Abstract
The COVID-19 pandemic is creating a havoc situation across the globe that modern society has ever seen. Despite of their paramount importance, the transmission routes of SARS-Cov-2 still remain debated among various sectors. Evidences compiled here strongly suggest that the COVID-19 could be transmitted via air in inadequately ventilated environments. Existing experimental data showed that coronavirus survival was negatively impacted by ozone, high temperature and low humidity. Here, regression analysis showed that the spread of SARS-Cov-2 was reduced by increasing ambient ozone concentration level from 48.83 to 94.67 μg/m3 (p-value = 0.039) and decreasing relative humidity from 23.33 to 82.67% (p-value = 0.002) and temperature from −13.17 to 19 °C) (p-value = 0.003) observed for Chinese cities during Jan-March 2020. Besides using these environmental implications, social distancing and wearing a mask are strongly encouraged to maximize the fight against the COVID-19 airborne transmission. At no other time than now are the scientists in various disciplines around the world badly needed by the society to collectively confront this disastrous pandemic.
Keywords: COVID-19, SARS-Cov-2, Viability, Environmental factors, Airborne transmission
Graphical abstract
1. Introduction
The COVID-19 pandemic has created a chaos across the globe. As of May 6, 2020 the confirmed cases exceeded a total of 3.5 million and over 240 thousand people lost their lives (WHO, 2020). Since Feb 21, China has initiated stringent control measures including the lockdown of Wuhan, travel ban, etc. (Tian et al., 2020). As of this writing, the COVID-19 pandemic is for now under good control for China and some other Asian countries. However, the pandemic continues to be spreading at an alarming speed for other parts of the world. Among many others, one of pressing things is to effectively control the extent of the pandemic spread. It is imperative that all possible SARS-Cov-2 transmission routes must be recognized and controlled in order to maximize the fight against the COVID-19 pandemic. Besides, it is equally important to identify potential environmental influencing factors on the spread of the COVID-19. Here, this communication is conducted to review the issues on airborne transmission of control of SARS-Cov-2, and to study the impacts of environmental factors for developing a viable control strategy for the pandemic.
2. Results and discussion
2.1. Airborne transmission of some known viruses such as SARS and influenza
Nearly 500 years ago, Girolamo Fracastoro (1478–1553) proposed in his book titled “(Contagion De Contagione et Contagiosis Morbis; 1546)” that tiny particles can cause epidemic diseases through direct or indirect contact or even without contact over a distance. It is no surprise that respiratory droplet and direct contact are both conceived to contribute to the infectious disease outbreaks (Stelzer-Braid et al., 2009; Ai and Melikov, 2018). However, for airborne transmission, there is a long lasting dispute over the years (Herfst et al., 2012). In the past, many studies were conducted to investigate the possibility of airborne transmission of infectious diseases. Back to 17 years ago for the SARS outbreak, there was a study conducted by scientists from Hong Kong showing the SARS can be transmitted via apartment building air (Yu et al., 2004). The SARS was also shown to be transmitted among people on an aircraft (Olsen et al., 2003), and it was detected in environmental samples including air (Booth et al., 2005). For other infectious viruses such as influenza, Norwalk-like virus, H5N1, and MERS, evidences are also accumulating to support that their airborne transmission does exist (Marks et al., 2003; Herfst et al., 2012; Leitmeyer and Adlhoch, 2016; Xiao et al., 2018; Yan et al., 2018). For influenza virus (2009 pandemic influenza A(H1N1), Kormuth et al. (2018) have demonstrated that its infectivity can be retained in fine aerosols and stationary droplets for up to 1 h under various relative humidity levels of from 20% to 98%. To simulate the emission by humans, they supplied human airway epithelial cells to the virus culture. These studies suggest that some known viruses are airborne and viable for their transmission. In addition to virus detection in air, many studies were also conducted to investigate the virus emission via breathing, coughing and sneezing (Stelzer-Braid et al., 2009; Shen et al., 2012; Gralton et al., 2013; Yan et al., 2018). According to one study, it was shown that 29 influenza viruses were emitted per uL of exhaled breath condensate (Shen et al., 2012). Besides emission of viruses, past studies also demonstrated that bacteria including human pathogens can be also emitted via breath (Xu et al., 2012; Zheng et al., 2018). In other studies, it was shown that the expiratory droplet size distribution had a peak size at 1.5 μm (Xu et al., 2017), and the exhaled droplet nuclei can be transmitted between occupants via airborne route (Ai et al., 2019). All these existing evidences in both air and exhaled breath indicate that airborne transmission plays a role in respiratory infection spread for some known viruses as mentioned above.
