To the Editor: Concern exists about COVID-19 in patients treated with systemic immunosuppressive or biologic therapy, or both.1 , 2 However, there are no data on the effects of COVID-19 infection in this population.1 , 3 We examined randomized controlled trials (RCTs), systematic reviews, and meta-analyses, focusing on RCTs for dermatologic conditions and respiratory infections where available.
The results are presented in Table I , and a detailed discussion is available in the Supplemental materials (via Mendeley at https://doi.org/10.17632/bsjcgsvvxw.2). Briefly, our findings suggest that upper respiratory infection (URI) rates associated with biologics were overall comparable with placebo before the COVID-19 outbreak; however, medication and disease-specific subtleties exist. Antitumor necrosis factor agents, for example, pose a risk for viral infections. However, differing results were seen in treatment of psoriasis vs hidradenitis suppurativa. Secukinumab was associated with increased URI but not the other IL-17 inhibitors. Tofacitinib treatment of patients with psoriasis resulted in an increased incidence of URIs compared with placebo. Cyclosporine is immunosuppressive; however, in the management of pyoderma gangrenosum, URIs were not reported in an RCT but 11% (n = 6) of patients on prednisolone experienced infections requiring hospital admissions compared with none in the cyclosporine arm. Placebo-controlled RCTs of other systemic immunosuppressive medications for dermatologic indications are lacking.
Table I.
Rate of infections with systemic immunosuppressive or biologic therapy, or both, for dermatologic indications
| Medication | Dermatologic indication (n) | URI drug/control, No. (%) | Viral infection drug/control No. (%) | Pneumonia drug/control, No. (%) | Infections, overall drug/control, No. (%) | Comments |
|---|---|---|---|---|---|---|
| Etanercept | Psoriasis (n = 583) | 51 (13)/25 (13)∗ ↔ | NR | NR | NR | … |
| Adalimumab | Psoriasis (n = 425) | NR | NR | 0 (0)/0 (0) ↔ | 14 (16.1)/59 (17.5) | … |
| Psoriasis (n = 1212) | 59 (7.2)/14 (3.5) ↑ | NR | NR | 235 (28.9)/89 (22.4) | … | |
| Psoriasis (n = 163) | NR | 0 (0)/1 (1.9) ↔ | NR | 51 (47.7)/23 (43.4) | … | |
| HS (n = 631) | NR | 0 (0)/1 (0.3) ↔ | NR | 79 (25)/96 (30.5)† | … | |
| Infliximab | Psoriasis (n = 129) | 7 (8.3)/2 (4.4) ↑ | NR | NR | NR | At week 26 |
| Psoriasis (n = 378) | 46 (15)/12 (16) ↔ | NR | NR | 125 (42)/30 (40) | … | |
| Psoriasis (n = 835) | 92 (14.7)/29 (14) ↔ | NR | NR | NR | … | |
| PG (n = 30) | NR↔ | 0 (0)/1 (6) | NR | NR | … | |
| HS (n = 33) | 4 (26.7)/5 (27.8) ↔ | 0 (0)/1 (5.6) | NR | NR | … | |
| Certolizumab | Psoriasis (n = 389) | 14 (4.2)/6 (10.5)∗ ↔ | NR | 1 (0.3)/0 (0)∗ | 82 (24.7)/16 (28.1)∗ | Reported as infections and infestations |
| Psoriasis (n = 460) | 24 (7)/5 (5) ↑ | NR | NR | 129 (36)/31 (31)∗,† | … | |
| Psoriasis (n = 175) | 4 (3.4)/4 (6.9)∗ ↔ | NR | NR | 43 (37)/24 (41)∗ | Reported as influenza. Other respiratory morbidities (tonsillitis, nasopharyngitis, rhinitis, bronchitis): 32 (27.4)/19 (32.8) | |
