ABSTRACT
Background
WHO guidelines recommend concurrent iron and antimalarial treatment in children with malaria and iron deficiency, but iron may not be well absorbed or utilized during a malaria episode.
Objectives
We aimed to determine whether starting iron 28 d after antimalarial treatment in children with severe malaria and iron deficiency would improve iron status and lower malaria risk.
Methods
We conducted a randomized clinical trial on the effect of immediate compared with delayed iron treatment in Ugandan children 18 mo–5 y of age with 2 forms of severe malaria: cerebral malaria (CM; n = 79) or severe malarial anemia (SMA; n = 77). Asymptomatic community children (CC; n = 83) were enrolled as a comparison group. Children with iron deficiency, defined as zinc protoporphyrin (ZPP) ≥ 80 µmol/mol heme, were randomly assigned to receive a 3-mo course of daily oral ferrous sulfate (2 mg · kg–1 · d–1) either concurrently with antimalarial treatment (immediate arm) or 28 d after receiving antimalarial treatment (delayed arm). Children were followed for 12 mo.
Results
All children with CM or SMA, and 35 (42.2%) CC, were iron-deficient and were randomly assigned to immediate or delayed iron treatment. Immediate compared with delayed iron had no effect in any of the 3 study groups on the primary study outcomes (hemoglobin concentration and prevalence of ZPP ≥ 80 µmol/mol heme at 6 mo, malaria incidence over 12 mo). However, after 12 mo, children with SMA in the delayed compared with the immediate arm had a lower prevalence of iron deficiency defined by ZPP (29.4% compared with 65.6%, P = 0.006), a lower mean concentration of soluble transferrin receptor (6.1 compared with 7.8 mg/L, P = 0.03), and showed a trend toward fewer episodes of severe malaria (incidence rate ratio: 0.39; 95% CI: 0.14, 1.12).
Conclusions
In children with SMA, delayed iron treatment did not increase hemoglobin concentration, but did improve long-term iron status over 12 mo without affecting malaria incidence.
This trial was registered at clinicaltrials.gov as NCT01093989.
Keywords: iron deficiency, malaria, severe malarial anemia, cerebral malaria, zinc protoporphyrin, inflammation, iron and malaria
Introduction
Iron supplementation makes children in malaria-endemic areas more susceptible to malaria and other infections (1–3). However, without sufficient iron, a child's brain and the hematologic and immune systems will not develop optimally (4, 5). Children with clinical malaria and iron deficiency (typically defined as low hemoglobin, an abnormal value of a more specific iron biomarker, or both) frequently present to health clinics in malaria-endemic regions. The current standard-of-care treatment for children with this presentation is to provide antimalarial treatment and start a 3-mo course of oral iron therapy concurrently (6). Studies following this regimen, however, have reported unresolved anemia, persistent iron deficiency, and more frequent episodes of clinical malaria (7–9).
A physiological explanation for these findings is that high concentrations of the hepatic protein hepcidin, which accompany the inflammatory response to malaria, block iron absorption from the gut and sequester iron that would normally be recycled from senescent RBCs in macrophages (10, 11). Thus, oral iron given when hepcidin concentrations remain high may not be well absorbed or utilized (12), and what small amount of iron is absorbed despite elevated hepcidin concentrations may benefit the malaria parasite. Studies have demonstrated that hepcidin normalizes ∼4 wk after successful antimalarial treatment (13, 14), suggesting that a 1-mo delay would improve iron absorption while minimizing the risk of exacerbating malaria infection in children recovering from malaria and iron deficiency.
To test this hypothesis, we conducted a randomized clinical trial that assessed whether delaying the start of a 3-mo course of oral iron therapy by 28 d, as compared with starting the same regimen immediately with antimalarial treatment, improved long-term iron status and affected the incidence of subsequent malaria episodes in iron-deficient Ugandan children with 2 forms of severe malaria [cerebral malaria (CM) or severe malarial anemia (SMA)] and in community children (CC) without severe malaria. As in many regions of the world, ∼1 in 4 Ugandan children <5 y of age are iron-deficient, half are anemic, and malaria is the leading cause of death (15, 16). Identification of a new timing sequence for iron and antimalarial treatment that safely improves iron status and reduces the risk of malaria thus may provide a better strategy to maintain optimal brain development and immune function for the millions of children who have both iron deficiency and severe malaria.
Methods
Study cohort enrollment and medical treatment
Between June 2010 and December 2013, the study (NCT01093989) enrolled 79 children with CM and 77 children with SMA, and 83 CC who were between the ages of 18 mo and 5 y. CC were enrolled from the same household or neighborhood as approximately every other child with CM or SMA to provide a 1:1:1 distribution among the 3 study groups (CM, SMA, CC). CC were enrolled to provide comparator population-specific values for iron and inflammatory markers in children in the local community who did not have severe malaria and to provide information on the background incidence of clinical malaria in this population.
