ABSTRACT
Background
Legumes are an excellent plant source of the limiting indispensable amino acid (IAA) lysine in vegetarian, cereal-based diets. However, their digestibility is poor largely because of their antiprotease content. Extrusion can enhance digestibility by inactivating trypsin inhibitors and thus potentially improve the protein quality of legumes.
Objective
We measured the digestibility of extruded chickpea and yellow pea protein with use of a dual stable isotope method in moderately stunted South Indian primary school children.
Methods
Twenty-eight moderately stunted children (height-for-age z scores <−2.0 SD and >−3.0 SD) aged 6–11 y from low to middle socioeconomic status were randomly assigned to receive a test protein (extruded intrinsically [2H]-labeled chickpea or yellow pea) along with a standard of U-[13C]-spirulina protein to measure amino acid (AA) digestibility with use of a dual stable isotope method. Individual AA digestibility in the test protein was calculated by the ratios of AA enrichments in the test protein to the standard protein in the food and their appearance in blood plasma collected at 6 and 6.5 h during the experiment, representing a plateau state.
Results
The mean AA digestibility of extruded chickpea and yellow pea protein in moderately stunted children (HAZ; −2.86 to −1.2) was high and similar in both extruded test proteins (89.0% and 88.0%, respectively, P = 0.83). However, lysine and proline digestibilities were higher in extruded chickpea than yellow pea (79.2% compared with 76.5% and 75.0% compared with 72.0%, respectively, P < 0.02).
Conclusion
Extruded chickpea and yellow pea protein had good IAA digestibility in moderately stunted children, which was 20% higher than an earlier report of their digestibility when pressure-cooked, measured by the same method in adults. Higher digestibility of lysine and proline highlights better retention of these AA in chickpea during extrusion-based processing. Extrusion might be useful for developing high-quality protein foods from legumes. This trial was registered at www.ctri.nic.in as CTRI/2018/03/012439.
Keywords: protein, digestibility, extrusion, legume, stable isotope
Introduction
Legumes are important plant-based sources of protein and indispensable amino acids (IAAs) such as lysine. They are commonly consumed in the cereal-based diets of many low and middle income country populations, where the consumption of animal-source foods can be constrained because of low availability, and economic and cultural reasons (1, 2). The beneficial effects of legumes for improved growth and gut health in Malawian infants and children has been highlighted (3, 4), but the digestibility of legumes is an important factor in the formulation of their diet. The nutritive value of legume proteins is limited by their digestibility, because of the complexity of plant cell walls and the presence of antinutritional factors (ANFs) such as phytates, select polyphenols, tannins, and trypsin inhibitors that can interfere with the digestive processes (5, 6). Furthermore, in a food matrix containing starch and protein, basic IAA such as lysine can be modified to unavailable forms when extended periods of high temperatures are used during preparation (7).
The digestibility of lysine from chickpea and yellow pea, measured by a dual stable isotope method in healthy South Indian adults, was found to be 60% and 62%, respectively, when pressure-cooked and eaten with rice (8). This low digestibility requires more than double the calculated quantity of legumes in predominantly cereal-based diets to meet the daily limiting IAA (lysine) requirement. Legumes are relatively expensive, and in many cases the cost of feeding programs could increase to unsustainable amounts if greater quantities of legumes were required. The poor digestibility of legumes can be improved by reducing the effect of ANFs through different types of processing and preparations, such as soaking, fermentation, germination, dehulling, boiling, pressure-cooking, and roasting (6, 9–11). Many of these processing methods are frequently used in home cooking, but they typically have a small impact (∼10%) on improving digestibility (11–15). Extrusion, a commonly used treatment in the food industry, is a short-time, high-temperature, high-pressure process that converts raw seeds into a fully cooked food, while largely eliminating most ANFs, with a potentially greater improvement in protein digestibility (16–18). It is important to note that even modest improvements in protein digestibility could be important in select foods, such as snacks, which are regularly consumed by target populations. As with cooking, extrusion also significantly removes microbial hazards, while the short-time and high-pressure conditions reduce the likelihood of IAA modifications, such as Maillard product formation (7).
While the diets of children living in poor environments are limited by the quality of protein (19), an additional consideration that could impact the digestion and absorption of dietary protein is environmental enteric dysfunction (EED). This can be characterized by the plasma kynurenine to tryptophan (KT) ratio, which serves as a biomarker of gastrointestinal inflammation and increased intestinal permeability, which is known to be associated with reduced linear growth in infants and children (20–25). The current study aimed to measure the AA digestibility of extruded chickpea and yellow pea proteins in primary school children with use of a dual stable isotope method (11). Because this method measures the digestibility and absorption of individual AA (26), the relation between the children's intestinal health (measured by the KT ratio) and AA digestibility, as well as their height-for-age (HAZ) and weight-for-age (WAZ) z scores, was also explored.
Methods
Primary school (6–11 y) children from low to middle class socio-economic status were recruited and the trial was conducted from June to August 2018. Their parents or guardian/caretaker provided written informed consent and each child provided their assent. All the experimental protocols and procedures were approved by the Institutional Ethics Committee of St. John's Medical College, Bangalore, India, and the trial was registered at www.ctri.nic.in as CTRI/2018/03/01,2439. Children who were on any prescribed medication or supplementation or had any history of serious illness or complications requiring hospitalization, were moderately anemic, under acute medical care within the last 3 mo, or currently participating in any sports program or a daily exercise routine of moderate or high intensity, or had any other disease reported by parents or found on clinical examination, or had any food allergies, specifically to legume-based foods, were excluded.