| Ustekinumab | Psoriasis (n = 9882) | 45 mg; 1.40 (1.09-1.81) | NR | NR | 45 mg; 1.09 (0.90-1.32) | Reported as RR by dose; 6 studies included. P > .2 for all data presented. Single study data for 3- and 5-year follow-up was similar |
| 90 mg; 1.02 (0.84-1.24) ↔ | 90 mg; 1.06 (0.93-1.21) | |||||
| Risankizumab | Psoriasis (n = 39) | 3 (10)/1 (13) ↔ | NR | NR | NR | … |
| Psoriasis (n = 171) | NR | NR | NR | NR | … | |
| Psoriasis (n = 997) | 30 (5)/8 (4) ↔ | NR | NR | 131 (22)/26 (13) | Reported as viral URI (other URI 4.7% and 2.0% for risankizumab and placebo, respectively) ↑ | |
| Tildrakizumab | Psoriasis (n = 2862) | 25 (2)/9 (1.9)∗,† ↔ | NR | NR | 3 (0.2)/1 (0.3) | Reported as severe infections requiring intravenous antibiotics |
| Guselkumab | Psoriasis (n = 837) | 25 (7.6)/9 (5.2) ↑ | NR | NR | 85 (25.8)/44 (25.3) | … |
| Psoriasis (n = 992) | 16 (3.2)/10 (4) ↔ | NR | NR | 106 (21.5)/46 (18.5) | … | |
| Secukinumab | Psoriasis (n = 2077) | 39 (2.8)/5 (0.7)† | 10 (0.7)/2 (0.3)† | NR | … | Viral infection with oral herpes |
| Psoriasis (n = 949) | Included in study immediately above | 31 (4.4)/3 (1.2)∗,† ↑ | NR | 378 (53.8)/48 (19.4) | Data included in study immediately above Reported separately due to description of influenza-like illness and overall infections | |
| Ixekizumab | Psoriasis (n = 1224) | 51 (3.5)/12 (3)∗,† ↔ | NR | NR | 381 (26)/74 (21)∗,† | … |
| Brodalumab | Psoriasis (n = 661) | 36 (8.2)/14 (6.4)∗ ↑ | NR | NR | 3 (0.7)/0 (0)∗ | … |
| Psoriasis (n = 195) | 13 (8)/2 (5)∗ ↑ | NR | NR | NR | … | |
| Psoriasis (n = 3089) | 112 (4.5)/40 (6.4)∗,† ↔ | NR | 1 (.04)/0 (0)∗,† ↔ | 11 (0.45)/2 (0.3)∗,† | … | |
| Anakinra | HS (n = 20) | … | NR | NR | 1 (5)/1 (5) | … |
| Omalizumab | Chronic urticaria (n = 322) | 7 (2.9)/7 (8.9) ↔ | NR | MR | 88 (36.2)/30 (38) | Additional outcomes, drug/control, No. (%): Viral URI: 6 (2.5)/1 (1.3); Flu: 10 (4.1)/4 (5.1) |
| Chronic urticaria (n = 335) | 18 (7.1)/2 (2.4) ↑ | NR | NR | 93 (36.9)/25 (30.1) | … | |
| Chronic urticaria (n = 318) | 7 (2.9)/3 (3.8) ↔ | NR | NR | 68 (28.6)/22 (27.5) | … | |
| Dupilumab | Atopic dermatitis (n = 2932) | 19 (1.0)/16 (1.5)∗ ↔ | 100 (5.4)/51 (4.7)∗ | NR | 739 (40.1)/453 (41.5)∗ | … |
| Rituximab | Pemphigus vulgaris (n = 91) | NR | NR | 3 (11)/1 (2) ↑ | NR | Control: oral prednisone (1-1.5 mg/kg/d) |
| Cyclosporine | Psoriasis (n = 217) | 10 (4.6%) ↑ | 4 (1.8) | NR | NR | Dose escalation study. No placebo control. |
| PG (n = 121) | NR | NR | NR | 4 (7)/5 (9) ↔ | Control: oral prednisolone (0.75 mg/kg/d) | |
| Azathioprine | Atopic dermatitis (n = 37) | 5 (13.5)/2 (5) ↑ | NR | NR | 1 (2.7)/1 (2.7) | … |
| Atopic dermatitis (n = 63) | 2 (5)/1 (5) ↔ | NR | NR | 2 (5)/0 (0) | Higher rates of lower respiratory infection in the treatment arm | |
| Tofacitinib | Psoriasis (n = 1106) | 10 (1.5)/0 (0) ↑ | NR | NR | 134 (20.3)/20 (18.7) | … |
| Methotrexate | Psoriasis, psoriatic arthritis (n = 221) | 31 (28.4)/25 (22.3) ↑ | NR | NR | NR | … |
HS, Hidradenitis suppurativa; NR, none reported; PG, pyoderma gangrenosum; RR, relative risk; URI, upper respiratory infection.
