Abstract
Given the major role of the mitochondrion in cellular homeostasis, dysfunctions of this organelle may lead to several common diseases in humans. Among these, maternal diseases linked to mitochondrial DNA (mtDNA) mutations are of special interest due to the unclear pattern of mitochondrial inheritance. Multiple copies of mtDNA are present in a cell, each encoding for 37 genes essential for mitochondrial function. In cases of mtDNA mutations, mitochondrial malfunctioning relies on mutation load, as mutant and wild-type molecules may co-exist within the cell. Since the mutation load associated with disease manifestation varies for different mutations and tissues, it is hard to predict the progeny phenotype based on mutation load in the progenitor. In addition, poorly understood mechanisms act in the female germline to prevent the accumulation of deleterious mtDNA in the following generations. In this review, we outline basic aspects of mitochondrial inheritance in mammals and how they may lead to maternally-inherited diseases. Furthermore, we discuss potential therapeutic strategies for these diseases, which may be used in the future to prevent their transmission.
Keywords: Oocyte, germline, mitochondrial dynamics, mtDNA, metabolism
Introduction
The mitochondrion gained its deserved reputation in cell biology due to its role as the cellular powerhouse, with most of the adenosine triphosphate (ATP) in eukaryotic cells being supplied by this organelle (Wallace, 2013). However, mitochondria play several functions in the cell that far exceed the role in ATP generation. These are linked with buffering of Ca+2 levels, innate immunity, apoptosis and biogenesis of iron-sulfur clusters (Yasukawa et al., 2009; Naon and Scorrano 2014; Stehling et al., 2014). Moreover, mitochondria closely interact with other organelles such as the endoplasmic reticulum (ER) and regulate several pathways in the cell (de Brito and Scorrano, 2009; Betz et al., 2013; Chen et al., 2014; Carreras-Sureda et al., 2017; Xu et al., 2017). As a result, perturbations in mitochondrial function may dramatically disturb cellular homeostasis, resulting in several common diseases in humans (Bach et al., 2003; Chen et al., 2007, 2010; Schaefer et al., 2008; Misko et al., 2012; Schon et al., 2012; Sebastian et al., 2012; Eschbach et al., 2013; Payne et al., 2013; Schneeberger et al., 2013; Pareyson et al., 2015; Ramírez et al., 2017).
Amongst mitochondria-associated diseases, those primarily linked to mitochondrial DNA (mtDNA) mutations have been a topic of great interest given their severe outcome and unclear pattern of inheritance (Craven et al., 2017). However, mtDNA mutations can also associate with nuclear mutations, leading to common diseases in humans such as cancer, diabetes, Alzheimer, and Parkinson (Wallace 2011; Schon et al., 2012; Stewart and Chinnery 2015). Thereby, recent findings have associated obesity with mitochondrial dysfunction in oocytes and increased risk of metabolic diseases in offspring (Wu et al., 2015; Saben et al., 2016). In mammals, mitochondria are uniparentally transmitted by females (Sutovsky et al., 1999). Thus, maternal mitochondria are replicated during early embryogenesis to colonize somatic and germline tissues (St John, 2019). As a result, mitochondrial abnormalities present in oocytes can be perpetuated and lead to disease in offspring (Payne et al., 2013; Saben et al., 2016; Craven et al., 2017; Wei et al., 2019). In this review, we outline basic aspects of mitochondrial transmission in mammalian germline and how they may lead to maternally inherited diseases. Furthermore, we discuss potential therapeutic strategies for these diseases, which may be used in the future to prevent their transmission.
Basic aspects of mitochondria
Mitochondria are double-membrane organelles with two distinct compartments, the inter-membrane space and the matrix. Most enzymes taking part in oxidative phosphorylation of energetic molecules (i.e., sugars, fats and proteins), including those of the Krebs cycle, are located in the mitochondrial matrix. The energy extracted from these molecules is then used by three (I, III and IV) out of four complexes imbedded in the inner mitochondrial membrane to pump H+ from the matrix to the inter-membrane space. This creates a difference in electric potential (the mitochondrial membrane potential – ΔΨm). In turn, a fifth complex (V) phosphorylates ADP into ATP using the electrochemical energy derived from the H+ return to the matrix.
Mitochondria harbor their own genome, the mtDNA, which in mammals is ~16.5-kb long and encodes for 13 mRNAs, 2 rRNAs, and 22 tRNAs. These genes are essential for ATP synthesis in mitochondria as the 13 mtDNA-encoded proteins play key roles in complexes I, III, IV, and V of the electron transport chain. However, nearly 1,200 different proteins are present in mitochondria (i.e., complexes I to V are composed of ~80 proteins), most of which are encoded in the nucleus, translated in the cytoplasm and imported by mitochondria. Proteins regulating mtDNA replication, transcription and repair are similarly derived from the nucleus. Therefore, although mtDNA-encoded proteins are essential for ATP production in mitochondria, the nucleus exerts a broader role in regulating mitochondrial function (Garesse and Vallejo, 2001; Scarpulla, 2002; Battersby et al., 2003).
Hundreds to thousands of mitochondria are present in each cell (Wassarman and Josefowicz, 1978; Jansen and De Boer, 1998; Motta et al., 2000). These are, albeit, not isolated from each other. Actually, through repeated cycles of fusion and fission, mitochondria exchange membranes, solutes, metabolites, proteins, RNAs, and mtDNAs, resulting in electrically coupled organelles. The balance of fusion to fission also regulates mitochondrial number, morphology, transport, function, and turnover, which is collectively known as mitochondrial dynamics (Mishra and Chan, 2014). Both, fusion- and fission-deficient cells exhibit mitochondrial heterogeneity and dysfunction (Eura et al., 2003; Chen et al., 2003, 2005; Ishihara et al., 2009; Udagawa et al., 2014; Wakai et al., 2014), supporting the importance of these events to mitochondrial health. In keeping with this, fragmentation of the mitochondrial network has been associated with a low bioenergetic state (i.e., in oocytes), while its elongation implies a high bioenergetic yielding, such as that of liver, muscle, and brain (Bach et al., 2003; Zorzano et al., 2015; Schrepfer and Scorrano 2016).
Several proteins regulate mitochondrial fission, with the Dynamin-related protein 1 (DRP1) being the best characterized (Ishihara et al., 2009). DRP1 is a cytosolic protein that is recruited to mitochondria by multiple receptors, including mitochondrial fission factor (MMF), mitochondrial dynamic proteins of 49 kDa (MID49) and 51 kDa (MID51), and fission 1 (FIS1) (Mishra and Chan, 2014; Schrepfer and Scorrano 2016). In turn, the optic atrophy 1 (OPA1) regulates inner membrane fusion and cristae remodeling (Olichon et al., 2002, 2003; Cipolat et al., 2004; Griparic et al., 2004; Pernas and Scorrano 2016), whereas mitofusins 1 (MFN1) and 2 (MFN2) regulate outer membrane fusion (Chen et al., 2003, 2005, 2007, 2010; Ishihara et al., 2004; Schrepfer and Scorrano, 2016). Mitochondrial fusion is initiated by homo and heterotypic interaction of MFN1 and MFN2 from two adjacent organelles (Ishihara et al., 2004; Schrepfer and Scorrano, 2016). Given that MFN2 is present on the ER membrane, it also regulates ER-mitochondria tethering (de Brito and Scorrano, 2008). This connection, known as ER mitochondria-associated membranes (MAMs), has been shown to play an essential role in the regulation of ER, mitochondrial, and cellular functions (Ngoh et al., 2012; Hamasaki et al., 2013; Schneeberger et al., 2013; Muñoz et al., 2014; Carreras-Sureda et al., 2017; Pathak and Trebak, 2018). MFN2 downregulation is associated with decreased expression of subunits of the Krebs cycle and electron transport chain, reduced oxygen consumption, lower ΔΨm, and increased reactive oxygen species (ROS) (Santel and Fuller, 2001; Yasukawa et al., 2009; Ngoh et al., 2012; Wakai et al., 2014; Filadi et al., 2015; Schrepfer and Scorrano, 2016). These effects of MFN2 seem to be more evident in muscle, liver and hypothalamic neurons, tissues in which expression of MFN2 is enhanced (Chen et al., 2007; Chen et al., 2010; Schneeberger et al., 2013; Schrepfer and Scorrano 2016). MFN2 expression has also been inversely linked with ER stress, insulin signaling and diabetes (Bach et al., 2003; Mingrone et al., 2005; Sebastian et al., 2012; Schneeberger et al., 2013; Zorzano et al., 2015; Sarparanta et al., 2017).
