Abstract
Signal transducers and activators of transcription 3 (STAT-3) is a transcription factor that regulates the gene expression of several target genes. These factors are activated by the binding of cytokines and growth factors with STAT-3 specific receptors on cell membrane. Few years ago, STAT-3 was considered an acute phase response element having several cellular functions such as inflammation, cell survival, invasion, metastasis and proliferation, genetic alteration, and angiogenesis. STAT-3 is activated by several types of inflammatory cytokines, carcinogens, viruses, growth factors, and oncogenes. Thus, the STAT3 pathway is a potential target for cancer therapeutics. Abnormal STAT-3 activity in tumor development and cellular transformation can be targeted by several genomic and pharmacological methodologies. An extensive review of the literature has been conducted to emphasize the role of STAT-3 as a unique cancer drug target. This review article discusses in detail the wide range of STAT-3 inhibitors that show antitumor effects both in vitro and in vivo. Thus, targeting constitutive STAT-3 signaling is a remarkable therapeutic methodology for tumor progression. Finally, current limitations, trials and future perspectives of STAT-3 inhibitors are also critically discussed.
Keywords: STAT-3, DNA binding domain, apoptosis, drug discovery STAT-3 inhibitors
Introduction
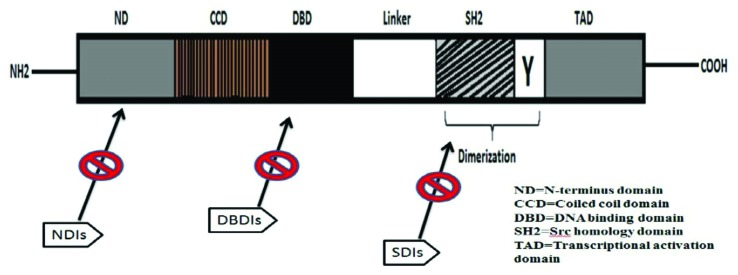
In 1994, STAT-3 was identified as a DNA transcription factor, bound with interleukin-6 responsive element in the promoter region of hepatic acute phase genes in response to IL-6 (Akira et al., 1994). Moreover, it was identified as a DNA binding protein that was found to be expressed in response to epidermal growth factor response (Zhong et al., 1994). The specific gene for encoding STAT-3 is located on the long arm of chromosome 17 at position 21 (17q21). This specific gene encodes a protein made up of 770 amino acids having molecular weight of up to 92 kDa. This protein structure can be divided into DNA binding domain, coiled coil domain (CCD), N-terminus domain (NTD), C-terminal domain (CTD) also called transactivation domain, and SH2 domain (Figure 1).
Figure 1. STAT-3 protein is segmental in organization. It contains an N-terminus domain (ND), coiled-coil domain (CCD), DNA-binding domain (DBD), Src homology 2 (SH2) domain, linker domain, Tyr (Y) residue, and the transactivation domain (TAD).
Serine and tyrosine are present at residues 727 and 705 positions respectively in cytosolic C-terminal or transactivation domain. These residues undergo phosphorylation after STAT-3 activation by cytokines and growth factors constituting Epithelial Growth Factor (EGF) (Cao et al., 1996), Platelet-Derived Growth Factors (PDGF) (Vignais et al., 1996), IL-6 (Akira et al., 1994) that will also stimulate the cytosolic proto-oncogenes and tyrosine protein kinases like Src (Yu et al., 1995), and Ras protein (Giordano et al., 1997; Aggarwal et al., 2009). Moreover, various other carcinogens have been recognized to initiate the expression of STAT-3 like cigarette smoke (Arredondo et al., 2006), polychlorinated biphenyls, and 7,12-dimethylbenz[a]anthracene(DMBA) (Tharappel et al., 2002; Chan et al., 2004).
STAT-3 signaling via IL6α receptor and Gp 130 subunit
Cancer progression and inflammatory responses are associated with cytokines activity mediated by STAT-3. The activation of STAT-3 is also carried out by other plasma membrane receptors, like tyrosine kinases comprising EGF and c-Met (Boccaccio et al., 1998; Gao et al., 2007; Quesnelle et al., 2007). Two of the functionally most important families of cytokines are IL6 and IL10. Glycoprotein 130 (Gp 130) is a general receptor subunit for interleukin-6 family of cytokines. Other ligands include cardiotrophin-1 (CT-1), ciliary neurotrophic factor (CNTF), IL-11, leukemia inhibitory factor (LIF), and oncostatin M (OSM), (Hibi et al., 1990; Hirano et al., 1997). Gp 130 arbitrates signals that are important in the immune, nervous, hematopoietic, cardiovascular and endocrine systems, bone metabolism, inflammation, plasmacytoma genesis, acute phase response, liver regeneration, osteoporosis, and hepatocyte maturation (Yamasaki et al., 1988; Suematsu et al., 1989, 1992; Lord et al., 1991; Brem and Thoenen, 1993; Escary et al., 1993; Kopf et al., 1994, 1998; Ramsay et al., 1994; Hilbert et al., 1995; Cressman et al., 1996; Romani et al., 1996; Yoshida et al., 1996; Kumanogoh et al., 1997; Betz et al., 1998; Hirota et al., 1999; Kamiya et al., 1999; Ohtani et al., 2000).
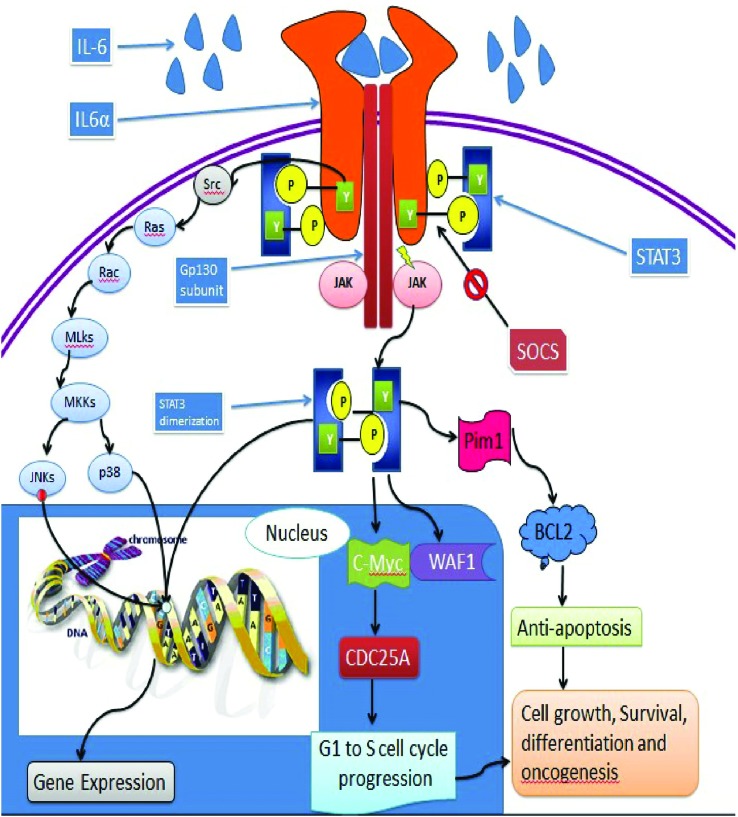
Activation of Gp 130 homo-dimerization is done by binding of IL6 and IL11 to IL6Rα and IL11Rα receptor subunits respectively, while activation of hetero-dimeric Gp 130 receptor complexes is done by some of the IL-6 family ligands (consisting of CNTF, LIF, CT-1, IL27, and oncostatin M) (Table 1) (Lacy et al., 2007). Binding of Gp 130 with ligands activates the cytosolic kinases, like Janus kinases (Jak1, Jak2, and Tyk2) (Guschin et al., 1995; Ernst et al., 1996) and cause the phosphorylation of tyrosine residues in the cytoplasmic region of the cytokine receptor (Figure 2). The activation of STAT-3 also requires cytoplasmic tail of Gp 130 having four distal membrane residues while its regulation requires binding of activated Gp 130 with SOCS3 that results in its proteosomal degradation.
Table 1. Activators and inhibitors of STAT-3.
| Activators | Inhibitors | |||||
|---|---|---|---|---|---|---|
| Cytokines | Growth factors | Carcinogens | Others | Synthetic | Natural | Others |
| Cardiotrophin-1 | EGF | Diesel & exhaust particles | Bile acids | AG 490 | Caffeic acid | EKB569 |
| IFN-γ | PDGF | Tobacco | Diazoxide | Aurothiomalate | Chalcone | Rituximab |
| IL-6 | TGF-α | UVB | Genistein3 | BMS-354825 | Cucurbitacin | GQ-ODN |
| IL-10 | G-CSF | HCV | Isoliquiritigenin | CADPE | Emodin | TKS –050 |
| IL-11 | GM- CSF | LPS | Leptin | Ethanol | Genistein | STA21 |
| IL-21 | RUNX1 | RSV | Morphine sulfate | PDP | Indirubin | Retinoic acid |
| IL-27 | ISRE | HPV | Olanzapine | PS-341 | Parthenolide | TGF-β1 |
| LIGHT | CRE | CNTF | Black soy peptides | S31-M2001 | Piceatannol | 17β-estradiol |
| MIP-1α | FGF1 | LIF | CaMKIIg Acid | Sodium | Resveratrol | COX-2 |
| RANTES | FGF2 | Sphingomyelinase Acid | Statin | Capsaicin | ||
| SLF25 | IGF-IR | Ceramidase | T40214 | CDDO-Me | ||
| MCP-1 | CSF1R | Colivelin | Atiprimod | Curcumin | ||
| CNTF | AgtR2 | Ruxolitinib Phosphate | Auranofin | EGCG | ||
| IL-5 | GPCRs | Stattic | Flavopiridol | |||
| IL-9 | Dobesilate | Ursolic acid | ||||
| IL-12 | Platinum compounds | Guggulsterone | ||||
| IL-22 | Y(p)LPQTV | Magnolol | ||||
| TNF-α | ||||||
Figure 2. Polypeptides bind to their associated receptors and trigger the tyrosine (Tyr) kinase (TK) events of the receptors, Src or JAKs. Underlying STAT-3 action is the stimulated receptor for phosphorylation on specific Tyr residue by TKs that causes STAT-3:STAT-3 dimerization and stimulation. STAT-3 mounts up in the nucleus, where it binds to specific DNA response elements in the promoters of target genes, which causes gene transcription.
The activated growth factor receptors Janus kinases (JAKs) or Src tyrosine kinase cause activation of STAT3 that begins with the phosphorylation of a critical tyrosine residue (Tyr705) on its Src homology 2 (SH2) domain. Upon activation, STAT3 forms dimers through a reciprocal phosphotyrosine (pTyr705):SH2 domain interaction that translocate to the nucleus where the dimers bind to the promoters of target genes and activate specific gene expression. The activated JAKs phosphorylate serine residues of the STAT-3 at position 727. Transcriptional activity of STAT-3 is modulated by phosphorylation of serine residue at position 727 of many proteins like protein kinases C (PKC) (Jain et al., 1999), CDK5, and mitogen activated protein kinases (Fu et al., 2004). PKC-ε binds with the STAT-3 and phosphorylates its serine residue at position 727, which enhances its transcriptional activity (Wen and Darnell, 1997; Yokogami et al., 2000; Aziz et al., 2007; Yue and Turkson, 2009).
