ABSTRACT
The food we consume and its interactions with the host and their gut microbiota affect normal gut function and health. Functional gut disorders (FGDs), including irritable bowel syndrome (IBS), can result from negative effects of these interactions, leading to a reduced quality of life. Certain foods exacerbate or reduce the severity and prevalence of FGD symptoms. IBS can be used as a model of perturbation from normal gut function with which to study the impact of foods and diets on the severity and symptoms of FGDs and understand how critical processes and biochemical mechanisms contribute to this impact. Analyzing the complex interactions between food, host, and microbial metabolites gives insights into the pathways and processes occurring in the gut which contribute to FGDs. The following review is a critical discussion of the literature regarding metabolic pathways and dietary interventions relevant to FGDs. Many metabolites, for example bile acids, SCFAs, vitamins, amino acids, and neurotransmitters, can be altered by dietary intake, and could be valuable for identifying perturbations in metabolic pathways that distinguish a “normal, healthy” gut from a “dysfunctional, unhealthy” gut. Dietary interventions for reducing symptoms of FGDs are becoming more prevalent, but studies investigating the underlying mechanisms linked to host, microbiome, and metabolite interactions are less common. Therefore, we aim to evaluate the recent literature to assist with further progression of research in this field.
Keywords: irritable bowel syndrome, functional gut disorder, gut microbiota, metabolites, diet
Introduction
The human gut is integral to well-being, with interactions between the diet, gut, and the resident microbiota resulting in beneficial or detrimental health effects. Functional gut disorders (FGDs) are characterized by bloating, pain, and stool inconsistency (1). Irritable bowel syndrome (IBS) is the most widespread example of an FGD with ∼11% of the population diagnosed worldwide, although there are variations in reported rates between geographical regions, in part as a result of language barriers that affect interpretation and communication of symptoms, and partly owing to the criteria used for diagnoses (2). IBS is broadly classified into 3 subtypes: constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), and mixed constipation and diarrhea IBS (IBS-M) (1). The mechanisms behind the onset of IBS symptoms remain unknown, and what differentiates a “normal, healthy” gut from a “dysfunctional, unhealthy” gut is difficult to define. Investigation of the gut microbial community and interactions between host and microbial metabolites may advance our understanding of mechanisms that differ between healthy individuals and those with IBS (3).
Metabolites are the products of biological pathways and enzymatic processes. Importantly, they can be measured using minimally invasive procedures to reflect the function of a tissue, organ, or system, and assist in distinguishing between disease phenotypes (3, 4). Many metabolites are signaling molecules that influence biological functions throughout the body. Alterations to metabolite production in the gut from either host, microbiota, or their interactions may link to FGD symptoms (5).
SCFAs, vitamins, bile acids (BAs), lipids, neurotransmitters, and amino acids are metabolites that can be produced or modified by the host or the gut microbiota (6). Excess or insufficient production of these metabolites, compared with normal homeostatic amounts, could signal disruptions to pathways important to gut and overall health (3). Metabolites produced can be used or further modified by the host or microorganisms, highlighting the complexity of the gut environment and the requirement for comprehensive measurement of these metabolites.
The European Food Safety Authority has recognized IBS as a relevant model of gut comfort and function that shows variation from the norm and applies to the general population (7). Because diet and nutrition are popular as interventions for alleviating FGDs, and because people with IBS are motivated to find solutions (including by modifying their food consumption), investigating the responses to dietary changes in this context is a useful approach for understanding mechanisms behind FGDs.
Metabolites Linked to IBS
BAs
BA profiles are affected by diet, host characteristics, age and life stages, and the resident microbiota, with recent research showing BA metabolism may be linked to IBS (8–13). There is evidence for variation in the concentration of primary and secondary BAs in plasma in IBS participants (12). Primary BAs are produced in the liver from cholesterol via the enzyme cholesterol 7-α-hydroxylase, to produce 7-α-hydroxy-4-cholesten-3-one (C4), which is then converted into the 2 primary Bas: chenodeoxycholic acid (CDCA) and cholic acid (CA) (Figure 1). These BAs are conjugated to either taurine or glycine, stored in the gallbladder, secreted into the gut lumen after digestion, and then unconjugated from taurine and glycine by bacterial bile salt hydrolase (BSH) enzymes (13, 14). Most BAs (95%) are reabsorbed and recycled via the hepatic circulation, which is controlled by fibroblast growth factor 15 (FGF15) and the BA receptor farnesoid X receptor (15). Five percent of BAs escape this process and undergo modification by microorganisms with the 7α-dehdroxylase enzyme, resulting in secondary BAs with altered structures that may interact differently with cellular receptors, potentially having impacts on the functionality of metabolites (13). It is unknown if BA concentrations fluctuate owing to differences or disruptions in the ileal epithelial transporter, FGF15 molecules, precursor mechanisms in the liver, or microbial modification of the metabolites.
FIGURE 1.
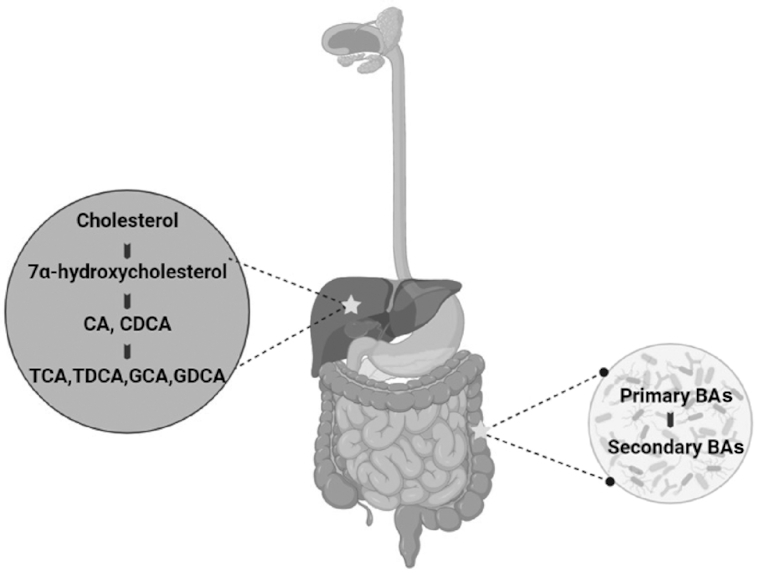
BA production and processes within the body. BAs are produced in the liver from cholesterol, followed by their storage in the gallbladder. After food intake, BAs are excreted out into the gut lumen, where they act as detergent molecules to aid in the absorption of nutrients. In the large intestine, they are converted to secondary BAs owing to the action of microbes possessing the bile salt hydrolase enzyme. Created with BioRender. BA, bile acid; CA, cholic acid; CDCA, chenodeoxycholic acid; GCA, glyco-cholic acid; GDCA, glyco-chenodeoxycholic acid; TCA, tauro-cholic acid; TDCA, tauro-chenodeoxycholic acid.