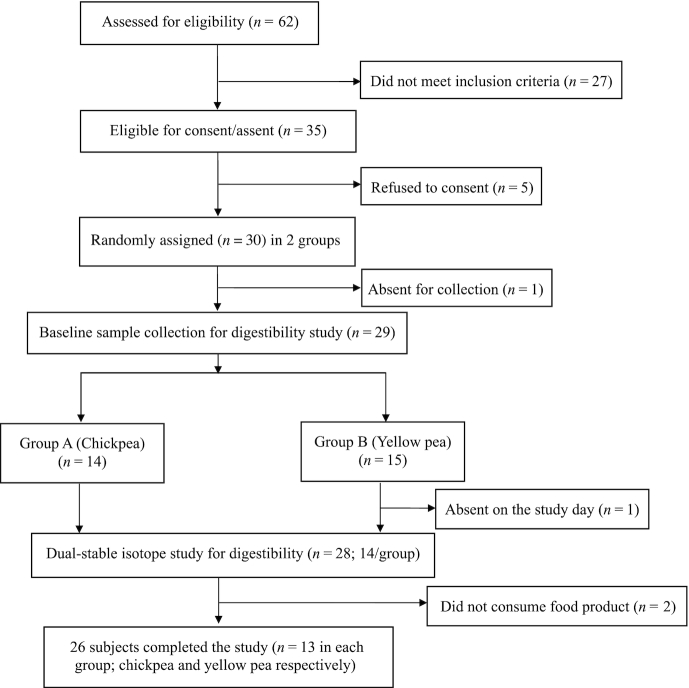
Height was measured with a precision of 0.1 cm without shoes through use of a floor model stadiometer (Seca), and body weight was measured without shoes on a standard weighing scale with a precision of 0.1 kg (Salter). Thirty children (18 girls and 12 boys) were screened for the study. Their weight and height-for-age z scores (WAZ and HAZ) were computed with WHO anthroplus software (version 1.0.4.21882 July 2015). A 2 mL blood sample was collected during screening to measure hemoglobin concentration. In those who were finally included in the study, plasma was stored for baseline isotopic abundance analysis of 13C and 2H, to reduce the burden of blood sampling on the study day. The enrolled children were randomly assigned as per the schedule provided by Biostatistician, to receive chickpea or yellow pea product, such that equal numbers were present in the 2 groups. The schedule was generated by https://www.sealedenvelope.com/simple-randomiser/v1/lists. The recruitment and screening flow chart for enrolling children for the study is provided in Figure 1.
FIGURE 1.
Screening and enrollment of moderately stunted school-age children.
Chickpea (Cicer arietinum var. KAK-2) also known as kabuli, was grown in the rabi season at the University of Agricultural Sciences, Bangalore, India. A standardized labeling protocol with deuterium oxide (2H2O, 99.9%, Sercon Ltd) was followed as described previously (11) in detail. The yellow pea (Pisum sativum var. Salamanca) was grown in spring season in a glasshouse at the James Hutton Institute, Dundee, United Kingdom. The methodology of the labeling protocol has been described in detail elsewhere (8). Chickpea and yellow pea seeds were harvested at maturity, air-dried, and stored for human experiments. A subsample of the legume seeds was ground to fine flour and subjected to gas-phase acid hydrolysis, cation exchange, and derivatization, before estimation of 2H enrichments of amino acids with gas chromatography-pyrolysis-isotope ratio mass spectrometry (GC-P-IRMS, Delta V Advantage, Thermo Fisher Scientific Inc.) as described previously (11).
The intrinsically labeled chickpea and yellow pea were processed with use of cooker extruder technology for microbial stabilization and to deliver a dual texture sensory experience. This technology transforms the raw materials from a solid phase to a rubbery state under elevated temperature, pressure, and shear processing conditions resulting in nucleation and, upon drying, an expanded crispy texture. The amount of extruded chickpea and yellow pea protein provided during the experimental protocol provided approximately one-third of the children's daily protein requirement. For palatability, legume flour was combined with vegetable oil and carbohydrate in the extruded food product. The extruded food product was made in the shape of a “tablet” that weighed ∼35 g each for chickpea and yellow pea. The composition, amino acid content, and safety report of the extruded food product (chickpea and yellow pea) are provided in Table 1. The extruded food product was supplied in sterile, prelabeled, color-coded containers. Both children and study personnel were blinded to the extruded product supplied.
TABLE 1.