Mitochondria in female germ cells
The earliest stages of embryogenesis are characterized by rapid cell division (i.e., cleavage) that gives rise to blastocysts. During these stages, the embryo relies on maternal factors inherited from the oocyte (i.e., mRNAs, proteins and mitochondria), as the embryonic genome is transcriptionally inactive. Also, in agreement with the “embryo silent” hypothesis, mitochondria show low activity during these stages to protect embryonic cells from oxidative damage (Leese, 2012). At the blastocyst stage, increased protein synthesis and blastocoel expansion is accompanied by upregulation of mitochondrial activity in cells that give rise to extraembryonic tissues (i.e., the trophectoderm) (Trimarchi et al., 2000; May-Panloup et al., 2005; Hashimoto et al., 2017; St John, 2019). Activation of mitochondrial function is postponed, however, in the inner cell mass that originates the embryo proper. Mitochondrial architecture and function seem to remain underdeveloped in cells committed with germline specification, and mtDNA replication is only resumed with primordial germ cell (PGC) differentiation (Wassarman and Josefowicz, 1978; Motta et al., 2000; Cree et al., 2008; Wai et al., 2008; St John et al., 2010; Floros et al., 2018; Chiaratti et al., 2018; St John, 2019).
Among the hundreds of cells in the developing fetus, PGCs originate from a few dozen located at the basis of allantois. Yet, after migration to the genital ridge, PGCs proliferate quickly to generate in females millions of oogonia (Leitch et al., 2013). After entering meiosis, these primary oocytes receive a cover layer of somatic pre-granulosa cells, giving rise to primordial follicles still during fetal life. These follicles constitute the ovarian reserve that females carry throughout their reproductive life (Oktem and Urman, 2010). After puberty, the ovary provides an adequate environment for follicle growth and maturation (Clarke, 2017). During this period, the oocyte stockpiles several molecules that are required later during embryogenesis. This includes a ~1,000-fold increase in mitochondria (Jansen and De Boer, 1998; Cree et al., 2008; Wai et al., 2008; St John, 2019), which accounts for the largest mitochondrial content amongst all cells in mammals. In spite of this, mitochondria display several characteristics that suggest they are immature and low functional in oocytes (Arhin et al., 2018). In fact, oocytes lacking the pyruvate dehydrogenase E1 alpha 1 (PDHA1), a key gene required for mitochondrial activity, successfully develop during most part of oogenesis and are ovulated (Johnson et al., 2007). Thus, although mitochondria do play an essential role during the final steps of oocyte development, the “embryo silent” hypothesis likely extends to oogenesis too (Arhin et al., 2018). Accordingly, somatic cells surrounding the oocyte (i.e., cumulus cells) provide the oocyte with several energetic molecules, including amino acids, cholesterol, pyruvate, AMP, and ATP (Su et al., 2007, 2009; Sugiura et al., 2007). Moreover, the adenosine salvage pathway seems to be a key source of ATP, giving it can be generated from abundant amounts of cyclic AMP (cAMP) present in oocytes (Scantland et al., 2014).
If mitochondria are not highly active in oocytes, why are they present in massive amounts before fertilization? This can be, at least, partially explained by downregulation of mitochondrial biogenesis during early embryogenesis; mitochondria are segregated among hundreds of embryonic cells without any increase in number up to the time of embryo implantation (Pikó and Taylor, 1987; Thundathil et al., 2005; Cree et al., 2008; Wai et al., 2008; St John, 2019). Therefore, a threshold number of mitochondria is necessary in oocytes to assure that every embryonic cell will inherit a minimum complement of mitochondria (Chiaratti and Meirelles, 2010; Wai et al., 2010). In keeping with this idea, extensive fragmentation of the mitochondrial network in oocytes allows for efficient segregation of mitochondria during early embryogenesis (Ashley et al., 1989; Cree et al., 2008; Ferreira et al., 2010; Lee et al., 2012b). Upregulation of pro-fission proteins (i.e., DRP1) and downregulation of MFN2 likely supports mitochondrial fragmentation during oogenesis (Udagawa et al., 2014; Machado et al., 2018; Hou et al., 2019; Zhang et al., 2019b). However, oocytes do retain fusion competence, as loss of DRP1 leads to mitochondrial elongation (Udagawa et al., 2014). Moreover, MFN1 is required for oocyte growth and ovulation; MFN1 loss impairs oocyte-somatic cell communication, disrupting folliculogenesis (Machado et al., 2018; Hou et al., 2019; Zhang et al., 2019a,b).
Mitochondrial diseases originated from mtDNA mutations
Diseases caused by mutations in mtDNA are mostly severe and affect ~1 in 4,300 people all over the world (Schaefer et al., 2008). In addition, almost every person (including healthy people) carries very low levels of mutant mtDNA (Payne et al., 2013) that may be passed down to following generations and associate with late-onset diseases, such as Parkinson disease, Alzheimer disease, and common cancers (Poulton et al., 2010;Wallace, 2011; Schon et al., 2012; Gorman et al., 2015; Stewart and Chinnery, 2015). With rare exceptions (Luo et al., 2018), mitochondria are inherited exclusively from the mother (Wallace and Chalkia, 2013). This uniparental pattern of inheritance is explained by the presence of several thousand mitochondria in the ovulated oocyte, against only dozens in the sperm (Wai et al., 2010). Additionally, the early embryo actively eliminates paternal mitochondria introduced into the oocyte during fertilization (Sutovsky et al., 1999; Rojansky et al., 2016). Although it is not clear why sperm mitochondria are excluded from the developing embryo, their elimination is in agreement with the “embryo silent” hypothesis, as sperm mitochondria are elongated, contain well-developed cristae and are highly active (Sutovsky et al., 1999; Ford, 2004; Ruiz-Pesini et al., 2007; Wai et al., 2010; Rojansky et al., 2016).
Mutations in mtDNA are much more frequent than in the nuclear DNA (Johnson and Johnson, 2001), which was initially thought to be explained by mtDNA proximity to ROS generation sites; the mitochondrial genome is attached to the inner mitochondrial membrane, close to complexes involved with the electron transport chain (Wallace, 2005). However, there is now data supporting that most mtDNA mutations originate from replication errors of the mtDNA polymerase (Kauppila et al., 2017). In humans, mice, and flies, for instance, transition mutations, which are indicative of replication errors, are more common than transversions, which often result from oxidative damage (Tomas, 1993; Zheng et al., 2006; Kennedy et al., 2013; Itsara et al., 2014). In fact, the machinery of DNA repair in the mitochondrion does not seem to be as effective as in the nucleus (Vermulst et al., 2008; Maynard et al., 2009; Kazak et al., 2012; Muftuoglu et al., 2014). Thus, intense replication of mtDNA during oogenesis makes it prone to replication errors (Wai et al., 2008; Mahrous et al., 2012; Wei et al., 2019).
The existence of a DNA repair machinery inside mitochondria is well established, but not fully characterized (Scheibye-Knudsen et al., 2015). Most genes encoding for factors involved in this machinery are shared with the nucleus; alternative variants of these genes allow for the protein to be targeted either to the nucleus or the mitochondrion (Muftuoglu et al., 2014; Scheibye-Knudsen et al., 2015). The best-known pathway of DNA repair in mitochondria is base excision repair (BER). Yet, several other enzymes involved with mismatch repair (MMR), non-homologous end joining (NHEJ), and direct repair have been reported in mitochondria (Maynard et al., 2009, 2010; Ruhanen et al., 2010; Halsne et al., 2012; Kazak et al., 2012; Sharma et al., 2014; Scheibye-Knudsen et al., 2015). Moreover, although homologous recombination (HR) has not been proved to contribute with mtDNA repair (Kazak et al., 2012; Hagström et al., 2014; Scheibye-Knudsen et al., 2015), mitochondria do import RAD51, one of the most prominent enzymes of HR (Sage et al., 2010; Chen, 2013). RAD51 has also been linked with mtDNA synthesis under replicative stress (Sage and Knight, 2013), and in oocytes RAD51 is required for mitochondrial function (Kim et al., 2016).