2.2. Evidences for airborne transmission of SARS-Cov-2 during the COVID-19 pandemic
As of this writing, evidences are still accumulating for possible airborne transmission of SARS-Cov-2. Santarpia et al. (2020) recently showed that 66.7% of their air samples collected from negative pressure equipped medical rooms housing 13 confirmed patients appeared to be positive with the SARS-Cov-2, and 100% of the personal air samples collected were positive with the SARS-Cov-2. In their study, the SARS-Cov-2 concentration levels were shown to reach 8688 copies/m3 for air sample and 67,164 copies/m3 for personal air samples (Santarpia et al., 2020). However, SARS-Cov-2 in these collected air samples have not been verified to be culturable (Santarpia et al., 2020). Similarly, Liu et al. (2020) also found that many air samples collected from the air of newly built hospital appeared to be positive with SARS-Cov-2 with a concentration level up to 42 copies/m3 as detected using a digital droplet PCR. Additionally, they have found floor samples collected from the hospital were positive with the SARS-Cov-2 (Liu et al., 2020). In contrast, another study conducted by scientists from Singapore showed the air sample collected was found to be negative with the virus, but for the surface sample collected from the ICU ventilation fan the virus was detected (Ong et al., 2020). This could be due to the deposition of airborne SARS-Cov-2 on the fan surface as a result from the ventilation process. Some of these studies indicated that SARS-Cov-2 was present with a high level up to 67,164 copies/m3 in the air of hospital environments that were housing the COVID-19 patients. Nonetheless, no evidence yet up to now was found for the presence of viable SARS-Cov-2, partially because the SARS-Cov-2 culturability was not investigated for many existing studies. However, one study from US NIH showed that the aerosolized SAR-Cov-2 from a liquid can survive at least 3 h in the air, and the SARS-Cov-2 was shown to have an equal half-life with the SARS in the air (van Doremalen et al., 2020). In another work, it was shown that SARS-Cov-2 can survive for many days on various surfaces, e.g., for up to 7 days on the outer surface of surgical mask (Chin et al., 2020). Belonging to the same virus family, the MERS was shown to be able to survive up to 1 h in the air at a relative humidity of 79% and a temperature of 25 °C (Pyankov et al., 2018). However, it started to decay rapidly at a lower humidity level (24%) and higher temperature (38 °C) (Pyankov et al., 2018). It is known that aerosolization process caused significant damages to microbes (Zhen et al., 2013), and using the same aerosolization process the NIH study still detected viable SARS-Cov-2 in the air even after 3 h (van Doremalen et al., 2020), which implies the SAR-Cov-2 had a strong survival ability in the air. Compared to the man-made aerosolization process, breathing, coughing or sneezing could have lower damaging effects since the virus residing lung environments are more favorable than the aerosolization liquid and the mechanical stress imparted on. For example, it was shown that exhaled influenza viruses retained their immuno-integrity as they could bind to the corresponding antibodies linked to the nanowire sensor (Shen et al., 2012). Similarly, aerosolized influenza viruses were also shown to retain their immuno-integrity (Shen et al., 2011). Previously, it was shown that some fraction of aerosolized MS2 virus, often used as a human model virus, could still survive even with microwave radiation and atmospheric cold plasma treatment, and about 20–90% of the treated MS2 were able to infect the host cell Escherichia coli under specific treatment conditions (Wu and Yao, 2014; Wu et al., 2015). Recently, National Research Council (2020) also stated that currently available research supports the possibility that SARS-CoV-2 could be spread via bioaerosols generated directly by patients' exhalation. The airborne transmission of COVID-19 could have been already happening in our daily life, e.g., the reported Washington State choir incident (three weeks later, 45 became ill out 60 attendants), and recently in a poorly ventilated restaurant (Li et al., 2020). Speaking itself was alos shown to emit a large amount of droplets, and different loudness resulted in different quantities (Anfinrud et al., 2020). Further to the problem, the COVID-19 transmission by asymptomatic patient was also found (Hoelscher et al., 2020). These undocumented or asymptomatic patient transmission add additionally to the mystery of SARS-Cov-2 transmission route, which otherwise can be well explained by an airborne route. In previous studies, for both controlled and natural indoor environments (classroom and subway), fine aerosol particles (around 1 μm) emitted by humans were shown to substantially predominate over coarse ones (Fan et al., 2017; Xu et al., 2017). For Beijing subway even with ventilation, the level of bioaerosol particle around 1 μm was still shown to increase significantly during the peak hour (Fan et al., 2017). The droplets in these environments were likely to evaporate very fast into fine ones. Accordingly, any viral particles if present should be largely in fine aerosol particles. Under controlled lab conditions, it was directly shown that humans emitted mainly fine aerosol particles (around 1.5 μm) during breathing (Xu et al., 2017). Given all these data above, it is highly likely that SARS-Cov-2 emitted by patients via fine aerosols into the air could be alive and able to replicate given conditions available. Accordingly, proper measures should be implemented to guard the airborne transmission route of the COVID-19 in not-well-ventilated indoor environments.