Compositional analysis, amino acid content, and safety report of extruded chickpea-based and yellow pea-based food products
| Parameters | Chickpea | Yellow pea |
|---|---|---|
| Proximate | ||
| Total carbohydrates, g/100 g | 51.60 | 55.17 |
| Protein, g/100 g | 24.11 | 23.30 |
| Crude fiber, g/100 g | 2.53 | 2.9 |
| Dietary fiber, g/100 g dry matter | 15.19 | 16.55 |
| Total sugar, g/100 g | 3.40 | 3.60 |
| Total fat, g/100 g | 22.45 | 18.7 |
| Saturated fatty acids, g/100 g | 5.57 | 5.76 |
| Total trans fatty acids, g/100 g | <0.10 | <0.10 |
| Total ash, g/100 g | 3.19 | 3.18 |
| Methionine, g/100 g | 0.14 | 0.11 |
| Lysine, g/100 g | 1.44 | 1.50 |
| Phenylalanine, g/100 g | 1.15 | 0.93 |
| Threonine, g/100 g | 1.06 | 1.03 |
| Leucine, g/100 g | 1.45 | 1.45 |
| Isoleucine, g/100 g | 0.97 | 0.95 |
| Valine, g/100 g | 0.97 | 1.04 |
| Proline, g/100 g | 0.87 | 0.88 |
| Alanine, g/100 g | 0.79 | 0.81 |
| Glycine, g/100 g | 1.62 | 1.74 |
| Serine, g/100 g | 0.82 | 0.75 |
| Tyrosine, g/100 g | 0.40 | 0.40 |
| Microbiological analysis | ||
| Bacillus cereus, cfu/g | <10 | <10 |
| Escherichia coli, per g | Absent | Absent |
| Salmonella spp, per 50 g | Absent | Absent |
| Stapylococcus aureus, cfu/g | <10 | <10 |
| Total plate count, cfu/g | <10 | <10 |
| Yeast and mold count, cfu/g | <10 | <10 |
| Heavy metals | ||
| Lead (Pb), mg/kg | <0.05 | <0.05 |
| Cadmium (Cd), mg/kg | <0.02 | <0.02 |
| Arsenic (As), mg/kg | <0.05 | <0.05 |
| Mercury (Hg), mg/kg | <0.01 | <0.01 |
| Chromium (Cr), mg/kg | 0.21 | 1.64 |
| Nickel (Ni), mg/kg | 0.79 | 0.45 |
The measurement of digestibility was based on a dual stable isotope approach with a plateau-feeding protocol that was developed for adults (11) and subsequently used in toddlers (27) to determine the protein digestibility of selected legumes and other foods (8, 28). On the study day, the children were brought at 07:30 to the metabolic unit of St. John's Medical College, in the fasted state. The extruded yellow pea and chickpea products were administered in mini-portions (obtained by dividing the entire meal into 9 equal aliquots for plateau feeding), following a primed plateau-feeding protocol. The study started with a priming dose (3 mini-portions), mixed homogenously with sodium-[13C]-bicarbonate (0.176 mg/kg, > 99% purity, Cambridge Isotope Laboratories) and uniformly labeled [13C]-spirulina (12 mg/kg, 97% purity; Cambridge Isotope Laboratories). Single mini-portions were then fed hourly for 5 h after the prime, such that 8 mini-portions were fed throughout the protocol; these 8 portions provided the required one-third of the daily protein requirement. The last mini-portion of the meal was retained for analysis of isotopic abundance. Blood samples were collected at 6 and 6.5 h, and breath was collected every hour for the entire duration of the protocol. The timing of these samples was based on previous experiments that demonstrated a plateau state for the isotopic enrichment of plasma amino acids is achieved from the fifth hour onwards (11). Whole blood was transferred into EDTA-coated vacutainers (Becton Dickinson) and centrifuged at 2588 × g for 10 min at 4°C to separate the plasma and stored at −80°C for isotopic analysis. For the baseline isotopic abundance measurement, plasma samples obtained during screening (as above) were used, while the 2 plateau-fed samples were used for analysis of isotopic abundances to calculate the isotopic enrichment over baseline.
For analysis of the isotopic abundance of the extruded yellow pea and chickpea proteins, these were lyophilized, followed by gas-phase acid hydrolysis before subsequent derivatization for analysis of 2H enrichments of amino acids with GC-P-IRMS, as described previously (11). The plasma samples were deproteinized with acetonitrile after spiking with an internal standard of norvaline (30 µL of 10 mM solution, Sigma Aldrich). In addition, to quantify the plasma KT ratio, an internal standard of 2H5-tryptophan (20 µL of 50 μmol/L solution) and 2H6-kynurenine (20 µL of 10 μmol/L solution, both from Cambridge Isotope Laboratories) was also spiked into the plasma samples. Along with the extruded protein hydrolysates, these were subjected to further purification by cation exchange columns (50WX8-100 ion exchange resin, Sigma Aldrich), and free amino acids were eluted with 4 M ammonium hydroxide. Purified eluates were dried and derivatized to their N-ethoxycarbonyl ethyl ester derivatives and analyzed by LC-MS/MS (6495 QQQ equipped with iFunnel Technology, Agilent Technologies) and GC-P-IRMS (Delta V Advantage, Thermo Scientific), to measure [13C] and [2H] isotopic abundances, respectively, in baseline and plateau plasma samples for enrichments of the amino acids for digestibility calculations (11). This procedure measures the appearance of only unmodified AA, thus if chemical modifications to IAA occurred during food preparation, these would not be measured in the determination of AA digestibility. The KT ratio was calculated within the same derivatization protocol in the baseline plasma samples, by correcting the individual analyte area ratios with their respective internal standards. Breath samples were analyzed for 13CO2 enrichment by monitoring ions at m/z ratios of 44 and 45 by isotope ratio mass spectrometry (Delta V Advantage, Thermo Scientific).
The digestibility for extruded chickpea and yellow pea protein was calculated with the equation:
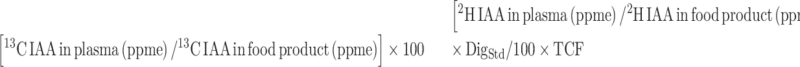 |
(1) |
where DigStd is the amino acid digestibility of standard spirulina protein that was earlier determined in healthy adults against a crystalline amino acid mixture (AA digestibility of 84.1%, 95.3%, 82.5%, 77.5%, 86.0%, 84.2%, 87.1%, and 41.4% for methionine, phenylalanine, threonine, lysine, leucine, isoleucine, valine, and proline, respectively) (11). A transamination correction factor (TCF; 1.058, 1.053, 1.016, 1.002, 1.081, 1.070, 1.048, 1.013 for methionine, phenylalanine, threonine, lysine, leucine, isoleucine, valine, and proline, respectively) measured in healthy adults previously (11) was used to correct for the loss of α-2H during transamination in the current study.