A meta-analysis of studies reporting on IBS-D symptoms showed that BA malabsorption (BAM) was evident in 16.9–35.3% of the individuals diagnosed with IBS-D (16). BAM is linked to diarrhea, where defective BA recycling or overproduction may increase colonic BA concentrations, leading to the onset of laxation (15, 17, 18). Conversely, a reduction in BA concentrations in the colonic mucosa may have the opposite effect, causing constipation and slowing colonic transit. In a study by Sadik et al. (18), BAM was positively associated with accelerated colonic transit in patients with chronic diarrhea (18). However, not all BAs have the same effect on the gut. Unlike CDCA and CA, which are predominantly recycled, the secondary BA lithocholic acid is poorly reabsorbed, instead passing through to the colon for further modification by bacteria and then excretion (19). The action of CDCA may be facilitated by activation of intracellular secretory channels, increased mucosal permeability, or decreased fluid and electrolyte absorption (20).
Differences in fecal and serum BA concentrations are observed in individuals with IBS-C and IBS-D and may correlate with visceral pain and colonic transit (17, 21, 22). In the feces of IBS-D individuals, primary BAs were higher and secondary BAs lower in concentration than in healthy controls, suggesting BAM and the inability of BAs to be modified by the gut microbiota (23). Positive correlations were evident between concentrations of C4 and FGF19, stool weight, and total BAs in IBS-D individuals, suggesting an increase of BA production to counteract BAs lost in fecal samples. Interestingly, the relative abundance of the fecal microbiota in IBS-D individuals was characterized by reduced concentrations of Bifidobacterium (<1 log10 difference) and Clostridium leptum (>1 log10 difference), bacteria possessing BSH enzymes involved in BA transformation (23).
David et al. (24) investigated how dietary intake over 5 d influenced the gut microbiota and metabolites. In this study, they showed that an animal-based diet, compared with a plant-based diet, increased the abundance of BAs in fecal samples, which they surmised was due to higher amounts of cholesterol (a precursor of BAs) in animal-based diets. Consequently, based on the relation between dietary patterns, BA metabolism, microbial enzymatic activities, host epithelium, hepatic portal circulation, and metabolism, BA fluctuations could provide valuable insight into understanding the mechanisms contributing to the onset and severity of IBS.
SCFAs
Carbohydrates that escape digestion in the stomach are passed intact to the small and large intestines where the gut microbiota ferments them into SCFAs (3, 25, 26). Acetate, propionate, and butyrate are the primary SCFAs produced in the gut (27). Approximately 80–90% of SCFAs produced in the colon are used by the body, with the rest excreted in feces (26).
Many bacteria can produce SCFAs, including butyrate. Some of the most common butyrate producers include Fecalibacterium prausnitzii,Roseburia spp., Eubacterium rectale, and Eubacterium hallii (28–30). Butyrate is produced via pathways utilizing lactate, acetate, sugars, and amino acids that may be by-products produced by other bacteria (29). Of the 3 pathways producing propionate, the succinate pathway is the most common and performed predominantly by Bacteroides spp. and Veillonella spp. (29). Acetate production pathways are more widespread, produced from a range of fermented carbohydrates and by a range of microbes (29, 31). Colonocytes predominantly use butyrate for energy, whereas propionate is utilized by the liver in gluconeogenesis and acetate circulates throughout the body (31, 32). Acetate and propionate are linked to the regulation of glucose homeostasis, fatty acid concentrations in the liver, and stimulating energy and appetite regulation, suggesting that relative proportions of specific SCFAs could be more important than total abundance (31).
Alterations in microbial composition and butyrate and propionate concentrations are evident in individuals with IBS compared with healthy controls (33, 34). Lower butyrate concentrations in IBS could indicate a disrupted energy supply to large intestinal colonocytes with consequences for IBS symptoms (34). A different study reported no difference in fecal acetate, propionate, butyrate, and lactate between controls and IBS participants, although total SCFA abundance was lower in the IBS-C subtype than in other subtypes (IBS-D, IBS-M) (35). Tana et al. (33) showed higher SCFA concentrations in fecal samples of IBS participants along with an increased relative abundance of Veillonella and Lactobacillus, a consistent observation because Lactobacillus prominently produces lactic and acetic acids, whereas Veillonella transforms lactic acid to acetic acid and propionic acid (33). There was a positive correlation between fecal SCFA concentration and symptom severity, signifying a possible association between metabolite production and gut discomfort (33).
The relation between SCFAs and IBS is inconsistent in the literature, because there is evidence for both higher and lower SCFA concentrations in IBS (5, 36, 37). A potential explanation for this variation is the functional redundancy of a microbial community where if one species is reduced in abundance, another species may fill the vacated niche, potentially contributing the same metabolites (e.g., SCFAs) to the system. Consequently, understanding the interaction between dietary patterns, SCFA concentration, host functions, and gut microbial activity, including species abundance, could be relevant to successfully elucidating a possible link to IBS (5, 33).
Vitamins
Perturbations in vitamin concentrations have been linked to IBS (38). Vitamins are obtained directly from dietary intake or are biosynthesized in the body. However, sufficient quantities required for the effective functioning of cellular processes may not be met by dietary intake and the host alone (39, 40). Some species of the human gut microbiota, for example lactic acid bacteria, can synthesize folate, thiamin, biotin, vitamin K, nicotinic acid, pantothenic acid, pyridoxine, and riboflavin, which may be utilized by the host (3, 39–42). These vitamins can have essential roles within the body, for example folate, which is vital in DNA replication (39). David et al. (24) noted subjects consuming animal-based dietary patterns had increased microbes with vitamin-synthesizing genes compared with those on plant-based dietary patterns, highlighting the potential role of diet, microbiota, and metabolome relations.
Vitamin B-6 (pyridoxine) has been linked to inflammatory conditions and, therefore, could be important in IBS (38, 43). In a study investigating the dietary intake of 17 individuals with IBS, a low vitamin B-6 concentration correlated to a high IBS symptom score (38). Consistent with other B-vitamins, vitamin B-6-producing pathways can be found in species from Actinobacteria, Bacteroidetes, and Proteobacteria phyla, with fewer synthesizing capabilities found in Firmicutes (40).
