Abstract
Introduction:
Although antidepressants are well known to cause sexual side effects in adults, the long-term effects of antidepressant use during development on adult sexual function is unknown.
Aim:
To explore differences in sexual desire and sexual behavior between adults who did vs did not use antidepressants during childhood or adolescence.
Methods:
An online survey of 610 young adults (66% women) assessed childhood and current mental health and use of antidepressants and other psychiatric medications before the age of 16 years and currently, partnered and solitary sexual desire, and frequency of masturbation and partnered sexual activity. Antidepressants were coded into either selective serotonin reuptake inhibitors (SSRIs) or non-SSRI antidepressants.
Main Outcome Measure:
Scores on the Sexual Desire Inventory, and self-reported frequency of masturbation and partnered sexual activity.
Results:
For women, childhood SSRI use was associated with significantly lower solitary sexual desire, desire for an attractive other, and frequency of masturbation. This was true even when controlling for childhood mental health concerns, current mental health, and current antidepressant use. However, there was no effect of childhood SSRI use on women’s partnered sexual desire or partnered sexual activity. There was no significant effect of childhood antidepressant use on men’s sexual desire or masturbation. However, in men, childhood use of non-SSRI antidepressants was associated with significantly higher frequency of partnered sexual activity. Childhood use of non-SSRI antidepressants, or non-antidepressant psychiatric medication, was not associated with adult sexual desire or behavior in either women or men.
Clinical Implications:
It is possible that SSRI use during childhood interrupts the normal development of sexual reward systems, which may be a risk factor for sexual desire dysfunction in adult women.
Strengths & Limitations:
Strengths include a large sample, use of attention checks and validated measures, and careful assessment of childhood mental health history; however, generalizability is limited by a predominantly white, young adult sample. These data are cross-sectional, and therefore, causal explanations for the association between childhood SSRI use and adult sexual well-being should be considered preliminary, warranting replication.
Conclusion:
These findings point to a critical need for well-controlled, prospective research on possible long-term effects of antidepressant use, particularly SSRI use, on the development of adult sexual well-being.
Keywords: Antidepressants, Sexual Function, Sexual Side Effects, Desire, Developmental
INTRODUCTION
It is well known that antidepressant use is associated with impairments of sexual functioning in both men and women, including negative effects on sexual desire above and beyond the negative effects of poor mental health.1 Sexual side effects are significant clinical issues because they contribute to difficulties with medication adherence2 and cause distress to the patient and their partners.3 Although antidepressants can impact all aspects of sexual function—from desire, to arousal and orgasm, to sexual satisfaction and pleasure—their effects on desire and pleasure are of particular importance in women as these are the most common sexual issues for which women seek clinical care4 and the most likely to cause significant distress.5,6 Of the antidepressant medications that are commonly used as monotherapies, serotonergic medications such as selective serotonin reuptake inhibitors (SSRIs) appear to have the most negative effects on sexual desire.7
Moreover, the negative effects of antidepressant use on sexual function can continue even after discontinuation,8 suggesting that these medications may have long-term effects that alter the structure or function of neural systems important for sexual functioning. One possibility is a very extended withdrawal effect; however, this would not explain why postdiscontinuation sexual side effects can persist even if the patient resumes the antidepressant treatment.8 Another possibility is that antidepressant medications may alter brain circuitry in areas relevant for sexual desire, such as sexual motivation or reward processing networks.9 Even in adults, there is evidence for significant neuroplasticity in reward networks, which contributes to the lasting therapeutic action of antidepressants after discontinuation 10-12 but also potentially to lasting sexual effects as well. Neuroplasticity is substantially higher during development, which raises the question: what is the effect of antidepressant use on the development of neural systems that are important to sexual functioning? The present study explored this question in a large, cross-sectional retrospective recall survey of childhood mental health and antidepressant use, and adult sexual desire.