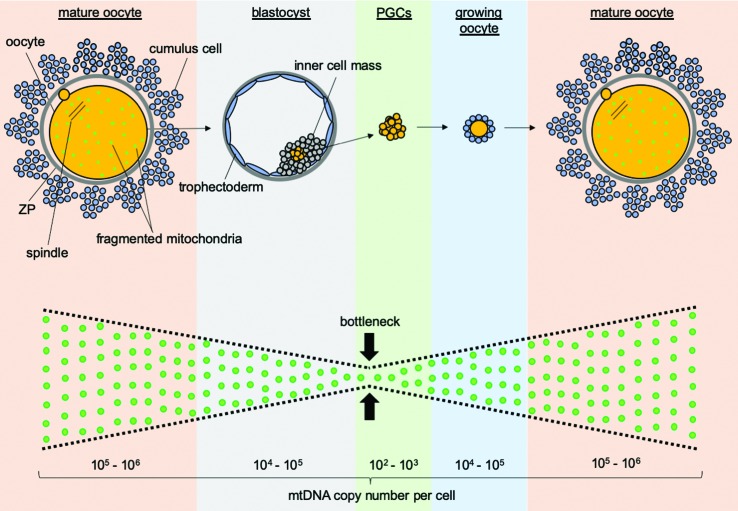
Given that most cells contain several mtDNA molecules, a de novo mutation creates a condition termed heteroplasmy, characterized by the co-existence of two or more mtDNA genotypes (i.e., wild-type and mutant mtDNAs) within the same cell or organelle. Heteroplasmy commonly protects the cell, as most mtDNA mutations are recessive. Unless the mutation level exceeds a critical threshold necessary to cause a biochemical defect (i.e., above 60-90%), the mutation effect will be masked by wild-type molecules (Schon et al., 2012; Aanen et al., 2014; Haig, 2016). In addition, a mechanism known as the mitochondrial genetic bottleneck (Hauswirth and Laipis, 1982; Olivo et al., 1983; Hauswirth et al., 1984; Jenuth et al., 1996; Burgstaller et al., 2018) acts in the germline to rapidly re-establish homoplasmy (i.e., the presence of a single mtDNA genotype). This mechanism is based on the absence of mtDNA replication during early embryogenesis, which forces wild-type and mutant mtDNAs to segregate. Also, few cells among the hundreds present in the embryo differentiate into PGCs, resulting in a sampling effect that efficiently selects one mtDNA genotype to populate the following generation (Stewart and Chinnery, 2015; Burgstaller et al., 2018). However, the selected genotype can be either wild-type or mutant, generating genetic variability to be put to test at the cellular, organismal, or population level (Figure 1).
Figure 1. Mitochondrial kinetics in the female germline. Throughout germline development, the number of mitochondrial DNA (mtDNA) molecules per cell varies from 105 - 106 in mature oocytes (before fertilization), 102 - 103 in primordial germ cells (PGCs) and 105 - 106 back to mature oocytes. This variation in copy number accounts for the mitochondrial genetic bottleneck, which forces segregation of mtDNA molecules. In line with this, the mitochondrial network is fragmented in oocytes, allowing efficient partitioning of mitochondria among hundreds of cells until embryonic implantation. In addition, only few cells in the embryo differentiate into PGCs, supporting a sampling effect towards selection of a single mtDNA genotype to populate the future oocyte.
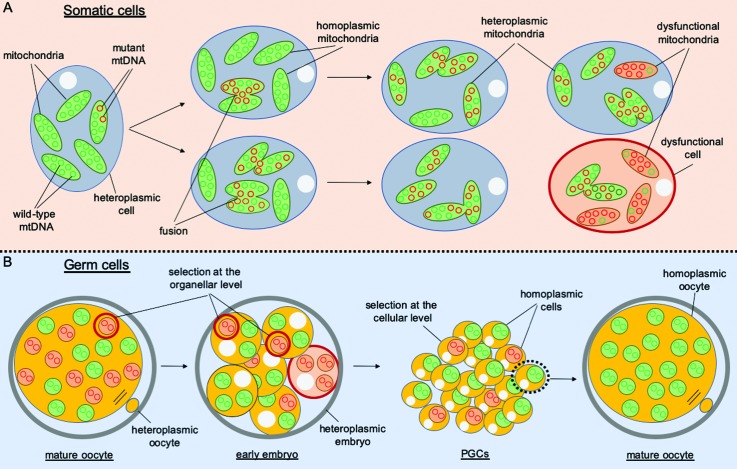
Mutations in mtDNA may vary considering their effect on mitochondrial function from neutral to deleterious. Among deleterious mutations, those affecting tRNA are the most frequent in humans. This is counter-intuitive though, as tRNA genes account for only 10% of the total coding capacity of mtDNA (Schon et al., 2012). However, in comparison with protein-coding genes, tRNA mutations are considered to be less severe, as higher levels (above 90%) are required to cause a biochemical defect (Yoneda et al., 1995). This finding is in agreement with several works that have provided evidence in support of purifying selection acting in germ cells against deleterious mtDNA mutations (Rand, 2008) (Figure 2). For instance, Stewart and colleagues have shown that mice with a burden of mtDNA mutations are less likely to transmit to offspring non-synonymous changes in protein-coding genes (Stewart et al., 2008). In contrast, synonymous substitutions in protein-coding genes and mutations in tRNAs and rRNAs were present at higher levels (Stewart et al., 2008). Similar observations have been reported for flies, mice, and humans (Sato et al., 2007; Fan et al., 2008; Freyer et al., 2012; Sharpley et al., 2012; Hill et al., 2014; Ma et al., 2014; Li et al., 2016; Floros et al., 2018; Wei et al., 2019), suggesting a conserved mechanism of purifying selection was established early during evolution. Accordingly, Lieber et al. (2019) recently reported that mitochondrial fragmentation is required to drive selective removal of deleterious mtDNA during early oogenesis in Drosophila. Fragmentation likely enhances association between mitochondrial genotype and phenotype, favoring one genotype over another (Aanen et al., 2014; Haig, 2016). Nonetheless, at least in Drosophila, this mechanism does not rely on autophagic elimination of mutant mtDNA. Instead, mitophagic proteins enable preferential replication of wild-type mtDNA to outcompete their mutant counterparts (Hill et al., 2014; Ma et al., 2014; Lieber et al., 2019).
Figure 2. Mitochondrial DNA inheritance in somatic and germ cells. Different mitochondria in a single somatic cell (A) are interconnected by constant events of fusion and fission, allowing them to share membranes, solutes, metabolites, proteins, RNAs and DNA (mitochondrial DNA – mtDNA). Hence, when a mutation in mtDNA arises, it can rapidly spread throughout the mitochondrial network. In this case, mutant (red circles) and wild-type (green circles) mtDNAs may co-exist, which is known as heteroplasmy. In comparison, homoplasmic mitochondria contain a single mtDNA genotype, either mutant or wild-type. Unless the mutation level exceeds a critical threshold necessary to cause a biochemical defect (i.e., above 60-90%; red mitochondria), the mutation effect will be masked by wild-type molecules (green mitochondria with both mutant and wild-type mtDNA). In germ cells (B), downregulation of fusion likely minimizes heteroplasmy within mitochondria, enhancing selection at the organellar level (i.e, stronger association between mitochondrial genotype and phenotype). In addition, decreased fusion leads to mitochondrial fragmentation, enhancing mtDNA segregation among embryonic cells. Hence, decreased levels of mtDNA in primordial germ cells (PGCs) makes possible selection at the cellular level (i.e., stronger association between mitochondrial genotype and cellular phenotype). Thus, as a result of selection against deleterious mutations, mature oocyte from the next generation may contain lower levels of mutant mtDNA.
In spite of the mounting evidence in support of a filter against mutant mtDNA in the female germline, this is not a resolved issue. Actually, there are conflicting data arguing against this filter, which has been generating much debate over the topic (Burr et al., 2018). Other questions involving the issue are: i) why would purifying selection be restricted to germline? ii) can one manipulate selection to avoid the accumulation of mutant mtDNA in somatic tissues? Whilst these questions remain unresolved, it is very likely that the purifying selection behaves differently for different mtDNA mutations and different nuclear genetic backgrounds.
Transmission of metabolic diseases linked to mitochondria dysfunction
Obesity and type II diabetes are currently recognized as the most endemic diseases in the human population. The frequency of these syndromes is increasing over the years; currently, nearly half of worldwide population suffers from obesity (Barnett, 2019; Blüher, 2019). Obesity and type II diabetes share similar metabolic alterations and are believed to be highly correlated (Volaco et al., 2018). Transmission of these diseases to the following generations can occur through both parents, yet the maternal contribution has been shown to be larger (Shankar et al., 2008; Jungheim et al., 2010; Rattanatray et al., 2010; Ruager-Martin et al., 2010; Luzzo et al., 2012). In humans, for instance, offspring body mass index (BMI) correlated through three generations with maternal but not paternal BMI (Murrin et al., 2012). Likewise, maternal overnutrition in mice leads to offspring that are glucose intolerant and present increased cholesterol and body fat (Jungheim et al., 2010). These alterations can last up to the third generation, even when pups are fed a regular diet (Saben et al., 2016). Although epigenetic alterations in the nucleus play a major role in the regulation of these effects (Agarwal et al., 2018; Wang et al., 2018), other maternal factors have also been taken into account (Wu et al., 2015; Saben et al., 2016).