Magnúsdóttir et al. (44) investigated the B-vitamin-producing capacity of 256 gut microbial genomes, finding 40–65% encoded for biosynthetic pathways necessary to synthesize 8 key vitamins (biotin, cobalamin, folate, niacin, pantothenate, pyridoxine, riboflavin, and thiamin) (44). Riboflavin, an activator of mucosal-associated invariant T cells and essential in cellular metabolism as a precursor to FAD and FMN, had synthesizing genes present in 166 of the 256 annotations (39, 45). Niacin synthesis was the second most prevalent pathway, present in 162 gene annotations (44). The biosynthesis of riboflavin was found predominantly in Bacteroidetes, Proteobacteria, and Fusobacteria, with low amounts in Firmicutes and no gene pathways in Actinobacteria, in contrast to niacin pathways which were uniformly distributed over the 5 phyla (44). Differences in vitamin-synthesizing capability across different taxa raise the potential for vitamin production to vary between healthy individuals and those with IBS (44). Interestingly, Firmicutes, often found in high abundance in IBS compared with healthy individuals, was the only phylum analyzed without all 8 vitamin synthesis pathways (27, 44, 46, 47). However, mathematical modeling indicated the gut microbiota could only produce 4 of the 8 vitamins in concentrations that could have clinical relevance (44). These estimates are based solely on computational modeling and, therefore, investigating the rate and source of both host and microbial vitamin production is required because the presence of vitamin-synthesizing genes does not necessarily correlate to clinical outcomes. In addition, investigating the absorption of host- and microbially produced vitamins using methods such as stable isotope probing is needed. A more in-depth understanding of host and microbial interactions could help to elucidate further roles for vitamins in IBS, and the clinical significance of microbially produced vitamins.
Amino acids
Tryptophan is an essential amino acid obtained from dietary patterns and is an important precursor for serotonin. Therefore, it has been hypothesized that tryptophan may be an important amino acid in IBS, owing to the relative importance of serotonin (5-HT) in FGDs (48). However, an examination of plasma tryptophan concentration in IBS individuals showed no difference to healthy controls (48). Two competitive pathways, the kynurenine and serotonin pathways, metabolize tryptophan into either the vitamin niacinamide or the neurotransmitters 5-HT/melatonin (48, 49). Although tryptophan concentrations may not differ, the balance between the kynurenine pathway and the serotonin pathway may be important because of the different biological functions of the resulting metabolites (48–51).
Investigation of urinary metabolites between individuals with IBS, ulcerative colitis (UC), and healthy controls found histidine, lysine, glutamine, proline, and glutamic acid concentrations varied between IBS and UC participants, but not from healthy controls (52). Ornithine, a metabolite of the urea cycle, was the only amino acid that varied between IBS and healthy controls, with a lower concentration in IBS participants (52). However, a dietary analysis was not completed in this study. Glutamine is involved in energy supply to the epithelial cells of the gut and consequently a depletion could be crucial in IBS symptomology. When given as an oral supplement (5 g 3 times/d), glutamine reduced IBS symptom severity in individuals with postinfectious IBS-D (53). In general, understanding the possible role of amino acid metabolism in IBS requires further research to investigate their clinical relevance.
Neurotransmitters
The neurotransmitter 5-HT is produced in the gut and affects neuronal signals in the brain, highlighting its importance in gut–brain responses (54). Ninety-five percent of 5-HT is produced by the enterochromaffin cells of the gut epithelium, whereas the other 5% is produced in serotonergic neurons (54–56). 5-HT in the gut is assumed to activate neurons linked to pain, sensitivity, and reflexes via enterochromaffin and enteroendocrine cells (57, 58). The biological activity of 5-HT is terminated by serotonin reuptake transporter (SERT), the recycling mechanism for 5-HT in the body (54, 58). An overproduction of 5-HT can lead to overactivation of nerve sensing mechanisms, causing increased hypersensitivity (55). It is possible that polymorphisms in SERT may influence IBS, although studies investigating the possible association between 5-HT, the SERT gene, and IBS subtypes have had varying results (56). Atkinson et al. (59) found a lack of 5-HT uptake was associated with IBS-D symptoms owing to the deletion of a base fragment (59), whereas others concluded there was no relation between the SERT polymorphism and IBS (60, 61).
In the gut of individuals with IBS, enterochromaffin cell counts and concentrations of 5-HT were higher than in healthy controls (54). However, such differences in enterochromaffin cells are not consistently observed in the literature (62, 63). Mast cell concentration was increased in the mucosal layer of the IBS group, suggesting activation of the immune system as a causal factor in the pain and discomfort associated with IBS. Supporting this finding, the authors noted the severity of visceral pain and hypersensitivity felt by IBS participants correlated to 5-HT release (54).
Dopamine and γ-aminobutyric acid (GABA) are key neurotransmitters which may be implicated in IBS. Dopamine, a neurotransmitter of the catecholamine family, is linked to depression and anxiety (64) and has been found at lower concentrations in individuals with IBS than in healthy controls (52). In addition, GABA, which exerts important anti-inflammatory effects, was reduced in IBS-D individuals compared with healthy controls (65).
Inflammatory molecules
Cytokines are metabolites linked to inflammatory responses (66). TNF-α, a proinflammatory molecule, and the anti-inflammatory cytokines IL-10 and transforming growth factor β1 (TGF-β1), have potential importance in IBS (66, 67). Polymorphisms in the genetic components encoding these cytokines may increase or decrease in concentration, causing disruptions to inflammatory responses (66). Gonsalkorale et al. (66) found an association between IBS symptoms and reduced IL-10 concentrations compared with healthy controls. Another investigation showed that the concentrations of IL-β1, IL-6, and TNF-α were higher in IBS-D participants than in healthy controls (65). A meta-analysis of 9 studies showed gender differences in TNF-α and IL-10 blood concentrations in patients with IBS (56, 68). However, results from another meta-analysis showed no correlation between TGF-β1 and IBS (69). In general, the importance and relevance of inflammatory molecules in IBS remain unclear but plausible.
Putative biomarkers of FGDs
A biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” (70). When considering diagnostic biomarker panels, both specificity and sensitivity are important for application in the clinical setting (71). Sensitivity refers to the true positive value where the biomarkers must accurately select for the true positives while ensuring false negatives are not selected (72). Conversely, specificity refers to the accurate selection of the true negative value, and no selection of false positives (72). Ideally, biomarkers need to be easy to measure and cost-effective in a clinical setting (8).
Studies have reported panels of metabolites in different biological matrixes between IBS and non-IBS participants with varying degrees of specificity and sensitivity (Table 1). In most cases, a high sensitivity and specificity is achieved by measuring a range of metabolic markers, and therefore the applicability for an effective and efficient diagnosis, although appropriate for research settings, would be limited in clinical settings. Current biomarker panels fail to address the underlying biochemical mechanisms and pathways that cause IBS, and therefore although diagnosis may be possible, effective treatment options remain elusive. Although biomarker panels are moving toward understanding IBS, there remains scope for significant improvement.
TABLE 1.