The vast majority of research on antidepressant sexual effects has been conducted in adults, despite increasing use of antidepressant therapy in children and adolescents.13 This may be due to an assumption that sexuality is not important to young people, despite empirical evidence that adolescents do experience lack of sexual response as distressing.14 If young people’s sexual functioning is impaired during adolescence, this may interfere with their ability to form their first sexual relationships and explore their sexual preferences. A consistent principle of neurodevelopment is “if you don’t use it, you lose it:” if young people are impaired during their attempts to engage in developmentally appropriate sexual experimentation, they may miss critical windows for shaping important neural areas relevant to sexual function. Moreover, research in animal models has suggested that the conditions under which an animal has its first sexual experiences can form a template that shapes its subsequent sexual development, and that changing those conditions later on can significantly impair adult sexual function.15-17
Even putting aside the cultural taboos against adolescents engaging in sexual activity, there are important questions about the possible long-term effects of antidepressant use on sexual functioning when these young people grow into adults. There is evidence in animal models that exposure to antidepressants during development appears to suppress the normal development of adult sexual behavior in both male and female rodents, long after the antidepressant has been stopped18,19 Female rodents treated with antidepressants during development show not only delayed and disorganized estrous cycling (an analog of menstrual cycling20) but also significantly reduced sexual receptivity in adulthood.21
To date, there is no such data available in humans, raising an important issue for informed consent. If antidepressant use during childhood or adolescence can cause long-term changes in the developing brain that reduces adult sexual function—potentially in ways that would be difficult or impossible to reverse—then patients and their families must weigh the possible benefits of improved mental health against the possible risks of loss of adult sexuality. That risk-benefit analysis will be different for every family and will depend on their individual circumstances and preferences—but to make a truly informed decision, we must have good data as to the likelihood of risks. The exploratory analysis reported here is a first step toward addressing this informed consent issue and is presented to support a call for more research on the long-term effects of childhood antidepressant use on adult sexual desire and sexual functioning more broadly.
MATERIALS AND METHODS
The present study is drawn from a large online survey of young adults. Participants were drawn from the psychology participant pools at the University of North Carolina, Charlotte and the University of Nebraska, Lincoln, and from social media and online bulletin boards such as Craigslist. All participants provided informed consent and were compensated with either credit toward research requirements or entry into a gift card raffle. Participants who failed attention checks or were missing data due to survey attrition were removed from further analysis. The final data set presented here includes 610 participants (66% female), with an average age of 20.05 years (SD = 3.26); most participants indicated either white (69%) or black (19%) race/ethnicity. In addition to the variables mentioned in the following section, the survey assessed a variety of other constructs presented elsewhere (eg, sexual self-schema22). Participants reporting female sex/gender also reported on their lifetime use of hormonal contraceptives, as some research suggests hormonal medications may moderate the negative effects of antidepressants on sexual functioning.23 As analyses investigating this subfactor are only relevant for participants reporting female sex/gender, they are presented in Supplementary Appendix A.
Mental Health History
Participants completed a screening battery that assessed for history of symptoms of the most common mood and anxiety disorders (eg, major depressive disorder; generalized anxiety disorder). For each condition, participants were asked if they had ever experienced those symptoms and if so, how long and at what age(s). This provided a very conservative estimate for possible childhood mental health problems for which antidepressants would be prescribed. From these data, the following codes were derived: no history of mental health concerns (40%); may have met criteria for mood or anxiety disorder before the age of 16 years (44%); and may have met criteria for mood or anxiety disorder after the age of 16 years (15%).
Antidepressant Use
Participants were presented with a list of the 48 most commonly prescribed psychiatric medications and asked if they had ever used any of these and at what age(s). These data were coded for use of selective serotonin reuptake inhibitors (SSRIs, eg, fluoxetine); non-SSRI antidepressants (eg, duloxetine); and other psychiatric medications (eg, antipsychotics, stimulants, sedatives). This was further broken down into use of SSRIs before the age of 16 years (n = 39), use of non-SSRI antidepressants before the age of 16 years (n = 13), current antidepressant use (n = 38), use of other psychiatric medications before the age of 16 years (n = 43), and current other psychiatric medication use (n = 38). Note that current and past use were not exclusive categories, and antidepressant and other psychiatric medication use were also not exclusive.
Sexual Desire and Behavior
Participants completed the Sexual Desire Inventory.24 This well-validated and widely used measure has 3 subscales supported by factor analysis: desire for an established sexual or romantic partner, desire for solitary sexual activity (masturbation), and desire for an attractive potential partner.25 Participants also indicated if they were in a sexually active relationship, the average number of times per month that they engaged in masturbation, and (for partnered participants) frequency of partnered sexual activity.
Analytic Plan
Group-wise differences in sexual desire variables were assessed using general linear models, one for each sex/gender, controlling for participants’ current age, history of childhood mental health concerns and current mental health, and current antidepressant use. As a robustness check, these models were run with and without controlling for history of other psychiatric medication use (both childhood and current); however, as this did not change the direction or significance of any effect, the models reported in the following section do not include these variables.
RESULTS
The effect of childhood antidepressant use was not significant in predicting adult men’s sexual desire or frequency of masturbation (all Ps > .05). In men, the use of non-SSRI antidepressants before the age of 16 years was associated with significantly higher levels of partnered sexual activity (F(1, 186) = 6.93, P = .009); however, this finding should be treated with caution as there was a very small number of men reporting non-SSRI antidepressant use before the age of 16 years (see Table 1).
Table 1.
Demographics and medication use patterns by sex/gender
| Demographic variables | Men (n = 209) | Women (n = 401) | Total (N = 610) |
|---|---|---|---|
| Age at time of taking survey, M (SD) | 20.05 (3.24) | 20.05 (3.28) | 20.05 (3.27) |
| Race/ethnicity, n (%) | |||
| White | 138 (66) | 232 (58) | 370 (61) |
| Black | 27 (13) | 68 (17) | 95 (16) |
| Latino/a | 15 (7) | 24 (6) | 39 (6) |
| Asian | 14 (7) | 28 (7) | 42 (7) |
| Multiracial/other | 13 (6) | 42 (10) | 55 (9) |
| Sexual orientation, n (%) | |||
| Exclusively heterosexual | 163 (78) | 257 (64) | 420 (69) |
| Mostly heterosexual | 23 (11) | 60 (15) | 83 (14) |
| Bisexual | 12 (6) | 56 (14) | 68 (11) |
| Mostly gay/lesbian or exclusively gay/lesbian | 9 (4) | 16 (4) | 25 (4) |
| Other | 2 (1) | 12 (3) | 14 (2) |
| Partnership status, n (%) | |||
| In a sexually active relationship | 67 (32) | 199 (50) | 266 (44) |
| Not in a sexually active relationship | 134 (64) | 189 (47) | 323 (53) |
| Data missing | 8 (4) | 13 (3) | |
| Mental health status, n (%) | |||
| No history of anxiety or depression | 104 (50) | 143 (36) | 247 (40) |
| History of anxiety and/or depression starting before the age of 16 years | 76 (36) | 193 (48) | 269 (44) |
| History of anxiety and/or depression starting after the age of 16 years | 29 (14) | 65 (16) | 94 (15) |
| Medication use patterns before the age of 16 years, n (%) | |||
| Selective serotonin reuptake inhibitor (SSRI) | 5 (2) | 34 (8) | 39 (6) |
| Non-SSRI antidepressant | 1 (0) | 12 (3) | 13 (2) |
| Stimulant | 7 (3) | 13 (3) | 20 (3) |
| Mood stabilizer | 1 (0) | 3 (1) | 4 (1) |
| Antipsychotic | 2 (1) | 5 (1) | 7 (1) |
| Sedative/hypnotic | 6 (3) | 15 (4) | 21 (3) |
| Other psychiatric medication (eg, antiepileptics) | 0 (0) | 0 (0) | 0 (0) |
| Medication use patterns after the age of 16 years, n (%) | |||
| Selective serotonin reuptake inhibitor (SSRI) | 13 (6) | 74 (18) | 87 (14) |
| Non-SSRI antidepressant | 3 (1) | 27 (7) | 30 (5) |
| Stimulant | 21 (10) | 32 (8) | 53 (9) |
| Mood stabilizer | 3 (1) | 11 (3) | 14 (2) |
| Antipsychotic | 2 (1) | 11 (3) | 13 (2) |
| Sedative/hypnotic | 22 (11) | 42 (10) | 64 (10) |
| Other psychiatric medication (eg, antiepileptics) | 4 (2) | 7 (2) | 11 (2) |
M = mean.
Medication use before and after the age of 16 years were coded separately and were not exclusive categories.
In women, there was a significant effect of childhood SSRI use on sexual desire among adult women (F(3, 560) = 4.30, P = .005). Specifically, women who used SSRIs before the age of 16 years reported significantly lower solitary sexual desire (F(1, 568) = 5.57, P = .019) and lower desire for an attractive other (F(1, 568) = 5.48, P = .020). Sexual desire for a partner was similar among women with and without a childhood SSRI use history (F(1, 568) = 0.643, P = .423; see Figure 1). Women who used antidepressants before the age of 16 years reported significantly lower rates of masturbation (F(1, 568) = 8.14, P = .005) but not partnered sexual activity (F(1, 568) = 0.79, P = .374). In contrast, there were no significant effects of childhood use of a non-SSRI antidepressant on any sexuality variable in women (all Ps > .05).
Figure 1.
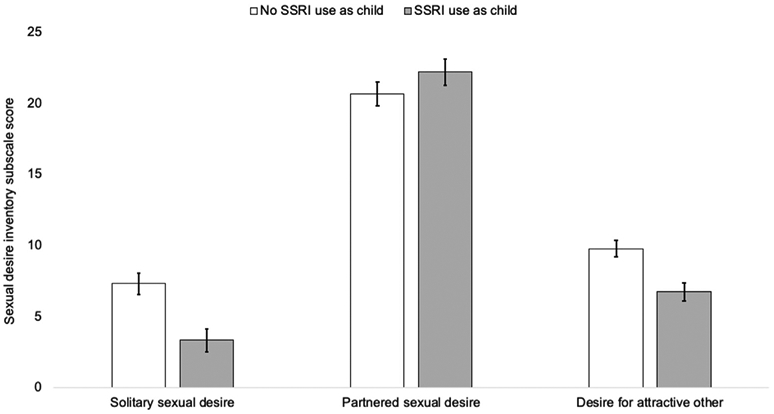
The effect of childhood selective serotonin reuptake inhibitor (SSRI) use on women's adult sexual desire, controlling for childhood and current mental health, current age, and current antidepressant use. Error bars indicate ± 1 SE. SE = standard error.
DISCUSSION
These findings suggest that childhood SSRI use may impair the development of women’s solitary sexual desire, above and beyond the deleterious effects of childhood or adult mental health concerns, or adult medication use. In other words, women who reported use of SSRIs during development had lower solitary sexual desire than other women who reported childhood mental health concerns but did not use SSRIs. Similarly, women who reported childhood SSRI use reported lower solitary sexual desire, regardless of whether or not they currently used antidepressants. These findings appeared to be unique to SSRIs as they were not replicated in non-SSRI antidepressants or other types of psychiatric medication use. In addition, these findings appeared to relate only to solitary desire and masturbation, as women’s partnered desire and activity was not related to childhood medication use.
In women, solitary sexual desire is thought to more closely reflect the physiologic underpinnings of sexual reward, separate from a desire for intimacy or emotional closeness.26 As such, these findings may reflect a specific effect of SSRI use on the development of sexual motivation systems, but not circuits related to interest in close relationships. Exposure to serotonergic medications during development has been shown to alter function of the ventral palladium, raphe nucleus, amygdala, and ventromedial hypothalamus, all areas important for sexual motivation and reward in women.27-29 In contrast, developmental exposure to non-SSRI antidepressants does not appear to hamper reward processing in these areas to the same extent, and in some cases increases reward activation in adults.30-32
Of relevance for understanding the divide between solitary and partnered sexual desire, SSRIs may have greater effect on reward anticipation than reward consummation33-35 — dulling the interest in seeking sexual rewards without dulling the actual pleasure experienced during sexual stimulation. This may result in more inhibition of spontaneous sexual desire (a baseline level of orientation toward seeking sexual rewards) than responsive desire (interest in sex that arises in response to external cues, such as partner initiation36,37). In other words, developmental SSRI exposure may dampen the intrinsic sexual drive in women, but not alter their sexual responsivity to partner-initiated sexual activity.