Inclusion criteria
CM was defined as 1) coma (Blantyre Coma Score ≤2 or Glasgow coma score ≤8); 2) Plasmodium falciparum on a blood smear; and 3) no other known cause of coma. SMA was defined as P. falciparum on a blood smear plus hemoglobin concentration ≤50 g/L. Eligibility criteria for CC were 1) age within 1 y of a child with CM or SMA; 2) within the same neighborhood or household as a child with CM or SMA; 3) currently healthy, with temperature <37.5°C; and 4) no illness requiring medical care within the previous 4 wk. Known chronic illness was an exclusion criterion for all study groups.
Medical treatment
Children with severe malaria (CM or SMA) were treated for malaria according to the Uganda national clinical guidelines: typically intravenous quinine from the start of the study through December 2012, and artesunate from January 2013 to study end (December 2014), with variation based on drug availability. This was followed by a 3-d oral dose of artemether/lumefantrine, once the child was able to take oral medication. Both children with CM and with SMA received the same course of antimalarial treatment. Children with SMA received a blood transfusion in addition to antimalarial treatment. If a CC had a positive blood smear for malaria, he/she was treated, but was not excluded from the study. All study children received an insecticide-treated bed net to prevent malaria. Blood and plasma samples were collected in purple-top (EDTA-coated) vacutainers, and plasma was stored on-site at −80°C until later shipment and testing for iron and inflammatory biomarkers at the University of Minnesota.
Iron deficiency testing and definition
Children were tested for zinc protoporphyrin (ZPP) via a front-face hematofluorometer (Aviv Biomedical). ZPP was chosen as the biomarker for iron deficiency primarily because it is a point-of-care test with immediate results. ZPP is a more sensitive marker of iron deficiency than hemoglobin because as a hemoglobin precursor that rises when iron is not available to the developing RBC, it increases before the onset of anemia (17). In a large iron supplementation study on malaria-endemic Pemba Island (1), children with ZPP ≥ 80 µmol/mol heme who received iron had significantly fewer hospitalizations and deaths than those who received placebo, suggesting a benefit of iron if given to children with ZPP ≥ 80 µmol/mol heme even in the context of malaria.
Random assignment and treatment
Children with CM or SMA who had ZPP ≥ 80 µmol/mol heme were randomly assigned to receive a 3-mo course of daily ferrous sulfate syrup at a dosage of 2 mg · kg–1 · d–1 that began immediately with antimalarial treatment on day 0 or as soon as oral medication could be given (immediate arm) or on day 28 (delayed arm). CC with ZPP ≥ 80 µmol/mol heme were randomly assigned to receive a 3-mo course of daily ferrous sulfate syrup at a dosage of 2 mg · kg–1 · d–1 that began immediately on day 0 (immediate arm) or on day 28 (delayed arm). All children (CM, SMA, CC) in the immediate arm received iron for the first, second, and third months of follow-up, whereas all children in the delayed arm received iron for the second, third, and fourth months of follow-up. Randomization assignment was based on simple randomization, stratified by study group, i.e., CM, SMA, and CC. Children without iron deficiency did not receive iron, so follow-up data from this group are not included in the current article.
Follow-up visits and home visits
Children returned to the clinic 6 and 12 mo after initiation of malaria treatment (day 0) for venous blood draw for reassessment of plasma iron and inflammatory biomarkers. Children who were iron-deficient (ZPP ≥ 80 µmol/mol heme) at the 6-mo follow-up visit were given an additional course of iron. Children were visited at home by study home visitors every 2 wk, starting 2 wk after enrollment and continuing until month 4, by which time all children had finished their randomly assigned iron therapy, and monthly thereafter.
Home visitors measured each child's temperature and referred any ill child to the study clinic for care. They also assessed the home environment (18), medication adherence, and side effects of iron treatment. Parents were asked to bring the child to the study clinic or hospital for any illness. Children with a history of fever on sick visits were tested for malaria. Malaria in follow-up was defined as the presence of P. falciparum on a blood smear in a child with an axillary temperature of ≥37.5°C at the time of a clinic visit.
Ethical review and approval
Written informed consent was obtained from parents or guardians of all study participants. Ethical approval was granted by the Institutional Review Boards for human studies at Makerere University School of Medicine and at the University of Minnesota, and regulatory approval by the Uganda National Council for Science and Technology, and the Uganda National Drug Authority.
Laboratory analysis
Plasma concentrations of ferritin (Ramco Laboratories Inc.), soluble transferrin receptor (sTfR) (Ramco Laboratories Inc.), and hepcidin (Bachem Holding AG) were measured by ELISA at the University of Minnesota. Plasma concentrations of C-reactive protein (CRP) were measured by Luminex immunoassay (Milliplex MAP kit, EMD Millipore) at the University of Minnesota.