Biomarker panels for discrimination of IBS1
| Biomarkers | Sample type | Sample cohort | Sample size | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|---|
|
Serum | IBS, IBD, celiac disease, HC | IBS, n = 876; IBD, n = 398; celiac disease, n = 57; HC, n = 235 | 50% | 88% | Lembo et al. (73) |
Ten original biomarkers from Lembo et al. (73) and24 additional biomarkers:
|
Serum | IBS, HC | IBS, n = 168; HC, n = 76 | 81% | 64% | (74) |
|
Fecal and plasma | IBS, HC | IBS, n = 196; HC, n = 160 | 88.1% | 86.5% | (71) |
|
Breath | IBS, HC | IBS, n = 170; HC, n = 153 | 89.4% | 73.3% | (75) |
HC, healthy control; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
Impact of Dietary Intake on IBS
Between 60% and 89% of individuals with an FGD found that dietary patterns exacerbate their symptoms, resulting in individuals excluding or including certain foods or even whole food groups from or into their diet (76–82). Caution must be taken in adopting dietary regimens for IBS because they can disrupt how and why specific metabolites are produced. In a study of 36,448 individuals from France (dietary data, Rome III Diagnostic Criteria), 1870 people diagnosed with IBS had different food consumption patterns than healthy controls (83). Reduced intake of protein and micronutrients (e.g., vitamins) was characteristic of IBS individuals, attributed to lower intakes of milk, yogurt, and fruit (83). The study's findings are consistent with previous results where people believed lactose intolerance was a contributing factor to their symptoms (84, 85).
Evidence for how the removal of whole food groups can affect microbial composition and successive metabolites has been shown in studies comparing predominantly animal- or plant-based dietary patterns in healthy cohorts. An animal-based diet increased the relative abundance of Alisipes,Bilophila, and Bacteroides and decreased proportions of microbes known to degrade plant compounds (e.g., Roseburia,E. rectale, and Ruminococcus bromii) (24). This study concluded a predominantly animal-based dietary intake rapidly altered microbial composition within 1 d, but the population returned to its original composition within 2 d after the withdrawal of the diet (24). Alterations to BA and SCFA profiles were observed with both diets (24), emphasizing dietary intake has the potential to affect the host and microbial metabolome. In addition, the microbial transcriptome in those consuming a predominantly animal-based compared with a predominantly plant-based dietary pattern showed increased expression of microbial genes involved in key metabolic pathways, for example, vitamin biosynthesis (24). A similar study comparing the microbiota of children from Italy and Africa found that Italian children who consumed more protein in their dietary intake had higher concentrations of Alisipes and Bacteroides (86, 87). In contrast, African children consuming more legumes and vegetables had higher counts of Prevotella and Succinivibrio microbes capable of degrading fiber and polysaccharides (87). Fiber intake between the 2 groups of children showed positive correlations to fecal SCFAs, highlighting metabolite production from the lower gut microbiota (87).
Microbial production of gases
Hydrogen gas, a product of microbial carbohydrate fermentation, is produced by numerous members of the gut microbiota (88, 89). Hydrogen is further used through cross-feeding to produce methane, hydrogen sulfide, and acetate by methanotrophic, sulfate-reducing, and acetogenic bacteria, respectively (90). These molecules are produced solely from the gut microbiota and reabsorbed into the body (88). An excess of hydrogen gas can cause discomfort for healthy and IBS individuals. Firmicutes are the primary hydrogen producers of the gut (88) and are often found in higher quantities in IBS patients with a corresponding decrease in Bacteroidetes, which may explain the common bloating and discomfort symptoms in IBS. This hypothesis is supported by the higher concentrations of breath hydrogen in IBS individuals than in healthy controls (91, 92). King et al. (92) and Dear et al. (93) both noted reducing consumption of foods known to promote hydrogen production decreased symptoms of IBS (92, 93). In addition, an increase in methane concentration is linked to a decrease in gut motility that is evident in IBS-C patients (94).
Fermentable oligo-di-monosaccharides and polyols
Fermentable oligo-di-monosaccharides and polyols (FODMAPs) are low-fermentable oligosaccharides (e.g., wheat, fructo-oligosaccharides), disaccharides (e.g., cheese, lactose), monosaccharides (e.g., honey, fructose), and polyols (e.g., certain fruits, sorbitol) that are assumed to be poorly digested and easily fermented. There is evidence that dietary regimens which exclude or reduce FODMAPs alleviate the pain and distension associated with IBS symptoms (78, 95). The association between reduced IBS symptoms and a low dietary FODMAP intake is well defined but primarily based on symptom improvement as the outcome measure, which can be subjective, rather than biochemical or mechanistic alterations (78, 96–99). Analysis by McIntosh et al. (100), where IBS participants were randomly assigned to either a low- or a high-FODMAP dietary intervention and then given a Kristalose® sachet, predominantly used as a lactulose supplement for constipation, used breath tests to measure volatile metabolites of microbial fermentation (100). Results showed an increase in hydrogen concentration in the high-FODMAP group compared with the low-FODMAP group from baseline over the 21-d period. In this study, methane concentration showed no variation, suggesting a low-FODMAP diet may not differentially alter microbial gas production (100). Both groups had similar baseline urine metabolite profiles, but after dietary intervention, 3 metabolites (histamine, azelaic acid, and p-hydroxybenzoic acid) showed variable differences (100). Urinary histamine, an immune response molecule, was higher in concentration (0.0085 µmol/mmol compared with 0.0008 µmol/mmol) in the high- than in the low-FODMAP intervention (100), in line with other findings that histamine is linked to hypersensitivity and immune activation (101, 102).
Dietary patterns, for example, normal dietary guidelines often given to IBS patients, are different to a low-FODMAP dietary regime because they involve the removal of specific foods, rather than whole food groups. Analysis of a low-FODMAP dietary intake compared with normal dietary guidelines often given to IBS patients for 4 wk showed a similar decrease in symptom severity (96). IBS dietary guidelines were focused mainly around the timing of meals, eating regular meals, avoidance of large meals, and reducing the intake of fat, caffeine, cabbage, beans, and onions (96). Further investigation into potential side-effects of a FODMAP dietary regime is required, because the removal of key food groups could present unfavorable conditions within the gut ecosystem and to the wider body. FODMAPs are often used as prebiotic supplements (103). Consequently, the widespread movement for their removal to reduce the symptoms of FGDs is paradoxical, considering the beneficial effects of prebiotics are mediated by microbial fermentation, yet the adverse effects of FODMAPs are also mediated by microbial fermentation. This is consistent with findings where Bifidobacteria, a butyrate-producing bacterium, was reduced after consumption of a 4-wk low-FODMAP diet (concentration 7.4 log10 cells/g feces) compared with normal dietary intake (concentration 8.2 log10 cells/g feces) (98, 103). Furthermore, SCFAs known to benefit the gut environment through a variety of mechanisms are produced from the fermentation of FODMAPs by the gut microbiota (103, 104). Although there is evidence to suggest a low-FODMAP diet is warranted in IBS, it is crucial that further studies aim to better understand the impact of FODMAP removal in the dietary pattern of healthy individuals compared with those with IBS.