2.3. Airborne transmission of SARS-Cov-2 is possibly influenced by environmental factors
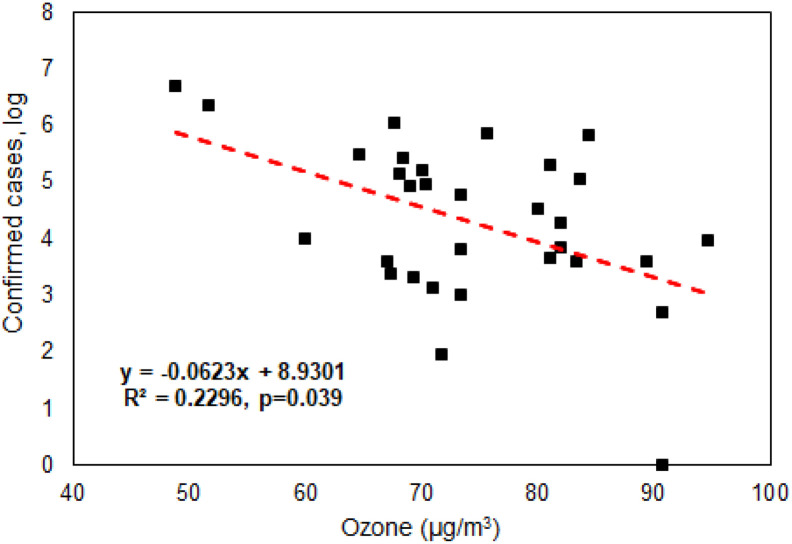
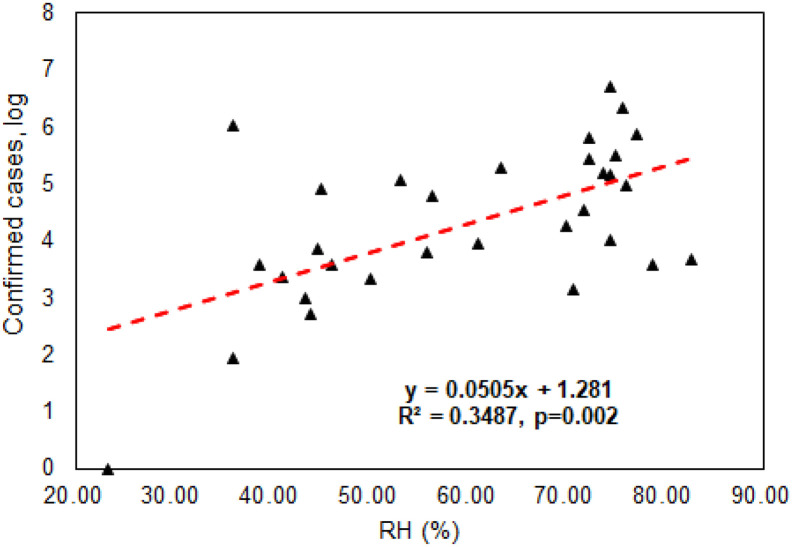
As discussed above, experimental evidences showed that the survival of aerosolized MERS in the air depends on the relative humidity and temperature, and the virus decay was much stronger for hot and dry air scenario with only 4.7% survival over 60 min period (Pyankov et al., 2018). In another work, it was demonstrated that when ambient ozone concentration level increased the transmission ability of influenza viruses (H1N1, H3N2, and Influenza B) decreased substantially (Ali et al., 2018). During a typical day in Beijing, it was also shown that when the ambient ozone concentration increased from late morning to early afternoon, the viability of biological particles decreased substantially (Wei et al., 2016). In considering existing evidences with respect to coronavirus, we performed Spearman's rank correlation analysis (data not normally distributed) using SPSS22.0 (IBM Corporation) and results as shown in Fig. 1 revealed that there was a statistically significant negative association between ambient average ozone levels (48.83–94.67 μg/m3) during Jan-March, 2020 and confirmed COVID-19 cases (log scale) for Chinese major cities (Supporting information File S1 and Fig. S1) (p-value = 0.039). In a previous work, it was shown that ozone water (4.86 mg/L) can completely inactivate SARS within 4 min (Zhang et al., 2004). On the other hand, ozone therapy was also used to treat many diseases (Elvis and Ekta, 2011) as it was described that an administrated dose of 30 and 55 μg/cc could induce greatest increase in the production of interferon as well as tumor necrosis factor and interleukin-2, triggering an entire cascade of subsequent immunological reactions (Elvis and Ekta, 2011). It seems that ozone has double effects with respect to virus transmission and infection control. In contrast, we have detected a statistically significant positive association between ambient average relative humidity (RH) levels (23.33–82.67%) and the confirmed COVID-19 cases (log scale) (p-value = .002) for the Chinese cities studied as shown in Fig. 2. Here, we also detected a possible association between the confirmed COVID-19 cases and the temperature (from −13 °C to 19 °C) (p-value = 0.003). The SARS-Cov-2 survival might have an optimal temperature, as some studies showed that above 56 °C would be lethal to the SARS-Cov-2 (Chin et al., 2020). In general, higher ozone level (>73 μg/m3) and lower RH level (<49%) as observed in Fig. S1 would lead to a lower number of the confirmed COVID-19 cases for a particular Chinese