Statistical analysis
All analyses were intention-to-treat. Primary study outcomes were compared within study groups (CM, SMA, CC) according to treatment arm (immediate compared with delayed iron). Primary study outcomes were 1) 6-mo hemoglobin concentrations, compared by t test; 2) the proportion of children iron-deficient by ZPP at 6 mo, compared by Pearson's chi-square test; and 3) incidence of clinical malaria over 12-mo follow-up, compared by negative binomial regression. A secondary outcome of time to first malaria episode was analyzed using Kaplan–Meier survival analysis.
We also examined the effect of immediate compared with delayed iron on iron and inflammatory markers over the course of the 12-mo follow-up period by averaging the value of each indicator at 6 and 12 mo and then modeling that average as the dependent variable in multiple linear regression equations performed separately by study group, with treatment arm (immediate or delayed iron) as the independent variable, adjusting for the baseline value of each indicator. We examined longitudinal changes in each indicator in each study group (CM, SMA, CC), using ANOVA to compare values of each indicator at baseline, 6-mo, and 12-mo follow-up, and using Tukey's post hoc test to account for pairwise comparisons between study groups. Iron and inflammatory biomarkers other than hemoglobin were log transformed before analysis.
We also examined the prevalence of iron deficiency based on alternative definitions, including those based on low ferritin (<12 μg/L or <30 μg/L if CRP > 10 mg/L) or high sTfR (>8.3 mg/L) (19). Potential confounding covariates, such as age, sex, anthropometric indexes, and proportion of children who received an additional course of iron, did not differ significantly between children in the immediate and the delayed treatment arms for each study group, so we did not adjust for these factors in the longitudinal biomarker or malaria incidence analyses.
We based sample size on the primary aim of hemoglobin value at 6 mo between the immediate and delayed iron arms in the 2 malaria groups (CM and SMA). Assuming an SD in hemoglobin at 6 mo follow-up of 1.5 g/dL for each group, and an α of 0.05, a sample size of 80 children in each study group had 80% power to detect a difference of ≥1.0 g/dL between the immediate and delayed arms. The sample size also provided 85% power to detect an average difference of 1.5 malaria episodes between the immediate and delayed arms in the CM and SMA groups, assuming 4 episodes/y in one arm and 2.5 episodes/y in the other. All analyses were done using STATA version 15.0 (StataCorp) and Prism 8 (GraphPad).
Results
Study cohort and baseline demographic, clinical, and laboratory characteristics
We enrolled a total of 239 children (Figure 1). All children with CM (n = 79), all children with SMA (n = 77), and 35 of 83 CC (42%) had ZPP ≥ 80 µmol/mol heme and were randomly assigned to receive immediate or delayed iron. Nine children with CM (1 child in the immediate arm and 8 children in the delayed arm) died before initial discharge from hospital and before receiving any study-specific supplement or treatment. Because randomization assignment was given at enrollment, rather than with hospital discharge, this disproportionate distribution of condition-specific mortality skewed the distribution of treatment arms (immediate and delayed iron) among children with CM. However, >90% of children initially discharged from the hospital completed the full 12-mo follow-up period (Figure 1). The median weight at baseline was 12 kg (IQR: 10.5–14 kg), making the typical daily dose of iron, based on 2 mg · kg–1 · d–1, 24 mg (IQR: 21–28 mg).
FIGURE 1.
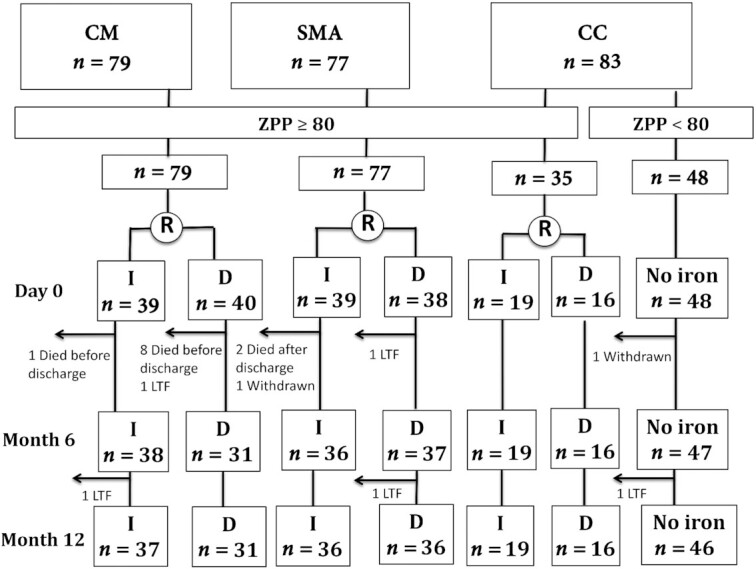
Study diagram. Children with severe malaria (CM or SMA) were enrolled from the Paediatric Acute Care Unit at Mulago Hospital, Kampala. Healthy CC from the same village or household compound as children with severe malaria were also enrolled. Any child with ZPP ≥ 80 μmol/mol heme was randomly assigned to start a 3-mo course of daily oral ferrous sulfate (2 mg/kg) at baseline (immediate arm, standard of care) or 28 d later (delayed arm). Children with ZPP < 80 μmol/mol heme were not randomly assigned to receive iron. CC, community children; CM, cerebral malaria; D, delayed; I, immediate; LTF, lost to follow-up; SMA, severe malarial anemia; ZPP, zinc protoporphyrin.
