wang et al., 2007, 2019), eventually leading to both AIH and SLE-like diseases on chronic exposure (Cai et al., 2008; Kondraganti et al., 2012). One of the oxidative metabolites of TCE, dichloroacetyl chloride (DCAC) with strong acylating capacity, has also the potential to induce autoimmune response in MRL+/+ mice at a much lower dose than the parent compound TCE (Khan et al., 1995, 1997; Wang et al., 2018). Furthermore, TCE-mediated autoimmunity is associated with increased oxidative stress, evidenced by oxidatively modified proteins (carbonylated and nitrated proteins) (Wang et al., 2009), and increased formation of lipid peroxidation-derived reactive aldehydes (ie, 4-hydroxynonenal [HNE] and malondialdehyde [MDA]), protein adducts and corresponding antibodies (Khan et al., 2001; Wang et al., 2012). However, the mechanism by which TCE-/DCAC-associated oxidative stress contributes to autoimmunity is not clearly understood.
Nuclear factor (erythroid-derived 2)-like2 (Nrf2) is an oxidative stress-related transcription factor which remains bound to its repressor protein Keap-1 and caspase-3 (Kelch-like ECH-associated protein 1) in the cytosol and subsequently undergoes degradation and ubiquitination by the proteasome (Jaiswal, 2004; Kaspar et al., 2009; Loboda et al., 2016; Zagorski et al., 2013). In response to endogenous or exogenous stresses caused by reactive oxygen species (ROS) and electrophiles, Nrf2 translocates to the nucleus and promotes transcription of its target genes responsible for antioxidant function and detoxification of toxic compounds (Jaiswal, 2004; Kaspar et al., 2009; Zagorski et al., 2013). A number of chemicals, antioxidants, and food preservatives, such as tert-butylhydroquinone (tBHQ), butylated hydroxyanisole, and sulforaphane (SFN), have the potential to activate Nrf2 (Guerrero-Beltrán et al., 2012; Zagorski et al., 2013; Zhao et al., 2010). Role of Nrf2 in inflammatory diseases is evident from vulnerability of Nrf2 knockout mice to develop inflammatory disorders, including SLE, AIH, and multiple sclerosis (Johnson et al., 2010; Kim et al., 2010; Pan et al., 2011). Nrf2 polymorphism is also shown to be associated with autoimmune nephritis in female SLE patients (Córdova et al., 2010). Despite the fact that TCE causes oxidative stress and induction of autoimmune response (Khan et al., 1995, 2001), the status of Nrf2 and its contribution to TCE-mediated autoimmunity is not known. Considering the antioxidant potential of Nrf2, it is necessary to establish its regulation and role in TCE-mediated autoimmunity. We hypothesize that TCE-mediated impairment in Nrf2 expression promotes a pro-oxidant signaling, leading to inflammatory and autoimmune response. Furthermore, Nrf2 activation provides protection by downregulating NF-κB (p65)-mediated inflammatory responses, thereby alleviating oxidant-induced inflammation and disease pathogenesis.
In previous studies, we have shown that chronic TCE exposure leads to reduction in the number and function of Kupffer cells (KCs) in the livers of MRL+/+ mice (Kondraganti et al., 2012). In response to any toxic insult, phagocytes provide the first line of defense and maintain cellular homeostasis. KCs control the hepatic innate immune response by phagocytosis, antigen presentation, and production of cytokines such as IL-6 and TNF-α (Ahmed et al., 2017; Wang et al., 2018). KCs are also involved in the activation of adaptive immune response via antigen presentation (Bilzer et al., 2006). In vitro studies with DCAC using KCs (Wang et al., 2018) further verified our in vivo findings (Kondraganti et al., 2012) and showed functional losses in KCs. Studies focused on hepatic lymphocytic changes following TCE exposure showed increased infiltration of CD3 T lymphocytes in the liver and also increased CD4+ T cell activation as assessed by IFN-γ secretion (Gilbert et al., 2009). Furthermore, splenic lymphocytes from TCE-treated mice generated greater proinflammatory response when incubated with MDA-/HNE-protein adducts (Wang et al., 2008). However, direct effect of TCE and/or its metabolites on Nrf2 signaling in KCs or T cells and its consequences in autoimmunity are not known.
Considering a significant immunomodulatory role of Nrf2 in regulating both innate and adaptive immune responses (Johnson et al., 2010), it is imperative to clearly understand the role of Nrf2 signaling in TCE-/DCAC-mediated autoimmunity. Therefore, in this study, we first delineated the status and role of Nrf2 and consequent inflammatory responses in vitro using both KCs and T cells. This was followed by validation of our in vitro findings on Nrf2 in vivo by conducting studies in female MRL+/+ mice following exposure to TCE with or without an antioxidant SFN.
MATERIALS AND METHODS
Cell culture
Immortalized Mouse Kupffer Cell Line was purchased from Millipore (Burlington, Massachusetts) and Human Jurkat T cells (T cells) were obtained from American Type Tissue Collection (ATCC, Manassas, Virginia). Both cell types were maintained according to the supplier’s guidelines. In brief, KCs and T cells were cultured separately in complete RPMI 1640 media, with 10% heat inactivated FBS and 1% penicillin and streptomycin. The cells were incubated at 37°C and treated with either DCAC alone (2 or 5 mM) (Sigma-Aldrich, St. Louis, Missouri) or treated first with/without tBHQ (5 µm) (Sigma-Aldrich) 1 h prior to incubation with DCAC for 24 h (Wang et al., 2018).
Animals and treatments
Female MRL/MpJ (MRL+/+) mice (5 weeks old) were obtained from the Jackson Laboratory (Bar Harbor, Maine). The animal protocols were approved by the Institutional Animal Care and Use Committee of University of Texas Medical Branch (UTMB). All the experiments were done based on National Institutes of Health (NIH) guidelines. Mice were maintained in an animal facility at the UTMB under controlled conditions of temperature and humidity with a 12 h light/dark cycle. Mice had free access to food and water ad libitum and acclimatized for a week before starting the treatments. Mice were randomly divided into 4 groups of 6 mice each and named control, TCE, SFN, or TCE+SFN treatment groups (TCE, 10 mmol/kg in corn oil, ip, every fourth day; sulforaphane [Sigma-Aldrich], 8 mg/kg in corn oil, ip, every other day) (Guerrero-Beltrán et al., 2012; Khan et al., 1995; Wang et al., 2019; Zhao et al., 2010). The control animals received an equal volume of corn oil only. The selection of the dose and duration of TCE or SFN exposure were based on previous studies (Guerrero-Beltrán et al., 2012; Khan et al., 1995, 2001; Wang et al., 2019; Zhao et al., 2010). The rationale behind selecting female mice was based on previous findings demonstrating 85% or more of AD patients were females (Cooper and Stroehla, 2003; Garabrant et al., 2003). MRL+/+ mice are an autoimmune-prone mouse model where most autoantibodies appear several months after their birth and SLE disease appears late in the second year of their lives (Khan et al., 1995, 2001). This slow development of the disease allows to evaluate autoimmune potential of specific agents in relatively young animals. After 6 weeks of treatment, the mice were euthanized under ketamine/xylazine anesthesia and blood was withdrawn from inferior vena cava. Then major organs were removed and weighed. Liver portions were snap-frozen in liquid nitrogen and stored at −80°C for future use. Sera obtained after blood clotting and centrifugation were stored in small aliquots at −80°C for further analysis.