Control group is placebo unless stated otherwise in comments. ↔Suggests no increased URI. ↑Suggests increased URI.
Combined doses reported as mean.
Data collected from 2 phase II-III trials and reported as mean.
Considering the data presented in this report, the vital protective function of the skin and mucosa, and the relatively lower doses used in dermatology, it is reasonable to conclude that patients with severe dermatologic conditions requiring systemic therapies are overall likely to benefit from improved intact cutaneous function afforded by these medications. In high-risk patients, consideration of stopping tofacitinib and secukinumab may be warranted.
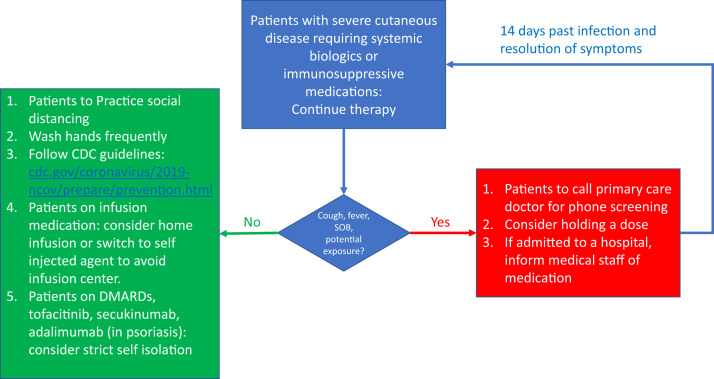
We encourage all patients to focus on infection prevention strategies. If symptoms arise, we advise a stepwise approach (Fig 1 ).4 Active infections remain a contraindication for systemic immunosuppressive and biologic therapy. Practical considerations should apply, including avoiding medications with frequent blood testing, switching to self-injected medication, and moving visits to telehealth. Discontinuation of systemic immunosuppressive and biologic therapy may result in disease exacerbation and loss of therapeutic response upon reintroduction. We hope these guidelines help dermatologists navigate therapy, as deemed appropriate by the patient and clinician during this dynamic period.
Fig 1.
University of Miami clinical considerations for managing patients on systemic immunosuppressive or biologic therapy, or both (SIBT), during COVID-19 pandemic. CDC, Centers for Disease Control and Prevention; DMARDs, disease-modifying antirheumatic drugs: azathioprine, cyclosporine, methotrexate; SOB, shortness of breath.
Acknowledgments
The authors thank Minhu Chen, MD, PhD, from the Department of Gastroenterology and Hepatology, The First Affiliated Hospital of Sun Yat-sen University, and from the Chinese Society of Gastroenterology, Guangzhou, China, for contributing unpublished data for this report.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
Reprints not available from the authors.
References
- 1.Mao R., Liang J., Shen J., et al. Chinese Society of IBD, Chinese Elite IBD Union; Chinese IBD Quality Care Evaluation Center Committee Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(5):426–428. doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-2019). People Who Are at Higher Risk for Severe Illness. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html Available at:
- 3.American College of Rheumatology A Message from the ACR about Coronavirus Disease 2019 (COVID-19) https://www.rheumatology.org/announcements Available at:
- 4.American Academy of Dermatology Coronavirus resource center. https://www.aad.org/member/practice/managing/coronavirus Available at: