Abstract
Introduction
We aimed to determine the diagnostic accuracy of the prostate cancer antigen 3 (PCA3) test before performing the first biopsy compared with prostate biopsy for the diagnosis of prostate cancer (PCa).
Methods
A systematic search was performed in MEDLINE, EMBASE, CENTRAL, LILACS, reference lists, specialized journals in urology and cancer, and unpublished literature. The population was adults with suspected PCa, and the intervention was the measurement of PCA3 in urine samples for the diagnosis of PCa. The quality of studies was evaluated with the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. The operative characteristics were determined, and a meta-analysis was performed.
Results
Nine studies of diagnostic tests were included based on a cutoff value of 35. The following overall values were obtained: the sensitivity was 0.69 (95% confidence interval [CI] 0.61–0.75); specificity was 0.65 (95% CI 0.553–0.733); the diagnostic odds ratio (DOR) was 4.244 (95% CI 3.487–5.166); and the area under the curve was 0.734 (95% CI 0.674–0.805), with a heterogeneity of 0%.
Conclusions
Urinary PCA3 has an acceptable diagnostic accuracy, aids in the study of patients with suspected PCa, and can be used as a guide for directing the performance of the first prostate biopsy and decreasing unnecessary biopsies.
Introduction
Prostate cancer (PCa) is the second most common type of cancer in the world’s male population. It is estimated that one in seven men will be diagnosed with PCa and one in 38 men will die as a result of the disease.1 The incidence of PCa in high/very high Human Development Index (HDI) is 37.5 age-standardized rate (ASR) per 100 000 compared with a lower data in medium/low HDI, which is 11.4 ASR per 100 000. Regarding the mortality, authors reported 8.0 and 6.3 ASR per 100 000, respectively.1
Since the 1980s, the use of prostate-specific antigen (PSA) has been implemented as an early detection test for PCa.2 However, PSA may be elevated in other benign pathologies, such as prostatitis and prostatic hyperplasia, leading to false positives that require an unnecessary prostate biopsy and may be accompanied by associated complications, as well as over-treatment and increased costs to the healthcare system.2
Given this situation, new diagnostic methods have emerged, such as prostate cancer-specific antigen 3 (PCA3), which is a marker that detects overexpression of the PCA3 gene by molecular techniques. As the name implies, PCA3 is specific for PCa and is expressed only in this disease; it is not affected by benign conditions, as occurs with PSA, thereby decreasing the risk of false positives.3
Although most studies to date have shown the usefulness of PCA3 for the early diagnosis of PCa and how it may contribute to the reduction of unnecessary biopsies to improve survival and quality of life, as well as optimizing the resources of the healthcare system, these studies were performed after a first biopsy.4–6 Importantly, there are no systematic reviews on the use of PCA3 in patients without previous biopsy. The objective of this study was to determine the diagnostic accuracy of the PCA3 test before performing the first biopsy compared to standard prostate biopsy for the diagnosis of PCa.
Methods
This systematic review was performed in compliance with the suggestions of the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) writing recommendations. The protocol was previously published in PROSPERO: CRD42018099528
Inclusion and exclusion criteria
The study population included adults (>18 years) with suspected PCa due to digital rectal examination (DRE) and/or an abnormal PSA and who did not have a previous biopsy. The index test for the diagnosis of PCa was the quantitative measurement of PCA3 in urine samples after prostate massage. The reference standard was prostate biopsy. Studies that included patients with a confirmed diagnosis of PCa and studies of patients with one or more negative prostate biopsies were excluded.
The outcome was the diagnosis of PCa with measures of sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), diagnostic odds ratio (DOR), and area under the curve.
Search strategy
A systematic search of studies of diagnostic tests was performed in the following databases, from their inception to the present time: MEDLINE; EMBASE; Cochrane Controlled Trials Register Center (CENTRAL); and Latin American and Caribbean Literature in Health Sciences (LILACS). Additional studies were also sought in the reference lists of selected articles, abstracts, theses, and conferences of the American Urological Association (AUA) and the European Association of Urology (EAU), as well as in specialized journals in urology and cancer, and in unpublished literature in databases, such as Open Gray, Google Scholar, and www.clinicaltrials.gov.
Study selection and data collection
Researchers performed the initial selection blindly and independently based on the title and abstract. The chosen studies were then reviewed based on the full text, applying the inclusion and exclusion criteria, which was also performed independently by two researchers, reaching the final selection.
