Abstract
A paucity of research exists to investigate whether the normal aging process influences the ability to adapt disparity vergence and phoria. Vergence eye movements and dissociated phoria were recorded from 49 healthy subjects (ages 20–70 years) using an objective eye movement tracking system. Four-degree vergence responses were modified using a double-step protocol. Dynamics of vergence were quantified via peak velocity. The phoria adaptation experiment measured the magnitude (net change in phoria level) and rate (magnitude divided by the time constant) of phoria adaption during 5 min of sustained fixation on a binocular target (40 cm/8.44° from midline). The magnitude of phoria adaptation decreased as a function of age (r =−0.33; p = 0.04). The ability to adapt vergence peak velocity and the rate of phoria adaptation showed no significant age-related influence (p > 0.05). The data suggest that the ability to modify the disparity vergence system and the rate of phoria adaptation are not dependent on age; whereas, the magnitude of phoria adaptation decreases as part of the normal adult aging process. These results have clinical and basic science implications because one should consider age when assessing the changes in the magnitude of phoria adaptation which can be abnormal in those with oculomotor dysfunctions.
Keywords: Age, Vergence, Phoria, Adaptation, Eye movements
1. Introduction
The human brain calibrates the motor control of eye movements for optimal performance in the presence of both intrinsic (i.e. trauma, diseases, development or aging) and extrinsic (i.e. environment) changes (Leigh & Zee, 2016). One remarkable trait of the oculomotor system is adaptation, or the ability to precisely plan, coordinate, and execute eye movements to continually varying visual stimuli. The mechanism of adaptation within the oculomotor system is well-studied because the output can be easily quantified while sensory inputs (visual stimuli) are changed (Dash, Catz, Dicke, & Thier, 2010; Iwamoto & Kaku, 2010; Leigh & Zee, 2016; Ono & Mustari, 2010; Schor, 2009; Schubert & Zee, 2010; Tian, Ethier, Shadmehr, Fujita, & Zee, 2009).
In everyday life, humans use vergence eye movements – the inward (convergence) or the outward (divergence) rotation of the eyes – to perceive objects located at various distances. One of the major inputs to the vergence system is retinal disparity. Disparity is the main binocular cue describing the visual mismatch between the visual scene observed by left and right eye. The horizontal vergence system adjusts the position of the eyes to track a visual target using the lateral and medial extraocular muscles (Leigh & Zee, 2016). Dissociated heterophoria or simply phoria is the latent deviation of the visual axes to fusion in the absence of visual input to one eye (i.e., occlusion) while the other eye fixates on a target (Casillas Casillas & Rosenfield, 2006; Coffey, Reichow, Colburn, & Clark, 1991; Han, Guo, Granger-Donetti, Vicci, & Alvarez, 2010; Rosenfield, Chun, & Fischer, 1997). Most clinicians measure phoria with a target along the subject’s midline. The occluded eye may maintain its position (orthophoria), rotate nasally (esophoria), rotate temporally (exophoria), rotate upward (hyperphoria) or rotate downward (hypophoria). A person’s phoria level may adapt in response to a visual demand, duration of a visual task, or the amount of time that the subject is visually dissociated (Kim, Granger-Donetti, Vicci, & Alvarez, 2010; Lee, Chen, & Alvarez, 2008; Rosenfield et al., 1997; Wilmer & Buchanan, 2009). Previous research indicates that a person’s phoria can be adapted or modified in order to reduce the load or amount of work expended by the vergence system (McCormack, 1985; North & Henson, 1981; Schor, 1979).
Other studies have demonstrated the malleability of the disparity vergence system (Alvarez, Bhavsar, Semmlow, Bergen, & Pedrono, 2005; Kim, Vicci, Granger-Donetti, & Alvarez, 2011; Munoz, Semmlow, Yuan, & Alvarez, 1999; Semmlow, Yuan, & Alvarez, 2002; Takagi et al., 2001). More specifically, double-step (Alvarez et al., 2005; Takagi et al., 2001) or step-ramp (Munoz et al., 1999) conditioning stimuli have been used to increase or decrease the gain of vergence eye movements as quantified by peak velocity. Similar to disparity vergence, the dissociated phoria level can also be adapted (Kim, Vicci, Granger-Donetti, & Alvarez, 2011). Sustained fixation of binocular targets placed at different distances (e.g. near, middle and far visual target locations), along with the use of lenses (e.g. plus or minus lenses) or prisms (e.g. base in and base out prisms), has been shown to significantly change a person’s phoria level (Cheng, Schmid, & Woo, 2008; Jiang, Tea, & O’Donnell, 2007; Kim et al., 2010). While these studies clearly demonstrate the malleability of disparity-vergence and phoria, the majority of them have chosen to focus specifically on a young adult population (18 to 35 years of age) and have not considered the potential influence of aging on these visual dynamics.
Aging of the visual system has been associated with a reduction in contrast sensitivity (Owsley, 2011), visual acuity (Chou, Dana, & Bougatsos, 2009), and accommodation (Polat et al., 2012). Similarly, some investigators have reported that aging decreases vergence peak velocity (Rambold, Neumann, Sander, & Helmchen, 2006) as well as the magnitude and the time constant of phoria adaptation (Winn, Gilmartin, Sculfor, & Bamford, 1994). Conversely, Kalsi, Heron, and Charman (2001) measured static and dynamic accommodation, accommodative convergence, vergence and convergence accommodation responses and reported that there were no age-related effects in the latency and maximum velocity of vergence and accommodative vergence (p > 0.11) (Kalsi et al., 2001). Yang et al. also reported no aging effects on the gain (the amplitude of the output vergence response divided by the amplitude of the input stimulus target), peak velocity and acceleration of vergence responses (p > 0.23) (Yang & Kapoula, 2008; Yang, Le, & Kapoula, 2009b). Based upon these aforementioned studies, the influence of age-related effects on vergence dynamics quantified as peak velocity and the ability to modify the vergence and phoria systems remain unresolved in vision research.
To date, a systematic study on the ability to adapt the disparity-vergence and phoria systems as a function of age using objective eye movement tracking has not been published. Thus, the purpose of this examination is to investigate whether the adaptability of the disparity-vergence and the phoria systems is maintained throughout adult life. It is well established that accommodation decreases with age (Leigh & Zee, 2016). Prior research supports that accommodation is an input to the vergence system (Maxwell, Tong, & Schor, 2010; Semmlow & Hung, 1980; Yuan, Semmlow, Alvarez, & Munoz, 1999). Since accommodation decreases with age and it does interact with the vergence system then it is possible that vergence performance may also decrease with age. This study will test the hypothesis that with the advancement of age, the ability to modify vergence peak velocity during a short-term modification experiment, as well as the magnitude and the rate of phoria adaptation, may decline with age. This knowledge is important because decreased phoria adaptation is common in some binocular dysfunctions such as convergence insufficiency (Brautaset & Jennings, 2005; Erkelens, Thompson, & Bobier, 2016; Sreenivasan & Bobier, 2015; Sreenivasan, Irving, & Bobier, 2008). If vergence and phoria adaptation are reduced with age, then it is important that clinicians and researchers take into account potential aging effects when studying the reduced ability to adapt vergence and phoria commonly observed in binocular dysfunctions.