Various types of post-translational modifications, besides Y705 phosphorylation, are required for the activation of STAT-3. S727 phosphorylation of STAT-3 by extracellular signal regulated kinase 1 and 2 (ERK1/2) induces full activation of STAT-3 (Couto et al., 2012). Dimerization of STAT-3 is mediated by a histone acetyltransferase p300 by acetylation at position K685, which is reversible by the type I histone deacetylase (HDAC) (Plaza-Menacho et al., 2007). K180 methylation of STAT-3 is also required for its activation, mediated by a lysine methyltransferase EZH2 included in the polycomb repressive complex 2 (PRC2) (Yuan et al., 2005).
Inhibition of STAT-3
There are several mechanisms that negatively regulate STAT-3 activity by the protein inhibitor of activated STAT (PIAS), suppressors of cytokine signaling (SOCS), ubiquitination dependent proteosomal degradation, and protein phosphatases (Table 1) (Stahl et al., 1995; Chung et al., 1997; Starr et al., 1997; Daino et al., 2000)
Inhibition by SOCS
Blocking of subsequent signaling, which requires phosphorylation and activation of STAT-3, is carried out by the binding of SOCS proteins with the JAK activation loop having SH2 domain (Zhang et al., 1999). Until now, there are eight different SOCS proteins that are identified with same structures (Yoshimura et al., 2007). In mouse skin wound healing, SOCS-3 modulates the Gp 130-STAT-3 signaling pathway, which shows that STAT-3 is essential for wound healing (Zhu et al., 2008). Similarly, tissue-specific SOCS3 aberrations in mice upregulate the signaling of ligand-dependent Gp 130, and substituting Y757F tyrosine-to-phenylalanine in the respective Gp 130Y757F knock-in mutant mice and results in hyperactivation of STAT-3 and STAT-1 (Tebbutt et al., 2002; Jenkins et al., 2005).
STAT-1 and STAT-3 are able to regulate each other in the framework of Gp 130 mediated STAT activation (Ernst et al., 2008; Musteanu et al., 2010). Likewise, in normal macrophages, inflammatory responses are generated when STAT-3 is stimulated by binding of IL-6 (Unver et al., 2018). However, in Gp 130 Y757F mutant macrophages, the induction of transcriptional repressor can inhibit inflammatory gene responses in STAT-3 signaling by IL-6 (El Kasmi et al., 2007; Murray, 2007). Therefore, an efficient anti-inflammatory feedback can be stimulated with continuous Gp 130 and STAT-3 activation in SOCS3-deficient macrophages (Croker et al., 2003; Lang et al., 2003; Yasukawa et al., 2003; Jenkins et al., 2005).
Inhibition by PIAS-3 and other deregulators
Another mechanism to inhibit the STAT-3 regulation is PIAS. PIAS-3 inhibits the process of transcription by collaborating with phosphorylated STAT-3 (Chung et al., 1997) that is produced by attachment of phosphate groups during normal regulation (Saini et al., 2018). In pancreatic cancerous cells, Smad4 inhibits the tyrosine phosphorylation of STAT-3 (Zhao et al., 2008). Activation of STAT-3 is also inhibited by various protein tyrosine phosphatases, comprising CD-45 (Irie-Sasaki et al., 2001), PTEN (Sun and Steinberg, 2002), SHP-1 (Migone et al., 1998), and SHP-2 (Schaper et al., 1998)
Inhibition via ubiquitin degradation
Another pathway to inhibit STAT-3 regulation is the ubiquitin-proteasome pathway. This pathway is essential for depletion of different transitory cellular proteins. It also controls the regulatory mechanism of cellular processes. STAT-3 degradation occurs through this pathway (Daino et al., 2000; Perry et al., 2004; Ulane et al., 2005). In IL-6-dependent KT-3 cells, proteosomal degradation of STAT3 occurs when it binds with the biotinylated ubiquitin, without effecting the expression of STAT1 and STAT5 (Daino et al., 2000). Moreover, caspases also inhibit STAT-3 (Darnowski et al., 2006). STAT-3 signaling inhibition is due to reduction in STAT-3 binding with DNA, STAT-3-driven reporter protein (luciferase) activity, STAT-3-dependent genes, and due to increased sensitivity to apoptotic stimuli.
STAT-3 is essential for many interconnected signaling pathways. Enhanced STAT-3 activity in cancer can be due to excess of growth factors and IL6-family cytokines in the cancer microenvironment. STAT-3 activation or secretion of inflammatory agents occurs by proto-oncogenes activation, tumor-suppressor genes, chromosomal rearrangement, and other genomic alterations in tumor cells. Unexpectedly, there is no inherent confirmation for triggering the mutations in STAT-3 itself. However, in many cases, frame deletion mutations in Gp 130 and point mutations in Jak2 (Morgan and Gilliland, 2008) promote the ligand-independent activation of STAT-3 found in hepatocellular carcinomas (Rebouissou et al., 2008). Under biological conditions, the stimulation of STAT-3 affects several inhibitory proteins, which also deregulate STAT-3 activity (Greenhalgh and Hilton, 2001). The abnormality in STAT-3 causes embryonic lethality, (Takeda et al., 1997) and tissue-specific abnormality that causes the destruction of liver cells (Niu et al., 2002), macrophages (Takeda et al., 1999), keratinocytes (Sano et al., 1999) and mammary or thymic epithelial cells (Chapman et al., 1999).
Different functions of STAT-3
Inflammation
STAT-3 is a mediator of inflammation, suggested by various lines of evidences (Pfitzner et al., 2004). Initially, STAT-3 was revealed as an acute-phase response protein, due to its inflammatory responses. Second, STAT-3 is activated mainly by pro-inflammatory agents, such as IL-6 that is a most important mediator of inflammation in the STAT-3 pathway (Zhong et al., 1994). Likewise, cigarette smoke, tumor promoters, and lipopolysaccharides also can trigger the STAT-3 pathway (Kobierski et al., 2000; Arredondo et al., 2006). Third, binding of acute-phase proteins on DNA promoter region compete with NF-κB, alternative pro-inflammatory transcription factor (Zhang and Fuller, 1997). Fourth, in accessory cells, NF-κB binding to the IL-12p40 promoter is controlled by STAT-3 (Hoentjen et al., 2005). Fifth, oncogenic transformation via IL-11 and its Gp 130 receptor in inflammation-associated gastric epithelial cell is triggered by and dependent on enhanced expression of STAT-3 (Ernst et al., 2008). Sixth, in various cell types IL-6-triggered STAT-3 activation has been shown to be dependent on cyclooxygenase 2 (Dalwadi et al., 2005). All this verification supports the function of the STAT-3 pathway in inflammation.
Transformation of cells
The stimulation of STAT-3 is mediated by various oncogenes having direct or indirect effects of STAT-3, protein tyrosine kinases, and viruses if transformed in to cells (Frank, 2007) proceeded by Src protein kinase (Yu et al., 1995; Bromberg et al., 1998). Similarly, in case of gastric cancer, the activation of STAT-3 by human T-cell lymphotropic virus I can transform T cells, involving a direct effects of STAT-3 and epithelial-mesenchymal transition (EMT). While the activation of cells in the microenvironment is dependent on indirect effects of STAT-3 (Migone et al., 1995). Activation of STAT-3 is triggered by polyoma virus middle T antigen (v-Fps) that triggers Src family kinases, or activation is triggered by v-Sis, acting as a ligand for PDGF-R (Garcia et al., 1997). STAT-3 signaling is also required for hepatocyte growth factor-Met mediated tumor genes factor. Cell growth in soft agar, cell transformation, and tumors in nude mice are stimulated by STAT-3. The activated form of STAT-3 detected in tumors confirms that it is an oncogene (Bromberg et al., 1999).
Apoptosis suppression
The activation of STAT-3 can be triggered by oncogenic transformation of the cells responsible for the survival signal. Conditional inactivation of STAT-3 has pro-apoptotic functions during mammary gland involution (Chapman et al., 1999). In most cells, STAT-3 activation can be suppressed by apoptosis. The induction of these special effects appears due to several gene products that are synchronized by STAT-3. These include BCL-2, (Zushi et al., 1998), survivin (Mahboubi et al., 2001), BCL-XL (Catlett-Falcone et al., 1999; Karni et al., 1999), MCLl-1 (Karni et al., 1999), CyclinD1 (Munoz et al., 2014), and CLap2 (Bhattacharya and Schindler, 2003). Furthermore, tumor cells exhibiting constitutive activation of STAT-3 also express cell survival genes (Aoki et al., 2003; Kanda et al., 2004). Suppression of STAT-3 activation also suppress the expression of all cell survival gene products, that potentiate apoptosis (Konnikova et al., 2003). Down-regulation of STAT-3 is promoted by apoptosis, leading to expression of FAS protein (Ivanov et al., 2001).
Cellular proliferation
STAT-3 activation can also be linked with proliferation of tumor cells, because it induces the expression of cyclin D1 (Masuda et al., 2002). STAT-3 increases the expression of numerous growth-promoting genes, like pim-1 (Kiuchi et al., 1999) and myc (Shirogane et al., 1999). Other reports suggested that STAT-3 can downregulate the expression of cell cycle inhibitor p21(waf1), showing abnormal cell production and cell cycle succession (Bellido et al., 1998) by means of p21 (Roninson, 2002). In cellular transformation, STAT-3, without varying the regulation of myc promoter, was found to impede the transcriptional stimulation of the p21 gene in the Akt pathway (Barré et al., 2003). Two stages of polymorphism have also been found to be vital in producing a negative response and in causing certain types of tumors (Xin-hua and Rui-yu, 2012).
Cellular invasion
Numerous reports indicated that STAT-3 activation plays the most significant function in tumor cell invasion, and its inhibition reduces invasion (Barré et al., 2003; Ma et al., 2007; Yakata et al., 2007; Xiong et al., 2008; Zhao et al., 2008). The expression of matrix metalloproteinase (MMP)-2 and MMP-1 is regulated by STAT-3 activation that initiates tumor invasion and metastasis (Xie et al., 2004; Itoh et al., 2005). Direct interaction of STAT-3 with MMP-2 up-regulates the transcription of MMP-2. In highly metastatic cells, invasiveness of the tumor cells is suppressed by obstruction of activated STAT-3, while metastasis of cutaneous squamous cell carcinoma is associated with overexpression of phosphorylated STAT-3 (Suiqing et al., 2005). Tissue inhibitors of metalloproteinase hinders the activity of metalloproteinases, which are upregulated by STAT-3, and decrease invasiveness in certain cancer cells (Dien et al., 2006). The expression of the MUC1 gene mediates tumor invasion and it is also controlled by STAT-3 (Gaemers et al., 2001). Thus, STAT-3 tumor invasion can be induced through several mechanisms.