The data collection was performed using a standardized format, which included the study design, participants, variables, interventions, comparisons, and results. Researchers confirmed the data entry and verified the data at least twice for accuracy.
Risk of bias
The evaluation of each included study was performed with the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool,7 which considers the risk of bias and applicability ratings. Each of the researchers rated these items. Disparities in the evaluation were resolved by joint review.
Synthesis of results
A 2×2 table was developed to determine the operative characteristics of sensitivity, specificity, LR+, LR−, and DOR, with their respective 95% confidence interval (CI). A meta-analysis was performed, estimating the summary measures of the included studies (sensitivity and specificity) by means of a bivariate random-effects model. For the meta-analysis, five of the nine studies were selected because they used a PCA3 cutoff value of 35, decreasing the risk of heterogeneity.
The model proposed by Rutter and Gatsonis was used for the estimation of summary receiver operating characteristic (SROC) curves for exploratory purposes. It is assumed that the validity and the positivity threshold are random effects, so their variances are estimated by the model.8
Review Manager 5.3 software (RevMan 5.3) was used to summarize the QUADAS-2 rating. R software was used for the estimates of sensitivity and specificity, as well as the generation of forest plots and SROC graphs9 using the metaanalysis of diagnostic accuracy (MADA) package and the descriptive statistics for meta-analysis of diagnostic accuracy (MADAD) function.
The heterogeneity was also assessed with the visual inspection of the forest plots and the SROC, and the heterogeneity was more objectively assessed with the I2 test, considering the interpretation that values of 25%, 50%, and 75% correspond to low, medium, and high levels of heterogeneity, respectively.10
Sensitivity analysis
Additionally, a sensitivity analysis was performed based on the exclusion of each of the included studies and those of smaller sample size.
Analysis by subgroup
A subgroup analysis was intended to be performed, but the data were not sufficient.
Publication bias
Implementation of publication bias was not possible given the small number of studies included in the meta-analysis.
Results
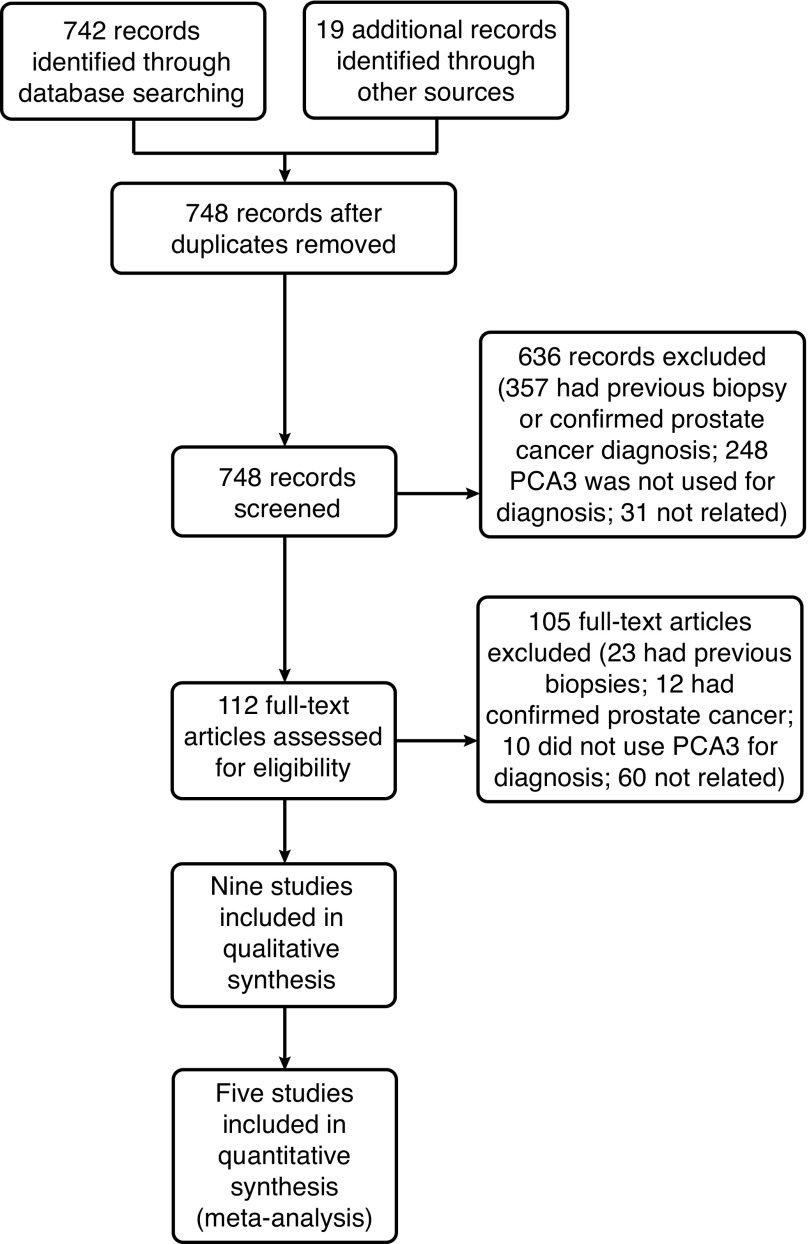
In the initial search, a total of 742 studies were found, which were reviewed by title and abstract. After the initial filter by full text, nine studies met the inclusion criteria: Salami, 201311; De Luca, 201412; Leyten, 201313; Ruffion, 201314; Ferro, 201315; Salagierski, 201316; van Gils, 200717; Dimitriadis, 201318; and Hansen, 201319 (Fig. 1).