Immunofluorescent detection of caspase-3 in KCs and T cells
Caspase-3 was quantified in mouse KCs and T cells using caspase-3/7 green fluorescent dye detection kit as described in the manufacturer’s instructions (Invitrogen, Carlsbad, California). KCs and T cells were seeded in 6-well plate overnight and then treated with DCAC (5 mM) alone or with tBHQ (5 µm) and were incubated at 37°C overnight. After 24 h of incubation, Keap-1 and caspase-3 green reagent was added and incubated for 30 min. Following that, fluorescent images were taken at ×10 magnification for each treatment group, ie, control, DCAC, or DCAC + tBHQ. ImageJ (v1.48, NIH) software was used to analyze the integrated density. Mean fluorescence along with the background was measured in the selected cells of interest. The total corrected cellular fluorescence (TCCF) = integrated density – (area of selected cell × mean fluorescence of background readings) was measured (McCloy et al., 2014; Mehlem et al., 2013).
Analysis of anti-dsDNA antibodies in MRL+/+ mice
The anti-dsDNA antibodies were analyzed in the sera by using a mouse-specific ELISA kit (Alpha Diagnostic Int’l, San Antonio, Texas) (Wang et al., 2015).
Real-time PCR
Total cellular/tissue RNA was extracted using RiboPure RNA Purification Kit (Invitrogen) from cells. cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California). The design of primer sequencing was done using Primer3Plus program. The primers were obtained from Integrated DNA Technologies (Coralville, Iowa) and mRNA expressions of Nrf2, HO-1, Keap-1, NF-kB (p65), and iNOS were determined. Glyceraldehyde 3-phosphate dehydrogenase was used as the endogenous loading control (Wang et al., 2015). Primer sequences are provided in Supplementary Table 1.
Western blotting
Proteins were extracted from cells using RIPA buffer (Cell Signaling Technology, Danvers, Massachusetts) with protease inhibitor (Sigma-Aldrich). Proteins from the liver tissues of MRL+/+ mice were isolated using nuclear and cytoplasmic extraction kits (Thermo Scientific, Rockford, Illinois). Protein lysates (30 μg) were used for Western blot analyses. The immunoblots were probed with primary antibodies against Nrf2, Keap-1, HO-1, iNOS, NF-kB (p65), pNF-κB (p-p65), and β-actin (Cell Signaling Technology) (Banerjee et al., 2013; Wang et al., 2015).The immunoblot images were quantified by densitometry and normalized using loading control protein β-actin (Wang et al., 2019).
Transfection assay
KCs were transfected with Nrf2 siRNA and nonspecific siRNA (ns small interfering RNA) by using lipofectamine 3000 reagent according to the manufacturer’s guidelines (Thermo Fisher, Waltham, Massachusetts). In brief, the cells were seeded in 6-well plates and incubated with negative control or Nrf2 siRNA (20 µm) for 4 h in serum-free OPTIMEM media (Invitrogen). The transfected cells were treated with DCAC for 24 h and the expressions of Nrf2 and NF-kB (p65) were analyzed (Banerjee et al., 2012; Luo et al., 2018).
Statistical analysis
The values are presented as means ± SEM. The data were analyzed using 1-way analysis of variance followed by Tukey-Kramer multiple comparisons test. The p values < .05 were statistically significant.
RESULTS
DCAC Treatment-induced Apoptotic Marker Caspase-3 in KCs and T Cells
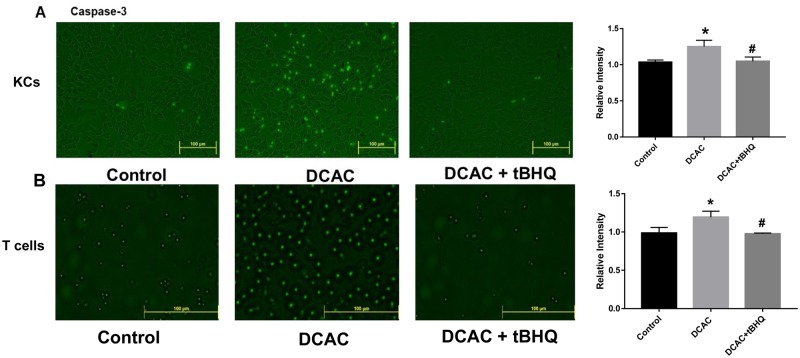
Oxidative stress leads to apoptosis which can play a critical role in the pathogenesis of ADs via generation of neoantigens (Wang et al., 2019). Previously, we have shown that TCE/DCAC induces oxidative stress, DNA damage, and upregulation of cleaved poly-ADP-ribose polymerase, which can initiate apoptosis through induction of caspases (Wang et al., 2019). Therefore, to understand the effect of DCAC on apoptotic response and protective role of antioxidant tBHQ, we determined the apoptotic marker caspase-3 in DCAC-treated KCs and T cells. Our data show that DCAC treatment led to increased caspase-3 expression in both KCs and T cells as compared with their respective controls (Figs. 1A and 1B). Interestingly, increased expression of caspase-3 in DCAC-treated group was ameliorated by tBHQ (Figs. 1A and 1B), suggesting that tBHQ provides protection against DCAC-induced apoptosis.Keap-1 and caspase-3
Figure 1.
Treatment of KCs and T cells with tBHQ attenuated DCAC-induced caspase-3 expression. KCs and T cells were pretreated with/without tBHQ (5 µm) 1 h prior to incubation with DCAC (5 mM) for 24 h. A, Fluorescent images of caspase-3 in KCs. B, Fluorescent images of caspase-3 in T cells. Bar diagrams on the right sides represent quantification of fluorescent intensity of caspase-3 in KCs and T cells; n = 3 in each group. *p < .05 (Control vs DCAC); #p < .05 (DCAC vs DCAC+ tBHQ).