2. Methods
2.1. Subjects
A total of forty-nine subjects participated in the study. All subjects signed a written informed consent form approved by the NJIT Institution Review Board in accordance with the Declaration of Helsinki. All subjects were instructed to look at the visual targets when presented and were naïve to the hypotheses of the study. The subjects were divided into three groups based upon age: 20 to 35 years (n = 10; “younger” group), 36 to 50 years (n = 28; “mid-aged” group), and 51 to 70 (n = 11; “older” group). All subjects had no prior experience with other oculomotor experiments and were naïve to the goals of the study. None of the subjects had neurological dysfunction or injury; ocular; oculomotor; or binocular abnormalities. Binocular function was assessed using a Randot Stereopsis Test (Bernell Corp., South Bend, IN, USA) and near point of convergence (NPC) using methods described in detail in a previous research (Alvarez, 2015; Alvarez et al., 2010; Jaswal, Gohel, Biswal, & Alvarez, 2014; Lee et al., 2008; Scheiman, Talasan, Mitchell, & Alvarez, 2016; Semmlow, Alvarez, & Pedrono, 2007; Talasan, Scheiman, Li, & Alvarez, 2016). An optometrist objectively measured refraction using static retinoscopy. Monocular amplitude of accommodation was assessed for the right eye with the Astron Accommodative Ruler with the printed Gulden fixation target of a column of 20/30 letters. The subject was instructed to keep the letters clear and tell the examiner when the letters first blur. The target was moved towards the subjects at a rate of about 1 cm/s until it appeared to blur. Subjects had normal or corrected-to-normal vision during the experiment. For incipient presbyopes and presbyopes, the vision parameters were measured through the near add of their spectacles. The mean and standard deviation of each group attributes are described in Table 1.
Table 1.
Mean and standard deviation of the three age group attributes.
| Group | Age (yrs) | Gender F = Female M = Male | Stereopsis (sec arc) | Amplitude of accommodation (D = Diopters) | Near point of Convergence (cm) | Mean right eye sphere (D = Diopters) | Mean left eye sphere (D = Diopters) |
|---|---|---|---|---|---|---|---|
| Young | 23.5 ± Z50 | 4 F and 6 M | 21.8 ±3.5 | 11.1 ± 1.8 | 5.0 ± 2.0 | −0.05 ± 0.98 | −0.13 ±0.94 |
| Mid-Aged | 46.1 ±2.60 | 16 F and 12 M | 35.2 ±15.7 | 3.2 ± 1.9 | 59 ±22 | −1.45 ± 2.22 | −1.02 ±1.93 |
| Older | 61.0 ±6.13 | 5 F and 6 M | 46 ±21.9 | 1.4 + 0.8 | 6.4 ± 32 | 0.30 ± 2.60 | 0.28 ± 2.63 |
2.2. Short-term vergence modification experiment
Eye movements were recorded using an infrared (λ = 950 nm) video-based ISCAN eye movement monitor which tracks both eyes simultaneously and independently. The manufacturer’s specification for accuracy was 0.3° over a ±20° horizontal range (ISCAN Inc., Burlington, MA, USA). The horizontal eye movements were analyzed by tracking the centroid of the pupil. Eye movements were sampled at 500 Hz using a custom LabVIEW™ program, VisualEyes, with the same 12-bit digital acquisition hardware card (Guo, Kim, & Alvarez, 2011).
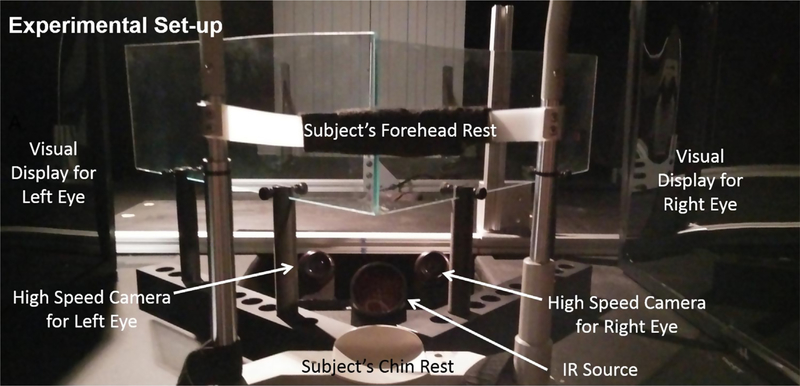
Before the experiment, all subjects were situated in a head and chin rest assembly to reduce the influence of the vestibular system (Khojasteh & Galiana, 2007). The stimuli were 40 cm away or 2.5D in a darkened room using a haploscopic experimental set-up, see Fig. 1. The haploscope kept the accommodative demand constant while changing the disparity visual cue to different vergence angles. Two computer monitors projected independent visual stimuli onto partially reflective mirrors situated along the subject’s midline. The subjects used a trigger button to initiate an experimental trial which consisted of a symmetrical convergence single-step or double-step stimulus. This allowed the subjects to blink between experimental trials and reduce the occurrence of blinking during data collection. Having the subject initiate the experimental trial also reduces the influence of fatigue (Alvarez, Semmlow, Yuan, & Munoz, 2000; Yuan & Semmlow, 2000). After the subject initiated an experimental trial, a random delay of 0.5–2 s was inserted prior to stimulus presentation to reduce the influence of anticipatory or predictive movements, which have been shown to influence the peak velocity of vergence eye movements (Alvarez, Semmlow, Yuan, & Munoz, 2002; Kumar, Han, Garbutt, & Leigh, 2002). Subjects were instructed to keep the visual stimuli single and clear.
Fig. 1.
Haploscope experimental set-up. Two monitors were used to project visual stimuli to two partially reflective mirrors so that the left and right eye each saw an independent visual image.
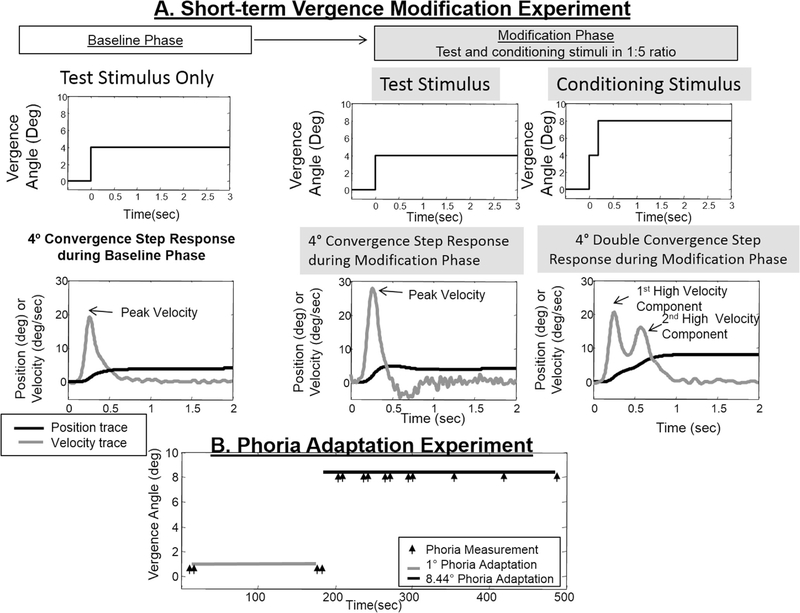
There were two phases (baseline and modification) to the experiment shown in Fig. 2A. There were two types of stimuli analyzed, termed the test and conditioning stimuli. During the baseline phase, only the test stimuli (4° convergence step) were presented to the subjects. During the modification phase, subjects viewed the conditioning stimuli (4° double step with a 200 ms delay between steps for a total change in disparity of 8°) and the test stimuli in a five-to-one ratio. The 200 ms delay is needed to reduce external visual feedback of the system. Subjects saw five times as many conditioning stimuli to the test stimuli. The purpose of the modification phase was to study how the conditioning stimuli (double step) influenced the test (single step) responses. The near visual target was presented for 3 s. Data were assessed using the peak velocity within the transient portion of the convergence movement.
Fig. 2.
A: An example of 4° single convergence step position (black) and velocity (gray) traces recorded during the baseline phase (left column) and modification (middle column) phase. Double steps have two high-velocity components termed the first high-velocity component and second high-velocity component (right column). The arrows in plot A indicate the peak velocity. During the conditioning phase, the peak velocity of the 4° vergence steps is greater compared to the baseline responses as a result of the short-term modification experiment. 2B: The phoria adaptation experimental protocol where each arrow represents a phoria measurement.