Further work may elucidate if these effects are due to the direct action of antidepressants on serotonergic systems relevant to sexual reward or sexual learning,9 or indirect effects (eg, through modulation of endocrine systems38). Further work is also needed to understand why these effects were only observed in women. To the extent that this represents a true sex/gender difference, it is likely that SSRIs cause changes in a combination of sex-specific physiologic factors, which act on behaviors in ways that manifest differently in people with different gender roles. For example, SSRIs appear to dampen sensitivity to tactile sexual stimulation, particularly in women39; it is possible that this lower sensitivity to pleasure during masturbation may interact with cultural taboos against female masturbation40 to disproportionately reduce girls’ solitary desire. Alternatively, SSRIs interact with neuropeptide synthesis to amplify the effects of oxytocin.41 Oxytocin is an important mediator of social bonding and appears to contribute to partnered, but not solitary sexual desire,42 particularly in women taking SSRIs.43 As oxytocin production is greater in women,44 the amplification by SSRIs may disproportionately shift women’s desire from solitary to partnered desire patterns. However, it is also possible that the lack of significant effects observed in men reflects the relatively smaller sample of men and lower power to detect small, but potentially meaningful effects. Regardless, the present study suggests that the relative effects of childhood SSRI use are greater in women.
There are several significant limitations to the current data: the participants were young adults whose sexual and romantic development was arguably not complete. Moreover, the sample was predominantly a convenience sample of undergraduate students, which may not be representative of the larger U.S. population. As this was a retrospective recall study, there was likely considerable variance in the accuracy of participants’ reports. To address this, the present study took a very conservative approach in the definition of variables of interest (eg, coding any evidence of possible childhood mental health disorders as “possible childhood mental health condition;” only coding antidepressant use in participants who reported specific medication types at specific ages, and not including participants who reported some medication use but could not recall which type). Thus, if anything, the observed effects are underestimates of the true longitudinal effect of adolescent antidepressant use on adult sexual function. Finally, there were no data on the length of medication treatment, dose, or number of unique episodes in which they took the same medication, all of which are important factors for future study.
Nevertheless, these data do point to a phenomenon worthy of more detailed study: namely, the possible role of childhood antidepressant use in decreased sexual desire in young adult women. Such future work must take into account not only replication and extension of these effects but also the mechanisms by which such effects may occur: such work is essential for developing better treatment plans. It may be possible to intervene in ways that do not impact the therapeutic efficacy of antidepressant treatment while still preserving typical development of sexual desire, such as use of non-SSRI antidepressants as a first line treatment option. In addition, there have been several studies noting a recent trend of lower sexual interest among teens and young adults45 and greater numbers of young adults identifying asexual orientation (ie, a lifelong lack of sexual desire46). It is worth investigating if the increasing number of antidepressant prescriptions being made to children and adolescents47 is in some way related to these larger national trends.
CONCLUSIONS
The present findings, while neither conclusive nor definitive, suggest the use of antidepressants in childhood may impair the normal development of women’s sexual desire, particularly solitary sexual desire. There is a critical need for prospective, well-controlled research on the effects of childhood and adolescent antidepressant use on the development of adult sexual functioning, so that our young patients and their families can make informed choices when balancing the known deleterious effects of untreated mental health concerns vs possible side effects of antidepressant use on their future development.
Supplementary Material
Acknowledgments
Funding: This research was carried out with support from the University of Nebraska-Lincoln Psychology Department and from the Nebraska Tobacco Settlement Biomedical Research Fund.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jsxm.2019.12.012.