city. These environmental parameters could not only influence the ambient air, but also indirectly affect those indoors via atmospheric dispersion and penetration. Thus, the survival and infectivity ability of SARS-Cov-2 emitted by COVID-19 patients into the air and onto various surfaces in indoor environments could be affected by these environmental factors. Nonetheless, the detected effects of ambient ozone, humidity and temperature on the COVID-19 could be modified or cofounded by social distancing and wearing a face mask as implemented during the epidemic period. Additionally, air toxicity was shown to vary from city to city on a global scale by using both chemical assay and animal model (Chen et al., 2018; Li et al., 2019). The differences in air toxicities among cities might also affect the viability of SARS-Cov-2, thus on its transmission ability in the air as both outdoor and indoor environments are closely connected via moving air. These data along with the literature evidences collectively support our hypothesis that environmental factors such as temperature, humidity as well as pollutants such as ozone would have impacts on the transmission ability of SARS-Cov-2 for different cities. Accordingly, different cities might have inherently different vulnerability to the SARS-Cov-2 spread in terms with environmental factors.
Fig. 1.
Negative association between the number of confirmed COVID-19 cases and ambient average ozone levels (48.83–94.67 μg/m3) observed during Jan and March, 2020 (City information including altitude, ozone letter, population density, ultraviolet irradiation, relative humidity, temperature and COVID-19 cases is listed in Supporting Information File S1). Spearman's rank correlation coefficient (data not normally distributed) was performed using SPSS22.0(IBM Corporation 2013).
Negative association between the number of confirmed COVID-19 cases and ambient average ozone levels (48.83–94.67 μg/m3) observed during Jan and March, 2020 (City information including altitude, ozone letter, population density, ultraviolet irradiation, relative humidity, temperature and COVID-19 cases is listed in Supporting Information File S1). Spearman's rank correlation coefficient (data not normally distributed) was performed using SPSS22.0(IBM Corporation 2013).
Fig. 2.
Positive association between the number of confirmed COVID-19 cases (log scale) and ambient average relative humidity levels (23.33–82.67%) observed for major Chinese cities during Jan and March, 2020 (City information is listed in Supporting information File S1). Spearman's rank correlation coefficient (data not normally distributed) was performed using SPSS22.0(IBM Corporation 2013).