A MATLAB™ program written within our laboratory was utilized for the data analysis. The left and right eye were subtracted for a new vergence response. The left and right eye were individually calibrated. Monocular calibration was collected to reduce fixation disparity which is important for subjects who may have a large exo or eso fixation disparity. The average voltage value was objectively measured at known distances from the subject for 500 ms which served as a calibration point. Two calibration points were recorded for each eye. The gain and offset were calculated for the left and for the right eye movement position. Eye movements with saccades within the transient were omitted from analysis because prior research has shown that saccades increase the peak velocity of vergence responses. (Kim & Alvarez, 2012b; Zee, Fitzgibbon, & Optican, 1992). Saccades are about an order of magnitude greater in velocity compared to vergence and hence are easily identified (Leigh & Zee, 2016). Saccades within the symmetrical vergence responses were detected by using a semi-automated custom software program written in MATLAB (Alvarez & Kim, 2013; Alvarez, Semmlow, Ciuffreda, Gayed, & Granger-Donetti, 2007; Kim & Alvarez, 2012b; Semmlow, Chen, Granger, Donnetti, & Alvarez, 2009; Semmlow, Chen, Pedrono, & Alvarez, 2008). Using the conjugate position trace, any saccades that were > 0.15° in magnitude were identified by the software. The responses were also manually inspected by the operator. Responses with blinks within the transient were also omitted from further data analysis because a peak velocity could not be measured. Blinks were rare within this dataset.
Convergence was plotted as a positive measurement. The two point central difference algorithm was used to compute convergence velocity (Bahill, Kallman, & Lieberman, 1982). The maximum velocity value was defined as the peak convergence velocity (Fig. 2A). An example of a typical position and velocity response stimulated from the conditioning 4 double step is shown in Fig. 2A (right column). The double steps contained two high-velocity components, which were quantified using the first and second peak velocities.
2.3. Phoria adaptation experiment
A haploscope was used during the phoria adaptation experiment so that the visual stimuli to both eyes could be controlled independently to measure phoria, Fig. 1. A green vertical line 2 mm in width and 2 cm in height presented on a black background was used as the visual stimulus. Two partially reflecting mirrors (50% reflectance and 50% transmission) projected the two green vertical lines from the computer screens to the subject. All visual stimuli on the computer monitors were calibrated with real world targets.
Within a dark room, the near dissociated phoria was measured where the subject’s left eye monocularly viewed a vertical line positioned 40 cm away (4.22° inward ocular rotation per eye) from the midline. Simultaneously, the right eye viewed a dark field; the right eye position rotated to the steady state phoria level. The right eye position was recorded for 15 s which our prior studies shows is ample time to objectively measure the phoria (Han et al., 2010). A prism diopter (Δ) is equal to 100 times the tangent of the vergence angle (Θ) in degrees (Δ = 100 tan (Θ)).
A four-point, right eye monocular calibration using a range of 16Δ exophoria to 7Δ esophoria was used to measure the right-eye movement response in prism diopters. This phoria calibration range was chosen because all of our subjects were within this range. The average of the last three sections of the right-eye response was used to measure the phoria level because all movements reached their steady state before 12 s. Phoria responses from our laboratory have been demonstrated in prior papers (Kim & Alvarez, 2012a, 2013; Kim et al., 2010; Kim, Vicci, Granger-Donetti, et al., 2011; Lee, Granger-Donetti, Chang, & Alvarez, 2009).
A schematic of the phoria adaptation experiment is shown in Fig. 2B. The phoria adaptation experiment used a cumulative time of 5 min of sustained fixation on an 8.44° binocular target (located 40 cm away from the subject’s midline) which is known to adapt the phoria (Han et al., 2010). Before the experiment, subjects were dark adapted for ten minutes to uncouple the accommodation and vergence systems (Wolf, Bedell, & Pedersen, 1990) and to reduce any prior visual adaptation stimulated from near or far visual work performed prior to the experiment. After dark adaptation, two phoria measurements were recorded (indicated using an arrow in Fig. 2B. Then, the subject fixated on a far target (a 1° binocular target along midline) for 3 min, after which two phoria were measured and averaged. Based upon our past research and that of others, a person’s baseline phoria can be influenced by the amount of near work conducted prior to the experiment (North & Henson, 1981). Specifically, if the person has been performing a lot of near work then the phoria will become more esophoric. We choose to adapt to a far position first and then to a near position to observe a greater change of the phoria level (Kim & Alvarez, 2012a; Kim, Vicci, Granger-Donetti, et al., 2011). Hence, all subjects were adapted to a 1° binocular target along midline to stimulate a greater change in the phoria adaptation measurements. A total of 13 phoria measurements (2 baseline and 11 adapted phoria measurements) were recorded during the five minutes of phoria adaptation to the 8.44° binocular target. After every 30 s of sustained fixation, two phoria measurements were recorded and averaged. The total fixation time was 2 min. Two phoria measurements were obtained after the following sequence: dark adaptation, 3 min of far adaptation, and four sampling periods of adaptation, each of which were 30 s of 8.44° sustained fixation (displayed as a double arrow in Fig. 2, plot B). To assess repeatability, the phoria measurement was recorded twice for each phoria measurement. For each pair of phoria measurements, significant differences between the pair were not observed (p > 0.1). Hence, each pair of phoria measurements was averaged. Last, the latter three phoria measurements were each recorded after 1 min of sustained fixation. Total adaptation time was 5 min. Then, the phoria measurements were fit with an exponential function and the time constant was computed. The time constant was the time needed for the response to reach 63% of the steady state. To compute the rate of phoria adaptation (Δ/min) the net change in phoria or magnitude was divided by the time constant. The rate of phoria adaptation was used to normalize data to allow comparisons between the data of different subjects.
2.4. Statistical analysis
The change in peak velocity was defined as the modification peak velocity minus the baseline peak velocity. The high-velocity ratio was calculated as the ratio of the peak velocity from the first high-velocity component, divided by the peak velocity from the second high-velocity component. To analyze the age-related effects, a one-way analysis of variance (ANOVA) was performed on the individual mean parameters (i.e. vergence peak velocities of the test (single step) and conditioning (double step) responses, magnitude and rate of phoria adaptation) with age (young, mid-aged and older groups) as the main factor, using NSC2004 (Kaysville, UT, USA). A repeated-measures ANOVA was performed to determine whether peak velocity from the 4° convergence steps was significantly different during the modification phase compared to the baseline phase.
Linear regression analyses were conducted between the parameters from the short-term vergence modification experiment and the phoria adaptation experiment on the data from seventeen subjects to study the relationship between the ability to adapt the vergence system and phoria. All linear regressions were quantified using a Pearson correlation coefficient, calculated using MATLAB™. Figures were generated using MATLAB™ and Microsoft™ Excel.