The data are expressed as mean and SD. Assuming that the difference in digestibility between 2 proteins was 7%, and that the CV of digestibility was 10%, the sample size required was 15 per group, with an α-error of 5% and power of 80%. The weighted AA digestibility was calculated from the relative measured AA content in the test protein, that is (AA content in mg/g of test protein)/(sum of measured AA content in mg/g in the test protein) × AA digestibility as measured by dual stable isotope method in the current study. An independent t test was used to determine significant differences in digestibility between the proteins. Pearson's correlation was used to evaluate the relation between the KT ratio and AA digestibility or the HAZ and WAZ score across pooled subjects. Differences and correlations were considered statistically significant if P < 0.05. All calculations were performed with SPSS (version 25, IBM Corp).
Results
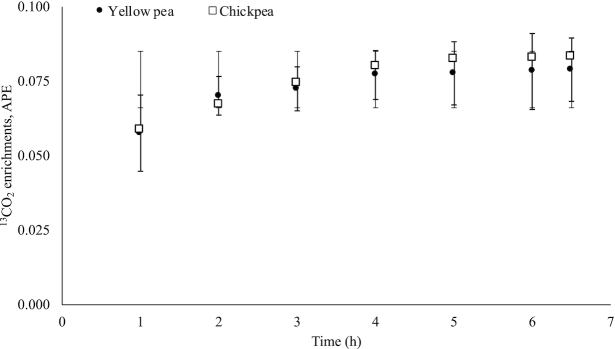
The baseline demography, anthropometry, and hemoglobin concentrations of the subjects are given in Table 2. The subjects in both protein groups were similar in their baseline characteristics. More than half (65%) in each group were stunted (HAZ <−2SD). The mean 2H enrichments of amino acids (AA) in raw intrinsically labeled chickpea and yellow pea protein, compared against unlabeled legumes grown in similar conditions, were 1261 and 944 parts per million excess (ppme), respectively (Figure 2). The postprandial plateau plasma AA enrichment of 13C and 2H (ppme), obtained with respect to the baseline plasma isotope abundance values (not shown), and in the extruded product that was fed, obtained with respect to isotope abundance values in unlabeled legume (not shown), are provided in Table 3. The AA digestibility of the extruded chickpea and yellow pea protein is provided in Table 4. The mean IAA digestibility was not different between the 2 groups of children who were fed chickpea or yellow pea protein (89.0% and 88.0%, P = 0.85). The weighted amino acid digestibility values, calculated from the relative amino acid content in the chickpea and yellow pea protein, were 88.4% and 86.4%, respectively. However, the digestibility of lysine and proline was significantly different between the 2 protein groups; it was higher in extruded chickpea compared to yellow pea protein (P < 0.02 for both, Table 4). The digestibility of phenylalanine was observed to be above 100% for both the test proteins. The breath 13CO2 enrichment showed that a plateau was achieved from the fifth hour onwards, as shown in Figure 3 for both protein groups. The enrichment achieved was similar in both groups, indicating similar use.
Table 2.
Demographic and anthropometric characteristics of moderately stunted school-age children at baseline1
| Chickpea | Yellow pea | ||
|---|---|---|---|
| Variables | (n = 14) | (n = 14) | P |
| Girls, n (%) | 8 (57) | 10 (71) | 0.45 |
| Age, y | 8.4 ± 0.9 | 8.7 ± 0.7 | 0.32 |
| Hemoglobin, g/dL | 12.7 ± 0.8 | 12.2 ± 1.5 | 0.24 |
| Height, cm | 118.6 ± 4.8 | 118.7 ± 4.7 | 0.94 |
| Weight, kg | 19.0 ± 2.3 | 19.1 ± 2.7 | 0.91 |
| Weight-for-age (z score) | −2.5 ± 0.6 | −2.5 ± 0.9 | 0.91 |
| Height-for-age (z score) | −2.0 ± 0.4 | −2.0 ± 0.5 | 0.65 |
| Plasma kynurenine: tryptophan ratio2 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.08 |
Values are means ± SDs and P value by t test.
Chickpea group, n = 10.
FIGURE 2.
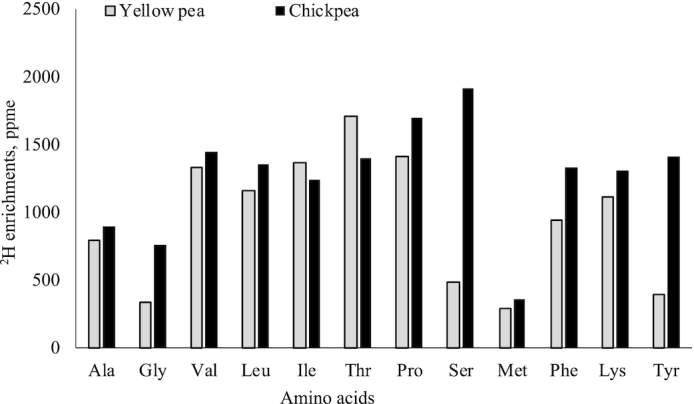
2H enrichments of intrinsically labeled yellow pea and chickpea seeds. Bars represents mean 2H enrichments of two replicates in ppme, parts per million excess.
TABLE 3.