Probiotics
Probiotics or foods with added beneficial bacteria have been investigated extensively for their ability to alleviate IBS symptoms, with the majority based on outcome measures of abdominal pain, bloating, and IBS symptoms (105–108). Two interventions showed improvement in symptoms after consumption of probiotics, where metabolic or microbial features were also recorded as outcome measures (109, 110). IBS-D participants given 100 g probiotic yogurt each day {7 log10Lactobacillus fermentum[American Type Culture Collection (ATCC) 14931] CFU per gram and 7 log10Lactobacillus plantarum (ATCC 14917) CFU per gram} for 4 wk showed beneficial changes to symptom scores, abdominal pain, and quality of life together with a reduction in fecal calprotectin from baseline (109). Fecal calprotectin is a marker of inflammation, prevalent at increased concentrations in inflammatory bowel disease. Yoon et al. (110) gave participants either a multistrain probiotic capsule [Bifidobacterium bifidum (KCTC12200BP), Bifidobacterium lactis (KCTC11904BP), Bifidobacterium longum (KCTC12200BP), Lactobacillus acidophilus (KCTC11906BP), Lactobacillus rhamnosus (KCTC12202BP), and Streptococcus thermophilus (KCTC11870BP); total 5 × 109 viable cells] or a placebo daily for 4 wk (110). Abdominal pain and bloating were both reduced in the probiotic group compared with the placebo group, although there was no difference in stool form or frequency in either group (110). Measurement of fecal microbiota showed 3 (B. lactis: 6.09 log10 cells/g feces to 7.57 log10 cells/g feces; L. rhamnosus: 2.80 log10 cells/g feces to 5.05 log10 cells/g feces; and S. thermophilus: 4.81 log10 cells/g feces to 5.35 log10 cells/g feces) probiotic species were increased after the intervention (110). These findings show modification and disturbances to the gut microbiome may be instrumental in understanding the underlying mechanisms linked to IBS.
High-fiber foods
There is an increasing awareness that some commonly consumed foods may reduce the symptoms and prevalence of IBS. Prunes, psyllium husk, wholegrain powders, and kiwifruit, which are all characterized by high dietary fiber content, have been investigated for their ability to beneficially alter IBS constipation symptoms (111–115). The soluble components of dietary fiber, for example fructans and inulin, are utilized by the gut microbiota as energy sources, promoting the growth of some beneficial bacteria, for example, Lactobacillus and Bifidobacteria (111, 116, 117). Insoluble fiber, for example, cellulose, is utilized less by the gut microbiota but is essential because it increases gut transit time by passing through the colon undissolved (117). Kiwifruit has a high nutritional value and for many years has been recommended to individuals with IBS-C (Figure 2). The high vitamin C content, actinidin (a unique protease abundant in kiwifruit), and amino acids (glutathione, arginine, and GABA) coupled with a high water-swelling capacity may be responsible for alleviating constipation symptoms (111). The effect of Hayward green kiwifruit (Actinida deliciosa var.) on individuals with IBS-C showed differences in symptom measures after the intervention (115). Consumption of 2 kiwifruit compared with 2 placebo capsules (glucose powder) per day showed decreased colonic transit time and increased weekly defecation in the kiwifruit-consuming participants (115). Prunes have also been shown to be effective in decreasing colonic transit and increasing stool consistency to treat chronic constipation (112). Forty participants with chronic constipation were given either prunes or psyllium (11 g twice daily) as part of a randomized crossover study (112). Both interventions improved complete spontaneous bowel movement compared with baseline, but consumption of prunes decreased colonic transit time compared with psyllium (112). Prunes also resulted in softer stool than did psyllium, with both interventions improving straining when trying to pass fecal matter compared with baseline. The improvement in symptoms from these studies highlights the relevance of using dietary interventions to understand better the mechanisms behind FGDs and their use in alleviating prevalence.
FIGURE 2.
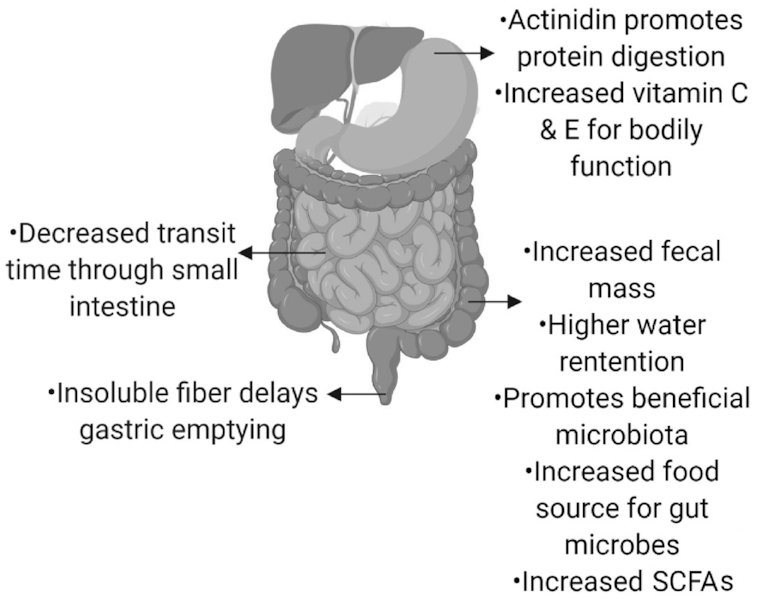
Potential beneficial effects of kiwifruit on healthy digestive progresses and on alleviating symptoms, including constipation, associated with IBS. Created with BioRender. IBS, irritable bowel syndrome.
Conclusions
The underlying mechanisms governing the interaction between dietary patterns, the gut microbiota, and the host are still unclear in IBS. New evidence suggests that research and clinical practices should move away from solely relying on symptoms as a diagnostic and results-based tool. Understanding variations and fluctuations in the concentrations of host- or microbial-derived metabolites that can be used to infer processes contributing to the symptoms and severity of IBS will provide important new insights for FGD research.
In this review, we have discussed 2 main themes, the first being critical metabolites linked to IBS, and the second being studies analyzing dietary interventions to reduce the symptoms and severity of IBS. There is increasing literature focused on the clinical aspect (including dietary solutions) of reducing IBS symptoms, but analyses that further investigate the mechanisms behind the success of interventions are less common. The metabolites discussed in this review are a few key metabolic groups potentially important in understanding IBS. A decrease or increase in production of these metabolites could theoretically disrupt metabolic processes throughout the body; however, further investigation is required. Data from the literature suggest that understanding the biochemical pathways and respective metabolic products will help to identify metabolic biomarkers that could be indicative of a “dysfunctional, unhealthy” gut.