3. Results
3.1. Short-term vergence modification experiment
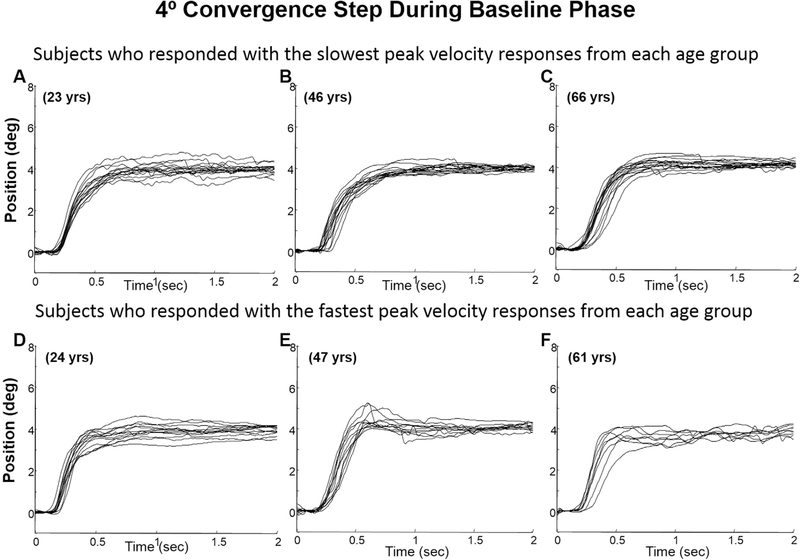
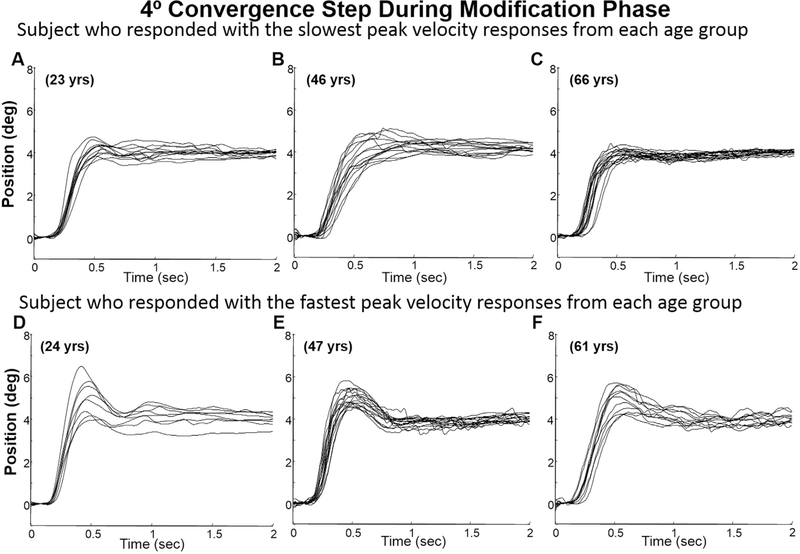
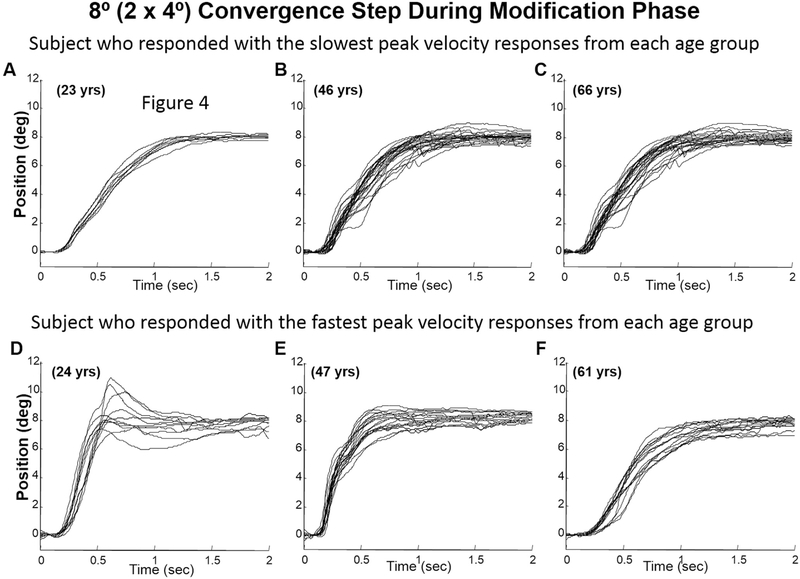
Examples of 4° convergence step responses of two subjects (the fastest and slowest subject) from each group (young, mid-aged, and older) during the baseline phase are shown in Fig. 3. Plots A, B, and C plot eye movements from the young, mid-aged and older groups where the subject chosen is the subject who had the slowest peak velocity within each age group. Plots D, E, and F are also eye movements from the young, middle again and older groups respective; however, these are the movements from the subject who exhibited the fastest eye movements within the designated age group. In all three age groups, a range of vergence peak velocities were observed. During the modification phase, all subjects demonstrated overshoots within the transient portion of the 4° convergence step movements, recorded during the modification phase compared to that subject’s baseline recordings. Note, that the retinal stimulus was the same for both the baseline and modification phases since the stimulus was the same 4° retinal disparity. As shown in Fig. 4, some subjects demonstrated small overshoots (plots A, B and C) while others demonstrated substantially large hypermetric responses (plots D, E, and F). This variability was observed within all three age groups. Examples of 4° convergence double-step responses consisting of two distinct high-velocity components are also shown in Fig. 5, plots A, B and C for the subject who had the slowest peak velocity within each age group and plots D, E, and F for the subject who had the fastest eye movement peak velocities within each age group.
Fig. 3.
Ensemble of several 4° convergence eye movements per subject who demonstrated slowest eye movements within each age group (plots A (young), B (mid-aged) and C (older)) as well as subjects who exhibited the fastest convergence eye movements (plots D (young), E (mid-aged), and F (older)) during the baseline phase of the vergence modification experiment. Each trace is an individual eye movement. The age of the subject is denoted on the plot.
Fig. 4.
Ensemble of several 4° convergence eye movements from the subject who demonstrated slowest (plots A, B and C) and fastest (plots D, E, and F) peak velocity convergence responses per age group during the modification phase of the vergence modification experiment. Each trace is an individual eye movement. The age of the subject is denoted on the plot.
Fig. 5.
Ensemble of several double 4° convergence eye movements (8° disparity) per subject who demonstrated the slowest (plots A, B and C) and fastest (plots D, E, and F) convergence responses from each age group during the modification phase of the vergence modification experiment. Each trace is an individual eye movement. The age of the subject is denoted on the plot.
Linear regression analyses revealed that no significant relationship between 4° convergence peak velocity and age during baseline (r = −0.04; p > 0.5) or modification (r = −0.05; p > 0.5) phases was observed. Change in peak velocity was calculated as the difference between the modification and baseline phase average peak velocity. There was no significant relationship between the change in peak velocity and age (r = −0.05; p > 0.5). The first high-velocity component (r = −0.04; p > 0.5) and the second high-velocity component (r = 0.11; p > 0.5) were also not dependent on age. The high-velocity ratio was calculated as the first high-velocity component divided by the second high-velocity component. Similarly, the high-velocity ratio was not dependent on age (r = 0.03; p > 0.5). A one-way ANOVA revealed no significant differences between the main factor of age groups compared to the mean convergence peak velocity during the baseline [F(2,22) = 2.08; p = 0.15] and modification [F(2,22) = 0.85; p = 0.44] phases, as well as the ability to change convergence peak velocity [F(2,22) = 0.24; p = 0.79]. Similarly, an one-way ANOVA showed no significant effect of age on the first high-velocity component [F(2,22) = 2.40; p = 0.11], the second high-velocity component [F(2,22) = 0.02; p = 0.98], or the high-velocity ratio [F(2,22) = 0.64; p = 0.54].