DCAC Modulated Expression of Nrf2/HO-1 in KCs
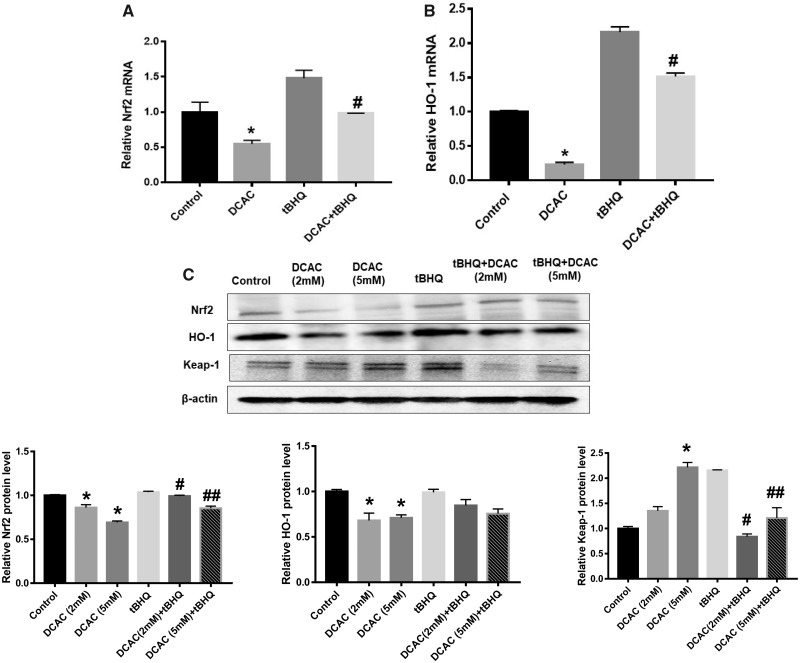
Nrf2 is an oxidative stress-responsive transcription factor which remains bound to its repressor protein Keap-1 and caspase-3 in cytosol (Loboda et al., 2016). Antioxidant tBHQ, which is used as a food preservative, interacts with thiol groups of cysteine molecules present on the Keap-1 and caspase-3 protein and inhibits the binding of Keap-1 and caspase-3 with Nrf2 (Zagorski et al., 2013). As a result, Nrf2 translocates to the nucleus where it binds to antioxidant responsive element (ARE) and induces transcription of antioxidant and cytoprotective genes (Ahmed et al., 2017; Liu et al., 2019; Zagorski et al., 2013). Nrf2 and its target genes, such as phase II detoxifying enzyme HO-1 and NQO1, are involved in xenobiotic detoxification and immune cell function, and Nrf2/HO-1 pathway could play a critical role in the pathogenesis of ADs (Kavian et al., 2018; Loboda et al., 2016). Previously, we have shown that exposure to TCE and its metabolite DCAC leads to oxidative stress (Khan et al., 2001; Wang et al., 2018). To further investigate the status and role of Nrf2 in TCE-/DCAC-mediated autoimmunity, we conducted studies with DCAC in vitro using KCs. DCAC (5 mM) significantly suppressed the mRNA expression of both Nrf2 and HO-1 (Figs. 2A and 2B). DCAC decreased Nrf2 protein expression, it also reduced HO-1 protein expression at both 2 and 5 mM concentrations (Figure 2C). Furthermore, DCAC treatment (5 mM) led to increased Keap-1 protein expression (Figure 2C) compared with the controls. Interestingly, tBHQ treatment attenuated the effect of DCAC in KCs which was evident from increased Nrf2 and HO-1 expression and decreased Keap-1 expression (Figs. 2A–C). These results suggest that DCAC suppresses Nrf2 expression and tBHQ provides protection against DCAC-induced oxidative stress in KCs.
Figure 2.
Treatment of KCs with tBHQ alleviated DCAC-mediated impairment of Nrf2 signaling. A and B, mRNA expression of Nrf2 and HO-1. C, Protein expression of Nrf2, HO-1, and Keap-1 and relative protein levels of Nrf2, HO-1, and Keap-1; n = 3 in each group. *p < .05 (Control vs DCAC); #p < .05 (DCAC [2 mM] vs DCAC [2 mM] + tBHQ); ##p < .05 (DCAC [5 mM] vs DCAC [5 mM] + tBHQ).
DCAC Modulated Inflammatory Markers in KCs
In response to toxic insult, especially oxidative stress, activated KCs secrete inflammatory cytokines, which could contribute to the pathogenesis of ADs (Wang et al., 2018). Therefore, we investigated the potential of DCAC in inducing inflammatory response in KCs and evaluated the role of Nrf2 in DCAC-mediated inflammation. The results are presented below.
Effect of DCAC on NF-κB (p65)
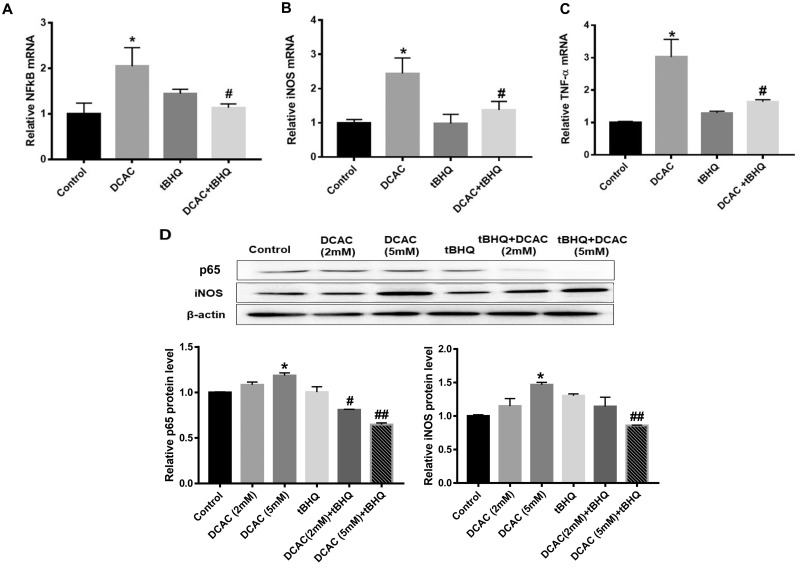
We determined the expression of NF-κB (p65), the downstream inflammatory target of Nrf2 following DCAC treatment. DCAC treatment (5 mM) led to increases in the NF-κB (p65) mRNA and its protein expression (Figs. 3A and 3D). tBHQ treatment ameliorated DCAC-mediated inflammatory response by downregulating NF-κB (p65) expression (Figs. 3A and 3D).
Figure 3.
Treatment of KCs with tBHQ ameliorated DCAC-induced inflammation. KCs were activated with LPS (100 ng/ml) for 4 h (for iNOS and TNF-α), and then pretreated with/without tBHQ (5 µm) 1 h prior to incubation with DCAC (2 or 5 mM) for 24 h. A–C, mRNA expression of p65, iNOS, and TNFα. D, Protein expression of p65 and iNOS and relative protein levels of p65 and iNOS; n = 3 in each group. *p < .05 (Control vs DCAC); #p < .05 (DCAC [2 mM] vs DCAC [2 mM] + tBHQ); ##p < .05 (DCAC [5 mM] vs DCAC [5 mM] + tBHQ).
Effect of DCAC on inflammatory markers
DCAC treatment (5 mM) of KCs also significantly increased the mRNA expression of inflammatory markers iNOS (with corresponding increase in its protein level) and TNF-α (Figs. 3B–D). tBHQ treatment attenuated the effect of DCAC and reduced the expression of iNOS and TNF-α (Figs. 3B–D), suggesting that tBHQ provides protection against DCAC-mediated inflammatory response (Figs. 3A–D).