Plasma appearance of 13C and 2H isotopic enrichments of amino acids in moderately stunted school-age children at 6 and 6.5 h (plateau) and in extruded chickpea-based and yellow pea-based food products1
| 13C enrichment (ppme) | 2H enrichment (ppme) | |||||
|---|---|---|---|---|---|---|
| Variables | 6.0 h | 6.5 h | Food product | 6.0 h | 6.5 h | Food product |
| Chickpea (n = 12) | ||||||
| Methionine | 33 ± 9 | 39 ± 27 | 337 ± 149 | 24 ± 7 | 24 ± 6 | 214 ± 38 |
| Lysine | 1150 ± 257 | 1020 ± 194 | 5870 ± 997 | 118 ± 28 | 104 ± 18 | 586 ± 34 |
| Phenylalanine | 1200 ± 183 | 1055 ± 174 | 6220 ± 888 | 179 ± 28 | 143 ± 21 | 819 ± 9 |
| Threonine | 40 ± 8 | 42 ± 10 | 692 ± 123 | 52 ± 8 | 53 ± 12 | 910 ± 106 |
| Leucine | 30 ± 13 | 27 ± 8 | 161 ± 53 | 177 ± 28 | 142 ± 19 | 892 ± 11 |
| Isoleucine | 32 ± 17 | 26 ± 10 | 121 ± 51 | 226 ± 48 | 186 ± 34 | 855 ± 17 |
| Valine | 26 ± 3 | 23 ± 3 | 115 ± 20 | 182 ± 23 | 160 ± 20 | 815 ± 18 |
| Proline | 31 ± 10 | 29 ± 7 | 333 ± 99 | 187 ± 22 | 164 ± 21 | 1049 ± 44 |
| Yellow pea (n = 13) | ||||||
| Methionine | 44 ± 18 | 45 ± 13 | 354 ± 106 | 25 ± 5 | 23 ± 6 | 208 ± 39 |
| Lysine | 1060 ± 216 | 1100 ± 272 | 5670 ± 992 | 90 ± 17 | 83 ± 16 | 464 ± 27 |
| Phenylalanine | 1250 ± 225 | 1140 ± 217 | 7950 ± 899 | 125 ± 22 | 110 ± 19 | 727 ± 20 |
| Threonine | 38 ± 12 | 46 ± 16 | 690 ± 161 | 48 ± 8 | 58 ± 16 | 921 ± 124 |
| Leucine | 25 ± 4 | 27 ± 8 | 168 ± 40 | 120 ± 23 | 107 ± 22 | 701 ± 25 |
| Isoleucine | 27 ± 7 | 28 ± 7 | 138 ± 42 | 156 ± 27 | 140 ± 27 | 735 ± 24 |
| Valine | 23 ± 5 | 22 ± 7 | 110 ± 26 | 138 ± 21 | 126 ± 21 | 650 ± 27 |
| Proline | 27 ± 4 | 28 ± 8 | 324 ± 77 | 122 ± 14 | 113 ± 17 | 788 ± 46 |
Values are means ± SDs, ppme (parts per million excess).
Table 4.
Amino acid digestibility of extruded chickpea and yellow pea product in moderately stunted school-age children1
| Digestibility (%) | |||
|---|---|---|---|
| Chickpea | Yellow pea | ||
| Variables | (n = 12)2 | (n = 13) | P |
| Methionine | 81.9 ± 2.8 | 80.4 ± 2.3 | 0.16 |
| Lysine | 79.2 ± 4.2 | 75.0 ± 4.2 | 0.02* |
| Phenylalanine | 105.8 ± 3.2 | 106.1 ± 3.8 | 0.80 |
| Threonine | 83.2 ± 5.3 | 80.5 ± 3.1 | 0.12 |
| Leucine | 92.4 ± 2.7 | 94.1 ± 2.9 | 0.14 |
| Isoleucine | 91.3 ± 3.2 | 90.6 ± 2.9 | 0.56 |
| Valine | 89.5 ± 3.4 | 89.5 ± 3.8 | 0.99 |
| Proline | 76.5 ± 4.4 | 72.0 ± 3.8 | 0.01* |
| Mean | 87.5 ± 9.4 | 86.0 ± 11.2 | 0.78 |
Values are means ± SDs.
Blood samples could not be collected from n = 1 subject for digestibility measurement.
indicates P < 0.05 by t test.
FIGURE 3.
Appearance of 13CO2 enrichments in breath from moderately stunted school-age children after consumption of extruded chickpea-based and yellow pea protein-based food products. Values are mean ± SD, n = 13. APE, atom % excess.
In addition to digestibility measurements, the KT ratio was measured from the baseline plasma samples. The KT ratio was not different (P = 0.08) between the 2 protein groups. Because mean digestibility values were not different between the 2 protein groups, these were pooled to assess the relation between the KT ratio and HAZ and WAZ score, as well as between the KT ratio and IAA digestibility. The KT ratio significantly and negatively correlated with HAZ (r = −0.60, P = 0.002), but not with WAZ. The KT ratio correlated positively only with proline digestibility (r = 0.50, P = 0.015). There were no other significant correlations.
Discussion
There have been no direct measurements of true ileal AA digestibility in school-age Indian children. Current estimates of protein quality are based on orofecal nitrogen balances (also called “fecal digestibility”) rather than true ileal AA digestibility at the level of the ileum. This study measured the AA digestibility of extruded legume (chickpea and yellow pea) proteins with an accurate and relatively noninvasive dual stable isotope method and found that the mean IAA digestibility values were similar and high for both proteins. The extruded protein's mean IAA digestibility was higher (by 18.7% and 22.2% for chickpea and yellow pea, respectively) compared with those of pressure-cooked whole chickpea and yellow pea protein in adults, measured with the same dual isotope method and reported previously (8). This is a relevant comparison, because earlier studies in Indian adults and toddlers (8, 27) showed comparable mung bean digestibility in both age groups. When compared to other methods of measuring digestibility, the mean IAA digestibility of extruded chickpea protein was 5% higher than estimates of orofecal N digestibility of cooked chickpea, whereas extruded yellow pea digestibility was comparable to dehulled yellow pea digestibility in a rat model (29). The findings from this study are also consistent with the other earlier estimates of extrusion-based processing that increased the apparent ileal digestibility of IAA and dispensable amino acids in pigs and chickens, by 6–16% (30, 31).