3.2. Phoria adaptation experiment
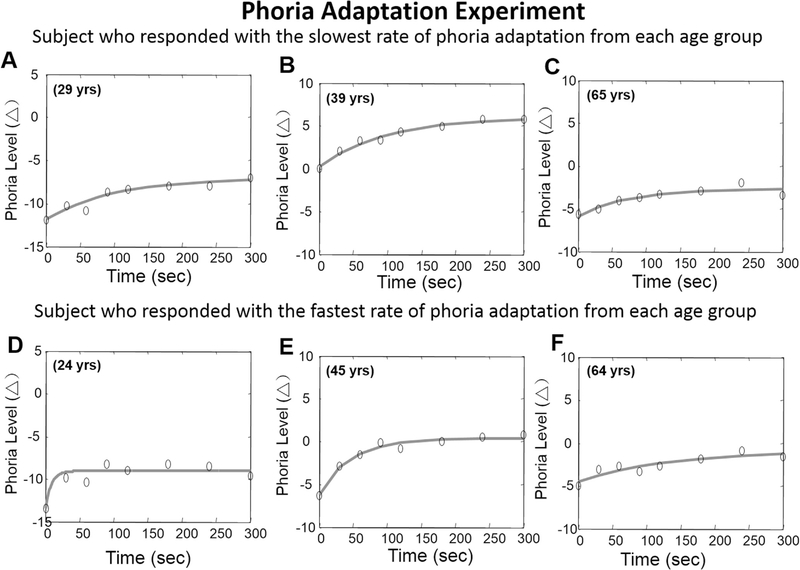
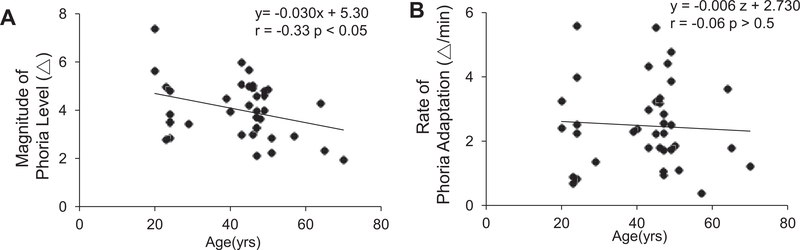
Fig. 6 shows a comparison of phoria adaptation to an 8.44° binocular target between the younger (plot A and D), mid-aged (plot B and E) and older (plot C and F) groups. Fig. 6 plots the subject who exhibited the slowest rate of phoria adaptation from each age group (upper plots) and the subject who exhibited the fastest rate of phoria adaptation from each age group (lower plots). A one-way ANOVA demonstrates that the change in the phoria magnitude was significantly different between groups [F(2, 37) = 4.99; p = 0.01]. The post hoc Bonferroni multiple comparison test confirmed that the older age group demonstrated significantly smaller magnitudes (net change in phoria) compared to the young and mid-aged groups. However, there was no significant difference between the young and mid-aged group. The change in the magnitude of phoria was significantly correlated with age (r = −0.33, p = 0.04) with approximately 0.3Δ reduction in the magnitude of phoria per year (Fig. 7, plot A). A one-way ANOVA demonstrates that the rate of phoria adaptation was not different between the age groups [F(2,37) = 1.67; p = 0.20]. There was no significant correlation between the rate of phoria adaptation and age (r = −0.06, p > 0.5) as shown in Fig. 7B.
Fig. 6.
Examples of phoria measurements (denoted as ‘o’) from six subjects where the first row are subjects who exhibited the slowest rate of phoria adaptation to the 40 cm (8.44°) binocular target for each age group. The second row are subjects who exhibited the fastest rate of phoria adaptation. The first, second and third column are from subjects within the young, mid-aged and older age groups, respectively. The age of the subject is denoted in the upper left portion of each plot. The phoria measurements were fit with an exponential curve denoted using a solid black line.
Fig. 7.
Linear regression analysis of the magnitude or change in the phoria during a phoria adaptation experiment (plot A) and the rate of phoria adaptation (plot B) plotted as a function of age.
3.3. Peak velocity of vergence versus rate and magnitude of phoria adaptation
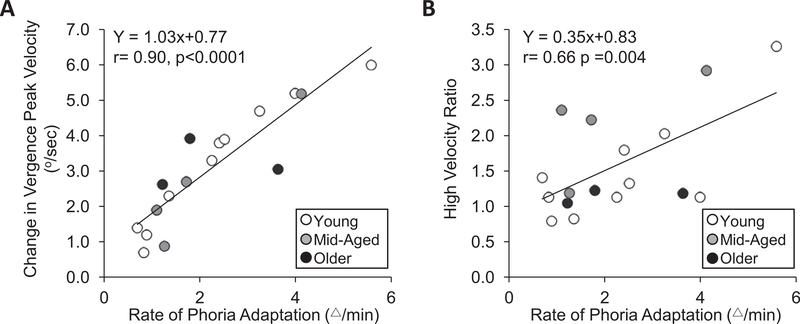
A total of seventeen subjects’ data were used to study the relationship between the vergence and phoria parameters. In summary, the data from three subjects who were categorized as older, four subjects who were categorized as middle-aged and ten subjects who were categorized as young were included within this analysis. The baseline and modification peak velocity were significantly correlated with rate of phoria adaptation (r > 0.59, p < 0.02). In addition, change in 4° convergence peak velocity was significantly correlated with rate of phoria adaptation (r = 0.90, p < 0.0001), Fig. 8, plot A. Note, that the rate of phoria adaptation and the high-velocity ratio were also significantly correlated (r = 0.66; p = 0.004) as shown in Fig. 8, plot B. The magnitude of phoria adaptation was not significantly correlated with the following vergence parameters: baseline peak velocity (r = 0.2, p = 0.95), modification peak velocity (r = 0.09, p = 0.72), first high-velocity component (r = −0.16, p = 0.54), second high-velocity component (r = −0.37, p = 0.13), change in peak velocity (r = 0.25, p = 0.54), and high-velocity ratio (r = 0.07, p = 0.78). Table 2 lists the summary of the linear regression analyses.
Fig. 8.
Change in convergence peak velocity (plot A) and high-velocity ratio (plot B) as a function of rate of phoria adaptation (Δ/min) shown for young (white), mid-aged (gray), and older (black) age groups.
Table 2.
Summary of linear regression analyses between short-term vergence modification and phoria adaptation experiment on seventeen subjects.
| Parameters denoted as Y in linear regression equation | Linear regression equation x = rate of phoria adaptation | R | P |
|---|---|---|---|
| Baseline peak velocity (°/sec) | Y = 1.6Ox + 13.11 | 0.59 | 0.013 |
| Modification peak velocity (°/sec) | Y = 2.43x + 13.89 | 0.77 | 0.0003 |
| Change in peak velocity (°/sec) | Y = 1.03x +0.77 | 0.90 | <0.0001 |
| First high-velocity component (°/sec) | Y = 1.83x + 17.13 | 0.41 | 0.10 |
| Second high-velocity Component (°/sec) | Y = –1.17 x + 16.47 | −0.58 | 0.02 |
| High-velocity ratio | Y = 0.3 5x + 0.83 | 0.66 | 0.004 |
4. Discussion
Three major insights were gained from these experiments. First, the ability to modify convergence peak velocity during the short-term vergence modification experiment was not influenced by age. Second, the magnitude of phoria adaptation decreased as a function of age. Third, the change in peak velocity from modification to baseline and the high-velocity ratio were significantly correlated to the rate of phoria adaptation.
4.1. Short-term vergence modification experiment
Previous studies have investigated the influence of age on vergence peak velocity (Heron, Charman, & Schor, 2001a, 2001b; Kalsi et al., 2001; Yang et al., 2009b). Specifically, Rambold and colleagues reported a significant decrease in the peak velocity of responses to 7° convergence steps with an initial vergence angle of 3° as well as an increase in latency as a function of increasing age (Rambold et al., 2006). Conversely, Heron and colleagues concluded that convergence peak velocity did not vary significantly with age (r2 = 0) (Heron et al., 2001b). Yang and colleagues also reported no significant differences in 6.3° and 8.5° vergence steps with initial vergence angles of 2.3° and 8.6° as a function of age (Yang, Le, & Kapoula, 2009a). Similar to the results of Heron et al. and Yang et al., the present study observed that 4° convergence average peak velocities during the baseline and modification phases did not significantly change as a function of age, suggesting that the dynamics of disparity-vergence is not dependent on age in the adult visual system.