Positive association between the number of confirmed COVID-19 cases (log scale) and ambient average relative humidity levels (23.33–82.67%) observed for major Chinese cities during Jan and March, 2020 (City information is listed in Supporting information File S1). Spearman's rank correlation coefficient (data not normally distributed) was performed using SPSS22.0(IBM Corporation 2013).
3. Control and protection against airborne SARS-Cov-2 exposure
Viral infection requires sufficient viral dose and viability as well as the immunity status of the exposed individual. For personal protection both for the medical professional and the public, wearing a mask is important and necessary to effectively minimize the exposure dose especially when entering or staying in a closed or semi-closed environments with patients inside or their past occupancy where the SARS-Cov-2 aerosol could accumulate over the time. However, different masks have different protection efficiencies and different breathability as studied by Zou and Yao (2015). Typically, N95, or surgical mask had higher overall protection efficiencies of >90% against particles of 0.3–4.5 μm, and reached about 100% for particles of larger than 4.5 μm (Zou and Yao, 2015). Therefore, these masks are sufficient for respiratory droplet transmission prevention. For example, a recent work showed that wearing a surgical face mask could prevent transmission of human coronaviruses and influenza viruses from symptomatic individuals (Leung et al., 2020). For doctor mask, it had a good breathability, but its practical protection efficiencies were lower than those of N95 types (Zou and Yao, 2015). On the other hand, different face masks have different degree of fitness. For example, because of its rigidity of N95 mask, it was found that nurses wearing a N95 mask still had an infection rate of 22.9% in a clinical setting (Loeb et al., 2009). Another study also found that wearing a doctor mask can help reduce more the emission of biological agents into the air than wearing a N95 type mask (Xu et al., 2017), as the doctor mask has better fitness thus preventing exhaled bio-particles from releasing into the air. Accordingly, for healthy individual protection in risky environments it is encouraged to wear a N95 type mask or surgical mask or similar types that are available; and for protecting the environments it is advised for the patients to wear a doctor mask as it would less contaminate the environment by exhaled air (Xu et al., 2017). As the pandemic is getting worse, many sectors are of shortage of face masks which are in high demand. Accordingly, re-generation of used mask is becoming necessary especially during an urgent need for the public and sometimes even for the medical professionals. Previously, it was shown that microwave irradiation can effectively kill airborne MS2 viruses (>90% for 1.7 min exposure at a power level of 700 W) (Wu and Yao, 2014), and also several log reductions for H1N1 viruses deposited on face mask were achieved (Heimbuch et al., 2011). And it was indicated that the A protein gene of MS2 was completely damaged upon the microwave exposure, thus losing the viral cell entry ability (Wu and Yao, 2014). The killing of virus using microwave exposure was due to the adsorption of microwave energy by the polar groups of the virus (Wu and Yao, 2014). Heimbuch et al., 2011 performed studies of using microwave-generated steam for re-generating six different types of face masks including N95 by effectively killing H1N1 virus (4-log reduction of viable H1N1 virus). Our preliminary data showed that microwave irradiation did not significantly affect the protection efficiencies for particles of larger than 0.5 μm of the tested face masks tested (data not shown). They can be also repeatedly microwaved without pronounced decline in their absolute protection efficiency of the mask material. However, using the microwave method is limited to those polypropylene face masks without metal-alike piece or it was removed before the microwave re-generation. More studies involving face mask types should be further conducted, however in some dire situations, microwave irradiation using a household unit can offer an immediate practical solution for solving the urgent need of a face mask for the general public in protecting the people health. As for reducing the SARS-Cov-2 levels in the air in closed or semi-enclosed environments, it is best to frequently provide full fresh air, and not to use re-circulated air in order to effectively dilute the virus concentration levels. The ventilation for indoor environments has been adequately discussed in the literature (Li et al., 2007).
4. Summary and outlook of SARS-Cov-2 pandemic
As often discussed for a potential pandemic by a new and unknown viruses, the real moment is now facing the less prepared world. The situation is increasingly getting worse, as the globally confirmed cases now exceeded 3.5 million. It is imperative to recognize all routes of possible transmission including direct contact, respiratory droplet and also by air (bioaerosol) in order to mount effective countermeasures to minimize the death toll and contain the pandemic earlier. Existing evidences for other viruses and emerging ones for SARS-Cov-2 indicated that the SARS-Cov-2 was present in air with a sufficient amount particularly in closed or semi-enclosed environments, and thus had the opportunity of infecting those healthy people staying for a prolonged time inside. As reported every day, the COVID-19 transmission varied from country to country, from region to region, and even from city to city. The analysis of the confirmed cases for different cities in China indicated that environmental factors such as ambient temperature, relative humidity and ozone concentration level observed during Jan-March 2020 could have potentially impacted the transmission ability of COVID-19. This observation, however, on the other hand, implies that these environmental parameters can be adjusted to mitigate the transmission of COVID-19, e.g., use of ozone generators inside hospitals or other risky environments for inactivating airborne SARS-Cov-2. Nonetheless, it should be noted that the SARS-Cov-2 survival dependence could vary with different ranges of the environmental parameters studied here. If economically feasible, all possible engineering solutions including proper ventilation, face mask and use of air sterilizer are strongly encouraged for protecting the people and environment in an effort of stopping and slowing down the pandemic before a vaccine for the COVID-19 is widely available. At no other time than now are the scientists in various disciplines around the world badly needed by the society to collectively confront this COVID-19 crisis.
The following are the supplementary data related to this article.
The number of confirmed COVID-19 cases (log scale), ambient average ozone levels(48.83–94.67 μg/m3) and average relative humidity levels (23.33–82.67%) for major Chinese cities during Jan-March,2020 (City information including altitude, ozone letter, population density, ultraviolet irradiation, relative humidity, temperature and COVID-19 cases is listed in Supporting information File S1).
City information
CRediT authorship contribution statement
Maosheng Yao: Formal analysis, Writing - original draft. Lu Zhang: Formal analysis, Writing - review & editing. Jianxin Ma: Writing - review & editing, Resources. Lian Zhou: Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by National Natural Science Foundation of China (NSFC) emergent COVID-19 grant (22040101). This work was also partially supported by NSFC Distinguished Young Scholars Fund Awarded to M.Y. (21725701).
References
- Ai Z.T., Melikov A.K. Airborne spread of expiratory droplet nuclei between the occupants of indoor environments: a review. Indoor Air. 2018;28(4):500–524. doi: 10.1111/ina.12465. [DOI] [PubMed] [Google Scholar]
- Ai Z.T., Huang T., Melikov A.K. Airborne transmission of exhaled droplet nuclei between occupants in a room with horizontal air distribution. Build. Environ. 2019;163 doi: 10.1016/j.buildenv.2019.106328. [DOI] [Google Scholar]
- Ali S.T., Wu P., Cauchemez S., He D., Fang V.J., Cowling B.J., Tian L. Ambient ozone and influenza transmissibility in Hong Kong [letter to the editor] Eur. Respir. J. 2018;51(5) doi: 10.1183/13993003.00369-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinrud P., Stadnytskyi V., Bax C.E., Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering [letter to the editor] N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastion N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B., Mederski B., White D., Low D.E., McGeer A., Simor A., Vearncombe M., Downey J., Jamieson F.B., Tang P., Plumme F. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Li J., Zhang X., Li X., Yao M., Zheng G. Automated in vivo nanosensing of breath-borne protein biomarkers. Nano Lett. 2018;18(8):4716–4726. doi: 10.1021/acs.nanolett.8b01070. [DOI] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H., Chan M.C.W., Malik Peiris J.S., Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions [letter to the editor] MedRxiv. 2020 doi: 10.1101/2020.03.15.20036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. Letter to the editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvis A.M., Ekta J.S. Ozone therapy: a clinical review. Journal of Natural Science, Biology and Medicine. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Li X., Deng J., Da G., Gehin E., Yao M. Time-dependent size-resolved bacterial and fungal aerosols in Beijing subway. Aerosol Air Qual. Res. 2017;17(3):799–809. doi: 10.4209/aaqr.2016.03.0114. [DOI] [Google Scholar]