Fig. 1.
Flowchart for included studies. PCA3: prostate cancer antigen 3.
The studies were published between 2007 and 2014, with an average of 336 participants per study and an age range between 44 and 87 years. With regard to the methodology, the design of all the studies was a prospective cohort in which patients were directed to have a prostate biopsy because of either an abnormal DRE or elevated PSA. Patients also had a urine sample taken after prostate massage to evaluate the PCA3 levels and to compare them with the results of the biopsy taken later, which was the reference standard in all the studies (Table 1).
Table 1.
Characteristics of the included studies
| Author, year | Country | Number of participants | Age | Design | Biomarker | Reference standard | Cutoff value |
|---|---|---|---|---|---|---|---|
| Salami S et al, 2013 | U.S. | 45 | 56–71 | Prospective cohort | PCA3 and TMPRSS2:ERG | Prostate biopsy | 35 |
| De Luca S et al, 2014 | Italy | 274 | 48–87 | Prospective cohort | PCA3, PHI, and PSA | Prostate biopsy | 35 |
| Leyten G et al, 2013 | Netherlands | 443 | 44–86 | Prospective cohort | PCA3 and TMPRSS2:ERG | Prostate biopsy | 35 |
| Ruffion A et al, 2013 | France | 594 | 58–67 | Prospective cohort | PCA3 and PSA | Prostate biopsy | 21 and 35 |
| Ferro M et al, 2013 | Italy | 300 | 50–73 | Prospective cohort | PCA3, PHI, and PSA | Prostate biopsy | 22 |
| Salagierski M et al, 2013 | Netherlands | 80 | 50–81 | Prospective cohort | PCA3 and PSA | Prostate biopsy | 10 and 35 |
| Hansen J et al, 2013 | Germany and U.S | 692 | 58–69 | Prospective cohort | PCA3 and PSA | Prostate biopsy | 17, 21, 24, and 35 |
| Dimitriadis E et al, 2013 | Greece | 66 | 45–83 | Prospective cohort | PCA3 and TMPRSS2:ERG | Prostate biopsy | 30 |
| Van Gils M et al, 2007 | Netherlands | 534 | 57–71 | Prospective cohort | PCA3 and PSA | Prostate biopsy | 58 |
PCA3: prostate cancer antigen 3; PHI: Prostate Health Index; PSA: prostate-specific antigen; TMPRSS2:ERG: transmembrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog.
Risk of bias assessment
All the included studies were evaluated with a low risk of bias with respect to the selection of patients, index test, and reference standard. However, with regard to the case of flow and timing, most of the studies had an unclear risk because they did not specify the followup time of the patients. One study had a high risk of bias regarding this aspect because it did not mention the reason for the loss of three patients during followup, and one study had a low risk (Fig. 2).
Fig. 2.
Risk of bias assessment (A) across studies; and (B) within studies.
Results of the individual studies
The diagnostic yield for each study showed sensitivity of 0.60–0.93 and specificity of 0.37–0.76. The positive likelihood ratio in all the studies was >1, with values ranging from 1.68–2.48; the LR− values ranged from 0.29–0.53 (Table 2).
Table 2.