This is the first study to conduct a short-term vergence modification experiment across a human adult lifespan (20–70 years of age). The results suggest that aging does not play a major role in one’s ability to modify peak velocity of disparity-vergence during a short-term modification protocol. The inter-individual variability was similar across all three age groups, suggesting that the ability to modify vergence peak velocity is subject-dependent rather than age-dependent. For example, some subjects may have an innately reduced ability to change their convergence peak velocity while others have a greater ability to adapt their convergence peak velocity. Similarly, Salman and colleagues report no age-related effects on a gain-decreasing adaptation protocol for saccades (Salman et al., 2006). Although the results presented here and by Salman and colleagues demonstrate that the ability to adapt vergence and saccades to different visual environments is not age dependent, a longitudinal within-subjects study may provide better insight into whether these functions decline with increasing age for a given subject.
4.2. Phoria adaptation experiment
To date, phoria adaptation has been studied extensively in the young adult population (typically between 18 to 35 years of age) (Fogt & Toole, 2001; Kim, Vicci, Granger-Donetti, et al., 2011; Rosenfield et al., 1997; Toole & Fogt, 2007) or the older population (ages 35 years and older) (Baker & Gilmartin, 2003; Rosenfield et al., 1997; Winn et al., 1994). Prior research has not studied the continuous age range of 20 to 70 years.
In this subject population, during a 5-min sustained fixation task (approximately 15Δ or fixating on a target located 40 cm away along the subject’s midline), the magnitude (the net change in phoria) significantly decreases as age increased. Other studies have also reported similar findings using a within-subjects design (Baker & Gilmartin, 2003) and between-subjects design (Winn et al., 1994). Winn and colleagues studied phoria adaptation using a 6Δ base in (divergent) and 6Δ base out (convergent) prism to a 5 m (1.2Δ) target for 225 s. They reported that older subjects (54–85 years) exhibited a significantly lower magnitude compared to the younger (18–37 years) subjects (Winn et al., 1994), suggesting that the magnitude of the phoria system decreases with normal aging. Aging has been implicated as one of the many potential causes of visual complaints during visual tasks (Wolska, 2003; Yang et al., 2012). The age dependent decline in the ability to change the magnitude of a person’s phoria should be further investigated as a potential cause of visual complaints.
No significant relationship between age and the rate of phoria adaptation, defined as the magnitude divided by the time constant, was observed. Other studies have only investigated the magnitude and not the rate of phoria adaptation (Baker & Gilmartin, 2003; Winn et al., 1994). This study reports large inter-variability was observed within the rate of phoria adaptation in the younger (0.7Δ/min to 5.6Δ/min), mid-aged (0.9Δ/min to 5.5Δ/min) and older (0.4Δ/min to 3.6Δ/min) groups. This suggests that the rate of phoria adaptation is subject-dependent rather than age-dependent, where some subjects have greater rates of adaptation compared to others regardless of the person’s age. This study concentrated on one type of phoria adaptation (near phoria adaptation to stimuli 40 cm along midline or 8.44° after being far adapted to a 1° vergence angle). Hence, it is beyond the scope of this study to comment whether similar results would be observed when the phoria was adapted using other visual cues or vergence angles.
While the baseline phoria level was subject dependent similar to our previous studies, the baseline phoria level was not an indicator of a subject’s ability to adapt. Our laboratory’s prior research showed that baseline phoria did not correlate to a subject’s ability to adapt to targets in near or far space (Kim, Vicci, Han, & Alvarez, 2011; Kim et al., 2010).
4.3. Rate of phoria adaptation is related to the high-velocity ratio
Interestingly, the high-velocity ratio was significantly correlated to the rate of phoria adaptation. Subjects with a lower rate of phoria adaptation also had similar magnitudes of their first and second high-velocity components during the modification phase. Conversely, subjects with a higher rate of phoria adaptation exhibited a substantially larger first high-velocity component compared to the second high-velocity component. During the experiment, subjects were five times more likely to view the conditioning, double-step stimulus compared to the test, single-step stimulus. Hence, most of the subjects performed 100–150 double-step trials during the modification phase. Repetitive presentation of the double-step stimuli may induce procedural learning – the ability to progressively produce smooth and efficient motor performance (Censor, Sagi, & Cohen, 2012; Mattar, Darainy, & Ostry, 2013). Subjects with an ability to modify their phoria and vergence peak velocity more quickly during the modification phase may be able to perceive the double-step stimuli as a single 8° convergence step target with repetitive exposure to the stimulus. Conversely, subjects with a reduced ability to modify their phoria and vergence peak velocity may have a more difficult time processing the double-step stimulus as a single 8° convergence step stimulus. Thus, the vergence system stimulates a second high-velocity component similar to the first high-velocity component.
5. Conclusion
This study has several novel findings. First, the ability to modify vergence peak velocity during a short-term vergence modification experiment showed no age-related effects on 4° convergence average peak velocities during baseline and modification phases. Second, the mean convergence peak velocities in young, mid-aged and older subject populations were similar. Third, the magnitude of phoria adaptation was significantly less within the older population as compared to the young adult population. Fourth, there was no significant relationship between the rate of phoria adaptation and age. Lastly, the rate of phoria adaptation was significantly correlated to the baseline and modification convergence peak velocity, the ability to modify convergence peak velocity and the convergence high-velocity ratio. This knowledge has clinical and basic science implications because reduction in the ability to adapt phoria is common in binocular dysfunctions. Hence, age related effects should be taken into account.
Acknowledgments
This work was supported by the National Institutes of Health [1R01EY023261], the National Science Foundation [MRI CBET 1428425] and Essilor International.
Footnotes
Declaration of interest
All authors do not have a potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, our work.