- Gralton J., Tovey E.R., McLaws M., Rawlinson W.D. Respiratory virus RNA is detectable in airborne and droplet particles. J. Med. Virol. 2013;85(12):2151–2159. doi: 10.1002/jmv.23698. [DOI] [PubMed] [Google Scholar]
- Heimbuch B.K., Wallace W.H., Kinney K., Lumley A.E., Wu C., Woo M., Wander J.D. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. AJIC: American Journal of Infection Control. 2011;39(1):e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Herfst S., Schrauwen E.J.A., Linster M., Chutinimitkul S., Wit E.D., Munster V.J., Sorrell E.M., Bestebroer T.M., Burke D.F., Smith D.J., Rimmelzwaan G.F., Osterhaus A.D.M.E., Fouchier R.A.M. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher M., Guggemos W., Vollmar P., Rothe C., Drosten C., Schunk M., Seilmaier M., Zwirglmaier K., Zange S., Sothmann P., Wölfel R., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. Letter to the editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormuth K.A., Lin K., Prussin A.J., Vejerano E.P., Tiwari A.J., Cox S.S., Myerburg M.M., Lakdawala S.S., Marr L.C. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J. Infect. Dis. 2018;218(5):739–747. doi: 10.1093/infdis/jiy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitmeyer K., Adlhoch C. Review article: influenza transmission on aircraft: a systematic literature review. Epidemiology. 2016;27(5):743–751. doi: 10.1097/EDE.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K., McDevitt J.J., Hau B.J.P., Yen H., Li Y., Ip D.K.M., Malik Peiris J.S., Seto W., Leung G.M., Milton D.K., Cowling B.J. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020 doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Leung G.M., Tang J.W., Yang X., Chao C.Y.H., Lin J.Z., Lu J.W., Nielsen P.V., Niu J., Qian H., Sleigh A.C., Su H.-J.J., Sundell J., Wong T.W., Yuen P.L. Role of ventilation in airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air. 2007;17(1):2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Li J., Chen H., Li X., Wang M., Zhang X., Cao J., Shen F., Wu Y., Xu S., Fan H., Da G., Huang R., Wang J., Chan C.K., De Jesus A.L., Morawska L., Yao M. Differing toxicity of ambient particulate matter (PM) in global cities. Atmos. Environ. 2019;212:305–315. doi: 10.1016/j.atmosenv.2019.05.048. [DOI] [Google Scholar]
- Li Y., Qian H., Hang J., Chen X., Hong L., Liang P., Li J., Xiao S., Wei J., Liu L., Kang M. 2020. Evidence for Probable Aerosol Transmission of SARS-CoV-2 in a Poorly Ventilated Restaurant. medRxiv preprint doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Ho K., Kan H., Fu Q., Lan K. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. BioRxiv. 2020 doi: 10.1101/2020.03.08.982637. [DOI] [Google Scholar]
- Loeb M., Dafoe N., Mahony J., John M., Sarabia A., Glavin V., Webby R., Smieja M., Earn D.J.D., Chong S., Webb A., Walter S.D. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302(17):1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- Marks P.J., Vipond I.B., Regan F.M., Wedgwood K., Fey R.E., Caul E.O. A school outbreak of norwalk-like virus: evidence for airborne transmission. Epidemiol. Infect. 2003;131(1):727–736. doi: 10.1017/S0950268803008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . 2020. Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic (April 1, 2020) [Google Scholar]
- Olsen S.J., Chang H., Cheung T.Y., Tang A.F., Fisk T.L., Ooi S.P., Kuo H., Jiang D.D., Chen T., Chen K., Lando J., Hsu K., Dowell S.F. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003;349(25):2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Sutjipto S., Chia P.Y., Young B.E., Gum M., Lau S.K., Chan M., Vasoo S., Mendis S., Toh B.K., Leong J., Barkham T., Ang B.S.P., Tan B.H., Leo Y., Marimuthu K., Wong M.S.Y., Ng O.T. Absence of contamination of personal protective equipment (PPE) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infect. Control Hosp. Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyankov O.V., Bodnev S.A., Pyankova O.G., Agranovski I.E. Survival of aerosolized coronavirus in the ambient air. J. Aerosol Sci. 2018;115:158–163. doi: 10.1016/j.jaerosci.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W., Crown K.K., Brett-Major D., Schnaubelt E., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. Medrxiv. 2020 doi: 10.1101/2020.03.23.20039446. [DOI] [Google Scholar]