There is overwhelming evidence to suggest the microbiome is involved in FGDs, although whether this is causative or correlative needs further investigation. Less research is focused on investigating the microbially produced metabolites, including those that could be utilized by other microbes in cross-feeding reactions or that could affect localized regions of the gut or be distributed throughout the body. Because metabolites are evidence that a process or pathway has occurred, measuring their fluctuation in individuals with FGDs and related interventions will give further insight into how dietary intake is linked to IBS. Specific metabolites, for example, SCFAs, have been extensively researched; however, the possible role of BAs, vitamins, neurotransmitters, and inflammatory molecules deserves more attention in FGDs because they can have metabolic properties that are directly or indirectly linked to symptoms of IBS.
Dietary intake undoubtedly plays a role in the severity of FGDs; however, future research needs to supplement clinical studies that aim to determine the underlying mechanisms. Defining an improvement in, for example, colonic transit time does little in improving our understanding of why some individuals develop FGDs when others do not, and how we can alleviate the prevalence of FGDs worldwide. For advancements to be made, investigations that undertake dietary interventions should be followed by a thorough analysis of the gut microbiota and both host and microbial metabolites. Critically, this will accommodate not just a better understanding of the epidemiology of FGDs but also recommendations for dietary intakes to alleviate symptoms. Dietary guidelines based on studies that lack mechanistic evidence may result in the adoption of dietary regimens that lead to beneficial outcomes, but equally may be detrimental after long-term adherence owing to unintentional impacts on other biological mechanisms.
Acknowledgements
The authors’ responsibilities were as follows—SCJ, WY, KF, NCR, and WCM: conceptualized the study; NCR and WCM: managed the resources and project administration; SCJ: prepared the original draft; WY, KF, NCR, and WCM: reviewed and edited the paper and performed supervision; and all authors: read and approved the final manuscript.
Notes
Supported by New Zealand Ministry of Business, Innovation and Employment High-Value Nutrition National Science Challenge grant UOAX1421 (to NCR) and the Tertiary Education Commission at The Riddet Institute Centre of Research Excellence.
Author disclosures:The authors report no conflicts of interest.
Abbreviations used: ATCC, American Type Culture Collection; BA, bile acid; BAM, bile acid malabsorption; BSH, bile salt hydrolase; CA, cholic acid; CDCA, chenodeoxycholic acid; C4, 7-α-hydroxy-4-cholesten-3-one; FGD, functional gut disorder; FGF, fibroblast growth factor; FODMAP, fermentable oligo-di-monosaccharide and polyol; GABA, γ-aminobutyric acid; IBS, irritable bowel syndrome; IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome; IBS-M, mixed constipation and diarrhea irritable bowel syndrome; SERT, serotonin reuptake transporter; TGF-β1, transforming growth factor β1; UC, ulcerative colitis; 5-HT, serotonin.
References
- 1. Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407.e5. [DOI] [PubMed] [Google Scholar]
- 2. Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M et al.. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66(6):1075–82. [DOI] [PubMed] [Google Scholar]
- 3. Vernocchi P, Del Chierico F, Putignani L. Gut microbiota profiling: metabolomics based approach to unravel compounds affecting human health. Front Microbiol. 2016;7:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Preter V, Verbeke K. Metabolomics as a diagnostic tool in gastroenterology. World J Gastrointest Pharmacol Ther. 2013;4(4):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajilić–Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–801. [DOI] [PubMed] [Google Scholar]
- 6. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7. [DOI] [PubMed] [Google Scholar]
- 7. EFSA Panel on Dietetic Products, Nutrition and Allergies. Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 2011;9(4):1984. [Google Scholar]
- 8. Camilleri M, Halawi H, Oduyebo I. Biomarkers as a diagnostic tool for irritable bowel syndrome: where are we?. Expert Rev Gastroenterol Hepatol. 2017;11(4):303–16. [DOI] [PubMed] [Google Scholar]
- 9. Camilleri M. What's new in functional and motility disorders in the lower GI tract?. Malta Med J. 2017;29(2):3–13. [Google Scholar]
- 10. Camilleri M, Shin A, Busciglio I, Carlson P, Acosta A, Bharucha AE, Burton D, Lamsam J, Lueke A, Donato LJ et al.. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol Motil. 2014;26(12):1677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camilleri M, Oduyebo I, Halawi H. Chemical and molecular factors in irritable bowel syndrome: current knowledge, challenges, and unanswered questions. Am J Physiol Gastrointest Liver Physiol. 2016;311(5):G777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Long SL, Gahan CGM, Joyce SA. Interactions between gut bacteria and bile in health and disease. Mol Aspects Med. 2017;56:54–65. [DOI] [PubMed] [Google Scholar]
- 13. Joyce SA, Gahan CGM. Bile acid modifications at the microbe-host interface: potential for nutraceutical and pharmaceutical interventions in host health. Annu Rev Food Sci Technol. 2016;7:313–33. [DOI] [PubMed] [Google Scholar]
- 14. Zheng X, Huang F, Zhao A, Lei S, Zhang Y, Xie G, Chen T, Qu C, Rajani C, Dong B et al.. Bile acid is a significant host factor shaping the gut microbiome of diet-induced obese mice. BMC Biol. 2017;15(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shin A, Camilleri M, Vijayvargiya P, Busciglio I, Burton D, Ryks M, Rhoten D, Lueke A, Saenger A, Girtman A. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(10):1270–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015;42(1):3–11. [DOI] [PubMed] [Google Scholar]
- 17. Wong BS, Camilleri M, Carlson P, McKinzie S, Busciglio I, Bondar O, Dyer RB, Lamsam J, Zinsmeister AR. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10(9):1009–15.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadik R, Abrahamsson H, Ung K-A, Stotzer P-O. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99(4):711–8. [DOI] [PubMed] [Google Scholar]
- 19. Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–6. [DOI] [PubMed] [Google Scholar]
- 20. Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, Burton D, Carlson P, Busciglio IA, Lamsam J, Singh R, Zinsmeister AR. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8(2):159–65.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dior M, Delagrèverie H, Duboc H, Jouet P, Coffin B, Brot L, Humbert L, Trugnan G, Seksik P, Sokol H et al.. Interplay between bile acid metabolism and microbiota in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28(9):1330–40. [DOI] [PubMed] [Google Scholar]
- 22. Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol. 2018;16(4):522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, Grondin V, Jouet P, Bouhassira D, Seksik P et al.. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea‐predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(6):513–e247. [DOI] [PubMed] [Google Scholar]