Submission Declaration and verification
The enclosed is not under consideration for publication elsewhere. The publication is approved by all authors, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
References
- Alvarez TL (2015). A pilot study of disparity vergence and near dissociated phoria in convergence insufficiency patients before vs. after vergence therapy. Frontiers in Human Neuroscience, 9, 419 10.3389/fnhum.2015.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez TL, Bhavsar M, Semmlow JL, Bergen MT, & Pedrono C (2005). Short-term predictive changes in the dynamics of disparity vergence eye movements. Journal of Vision, 5(7), 640–649. 10.1167/5.7.4 [DOI] [PubMed] [Google Scholar]
- Alvarez TL, Semmlow JL, Ciuffreda KJ, Gayed B, & Granger-Donetti B (2007). Vergence transient component: an index to oculomotor learning modification. Conference Proceeding: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society. Annual Conference, 2007, 4850–3. 10.1109/IEMBS.2007.4353426. [DOI] [PubMed] [Google Scholar]
- Alvarez TL, & Kim EH (2013). Analysis of saccades and peak velocity to symmetrical convergence stimuli: Binocularly normal controls compared to convergence insufficiency patients. Investigative Ophthalmology & Visual Science, 54(6), 4122–4135. 10.1167/iovs.13-11797. [DOI] [PubMed] [Google Scholar]
- Alvarez TL, Semmlow JL, Yuan W, & Munoz P (2000). Disparity vergence double responses processed by internal error. Vision Research, 40(3), 341–347. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10793906. [DOI] [PubMed] [Google Scholar]
- Alvarez TL, Semmlow JL, Yuan W, & Munoz P (2002). Comparison of disparity vergence system responses to predictable and non- predictable stimulations. Current Psychology of Cognition, 21 (3), 243–261. [Google Scholar]
- Alvarez TL, Vicci VR, Alkan Y, Kim EH, Gohel S, Barrett AM, & Ellipsis Biswal BB (2010). Vision therapy in adults with convergence insufficiency: Clinical and functional magnetic resonance imaging measures. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 87(12), E985–E1002. 10.1097/OPX.0b013e3181fef1aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, Kallman JS, & Lieberman JE (1982). Frequency limitations of the two-point central difference differentiation algorithm. Biological Cybernetics, 45 (1), 1–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7126687. [DOI] [PubMed] [Google Scholar]
- Baker FJ, & Gilmartin B (2003). A longitudinal study of vergence adaptation in incipient presbyopia. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 23(6), 507–511. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14622353. [DOI] [PubMed] [Google Scholar]
- Brautaset RL, & Jennings JAM (2005). Distance vergence adaptation is abnormal in subjects with convergence insufficiency. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 25 (3), 211–214. 10.1111/j.1475-1313.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- Casillas Casillas E, & Rosenfield M (2006). Comparison of subjective heterophoria testing with a phoropter and trial frame. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 83(4), 237–241. 10.1097/01.opx.0000214316.50270.24. [DOI] [PubMed] [Google Scholar]
- Censor N, Sagi D, & Cohen LG (2012). Common mechanisms of human perceptual and motor learning. Nature Reviews Neuroscience, 13(9), 658–664. 10.1038/nrn3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Schmid KL, & Woo GC (2008). The effect of positive-lens addition and base-in prism on accommodation accuracy and near horizontal phoria in Chinese myopic children. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 28(3), 225–237. 10.1111/j.1475-1313.2008.00560.x. [DOI] [PubMed] [Google Scholar]
- Chou R, Dana T, & Bougatsos C (2009). Screening older adults for impaired visual acuity: A review of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 151(1), 44–58. W11–20. Retrieved from <http://www.ncbi.nlm.nih.gov/pubmed/19581646>. [DOI] [PubMed] [Google Scholar]
- Coffey B, Reichow AW, Colburn PB, & Clark DL (1991). Influence of ocular gaze and head position on 4 m heterophoria and fixation disparity. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 68 (11), 893–898. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1766653. [DOI] [PubMed] [Google Scholar]
- Dash S, Catz N, Dicke PW, & Thier P (2010). Specific vermal complex spike responses build up during the course of smooth-pursuit adaptation, paralleling the decrease of performance error. Experimental Brain Research, 205(1), 41–55. 10.1007/s00221-010-2331-2. [DOI] [PubMed] [Google Scholar]
- Erkelens IM, Thompson B, & Bobier WR (2016). Unmasking the linear behaviour of slow motor adaptation to prolonged convergence. European Journal of Neuroscience, 43(12), 1553–1560. 10.1111/ejn.13240. [DOI] [PubMed] [Google Scholar]
- Fogt N, & Toole AJ (2001). The effect of saccades and brief fusional stimuli on phoria adaptation. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 78(11), 815–824. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11763255. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kim EH, & Alvarez TL (2011). VisualEyes: A modular software system for oculomotor experimentation. Journal of Visualized Experiments : JoVE, 49, 1–6. 10.3791/2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Guo Y, Granger-Donetti B, Vicci VR, & Alvarez TL (2010). Quantification of heterophoria and phoria adaptation using an automated objective system compared to clinical methods. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 30 (1), 95–107. 10.1111/j.1475-1313.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- Heron G, Charman WN, & Schor C (2001a). Dynamics of the accommodation response to abrupt changes in target vergence as a function of age. Vision Research, 41(4), 507–519. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11166053. [DOI] [PubMed] [Google Scholar]
- Heron G, Charman WN, & Schor CM (2001b). Age changes in the interactions between the accommodation and vergence systems. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 78(10), 754–762. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11700969. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, & Kaku Y (2010). Saccade adaptation as a model of learning in voluntary movements. Experimental Brain Research, 204(2), 145–162. 10.1007/s00221-010-2314-3. [DOI] [PubMed] [Google Scholar]
- Jaswal R, Gohel S, Biswal BB, & Alvarez TL (2014). Task-modulated coactivation of vergence neural substrates. Brain Connectivity, 4(8), 595–607. 10.1089/brain.2013.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B-C, Tea YC, & O’Donnell D (2007). Changes in accommodative and vergence responses when viewing through near addition lenses. Optometry (St. Louis, Mo.), 78(3), 129–134. 10.1016/j.optm.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Kalsi M, Heron G, & Charman WN (2001). Changes in the static accommodation response with age. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 21(1), 77–84. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11220044. [DOI] [PubMed] [Google Scholar]
- Khojasteh E, & Galiana HL (2007). Modulation of vergence during the vestibuloocular reflex. In 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (Vol. 2007, pp. 5377–5380). IEEE. 10.1109/IEMBS.2007.4353557. [DOI] [PubMed] [Google Scholar]
- Kim EH, & Alvarez TL (2012a). The changes in phoria and convergence to divergence peak velocity ratio are correlated. Current Eye Research, 37(11), 1054–1065. 10.3109/02713683.2012.694551. [DOI] [PubMed] [Google Scholar]
- Kim EH, & Alvarez TL (2012b). The frequency of horizontal saccades in near and far symmetrical disparity vergence. Vision Research, 63, 9–19. 10.1016/j.visres.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Kim EH, & Alvarez TL (2013). The horizontal dark oculomotor rest position. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht von Graefes Archiv Für Klinische Und Experimentelle Ophthalmologie, 251(9), 2119–2130. 10.1007/s00417-013-2379-3. [DOI] [PubMed] [Google Scholar]
- Kim EH, Granger-Donetti B, Vicci VR, & Alvarez TL (2010). The relationship between phoria and the ratio of convergence peak velocity to divergence peak velocity. Investigative Ophthalmology & Visual Science, 51(8), 4017–4027. 10.1167/iovs.09-4560. [DOI] [PubMed] [Google Scholar]
- Kim EH, Vicci VR, Granger-Donetti B, & Alvarez TL (2011). Short-term adaptations of the dynamic disparity vergence and phoria systems. Experimental Brain Research, 212(2), 267–278. 10.1007/s00221-011-2727-7. [DOI] [PubMed] [Google Scholar]
- Kim EH, Vicci VR, Han SJ, & Alvarez TL (2011). Sustained fixation induced changes in phoria and convergence peak velocity. PloS One, 6(6), e20883 10.1371/journal.pone.0020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AN, Han Y, Garbutt S, & Leigh RJ (2002). Properties of anticipatory vergence responses. Investigative Ophthalmology & Visual Science, 43(8), 2626–2632. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12147595. [PubMed] [Google Scholar]
- Lee YY, Chen T, & Alvarez TL (2008). Quantitative assessment of divergence eye movements. Journal of Vision, 8(12). 10.1167/8.12.5.5.1-13. [DOI] [PubMed] [Google Scholar]
- Lee YY, Granger-Donetti B, Chang C, & Alvarez TL (2009). Sustained convergence induced changes in phoria and divergence dynamics. Vision Research, 49(24), 2960–2972. 10.1016/j.visres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, & Zee DS (2016). The Neurology of Eye Movements. Retrieved from https://books.google.com/books?hl=en&lr=&id=v2s0BwAAQBAJ&oi=fnd&pg=PP1&dq=zee+and+leigh&ots=4pwi0gySwN&sig=TWfChdSm-5ch3SL67rpJl2kZq6E#v=onepage&q=zeeandleigh&f=false.