- Shen F., Tan M., Wang Z., Yao M., Xu Z., Wu Y., Wang J., Guo X., Zhu T. Integrating silicon nanowire field effect transistor, microfluidics and air sampling techniques for real-time monitoring biological aerosols. Environmental Science & Technology. 2011;45(17):7473–7480. doi: 10.1021/es1043547. [DOI] [PubMed] [Google Scholar]
- Shen F., Wang J., Xu Z., Wu Y., Chen Q., Li X., Jie X., Li L., Yao M., Guo X., Zhu T. Rapid flu diagnosis using silicon nanowire sensor. Nano Lett. 2012;12(7):3722–3730. doi: 10.1021/nl301516z. [DOI] [PubMed] [Google Scholar]
- Stelzer-Braid S., Oliver B.G., Blazey A.J., Argent E., Newsome T.P., Rawlinson W.D., Tovey E.R. Exhalation of respiratory viruses by breathing, coughing, and talking. J. Med. Virol. 2009;81(9):1674–1679. doi: 10.1002/jmv.21556. [DOI] [PubMed] [Google Scholar]
- Tian H., Liu Y., Li Y., Wu C., Chen B., Kraemer M.U.G., Li B., Cai J., Xu B., Yang Q., Wang B., Yang P., Cui Y., Song Y., Zheng P., Wang Q., Bjornstad O.N., Yang R., Grenfell B.T., Pybus O.G., Dye C. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020 doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K., Zou Z., Zheng Y., Li J., Shen F., Wu C., Wu Y., Hu M., Yao M. Ambient bioaerosol particle dynamics observed during haze and sunny days in Beijing. Sci. Total Environ. 2016;550:751–759. doi: 10.1016/j.scitotenv.2016.01.137. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization (WHO); Geneva: 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 96. https://www.who.int/docs/default-source/coronaviruse/situation-reports/ (Accessed on April 26, 2020) [Google Scholar]
- Wu Y., Yao M. In situ airborne virus inactivation by microwave irradiation. Chin. Sci. Bull. 2014;59(13):1438–1445. doi: 10.1007/s11434-014-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Liang Y., Wei K., Li W., Yao M., Zhang J., Grinshpun S.A. MS2 virus inactivation by atmospheric-pressure cold plasma using different gas carriers and power levels. Appl. Environ. Microbiol. 2015;81(3):996–1002. doi: 10.1128/AEM.03322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Li Y., Sung M., Wei J., Yang Z. A study of the probable transmission routes of MERS-CoV during the first hospital outbreak in the republic of Korea. Indoor Air. 2018;28(1):51–63. doi: 10.1111/ina.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shen F., Li X., Wu Y., Chen Q., Jie X., Yao M. Molecular and microscopic analysis of bacteria and viruses in exhaled breath collected using a simple impaction and condensing method. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Wu C., Yao M. Fluorescent bioaerosol particles resulting from human occupancy with and without respirators. Aerosol Air Qual. Res. 2017;17(1):198–208. doi: 10.4209/AAQR.2016.09.0400. [DOI] [Google Scholar]
- Yan J., Grantham M., Pantelic J., Bueno de Mesquita P.J., Albert B., Liu F., Ehrman S., Milton D.K. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. U. S. A. 2018;115(5):1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W., Leung D.Y.C., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zheng C., Xiao G., Zhou Y., Gao R. Examination of the efficacy of ozone solution disinfectant in inactivating SARs virus. Chinese Journal of Disinfection. 2004;21(1):27–28. doi: 10.3969/j.issn.1001-7658.2004.01.009. [DOI] [Google Scholar]
- Zhen H., Han T., Fennell D.E., Mainelis G. Release of free DNA by membrane-impaired bacterial aerosols due to aerosolization and air sampling. Appl. Environ. Microbiol. 2013;79(24):7780–7789. doi: 10.1128/AEM.02859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Chen H., Yao M., Li X. Bacterial pathogens were detected from human exhaled breath using a novel protocol. J. Aerosol Sci. 2018;117:224–234. doi: 10.1016/j.jaerosci.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z., Yao M. Airflow resistance and bio-filtering performance of carbon nanotube filters and current facepiece respirators. J. Aerosol Sci. 2015;79:61–71. doi: 10.1016/j.jaerosci.2014.10.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The number of confirmed COVID-19 cases (log scale), ambient average ozone levels(48.83–94.67 μg/m3) and average relative humidity levels (23.33–82.67%) for major Chinese cities during Jan-March,2020 (City information including altitude, ozone letter, population density, ultraviolet irradiation, relative humidity, temperature and COVID-19 cases is listed in Supporting information File S1).
City information