DCAC Modulated Expression of Nrf2 and Its Downstream Inflammatory Marker in T Cells
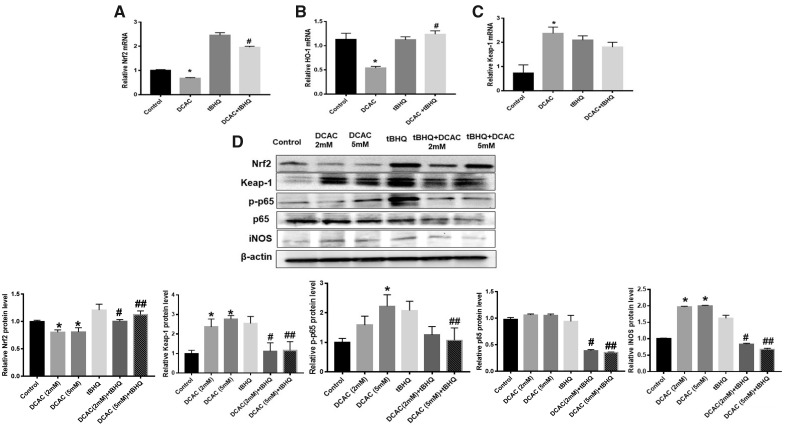
Because both KCs and T cells contribute to the pathogenesis of ADs (Wang et al., 2008, 2018; Zagorski et al., 2013, 2017), it was deemed necessary to also determine the response of T cells to DCAC, especially its impact on Nrf2 pathway. DCAC treatment in T cells also significantly suppressed the mRNA expressions of Nrf2 and HO-1 (Figs. 4A and 4B), whereas Keap-1 mRNA expression was upregulated (Figure 4C). DCAC treatment also resulted in reduction in Nrf2 protein expression (Figure 4D). However, DCAC treatment increased the protein expression of phospho-NF-kB (p-p65) and iNOS (Figure 4D) but no change was observed in NF-kB (p65) expression (Figure 4D). Interestingly, tBHQ treatment attenuated the effects of DCAC in T cells (Figs. 4A–D). It is thus clear from our data that tBHQ also provides protection against DCAC-mediated oxidative and inflammatory responses in T cells.
Figure 4.
tBHQ treatment of T cells attenuated DCAC-mediated impairment of Nrf2 signaling and inflammatory responses. T cells were pretreated with/without tBHQ (5 µm) 1 h prior to incubation with DCAC (2 or 5 mM) for 24 h. A–C, mRNA expression of Nrf2, HO-1, and Keap-1. D, Protein expression of Nrf2, Keap-1, p-p65, p65, and iNOS and relative protein levels ofNrf2, Keap-1, p-p65, p65, and iNOS; n = 3 in each group. *p < .05 (Control vs DCAC); #p < .05 (DCAC [2 mM] vs DCAC [2 mM] + tBHQ); ##p < .05 (DCAC [5 mM] vs DCAC [5 mM] + tBHQ).
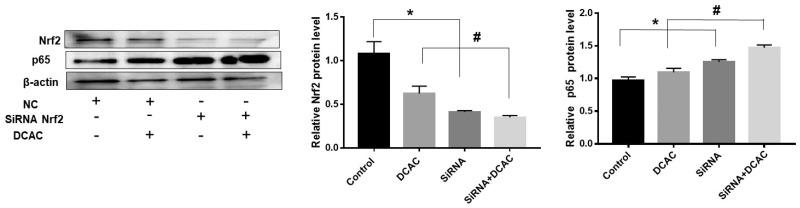
Nrf2 siRNA Modulated the Effects of DCAC
To further validate and support the contribution of the Nrf2 signaling in DCAC-mediated toxic responses, we knocked down the Nrf2 gene by transfecting KCs with Nrf2 siRNA. Because our purpose was to establish the contribution of Nrf2 signaling, these transfection studies were conducted in KCs. Our results showed significant reduction in Nrf2 expression but induction in NF-kB (p65) expression following Nrf2 siRNA transfection (Figure 5). DCAC-mediated reduction in the expression of Nrf2 (Figure 5) was also associated with concurrent increase in NF-κB (p65) expression following Nrf2 siRNA transfection (Figure 5). These transfection results further validate the contribution of Nrf2 in DCAC-mediated inflammatory responses.
Figure 5.
Nrf2 siRNA modulated the effect of DCAC in KCs. KCs were transfected with Nrf2 siRNA (20 µm) for 4 h and then exposed to DCAC (5 mM) for 24 h. Protein expression ofNrf2 and p65 and relative protein levels of Nrf2 and p65; n = 3 in each group. *p < .05 (Control vs siRNA); #p < .05 (DCAC vs siRNA + DCAC).
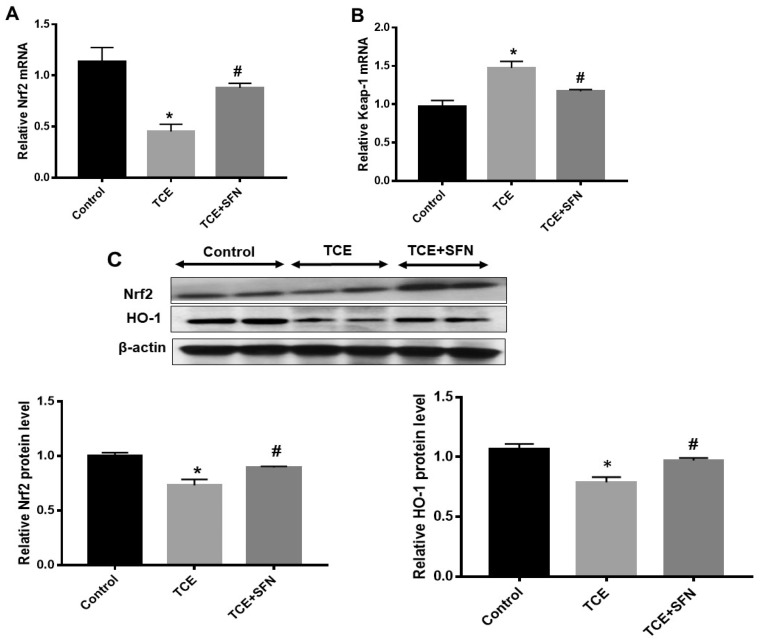
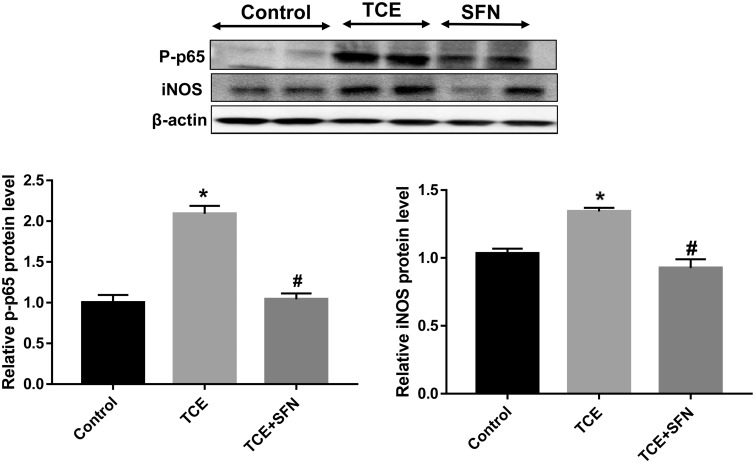
TCE Treatment in MRL+/+ Mice Modulated Nrf2/Keap-1/HO-1 Expression and Inflammatory Markers: Protective Effect of SFN
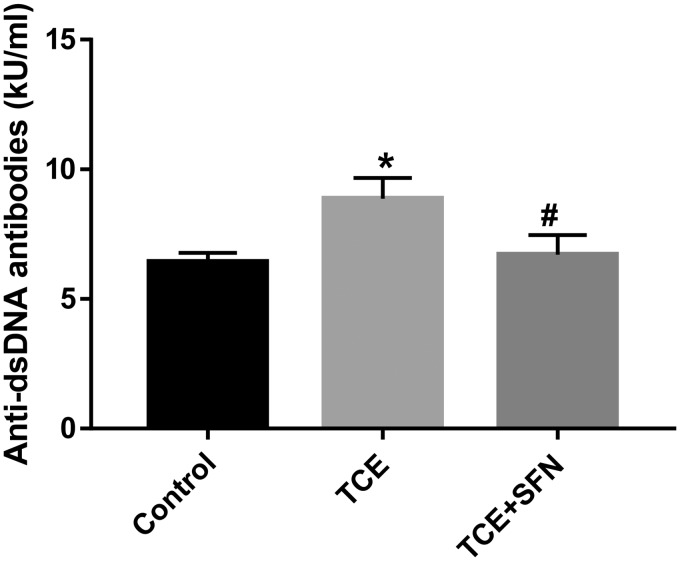
Previously we have shown the involvement of oxidative stress in TCE-/DCAC-mediated autoimmune response in MRL+/+ mice (Khan et al., 1995, 2001; Wang et al., 2019). Furthermore, our in vitro studies with DCAC in KCs and T cells showed significant reduction in Nrf2 signaling. Therefore, to further establish the contribution of Nrf2 signaling in TCE-mediated autoimmunity, we conducted studies in MRL+/+ mice exposed to TCE with or without antioxidant SFN. TCE exposure in MRL+/+ mice in this study led to significantly reduced Nrf2 mRNA expression (Figure 6A) with corresponding reduction in Nrf2 protein level (Figure 6C) in the livers. TCE exposure led to increased Keap-1 mRNA expression (Figure 6B), whereas it reduced HO-1 protein expression (Figure 6C). Furthermore, TCE