Results of the individual studies
| Study | True positives | False positives | False negatives | True negatives | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| De Luca, 2014 | 86 | 6 | 21 | 12 | 0.80 (0.72, 0.87) | 0.67 (0.41, 0.87) |
| Dimitriadis, 2013 | 11 | 17 | 3 | 35 | 0.79 (0.49, 0.95) | 0.67 (0.53, 0.80) |
| Ferro, 2013 | 97 | 116 | 11 | 76 | 0.90 (0.83, 0.95) | 0.40 (0.33, 0.47) |
| Hansen, 2013 | 190 | 90 | 128 | 284 | 0.60 (0.54, 0.65) | 0.76 (0.71, 0.80) |
| Leyten, 2014 | 134 | 98 | 62 | 149 | 0.68 (0.61, 0.75) | 0.60 (0.54, 0.66) |
| Ruffion, 2013 | 175 | 90 | 101 | 228 | 0.63 (0.57, 0.69) | 0.72 (0.66, 0.77) |
| Salagierski, 2013 | 18 | 24 | 6 | 32 | 0.75 (0.53, 0.90) | 0.57 (0.43, 0.70) |
| Salami, 2013 | 14 | 19 | 1 | 11 | 0.93 (0.68, 1.00) | 0.37 (0.20, 0.56) |
| Van Gils, 2007 | 113 | 122 | 61 | 238 | 0.65 (0.57, 0.72) | 0.66 (0.61, 0.71) |
CI: confidence interval.
The meta-analysis was developed with five studies — De Luca, 201412; Leyten, 201313; Ruffion, 201314; Salagierski, 201316; Hanssen, 201319—in which the PCA3 had a cutoff value of 35. A bivariate random-effects model and estimation of SROC curves indicated that the overall sensitivity was 0.69 (95% CI 0.61–0.75), I2= 0%, and that the overall specificity was 0.65 (95% CI 0.553–0.733), I2=0%. For the DOR, the overall result was 4.244 (95% CI 3.487–5.166), I2=0%, and the area under the curve was 0.734 (95% CI 0.674–0.805), I2=0%. Thus, these results demonstrated a good discriminatory capacity of the index test (Figs. 3, 4).
Fig. 3.

Summary receiver operating characteristic curve (bivariate model) for prostate cancer antigen 3 data.
Fig. 4.
Forest plot of diagnostic odds ratio.
Discussion
Summary of the principal findings
With a cutoff value of 35, PCA3 presented an overall sensitivity of 0.69 (95% CI 0.61–0.75) and an overall specificity of 0.65 (95% CI 0.553–0.733). Additionally, the overall DOR was 4.244 (95% CI 3.487–5.166), and the area under the curve was 0.734 (95% CI 0.674–0.805).
Comparison with the literature
Currently, PSA is the marker that guides whether or not to have a prostate biopsy in patients with suspected PCa, but the low specificity of this test has led to the search for new markers, such as PCA3. PCA3 reveals better results, suggesting a greater usefulness for PCA3 in directing patients to prostate biopsy. Due to the better specificity, only those patients who really need this procedure would be referred, thereby reducing biopsies in patients without cancer. Moreover, PSA is affected by benign conditions, such as prostatitis and prostatic hyperplasia, which is not the case with PCA3.
When comparing this meta-analysis with other studies, the overall results were in the range of 50–70% for sensitivity and specificity. Luo et al20 reported a PCA3 cutoff value of 35, a sensitivity of 75%, and a specificity of 57%. Another meta-analysis developed by Xue et al21 found an overall sensitivity of 62% and a specificity of 75%, similar to the values found in the present meta-analysis, with an overall sensitivity and specificity that did not exceed 70%. Regarding the DOR and the values of the area under the curve, Luo et al20 reported values of 4.11 and 0.69, respectively, while Xue et al21 reported values of 5.49 and 0.75, respectively, which are in the range of the present results, indicating a good discriminatory capacity of PCA3.
Strengths and limitations
The strengths of this analysis included the selection of patients, the index test, and the reference standard, as there was a low risk of bias related to these aspects in all the included studies. Moreover, the homogeneity of the studies was 100%, including comparison with the studies available in the literature, which report some degree of heterogeneity. The lack of heterogeneity in the present meta-analysis is a positive aspect that favors the conclusions regarding the diagnostic accuracy of PCA3.
One of the major limitations of the analysis was the flow and timing of the included studies, with risk of unclear bias, as they did not describe the reason for the loss of some patients.