Baseline demographic, socioeconomic (20), and clinical findings in children in the immediate and the delayed arms in each study group are shown in Table 1 in the full intention-to-treat cohort, and in Supplemental Table 1 in the children who received randomly assigned treatment.
TABLE 1.
Demographic and clinical characteristics of randomly assigned study children by study group and treatment arm1
| CM | SMA | CC | ||||
|---|---|---|---|---|---|---|
| Characteristic | Immediate | Delayed | Immediate | Delayed | Immediate | Delayed |
| n | 39 | 40 | 39 | 38 | 19 | 16 |
| Age, y | 3.1 ± 1.0 | 3.1 ± 1.0 | 2.9 ± 1.1 | 2.7 ± 0.9 | 2.9 ± 1.0 | 3.0 ± 0.7 |
| Female | 16 (41) | 21 (53) | 17 (44) | 14 (37) | 11 (58) | 13 (81) |
| Weight-for-age z score | −1.2 ± 1.1 | −1.4 ± 0.8 | −1.6 ± 1.1 | −1.5 ± 1.1 | −1.0 ± 0.8 | −1.2 ± 1.1 |
| Height-for-age z score | −0.7 ± 1.6 | −1.3 ± 1.2 | −1.2 ± 1.8 | −1.5 ± 1.3 | −1.0 ± 0.8 | −1.5 ± 0.9 |
| Weight-for-height z score2 | −0.9 ± 1.2 | −0.8 ± 1.3 | −1.1 ± 1.3 | −0.8 ± 1.0 | −0.7 ± 0.8 | −0.3 ± 1.1 |
| Socioeconomic status score3 | 10.3 ± 3.6 | 10.0 ± 3.4 | 9.6 ± 2.9 | 9.0 ± 2.7 | 10.0 ± 2.6 | 10.6 ± 2.9 |
| Home environment z score | 0.1 ± 1.1 | 0.0 ± 1.1 | −0.2 ± 0.8 | −0.1 ± 1.1 | 0.0 ± 0.8 | 0.1 ± 1.0 |
| Maternal education level | ||||||
| Primary 6 or lower | 12 (31) | 18 (45) | 15 (38) | 15 (39) | 6 (31) | 3 (19) |
| Primary 7 | 8 (21) | 5 (12) | 9 (23) | 6 (16) | 7 (37) | 6 (37) |
| Secondary or higher | 18 (46) | 8 (20) | 14 (36) | 16 (42) | 6 (31) | 7 (44) |
| Not known | 1 (2) | 9 (23) | 1 (3) | 1 (3) | — | — |
| Paternal education level | ||||||
| Primary 6 or lower | 7 (18) | 5 (12) | 7 (18) | 12 (32) | 3 (16) | 2 (12) |
| Primary 7 | 6 (15) | 7 (18) | 11 (28) | 2 (5) | 5 (26) | 2 (12) |
| Secondary or higher | 18 (46) | 14 (35) | 18 (46) | 20 (53) | 10 (53) | 10 (63) |
| Not known | 8 (21) | 14 (35) | 3 (8) | 4 (10) | 1 (5) | 2 (12) |
| Child with preschool education | 9 (24) | 8 (25) | 6 (16) | 6 (17) | 3 (16) | 2 (13) |
| HIV-positive at baseline | 1 (3) | 0 (0) | 0 (0) | 2 (5) | 0 (0) | 0 (0) |
| Plasmodium falciparum on admission blood smear | 39 (100) | 40 (100) | 39 (100) | 38 (100) | 2 (11) | 2 (13) |
Values are means ± SDs or n (%) unless otherwise indicated. CC, community children; CM, cerebral malaria; SMA, severe malarial anemia.
n = 37 for SMA delayed.
n = 38 for CM immediate, n = 38 for SMA immediate; n = 32 for CM delayed, n = 37 for SMA delayed, n = 16 for CC delayed.
Iron biomarker values in each study group (CM, SMA, or CC) at baseline, 6 mo, and 12 mo, according to immediate compared with delayed iron treatment
Concentrations of all iron biomarkers were similar between children in the 2 iron treatment arms at baseline in each study group (P > 0.05 for all comparisons), whether in the full sample (Table 2) or among children who received randomly assigned treatment (data not shown). The markers reflected profound inflammation (high CRP, ferritin, and hepcidin) and consequent limited availability of iron to the bone marrow (low hemoglobin, high ZPP) in both severe malaria groups (CM and SMA; Table 2). STfR was greater in children with SMA than in children with CM, potentially reflecting hemolysis or greater pre-existing iron deficiency.