Among the factors that contribute with maternal transmission of metabolic diseases, mitochondria are a main candidate giving their maternal-exclusive inheritance. In fact, mitochondrial defects in somatic tissues have been associated with obesity, diabetes and cardiovascular disease (Silva et al., 2000; Sarparanta et al., 2017; Ferey et al., 2019). For instance, mtDNA mutations impacting mitochondrial function and ATP production link with abnormal insulin release and β-cell development, insulin resistance, and diabetes (Poulton et al., 1998; Silva et al., 2000; Kaufman et al., 2015). In this context, Tanaka et al. (2002) demonstrated that single nucleotide polymorphisms in mtDNA (mtSNPs) may result in decreased energy expenditure, leading to obesity. Moreover, several studies have associated mtSNPs with type II diabetes and obesity (Rivera et al., 1999; Fuku et al., 2002; Okura et al., 2003; Guo et al., 2005). These mtSNPs can be located in genes coding for rRNAs, tRNAs, mRNAs (i.e., MT-CYB or MT-ATP6), and even in the non-coding region of mtDNA, the D-loop. Similarly, it was recently described that several mtDNA mutations in tRNAs lead to polycystic ovarian syndrome and metabolic alterations (Ding et al., 2018), both closely related to type II diabetes and obesity. Altogether, these findings provide evidence that mtDNA mutations may underpin maternal transmission of metabolic diseases.
Apart from mtDNA mutations, mitochondrial damage in oocytes has also been linked with increased risk of metabolic diseases in offspring. Obesity leads to increased lipid content in the follicular fluid, cumulus cells, and oocytes, which in turn damage organelles such as mitochondria and the ER (Wang et al., 2009; Wu et al., 2010; Fullston et al., 2015; Ruebel et al., 2017). Impaired ER function can lead to activation of the unfolded protein response (UPR) and Ca+2 release, further disrupting mitochondrial function (i.e., decreased Δψm and increased ROS) and oocyte homeostasis (Wu et al., 2010, 2015; Luzzo et al., 2012; Hou et al., 2016). Besides impacting oocyte competence and fertility (Wu et al., 2015; Pasquariello et al., 2019), these mitochondrial abnormalities can be passed down to the following generations, increasing their risk to develop metabolic diseases (Saben et al., 2016). Hence, mice born to pregnant females under a high-fat/high-sucrose diet have impaired peripheral insulin signaling which associates with abnormal mitochondrial function and dynamics in skeletal muscle up to the third generation (Saben et al., 2016). Similar mitochondrial abnormalities were present in oocytes from the first and second generations, even though these were fed a regular diet (Saben et al., 2016). Therefore, apart from epigenetic alterations in the nucleus, mitochondria also contribute with the metabolic programing resulting from maternal overnutrition. Given that epigenetic marks in mtDNA regulate expression of this genome (Kobayashi et al., 2012; Sun et al., 2013; Sirard, 2019), it remains to be investigated whether these can also explain maternal transmission of metabolic diseases.
Treatment options for preventing mitochondrial disease transmission
Due to the poor understanding of the mechanisms regulating transmission of mitochondria-related diseases, there are few treatment options available to prevent their inheritance to the following generations (Craven et al., 2017). With respect to non-genetic alterations in mitochondria, the oocyte might benefit from treatments performed before fertilization, during the in vitro maturation. The idea is to expose the oocyte for a period of ~24 h to drugs such as L-carnitine, rosiglitazone, salubrinal, or BGP-15, which potentially enhance mitochondria activity, decrease lipid content, and mitigate ER stress. In fact, treatments involving one or more of these drugs have been shown to mitigate the defects in the oocyte and the next generation (Wu et al., 2010, 2015; Dunning and Robker, 2012; Liang et al., 2017). However, a major challenge in making these treatments available is to overcome the side effects of in vitro maturation (Lonergan and Fair, 2016; Yang and Chian, 2017). Given this is a critical period of oocyte development, which encompasses meiotic resumption from prophase I (dictyate) to metaphase II, any perturbation in oocyte homeostasis may lead to mis-segregation of chromosomes and aneuploidy (Greaney et al., 2017; Danadova et al., 2017). In addition, in vitro maturation on its own leads to metabolic alterations that mimic those of oocytes from obese donors (i.e., mitochondrial dysfunction and increased lipid content), potentially impacting the next generation (Farin et al., 2006; Li et al., 2014; del Collado et al., 2017a; del Collado et al., 2017b; Wang et al., 2018). Thus, these alternatives are not currently available in humans.
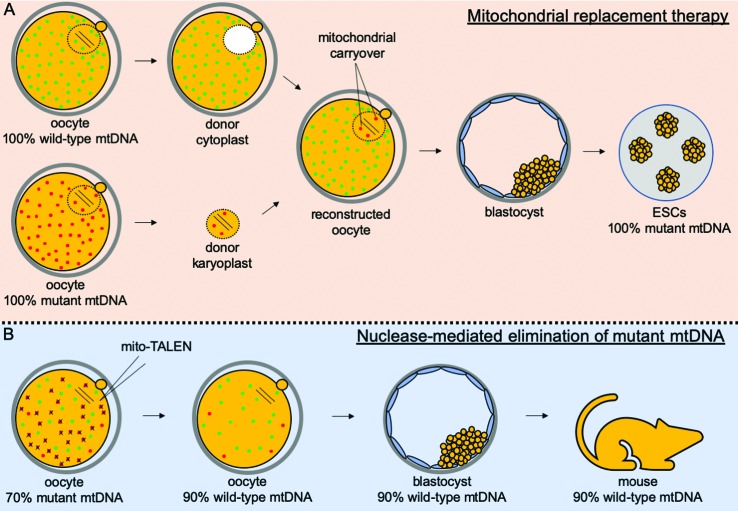
An alternative option to treat oocytes harboring mitochondria abnormalities, particularly those caused by mtDNA mutations, is known as mitochondrial replacement therapy (MRT; Figure 3). This method involves replacement of abnormal mitochondria in the oocyte by functional ones provided by a donated oocyte (Wolf et al., 2017). More specifically, ovulated oocytes at the metaphase-II stage are collected from both the patient and a “healthy” donor not containing mitochondrial abnormalities. With the aid of a micromanipulation set, the spindle from the donated oocyte is replaced by the patient’s spindle. The resulting oocyte containing the patient’s spindle and donated mitochondria is then fertilized to allow development to term. Provided that the large majority of mitochondria is replaced by donated ones, MRT has virtually the potential to prevent transmission of mitochondrial diseases. Yet, ~1% of mitochondria from the patient’s oocyte are transferred along with the spindle. This level can be even higher (up to 4%) when pronuclear zygotes are used instead of metaphase-II oocytes, which can lead in ~15% of cases to a reversal back to the patient’s mtDNA (Hyslop et al., 2016; Kang et al., 2016). Although hard to explain, rapid mtDNA segregation and bottleneck during preimplantation development might account for these quick shifts in mtDNA genotype (Lee et al., 2012a; Freyer et al., 2012). Alternatively, it has been proposed that a specific population of mtDNA is tagged in oocytes (i.e., from spindle-surrounding mitochondria) for replication during early development (Wolf et al., 2017). No matter the mechanism underlying these unexpected results, they highlight the need for careful studies before the clinical practice of MRT (Wolf et al., 2017; Craven et al., 2018).
Figure 3. New technologies for preventing inheritance of mitochondrial diseases. The mitochondrial replacement therapy (MRT; A) proposes the replacement of a patient’s mitochondria in oocytes by donor mitochondria. Towards that, mature oocytes arrested at the metaphase-II stage are collected from the patient and a donor. While the patient’s oocytes are supposed to contain mutant (red) mitochondrial DNA (mtDNA), donor oocytes should contain only wild-type (green) mtDNA. Next, the spindle is removed from the patient’s oocyte (donor karyoplast) to be injected into the donor oocyte from which the spindle was previously removed (donor cytoplast). Fertilization of the reconstructed oocyte should lead to a blastocyst, which can be used for embryonic stem cell (ESC) derivation. Although MRT allows transplantation of karyoplast with minimal carryover (~1%) of mutant mtDNA, recent data have provided evidence of a reversal in ESCs back to 100% mutant mtDNA (Hyslop et al., 2016; Kang et al., 2016). An alternative strategy to MRT is the nuclease-mediated elimination of mutant mtDNA (B), which relies on the use of mitochondrial-targeted restriction endonucleases (mito-TALENs). These nucleases are designed to selectively cut mutant mtDNA, but not wild-type molecules. However, ~10% of targeted molecules were shown to be left uncut in newborns after use of mito-TALENs (Reddy et al., 2015).
With the advances in genome editing technologies, another potential strategy to prevent transmission of mitochondrial abnormalities is the targeted elimination of mutant mtDNA in oocytes or early embryos (Figure 3). As a proof of concept, Reddy et al. (2015) used mitochondrial-targeted restriction endonucleases (mito-TALENs) to selectively eliminate mutant mtDNA in mice and humans. Although this strategy proved efficient, ~10% of targeted molecules (i.e., mutant mtDNA) were left in oocytes, embryos and offspring produced after the use of mito-TALENs. Moreover, given that the mtDNA is not replicated during early embryogenesis (Pikó and Taylor, 1987; Thundathil et al., 2005; Cree et al., 2008), the use of mito-TALENs resulted in mtDNA-depleted embryos (Reddy et al., 2015). Although in the newborns the content of mtDNA was normal (Reddy et al., 2015), the lower levels of mtDNA (and likely of mitochondria too) in oocytes and embryos could lead to poorer developmental rates (Wai et al., 2010). Based on these uncertainties, mito-TALENs are not currently taken as a viable alternative to prevent transmission of mtDNA-linked diseases (Wolf et al., 2017).
Final considerations
Mitochondrial abnormalities have been linked with maternal transmission of important diseases in humans. Among these, mtDNA mutations in oocytes can be transmitted to the following generations and cause severe diseases. In addition, maternal obesity damages mitochondria in oocytes, leading to poor fertility and increased risk of metabolic diseases in offspring. Understanding how mitochondrial abnormalities are established and transmitted are of fundamental importance to mitigate their incidence in the human population. Moreover, treatment options involving manipulation of oocytes and early embryos are currently under consideration and may become available in the future to prevent transmission of mitochondria-associated diseases.
Acknowledgments
We would like to thank the São Paulo Research Foundation (FAPESP – grant # 2016/07868-4, 2017/05899-2, 2017/19825-0, 2017/25916-9 and 2018/13155-6) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES – finance code 001).
Footnotes
Associate Editor: Carlos R. Machado
Conflict of Interests
The authors declare that there is no conflict of interest that could be perceived as prejudicial to the impartiality of the reported research.
Author contributions
MRC and MDC conceived the study. MRC and MDC reviewed previous publications. MRC, CHM, JDAN, MPG, AKP, FP and MDC wrote the manuscript. All authors read and approved the final version.
References
- Aanen DK, Spelbrink JN, Beekman M. What cost mitochondria? The maintenance of functional mitochondrial DNA within and across generations. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130438. doi: 10.1098/rstb.2013.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 2018;55:71–101. doi: 10.1080/10408363.2017.1422109. [DOI] [PubMed] [Google Scholar]
- Arhin SK, Lu J, Xi H, Jin X. Energy requirements in mammalian oogenesis. Cell Mol Biol. 2018;64:12–19. [PubMed] [Google Scholar]
- Ashley MV, Laipis PJ, Hauswirth WW. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res. 1989;17:7325–7331. doi: 10.1093/nar/17.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism: A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. doi: 10.1074/jbc.M212754200. [DOI] [PubMed] [Google Scholar]
- Barnett R. Type 2 diabetes. Lancet. 2019;394:557. doi: 10.1016/S0140-6736(19)31728-3. [DOI] [PubMed] [Google Scholar]
- Battersby BJ, Loredo-Osti JC, Shoubridge EA. Nuclear genetic control of mitochondrial DNA segregation. Nat Genet. 2003;33:183–186. doi: 10.1038/ng1073. [DOI] [PubMed] [Google Scholar]
- Betz C, Stracka D, Prescianotto-baschong C, Frieden M, Demaurex N. mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Nat Acad Sci U S A. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- Burgstaller JP, Kolbe T, Havlicek V, Hembach S, Poulton J, Piálek J, Steinborn R, Rülicke T, Brem G, Jones NS, et al. Large-scale genetic analysis reveals mammalian mtDNA heteroplasmy dynamics and variance increase through lifetimes and generations. Nat Commun. 2018;9:1–12. doi: 10.1038/s41467-018-04797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr SP, Pezet M, Chinnery PF. Mitochondrial DNA heteroplasmy and purifying selection in the mammalian female germ line. Dev Growth Differ. 2018;60:21–32. doi: 10.1111/dgd.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Sureda A, Pihán P, Hetz C. The unfolded protein response: At the intersection between endoplasmic reticulum function and mitochondrial bioenergetics. Front Oncol. 2017;7:1–7. doi: 10.3389/fonc.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–62. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–9. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Dasgupta A, Ding J, Indig FE, Ghosh P, Longo DL. Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J. 2014;28:382–394. doi: 10.1096/fj.13-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ. Mechanism of homologous recombination and implications for aging-related deletions in mitochondrial DNA. Microbiol Mol Biol Rev. 2013;77:476–496. doi: 10.1128/MMBR.00007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaratti MR, Meirelles FV. Mitochondrial DNA copy number, a marker of viability for oocytes. Biol Reprod. 2010;83:1–2. doi: 10.1095/biolreprod.110.084269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaratti MR, Garcia BM, Carvalho KF, Machado TS, Ribeiro FKDS, Macabelli CH. The role of mitochondria in the female germline: Implications to fertility and inheritance of mitochondrial diseases. Cell Biol Int. 2018;42:1–39. doi: 10.1002/cbin.10947. [DOI] [PubMed] [Google Scholar]
- Cipolat S, de Brito OM, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Nat Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HJ. Regulation of germ cell development by intercellular signaling in the mammalian ovarian follicle. Wiley Interdiscip Rev Dev Biol. 2017;7:e294. doi: 10.1002/wdev.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven L, Alston CL, Taylor RW, Turnbull DM. Recent advances in mitochondrial disease. Annu Rev Genomics Hum Genet. 2017;18:257–275. doi: 10.1146/annurev-genom-091416-035426. [DOI] [PubMed] [Google Scholar]
- Craven L, Murphy J, Turnbull DM, Taylor RW, Gorman GS, McFarland R. Scientific and ethical issues in mitochondrial donation. New Bioeth. 2018;24:57–73. doi: 10.1080/20502877.2018.1440725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree LM, Samuels DC, Sousa Lopes SC, Rajasimha HK, Wonnapinij P, Mann JR, Dahl H-HM, Chinnery PF. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet. 2008;40:249–54. doi: 10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- Danadova J, Matijescukova N, Danylevska AMG, Anger M. Increased frequency of chromosome congression defects and aneuploidy in mouse oocytes cultured at lower temperature. Reprod Fertil Dev. 2017;29:968. doi: 10.1071/RD15306. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin-2 regulates mitochondrial and endoplasmic reticulum morphology and tethering: The role of Ras. Mitochondrion. 2009;9:222–226. doi: 10.1016/j.mito.2009.02.005. [DOI] [PubMed] [Google Scholar]
- del Collado M, da Silveira JC, Oliveira MLF, Alves BMSM, Simas RC, Godoy AT, Coelho MB, Marques LA, Carriero MM, Nogueira MFG, et al. In vitro maturation impacts cumulus–oocyte complex metabolism and stress in cattle. Reproduction. 2017;154:881–893. doi: 10.1530/REP-17-0134. [DOI] [PubMed] [Google Scholar]
- del Collado M, da Silveira JC, Sangalli JR, Andrade GM, Sousa LRDS, Silva LA, Meirelles FV, Perecin F. Fatty acid binding protein 3 and transzonal projections are involved in lipid accumulation during in vitro maturation of bovine oocytes. Sci Rep. 2017;7:2645. doi: 10.1038/s41598-017-02467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene. 2018;642:299–306. doi: 10.1016/j.gene.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Dunning KR, Robker RL. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim Reprod Sci. 2012;134:69–75. doi: 10.1016/j.anireprosci.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Eschbach J, Sinniger J, Bouitbir J, Fergani A, Schlagowski A-I, Zoll J, Geny B, René F, Larmet Y, Marion V, et al. Dynein mutations associated with hereditary motor neuropathies impair mitochondrial morphology and function with age. Neurobiol Dis. 2013;58:220–30. doi: 10.1016/j.nbd.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134:333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- Fan W, Waymire KG, Narula N, Li P, Rocher C, Coskun PE, Vannan MA, Narula J, Macgregor GR, Wallace DC. A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science. 2008;319:958–62. doi: 10.1126/science.1147786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin PW, Piedrahita JA, Farin CE. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology. 2006;65:178–91. doi: 10.1016/j.theriogenology.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Ferey JLA, Boudoures AL, Reid M, Drury A, Scheaffer S, Modi Z, Kovacs A, Pietka T, DeBosch BJ, Thompson MD, et al. A maternal high-fat, high-sucrose diet induces transgenerational cardiac mitochondrial dysfunction independently of maternal mitochondrial inheritance. Am J Physiol Heart Circ Physiol. 2019;316:H1202–H1210. doi: 10.1152/ajpheart.00013.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CR, Burgstaller JP, Perecin F, Garcia JM, Chiaratti MR, Méo SC, Müller M, Smith LC, Meirelles FV, Steinborn R. Pronounced segregation of donor mitochondria introduced by bovine ooplasmic transfer to the female germ-line. Biol Reprod. 2010;82:563–71. doi: 10.1095/biolreprod.109.080564. [DOI] [PubMed] [Google Scholar]
- Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Nat Acad Sci U S A. 2015;112:E2174–81. doi: 10.1073/pnas.1504880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floros VI, Pyle A, Dietmann S, Wei W, Tang WCW, Irie N, Payne B, Capalbo A, Noli L, Coxhead J, et al. Segregation of mitochondrial DNA heteroplasmy through a developmental genetic bottleneck in human embryos. Nat Cell Biol. 2018;20:144–151. doi: 10.1038/s41556-017-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford WCL. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10:387–399. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- Freyer C, Cree LM, Mourier A, Stewart JB, Koolmeister C, Milenkovic D, Wai T, Floros VI, Hagström E, Chatzidaki EE, et al. Variation in germline mtDNA heteroplasmy is determined prenatally but modified during subsequent transmission. Nat Genet. 2012;44:1282–1285. doi: 10.1038/ng.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuku N, Oshida Y, Takeyasu T, Guo LJ, Sato Y, Fuku N, Oshida Y, Takeyasu T, Guo LJ, Sato Y, et al. Mitochondrial ATPase subunit 6 and cytochrome b gene polymorphisms in young obese adults. Biochem Biophys Res Commun. 2002;290:1199–1205. doi: 10.1006/bbrc.2002.6330. [DOI] [PubMed] [Google Scholar]
- Fullston T, Shehadeh H, Sandeman LY, Kang WX, Wu LL, Robker RL, McPherson NO, Lane M. Female offspring sired by diet induced obese male mice display impaired blastocyst development with molecular alterations to their ovaries, oocytes and cumulus cells. J Assist Reprod Genet. 2015;32:725–735. doi: 10.1007/s10815-015-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garesse R, Vallejo CG. Animal mitochondrial biogenesis and function: A regulatory cross-talk between two genomes. Gene. 2001;263:1–16. doi: 10.1016/s0378-1119(00)00582-5. [DOI] [PubMed] [Google Scholar]
- Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015;77:753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney J, Wei Z, Homer H. Regulation of chromosome segregation in oocytes and the cellular basis for female meiotic errors. Hum Reprod Update 10.1093/humupd/dmx035. 2017 doi: 10.1093/humupd/dmx035. [DOI] [PubMed] [Google Scholar]
- Griparic L, Van Der Wel NN, Orozco IJ, Peters PJ, Van Der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- Guo LJ, Oshida Y, Fuku N, Takeyasu T, Fujita Y, Kurata M, Sato Y, Ito M, Tanaka M. Mitochondrial genome polymorphisms associated with type-2 diabetes or obesity. Mitochondrion. 2005;5:15–33. doi: 10.1016/j.mito.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hagström E, Freyer C, Battersby BJ, Stewart JB, Larsson NG. No recombination of mtDNA after heteroplasmy for 50 generations in the mouse maternal germline. Nucleic Acids Res. 2014;42:1111–1116. doi: 10.1093/nar/gkt969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D. Intracellular evolution of mitochondrial DNA (mtDNA) and the tragedy of the cytoplasmic commons. BioEssays. 2016;38:549–555. doi: 10.1002/bies.201600003. [DOI] [PubMed] [Google Scholar]
- Halsne R, Esbensen Y, Wang W, Scheffler K, Suganthan R, Bjørås M, Eide L. Lack of the DNA glycosylases MYH and OGG1 in the cancer prone double mutant mouse does not increase mitochondrial DNA mutagenesis. DNA Repair. 2012;11:278–285. doi: 10.1016/j.dnarep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, et al. Autophagosomes form at ER–mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Morimoto N, Yamanaka M, Matsumoto H, Yamochi T, Goto H, Inoue M, Nakaoka Y, Shibahara H, Morimoto Y. Quantitative and qualitative changes of mitochondria in human preimplantation embryos. J Assist Reprod Genet. 2017;34:573–580. doi: 10.1007/s10815-017-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Laipis PJ. Mitochondrial DNA polymorphism in a maternal lineage of Holstein cows. Proc Natl Acad Sci U S A. 1982;79:4686–4690. doi: 10.1073/pnas.79.15.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Van de Walle MJ, Laipis PJ, Olivo PD. Heterogeneous mitochondrial DNA D-loop sequences in bovine tissue. Cell. 1984;37:1001–1007. doi: 10.1016/0092-8674(84)90434-3. [DOI] [PubMed] [Google Scholar]
- Hill JH, Chen Z, Xu H. Selective propagation of functional mitochondrial DNA during oogenesis restricts the transmission of a deleterious mitochondrial variant. Nat Genet. 2014;46:389–92. doi: 10.1038/ng.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Zhu S, Zhang H, Li C, Qiu D, Ge J, Guo X, Wang Q. Mitofusin1 in oocyte is essential for female fertility. Redox Biol. 2019;21:101110. doi: 10.1016/j.redox.2019.101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun SC. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:1–10. doi: 10.1038/srep18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop LA, Blakeley P, Craven L, Richardson J, Fogarty NME, Fragouli E, Lamb M, Wamaitha SE, Prathalingam N, Zhang Q, et al. Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature. 2016;534:383–386. doi: 10.1038/nature18303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y-I, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–66. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Itsara LS, Kennedy SR, Fox EJ, Yu S, Hewitt JJ, Sanchez-Contreras M, Cardozo-Pelaez F, Pallanck LJ. Oxidative stress is not a major contributor to somatic mitochondrial DNA mutations. PLoS Genet. 2014;10:1003974. doi: 10.1371/journal.pgen.1003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RPS, De Boer K. The bottleneck: Mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cellular Endocrinol. 1998;145:81–88. doi: 10.1016/s0303-7207(98)00173-7. [DOI] [PubMed] [Google Scholar]
- Jenuth J, Peterson A, Fu K, Shoubridge E. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat Genet. 1996;14:146–151. doi: 10.1038/ng1096-146. [DOI] [PubMed] [Google Scholar]
- Johnson AA, Johnson KA. Exonuclease proofreading by human mitochondrial DNA polymerase. J Biol Chem. 2001;276:38097–107. doi: 10.1074/jbc.M106046200. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77:2–8. doi: 10.1095/biolreprod.106.059899. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Wu J, Gutierrez NM, Koski A, Tippner-Hedges R, Agaronyan K, Platero-Luengo A, Martinez-Redondo P, Ma H, Lee Y, et al. Mitochondrial replacement in human oocytes carrying pathogenic mitochondrial DNA mutations. Nature. 2016;540:270–275. doi: 10.1038/nature20592. [DOI] [PubMed] [Google Scholar]
- Kaufman BA, Li C, Soleimanpour SA. Mitochondrial regulation of β-cell function: Maintaining the momentum for insulin release. Mol Aspects Med. 2015;42:91–104. doi: 10.1016/j.mam.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila TES, Kauppila JHK, Larsson NG. Mammalian mitochondria and aging: An update. Cell Metab. 2017;25:57–71. doi: 10.1016/j.cmet.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Kazak L, Reyes A, Holt IJ. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol. 2012;13:659–671. doi: 10.1038/nrm3439. [DOI] [PubMed] [Google Scholar]
- Kennedy SR, Salk JJ, Schmitt MW, Loeb LA. Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet. 2013;9:e1003794. doi: 10.1371/journal.pgen.1003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Park JH, Kim EY, Ko JJ, Park KS, Lee KA. The role of Rad51 in safeguarding mitochondrial activity during the meiotic cell cycle in mammalian oocytes. Sci Rep. 2016;6:34110. doi: 10.1038/srep34110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Sakurai T, Imai M, Takahashi N, Fukuda A, Yayoi O, Sato S, Nakabayashi K, Hata K, Sotomaru Y, et al. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish Oocyte-specific heritable marks. PLoS Genet. 2012;8:e1002440. doi: 10.1371/journal.pgen.1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ma H, Juanes RC, Tachibana M, Sparman M, Woodward J, Ramsey C, Xu J, Kang EJ, Amato P, et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012;1:506–15. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ma H, Juanes R, Tachibana M, Sparman M, Woodward J, Ramsey C, Xy J, Kand EJ, Amato P, et al. Rapid mitochondrial DNA segregation in primate preimplantation embryos precedes somatic and germline bottleneck. Cell Rep. 2012;1:506–515. doi: 10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ. Metabolism of the preimplantation embryo: 40 Years on. Reproduction. 2012;143:417–427. doi: 10.1530/REP-11-0484. [DOI] [PubMed] [Google Scholar]
- Leitch HG, Tang WWC, Surani MA. Primordial germ-cell development and epigenetic reprogramming in mammals. Curr Top Dev Biol. 2013;104:149–187. doi: 10.1016/B978-0-12-416027-9.00005-X. [DOI] [PubMed] [Google Scholar]
- Li H, Jia GH, Lu XL, Zhang G, Tian KY, Li JT, Zhang JM. In vitro maturation of oocytes is not a risk factor for adult metabolic syndrome of mouse offspring. Eur J Obstet Gynecol Reprod Biol. 2014;174:96–99. doi: 10.1016/j.ejogrb.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Li M, Rothwell R, Vermaat M, Wachsmuth M, Schröder R, Laros JFJ, van Oven M, de Bakker PIW, Bovenberg JA, van Duijn CM, et al. Transmission of human mtDNA heteroplasmy in the genome of the Netherlands families: Support for a variable-size bottleneck. Genome Res. 2016;26:417–26. doi: 10.1101/gr.203216.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang LF, Qi ST, Xian YX, Huang L, Sun XF, Wang WH. Protective effect of antioxidants on the pre-maturation aging of mouse oocytes. Sci Rep. 2017;7:1434. doi: 10.1038/s41598-017-01609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T, Jeedigunta SP, Palozzi JM, Lehmann R, Hurd TR. Mitochondrial fragmentation drives selective removal of deleterious mtDNA in the germline. Nature. 2019;570:380–384. doi: 10.1038/s41586-019-1213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan P, Fair T. Maturation of oocytes in vitro . Ann Rev Anim Biosci. 2016;4:255–268. doi: 10.1146/annurev-animal-022114-110822. [DOI] [PubMed] [Google Scholar]
- Luo S, Valencia CA, Zhang J, Lee NC, Slone J, Gui B, Wang X, Li Z, Dell S, Brown J, et al. Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci U S A. 2018;115:13039–13044. doi: 10.1073/pnas.1810946115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: Oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e0049217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Xu H, O’Farrell PH. Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster. Nat Genet. 2014;46:393–7. doi: 10.1038/ng.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado TS, Carvalho KF, Garcia BM, Zangirolamo AF, Macabelli CH, Sugiyama FHC, Grejo MP, Augusto JD, Neto, Ribeiro FKS, Sarapiao FD, et al. Mitofusin 1 is required for the oocyte-granulosa cell communication that regulates oogenesis. bioRxiv 10.1101/498642 2018 [Google Scholar]
- Mahrous E, Yang Q, Clarke HJ. Regulation of mitochondrial DNA accumulation during oocyte growth and meiotic maturation in the mouse. Reproduction. 2012;144:177–185. doi: 10.1530/REP-12-0113. [DOI] [PubMed] [Google Scholar]
- May-Panloup P, Vignon X, Chrétien MF, Heyman Y, Tamassia M, Malthièry Y, Reynier P. Increase of mitochondrial DNA content and transcripts in early bovine embryogenesis associated with upregulation of mtTFA and NRF1 transcription factors. Reprod Biol Endocrinol. 2005;3:65. doi: 10.1186/1477-7827-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard S, de Souza-Pinto NC, Scheibye-Knudsen M, Bohr VA. Mitochondrial base excision repair assays. Methods. 2010;51:416–25. doi: 10.1016/j.ymeth.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingrone G, Manco M, Calvani M, Castagneto M, Naon D, Zorzano A. Could the low level of expression of the gene encoding skeletal muscle mitofusin-2 account for the metabolic inflexibility of obesity? Diabetologia. 2005;48:2108–2114. doi: 10.1007/s00125-005-1918-9. [DOI] [PubMed] [Google Scholar]
- Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15:634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko AL, Sasaki Y, Tuck E, Milbrandt J, Baloh RH. Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. J Neurosci. 2012;32:4145–4155. doi: 10.1523/JNEUROSCI.6338-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod 15 Suppl. 2000;2:129–147. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- Muftuoglu M, Mori MP, de Souza-Pinto NC. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion. 2014;17:164–181. doi: 10.1016/j.mito.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Muñoz JP, Ivanova S, Sánchez-Wandelmer J, Martínez-Cristóbal P, Noguera E, Sancho A, Díaz-Ramos A, Hernández-Alvarez MI, Sebastián D, Mauvezin C, et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 2013;32:2348–2361. doi: 10.1038/emboj.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin CM, Kelly GE, Tremblay RE, Kelleher CC. Body mass index and height over three generations: Evidence from the Lifeways cross-generational cohort study. BMC Public Health. 2012;12:81. doi: 10.1186/1471-2458-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naon D, Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta. 2014;1843:2184–2194. doi: 10.1016/j.bbamcr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Papanicolaou KN, Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. J Biol Chem. 2012;287:20321–20332. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem O, Urman B. Understanding follicle growth in vivo . Hum Reprod. 2010;25:2944–2954. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Tanaka M, Shimokata H. Association of the mitochondrial DNA 15497G/A polymorphism with obesity in a middle-aged and elderly Japanese population. Hum Genet. 2003;113:432–6. doi: 10.1007/s00439-003-0983-8. [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- Olivo PD, Van de Walle MJ, Laipis PJ, Hauswirth WW. Nucleotide sequence evidence for rapid genotypic shifts in the bovine mitochondrial DNA D-loop. Nature. 1983;306:400–402. doi: 10.1038/306400a0. [DOI] [PubMed] [Google Scholar]
- Pareyson D, Saveri P, Sagnelli A, Piscosquito G. Mitochondrial dynamics and inherited peripheral nerve diseases. Neurosci Lett. 2015;596:66–77. doi: 10.1016/j.neulet.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Pasquariello R, Ermisch AF, Silva E, McCormick S, Logsdon D, Barfield JP, Schoolcraft WB, Krisher RL. Alterations in oocyte mitochondrial number and function are related to spindle defects and occur with maternal aging in mice and humans. Biol Reprod. 2019;100:971–981. doi: 10.1093/biolre/ioy248. [DOI] [PubMed] [Google Scholar]
- Pathak T, Trebak M. Mitochondrial Ca2+ signaling. Pharmacol Ther. 2018;192:112–123. doi: 10.1016/j.pharmthera.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BAI, Wilson IJ, Yu-Wai-Man P, Coxhead J, Deehan D, Horvath R, Taylor RW, Samuels DC, Santibanez-Koref M, Chinnery PF. Universal heteroplasmy of human mitochondrial DNA. Hum Mol Genet. 2013;22:384–390. doi: 10.1093/hmg/dds435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas L, Scorrano L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- Pikó L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–374. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Poulton J, Scott Brown M, Cooper A, Marchington DR, Phillips DIW. A common mitochondrial DNA variant is associated with insulin resistance in adult life. Diabetologia. 1998;41:54–58. doi: 10.1007/s001250050866. [DOI] [PubMed] [Google Scholar]
- Poulton J, Chiaratti MR, Meirelles FV, Kennedy S, Wells D, Holt IJ. Transmission of mitochondrial DNA diseases and ways to prevent them. PLoS Genet. 2010;6:e1001066. doi: 10.1371/journal.pgen.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez S, Gómez-Valadés AG, Schneeberger M, Varela L, Haddad-Tóvolli R, Altirriba J, Noguera E, Drougard A, Flores-Martínez Á, Imbernón M, et al. Mitochondrial dynamics mediated by Mitofusin 1 is required for POMC neuron glucose-sensing and insulin release control. Cell Metab. 2017;25:1390–1399.e6. doi: 10.1016/j.cmet.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Rand DM. Mitigating mutational meltdown in mammalian mitochondria. PLoS Biol. 2008;6:e35. doi: 10.1371/journal.pbio.0060035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanatray L, MacLaughlin SM, Kleemann DO, Walker SK, Muhlhausler BS, McMillen IC. Impact of maternal periconceptional overnutrition on fat mass and expression of adipogenic and lipogenic genes in visceral and subcutaneous fat depots in the postnatal lamb. Endocrinology. 2010;151:5195–5205. doi: 10.1210/en.2010-0501. [DOI] [PubMed] [Google Scholar]
- Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, Sugawara A, Okamura D, Tsunekawa Y, Wu J, et al. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161:459. doi: 10.1016/j.cell.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera MA, Pérusse L, Gagnon J, Dionne FT, Leon AS, Rao DC, Skinner JS, Wilmore JH, Sjöström L, Bouchard C. A mitochondrial DNA D-loop polymorphism and obesity in three cohorts of women. Int J Obes Relat Metab Disord. 1999;23:666–668. doi: 10.1038/sj.ijo.0800900. [DOI] [PubMed] [Google Scholar]
- Rojansky R, Cha MY, Chan DC. Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. eLife. 2016;5:1–18. doi: 10.7554/eLife.17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruager-Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev. 2010;86:715–722. doi: 10.1016/j.earlhumdev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Ruebel ML, Cotter M, Sims CR, Moutos DM, Badger TM, Cleves MA, Shankar K, Andres A. Obesity modulates inflammation and lipidmetabolism oocyte gene expression: A single-cell transcriptome perspective. J Clin Endocrinol Metab. 2017;102:2029–2038. doi: 10.1210/jc.2016-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhanen H, Borrie S, Szabadkai G, Tyynismaa H, Jones AWE, Kang D, Taanman JW, Yasukawa T. Mitochondrial single-stranded DNA binding protein is required for maintenance of mitochondrial DNA and 7S DNA but is not required for mitochondrial nucleoid organisation. Biochim Biophys Acta. 2010;1803:931–939. doi: 10.1016/j.bbamcr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA. The role of the mitochondrion in sperm function: Is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- Saben JL, Boudoures AL, Asghar Z, Cusumano A, Scheaffer S, Moley KH, Saben JL, Boudoures AL, Asghar Z, Thompson A, et al. Mitochondrial dysfunction via germline changes across three generations maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 2016;16:1–8. doi: 10.1016/j.celrep.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage JM, Gildemeister OS, Knight KL. Discovery of a novel function for human. RadJ Biol Chem. 2010;285:18984–18990. doi: 10.1074/jbc.M109.099846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage JM, Knight KL. Human Rad51 promotes mitochondrial DNA synthesis under conditions of increased replication stress. Mitochondrion. 2013;13:350–356. doi: 10.1016/j.mito.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- Sarparanta J, García-Macia M, Singh R. Autophagy and mitochondria in obesity and type 2 diabetes. Curr Diabetes Rev. 2017;13:352–369. doi: 10.2174/1573399812666160217122530. [DOI] [PubMed] [Google Scholar]