Implications for practice
The results obtained in this meta-analysis are not sufficient to recommend the replacement of prostate biopsy by PCA3 as a reference standard for the diagnosis of PCa; more studies are warranted. However, these results may be a useful guide for directing patients who require this confirmatory test, as PCA3 has few false positives and a better specificity compared to PSA, especially in the gray area range. Adding PCA3 to flow charts for the diagnosis of PCa would optimize the study of these patients, thus avoiding unnecessary prostate biopsies.
Conclusions
Urinary PCA3 with a cutoff value of 35 has an acceptable diagnostic yield, aids in the study of patients with suspected PCa, and can be used as a guide for directing the performance of the first prostate biopsy and decreasing unnecessary biopsies.
Footnotes
Competing interests: The authors report no competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Bray F, Ferlay J, Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ministerio de la Protección Social. [Guía de práctica clínica ( GPC ) para la detección temprana, seguimiento y rehabilitación del cáncer de próstata]. Bogota, Colombia: 2013. [Google Scholar]
- 3.Ruiz-Aragón J, Márquez-Peláez S. [Evaluación del test PCA3 para el diagnóstico de cáncer de próstata: revisión sistemática y metanálisis]. Actas Urológicas Españolas. 2010;34:346–55. doi: 10.1016/j.acuro.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Wei JT, Feng Z, Partin AW, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol. 2014;32:4066–72. doi: 10.1200/JCO.2013.52.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auprich M, Bjartell A, Chun FK, et al. Contemporary role of prostate cancer antigen 3 in the management of prostate cancer. Eur Urol. 2011;60:1045–54. doi: 10.1016/j.eururo.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Groskopf J, Aubin SM, Deras IL, et al. APTIMA PCA3 molecular urine test: Development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089–95. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]
- 7.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Macaskill P, Gatsonis C, Deeks J, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. December. The Cochrane Collaboration; 2010. Chapter 10: Analyzing and presenting results; pp. 1–61. [Google Scholar]
- 9.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. [Google Scholar]
- 10.Sobre I, Manual EL, Manual S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. pp. 1–639. [Google Scholar]
- 11.Salami SS, Schmidt F, Laxman B, et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. 2015;31:213–23. doi: 10.1016/j.urolonc.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca S, Passera R, Bollito E, et al. Comparison of prostate cancer gene 3 score, prostate health index and percentage free prostate-specific antigen for differentiating histological inflammation from prostate cancer and other non-neoplastic alterations of the prostate at initial biopsy. Anticancer Res. 2014;34:7159–65. [PubMed] [Google Scholar]
- 13.Leyten G, Hessels D, Jannink SA, et al. Prospective multicenter evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–42. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Ruffion A, Devonec M, Champetier D, et al. PCA3 and PCA3-based nomograms improve diagnostic accuracy in patients undergoing first prostate biopsy. Int J Mol Sci. 2013;14:17767–80. doi: 10.3390/ijms140917767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferro M, Bruzzese D, Perdonà S, et al. Prostate health index (PHI) and prostate cancer antigen 3 (PCA3) significantly improve prostate cancer detection at initial biopsy in a total PSA range of 2–10 ng/ml. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0067687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salagierski M, Mulders P, Schalken JA. Predicting prostate biopsy outcome using a PCA3-based nomogram in a Polish cohort. Anticancer Res. 2013;33:553–8. [PubMed] [Google Scholar]
- 17.van Gils MP, Hessels D, van Hooij O, et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin Cancer Res. 2007;13:939–43. doi: 10.1158/1078-0432.CCR-06-2679. [DOI] [PubMed] [Google Scholar]
- 18.Dimitriadis E, Kalogeropoulos T, Velaeti S, et al. Study of genetic and epigenetic alterations in urine samples as diagnostic markers for prostate cancer. Anticancer Res. 2013;33:191–8. [PubMed] [Google Scholar]
- 19.Hansen J, Auprich M, Ahyai SA, et al. Initial prostate biopsy: Development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol. 2013;63:201–9. doi: 10.1016/j.eururo.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Gou X, Huang P, et al. The PCA3 test for guiding repeat biopsy of prostate cancer and its cutoff score: A systematic review and meta-analysis. Asian J Androl. 2014;16:487–92. doi: 10.4103/1008-682X.125390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue WJ, Jiang JH, Xu YH. Prostate cancer antigen 3 as a biomarker in the urine for prostate cancer diagnosis: A meta-analysis. J Cancer Res Ther. 2014;10:1–4. doi: 10.4103/0973-1482.145881. [DOI] [PubMed] [Google Scholar]