TABLE 2.
Baseline values of iron and inflammatory markers by study group and treatment arm1
| CM | Severe malarial anemia | Community children | ||||
|---|---|---|---|---|---|---|
| Immediate | Delayed | Immediate | Delayed | Immediate | Delayed | |
| n | 39 | 40 | 39 | 38 | 19 | 16 |
| Hemoglobin, g/dL | 7.0 ± 1.9 | 7.0 ± 1.9 | 3.6 ± 0.87 | 3.9 ± 1.0 | 11.0 ± 1.5 | 11.4 ± 1.2 |
| ZPP, μmol/mol heme | 261 (225, 303) | 263 (219, 316) | 396 (324, 484) | 357 (286, 445) | 134 (111, 163) | 117 (98, 139) |
| Ferritin, μg/L | 984 (754, 1284)2 | 1162 (798, 1691) | 912 (644, 1291) | 717 (487, 1054) | 45.0 (28.2, 71.9) | 30.5 (14.7, 63.0) |
| sTfR, mg/L | 3.7 (3.3, 4.3)2 | 4.4 (3.9, 4.9) | 6.6 (5.6, 7.9) | 7.5 (6.3, 8.9) | 6.5 (5.3, 7.3) | 5.5 (4.4, 6.8) |
| Hepcidin, ng/mL | 135 (97.1, 188) | 145 (109, 193) | 47.6 (29.2, 77.6) | 67.8 (44.7, 103) | 21.9 (13.1, 36.7) | 18.3 (11.5, 28.9) |
| CRP, mg/L | 686 (582, 807)2 | 816 (647, 1028) | 474 (368, 610) | 532 (414, 684) | 5.6 (2.0, 15.6) | 2.1 (0.38, 11.6) |
Values are means ± SDs or geometric means (95% CIs) unless otherwise indicated. CM, cerebral malaria; CRP, C-reactive protein; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin.
n = 38 for CM immediate group.
The 2 primary iron status outcomes, hemoglobin concentration and prevalence of iron deficiency (ZPP ≥ 80 µmol/mol heme) at 6-mo follow-up, did not differ significantly between the immediate and delayed treatment arms in any study group (Table 3). At 12 mo follow-up, children with SMA who received delayed compared with immediate iron treatment had significantly lower ZPP and sTfR concentrations, both reflective of better iron status and greater availability of iron to the bone marrow (19) (Table 4). Further, at 12 mo, the prevalence of iron deficiency as defined by ZPP among children with SMA who received delayed iron (10 of 34, 29.4%) was lower than that among children with SMA who received immediate iron (21 of 32, 65.6%, P = 0.006; Supplemental Table 2). The prevalence of iron deficiency as defined by sTfR or ferritin did not differ between treatment arms at 6 or 12 mo. At 12 mo, increased hepcidin concentrations were seen in children in the CC group who received delayed iron compared with immediate iron (Table 4).
TABLE 3.
Hemoglobin concentration and presence of iron deficiency (defined by a zinc protoporphyrin value ≥80 µmol/mol heme) at 6-mo follow-up, according to treatment with immediate or delayed iron1
| CM | SMA | CC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Immediate | Delayed | P 2 | Immediate | Delayed | P 2 | Immediate | Delayed | P 2 | |
| Hemoglobin,3 g/L | 12.4 ± 1.4 | 11.8 ± 1.3 | 0.13 | 11.1 ± 2.2 | 11.7 ± 1.1 | 0.16 | 12.0 ± 1.1 | 12.1 ± 1.1 | 0.79 |
| Iron deficiency | 8/37 (21.6) | 10/31 (32.3) | 0.32 | 19/34 (55.9) | 14/36 (38.9) | 0.15 | 9/19 (47.4) | 5/16 (31.3) | 0.33 |
Values are means ± SDs or n/N (%). CC, community children; CM, cerebral malaria; SMA, severe malarial anemia.
t Test for comparison of hemoglobin concentration; chi-square test for comparison of prevalence of iron deficiency.
CM immediate n = 31, delayed n = 27; SMA immediate n = 28, delayed n = 33; CC immediate n = 18, delayed n = 16. Some children who were seen at the 6-mo time point did not have hemoglobin tested owing to study errors in sample collection at this time point.
TABLE 4.