exposure in these mice led to significant induction of Nrf2 downstream inflammatory targets phospho-NF-kB (p-p65) and iNOS protein expression (Figure 7). Remarkably, treatment with antioxidant SFN, which is found in broccoli sprouts and also available as a dietary supplement (Greaney et al., 2016), ameliorated the effects of TCE in MRL+/+ mice as evident from upregulation of Nrf2 and decreased expression of phospho-NF-kB (p-p65) and iNOS (Figs. 6A–C and 7) in the livers. Consistent with our previous findings (Wang et al., 2015), TCE exposure in this study also led to induction of autoantibodies, as evident from increased levels of anti-dsDNA antibodies (Figure 8). Appreciably, the TCE-mediated increases in anti-dsDNA antibodies were also attenuated following SFN treatment. These findings thus suggest that SFN provides effective protection against TCE-mediated oxidative stress, inflammation and autoimmune response in MRL+/+ mice.
Figure 6.
SFN treatment ameliorated the effect of TCE on Nrf2/HO-1 pathway in the livers of MRL+/+ mice. Mice were exposed to TCE or TCE+SFN for 6 weeks. Nrf2, Keap-1, and HO-1 expression were analyzed. A and B, mRNA expression of Nrf2 and Keap-1. C, Protein expression of Nrf2 and HO-1 and relative protein levels of Nrf2 and HO-1; n = 4 in each group. *p < .05 (Control vs TCE); #p < .05 (TCE vs TCE + SFN).
Figure 7.
SFN exposure ameliorated the effect of TCE on inflammatory markers p-p65 and iNOS in the livers of MRL+/+ mice. Mice were exposed to TCE or TCE + SFN for 6 weeks. p-p65 and iNOS were analyzed. Protein expression of p-p65 and iNOS and relative protein levels of pNF-κB (p65) and iNOS; n = 4 in each group. *p < .05 (Control vs TCE); #p < .05 (TCE vs TCE + SFN).
Figure 8.
SFN treatment attenuated TCE-mediated increases in anti-dsDNA antibodies in the sera of MRL+/+ mice. Mice were exposed to TCE or TCE+SFN for 6 weeks and anti-dsDNA antibodies were analyzed in the sera of these mice; n = 4 in each group. *p < .05 (Control vs TCE); #p < .05 (TCE vs TCE + SFN).
DISCUSSION
TCE, a widely used industrial agent and a common environmental contaminant, is associated with development of autoimmune response in both humans and experimental animals (Cooper et al., 2009; Flindt-Hansen and Isager, 1987; Garabrant et al., 2003; Gilbert et al., 2009, 2014; Gist and Burg, 1995; Khan et al., 1995, 2001). Similarly, DCAC, one of the TCE metabolites with strong acylating property, induces an autoimmune response in MRL+/+ mice at a much lower dose than the parent compound TCE (Khan et al., 1995, 1997). Earlier studies also showed that TCE-mediated autoimmune response was associated with increased oxidative stress, as evident from increased protein carbonylation and nitration (Wang et al., 2009), and formation of HNE- and MDA-protein adducts and their respective antibodies (Khan et al., 2001). However, the mechanism by which TCE-/DCAC-associated oxidative stress contributes to the development of autoimmunity is not clearly understood. Nrf2 plays a critical role in cellular defense against oxidative stress, and regulates several downstream genes such as HO-1 and NQO1 that control cell homeostasis, cell growth, and functions (Ahmed et al., 2017; Cuadrado et al., 2019; Geisel et al., 2014; Liang et al., 2018; Ma, 2013). To understand the potential role of an oxidative stress-related transcription factor Nrf2 in TCE-/DCAC-mediated autoimmune response, we investigated the biomarkers of apoptosis, inflammation, and oxidative stress, with a major focus on Nrf2 and its downstream targets, by first conducting studies using KCs and T cells and then validating the in vitro findings in vivo using MRL+/+ mice.
