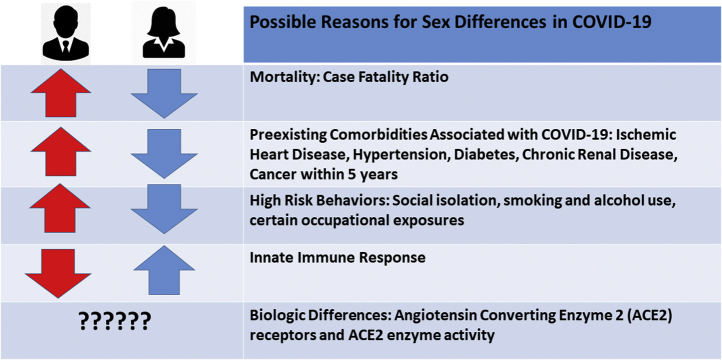
Graphical abstract
Key Words: COVID-19, mortality, sex differences
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease-2019; CVD, cardiovascular disease; SARS-CoV, severe acute respiratory syndrome-coronavirus
Collecting sex-disaggregated data is essential to understanding the risk factors of poor health, early death, and health inequities. Compared to women, men infected with the 2019 novel coronavirus disease-2019 (COVID-19) have more severe disease and a higher mortality (1, 2, 3). The COVID-19 pandemic is a global health emergency that has changed the world in an unprecedented way. Public health policies need to address the sex impact of this pandemic so that targeted treatment strategies can be implemented, such as early recognition and aggressive testing in subgroups, and to prevent bias in treating men and women. As the world responds to this extraordinary crisis, we need to have sex-specific analyses of the outbreak by health agencies and governments to better inform public health policies. We also need to recognize the phenotypical differences in severe case manifestations of COVID-19 in men and women as a fundamental step to understanding the effects of this health emergency on different individuals, families, and communities and for creating sustainable and equitable interventions.
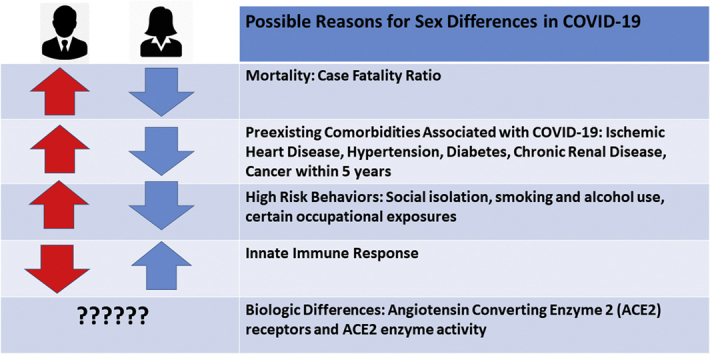
This analysis summarizes what is known so far about the sex differences in mortality, the contributions of underlying cardiovascular disease (CVD) risk factors, plausible biological reasons for this observed sex difference in COVID-19, and guidance to the cardiovascular community in conducting sex-specific research regarding COVID-19 disease severity and reasons for recovery (Figure 1).
Figure 1.
Sex Differences in Risk Factors and Mortality From the COVID-19 Pandemic
Global Health 5050, an organization that promotes gender equality in health care, has published the total and partial publicly available sex-disaggregated data from government sources (1). Sex-disaggregated data from 26 countries indicate that the overall case fatality ratio in men is higher than in women (1). The proportional male-to-female death ratio in confirmed cases is higher in all the countries with available data, with the largest male-to-female ratios seen in the Dominican Republic, the Netherlands, Denmark, and the Philippines of 2.8, 2.1, 1.9, and 1.8, respectively (as of April 17, 2020) (1). The United States, despite having the largest reported outbreak of COVID-19 in the world, has only partial sex-disaggregated data because all states and counties do not uniformly report these data to the Centers for Disease Control and Prevention. This disproportionate death ratio in men may partly be explained by their relatively higher contribution of pre-existing diseases (i.e., CVD, hypertension, diabetes, and chronic lung disease), higher risk behaviors (i.e., smoking and alcohol use), and occupational exposure (1). There may be other behavioral and social differences that favor women, with prior studies suggesting women are more likely than men to follow hand hygiene practices (4) and seek preventive care (5).
Regarding the impact of prevalent CVD and its risk factors, patients in Wuhan, China, with comorbid conditions had much higher death rates—10.5% for those with CVD, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer (6). In 1 case series from China, 75% of the COVID-19 deaths were among men (2). A report on 3,200 COVID-19–related deaths from Italy also showed higher deaths for men than women across all age groups, with women accounting for only 29.4% of these deaths (7). Overall, women dying from COVID-19 infection were older than men (median age: 82 vs. 79 years for women vs. men, respectively). Among the 9 deaths of patients younger than 40 years, 8 were men (7). Similar to the Chinese experience, the most common comorbidities diagnosed before COVID-19 infection in Italy were hypertension (73.8%), diabetes (33.9%), ischemic heart disease (30%), and atrial fibrillation (20%) (7).
It should be noted that similar coronaviruses, the Middle East respiratory syndrome coronavirus and the severe acute respiratory syndrome-coronavirus (SARS-CoV), were found to infect more men than women (8). In a mouse model study of SARS-CoV infection, male mice were more susceptible to infection than female mice. The enhanced susceptibility of male mice to SARS-CoV correlated with a moderate increase in virus titer and extensive alveolar macrophages and neutrophil accumulation in the lungs (9). Furthermore, although gonadectomy did not affect disease outcome in male mice, oophorectomy or treating female mice with an estrogen receptor antagonist did result in an increased mortality to SARS-CoV infection. These findings suggest that estrogen signaling protects female mice from a lethal infection (9). Sex chromosome genes and sex hormones, including estrogens, progesterone, and androgens, contribute to the differential regulation of immune responses between the sexes. Sex-specific disease outcomes following virus infections are attributed to sex-dependent production of steroid hormones, different copy numbers of immune response X-linked genes, and the presence of disease susceptibility genes in males and females (10,11).