Adjusted mean values of iron and inflammatory markers over 12 mo follow-up in each study group, according to immediate and delayed iron treatment1
| Cerebral malaria | Severe malarial anemia | Community children | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediate | Delayed | Mean effect2 | P | Immediate | Delayed | Mean effect | P | Immediate | Delayed | Mean effect | P | |
| Hemoglobin,3 g/dL | 12.4 ± 0.2 | 12.1 ± 0.2 | 0.33 (−0.2, 0.8) | 0.20 | 11.3 ± 0.3 | 11.7 ± 0.3 | −0.4 (−1.1, 0.3) | 0.28 | 12.2 ± 0.2 | 11.9 ± 0.2 | 0.34 (−0.2, 0.9) | 0.20 |
| ZPP,4 μmol/mol heme | 71 ± 1.0 | 73 ± 1.1 | 0.98 (0.9, 1.1) | 0.72 | 108 ± 1.1 | 82 ± 1.1 | 1.3 (1.1, 1.6) | 0.01 | 90 ± 1.1 | 76 ± 1.1 | 1.2 (0.9, 1.5) | 0.16 |
| Ferritin,4 μg/L | 70.2 ± 1.1 | 81.5 ± 1.1 | 0.86 (0.63, 1.2) | 0.36 | 127 ± 1.2 | 108 ± 1.2 | 1.2 (0.8, 1.8) | 0.47 | 51.2 ± 1.2 | 50.9 ± 1.2 | 1.0 (0.6, 1.7) | 0.99 |
| sTfR,4 mg/L | 5.6 ± 1.1 | 5.6 ± 1.1 | 0.99 (0.84, 1.2) | 0.91 | 7.8 ± 1.1 | 6.1 ± 1.1 | 1.3 (1.0, 1.6) | 0.03 | 6.4 ± 1.1 | 5.7 ± 1.1 | 1.1 (0.9, 1.4) | 0.24 |
| Hepcidin,4 ng/mL | 23.9 ± 1.1 | 27.8 ± 1.1 | 0.86 (0.6, 1.2) | 0.40 | 25.5 ± 1.2 | 34.1 ± 1.2 | 0.75 (0.5, 1.2) | 0.19 | 15.6 ± 1.2 | 30.9 ± 1.3 | 0.50 (0.3, 1.0) | 0.04 |
| CRP,4 mg/L | 12.1 ± 1.4 | 16.3 ± 1.4 | 0.74 (0.3, 1.7) | 0.49 | 42.1 ± 1.3 | 25.5 ± 1.3 | 1.7 (0.7, 3.7) | 0.21 | 12.9 ± 1.4 | 29.5 ± 1.5 | 0.44 (0.1, 1.3) | 0.14 |
The value of each indicator at 6 and 12 mo was averaged and then modeled as the dependent variable in multiple linear regression equations performed separately by study group, which adjusted for treatment arm (immediate or delayed) and the baseline value of the indicator. The averaged value of all variables other than hemoglobin was log transformed before analysis. CRP, C-reactive protein; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin.
Immediate minus delayed for hemoglobin (95% CI); ratio of immediate-to-delayed (95% CI) for all other indicators.
Values are arithmetic means ± SEs.
Values are geometric means ± SEs.
Malaria incidence and serious adverse events in each study group (CM, SMA, or CC), according to immediate compared with delayed iron treatment
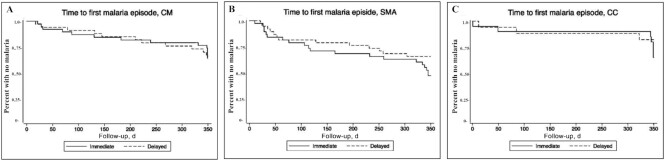
The primary clinical outcome of malaria incidence (inpatient or outpatient) did not differ significantly between the immediate and delayed treatment arms in any study group (Table 5), although a trend toward decreased inpatient malaria incidence was seen in children with SMA in the delayed compared with the immediate iron treatment arm [incidence rate ratio (IRR): 0.39; 95% CI: 0.14, 1.12]. The time to first malaria episode also did not differ significantly between the immediate and delayed iron treatment arms within any study group (HR delayed compared with immediate: CM: 1.15; 95% CI: 0.43, 3.07; P = 0.78; SMA: 0.75; 95% CI: 0.35, 1.61; P = 0.46; CC: 1.67; 95% CI: 0.28, 9.98; P = 0.58) (Figure 2).
TABLE 5.