Under basal condition, Keap-1 suppresses transcriptional activity of Nrf2 and undergoes ubiquitination and proteasomal degradation (Ahmed et al., 2017; Cuadrado et al., 2019). In response to stress caused by ROS and electrophiles, Nrf2 translocates to the nucleus and involves in transcriptional regulation of phase II detoxifying and antioxidant genes such as HO-1 and NQO1 (Ahmed et al., 2017; Geisel et al., 2014; Liang et al., 2018; Zagorski et al., 2013). Reduced Nrf2 expression and its dependent gene HO-1 modulate the immune function that may contribute toward pathogenesis of ADs (Ahmed et al., 2017; Cuadrado et al., 2019; Geisel et al., 2014; Liang et al., 2018; Ma, 2013). Considering immunomodulatory role of Nrf2 in regulating both innate and adaptive immune systems (Johnson et al., 2010), we used both KCs and T cells to evaluate the impact of DCAC on Nrf2 regulation. Our results in this study show that DCAC suppressed the expression of Nrf2 and its dependent gene HO-1, whereas it increased the expression of Keap-1, suggesting that one of the mechanisms by which DCAC exerts its toxic effects is by modulating oxidative stress-responsive Nrf2 transcription factor. Furthermore, the amelioration of DCAC-mediated effects on Nrf2 by tBHQ also validates the role of DCAC-mediated oxidative stress and the potential role of Nrf2/HO-1 pathway in TCE-/DCAC-mediated inflammatory response. It is not surprising that detoxification enzymes are impaired in the livers of Nrf2 knocked out mice, making them more susceptible to wide range of toxicants leading to oxidative stress and such ADs as lupus-like autoimmune nephritis (Ma, 2013; Yoh et al., 2001). Similarly, HO-1 knockout mice also develop systematic inflammation, and autoimmune response, and SLE (Liu et al., 2019; Loboda et al., 2016). To further understand the role of Nrf2/HO-1 in DCAC-mediated inflammation and autoimmunity, we knocked down Nrf2 by using Nrf2 siRNA and found that DCAC-mediated inhibition of Nrf2 and HO-1 was further suppressed following Nrf2 siRNA transfection, which subsequently also resulted in the induction of NF-κB expression. Our findings from these in vitro studies thus further support that DCAC suppresses the Nrf2 signaling and leads to NF-κB upregulation.
Studies have shown that activation of Nrf2 is involved in the inhibition of proinflammatory cytokines and inflammatory markers (Ahmed et al., 2017; Liu et al., 2019). Nrf2-dependent antioxidant genes HO-1 and NQO1 inhibit TNF-α production and also inhibit its downstream NF-κB signaling (Ahmed et al., 2017), suggesting an inverse relation between Nrf2/HO-1 with proinflammatory cytokine production. Our laboratory previously showed that TCE exposure caused iNOS induction (nitrosative stress) (Wang et al., 2014) and iNOS knockout MRL+/+ mice were less susceptible to TCE-mediated autoimmunity (Wang et al., 2015). In this study, we observed that DCAC treatment increased the expression of TNF-α and NF-κB (p-65) in KCs, and iNOS in both KCs and T cells. Our results with tBHQ suggest that increased expression of Nrf2 can upregulate HO-1 production, which can further increase cellular antioxidant and cytoprotective defense system (Ahmed et al., 2017; Liu et al., 2019) and inhibit NF-κB (p-65) expression, thereby reducing inflammatory response (Ahmed et al., 2017; Chi et al., 2015), and eventually may provide protection against DCAC-/TCE-mediated autoimmunity. DCAC-mediated suppression of Nrf2 in both KCs and T cells and its reversal by tBHQ suggests an immunomodulatory role of Nrf2 and could provide protection against TCE-mediated autoimmunity. In addition, in this study, we also observed an increase in caspase-3 in DCAC-treated cells, which is consistent with earlier findings illustrating that TCE/DCAC increased hepatic apoptosis through induction of caspase protein and may contribute to the pathogenesis of ADs (Wang et al., 2019). Induction of apoptosis by DCAC was inversely correlated with the Nrf2 regulation which further supports a role of Nrf2 in the protection against TCE-/DCAC-mediated apoptosis and autoimmune response (He et al., 2007; Wang et al., 2019).
To validate our in vitro findings, we conducted in vivo studies by treating female MRL+/+ mice with TCE or TCE along with antioxidant SFN. SFN is known to oxidize reactive thiol groups on small molecules such as glutathione (GSH), and provide protection against oxidative stress and autoimmune response (Geisel et al., 2014; Guerrero-Beltrán et al., 2012; Patel et al., 2018; Singh et al., 2015). SFN is known to activate Nrf2 via modification of cysteine residue of Keap-1, as a result Nrf2 translocates to the nucleus where it binds to ARE and induces transcription of antioxidant and cytoprotective genes such as HO-1 (Hu et al., 2011). Apparently, intracellular GSH levels and GSH-dependent enzymes are increased by Nrf2 induction which then lead to NF-κB suppression (Cuadrado et al., 2018; Wardyn et al., 2015). Furthermore, Nrf2-deficient mice show increased NF-κB-mediated proinflammatory reactions (Cuadrado et al., 2018; Li et al., 2008; Wardyn et al., 2015).
In this study, SFN-induced Nrf2-dependent antioxidant defense pathway and inhibited inflammatory marker NF-κB expression. It is thus apparent that SFN modulates the cross-talk between the 2 redox-sensitive transcription factors Nrf2 and NF-κB (Patel et al., 2018). Several studies have shown the therapeutic potential of Nrf2, which is one of the major targets for multiple drugs (Cuadrado et al., 2018, 2019). From a clinical perspective, it is very important to understand the significance and role of Nrf2 in chronic diseases. This is also of great importance in developing therapeutic approaches via modulation of the Nrf2/HO-1 pathway (Cuadrado et al., 2018, 2019) which can further suppress the inflammatory and oxidative stress-mediated chronic diseases.
Interestingly, in our in vivo study, we observed that TCE suppressed the expression of Nrf2 and its dependent gene HO-1 and increased the expression of Keap-1. However, TCE treatment led to increase expression of NF-κB (phosphorylated p65) and iNOS in the livers of MRL+/+ mice. More importantly, antioxidant SFN ameliorated the effects of TCE-induced oxidative stress and inflammatory response by restoring Nrf2 expression and reducing NF-κB (p65) expression in MRL+/+ mice. Usefulness of SFN was even more apparent with our finding of diminution of TCE-mediated anti-dsDNA antibodies, suggesting a therapeutic potential of SFN in inflammatory diseases.