The digestibility of legume protein is known to be poor because of the plant cell matrix and the presence of protease inhibitors, haemagglutinins, tannins, and alkaloids. Furthermore, ANFs can also reduce the digestibility of other macronutrient classes as well as absorption of micronutrients (e.g., phytic acid–mineral complexes). These compounds may be beneficial for pest resistance or plant survival, but decrease the digestibility and palatability when consumed without any processing. Because legumes continue to serve as the primary source of quality protein in many parts of the world where animal-based food is not accessible, or the intake is minimal, enhancing their nutritional value by increasing their IAA content by varietal selection or by increasing IAA digestibility is important. Extrusion-based processing of legumes is 1 such method to increase digestibility, and in a recent protein quality study of different beans, extrusion was found to be the most beneficial method for processing kidney beans (32). The beneficial effect of extrusion, particularly in legumes, is thought to be related to increase in protein solubility (as well as fragmenting the long polymer chains of intact protein structures) and starch digestibility in faba beans and peas, while other methods such as toasting and pressurized cooking are also effective in the improvement of starch as well as protein digestibility (13, 33, 34). The cell wall structure and seed components could also affect the digestibility as well as solubility of the legume proteins; possibly because of the heat and shear related to the degradation of the protein complexes within the extrudates causing alterations in the protein structure and making it more susceptible to degradation and thereby increasing release of the digestion products, to enhance the protein bioavailability (2). These studies imply that extrusion can lead to substantial changes in protein structure, thereby improving the digestibility of legume protein. The higher digestibility of lysine and proline in chickpea compared to yellow pea also deserves mention. The small but statistically significant increase of about 6% in digestibility of both AA is not immediately clear, but could be linked to a different peptide motif or different reducing sugar content (monosaccharides and disaccharides) of the legumes. The latter was higher in the extruded yellow pea in comparison to extruded chickpea, such that the ratio of reducing sugar to protein content was about 10% higher in the former, and could lead to increased Maillard reactions that occur between reducing sugars and free amino acids and peptides when heated. This could have possibly decreased the digestibility of lysine and proline by a greater formation of lysine and proline-specific Maillard reaction products in yellow pea protein, which would reduce the appearance of unmodified amino acids as measured (6). Regardless of the mechanisms involved, a key take-home message from the current study is that extrusion, a commonly used process in the food industry, may be useful in improving the nutritional value of numerous food sources, including snacks, that contain the proteins under study. This needs to be further explored and pursued.
Poor hygiene and absence of adequate sanitation may further induce gut dysfunction (35, 36), which is known to impact nutritional status in early childhood through impaired absorption of nutrients as well as through subclinical and recurrent infections, which may further lead to EED, a subclinical condition that can lead to growth-faltering (37, 38). Tryptophan, along with its metabolite kynurenine, is associated with gut permeability as assessed by the lactulose to mannitol (L:M) ratio (23), and may have utility in the assessment of intestinal function and its relation to linear growth (39, 40). The KT ratio, linked to the IDO1 (indoleamine 2,3-dioxygenase 1) pathway activity in tryptophan metabolism, is associated with systemic markers of inflammation, supporting its role as a potential biomarker for intestinal health and its relation to growth in infants and children (21). The negative association of the KT ratio with HAZ in the present study indicates its potential use as a biomarker for growth in the context of environmental enteropathy (25).
The positive relation of the KT ratio with proline digestibility suggests that proline could also act as a stress biomarker, as it is also considered to be a special microenvironmental stress substrate. The enzyme proline oxidase/proline dehydrogenase responds to genotoxic, inflammatory, and nutrient stress by inducing matrix metalloproteinases (41). These proteinases (carboxypeptidases) are present at the brush border of the ileal mucosa and possibly other regions of the small intestine (42) and play a dual role in pathophysiological digestion of extracellular matrix including proline-rich peptides through direct cleavage and activation of inactive zymogens like pro-matrix metalloproteinase-2 (43, 44). This finding may help in understanding the important role of proline in the homeostasis of intestinal epithelial cells and help in identifying novel targets for therapeutic interventions of diseases associated with dysfunctional intestinal epithelial cells, such as EED.
The strength of this study lies in the use of a relatively noninvasive, dual stable isotope technique to measure the digestibility of extruded legume proteins. A limitation of this study is that AA digestibility was calculated against a standard protein (spirulina), whose digestibility values and transamination correction factors had been obtained in an earlier study in adults (11). In the earlier study, the spirulina protein AA digestibility values were less than 100%, with an interindividual SD of 3–9% for different AA (11). Thus, the use of a single standard AA digestibility value (as measured in the earlier study) as a correction factor in the current study, could result in uncertainty of the present AA digestibility estimates, equivalent to the SD of each respective AA digestibility in the earlier study (11). The specific determination of these values for the children was not possible in the frame of this study because of the experimental burden this would have imposed. For the same reason, there was no direct comparison of the digestibility of pressure-cooked whole legumes. Thus, comparisons were made with earlier estimations in adults (albeit with the same method) as no data in children were available. Another limitation was the high (>100%) digestibility of phenylalanine in both extruded chickpea and yellow pea. While this could be because several digestive enzymes have a higher affinity towards aromatic amino acids, it is also possible to obtain values that are greater than 100% relative to the standard; this has been observed previously as well, in ileostomy-based balance measurements of ileal IAA digestibility, where the digestibility of threonine and proline was also reported to be over 100% (45). In conclusion, this study demonstrated an increase in the AA digestibility of extruded chickpea and yellow pea protein to amounts that approached the digestibility of animal-source proteins, which could allow for the development of processing and preparation methods for higher-quality legume-based foods.
