Supplemental Digital Content is available in the text.
Keywords: tuberculosis, children, diagnosis, mycobacterial blood culture
Abstract
Diagnosis of pediatric tuberculosis is notoriously difficult. We investigated the additional yield of blood culture in hospitalized children in Vietnam. Among 554 enrolled clinically suspected patients, an additional 6 cases were diagnosed, while the incremental cost per case was USD500. Addition of blood culture is therefore not recommended for our total patient population, but may be considered in specific groups.
Diagnosis of pediatric tuberculosis (TB) is difficult because of nonspecific clinical presentation and the difficulty of sampling and microbiologic confirmation. Young children are at higher risk of mycobacteremia due to an immature immune system, but there is limited evidence of the utility of blood culture for diagnostics. We undertook a prospective study to investigate the additional yield of mycobacterial blood culture for TB diagnosis in children at 2 tertiary hospitals in Hanoi, Vietnam: Vietnam National Children Hospital, the largest general hospital for children in northern Vietnam and the National Lung Hospital, the designated referral hospital for treatment of TB including pediatric TB.
Children <15 years of age presenting during 2011 to 2014 with suspected TB and not receiving TB treatment for more than 1 week were screened for meeting at least 2 inclusion criteria: unexplained fever (>2 weeks), unexplained cough (>2 weeks), chest radiograph suspicious for TB, evidence of malnutrition, failure to thrive/loss of weight, lymphadenopathy, suspected meningitis (>1 week), HIV infection and contact with TB cases. One blood sample (3–5 mL) was collected, and other diagnostic samples were collected as per standard of care. Clinical categories were determined retrospectively from the standardized case definition for intrathoracic TB in children published by Graham et al.1 Three categories were defined: confirmed, unconfirmed and unlikely TB. “Confirmed TB” was defined as microbiologically confirmed TB with at least one positive culture or WHO-endorsed nucleic acid amplification test (Xpert MTB/RIF) in any sample. “Unconfirmed TB” was defined as meeting at least 2 of the following criteria: defined signs or symptoms suggestive of TB; chest radiograph consistent with TB; close TB exposure or immunologic evidence of TB; and positive response to treatment. “Unlikely TB” was defined as not meeting criteria for confirmed and unconfirmed TB. Disseminated TB was defined as: (1) isolation of MTB from blood or ≥2 noncontiguous organs or (2) isolation of MTB from any organ with miliary pattern on the chest radiograph.
Blood was collected in BACTEC Myco/F Lytic culture bottles and incubated in the Bactec 9050 (Becton Dickinson, Sparks, MD) for 42 days following the manufacturer’s protocol. The added volume was not checked. Positive bottles were checked by Ziehl–Neelsen smear and susceptibility testing for isoniazid and rifampicin (GenoType MTBDRplus assay, Hain Lifescience, Nehren, Germany). All samples, except cerebrospinal fluid, were decontaminated using the NaOH protocol. Pellets were split for microscopy, microscopic observation drug susceptibility assay,2 and liquid culture (MGIT). If insufficient volume was available, repeated sampling was attempted. All children’s parents/guardians gave written informed consent. The study protocol was approved by the Vietnam National Children Hospital Institutional Review Board (IRB-2010) and the Oxford Tropical Research Ethics Committee (13-10).
Two separate definitions of gold standard for pediatric TB: “confirmed TB gold standard” and “clinical gold standard” were used. The “confirmed TB gold standard” usually overestimates sensitivity and underestimates specificity of an evaluated test due to lack of sensitivity and this is the other way around with the “clinical gold standard” that lacks in specificity. We compared demographic and clinical characteristics of patients among diagnosed categories of TB using the Fisher’s exact test for categorical variables and the Kruskal Wallis test for continuous variables. All analyses were done using SPSS version 21 (IBM Inc, Armonk, NY). Two sided P-values ≤ 0.05 were regarded as statistically significant.
Our study population represents the diversity of pediatric TB in 2 large referral hospitals in an endemic setting, where approximately half of children have extrapulmonary TB.2,3 Five hundred forty-four children, among whom 11 (11/554, 2%) were receiving treatment <1 week, were enrolled. 92 children (16.6%, n = 92/554) were classified as “confirmed TB,” 345 (62.3%, n = 345/554) as “unconfirmed TB” and 73 (12.3% n = 73/554) as “unlikely TB.” Forty-four children from whom no routine samples were collected, were classified separately and were excluded from the general analysis (Fig. 1). One thousand twenty-eight routine samples were collected from 510 patients: sputum (n = 81), gastric aspirates (n = 831) and others (n = 116). One samples was taken from 125 patients, 2 from 263 and 3–4 from 122.