Metastasis and angiogenesis
A STAT-3 link to angiogenesis was revealed by factor-induced angiogenetic action in chick chorioallantoic membrane stimulated from granulocyte-macrophage colony (Valdembri et al., 2002). Tumor angiogenesis and Vascular Endothelial Growth Factor appearance is also due to STAT-3 upregulation (Niu et al., 2002). Activated STAT-3 in the majority of cancer cells has an effect on vascular endothelial growth factor (VEGF) (Wei et al., 2003a,b). Consequently, downregulation of STAT-3 can suppress the appearance of VEGF and reduce angiogenesis. Metastasis of human hepatocellular carcinoma and inhibition of growth was found by antisense oligonucleotide targeting of STAT-3 (Li et al., 2006). STAT-3 activation was also associated with metastasis of human melanoma to brain (Xie et al., 2006). Moreover, VEGF and TWIST, another mediator of tumor metastasis, were synchronized by STAT-3 in an in-vivo analysis, whereas chemoresistance, angiogenesis, and cell survival were induced in an in-vitro study (Cheng et al., 2008)
Carcinogenesis
The stages of carcinogenesis, such as tumor initiation and tumor progression, are enhanced by irregularities in the STAT-3 signaling pathway. Skin cancer can be suppressed by blocking aberrant expression of STAT-3 (Nagpal et al., 2002; Chan et al., 2004; Ahsan et al., 2005). When 12-O-tetradecanolyphorbol-13-acetate was used as the promoter and 9,10- dimethylbenz-[a-]anthracene was used as an activator, development of skin tumor was entirely opposed in STAT-3-deficient mice (Dvorak et al., 2007). The activation of STAT-3 is an early event in oral carcinogenesis by tobacco consumption (Jang et al., 2008). The triggering of STAT-3 has also been associated with hepatocarcinogenesis (Ahn et al., 2008).
Radioresistance and chemoresistance
The aberrant activation of STAT3 in cancerous cells is also associated with chemo- and radioresistance (Real et al., 2002; Bharti et al., 2004; Boehm et al., 2008). This resistance was shown by the up-regulation of anti-apoptotic genes activated by STAT-3 (Bhardwaj et al., 2007). Therefore, it is suggested that chemoresistance can be stroked by modulating STAT-3 activation (Otero et al., 2006). Several studies indicated that the deletion of STAT-3 causes B cells to become hyper responsive to irradiation (Drews, 2000). In vivo studies on gene-targeted mice showed that binding of IL-6 and IL-10 and some other BCR ligands to the receptors is associated with B1 cell radioresistance.
Epithelial mesenchymal transition (EMT)
EMT is the binding of epithelial cells with mesenchymal cancer-associated fibroblasts (CAFs), which results in loss of adhesion among cells, causing tumor progression (Larue and Bellacosa, 2005). In cancers mediated by STAT-3, EMT gets involved in cancer progression by the IL-6 and JAK-STAT-3 pathway. In cases of gastric cancer, CAFs promote EMT in cells by secreting enough IL-6 that in turns activates the STAT-3 pathway. The minimization effect of IL-6 and use of AG490 inhibits STAT-3 that results in tumor metastasis induced by CAFs in vivo. CAFs are important in cancer progression by indirect inhibition of the JAK/STAT pathway in a microenvironment (Wu et al., 2017a; Karakasheva et al., 2018).
Prevention of ROS production in mitochondria during stress
STAT-3 expression has a crucial role in cardiac protection against stresses. STAT-3 influences the activity of cytochrome I and II in mitochondria. The deletion of STAT-3 in mice cardiomyocytes was found to reduce the activity of complex I and II up to 50%. But in the case of overexpression of transcriptionally inactive STAT-3, the activity of both complexes was reduced only by 20%. Moreover, the overexpressed STAT-3 in mitochondria in comparison to wild type mitochondria showed protection from ischemia. Ischemia causes the production of reactive oxygen species (ROS) from complex I in case of wild type mitochondria, which delocalizes cytochrome c from the inner mitochondrial membrane and eventually results in its release from mitochondria causing apoptosis. On the contrary, overexpressed STAT-3 mitochondria blocked ROS production, preventing cytochrome c release and hence apoptosis (Szczepanek et al., 2011, 2012).
Embryonic development
OCT4 is a transcriptional factor responsible for the regulation of several genes, including NANOG and SOX2, and maintenance of embryonic stem cells pluripotency. STAT-3 is also expressed in the embryo and is involved in preserving the pluripotency of ESCs by interacting with OCT4 and NANOG through regulating klf4 (Do et al., 2013). Elimination of STAT-3 in mouse embryonic cells showed the importance of STAT-3 in retaining inner cell mass (ICM) pluripotency, but the phosphorylation of STAT-3 by β-catenin and E cadherin is essential for this role. The blockage of E cadherin inhibits STAT-3 phosphorylation, disabling pluripotency preservation. Alternatively, N cadherin can also perform the E cadherin task (Hawkins et al., 2012).
Axonal degeneration
Degeneration of axons starts with changes in distal axon and presynaptic terminals, leading to irreparable damage and eventually death of brain cells. In axon regeneration, plasticity is maintained by neurotrophic factors released by the surrounding cells, especially Schwann cells. Ciliary neurotrophic factor (CNTF) and other neurotrophic factors are produced by Schwann cells for this function. CNTF activates STAT-3, which in turn interacts with stathmin. Stathmin is a protein that binds with the α/β-tubulin heterodimers, preventing the assembly of MT. STAT-3 resultantly promotes the regeneration of microtubules, preventing MT from destabilization, as studied in pmn mutant motoneurons (Selvaraj et al., 2012).
Strategies to target STAT-3 for novel cancer drugs
Understanding the mechanisms of STAT-3 activation and transcriptional events from the receptor on cell surface to the nucleus may provide different approaches to target STAT-3 in cancer therapeutics. Since the objective for drug discovery is to target a disease, it is essential to determine whether the proposed target can effectively bind with a specific drug. Drug affinity with a target depends on disease development, mode of action of the drug, and consequences of the approach of drug target. Research showed strong association of the location of the target relative to the disease (Gibbs, 2000; Turkson, 2004). It is well-known that STAT-3 plays a significant role as a principal regulator of biological and molecular actions. In addition, deregulation of STAT-3 leads to tumorigenesis (Yu and Jove, 2004; Darnell, 2005; Siddiquee et al., 2007a). Therefore, targeting abnormal STAT-3 action by pharmacological or genetic approaches triggered apoptosis and growth arrest of tumor cells in vitro and tumor reversion in vivo (Darnell, 2005; Jing et al., 2006; Leeman et al., 2006; Siddiquee et al., 2007b; Weerasinghe et al., 2007). Actually, various studies showed STAT-3 as a promising cancer drug target, so a rationale can be developed for the discovery and design of anti-cancerous drugs (Table 2).
Table 2. Targets and Inhibitors of the STAT-3 signaling pathway.
| Site of action | Class | Preclinical Data | Clinical Data | Challenges |
|---|---|---|---|---|
| Inhibitors of STAT-3 stimulation | JAK1/2 inhibitor Ruxolitinib | Tumor xenograft models | primary myelofibrosis (phase III) | Unfeasible in most cancers |
| Small-molecule JAK inhibitors (LS-104, AG490, CEP701and ICNB18424) | (phase II) | Insignificant myelosuppression and a minor but recurrent GI toxicity | ||
| SH2 domain dimerization inhibitors | Peptides | Src-transformed | Nil | Poor cellular permeability in vivo stability |
| (XpYL) | fibroblasts | |||
| Peptidomimetics | Breast, NSCLC | |||
| (ISS610) | Src-transformed | |||
| fibroblasts | ||||
| Small molecule | Breast, sarcoma, Nil | Lack of potency and specificity | ||
| inhibitor | GBM | |||
| STA-21 and | GBM | |||
| analogues | Breast | |||
| LLL-3 | Breast, HCC, GI | |||
| S3I-201 | Breast | |||
| Static | CRC | |||
| OPB-31121 | Liver | |||
| OPB-51602 | Lung | |||
| Natural | RCC | Advanced hematologic and solid tumors (Phase I) | Unexpected toxicities Unpredictable PK properties | |
| compounds | Breast | |||
| Curcumin | Pancreas, HCC, | |||
| Curcumin | GI | |||
| analogues | NSCLC | |||
| (FLLL11, FLLL12, FLLL32) | ||||
| N terminal domain inhibitors | Synthetic analogues for STAT-3 helix 2 | Inhibited cell growth and apoptosis of human MCF-7, MDA-MB-435 and MDA-MB-231 breast cancer cells | Peptides interaction with N-terminal domain and their mechanisms of action are still unknow | |
| Synthetic peptide | ||||
| ST3-H2A2 | Cause blockage of STAT-3 dimerization and apoptosis in prostate cancer cell lines | |||
| Upstream tyrosine kinase inhibitors | Small molecule | EGFR NSCLC | Neurologic dose-limiting toxicities | |
| inhibitors | NSCLC | Phase I | Toxicity and lack of efficacy | |
| AZD1480 | HNSCC | Phase II | Lack of potency and specificity | |
| (JAK1/2) | ||||
| Dasatinib (Src) | ||||
| Natural | HCC, breast, | Nil | ||
| compounds | pancreas | |||
| Butein Capsaicin | Prostate, gastric | |||
| Inhibitors of Nuclear Translocation | Karyostatin | Nil | Inhibition of general trafficking through the nuclear membrane is probable to be harmful | |
| 1ARajtadoneB | Human lung | |||
| Leptomycin | carcinoma cell | |||
| Bimax1, bimax2 | lines | |||
| mouse | ||||
| neuroblastoma | ||||
| cell lines | ||||
| HeLa cell lines | ||||
| STAT3 pathway oligonucleotides | Antisense oligonucleotide | Lymphoma | Phase I/II | Rapid degradation, not amenable to systemic administration |
| AZD9150 | ||||
| Phase 0 | ||||
| STAT3 decoy oligonucleotide | NSCLC | |||
| (DNA-binding) | NSCLC, colorectal | |||
| glioma | ||||
| STAT3 post-transcriptional | Breast, brain, SCC | |||
| (siRNA) | ||||
| STAT3 DNA-binding domain | Platinum IV compounds, | Breast | ||
| Colon | Nil | Lack of specificity | ||
| IS3295, | NSCLC | |||
| CPA-1, | ||||
| CPA-7, |
Abbreviations: RSV: Respiratory syncytial virus, ISRE: interferon-stimulated responsive element, CRE: cyclic AMP-responsive element, FGF: fibroblast growth factor, HPV: human papillomavirus infection, IGF-IR: insulin-like growth factor I receptor, VEGF: vascular endothelial growth factor, CNTF: Ciliary neurotrophic factor, LIF: Leukemia inhibitory factor, CSF1R: Colony Stimulating Factor-1 Receptor, GPCRs: G-Protein Coupled Receptors, : Angiotensin-II Receptor.
Strategies to prevent STAT-3 stimulation
Targeting JAK/STAT signaling is the most used therapy in patients with myeloproliferative disorders, majority of which show the oncogenicJAK2V617F mutation. These gain-of-function mutations generate constitutive activation of JAK/STAT signaling, especially through STAT-3 and STAT-5. According to a phase III randomized-controlled study of the JAK1/2 inhibitor ruxolitinib, a prolonged survival was found with the inhibitor in comparison to the best available therapy in primary myelofibrosis (Harrison et al., 2012). One way to prevent STAT-3 activation is to inhibit the tyrosine kinase events at receptor level, which is unfeasible in most cancers. (Jing and Tweardy, 2005; Quesnelle et al., 2007; Boehm et al., 2008; Wilks, 2008; Egloff and Grandis, 2009; Sen et al., 2009a; Santos et al., 2010; Verstovsek et al., 2010).