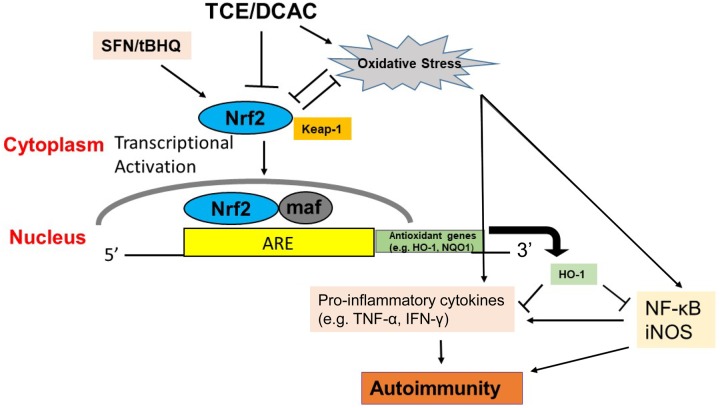
In conclusion, our studies define the status and protective role of Nrf2 in TCE-/DCAC-induced autoimmunity. Studies with TCE metabolite DCAC showed suppressed Nrf2 expression and its dependent gene HO-1 in KCs and T cells, whereas TCE exposure in MRL+/+ mice also validated our in vitro findings. TCE/DCAC insult thus leads to suppression in Nrf2 expression and upregulation of inflammatory NF-κB, and this imbalance between the 2 transcriptional pathways contributes to a prooxidative and proinflammatory outcome and thus leads to an autoimmune response/disease progression. Our findings provide the basis of underlying mechanism of Nrf2 and support the notion that Nrf2 action can modulate the TCE-/DCAC-mediated autoimmunity and SFN/tBHQ supplementation can provide protection as depicted in Figure 9. Our findings, especially the protective role of Nrf2 in TCE-mediated autoimmunity, provide the basis to develop novel therapeutic strategies against oxidative stress-induced ADs.
Figure 9.
Schematic presentation of the effects of TCE/DCAC on the Nrf2/HO-1 signaling pathway. TCE/DCAC exposure-mediated impairment of Nrf2 signaling, resulting in a proinflammatory response. In the presence of antioxidants (tBHQ/SFN), activated Nrf2 translocates to the nucleus where it binds to ARE and induces transcription of cytoprotective gene HO-1, and also suppresses downstream inflammatory targets NF-κB (p65), iNOS, and TNF-α. Antioxidant SFN/tBHQ supplementation can thus provide protection against TCE-/DCAC-mediated autoimmunity by modulating Nrf2/HO-1 signaling pathway.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
FUNDING
The National Institute of Environmental Health Sciences (NIEHS) (R01 ES016302 and ES026887), NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
DECLARATION OF CONFLICTING INTERESTS
The author/authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Ahmed S. M., Luo L., Namani A., Wang X. J., Tang X. (2017). Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 585–597. [DOI] [PubMed] [Google Scholar]
- Banerjee N., Kim H., Talcott S., Mertens-Talcott S. (2013). Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci and inflammation: Possible role of miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR. Carcinogenesis 34, 2814–2822. [DOI] [PubMed] [Google Scholar]
- Banerjee N., Talcott S., Safe S., Mertens-Talcott S. U. (2012). Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: Potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res. Treat. 136, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilzer M., Roggel F., Gerbes A. L. (2006). Role of Kupffer cells in host defense and liver disease. Liver Int. 10, 1175–1186. [DOI] [PubMed] [Google Scholar]
- Cai P., König R., Boor P. J., Kondraganti S., Kaphalia B. S., Khan M. F., Ansari G. A. (2008). Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 228, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yao W., Xia H., Jin Y., Li X., Cai J., Hei Z. (2015). Elevation of HO-1 expression mitigates intestinal ischemia-reperfusion injury and restores tight junction function in a rat liver transplantation model. Oxid. Med. Cell. Longev. 986075, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W. A., Jinot J., Scott C. S., Makris S. L., Cooper G. S., Dzubow R. C., Bale A. S., Evans M. V., Guyton K. Z., Keshava N., et al. (2013). Human health effects of trichloroethylene: Key findings and scientific issues. Environ. Health Perspect. 121, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. S., Makris S. L., Nietert P. J., Jinot J. (2009). Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Perspect. 117, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. S., Stroehla B. C. (2003). The epidemiology of autoimmune diseases. Autoimmun. Rev. 3, 119–125. [DOI] [PubMed] [Google Scholar]
- Córdova E. J., Velázquez-Cruz R., Centeno F., Baca V., Orozco L. (2010). The Nrf2 gene variant, -653G/A, is associated with nephritis in childhood-onset systemic lupus erythematosus. Lupus 19, 1237–1242. [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Manda G., Hassan A., Alcaraz M. J., Barbas C., Daiber A., Ghezzi P., León R., López M. G., Oliva B., et al. (2018). Transcription factor Nrf2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 70, 348–383. [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Rojo A. I., Wells G., Hayes J. D., Cousin S. P., Rumsey W. L., Attucks O. C., Franklin S., Levonen A. L., Kensler T. W., et al. (2019). Therapeutic targeting of the Nrf2 and Keap1 partnership in chronic diseases. Nat. Rev. Drug Discov. 4, 295–317. [DOI] [PubMed] [Google Scholar]
- Flindt-Hansen H., Isager H. (1987). Scleroderma after occupational exposure to trichloroethylene and trichloroethane. Acta Derm. Venereol. 67, 263–264. [PubMed] [Google Scholar]
- Garabrant D. H., Lacey J. V., Laing T. J., Gillespie B. W., Mayes M. D., Cooper B. C., Schottenfeld D. (2003). Scleroderma and solvent exposure among women. Am. J. Epidemiol. 157, 493–500. [DOI] [PubMed] [Google Scholar]
- Geisel J., Brück J., Glocova I., Dengler K., Sinnberg T., Rothfuss O., Walter M., Schulze-Osthoff K., Röcken M., Ghoreschi K. (2014). Sulforaphane protects from T cell-mediated autoimmune disease by inhibition of IL-23 and IL-12 in dendritic cells. J. Immunol. 192, 3530–3539. [DOI] [PubMed] [Google Scholar]
- Gilbert K. M., Przybyla B., Pumford N. R., Han T., Fuscoe J., Schnackenberg L. K., Holland R. D., Doss J. C., Macmillan-Crow L. A., Blossom S. J. (2009). Delineating liver events in trichloroethylene-induced autoimmune hepatitis. Chem. Res. Toxicol. 22, 626–632. [DOI] [PubMed] [Google Scholar]
- Gilbert K. M., Reisfeld B., Zurlinden T. J., Kreps M. N., Erickson S. W., Blossom S. J. (2014). Modeling toxicodynamic effects of trichloroethylene on liver in mouse model of autoimmune hepatitis. Toxicol. Appl. Pharmacol. 279, 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gist G. L., Burg J. R. (1995). Trichloroethylene—A review of the literature from a health effects perspective. Toxicol. Ind. Health 11, 253–307. [DOI] [PubMed] [Google Scholar]
- Greaney A. J., Maier N. K., Leppla S. H., Moayeri M. (2016). Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 99, 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Beltrán C. E., Calderón-Oliver M., Pedraza-Chaverri J., Chirino Y. I. (2012). Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 64, 503–508. [DOI] [PubMed] [Google Scholar]
- He X., Lin G. X., Chen M. G., Zhang J. X., Ma Q. (2007). Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol. Sci. 98, 298–309. [DOI] [PubMed] [Google Scholar]