FIGURE 1.
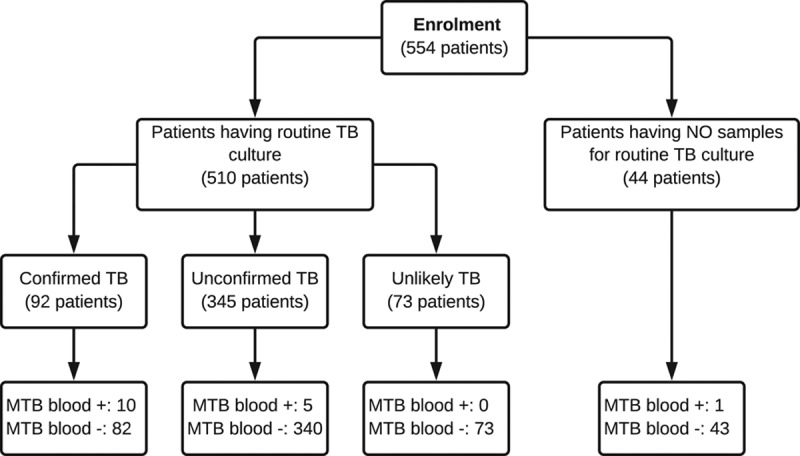
Flowchart of diagnosis for patients included in the study
Children’s baseline characteristics are shown in Table, Supplemental Digital Content 1, http://links.lww.com/INF/D600. The median age was 29.9 months (IQR: 10.9–85.2). Evidence of past BCG vaccination was observed or reported from 92.2% (n = 470/510); 25.3% (n = 129/510) had a TB contact within the previous year, 12% (n = 15/129) of whom were household members. The clinical findings included fever (79.4%), persistent cough (82.4%), weight loss (44.2%), failure to thrive (54.9%), lymphadenopathy (11.5%) and chest radiograph consistent with TB (61.7%) (Table, Supplemental Digital Content 2, http://links.lww.com/INF/D601). The median history of illness was 30 days (IQR 6.45–90).
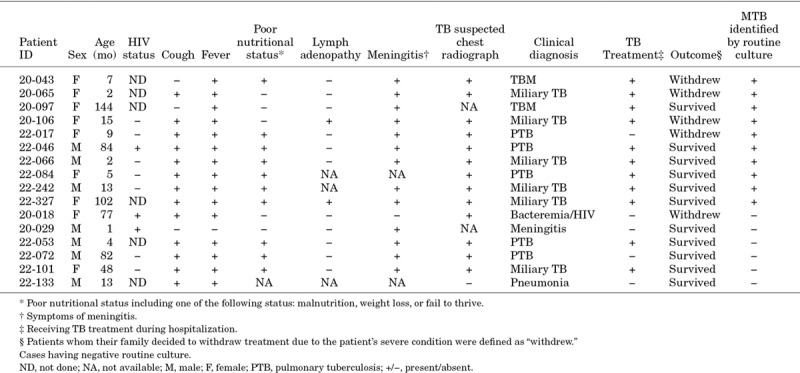
Thirty-two among 554 children had a positive blood culture, among whom 16 were confirmed MTB (2.9%) (Table, Supplemental Digital Content 3, http://links.lww.com/INF/D602). Fifteen of these 16 children had other samples collected (Table, Supplemental Digital Content 4, http://links.lww.com/INF/D603), two were AFB smear positive and 5 had negative routine cultures (Fig. 1). Thus, mycobacterial blood culture increased the number of confirmed cases from 92 to 97. The 5 additional cases were susceptible to rifampicin and isoniazid. Among 28 disseminated TB cases, as determined by clinical diagnosis, the yield of MTB blood culture was lower than that of routine liquid culture (6/28, 21.4% vs. 21/28, 75%). Among the 15 mycobacteremia cases with routine samples available, the median time to detection was 26 days (IQR: 13.0–31.0) for mycobacterial blood culture and 14 days (IQR: 8.8–16.0) for routine culture. The demographic and clinical characteristics of patients with MTB isolated from blood are summarized in Table 1. Among 16 cases with MTB bacteremia, 10 presented with disseminated TB (3 with tuberculous meningitis and 7 with miliary TB). Two patients were HIV infected. In the bivariate analysis, HIV infection did not showed any association with mycobacteremia (22.2% vs. 5.6%, P = 0.105). Unfavorable outcome (death or palliative discharge) was more frequent in patients with mycobacteremia (26.7% vs. 5.5%, P = 0.01) (Table, Supplemental Digital Content 5, http://links.lww.com/INF/D604). One of 16 cases with mycobacteremia had no routine cultures taken. Among the other 5 cases with mycobacterial blood culture as the sole source of MTB, there were 2 cases who already received TB treatment with a final clinical diagnosis of miliary TB when the culture became positive. The remaining 3 cases had not received TB treatment at the time of discharge before the positive MTB growth in the blood sample was released.
TABLE 1.
Characteristics of 16 Patients With Mycobacteremia
The low culture confirmation rate (28.4%, 98/345) was similar to other studies in children.4,5 In a recent study in a TB hospital in southern Vietnam, there were also ~25% culture-confirmed cases.6 Adding blood culture to routine diagnostics only marginally increased the yield of TB confirmation and the added yield was small compared with the substantial additional cost: the costs of Myco F/Lytic bottles alone per additional case detected was 525 USD.
Blood specimens are considered an attractive sample for diagnosis of pediatric TB because of the higher frequency of disseminated disease. However, here, only 21.4% cases were detected using blood culture when compared with 75% from respiratory samples using routine culture. An explanation may be that the mycobacteremia is transient and occurs before presentation at the hospital and blood collection, or that the mycobacteremia only occurs in a minority of children or that volume of blood collected for mycobacterial culture was not optimal.
In previous studies in adults,7,8 blood culture positivity for mycobacteria was more frequently found in HIV-infected patients but this difference was not statistically significant in our study population (2/15 in HIV positive vs. 5.6% in HIV negative (11/422), P = 0.105). This could be explained by the fact that not all children were tested and the number of children with confirmed HIV infection was small (n = 13/223 tests).
The time to positivity of mycobacterial blood culture in children with suspected TB in this study was long [median turnaround time (TAT): 25 days], similar to studies in adults.7,9 Recently, it was found that detection of MTB in blood using GeneXpert MTB/RIF could produce results within a day. The sensitivity of GeneXpert MTB/RIF was found to be similar to that of mycobacterial blood culture in a study of 104 HIV infected adults,10 but the high volume of blood used here (20 mL) could be problematic in small children.
Despite several limitations, our study demonstrates the utility of mycobacterial blood culture for TB detection in children: mycobacterial blood culture is able to detect more TB cases, but contributes little to the overall diagnostic yield in children.
Supplementary Material
Footnotes
Supported by the Wellcome Trust of Great Britain.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis. 2015;61suppl 3S179–S187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran ST, Renschler JP, Le HT, et al. Diagnostic accuracy of microscopic Observation Drug Susceptibility (MODS) assay for pediatric tuberculosis in Hanoi, Vietnam. PLoS One. 2013;8:e72100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blount RJ, Tran B, Jarlsberg LG, et al. Childhood tuberculosis in northern Viet Nam: a review of 103 cases. PLoS One. 2014;9:e97267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas TA, Heysell SK, Moodley P, et al. Intensified specimen collection to improve tuberculosis diagnosis in children from Rural South Africa, an observational study. BMC Infect Dis. 2014;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabi I, Kabyemera R, Mshana SE, et al. Pulmonary TB bacteriologically confirmed by induced sputum among children at Bugando Medical Centre, Tanzania. Int J Tuberc Lung Dis. 2016;20:228–234. [DOI] [PubMed] [Google Scholar]
- 6.Giang do C, Duong TN, Ha DT, et al. Prospective evaluation of GeneXpert for the diagnosis of HIV- negative pediatric TB cases. BMC Infect Dis. 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heysell SK, Thomas TA, Gandhi NR, et al. Blood cultures for the diagnosis of multidrug-resistant and extensively drug-resistant tuberculosis among HIV-infected patients from rural South Africa: a cross-sectional study. BMC Infect Dis. 2010;10:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Gottberg A, Sacks L, Machala S, et al. Utility of blood cultures and incidence of mycobacteremia in patients with suspected tuberculosis in a South African infectious disease referral hospital. Int J Tuberc Lung Dis. 2001;5:80–86. [PubMed] [Google Scholar]
- 9.Nguyen DN, Nguyen TV, Dao TT, et al. One year experience using mycobacterial blood cultures to diagnose tuberculosis in patients with prolonged fever in Vietnam. J Infect Dev Ctries. 2014;8:1620–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feasey NA, Banada PP, Howson W, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. J Clin Microbiol. 2013;51:2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]