REFERENCES
- [1].Lorenz T, Rullo J, Faubion S Antidepressant-induced female sexual dysfunction. Mayo Clin Proc 2016;91:1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montejo AL, Calama J, Rico-Villademoros F, et al. A real-world study on antidepressant-associated sexual dysfunction in 2144 outpatients: The SALSEX I Study. Arch Sex Behav 2019;48:923–933. [DOI] [PubMed] [Google Scholar]
- 3.Montejo AL, Montejo L, Baldwin DS. The impact of severe mental disorders and psychotropic medications on sexual health and its implications for clinical management. World Psychiatry 2018;17:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clayton AH, Goldstein I, Kim NN, et al. The International Society for the Study of Women’s Sexual Health process of care for management of hypoactive sexual desire disorder in women. Mayo Clin Proc 2018;93:467–487. [DOI] [PubMed] [Google Scholar]
- 5.Worsley R, Bell RJ, Gartoulla P, et al. Prevalence and predictors of low sexual desire, sexually related personal distress, and hypoactive sexual desire dysfunction in a community-based sample of midlife women. J Sex Med 2017;14:675–686. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson KR, Meston CM. Why is impaired sexual function distressing to women? The primacy of pleasure in female sexual dysfunction. J Sex Med 2015;12:728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serretti A, Chiesa A. Sexual side effects of pharmacological treatment of psychiatric diseases. Clin Pharmacol Ther 2011;89:142–147. [DOI] [PubMed] [Google Scholar]
- 8.Bala A, Nguyen HMT, Hellstrom WJ. Post-SSRI sexual dysfunction: A literature review. Sex Med Rev 2018;6:29–34. [DOI] [PubMed] [Google Scholar]
- 9.Pfaus JG. Reviews: Pathways of sexual desire. J Sex Med 2009;6:1506–1533. [DOI] [PubMed] [Google Scholar]
- 10.Tardito D, Perez J, Tiraboschi E, et al. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: A critical overview. Pharmacol Rev 2006;58:115–134. [DOI] [PubMed] [Google Scholar]
- 11.Hayley S, Litteljohn D Neuroplasticity and the next wave of antidepressant strategies. Front Cell Neurosci 2013;7:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björkholm C, Monteggia LM. BDNF–a key transducer of antidepressant effects. Neuropharmacology 2016;102:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann CJ, Aagaard L, Burcu M, et al. Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005–2012. Eur Neuropsychopharmacol 2016;26:411–419. [DOI] [PubMed] [Google Scholar]
- 14.O’Sullivan LF, Byers ES, Brotto LA, et al. A longitudinal study of problems in sexual functioning and related sexual distress among middle to late adolescents. J Adolesc Health 2016;59:318–324. [DOI] [PubMed] [Google Scholar]
- 15.Quintana GR, Guizar A, Rassi S, et al. First sexual experiences determine the development of conditioned ejaculatory preference in male rats. Learn Mem 2018;25:522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quintana GR, Jackson M, Nasr M, et al. Effect of CS preexposure on the conditioned ejaculatory preference of the male rat: behavioral analyses and neural correlates. Learn Mem 2018;25:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintana GR, Desbiens S, Marceau S, et al. Conditioned partner preference in male and female rats for a somatosensory cue. Behav Neurosci 2019;133:188. [DOI] [PubMed] [Google Scholar]
- 18.Iñiguez SD, Warren BL, Bolaños-Guzmán CA. Short-and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry 2010;67:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayen I, Steinbusch HW, Charlier TD, et al. Developmental fluoxetine exposure facilitates sexual behavior in female offspring. Psychopharmacology (Berl) 2014;231:123–133. [DOI] [PubMed] [Google Scholar]
- 20.dos Santos AH, Vieira ML, de Azevedo Camin N, et al. In utero and lactational exposure to fluoxetine delays puberty onset in female rats offspring. Reprod Toxicol 2016;62:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Molina-Jiménez T, Limón-Morales O, Bonilla-Jaime H Early postnatal treatment with clomipramine induces female sexual behavior and estrous cycle impairment. Pharmacol Biochem Behav 2018;166:27–34. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz TK. Brief report: Sexual wellbeing in heterosexual, mostly heterosexual, and bisexually attracted men and women. Int J Sex Health 2019;31:339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casado-Espada NM, de Alarcón R, de la Iglesia-Larrad JI, et al. Hormonal contraceptives, female sexual dysfunction, and managing strategies: A review. J Clin Med 2019;8:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spector IP, Carey MP, Steinberg L The sexual desire inventory: Development, factor structure, and evidence of reliability. J Sex Marital Ther 1996;22:175–190. [DOI] [PubMed] [Google Scholar]