Small-molecule JAK inhibitors (for example, LS-104, AG490, CEP701, and ICNB18424) have been tried in tumor xenograft models before several clinical trials (Sen et al., 2009a; Santos et al., 2010; Verstovsek et al., 2010). Equally in in vivo and in vitro, AG490 inhibits the action of JAK2, decreases STAT-3 levels, stops STAT-3 DNA binding, and decreases leukemic cells growth (Fletcher et al., 2009; Santos et al., 2010). Its analog LS-104 was developed for acute lymphoblastic leukemia in a level II clinical trial (Santos et al., 2010). INCB1824 suppresses phosphorylated STAT-3, which leads to V617F JAK2 gain-of-function mutation (Sen et al., 2009a; Santos et al., 2010). CEP-701, which is a JAK2 inhibitor, decreases the phosphorylation of STAT-3 in patients under therapy; however, its modest effectiveness in myelofibrosis patients is related with an insignificant myelosuppression and a minor but recurrent GI toxicity (Verstovsek et al., 2010).
The comparatively modest feedback of agents targeting JAKs from cancer patients demonstrates that single pathways possibly will not satisfactory inhibit the activation of STAT-3. Unfortunately, early phase clinical trials of JAK1/2 inhibitors (e.g. AZD1480) and Src inhibitors (e.g. dasatinib) have revealed limited efficacy or excessive toxicity in advanced solid tumors (Murakami et al., 2014). Possible explanations for toxicities and off-target adverse events are pathway redundancy and pathway cross-talk.
Strategies to prevent protein–protein interaction
Targeting protein-protein interaction involves a scattered and huge surface area in contrast to the easily `druggable’ classic binding pocket found in receptor tyrosine kinases or other enzymatic targets (Fontaine et al., 2015). Furthermore, STAT proteins share a highly homologous domain structure, making the specific targeting of STAT-3 more challenging. The main targets to prevent protein-protein interaction/dimerization in STAT-3 are SH2-domain and N-terminal domain, for which various drugs have been designed as discussed briefly below.
Targeting the STAT-3 SH-2 domain
The first successful attempt at disrupting STAT-3:STAT-3 dimerization and its downstream transcription was the discovery of a phospho-peptide inhibitor (PY*LKTK), which is obtained from the STAT-3-SH2 domain-binding peptide sequence. However, the intrinsic pharmacokinetic properties of peptides, including poor cellular permeability and lack of stabilityin vivo, have curtailed their further development. Even second-generation peptidomimetics have failed to overcome these limitations (Turkson et al., 2004).
Various approaches have been developed to inhibit protein-protein interaction. One of them is the use of pY-containing peptide. Researchers have produced pY-containing peptide structures to target the SH-2 domain of STAT-3, preventing recruitment of STAT-3 to stimulated receptors and homo-dimerization of STAT-3 (Germain and Frank, 2007; Quesnelle et al., 2007; Fletcher et al., 2009). The peptide containing pY residue was investigated to study the mechanism of STAT-3 inhibition for the first time. Inhibition activity of STAT-3 was based on the synthesis of a Y705 residue. This residue is phosphorylated, which promotes homodimerization of STAT-3, which in turn inhibits the binding of STAT-3 to the DNA in vitro (Table 3) (Germain and Frank, 2007; Fletcher et al., 2009).
Table 3. Domains of STAT-3.
| Domain | Function |
|---|---|
| N-terminus domain | The N-terminus comprises a domain (ND) that facilitates STAT dimer–dimer communications in tetramer establishment that is vital to stabilize the dimers binding to DNA. |
| Coiled-coil domain | The coiled-coil domain (CCD) connects the (ND) N-terminus domain to the (DBD) DNA-binding domain and also take part in the communications with other proteins |
| The DNA-binding Domain | The DBD creates physical connection with SRE in target genes promoters and is associated to the Src homology 2 domains via the linker domain (Linker). |
| SH2 domain | The SH2 domain is central for dimerization of two STAT3 monomers. |
| Tyr (Y) residue | On phosphorylation of the specific Tyr (Y) residue in the transcriptional activation domain (TAD), the pTyr residue of one monomer and the Src homology 2 domains of a different monomer involve in shared pTyr-SH2 domain interaction |
| The transactivation domain | The transactivation domain assists the transcriptional stimulation of target genes and can comprise a serine residue essential for utmost transcriptional activity in the circumstance of some STAT proteins, including STAT3. |
This methodology has been extended upon by a number of groups to take account of pY-containing peptide structures from further proteins that act together with SH-2 domains of STAT-3 (Germain and Frank, 2007; Fletcher et al., 2009). pY-containing motifs with the SH-2 domain of STAT-3 have been explored. These motifs contain the four distinctive pY-containing (pYXXQ) motifs inside the signal-transducing subunits of Gp 130 of the activated IL-6 receptor complex with numerous pY-containing motifs after cytokine receptor chains (for example, IL-10 receptor, granulocyte colony-stimulating factor receptor, and leukemia inhibitory factor receptor) (Frank, 2007).
Unfortunately, peptide inhibitors have poor cell absorptive capacity and metabolic solidity, which encouraged a quest for small-molecule derivatives and peptidomimetics (Germain and Frank, 2007; Fletcher et al., 2009). Numerous small-molecule inhibitors were identified by peptidomimetics and peptide-inspired coherent strategy together with in silico computational methodologies and in vitro high-output selection. In spite of developments in detecting small molecules and peptides that target the SH-2 domain of STAT-3, molecules with satisfying anticancer activity have yet to be revealed.
Another approach that blocks STAT-3-protein interactions via targeting SH-2 domain is using G-rich oligodeoxynucleotides, which produce potassium-dependent four-stranded assemblies to reside inside the SH-2 domains of STAT (Heinrich et al., 2003; Germain and Frank, 2007; Quesnelle et al., 2007; Fletcher et al., 2009). G quartet oligodeoxynucleotides may interrupt the homo-dimerization of STAT-3 activity. The large size and potassium dependence of G quartet oligodeoxynucleotide make the cell less permeable, hence providing a great challenge for in vivo studies (Fletcher et al., 2009).
Novel small molecular inhibitors that target the STAT-3-SH2 domain have been discovered through virtual screening and have demonstrated physiochemical properties, indicating their potential for clinical use. These constitute the largest class of STAT-3 inhibitors. Numerous preclinical studies have confirmed their mode of action and downstream effects on tumor cell inhibition in an array of animal models and cell lines. However, most of these compounds have yet to be explored in clinical studies due to concerns with their relative lack of potency and selectivity (Furqan et al., 2013).
OPB-51602 and OPB-31121 are the only agents in this class to have reached early phase clinical trials in advanced solid malignancies. Although signals of efficacy were observed in tyrosine kinase inhibitor (TKI)-resistant EGFR-mutant NSCLC and gastrointestinal malignancies, further development of these compounds was limited because of the concerns about unpredictable pharmacokinetics profiles and potentially severe toxicities including lactic acidosis, peripheral neuropathy, and susceptibility to opportunistic infections (Wong et al., 2015). A possible explanation for this unusual side effect profile is the ubiquitous expression of STAT-3 within the body and its diverse physiological roles, including modulation of mitochondrial metabolism and the immune system (Myers, 2009).
Targeting STAT-3 N-terminal domain
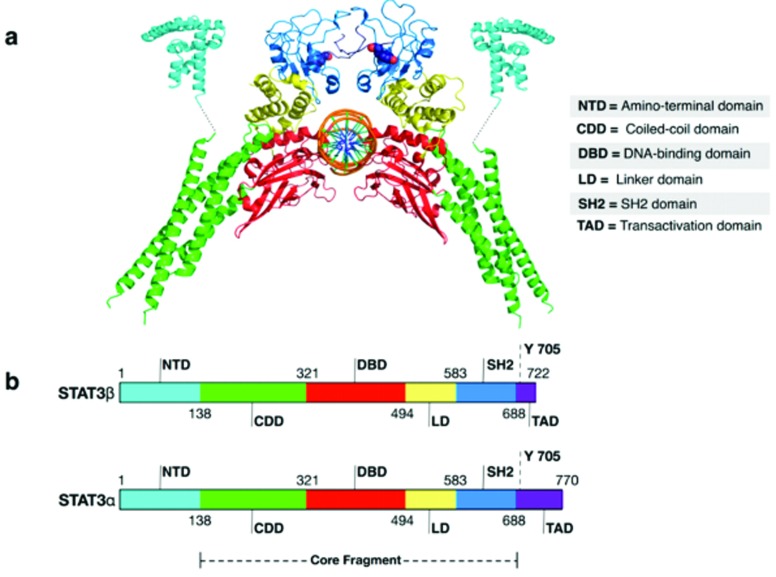
N-terminal domain is pivotal for the interaction of dimers of STAT-3 for the formation of tetramer, interaction with other regulators and the attachment of STAT-3 dimers to the sites of DNA, and protein interaction (Figure 3) (Sgrignani et al., 2018). The N-terminal domain has a leading role in dimer formation, so it is a captivating approach to inhibit the function of STAT-3. The N-terminal domain of STAT-3 protein comprises eight helices with 130 amino acids (Blaskovich et al., 2003; Timofeeva et al., 2007). Studies found that synthetic analog of STAT-4’s helix 2 can target and disturb the structure of N-terminal. Based on the knowledge of the N-terminal domain of STAT-4, a synthetic analogue for STAT-3 helix 2 was designed that specifically binds to STAT-3 instead of binding with other members of STAT. As a result, transcriptional activity of STAT-3 is inhibited without affecting phosphorylation.
Figure 3. Three dimensional structure of STAT 3 indicating its active and inhibitory sites. (a) 4Z1A PDB hit of STAT 3. (b) Different domains of STAT 3 present in loops of STAT 3.
By fusing alpha helix 2 with ε-palmitoyl lysine and three residues of penetratin, a cell permeable form of the alpha helix was produced that retards cell growth and apoptosis of human MDA-MB-435, MCF-7, and MDA-MB-231 breast cancer cells. However, details of how these peptides interact with the N-terminal domain of STAT-3 and their mechanisms of action are still unknown (Timofeeva et al., 2007). Another approach developed to inhibit dimerization via targeting the N-terminal domain is ST3-H2A2, a synthetic peptide. ST3-H2A2 selectively targets the N-terminal domain of STAT-3 and inhibits dimerization (Wake and Watson, 2015). In preclinical trials, ST3-H2A2 was found to inhibit STAT-3 N-terminal domain, resulting in the blockage of STAT-3 dimerization and apoptosis in prostate cancer cell lines (Timofeeva et al., 2013).
Strategies to prevent nuclear translocation
The function of stimulated STAT-3 depends on the transfer of homodimers from underneath the plasma membrane into the nucleus (Heinrich et al., 2003; Liu et al., 2005; Frank, 2007; Herrmann et al., 2007; Lui et al., 2007; Quesnelle et al., 2007; Fletcher et al., 2009). Stopping the transfer of STAT-3 dimers via the nuclear pore complex can be a strategy to block transcriptional activity of STAT-3 (Liu et al., 2005; Lui et al., 2007). Yet, the thorough process and uniqueness of the mechanisms that facilitate the shifting of STAT-3 from the periphery of the cell to the nucleus has to be clear. Large protein multiplexes perhaps move into the nucleus via the nuclear multiplex pore from side to side the nuclear pore composite, in a progression aided by α and β proteins (Liu et al., 2005; Lui et al., 2007). Importin β along with importins α5 and α7 have been involved in the channeling of phosphorylated STAT-3 through the nuclear pore (Liu et al., 2005).