Incidence of malaria according to iron treatment arm, by study group1
| Malaria incidence per 100 person-years (95% CI) | Delayed to immediate incidence rate ratio (95% CI) | |||
|---|---|---|---|---|
| Study group | Delayed | Immediate | P | |
| Cerebral malaria | ||||
| n | 40 | 39 | ||
| All visits | 30.4 (15.6, 59.2) | 28.5 (15.2, 53.6) | 1.07 (0.43, 2.67) | 0.89 |
| Inpatient | 10.1 (3.3, 31.4) | 8.6 (2.8, 26.5) | 1.18 (0.24, 5.87) | 0.84 |
| Outpatient | 20.3 (9.1, 45.1) | 20.0 (9.5, 41.9) | 1.02 (0.34, 3.02) | 0.98 |
| Severe malarial anemia | ||||
| n | 38 | 39 | ||
| All visits | 47.0 (27.6, 79.8) | 63.2 (38.8, 102.9) | 0.74 (0.36, 1.53) | 0.42 |
| Inpatient | 14.7 (6.1, 35.3) | 37.3 (21.2, 65.6) | 0.39 (0.14, 1.12) | 0.08 |
| Outpatient | 32.2 (16.4, 63.6) | 25.3 (11.6, 54.6) | 1.28 (0.46, 3.58) | 0.64 |
| Community children | ||||
| n | 16 | 19 | ||
| All visits | 32.7 (8.6, 123.9) | 16.5 (3.8, 71.0) | 1.98 (0.28, 14.27) | 0.50 |
| Inpatient | 13.1 (1.6, 105.4) | 5.5 (0.50, 62.3) | 2.38 (0.10, 58.4) | 0.60 |
| Outpatient | 19.6 (5.1, 75.2) | 11.0 (2.4, 51.2) | 1.78 (0.23, 13.7) | 0.58 |
Malaria defined as Plasmodium falciparum on a blood smear with measured axillary temperature ≥37.5°C.
FIGURE 2.
Kaplan–Meier survival plots reflecting time to first malaria episodes in children with CM (n = 79) (A), SMA (n = 77) (B), and CC (n = 35) (C), who received immediate or 28-d delayed iron therapy after treatment of malaria and were followed for 12 mo (350 d). CC, community children; CM, cerebral malaria; SMA, severe malarial anemia.
Incidence of serious adverse events (postdischarge death, life-threatening event, hospitalization, or iron overdose) in children in all study groups who were randomly assigned to iron treatment did not differ significantly between children in the delayed and the immediate treatment arms (number of serious adverse events delayed and immediate: 34 and 48, respectively; IRR: 0.79; 95% CI: 0.45, 1.38). Both deaths in follow-up occurred in children with SMA in the immediate treatment arm.
Changes in iron biomarkers in each study group (CM, SMA, or CC) over time
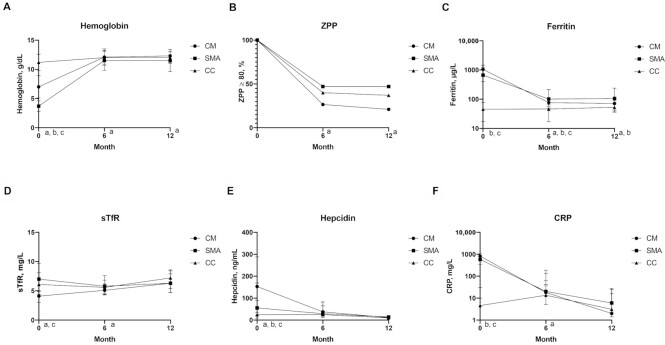
To assess whether changes in iron status over time were affected by the form of severe malaria, we compared longitudinal trajectories in iron biomarkers by study group (CM, SMA, CC), including all children in the groups, regardless of treatment arm, over the 12-mo follow-up period (Figure 3). Iron biomarkers improved in all 3 study groups (CM, SMA, CC). Among children with severe malaria (CM and SMA groups), inflammation declined, as reflected by prompt declines in CRP, ferritin, and hepcidin. At 6 mo, CRP concentrations were higher among children with SMA than among children in the CM group (Figure 3F). Hepcidin was not different between the 3 study groups after day 0 (Figure 3E), and ferritin concentrations were higher in children with SMA than with CM at both 6- and 12-mo follow-up (Figure 3C).
FIGURE 3.
Value of biomarkers or proportion by month and study group for all children who were randomly assigned to iron [all CM (n = 79), all SMA (n = 77), and CC who were iron-deficient at day 0 (n = 35)]. (A) Hemoglobin, (B) ZPP, (C) ferritin, (D) sTfR, (E) hepcidin, (F) CRP. Hemoglobin values are presented as the mean value at each time point; bars represent SD. Other biomarker values are the median value; bars represent IQR. Superscripts reflect significant differences (P < 0.05) at each time point as assessed by ANOVA for continuous outcomes, with Tukey's test for pairwise comparisons. All values aside from hemoglobin were log transformed before ANOVA. Differences in the proportion iron-deficient by ZPP (B) were determined by chi-square test. aDifference between SMA and CM; bdifference between SMA and CC; cdifference between CM and CC. CC, community children; CM, cerebral malaria; CRP, C-reactive protein; SMA, severe malarial anemia; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin.
Concomitant with the resolution of inflammation, hemoglobin rose and ZPP declined in all 3 study groups, presumably reflecting increased iron availability to the bone marrow (Figure 3A, B). However, children with SMA had lower hemoglobin and a greater prevalence of ZPP ≥ 80 µmol/mol heme at both 6 and 12 mo than children with CM, whereas both measures were similar at these time points between the CM and CC groups (Figure 3A, B, Supplemental Table 2). Nearly half of children in the SMA group (47%) remained iron-deficient by ZPP at both 6 and 12 mo, despite treatment of malaria, initial provision via transfusion of a 3-mo course of iron, and retreatment with iron if iron-deficient by ZPP at 6 mo.