Women are functional mosaics for X-lined genes, and the X chromosome contains a high density of immune-related genes; therefore, women generally mount stronger innate and adaptive immune responses than men (12). This results in faster clearance of pathogens and greater vaccine efficacy in females than in males but also contributes to their increased susceptibility to inflammatory and autoimmune diseases. The contribution of immunologic response mounted in women compared with men and its association with decreased mortality is certainly worth investigating.
Another important observation is that there does not appear to be increased risk for severe COVID-19 cases in pregnant women (13). In a study from Wuhan, China, of 118 pregnant women with COVID-19, the authors reported that 92% had mild disease and only 8% had severe hypoxemia, of whom 1 individual was critically ill, requiring mechanical ventilation. Among those women who had severe disease, 6 out of 9 developed this after birth. As of March 20, 2020, there have been no deaths among these pregnant women, and 94% have been discharged home, including all the women with severe or critical disease (13). Although this is reassuring and confirms the prior data of less severe disease in women and younger patients (3), this is a very small sample size of pregnant women, and extrapolations from these data should be made cautiously. When sex-disaggregated data were collected during the H1N1 pandemic, it was clear that pregnant women were actually at a higher risk of contracting the virus, and as such, were aggressively offered the vaccine.
Another biological difference may relate to sex differences in angiotensin-converting enzyme 2 (ACE2) receptors. The primary route of infection is via the ACE2 receptors, which is found in high levels throughout the lungs, myocardium, kidneys, and gastrointestinal system (14). Interestingly, there are marked differences in the density of ACE2 receptors in the reproductive organs: the testes have much higher levels of ACE2 than the ovaries (15). On one hand, ACE2 serves as a gateway for the virus’s entry into tissue. On the other hand, ACE2 plays an anti-inflammatory role and is thought to protect against lung injury. These 2 functions may be due to differences in the location of the ACE2 proteins, transmembrane or in the plasma (16). The ACE2 gene is located on the X chromosome, which suggests that women might have higher ACE2 levels and thus be protected against more severe disease compared to men (17). Further investigation into the association of ACE2 enzyme activity in severe myocardial injury and its correlation with sex is warranted. This will help us identify treatment targets and better understand the role of commonly used renin-angiotensin-aldosterone system medications in this disease. It is important to understand if sex is an independent risk factor for disease susceptibility and poorer outcomes.
In sum, evidence thus far tells us that sex is an important driver of risk of mortality and response to the COVID-19 pandemic. To develop a better understanding of the true biological differences in disease propagation and adverse outcomes, further research is warranted to investigate hormonal, inflammatory, immunologic, and phenotypical differences in severe COVID-19 disease presentations such as acute myocardial infarction, myocarditis, and pulmonary edema with diastolic dysfunction. Sex-disaggregated data are essential for understanding the distributions of risk, infection, and disease in the population and the extent to which sex affects clinical outcomes. Most importantly, as the COVID-19 pandemic unfolds, sex-disaggregated data can help guide clinical care and therapeutics and address questions such as whether older men with comorbidities require additional prevention, surveillance, or earlier intensive intervention than women or younger people or those without comorbidities.
Failing to integrate sex differences into COVID-19 research may lead us to neglect an important determinant of reducing the effectiveness of implementation interventions, inadvertently reinforcing sex-neutral claims, and possibly creating or increasing health inequities in care. Only by consistently investigating sex differences in a critical and reflective manner that addresses underlying inequities can we meet the requirements of scientific rigor, excellence, and maximal impact. Understanding sex differences of cardiac manifestations in COVID-19 disease should not be optional but must be considered as a core component of an equitable national response to this pandemic.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Global Health 5050 COVID-19 Sex-Disaggregated Data Tracker. http://globalhealth5050.org/covid19 Available at:
- 2.Xie J., Tong Z., Guan X., Du B., Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson H.D., Sholcosky D., Gabello K., Ragni R., Ogonosky N. Sex differences in public restroom handwashing behavior associated with visual behavior prompts. Percept Mot Skills. 2003;97:805–810. doi: 10.2466/pms.2003.97.3.805. [DOI] [PubMed] [Google Scholar]
- 5.Bertakis K.D., Azari R., Helms L.J., Callahan E.J., Robbins J.A. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–152. [PubMed] [Google Scholar]
- 6.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 7.Characteristics of COVID-19 Patients Dying in Italy. https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf Available at:
- 8.Alghamdi I.G., Hussain, Almalki S.S., Alghamdi M.S., Alghamdi M.M., El-Sheemy M.A. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 11.Robinson D.P., Huber S.A., Moussawi M. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ. 2011;2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurz H., Salie M., Tromp G., Hoal E.G., Kinnear C.J., Möller M. The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics. 2019;13:2. doi: 10.1186/s40246-018-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L., Li Q., Zheng D. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382:e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020 Mar 26 doi: 10.1161/CIRCULATIONAHA.120.047049. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci. 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia K., Zimmerman M.A., Sullivan J.C. Sex differences in angiotensin-converting enzyme modulation of Ang (1-7) levels in normotensive WKY rats. Am J Hypertens. 2013;26:591–598. doi: 10.1093/ajh/hps088. [DOI] [PMC free article] [PubMed] [Google Scholar]