Nonetheless, it is reported that nuclear import of STAT-3 might be self-determining for tyrosine phosphorylation and intervened through importin α3 (Lui et al., 2007). The nucleocytoplasmic transfer of STAT-3 reveals a forceful stable state among proportions of export and import (Liu et al., 2005; Lui et al., 2007). But interestingly, phosphorylated STAT-3 enters more quickly than non-activated STAT-3 (Liu et al., 2005). Inside the nucleus, dephosphorylation of STAT-3 is carried out by the nuclear PTP-TC45, a suitable substrate to facilitated transfer of exportin-1 (Lui et al., 2007). Up to present time, small-molecule inhibitors of importins α3, α5, or α7 have not been recognized. In addition to effects of Karyostatin 1A, a newly recognized importin β inhibitor, have so far to be described with respect to nuclear import of STAT-3 (Liu et al., 2005; Lui et al., 2007). This importin β inhibitor was trialed on HeLa cell lines (Soderholm et al., 2011).
By using Ratjadone A or Leptomycin B, inhibition of exportin 1 hinders nuclear import of STAT-3, as well as diminishing intensities of STAT-3 phosphorylation and STAT-3-facilitated transcription, which leads to increased apoptosis (Liu et al., 2005). Rajtadone-A was tested on N2A (mouse neuroblastoma) cell lines, while Leptomycin B was examined on human lung carcinoma cell lines (A549). In both of these trials, green fluorescence protein encoding marker genes were also transfected along with the inhibitors and then analyzed under the fluorescence microscope (Köster et al., 2003; Rahmani and Dean 2017). The importins have specific NLS (nuclear localization signals) receptors, and these importins are expressed after the stimulation of cytokines. The elements Arg214/215 and Arg414/417 are essential for the binding of STAT-3 dimer with importins.
Artificially designed inhibitor proteins bimax1 and bimax2 have strong affinity for NLS receptors ultimately inhibiting the nuclear translocation of STAT-3 (Kosugi et al., 2008; Ma and Cao 2006). For the assessment of bimax1 and bimax2, designed recombinant vectors (pDs-Red-bimax) were transfected into HeLa cell lines. After that, fluorescent microscopy was used to examine the location of red fluorescent protein. This indicated the successful transfection of bimax1 and bimax2, inhibiting the proliferation of HeLa cells (Wu et al., 2017b). Regardless, inhibition of general trafficking by any small-molecule inhibitor through the nuclear membrane is probably harmful (Liu et al., 2005). In future studies, scientists must design competitive inhibitors of importins. The cytokine stimulus for expression of importins must be blocked, or the elements Arg214/217 and Arg414/417 must be modified to prevent the nuclear translocation of STAT-3 dimer.
Indirect inhibition of the STAT-3 signaling pathway
The activation of Tyr kinases receptors is linked to the STAT-3 pathway. The transcription of STAT-3 targeted genes in tumor cells can be blocked by EGFR and Src (upstream tyrosine kinase inhibitor). Beside these inhibitors, there are other small molecules that affect upstream tyrosine kinase and hence are indirect inhibitors of the STAT-3 signaling pathway, e.g. JSI-124 (van Kester et al., 2008). This molecule suppresses phosphorylation of STAT-3 in tumor cells of humans and mouse by inhibiting upstream kinase.
Growth retardation and apoptosis in v-Src-transformed mouse fibroblast, lung and colon carcinomas, and lymphoma cell lines is observed by the administration of JSI-124 at a concentration of 10 μM (Huang et al., 2012; Nefedova et al., 2005; Su et al., 2008; Sun et al., 2005; van Kester et al., 2008). Antitumor immune response of dendritic cells is also promoted by this inhibitor (Sun et al., 2005). Other agents that have a similar mechanism of antitumor activity have also been discovered (Levitzki 1992; Liu et al., 2008; Tannin-Spitz et al., 2007). Among these, a natural product available in the NCI DTP repository has the ability to inhibit activation and phosphorylation of STAT-3 selectively, which leads to apoptosis of tumor cells (Zhang et al., 2007).
Studies showed that, although these analogs may be more effective inhibitors of the STAT-3 signaling pathway, their exact mechanism of action is still unknown. Tyrphostins (Ferrajoli et al., 2007), resveratrol (Nam et al., 2005), AG490, WP1066 (Iwamaru et al., 2006; Pardanani et al., 2007) and TG101209 (Kotha et al., 2006), indirubin (Lee et al., 2008; Natarajan and Bright 2002), as well as curcumin (Bharti et al., 2003; Kagialis-Girard et al., 2007) act as inhibitors of tyrosine kinase by modulating STAT-3 signaling. Mechanisms of action of these inhibitors are clearly known. However, the use of these agents as specific STAT-3 inhibitors is limited because of their effects in many signal transduction pathways. Second-generation OPB compounds with more favorable toxicity profiles have been identified and are currently being evaluated in early phase clinical trials. The inhibition of upstream tyrosine kinases has led to downstream abrogation of STAT-3 signaling with antitumor effects in multiple preclinical models, including prostate cancer (Gu et al., 2014).
Strategies to prevent STAT-3-DNA binding
In order to imitate the action of cis-regulatory elements present in genes, double-stranded oligodeoxynucleotides are synthesized. These dsODN inhibit the stimulation of STAT-3 dimers; as a result, gene expression activated by STAT-3 and growth of tumor cells is inhibited (Becker et al., 1998; Sen et al., 2009b; Zhang et al., 2007). STAT-3-specific dsODN are also used in mouse to inhibit expression of STAT-3 responsive genes and ultimately prevent tumor cell growth. In one trial, 3.2 mg/kg of a STAT-3 dsODN decoy was injected intramuscularly in cynomolgus monkeys and no apparent adverse effect was observed, in spite of reduced gene transcription of STAT-3 activated genes.
An experimental study was carried out to check biological effects of STAT-3 dsODN decoy when injected intratumorally in HNSCC patients. Although initial results of this study indicate the inhibition of genes targeted by STAT-3, the decoy degrades rapidly in serum and is amenable when injected systemically. Metabolic stability of the double-stranded oligodeoxynucleotides can be improved by chemical modification, but in case of systemic administration, the successful delivery of chemically modified dsODNs remains a considerable challenge. Peptide aptamers were identified in modified yeast and have the ability to inhibit binding of DNA and STAT-3, but they are not commonly used inhibitors because of metabolic stability and cell permeability.
STAT-3 pathway oligonucleotides
Abnormal stimulation of STAT-3 in various cancerous cells led to the development of STAT-3 inhibitors as an anti-cancer defense (Chiba, 2016). Novel and promising strategies targeting transcription factors have recently emerged. These include the inhibition of transcription factor gene expression using antisense oligonucleotides, inhibition of the STAT-3-DNA binding domain using decoy oligonucleotides, or post-transcriptional gene-silencing using small interfering RNA (Turkson et al., 2004). The inhibitors of STAT-3 contain decoy oligodeoxynucleotides (ODN), which has a great influence on DBD.
ODN is an oligonucleotide with usually 10–20 base pair sequences that is incorporated in cells. ODN attaches to the STAT-3-DNA binding domain and helps to block their binding with the responsive elements exhibiting transcription factors, and hence prevent the process of transcription. In this way, this phenomenon helps reduce gene expression (Furqan et al., 2013; Palma et al., 2015). An ODN targeting of the STAT-3 DNA-binding domain showed a desired pharmacodynamic effects when injected into head and neck malignancies (Sen et al., 2012). ODNs and ASOs block the STAT-3 DNA-binding domain and STAT mRNA, respectively (Palma et al., 2015).
AZD9150, an antisense oligonucleotide inhibitor of STAT-3, was well-tolerated and demonstrated single-agent antitumor activity against the treatment-refractory lymphomas and NSCLC in a phase I clinical trial (Hong et al., 2015). This compound has since progressed to phase II clinical evaluation. This decoy shows similarity with STAT-3 genes and helps prevent the signaling of STAT-3 by blocking the activation of STAT-3 molecules. By using this approach, the expression of STAT 3 genes was reduced without showing any toxicity (Dutzmann et al., 2015).
However, ODNs and siRNA are unsuitable for systemic administration because of their rapid degradation. Studies have also confirmed the in vitro efficacy of other STAT-3 DNA-binding domain inhibitors, including platinum (IV) compounds such as CPA-1, CPA-7, and IS3295 (Turkson et al., 2004). However, these compounds lack specificity to STAT-3, and studies informing on their pharmacology as well as suitable therapeutic doses are lacking. These inhibitors, therefore, enhance apoptosis and retard the growth of cells in several types of human cancers (Wong et al., 2017).
Galiellalactone, obtained from Galiella rufa, is another inhibitor of the STAT-3 DNA binding domain. Administration of Galiellalactone in mouse xenograft by intraperitoneal injection helps to generate apoptosis in prostate cancer cells. Galiellalactone also helps to decrease the expression of mRNA and blocks luciferase activity (Hellsten et al., 2008). There are also different types of drugs that bind to STAT-3. These drugs are made up of DNA-binding inhibitors and include various peptide conjugates, peptide aptamers, and metal-chelating compounds (Szelag et al., 2016). First, a small inhibitor named as STA-21 has been identified by the method of virtual screening. This inhibitor suppresses the activity of STAT-3 luciferase and STAT-3 DNA binding in breast cancer (Wake and Watson, 2015).
Another inhibitor is S3I-20, which blocks the STAT-3 DNA binding, promotes apoptosis, and inhibits growth in human breast cancer (Palma et al., 2015). Another small molecule known as InS3–54 has also been identified by virtual screening, and helps retard the transcriptional activity of the STAT-3 DNA-binding domain (Huang et al., 2016). InS3-54A18 suppresses STAT-3 DNA binding and inhibits gene expression of STAT-3 (Huang et al., 2014). It also generates apoptosis and inhibits the survival, migration, and spreading of cancer cells in the body. Hence, InS3-54 is an effective treatment that helps to promote various inhibitors of the STAT-3 DNA binding domain and could be a novel anti-cancer therapy (Huang et al., 2014). Other novel strategies involving the activation of endogenous negative regulators of STAT-3 (SOCS and PTPs) are also being explored, but are still incipient.
Future perspectives
Various methodologies have been discovered to suppress the action of the STAT-3-DNA binding. In mouse models, different techniques have been developed to influence apoptosis, multiplication of cancer cells, and tumor growth (Thomas et al., 2015). To regulate gene expression, STAT-3-DNA binding is mandatory. Hence, the inhibition of the STAT-3-DNA binding domain will invalidate the function of STAT-3, and therefore will help to block the regulation of gene expression. In B16 cells of murine melanoma, DBD-1, which is a small peptide, identifies the STAT-3-DNA binding domain and shows remarkable apoptosis. Therefore, inhibitors of the STAT-3-DNA-binding domain exhibit various anti-cancer effects (Chai et al., 2016).
Recent studies suggest that various STAT-3 inhibitors, such as natural agents, synthetic products, and ODNs can gain clinical application in the future (Huang et al., 2016). It is important to know the mechanism of STAT-3 to help regulate the various signaling pathways for the recognition of different therapies. However, the suppression of STAT3 is not an effective treatment by itself. For this purpose, different therapies may be developed to block the activity of STAT-3, as well as signaling pathway by inhibiting the proteins and DBD involved in this pathway (Banerjee and Resat 2016). Among all the strategies mentioned above to target STAT-3, the most appropriate is the inhibition of the DNA binding domain of STAT-3, as it will directly inhibit gene expression of the anti-apoptotic protein.