Acknowledgements
We acknowledge the expert assistance of Mr. Kashiraya DB, Mr. Vincent D, Mr. Praveen MS, Ms. Pushpaveni CR, Ms. Roshni P, Mr. Charles, Ms. Lobsang D, Ms. Bincy A, and Dr. Nirupama S (St. John's Medical College and Research Institute, Bangalore, India) in conducting the human experimental protocols, blood collection, and sample preparation for successful completion of the study. We gratefully acknowledge the expertise of Ms. Alexandra Small (SUERC, University of Glasgow, UK) in producing intrinsically labeled yellow pea. The authors' responsibilities were as follows—AVK, SD, TP, LHA, and CLK: conceived the project and its design; TP, MSS, and SD: designed the intrinsic labeling experiments; SD and AV: conducted the human experimental protocol, sample collection, and data analysis; SD, AV, MD, and RRH: analyzed the data; SD and AVK: were involved with interpretation of results and wrote the first draft of the manuscript and had primary responsibility for the final content of the draft; and all authors: read and approved the final manuscript.
Notes
Supported by the Tata Trusts, a philanthropic organization. The food products that were developed by Mars International India Private Limited were an unrestricted gift. AVK was supported by the Wellcome Trust/DBT India Alliance Margdarshi Fellowship (IA/M/14/1/501681).
Author disclosures: MD has a consultant relation with MARS, Inc. CLK is the Mars Chair in Developmental Nutrition. AVK is an advisor to the Tata Trusts. All other authors report no conflicts of interest.
CLK and AVK are joint last authors.
Abbreviations used: AA, amino acid; ANF, antinutritional factor; EED, environmental enteric dysfunction; HAZ, height-for-age z score; IAA, indispensable amino acid; KT, kynurenine to tryptophan ratio; WAZ, weight-for-age z score.
References
- 1. Tharanathan R, Mahadevamma S. Grain legumes—a boon to human nutrition. Trends Food Sci Technol. 2003;14:507–18. [Google Scholar]
- 2. Patil S, Brennan M, Mason S, Brennan C. The effects of fortification of legumes and extrusion on the protein digestibility of wheat based snack. Foods. 2016;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stephenson KB, Agapova SE, Divala O, Kaimila Y, Maleta KM, Thakwalakwa C, Ordiz MI, Trehan I, Manary MJ. Complementary feeding with cowpea reduces growth faltering in rural Malawian infants: a blind, randomized controlled clinical trial. Am J Clin Nutr. 2017;106:1500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agapova SE, Stephenson KB, Divala O, Kaimila Y, Maleta KM, Thakwalakwa C, Ordiz MI, Trehan I, Manary MJ. Additional common bean in the diet of Malawian children does not affect linear growth, but reduces intestinal permeability. J Nutr. 2018;148:267–74. [DOI] [PubMed] [Google Scholar]
- 5. Avilés-Gaxiola S, Chuck-Hernández C, Serna Saldívar SO. Inactivation methods of trypsin inhibitor in legumes: a review. J Food Sci. 2018;83:17–29. [DOI] [PubMed] [Google Scholar]
- 6. Sarwar Gilani G, Wu Xiao C, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr. 2012;108:S315–32. [DOI] [PubMed] [Google Scholar]
- 7. Lund MN, Ray CA. Control of Maillard reactions in foods: strategies and chemical mechanisms. J Agric Food Chem. 2017;65:4537–52. [DOI] [PubMed] [Google Scholar]
- 8. Kashyap S, Varkey A, Shivakumar N, Devi S, Reddy BHR, Thomas T, Preston T, Sreeman S, Kurpad AV. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am J Clin Nutr. 2019;110:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilani GS, Cockell KA, Sepehr E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J AOAC Int. 2005;88:967–87. [PubMed] [Google Scholar]
- 10. Kumar V, Sinha AK, Makkar HPS, Becker K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010;120:945–59. [Google Scholar]
- 11. Devi S, Varkey A, Sheshshayee MS, Preston T, Kurpad AV. Measurement of protein digestibility in humans by a dual-tracer method. Am J Clin Nutr. 2018;107:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mubarak AE. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005;89:489–95. [Google Scholar]
- 13. Linsberger-Martin G, Weiglhofer K, Thi Phuong TP, Berghofer E. High hydrostatic pressure influences antinutritional factors and in vitro protein digestibility of split peas and whole white beans. LWT Food Sci Technol. 2013;51:331–6. [Google Scholar]
- 14. Khatoon N, Prakash J. Nutrient retention in microwave cooked germinated legumes. Food Chem. 2006;97:115–21. [Google Scholar]
- 15. Steel CJ, Vernaza Leoro MG, Schmiele M, Ferreira RE, Chang YK. Thermoplastic Elastomers. Rijeka, Croatia: InTech; 2012. [Google Scholar]
- 16. Steel CJ, Vernaza Leoro MG, Schmiele M, Ferreira RE, Chang YK. Thermoplastic extrusion in food processing. Chapter 13 in: Thermoplastic Elastomers. 2012; [Internet] accessed on 19 December, 2018. Available from: https://www.intechopen.com/books/thermoplastic-elastomers/thermoplastic-extrusion-in-food-processing. [Google Scholar]
- 17. Abd El-Hady E., Habiba R. Effect of soaking and extrusion conditions on antinutrients and protein digestibility of legume seeds. LWT Food Sci Technol. 2003;36:285–93. [Google Scholar]
- 18. Smith J, Hardacre A. Development of an extruded snack product from the legume Vicia faba minor. Procedia Food Sci. 2011;1:1573–80. [Google Scholar]