- Mattar AAG, Darainy M, & Ostry DJ (2013). Motor learning and its sensory effects: Time course of perceptual change and its presence with gradual introduction of load. Journal of Neurophysiology, 109(3), 782–791. 10.1152/jn.00734.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J, Tong J, & Schor CM (2010). The first and second order dynamics of accommodative convergence and disparity convergence. Vision Research, 50 (17), 1728–1739. 10.1016/j.visres.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack GL (1985). Vergence adaptation maintains heterophoria in normal binocular vision. American Journal of Optometry and Physiological Optics, 62(8), 555–561. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4037062. [DOI] [PubMed] [Google Scholar]
- Munoz P, Semmlow JL, Yuan W, & Alvarez TL (1999). Short term modification of disparity vergence eye movements. Vision Research, 39(9), 1695–1705. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10343861. [DOI] [PubMed] [Google Scholar]
- North R, & Henson DB (1981). Adaptation to prism-induced heterophoria in subjects with abnormal binocular vision or asthenopia. American Journal of Optometry and Physiological Optics, 58(9), 746–752. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7294146. [DOI] [PubMed] [Google Scholar]
- Ono S, & Mustari MJ (2010). Visual error signals from the pretectal nucleus of the optic tract guide motor learning for smooth pursuit. Journal of Neurophysiology, 103(5), 2889–2899. 10.1152/jn.01024.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C (2011). Aging and vision. Vision Research, 51(13), 1610–1622. 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Schor C, Tong J-L, Zomet A, Lev M, Yehezkel O, & Ellipsis Levi DM (2012). Training the brain to overcome the effect of aging on the human eye. Scientific Reports, 2, 278 10.1038/srep00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold H, Neumann G, Sander T, & Helmchen C (2006). Age-related changes of vergence under natural viewing conditions. Neurobiology of Aging, 27(1), 163–172. 10.1016/j.neurobiolaging.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Rosenfield M, Chun TW, & Fischer SE (1997). Effect of prolonged dissociation on the subjective measurement of near heterophoria. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 17 (6), 478–482. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9666921. [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Eizenman M, Lillakas L, Westall C, To T, & Ellipsis Steinbach MJ (2006). Saccades in children. Vision Research, 46(8–9), 1432–1439. 10.1016/j.visres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Scheiman MM, Talasan H, Mitchell GL, & Alvarez TL (2016). Objective Assessment of vergence after treatment of concussion-related CI: A pilot study. Optometry and Vision Science : Official Publication of the American Academy of Optometry. 10.1097/OPX.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor CM (1979). The relationship between fusional vergence eye movements and fixation disparity. Vision Research, 19(12), 1359–1367. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/532102. [DOI] [PubMed] [Google Scholar]
- Schor CM (2009). Neuromuscular plasticity and rehabilitation of the ocular near response. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 86(7), E788–E802. 10.1097/OPX.0b013e3181ae00a5. [DOI] [PubMed] [Google Scholar]
- Schubert MC, & Zee DS (2010). Saccade and vestibular ocular motor adaptation. Restorative Neurology and Neuroscience, 28(1), 9–18. 10.3233/RNN-2010-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmlow JL, Alvarez TL, & Pedrono C (2007). Dry dissection of disparity divergence eye movements using independent component analysis. Computers in Biology and Medicine, 37(7), 910–918. 10.1016/j.compbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Semmlow JL, Chen J, Granger Y-F, Donnetti B, & Alvarez TL (2009). Correction of saccade-induced midline errors in responses to pure disparity Vergence stimuli. Journal of Eye Movement Research Semmlow, 21(5), 1–13 10.16910/jemr.2.5.1. [DOI] [Google Scholar]
- Semmlow JL, Chen Y-F, Pedrono C, & Alvarez T (2008). Saccadic behavior during the response to pure disparity Vergence stimuli I: General properties. Journal of Eye Movement Research Journal of Eye Movement Research Semmlow J, 11(2), 1–11 10.16910/jemr.1.2.1. [DOI] [Google Scholar]
- Semmlow JL, & Hung GK (1980). Binocular interactions of vergence components. American Journal of Optometry and Physiological Optics, 57(9), 559–565. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7425078. [DOI] [PubMed] [Google Scholar]
- Semmlow JL, Yuan W, & Alvarez T (2002). Short-term adaptive control processes in vergence eye movement. Current Psychology of Cognition, 21 (45), 343–375. [Google Scholar]
- Sreenivasan V, & Bobier WR (2015). Increased onset of vergence adaptation reduces excessive accommodation during the orthoptic treatment of convergence insufficiency. Vision Research, 111(Pt A), 105–113. 10.1016/j.visres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Sreenivasan V, Irving EL, & Bobier WR (2008). Binocular adaptation to near addition lenses in emmetropic adults. Vision Research, 48(10), 1262–1269. 10.1016/j.visres.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Takagi M, Oyamada H, Abe H, Zee DS, Hasebe H, Miki A, & Ellipsis Bando T (2001). Adaptive changes in dynamic properties of human disparity-induced vergence. Investigative Ophthalmology & Visual Science, 42(7), 1479–1486. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11381050. [PubMed] [Google Scholar]
- Talasan H, Scheiman M, Li X, & Alvarez TL (2016). Disparity vergence responses before versus after repetitive vergence therapy in binocularly normal controls. Journal of Vision, 16(1), 7 10.1167/16.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Ethier V, Shadmehr R, Fujita M, & Zee DS (2009). Some perspectives on saccade adaptation. Annals of the New York Academy of Sciences, 1164(1), 166–172. 10.1111/j.1749-6632.2009.03853.x. [DOI] [PubMed] [Google Scholar]
- Toole AJ, & Fogt N (2007). The forced vergence cover test and phoria adaptation. Ophthalmic & Physiological Optics : The Journal of the British College of Ophthalmic Opticians (Optometrists), 27(5), 461–472. 10.1111/j.1475-1313.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Wilmer JB, & Buchanan GM (2009). Nearpoint phorias after nearwork predict ADHD symptoms in college students. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 86(8), 971–978. 10.1097/OPX.0b013e3181b2f403. [DOI] [PubMed] [Google Scholar]
- Winn B, Gilmartin B, Sculfor DL, & Bamford JC (1994). Vergence adaptation and senescence. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 71(12), 797–800. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7898888. [DOI] [PubMed] [Google Scholar]
- Wolf KS, Bedell HE, & Pedersen SB (1990). Relations between accommodation and vergence in darkness. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 67(2), 89–93. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2336261. [DOI] [PubMed] [Google Scholar]
- Wolska A (2003). Visual strain and lighting preferences of VDT users under different lighting systems. International Journal of Occupational Safety and Ergonomics, 9(4), 431–440. 10.1080/10803548.2003.11076580. [DOI] [PubMed] [Google Scholar]
- Yang Q, & Kapoula Z (2008). Aging does not affect the accuracy of vertical saccades nor the quality of their binocular coordination: A study of a special elderly group. Neurobiology of Aging, 29(4), 622–638. 10.1016/j.neurobiolaging.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Yang Q, Le T-T, & Kapoula Z (2009a). Aging effects on the visually driven part of vergence movements. Investigative Opthalmology & Visual Science, 50(3), 1145 10.1167/iovs.08-2474. [DOI] [PubMed] [Google Scholar]
- Yang Q, Le T-T, & Kapoula Z (2009b). Aging effects on the visually driven part of vergence movements. Investigative Ophthalmology & Visual Science, 50(3), 1145–1151. 10.1167/iovs.08-2474. [DOI] [PubMed] [Google Scholar]
- Yang S, Schlieski T, Selmins B, Cooper SC, Doherty RA, Corriveau PJ, & Sheedy JE (2012). Stereoscopic viewing and reported perceived immersion and symptoms. Optometry and Vision Science : Official Publication of the American Academy of Optometry, 89(7), 1068–1080. 10.1097/OPX.0b013e31825da430. [DOI] [PubMed] [Google Scholar]
- Yuan W, & Semmlow JL (2000). The influence of repetitive eye movements on vergence performance. Vision Research, 40(22), 3089–3098. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10996613. [DOI] [PubMed] [Google Scholar]
- Yuan W, Semmlow JL, Alvarez TL, & Munoz P (1999). Dynamics of the disparity vergence step response: A model-based analysis. IEEE Transactions on Bio-Medical Engineering, 46(10), 1191–1198. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10513123. [DOI] [PubMed] [Google Scholar]
- Zee DS, Fitzgibbon EJ, & Optican LM (1992). Saccade-vergence interactions in humans. Journal of Neurophysiology, 68(5), 1624–1641. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1479435. [DOI] [PubMed] [Google Scholar]