Conclusion
STAT-3 activation plays a major role in carcinogenesis. The constitutive activation of STAT-3 with malignant transformation has been known for 13 years. Since then, various studies have been carried out, confirming STAT-3 as a cancer drug target, and significant work has focused on the discovery of novel STAT-3 inhibitors. A huge number of STAT-3 inhibitors is known to date, as illustrated in this review. Most inhibitors are at the trial phase and not yet used in clinical practice. The chemo-preventive agents used against cancer cells in vitro and (in mouse cancer models) in vivo should be relatively non-toxic to normal cells and exhibit significant bioavailability. The current review may be a platform to critically evaluate and analyze the global methodologies for STAT-3 targeting and for the emergence of clinically favorable direct STAT-3 inhibitors as innovative anticancer agents.
Conflict of Interest
The authors confirm that there is no conflict of interest.
Author contributions
SA conceived and designed this manuscript, MN formulated and supervised the study, MU, KJ, AB did wrote up of manuscript, MK and FA proofread the manuscript technically. All authors read and approved the final version.
Footnotes
Associate Editor: Anamaria Aranha Camargo
References
- Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer. Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KS, Sethi G, Sung B, Goel A, Ralhan R, Aggarwal BB. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–4415. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Aziz MH, Ahmad N. Ultraviolet B exposure activates Stat3 signaling via phosphorylation at tyrosine 705 in skin of SKH1 hairless mouse: A target for the management of skin cancer? Biochem Biophys Res Commun. 2005;333:241–246. doi: 10.1016/j.bbrc.2005.05.106. [DOI] [PubMed] [Google Scholar]
- Akira S, Nishio Y, Inoue M, Wang X-J, We S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of α7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–2101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase Cε interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer. 2016;138:2570–2578. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré B, Avril S, Coqueret O. Opposite regulation of Myc and p21 waf1 transcription by STAT3 proteins. J Biol Chem. 2003;278:2990–2996. doi: 10.1074/jbc.M210422200. [DOI] [PubMed] [Google Scholar]
- Becker S, Groner B, Müller CW. Three-dimensional structure of the Stat3β homodimer bound to DNA. Nature. 1998;394:145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- Bellido T, O’Brien CA, Roberson PK, Manolagas SC. Transcriptional activation of the p21(WAF1,CIP1,SDI1) gene by interleukin-6 type cytokines. A prerequisite for their pro-differentiating and anti-apoptotic effects on human osteoblastic cells. J Biol Chem. 1998;273:21137–21144. doi: 10.1074/jbc.273.33.21137. [DOI] [PubMed] [Google Scholar]
- Betz UA, Bloch W, Van Den Broek M, Yoshida K, Taga T, Kishimoto T, Addicks K, Rajewsky K, Müller W. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955–1965. doi: 10.1084/jem.188.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB–regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB. Nuclear factor–κB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Schindler C. Regulation of Stat3 nuclear export. J Clin Invest. 2003;111:553–559. doi: 10.1172/JCI15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- Boccaccio C, Andò M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Boehm AL, Sen M, Seethala R, Gooding WE, Freilino M, Wong SMY, Wang S, Johnson DE, Grandis JR. Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-XL enhances antitumor effects in squamous cell carcinoma of the head and neck. Mol Pharmacol. 2008;73:1632–1642. doi: 10.1124/mol.107.044636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem G, Thoenen BH. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:2. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an Oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Cao X, Tay A, Guy GR, Tan Y. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- Chai EZP, Shanmugam MK, Arfuso F, Dharmarajan A, Wang C, Kumar AP, Samy RP, Lim LH, Wang L, Goh BC. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharmacol Ther. 2016;162:86–97. doi: 10.1016/j.pharmthera.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Chan KS, Sano S, Kiguchi K, Anders J, Komazawa N, Takeda J, DiGiovanni J. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–728. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999;13:2604–2616. doi: 10.1101/gad.13.19.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GZ, Zhang W, Sun M, Wang Q, Coppola D, Mansour M, Xu L, Costanzo C, Cheng JQ, Wang L-H. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T. STAT3 inhibitors for cancer therapy-the rationale and remained problems. EC Cancer. 2016;1:S1. [Google Scholar]
- Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- Couto JP, Daly L, Almeida A, Knauf JA, Fagin JA, Sobrinho-Simões M, Lima J, Máximo V, Soares P, Lyden D. STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci USA. 2012;109:e2361–e2370. doi: 10.1073/pnas.1201232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang J-G, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Daino H, Matsumura I, Takada K, Odajima J, Tanaka H, Ueda S, Shibayama H, Ikeda H, Hibi M, Machii T. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood. 2000;95:2577–2585. [PubMed] [Google Scholar]
- Dalwadi H, Krysan K, Heuze-Vourc’h N, Dohadwala M, Elashoff D, Sharma S, Cacalano N, Lichtenstein A, Dubinett S. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non–small cell lung cancer. Clin Cancer Res. 2005;11:7674–7682. doi: 10.1158/1078-0432.CCR-05-1205. [DOI] [PubMed] [Google Scholar]
- Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- Darnowski JW, Goulette FA, Guan YJ, Chatterjee D, Yang ZF, Cousens LP, Chin YE. Stat3 cleavage by caspases impact on full-length Stat3 expression, fragment formation, and transcriptional activity. J Biol Chem. 2006;281:17707–17717. doi: 10.1074/jbc.M600088200. [DOI] [PubMed] [Google Scholar]
- Dien J, Amin HM, Chiu N, Wong W, Frantz C, Chiu B, Mackey JR, Lai R. Signal transducers and activators of transcription-3 up-regulates tissue inhibitor of metalloproteinase-1 expression and decreases invasiveness of breast cancer. Am J Pathol. 2006;169:633–642. doi: 10.2353/ajpath.2006.051109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DV, Ueda J, Messerschmidt DM, Lorthongpanich C, Zhou Y, Feng B, Guo G, Lin PJ, Hossain MZ, Zhang W. A genetic and developmental pathway from STAT3 to the OCT4–NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev. 2013;27:1378–1390. doi: 10.1101/gad.221176.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J. Drug discovery: a historical perspective. Science. 2000;287:1960–1964. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- Dutzmann J, Daniel JM, Bauersachs J, Hilfiker-Kleiner D, Sedding DG. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc Res. 2015;106:365–374. doi: 10.1093/cvr/cvv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B, Dvorak B, Bernstein H, Holubec H, Sampliner RE. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to Barrett’s esophagus. Clin Cancer Res. 2007;13:5305–5313. doi: 10.1158/1078-0432.CCR-07-0483. [DOI] [PubMed] [Google Scholar]
- Egloff AM, Grandis JR. Improving response rates to EGFR-targeted therapies for head and neck squamous cell carcinoma: candidate predictive biomarkers and combination treatment with Src inhibitors. J Oncol. 2009;2009 doi: 10.1155/2009/896407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kasmi KC, Smith AM, Williams L, Neale G, Panopolous A, Watowich SS, Häcker H, Foxwell BM, Murray PJ. Cutting edge: A transcriptional repressor and corepressor induced by the STAT3-regulated anti-inflammatory signaling pathway. J Immunol. 2007;179:7215–7219. doi: 10.4049/jimmunol.179.11.7215. [DOI] [PubMed] [Google Scholar]
- Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J Biol Chem. 1996;271:30136–30143. doi: 10.1074/jbc.271.47.30136. [DOI] [PubMed] [Google Scholar]
- Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR. STAT3 and STAT1 mediate IL-11–dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escary JL, Perreau J, Duménil D, Ezine S, BrÛlet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993;363:361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;67:11291–11299. doi: 10.1158/0008-5472.CAN-07-0593. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Drewry JA, Shahani VM, Page BD, Gunning PT. Molecular disruption of oncogenic signal transducer and activator of transcription 3 (STAT3) protein. Biochem Cell Biol. 2009;87:825–833. doi: 10.1139/o09-044. [DOI] [PubMed] [Google Scholar]
- Fontaine F, Overman J, François M. Pharmacological manipulation of transcription factor protein-protein interactions: opportunities and obstacles. Cell Regen. 2015;4:2. doi: 10.1186/s13619-015-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251:199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci USA. 2004;101:6728–6733. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furqan M, Akinleye A, Mukhi N, Mittal V, Chen Y, Liu D. STAT inhibitors for cancer therapy. J Hematol Oncol. 2013;6:90. doi: 10.1186/1756-8722-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaemers IC, Vos HL, Volders HH, van der Valk SW, Hilkens J. A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J Biol Chem. 2001;276:6191–6199. doi: 10.1074/jbc.M009449200. [DOI] [PubMed] [Google Scholar]
- Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Yu C, Hudnall A, Catlett R, Nelson K, Smithgall T, Fujita D, Ethier S, Jove R. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267–1276. [PubMed] [Google Scholar]
- Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- Gibbs JB. Mechanism-based target identification and drug discovery in cancer research. Science. 2000;287:1969–1973. doi: 10.1126/science.287.5460.1969. [DOI] [PubMed] [Google Scholar]
- Giordano V, De Falco G, Chiari R, Quinto I, Pelicci P, Bartholomew L, Delmastro P, Gadina M, Scala G. Shc mediates IL-6 signaling by interacting with gp130 and Jak2 kinase. J Immunol. 1997;158:4097–4103. [PubMed] [Google Scholar]
- Greenhalgh CJ, Hilton DJ. Negative regulation of cytokine signaling. J Leukocyte Biol. 2001;70:348–356. [PubMed] [Google Scholar]
- Gu L, Talati P, Vogiatzi P, Romero-Weaver AL, Abdulghani J, Liao Z, Leiby B, Hoang DT, Mirtti T, Alanen K. Pharmacological suppression of JAK1/2 by JAK1/2 inhibitor AZD1480 potently inhibits IL-6-induced prostate cancer metastases formation. Mol Cancer Ther. 2014;13:1246–1258. doi: 10.1158/1535-7163.MCT-13-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark G. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- Hawkins K, Mohamet L, Ritson S, Merry CL, Ward CM. E-cadherin and, in its absence, N-cadherin promotes Nanog expression in mouse embryonic stem cells via STAT3 phosphorylation. Stem Cells. 2012;30:1842–1851. doi: 10.1002/stem.1148. [DOI] [PubMed] [Google Scholar]
- Heinrich P, Behrmann I, Haan S, Hermanns H, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten R, Johansson M, Dahlman A, Dizeyi N, Sterner O, Bjartell A. Galiellalactone is a novel therapeutic candidate against hormone-refractory prostate cancer expressing activated Stat3. Prostate. 2008;68:269–280. doi: 10.1002/pros.20699. [DOI] [PubMed] [Google Scholar]
- Herrmann A, Vogt M, Mönnigmann M, Clahsen T, Sommer U, Haan S, Poli V, Heinrich PC, Müller-Newen G. Nucleocytoplasmic shuttling of persistently activated STAT3. J Cell Sci. 2007;120:3249–3261. doi: 10.1242/jcs.03482. [DOI] [PubMed] [Google Scholar]
- Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hilbert DM, Kopf M, Mock BA, Köhler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med. 1995;182:243–248. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, Müller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-κB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- Hong D, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, Fowler N, Zhou T, Schmidt J, Jo M. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med. 2015;7:314ra185. doi: 10.1126/scitranslmed.aac5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Lu JJ, Huang MQ, Bao JL, Chen XP, Wang YT. Terpenoids: natural products for cancer therapy. Expert Opin Investig Drugs. 2012;21:1801–1818. doi: 10.1517/13543784.2012.727395. [DOI] [PubMed] [Google Scholar]
- Huang W, Dong Z, Chen Y, Wang F, Wang C, Peng H, He Y, Hangoc G, Pollok K, Sandusky G. Small-molecule inhibitors targeting the DNA-binding domain of STAT3 suppress tumor growth, metastasis and STAT3 target gene expression in vivo. Oncogene. 2016;35:783–792. doi: 10.1038/onc.2015.215. [DOI] [PubMed] [Google Scholar]
- Huang W, Dong Z, Wang F, Peng H, Liu JY, Zhang JT. A small molecule compound targeting STAT3 DNA-binding domain inhibits cancer cell proliferation, migration, and invasion. ACS Chem Biol. 2014;9:1188–1196. doi: 10.1021/cb500071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, Griffiths E, Krawczyk C, Richardson CD, Aitken K. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–354. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- Itoh M, Murata T, Suzuki T, Shindoh M, Nakajima K, Imai K, Yoshida K. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2005;25:1195–1204. doi: 10.1038/sj.onc.1209149. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, Horvath CM, Ronai Ze. Cooperation between STAT3 and c-Jun suppresses Fas transcription. Mol Cell. 2001;7:517–528. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2006;26:2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- Jain N, Zhang T, Kee WH, Li W, Cao X. Protein kinase C δ associates with and phosphorylates Stat3 in an interleukin-6-dependent manner. J Biol Chem. 1999;274:24392–24400. doi: 10.1074/jbc.274.34.24392. [DOI] [PubMed] [Google Scholar]
- Jang E, Moon J, Ko J, Ahn C, Lee H, Shin J, Park C, Kang J. Novel black soy peptides with antiobesity effects: activation of leptin-like signaling and AMP-activated protein kinase. Int J Obes. 2008;32:1161–1170. doi: 10.1038/ijo.2008.60. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-β signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- Jing N, Zhu Q, Yuan P, Li Y, Mao L, Tweardy DJ. Targeting signal transducer and activator of transcription 3 with G-quartet oligonucleotides: a potential novel therapy for head and neck cancer. Mol Cancer Ther. 2006;5:279–286. doi: 10.1158/1535-7163.MCT-05-0302. [DOI] [PubMed] [Google Scholar]
- Kagialis-Girard S, Mialou V, Chebel A, Chien WW, Tigaud I, Mokdad F, Badiou C, Ffrench M. Inhibition of normal lymphocyte proliferation by Indirubin-3’-monoxime: A multifactorial process. Leuk Lymphoma. 2007;48:605–615. doi: 10.1080/10428190601059696. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127–2136. doi: 10.1093/emboj/18.8.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, Klein-Szanto AJ, Sahu V, Basu D, Ohashi S. IL-6 mediates cross-talk between activated fibroblasts and tumor cells in the tumor microenvironment. Cancer Res. 2018;78:4957–4970. doi: 10.1158/0008-5472.CAN-17-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni R, Jove R, Levitzki A. Inhibition of pp 60 c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene. 1999;18:4654–4662. doi: 10.1038/sj.onc.1202835. [DOI] [PubMed] [Google Scholar]
- Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobierski LA, Srivastava S, Borsook D. Systemic lipopolysaccharide and interleukin-1 β activate the interleukin 6: STAT intracellular signaling pathway in neurons of mouse trigeminal ganglion. Neurosci Lett. 2000;281:61–64. doi: 10.1016/s0304-3940(99)00953-2. [DOI] [PubMed] [Google Scholar]
- Konnikova L, Kotecki M, Kruger MM, Cochran BH. Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer. 2003;3:23. doi: 10.1186/1471-2407-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster M, Lykke-Andersen S, Elnakady YA, Gerth K, Washausen P, Höfle G, Sasse F, Kjems J, Hauser H. Ratjadones inhibit nuclear export by blocking CRM1/exportin 1. Exp Cell Res. 2003;286:321–331. doi: 10.1016/s0014-4827(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Hasebe M, Entani T, Takayama S, Tomita M, Yanagawa H. Design of peptide inhibitors for the importin α/β nuclear import pathway by activity-based profiling. Chem Biol. 2008;15:940–949. doi: 10.1016/j.chembiol.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Marukawa S, Kumanogoh T, Hirota H, Yoshida K, Lee IS, Yasui T, Yoshida K, Taga T, Kishimoto T. Impairment of antigen-specific antibody production in transgenic mice expressing a dominant-negative form of gp130. Proc Natl Acad Sci USA. 1997;94:2478–2482. doi: 10.1073/pnas.94.6.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy HA, Smith A, Tissire H, Barry M, Crowley D, Bronson R, Roes J, Savill J, Hynes R. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial–mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Kim MY, Mo JS, Ann EJ, Seo MS, Hong J, Kim YC, Park HS. Indirubin-3’-monoxime, a derivative of a Chinese anti-leukemia medicine, inhibits Notch1 signaling. Cancer Lett. 2008;265:215–225. doi: 10.1016/j.canlet.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Leeman RJ, Lui VWY, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6:231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J. 1992;6:3275–3282. doi: 10.1096/fasebj.6.14.1426765. [DOI] [PubMed] [Google Scholar]
- Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, Karras JG, Zhang H. Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res. 2006;12:7140–7148. doi: 10.1158/1078-0432.CCR-06-0484. [DOI] [PubMed] [Google Scholar]
- Liu L, McBride KM, Reich NC. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-α3. Proc Natl Acad Sci USA. 2005;102:8150–8155. doi: 10.1073/pnas.0501643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang M, Zhang H, Sun C, Yang X, Deng Y, Ji W. Combined antitumor activity of cucurbitacin B and docetaxel in laryngeal cancer. Eur J Pharmacol. 2008;587:78–84. doi: 10.1016/j.ejphar.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Lord K, Abdollahi A, Thomas S, DeMarco M, Brugge J, Hoffman-Liebermann B, Liebermann D. Leukemia inhibitory factor and interleukin-6 trigger the same immediate early response, including tyrosine phosphorylation, upon induction of myeloid leukemia differentiation. Mol Cell Biol. 1991;11:4371–4379. doi: 10.1128/mcb.11.9.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui VWY, Boehm AL, Koppikar P, Leeman RJ, Johnson D, Ogagan M, Childs E, Freilino M, Grandis JR. Antiproliferative mechanisms of a transcription factor decoy targeting signal transducer and activator of transcription (STAT) 3: the role of STAT1. Mol Pharmacol. 2007;71:1435–1443. doi: 10.1124/mol.106.032284. [DOI] [PubMed] [Google Scholar]
- Ma J, Cao X. Regulation of Stat3 nuclear import by importin α5 and importin α7 via two different functional sequence elements. Cell Signal. 2006;18:1117–1126. doi: 10.1016/j.cellsig.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Ma P, Tretiakova M, Nallasura V, Jagadeeswaran R, Husain A, Salgia R. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97:368–377. doi: 10.1038/sj.bjc.6603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y, Carroll JM, Elias JA, Altieri DC, Pober JS. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab Invest. 2001;81:327–334. doi: 10.1038/labinvest.3780241. [DOI] [PubMed] [Google Scholar]
- Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- Migone TS, Cacalano NA, Taylor N, Yi T, Waldmann TA, Johnston JA. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci USA. 1998;95:3845–3850. doi: 10.1073/pnas.95.7.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone TS, Lin JX, Cereseto A, Mulloy JC, O’Shea JJ, Franchini G, Leonard WJ. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- Morgan KJ, Gilliland DG. A role for JAK2 mutations in myeloproliferative diseases. Annu Rev Med. 2008;59:213–222. doi: 10.1146/annurev.med.59.061506.154159. [DOI] [PubMed] [Google Scholar]
- Munoz J, Dhillon N, Janku F, Watowich SS, Hong DS. STAT3 inhibitors: finding a home in lymphoma and leukemia. Oncologist. 2014;19:536–544. doi: 10.1634/theoncologist.2013-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Takigawa N, Ninomiya T, Ochi N, Yasugi M, Honda Y, Kubo T, Ichihara E, Hotta K, Tanimoto M. Effect of AZD1480 in an epidermal growth factor receptor-driven lung cancer model. Lung Cancer. 2014;83:30–36. doi: 10.1016/j.lungcan.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S, Esterbauer H, Mueller M, Casanova E, Kenner L. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology. 2010;138:1003–1011. doi: 10.1053/j.gastro.2009.11.049. [DOI] [PubMed] [Google Scholar]
- Myers MG. Moonlighting in mitochondria. Science. 2009;323:723–724. doi: 10.1126/science.1169660. [DOI] [PubMed] [Google Scholar]
- Nagpal JK, Mishra R, Das BR. Activation of Stat-3 as one of the early events in tobacco chewing-mediated oral carcinogenesis. Cancer. 2002;94:2393–2400. doi: 10.1002/cncr.10499. [DOI] [PubMed] [Google Scholar]
- Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus kinase-STAT pathway in T lymphocytes. J Immunol. 2002;168:6506–6513. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–9535. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, Itoh S, Narimatsu M, Maeda H, Fukada T. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3-and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- Otero DC, Poli V, David M, Rickert RC. Cutting edge: inherent and acquired resistance to radiation-induced apoptosis in B cells: a pivotal role for STAT3. J Immunol. 2006;177:6593–6597. doi: 10.4049/jimmunol.177.10.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma G, D’Aiuto M, Petrillo A, Dallemagne P, Sinicropi M, Rodriquez M, Longo P, Mariconda A, Arra C, De Martino F. Targeting STAT3 in cancer inhibition. Pharmacologyonline. 2015;1:50–66. [Google Scholar]
- Pardanani A, Hood J, Lasho T, Levine R, Martin M, Noronha G, Finke C, Mak C, Mesa R, Zhu H. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia. 2007;21:1658–1668. doi: 10.1038/sj.leu.2404750. [DOI] [PubMed] [Google Scholar]
- Perry E, Tsruya R, Levitsky P, Pomp O, Taller M, Weisberg S, Parris W, Kulkarni S, Malovani H, Pawson T. TMF/ARA160 is a BC-box-containing protein that mediates the degradation of Stat3. Oncogene. 2004;23:8908–8919. doi: 10.1038/sj.onc.1208149. [DOI] [PubMed] [Google Scholar]
- Pfitzner E, Kliem S, Baus D, Litterst M. The role of STATs in inflammation and inflammatory diseases. Curr Pharm Des. 2004;10:2839–2850. doi: 10.2174/1381612043383638. [DOI] [PubMed] [Google Scholar]
- Plaza-Menacho I, van der Sluis T, Hollema H, Gimm O, Buys CH, Magee AI, Isacke CM, Hofstra RM, Eggen BJ. Ras/ERK1/2-mediated STAT3 Ser727 phosphorylation by familial medullary thyroid carcinoma-associated RET mutants induces full activation of STAT3 and is required for c-fos promoter activation, cell mitogenicity, and transformation. J Biol Chem. 2007;282:6415–6424. doi: 10.1074/jbc.M608952200. [DOI] [PubMed] [Google Scholar]
- Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–319. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- Rahmani K, Dean DA. Leptomycin B alters the subcellular distribution of CRM1 (Exportin 1) Biochem Biophys Res Commun. 2017;488:253–258. doi: 10.1016/j.bbrc.2017.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- Real PJ, Sierra A, de Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]
- Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2008;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans . J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson IB. Oncogenic functions of tumour suppressor p21Waf1/Cip1/Sdi1: association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179:1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- Saini U, Suarez AA, Naidu S, Wallbillich JJ, Bixel K, Wanner R, Bice J, Kladney RD, Lester J, Karlan BY. STAT3/PIAS3 levels serve as" early signature" genes in the development of high-grade serous carcinoma from the fallopian tube. Cancer Res. 2018;78:1739–1750. doi: 10.1158/0008-5472.CAN-17-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos FP, Kantarjian HM, Jain N, Manshouri T, Thomas DA, Garcia-Manero G, Kennedy D, Estrov Z, Cortes J, Verstovsek S. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115:1131–1136. doi: 10.1182/blood-2009-10-246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper F, Gendo C, Eck M, Schmitz J, Grimm C, Anhuf D, Kerr I, Heinrich P. Activation of the protein tyrosine phosphatase SHP2 via the interleukin-6 signal transducing receptor protein gp130 requires tyrosine kinase Jak1 and limits acute-phase protein expression. Biochem J. 1998;335:557–565. doi: 10.1042/bj3350557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj BT, Frank N, Bender FL, Asan E, Sendtner M. Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease. J Cell Biol. 2012;199:437–451. doi: 10.1083/jcb.201203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src Inhibition Results in Signal Transducer and Activator of Transcription 3 (STAT3) Activation and Cancer Cell Survival via Altered Janus-Activated Kinase–STAT3 Binding. Cancer Res. 2009;69:1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Tosca PJ, Zwayer C, Ryan MJ, Johnson JD, Knostman KA, Giclas PC, Peggins JO, Tomaszewski JE, McMurray TP. Lack of toxicity of a STAT3 decoy oligonucleotide. Cancer Chemother Pharmacol. 2009;63:983–995. doi: 10.1007/s00280-008-0823-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgrignani J, Garofalo M, Matkovic M, Merulla J, Catapano CV, Cavalli A. Structural biology of STAT3 and its implications for anticancer therapies development. Int J Mol Sci. 2018;19:1591. doi: 10.3390/ijms19061591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirogane T, Fukada T, Muller JM, Shima DT, Hibi M, Hirano T. Synergistic roles for Pim-1 and c-Myc in STAT3-mediated cell cycle progression and antiapoptosis. Immunity. 1999;11:709–719. doi: 10.1016/s1074-7613(00)80145-4. [DOI] [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip MR, Jove R, McLaughlin MM, Lawrence NJ. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquee KA, Gunning PT, Glenn M, Katt WP, Zhang S, Schroeck C, Sebti SM, Jove R, Hamilton AD, Turkson J. An oxazole-based small-molecule Stat3 inhibitor modulates Stat3 stability and processing and induces antitumor cell effects. ACS Chem Biol. 2007;2:787–798. doi: 10.1021/cb7001973. [DOI] [PubMed] [Google Scholar]
- Soderholm JF, Bird SL, Kalab P, Sampathkumar Y, Hasegawa K, Uehara-Bingen M, Weis K, Heald R. Importazole, a small molecule inhibitor of the transport receptor importin-β. ACS Chem Biol. 2011;6:700–708. doi: 10.1021/cb2000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell J, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray L, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Su Y, Li G, Zhang X, Gu J, Zhang C, Tian Z, Zhang J. JSI-124 inhibits glioblastoma multiforme cell proliferation through G. Cancer Biol Ther. 2008;7:1243–1249. doi: 10.4161/cbt.7.8.6263. [DOI] [PubMed] [Google Scholar]
- Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K-i, Hirano T, Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Generation of plasmacytomas with the chromosomal translocation t (12; 15) in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suiqing C, Min Z, Lirong C. Overexpression of phosphorylated-STAT3 correlated with the invasion and metastasis of cutaneous squamous cell carcinoma. J Dermatol. 2005;32:354–360. doi: 10.1111/j.1346-8138.2005.tb00906.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- Sun S, Steinberg BM. PTEN is a negative regulator of STAT3 activation in human papillomavirus-infected cells. J Gen Virol. 2002;83:1651–1658. doi: 10.1099/0022-1317-83-7-1651. [DOI] [PubMed] [Google Scholar]
- Szczepanek K, Chen Q, Derecka M, Salloum FN, Zhang Q, Szelag M, Cichy J, Kukreja RC, Dulak J, Lesnefsky EJ. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286:29610–29620. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanek K, Chen Q, Larner AC, Lesnefsky EJ. Cytoprotection by the modulation of mitochondrial electron transport chain: the emerging role of mitochondrial STAT3. Mitochondrion. 2012;12:180–189. doi: 10.1016/j.mito.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelag M, Wesoly J, AR Bluyssen H. Advances in peptidic and peptidomimetic-based approaches to inhibit STAT signaling in human diseases. Curr Protein Pept Sci. 2016;17:135–146. doi: 10.2174/1389203716666151102103706. [DOI] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73:56–67. doi: 10.1016/j.bcp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- Tharappel JC, Lee EY, Robertson LW, Spear BT, Glauert HP. Regulation of cell proliferation, apoptosis, and transcription factor activities during the promotion of liver carcinogenesis by polychlorinated biphenyls. Toxicol Appl Pharmacol. 2002;179:172–184. doi: 10.1006/taap.2001.9360. [DOI] [PubMed] [Google Scholar]
- Thomas S, Snowden J, Zeidler M, Danson S. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Gaponenko V, Lockett SJ, Tarasov SG, Jiang S, Michejda CJ, Perantoni AO, Tarasova NI. Rationally designed inhibitors identify STAT3 N-domain as a promising anticancer drug target. ACS Chem Biol. 2007;2:799–809. doi: 10.1021/cb700186x. [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Tarasova NI, Zhang X, Chasovskikh S, Cheema AK, Wang H, Brown ML, Dritschilo A. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci USA. 2013;110:1267–1272. doi: 10.1073/pnas.1211805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–422. doi: 10.1517/14728222.8.5.409. [DOI] [PubMed] [Google Scholar]
- Turkson J, Kim JS, Zhang S, Yuan J, Huang M, Glenn M, Haura E, Sebti S, Hamilton AD, Jove R. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol Cancer Ther. 2004;3:261–269. [PubMed] [Google Scholar]
- Ulane CM, Kentsis A, Cruz CD, Parisien JP, Schneider KL, Horvath CM. Composition and assembly of STAT-targeting ubiquitin ligase complexes: paramyxovirus V protein carboxyl terminus is an oligomerization domain. J Virol. 2005;79:10180–10189. doi: 10.1128/JVI.79.16.10180-10189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver N, Delgado O, Zeleke K, Cumpian A, Tang X, Caetano MS, Wang H, Katayama H, Yu H, Szabo E. Reduced IL-6 levels and tumor-associated phospho-STAT 3 are associated with reduced tumor development in a mouse model of lung cancer chemoprevention with myo-inositol. Int J Cancer. 2018;142:1405–1417. doi: 10.1002/ijc.31152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdembri D, Serini G, Vacca A, Ribatti D, Bussolino F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. FASEB J. 2002;16:225–227. doi: 10.1096/fj.01-0633fje. [DOI] [PubMed] [Google Scholar]
- van Kester MS, Out-Luiting JJ, von dem Borne PA, Willemze R, Tensen CP, Vermeer MH. Cucurbitacin I inhibits Stat3 and induces apoptosis in Sezary cells. J Invest Dermatol. 2008;128:1691–1695. doi: 10.1038/sj.jid.5701246. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, Estrov Z, Fridman JS, Bradley EC, Erickson-Viitanen S. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. New Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignais ML, Sadowski HB, Watling D, Rogers NC, Gilman M. Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol. 1996;16:1759–1769. doi: 10.1128/mcb.16.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake MS, Watson CJ. STAT3 the oncogene–still eluding therapy? FEBS J. 2015;282:2600–2611. doi: 10.1111/febs.13285. [DOI] [PubMed] [Google Scholar]
- Weerasinghe P, Garcia GE, Zhu Q, Yuan P, Feng L, Mao L, Jing N. Inhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotides. Int J Oncol. 2007;31:129–136. [PubMed] [Google Scholar]
- Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Wen Z, Darnell JE. Mapping of Stat3 serine phosphorylation to a single residue (727) and evidence that serine phosphorylation has no influence on DNA binding of Stat1 and Stat3. Nucleic Acids Res. 1997;25:2062–2067. doi: 10.1093/nar/25.11.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks AF. The JAK kinases: not just another kinase drug discovery target. Semin Cell Dev Biol. 2008;19:319–328. doi: 10.1016/j.semcdb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Wong A, Soo RA, Tan D, Lee SC, Lim J, Marban P, Kong LR, Lee Y, Wang L, Thuya WL. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann Oncol. 2015;26:998–1005. doi: 10.1093/annonc/mdv026. [DOI] [PubMed] [Google Scholar]
- Wong AL, Hirpara JL, Pervaiz S, Eu JQ, Sethi G, Goh BC. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin Investig Drugs. 2017;26:883–887. doi: 10.1080/13543784.2017.1351941. [DOI] [PubMed] [Google Scholar]
- Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li J, Li C, Yan M, Zhu Z. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741–20750. doi: 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yan R, Liu J, Che X, Xiong J. Construction of vectors expressing inhibiting peptides for nuclear import and its effects on growth and migration of HeLa cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:824–827. [PubMed] [Google Scholar]
- Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F, Sawaya R, Huang S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–3560. doi: 10.1038/sj.onc.1207383. [DOI] [PubMed] [Google Scholar]
- Xin–hua B, Rui–yu L. Progress in research on correlation among STAT3, CyclinD1, P21 genes and tumors. J Otol. 2012;7:19–24. [Google Scholar]
- Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- Yamasaki K, Taga T, Hirata Y, Yawata H, Kawanishi Y, Seed B, Taniguchi T, Hirano T, Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Wang L, Wei H, Tian Z. Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer. 2007;7:149. doi: 10.1186/1471-2407-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fuller GM. The competitive binding of STAT3 and NF-κB on an overlapping DNA binding site. Biochem Biophys Res Commun. 1997;237:90–94. doi: 10.1006/bbrc.1997.7082. [DOI] [PubMed] [Google Scholar]
- Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, Freeman JW. Inhibition of STAT3Tyr705 phosphorylation by Smad4 suppresses transforming growth factor β–mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68:4221–4228. doi: 10.1158/0008-5472.CAN-07-5123. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell J. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Zhu BM, Ishida Y, Robinson GW, Pacher-Zavisin M, Yoshimura A, Murphy PM, Hennighausen L. SOCS3 negatively regulates the gp130–STAT3 pathway in mouse skin wound healing. J Invest Dermatol. 2008;128:1821–1829. doi: 10.1038/sj.jid.5701224. [DOI] [PubMed] [Google Scholar]
- Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S, Sugimachi M, Higashimoto Y, Kanayama S, Matsuzawa Y. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–330. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]