- 24. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huda-Faujan N, Abdulamir A, Fatimah A, Anas OM, Shuhaimi M, Yazid A, Loong Y. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J. 2010;4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayer EA, Savidge T, Shulman RJ. Brain–gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146(6):1500–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vital M, Karch A, Pieper DH. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems. 2017;2(6):e00130–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbara G, Feinle-Bisset C, Ghoshal UC, Santos J, Vanner SJ, Vergnolle N, Zoetendal EG, Quigley EM. The intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1305–18.e8. [DOI] [PubMed] [Google Scholar]
- 30. Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl‐CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12(2):304–14. [DOI] [PubMed] [Google Scholar]
- 31. Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22(5):512–e115. [DOI] [PubMed] [Google Scholar]
- 34. Farup PG, Rudi K, Hestad K. Faecal short-chain fatty acids—a diagnostic biomarker for irritable bowel syndrome?. BMC Gastroenterol. 2016;16(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015;110(9):1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mortensen P, Andersen J, Arffmann S, Krag E. Short-chain fatty acids and the irritable bowel syndrome: the effect of wheat bran. Scand J Gastroenterol. 1987;22(2):185–92. [DOI] [PubMed] [Google Scholar]
- 37. Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23(3):280–6. [DOI] [PubMed] [Google Scholar]
- 38. Ligaarden SC, Farup PG. Low intake of vitamin B6 is associated with irritable bowel syndrome symptoms. Nutr Res. 2011;31(5):356–61. [DOI] [PubMed] [Google Scholar]
- 39. LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24(2):160–8. [DOI] [PubMed] [Google Scholar]
- 40. Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Connor E, Barrett E, Fitzgerald G, Hill C, Stanton C, Ross R. Production of vitamins, exopolysaccharides and bacteriocins by probiotic bacteria. In: Tamime A, editor. Probiotic dairy products. Oxford: Blackwell; 2005. pp. 167–94. [Google Scholar]
- 42. Hill M. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;6(Suppl 1):S43–5. [DOI] [PubMed] [Google Scholar]
- 43. Saibeni S, Cattaneo M, Vecchi M, Zighetti ML, Lecchi A, Lombardi R, Meucci G, Spina L, De Franchis R. Low vitamin B6 plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98(1):112–7. [DOI] [PubMed] [Google Scholar]
- 44. Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–23. [DOI] [PubMed] [Google Scholar]
- 46. Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997–1006. [DOI] [PubMed] [Google Scholar]
- 47. Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berstad A, Raa J, Valeur J. Tryptophan: ‘essential’ for the pathogenesis of irritable bowel syndrome?. Scand J Gastroenterol. 2014;49(12):1493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heitkemper MM, Han CJ, Jarrett ME, Gu H, Djukovic D, Shulman RJ, Raftery D, Henderson WA, Cain KC. Serum tryptophan metabolite levels during sleep in patients with and without irritable bowel syndrome (IBS). Biol Res Nurs. 2016;18(2):193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG. Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol. 2009;9(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol. 2012;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keshteli AH, Madsen KL, Mandal R, Boeckxstaens GE, Bercik P, De Palma G, Reed DE, Wishart D, Vanner S, Dieleman LA. Comparison of the metabolomic profiles of irritable bowel syndrome patients with ulcerative colitis patients and healthy controls: new insights into pathophysiology and potential biomarkers. Aliment Pharmacol Ther. 2019;49(6):723–32. [DOI] [PubMed] [Google Scholar]
- 53. Zhou Q, Verne ML, Fields JZ, Lefante JJ, Basra S, Salameh H, Verne GN. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019;68(6):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De Ponti F et al.. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106(6):1290–8. [DOI] [PubMed] [Google Scholar]
- 55. Yeo A, Boyd P, Lumsden S, Saunders T, Handley A, Stubbins M, Knaggs A, Asquith S, Taylor I, Bahari B. Association between a functional polymorphism in the serotonin transporter gene and diarrhoea predominant irritable bowel syndrome in women. Gut. 2004;53(10):1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Makker J, Chilimuri S, Bella JN. Genetic epidemiology of irritable bowel syndrome. World J Gastroenterol. 2015;21(40):11353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414. [DOI] [PubMed] [Google Scholar]
- 58. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130(1):34–43. [DOI] [PubMed] [Google Scholar]
- 60. Pata C, Erdal ME, Derici E, Yazar A, Kanık A, Ulu O. Serotonin transporter gene polymorphism in irritable bowel syndrome. Am J Gastroenterol. 2002;97(7):1780–4. [DOI] [PubMed] [Google Scholar]
- 61. Lee DY, Park H, Kim WH, Lee SI, Seo YJ, Choi YC. Serotonin transporter gene polymorphism in healthy adults and patients with irritable bowel syndrome. Korean J Gastroenterol. 2014;64(1):18–22. [PubMed] [Google Scholar]
- 62. Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139(1):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM et al.. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126(7):1657–64. [DOI] [PubMed] [Google Scholar]
- 64. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–37. [DOI] [PubMed] [Google Scholar]
- 65. Aggarwal S, Ahuja V, Paul J. Dysregulation of GABAergic signalling contributes in the pathogenesis of diarrhea-predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2018;24(3):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gonsalkorale W, Perrey C, Pravica V, Whorwell P, Hutchinson I. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component?. Gut. 2003;52(1):91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Komuro H, Sato N, Sasaki A, Suzuki N, Kano M, Tanaka Y, Yamaguchi-Kabata Y, Kanazawa M, Warita H, Aoki M et al.. Corticotropin-releasing hormone receptor 2 gene variants in irritable bowel syndrome. PLoS One. 2016;11(1):e0147817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bashashati M, Rezaei N, Shafieyoun A, McKernan DP, Chang L, Öhman L, Quigley EM, Schmulson M, Sharkey KA, Simrén M. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta‐analysis. Neurogastroenterol Motil. 2014;26(7):1036–48. [DOI] [PubMed] [Google Scholar]
- 69. Bashashati M, Rezaei N, Bashashati H, Shafieyoun A, Daryani NE, Sharkey KA, Storr M. Cytokine gene polymorphisms are associated with irritable bowel syndrome: a systematic review and meta‐analysis. Neurogastroenterol Motil. 2012;24(12):1102–e566. [DOI] [PubMed] [Google Scholar]
- 70. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. [DOI] [PubMed] [Google Scholar]
- 71. Mujagic Z, Tigchelaar EF, Zhernakova A, Ludwig T, Ramiro-Garcia J, Baranska A, Swertz MA, Masclee AAM, Wijmenga C, Van Schooten FJ et al.. A novel biomarker panel for irritable bowel syndrome and the application in the general population. Sci Rep. 2016;6:26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Parikh R, Mathai A, Parikh S, Chandra Sekhar G, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56(1):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lembo AJ, Neri B, Tolley J, Barken D, Carroll S, Pan H. Use of serum biomarkers in a diagnostic test for irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29(8):834–42. [DOI] [PubMed] [Google Scholar]