- 25.Moyano N, Vallejo-Medina P, Sierra JC. Sexual desire inventory: Two or three dimensions? J Sex Res 2017;54:105–116. [DOI] [PubMed] [Google Scholar]
- 26.van Anders S Testosterone and sexual desire in healthy women and men. Arch Sex Behav 2012;41:1471–1484. [DOI] [PubMed] [Google Scholar]
- 27.DiBenedictis BT, Cheung HK, Nussbaum ER, et al. Involvement of ventral pallidal vasopressin in the sex-specific regulation of sociosexual motivation in rats. Psychoneuroendocrinology 2020;111:104462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudzinskas SA, Williams KM, Mong JA, et al. Sex, drugs, and the medial amygdala: A model of enhanced sexual motivation in the female rat. Front Behav Neurosci 2019;13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sano K, Morimoto C, Nataka M, et al. The role of estrogen receptor β in the Dorsal raphe nucleus on the expression of female sexual behavior in c57Bl/6J mice. Front Endocrinol (Lausanne) 2018;9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su S-W, Cherng C, Lin YC, et al. Prenatal exposure of bupropion may enhance agitation, anxiety responses, and sensitivity to cocaine effects in adult mice. Chin J Physiol 2007;50:1–8. [PubMed] [Google Scholar]
- 31.Jeffery E, Nomme K, Deane T, et al. Investigating the role of an inquiry-based biology lab course on student attitudes and views toward science. CBE–Life Sci Education 2016;15:ar61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abler B, Seeringer A, Hartmann A, et al. Neural correlates of antidepressant-related sexual dysfunction: A placebocontrolled fMRI study on healthy males under subchronic paroxetine and bupropion. Neuropsychopharmacology 2011; 36:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkhouse KL, Gorka SM, Klumpp H, et al. Neural responsiveness to reward as an index of depressive symptom change following cognitive-behavioral therapy and selective serotonin reuptake inhibitor treatment. J Clin Psychiatry 2018; 79:17m11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macoveanu J Serotonergic modulation of reward and punishment: evidence from pharmacological fMRI studies. Brain Res 2014;1556:19–27. [DOI] [PubMed] [Google Scholar]
- 35.Eshel N, Roiser JP. Reward and punishment processing in depression. Biol Psychiatry 2010;68:118–124. [DOI] [PubMed] [Google Scholar]
- 36.Basson R The female sexual response: A different model. J Sex Marital Ther 2000;26:51–65. [DOI] [PubMed] [Google Scholar]
- 37.Chivers ML, Brotto LA. Controversies of women’s sexual arousal and desire. Eur Psychol 2017;22:5–26. [Google Scholar]
- 38.Müller JC, Imazaki PH, Boareto AC, et al. In vivo and in vitro estrogenic activity of the antidepressant fluoxetine. Reprod Toxicol 2012;34:80–85. [DOI] [PubMed] [Google Scholar]
- 39.Frohlich P, Meston CM. Fluoxetine-induced changes in tactile sensation and sexual functioning among clinically depressed women. J Sex Marital Ther 2005;31:113–128. [DOI] [PubMed] [Google Scholar]
- 40.Kaestle CE, Allen KR. The role of masturbation in healthy sexual development: Perceptions of young adults. Arch Sex Behav 2011;40:983–994. [DOI] [PubMed] [Google Scholar]
- 41.Gołyszny M, Obuchowicz E. Are neuropeptides relevant for the mechanism of action of SSRIs? Neuropeptides 2019;75:1–17. [DOI] [PubMed] [Google Scholar]
- 42.Behnia B, Heinrichs M, Bergmann W, et al. Differential effects of intranasal oxytocin on sexual experiences and partner interactions in couples. Horm Behav 2014;65:308–318. [DOI] [PubMed] [Google Scholar]
- 43.Abbasinazari M, Heidari-Kord M, Mazaheri-Meybodi A, et al. Plasma oxytocin level and sexual dysfunction in depressed women treated by either fluoxetine or citalopram: A pilot clinical trial. Iranian J Pharm Res 2018;17:408. [PMC free article] [PubMed] [Google Scholar]
- 44.Marazziti D, Baroni S, Mucci F, et al. Sex-related differences in plasma oxytocin levels in humans. Clin Pract Epidemiol Ment Health 2019;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kann L, McManus T, Harris WA, et al. Youth risk behavior surveillance–United States, 2017. MMWR Surveill Summ; 2018;67:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brotto LA, Yule M Asexuality: Sexual orientation, paraphilia, sexual dysfunction, or none of the above? Arch Sex Behav 2017;46:619–627. [DOI] [PubMed] [Google Scholar]
- 47.Sarginson J, Webb RT, Stocks SJ, et al. Temporal trends in antidepressant prescribing to children in UK primary care, 2000–2015. J Affect Disord 2017;210:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