- Sato A, Nakada K, Shitara H, Kasahara A, Yonekawa H, Hayashi JI. Deletion-mutant mtDNA increases in somatic tissues but decreases in female germ cells with age. Genetics. 2007;177:2031–2037. doi: 10.1534/genetics.107.081026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantland S, Tessaro I, Macabelli CH, Macaulay AD, Cagnone G, Fournier É, Luciano AM, Robert C. The adenosine salvage pathway as an alternative to mitochondrial production of ATP in maturing mammalian oocytes. Biol Reprod. 2014;91:1–11. doi: 10.1095/biolreprod.114.120931. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene. 2002;286:81–9. doi: 10.1016/s0378-1119(01)00809-5. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, Chinnery PF, Turnbull DM. Prevalence of mitochondrial DNA disease in adults. Ann Neurol. 2008;63:35–9. doi: 10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM, Bohr VA. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25:158–170. doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodríguez IC, Bortolozzi A, et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878–90. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar K, Harrell A, Liu X, Gilchrist JM, Ronis MJJ, Badger TM. Maternal obesity at conception programs obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2008;294:528–538. doi: 10.1152/ajpregu.00316.2007. [DOI] [PubMed] [Google Scholar]
- Sharma NK, Lebedeva M, Thomas T, Kovalenko OA, Stumpf JD, Shadel GS, Santos JH. Intrinsic mitochondrial DNA repair defects in Ataxia Telangiectasia. DNA Repair. 2014;13:22–31. doi: 10.1016/j.dnarep.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, Waymire K, Lin CS, Masubuchi S, Friend N, Koike M, et al. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–43. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Köhler M, Graff C, Oldfors A, Magnuson MA, Berggren PO, Larsson NG. Impaired insulin secretion and β-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nat Genet. 2000;26:336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- Sirard MA. Distribution and dynamics of mitochondrial DNA methylation in oocytes, embryos and granulosa cells. Sci Rep. 2019;9:11937. doi: 10.1038/s41598-019-48422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John JC. Mitochondria and female germline stem cells - a mitochondrial DNA perspective. Cells. 2019;8:852. doi: 10.3390/cells8080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: A journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16:488–509. doi: 10.1093/humupd/dmq002. [DOI] [PubMed] [Google Scholar]
- Stehling O, Wilbrecht C, Lill R. Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie. 2014;100:61–77. doi: 10.1016/j.biochi.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Stewart JB, Chinnery PF. The dynamics of mitochondrial DNA heteroplasmy: Implications for human health and disease. Nat Rev Genet. 2015;16:530–42. doi: 10.1038/nrg3966. [DOI] [PubMed] [Google Scholar]
- Stewart JB, Freyer C, Elson JL, Wredenberg A, Cansu Z, Trifunovic A, Larsson NG. Strong purifying selection in transmission of mammalian mitochondrial DNA. PLoS Biol. 2008;6:e10. doi: 10.1371/journal.pbio.0060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: Paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2007;135:111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- Sun Z, Terragni J, Borgaro JG, Liu Y, Yu L, Guan S, Wang H, Sun D, Cheng X, Zhu Z, et al. High-resolution enzymatic mapping of genomic 5-Hydroxymethylcytosine in mouse embryonic stem cells. Cell Rep. 2013;3:567–576. doi: 10.1016/j.celrep.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitin tag for sperm mitochondria. Nature. 1999;402:371–372. doi: 10.1038/46466. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Fuku N, Takeyasu T, Guo LJ, Hirose R, Kurata M, Borgeld HJW, Yamada Y, Maruyama W, Arai Y, et al. Golden mean to longevity: Rareness of mitochondrial cytochrome b variants in centenarians but not in patients with Parkinson’s disease. J Neurosci Res. 2002;70:347–355. doi: 10.1002/jnr.10444. [DOI] [PubMed] [Google Scholar]
- Thundathil J, Filion F, Smith LC. Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev. 2005;71:405–13. doi: 10.1002/mrd.20260. [DOI] [PubMed] [Google Scholar]
- Tomas L. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL. Oxidative phosphorylation-dependent and -independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod. 2000;62:1866–74. doi: 10.1095/biolreprod62.6.1866. [DOI] [PubMed] [Google Scholar]
- Udagawa O, Ishihara T, Maeda M, Matsunaga Y, Tsukamoto S, Kawano N, Miyado K, Shitara H, Yokota S, Nomura M, et al. Mitochondrial Fission Factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr Biol. 2014;24:2451–2458. doi: 10.1016/j.cub.2014.08.060. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Volaco A, Cavalcanti AM, RP, Filho, Precoma DB. Socioeconomic status: The missing link between obesity and diabetes mellitus? Curr Diabetes Rev. 2018;14:321–326. doi: 10.2174/1573399813666170621123227. [DOI] [PubMed] [Google Scholar]
- Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet. 2008;40:1484–1488. doi: 10.1038/ng.258. [DOI] [PubMed] [Google Scholar]
- Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Harada Y, Miyado K, Kono T. Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Mol Hum Reprod. 2014;20:1090–1100. doi: 10.1093/molehr/gau064. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Bioenergetic origins of complexity and disease. Cold Spring Harb Symp Quantit Biol. 2011;76:1–16. doi: 10.1101/sqb.2011.76.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Bioenergetics in human evolution and disease: implications for the origins of biological complexity and the missing genetic variation of common diseases. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120267. doi: 10.1098/rstb.2012.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC, Chalkia D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb Perspect Biol. 2013;5:a021220. doi: 10.1101/cshperspect.a021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–1612. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tang SB, Song XB, Deng TF, Zhang TT, Yin S, Luo SM, Shen W, Zhang CL, Ge ZJ. High-glucose concentrations change DNA methylation levels in human IVM oocytes. Hum Reprod. 2018;33:474–481. doi: 10.1093/humrep/dey006. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Josefowicz WJ. Oocyte development in the mouse: An ultrastructural comparison of oocytes isolated at various stages of growth and meiotic competence. J Morphol. 1978;156:209–235. doi: 10.1002/jmor.1051560206. [DOI] [PubMed] [Google Scholar]
- Wei W, Tuna S, Keogh MJ, Smith KR, Aitman TJ, Beales PL, Bennett DL, Gale DP, Bitner-Glindzicz MAK, Black GC, et al. Germline selection shapes human mitochondrial DNA diversity. Science. 2019;364:eaau6520. doi: 10.1126/science.aau6520. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Hayama T, Mitalipov S. Mitochondrial genome inheritance and replacement in the human germline. EMBO J. 2017;36:2177–2181. doi: 10.15252/embj.201797606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus - oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC, Norman RJ, Febbraio MA, Carroll J, Robker RL. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681–691. doi: 10.1242/dev.114850. [DOI] [PubMed] [Google Scholar]
- Xu K, Chen G, Li X, Wu X, Chang Z, Xu J, Zhu Y, Yin P, Liang X, Dong L. MFN2 suppresses cancer progression through inhibition of mTORC2/Akt signaling. Sci Rep. 2017;7:41718. doi: 10.1038/srep41718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Chian RC. Development of invitro maturation techniques for clinical applications. Fertil Steril. 2017;108:577–584. doi: 10.1016/j.fertnstert.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Yasukawa K, Oshiumi H, Takeda M, Ishihara N, Yanagi Y, Seya T, Kawabata S, Koshiba T. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Miyatake T, Attardi G. Heteroplasmic mitochondrial tRNA(Lys) mutation and its complementation in MERRF patient-derived mitochondrial transformants. Muscle Nerve Suppl. 1995;3:S95–101. doi: 10.1002/mus.880181420. [DOI] [PubMed] [Google Scholar]
- Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, III, Horvath T, Seli E. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019;10:560. doi: 10.1038/s41419-019-1799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, Horvath T, Seli E. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging. 2019;11:3919–3938. doi: 10.18632/aging.102024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Khrapko K, Coller HA, Thilly WG, Copeland WC. Origins of human mitochondrial point mutations as DNA polymerase γ-mediated errors. Mut Res. 2006;599:11–20. doi: 10.1016/j.mrfmmm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Zorzano A, Hernández-Alvarez MI, Sebastián D, Muñoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal. 2015;22:1020–31. doi: 10.1089/ars.2014.6208. [DOI] [PubMed] [Google Scholar]