- 74. Jones M, Chey W, Singh S, Gong H, Shringarpure R, Hoe N, Chuang E, Talley N. A biomarker panel and psychological morbidity differentiates the irritable bowel syndrome from health and provides novel pathophysiological leads. Aliment Pharmacol Ther. 2014;39(4):426–37. [DOI] [PubMed] [Google Scholar]
- 75. Baranska A, Mujagic Z, Smolinska A, Dallinga J, Jonkers D, Tigchelaar E, Dekens J, Zhernakova A, Ludwig T, Masclee A et al.. Volatile organic compounds in breath as markers for irritable bowel syndrome: a metabolomic approach. Aliment Pharmacol Ther. 2016;44(1):45–56. [DOI] [PubMed] [Google Scholar]
- 76. Tuck CJ, Vanner SJ. Dietary therapies for functional bowel symptoms: recent advances, challenges, and future directions. Neurogastroenterol Motil. 2018;30(1):e13238. [DOI] [PubMed] [Google Scholar]
- 77. Hayes P, Corish C, O'Mahony E, Quigley E. A dietary survey of patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27(s2):36–47. [DOI] [PubMed] [Google Scholar]
- 78. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75.e5. [DOI] [PubMed] [Google Scholar]
- 79. Halpert A, Dalton C, Palsson O, Morris C, Hu Y, Bangdiwala S, Hankins J, Norton N, Drossman D. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ). Am J Gastroenterol. 2007;102(9):1972–82. [DOI] [PubMed] [Google Scholar]
- 80. Böhn L, Störsrud S, Törnblom H, Bengtsson U, Simrén M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. [DOI] [PubMed] [Google Scholar]
- 81. Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome – etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667–72. [DOI] [PubMed] [Google Scholar]
- 82. Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals?. Therap Adv Gastroenterol. 2012;5(4):261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Torres MJ, Sabate J-M, Bouchoucha M, Buscail C, Hercberg S, Julia C. Food consumption and dietary intakes in 36,448 adults and their association with irritable bowel syndrome: Nutrinet-Santé study. Therap Adv Gastroenterol. 2018;11:1756283X17746625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 2015;7(9):8020–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, Misselwitz B, Fried M, Dai N, Fox M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(3):262–8.e1. [DOI] [PubMed] [Google Scholar]
- 86. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH et al.. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9(9):504–18. [DOI] [PubMed] [Google Scholar]
- 89. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. [DOI] [PubMed] [Google Scholar]
- 90. Smith NW, Shorten PR, Altermann EH, Roy NC, McNabb WC. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes. 2019;10(3):270–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kumar S, Misra A, Ghoshal UC. Patients with irritable bowel syndrome exhale more hydrogen than healthy subjects in fasting state. J Neurogastroenterol Motil. 2010;16(3):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. King T, Elia M, Hunter J. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352(9135):1187–9. [DOI] [PubMed] [Google Scholar]
- 93. Dear KL, Elia M, Hunter JO. Do interventions which reduce colonic bacterial fermentation improve symptoms of irritable bowel syndrome?. Dig Dis Sci. 2005;50(4):758–66. [DOI] [PubMed] [Google Scholar]
- 94. Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48(1):86–92. [DOI] [PubMed] [Google Scholar]
- 95. Gibson P, Shepherd S. Personal view: food for thought – Western lifestyle and susceptibility to Crohn's disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21(12):1399–409. [DOI] [PubMed] [Google Scholar]
- 96. Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, Simrén M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–407.e2. [DOI] [PubMed] [Google Scholar]
- 97. Staudacher HM, Lomer MCE, Farquharson FM, Louis P, Fava F, Franciosi E, Scholz M, Tuohy KM, Lindsay JO, Irving PM et al.. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores bifidobacterium species: a randomized controlled trial. Gastroenterology. 2017;153(4):936–47. [DOI] [PubMed] [Google Scholar]
- 98. Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142(8):1510–18. [DOI] [PubMed] [Google Scholar]
- 99. Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106(10):1631–9. [DOI] [PubMed] [Google Scholar]
- 100. McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241–51. [DOI] [PubMed] [Google Scholar]
- 101. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G775–85. [DOI] [PubMed] [Google Scholar]
- 102. Barbara G, Wang B, Stanghellini V, De Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. [DOI] [PubMed] [Google Scholar]
- 103. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. [DOI] [PubMed] [Google Scholar]
- 104. Zhang Y, Ma ZF, Zhang H, Pan B, Li Y, Majid HA, Lee YY. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polypols diet and irritable bowel syndrome in Asia. JGH Open. 2019;3(2):173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18(30):4012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, Desreumaux P. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47(2):119–24. [DOI] [PubMed] [Google Scholar]
- 107. Jafari E, Vahedi H, Merat S, Momtahen S, Riahi A. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med. 2014;17(7):466–70. [PubMed] [Google Scholar]
- 108. Lorenzo-Zuniga V, Llop E, Suarez C, Alvarez B, Abreu L, Espadaler J, Serra J. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol. 2014;20(26):8709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noorbakhsh H, Yavarmanesh M, Mortazavi SA, Adibi P, Moazzami AA. Metabolomics analysis revealed metabolic changes in patients with diarrhea-predominant irritable bowel syndrome and metabolic responses to a synbiotic yogurt intervention. Eur J Nutr. 2019;58(8):3109–19. [DOI] [PubMed] [Google Scholar]
- 110. Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS et al.. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double‐blind, placebo‐controlled trial. J Gastroenterol Hepatol. 2014;29(1):52–9. [DOI] [PubMed] [Google Scholar]
- 111. Bayer SB, Gearry RB, Drummond LN. Putative mechanisms of kiwifruit on maintenance of normal gastrointestinal function. Crit Rev Food Sci Nutr. 2018;58(14):2432–52. [DOI] [PubMed] [Google Scholar]
- 112. Attaluri A, Donahoe R, Valestin J, Brown K, Rao SSC. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33(7):822–8. [DOI] [PubMed] [Google Scholar]
- 113. Cheskin L, Mitola A, Ridoré M, Kolge S, Hwang K, Clark B. A naturalistic, controlled, crossover trial of plum juice versus psyllium versus control for improving bowel function. Internet J Nutr Wellness. 2009;7(2):5447. [Google Scholar]
- 114. Woo H-I, Kwak SH, Lee Y, Choi JH, Cho YM, Om A-S. A controlled, randomized, double-blind trial to evaluate the effect of vegetables and whole grain powder that is rich in dietary fibers on bowel functions and defecation in constipated young adults. J Cancer Prev. 2015;20(1):64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chang C-C, Lin Y-T, Lu Y-T, Liu Y-S, Liu J-F. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac J Clin Nutr. 2010;19(4):451–7. [PubMed] [Google Scholar]
- 116. Brownlee IA. The physiological roles of dietary fibre. Food Hydrocolloids. 2011;25(2):238–50. [Google Scholar]
- 117. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