- Hu C., Eggler A. L., Mesecar A. D., van Breemen R. B. (2011). Modification of keap1 cysteine residues by sulforaphane. Chem. Res. Toxicol. 24, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K. (2004). Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 36, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Amirahmadi S., Ward C., Fabry Z., Johnson J. A. (2010). The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol. Sci. 114, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar J. W., Niture S. K., Jaiswal A. K. (2009). Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 47, 1304–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavian N., Mehlal S., Jeljeli M., Saidu N. E. B., Nicco C., Cerles O., Chouzenoux S., Cauvet A., Camus C., Ait-Djoudi M., et al. (2018). The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 9, 1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. F., Kaphalia B. S., Ansari G. A. (1997). Time-dependent autoimmune response of dichloroacetyl chloride in female MRL +/+ mice. Immunopharmacol. Immunotoxicol. 19, 265–277. [DOI] [PubMed] [Google Scholar]
- Khan M. F., Kaphalia B. S., Prabhakar B. S., Kanz M. F., Ansari G. A. (1995). Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 134, 155–160. [DOI] [PubMed] [Google Scholar]
- Khan M. F., Wang G. (2018). Environmental agents, oxidative stress and autoimmunity. Curr. Opin. Toxicol. 7, 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. F., Wu X., Ansari G. A. (2001). Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: Possible role of lipid peroxidation in autoimmunity. Toxicol. Appl. Pharmacol. 170, 88–92. [DOI] [PubMed] [Google Scholar]
- Kim J., Cha Y. N., Surh Y. J. (2010). A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 690, 12–23. [DOI] [PubMed] [Google Scholar]
- Kondraganti S., König R., Boor P. J., Khan S., Kaphalia B. S., Khan M. K., Ansari G. A. (2012). Mechanistic evaluation of trichloroethene-mediated autoimmune hepatitis-like disease in female MRL+/+ mice. Open Toxicol. J. 5, 1–10. [Google Scholar]
- Lash L. H., Putt D. A., Parker J. C. (2006). Metabolism and tissue distribution of orally administered trichloroethylene in male and female rats: Identification of glutathione- and cytochrome P-450-derived metabolites in liver, kidney, blood, and urine. J. Toxicol. Environ. Health A 69, 1285–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Khor T. O., Xu C., Shen G., Jeong W. S., Yu S., Kong A. N. (2008). Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 76, 1485–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Jahraus B., Balta E., Ziegler J. D., Hübner K., Blank N., Niesler B., Wabnitz G. H., Samstag Y. (2018). Sulforaphane inhibits inflammatory responses of primary human T-cells by increasing ROS and depleting glutathione. Front. Immunol. 9, 2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. T., Lin Z. M., He S. J., Zuo J. P. (2019). Heme oxygenase-1 as a potential therapeutic target in rheumatic diseases. Life Sci. 218, 205–212. [DOI] [PubMed] [Google Scholar]
- Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73, 3221–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. F., Shen X. Y., Lio C. K., Dai Y., Cheng C. S., Liu J. X., Yao Y. D., Yu Y., Xie Y., Luo P., et al. (2018). Activation of Nrf2/HO-1 pathway by nardochinoid C inhibits inflammation and oxidative stress in lipopolysaccharide-stimulated macrophages. Front. Pharmacol. 9, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. (2013). Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloy R. A., Rogers S., Caldon C. E., Lorca T., Castro A., Burgess A. (2014). Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlem A., Hagberg C. E., Muhl L., Eriksson U., Falkevall A. (2013). Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8, 1149–1154. [DOI] [PubMed] [Google Scholar]
- Pan H., Wang H., Zhu L., Mao L., Qiao L., Su X. (2011). Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem. Res. 36, 2434–2441. [DOI] [PubMed] [Google Scholar]
- Patel B., Mann G. E., Chapple S. (2018). Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic. Biol. Med. 122, 150–160. [DOI] [PubMed] [Google Scholar]
- Singh P., Sharma R., McElhanon K., Allen C. D., Megyesi J. K., Beneš H., Singh S. P. (2015). Sulforaphane protects the heart from doxorubicin-induced toxicity. Free Radic. Biol. Med. 86, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Cai P., Ansari G. A., Khan M. F. (2007). Oxidative and nitrosative stress in trichloroethene-mediated autoimmune response. Toxicology 229, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., König R., Ansari G. A., Khan M. F. (2008). Lipid peroxidation-derived aldehyde-protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic. Biol. Med. 44, 1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Ma H., Wang J., Khan M. F. (2019). Contribution of poly(ADP-ribose)polymerase-1 activation and apoptosis in trichloroethene-mediated autoimmunity. Toxicol. Appl. Pharmacol. 362, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wakamiya M., Wang J., Ansari G. A., Khan M. F. (2015). iNOS null MRL+/+ mice show attenuation of trichloroethene-mediated autoimmunity: Contribution of reactive nitrogen species and lipid-derived reactive aldehydes. Free Radic. Biol. Med. 89, 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wang J., Fan X., Ansari G. A., Khan M. F. (2012). Protein adducts of malondialdehyde and 4-hydroxynonenal contribute to trichloroethene-mediated autoimmunity via activating Th17 cells: Dose- and time-response studies in female MRL+/+ mice. Toxicology 292, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wang J., Luo X., Ansari G. A., Khan M. F. (2014). Nitrosative stress and nitrated proteins in trichloroethene-mediated autoimmunity. PLoS One 9, e98660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wang J., Ma H., Khan M. F. (2009). Increased nitration and carbonylation of proteins in MRL+/+ mice exposed to trichloroethene: Potential role of protein oxidation in autoimmunity. Toxicol. Appl. Pharmacol. 237, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wang G., Ansari G. A. S., Khan M. F. (2018). Trichloroethene metabolite dichloroacetyl chloride induces apoptosis and compromises phagocytosis in Kupffer cells: Activation of inflammasome and MAPKs. PLoS One 13, e0210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardyn J. D., Ponsford A. H., Sanderson C. M. (2015). Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 43, 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh K., Itoh K., Enomoto A., Hirayama A., Yamaguchi N., Kobayashi M., Morito N., Koyama A., Yamamoto M., Takahashi S. (2001). Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 60, 1343–1353. [DOI] [PubMed] [Google Scholar]
- Zagorski J. W., Maser T. P., Liby K. T., Rockwell C. E. (2017). Nrf2-dependent and -independent effects of tert-butylhydroquinone, CDDO-Im, and H2O2 in human Jurkat T cells as determined by CRISPR/Cas9 gene editing. J. Pharmacol. Exp. Ther. 361, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorski J. W., Turley A. E., Dover H. E., VanDenBerg K. R., Compton J. R., Rockwell C. E. (2013). The Nrf2 activator, tBHQ, differentially affects early events following stimulation of Jurkat cells. Toxicol. Sci. 136, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. D., Zhang F., Shen G., Li Y. B., Li Y. H., Jing H. R., Ma L. F., Yao J. H., Tian X. F. (2010). Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J. Gastroenterol. 16, 3002–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.