- 19. Semba RD, Shardell M, Sakr Ashour FA, Moaddel R, Trehan I, Maleta KM, Ordiz MI, Kraemer K, Khadeer MA, Ferrucci L et al.. Child stunting is associated with low circulating essential amino acids. EBioMedicine. 2016;6:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crane RJ, Jones KDJ, Berkley JA. Environmental enteric dysfunction: An overview. Food Nutr Bull. 2015;36:S76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisz AJ, Manary MJ, Stephenson K, Agapova S, Manary FG, Thakwalakwa C, Shulman RJ, Manary MJ. Abnormal gut integrity is associated with reduced linear growth in rural Malawian children. J Pediatr Gastroenterol Nutr. 2012;55:747–50. [DOI] [PubMed] [Google Scholar]
- 22. Lunn P, Northrop-Clewes C, Downes R. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–10. [DOI] [PubMed] [Google Scholar]
- 23. Semba RD, Shardell M, Trehan I, Moaddel R, Maleta KM, Ordiz MI, Kraemer K, Khadeer M, Ferrucci L, Manary MJ. Metabolic alterations in children with environmental enteric dysfunction. Sci Rep. 2016;6:28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl Trop Dis. 2018;12:e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kosek MN, Mduma E, Kosek PS, Lee GO, Svensen E, Pan WKY, Olortegui MP, Bream JH, Patil C, Asayag CR et al.. Plasma tryptophan and the kynurenine-tryptophan ratio are associated with the acquisition of statural growth deficits and oral vaccine underperformance in populations with environmental enteropathy. Am J Trop Med Hyg. 2016;95:928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Research approaches and methods for evaluating the protein quality of human foods: report of a FAO Expert Working Group, Bangalore, India. [Internet] Rome: Food and Agriculture Organization of the United Nations; 2014. Available from: http://www.fao.org/3/a-i4325e.pdf. [Google Scholar]
- 27. Shivakumar N, Kashyap S, Kishore S, Thomas T, Varkey A, Devi S, Preston T, Jahoor F, Sheshshayee MS, Kurpad AV. Protein-quality evaluation of complementary foods in Indian children. Am J Clin Nutr. 2019;109:1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kashyap S, Shivakumar N, Varkey A, Duraisamy R, Thomas T, Preston T, Devi S, Kurpad AV. Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr. 2018;108:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nosworthy MG, Neufeld J, Frohlich P, Young G, Malcolmson L, House JD. Determination of the protein quality of cooked Canadian pulses. Food Sci Nutr. 2017;5:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rojas OJ, Vinyeta E, Stein HH. Effects of pelleting, extrusion, or extrusion and pelleting on energy and nutrient digestibility in diets containing different levels of fiber and fed to growing pigs. J Anim Sci. 2016;94:1951. [DOI] [PubMed] [Google Scholar]
- 31. Jahanian R, Rasouli E. Effect of extrusion processing of soybean meal on ileal amino acid digestibility and growth performance of broiler chicks. Poult Sci. 2016;95:2871–8. [DOI] [PubMed] [Google Scholar]
- 32. Nosworthy M, Medina G, Franczyk A, Neufeld J, Appah P, Utioh A, Frohlich P, House J. Effect of processing on the in vitro and in vivo protein quality of beans (Phaseolus vulgaris and Vicia faba). Nutrients. 2018;10:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masoero F, Pulimeno AM, Rossi F. Effect of extrusion, expansion and toasting. on the nutritional value of peas, faba beans and lupins. Ital J Anim Sci. 2005;4:177–89. [Google Scholar]
- 34. Goelema J, Smits A, Vaessen L, Wemmers A. Effects of pressure toasting, expander treatment and pelleting on in vitro and in situ parameters of protein and starch in a mixture of broken peas, lupins and faba beans. Anim Feed Sci Technol. 1999;78:109–26. [Google Scholar]
- 35. Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JP et al.. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mbuya MNN, Humphrey JH. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: An opportunity for stunting reduction in developing countries. Matern Child Nutr. 2016;12(Suppl 1):106–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ngure FM, Reid BM, Humphrey JH, Mbuya MN, Pelto G, Stoltzfus RJ. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Ann N Y Acad Sci. 2014;1308:118–28. [DOI] [PubMed] [Google Scholar]
- 39. Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr. 2003;133:1332–8. [DOI] [PubMed] [Google Scholar]
- 40. Campbell DI, Lunn PG, Elia M. Age-related association of small intestinal mucosal enteropathy with nutritional status in rural Gambian children. Br J Nutr. 2002;88:499. [DOI] [PubMed] [Google Scholar]
- 41. Phang JM, Pandhare J, Liu Y. The metabolism of proline as microenvironmental stress substrate. J Nutr. 2008;138:2008S–15S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lyons PJ, Fricker LD. Carboxypeptidase O is a glycosylphosphatidylinositol-anchored intestinal peptidase with acidic amino acid specificity. J Biol Chem. 2011;286:39023–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–51. [DOI] [PubMed] [Google Scholar]
- 44. Yoshioka M, Erickson RH, Kim YS. Digestion and assimilation of proline-containing peptides by rat intestinal brush border membrane carboxypeptidases. Role of the combined action of angiotensin-converting enzyme and carboxypeptidase P. J Clin Invest. 1988;81:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Darragh AJ, Hodgkinson SM. Quantifying the digestibility of dietary protein. J Nutr. 2000;130:1850S–6S. [DOI] [PubMed] [Google Scholar]