Discussion
Our primary finding was that delaying the start of a 3-mo course of daily oral iron therapy by 28 d after antimalarial treatment in iron-deficient Ugandan children with CM or SMA, and in CC, did not improve iron status outcomes at 6 mo (hemoglobin concentration; prevalence of ZPP ≥ 80 µmol/mol heme at 6 mo) or affect malaria incidence over 12 mo follow-up as compared with starting the same iron treatment regimen concurrently with antimalarial treatment per the standard of care. However, children with SMA who received delayed iron had lower concentrations of ZPP and sTfR over the 12-mo follow-up period and were much less likely to be iron-deficient at 12 mo than children with SMA who received immediate iron. In addition, children with SMA who were treated with delayed iron showed a trend toward decreased inpatient malaria incidence.
Although multiple studies have demonstrated that iron supplementation may increase malaria risk in children living in areas of high infection burden (1–3), iron deficiency limits developmental potential and immune function, necessitating strategies to optimize iron status safely in malaria-endemic areas (4, 5). The WHO's standard-of-care treatment for any child with malaria and anemia or an abnormal iron biomarker is to treat the malaria and start a standard 3-mo course of iron therapy concurrently (6). However, the suboptimal absorption of dietary iron and poor distribution of iron to the bone marrow induced by high hepcidin during acute malarial infection make providing iron at this time ineffective (10, 11, 21, 22). Iron given at this time may also increase the risk of subsequent malaria infection (12). The 28-d delayed-iron design in the current study permitted provision of iron at the time that hepcidin is lowest after treatment of malaria (13, 14, 22). As previously reported in this cohort, hepcidin was significantly lower among children in the delayed iron treatment arm in all malarial study groups at day 28 (23). This acute finding set the stage for the current assessment of long-term response to delayed iron therapy in all groups. In this study, we observed improved long-term iron status (lower ZPP and lower sTfR, both indicating better availability of iron to erythroblasts) with delayed iron only among children with SMA.
The consistent evidence of better iron status among children with SMA who received delayed iron is perhaps due to the dual contribution of lower hepcidin at the time of initial iron treatment together with a greater degree of pre-existing dietary iron deficiency in children with SMA than in children with CM. This latter hypothesis is supported by 1) consistently higher ZPP among children in the SMA group at 6 and 12 mo; 2) higher sTfR concentrations among children with SMA than among children with CM; and 3) the relative similarity of iron biomarkers between the CM and CC groups at 6 and 12 mo. The latter finding suggests that disruption of iron homeostasis among children with CM may have been more rapid and readily reversible than among children with SMA, who may have had a pre-existing dietary deficiency and/or a resumption of deficiency that continued in the current study after the supplementation period.
The differences in iron biomarkers and hemoglobin concentrations during follow-up between children with SMA and children with CM were also informative. Ferritin, an important iron and inflammatory marker whose interpretation is affected by both dietary iron status and inflammation (19), was not significantly affected by immediate or delayed iron. Ferritin was highest among children with SMA, but it was likely not reflecting dietary iron status, because it was much higher than published normal values for children of the same age in this setting. Rather, the elevated ferritin, higher prevalence of ZPP ≥ 80 µmol/mol heme, and lower hemoglobin in SMA compared with CM throughout the follow-up period more likely reflect chronic, low-level inflammation in children with SMA. This chronic inflammation may have led to hepcidin-driven disruption in iron absorption and utilization and this could in turn have contributed to persistence of iron deficiency and lower hemoglobin in the children with SMA. The chronic inflammation could also potentially contribute to restriction of iron to the developing brain. These novel findings of chronically higher ferritin, higher ZPP, and lower hemoglobin in children with SMA than with CM may also explain in part why children with SMA are at increased risk of readmission compared with children with CM (24), because ongoing lower hemoglobin concentrations and inflammation could lead to increased risk of severe anemia with subsequent malaria infections.
Malaria incidence did not differ significantly between delayed and immediate treatment in any study group. However, children with SMA who received delayed iron showed a trend toward a lower rate of subsequent inpatient admissions for malaria. Subsequent malaria incidence was relatively low in this cohort, so only large differences in incidence could be detected between treatment arms. For other study groups, and for outpatient incidence, there was no trend toward protection with delayed iron treatment. Given the benefits of delayed iron treatment on iron status in children with SMA, future studies should confirm whether delayed iron treatment also protects against recurrent severe malaria (i.e., malaria requiring inpatient admission).
Limitations of the current study include that randomization assignment was given on enrollment rather than at hospital discharge. Nine of the 79 children with CM died before discharge, a case fatality rate of 11.3%, which is lower than some published estimates (25). Eight of the 9 children were in the delayed iron arm, although none of the 9 children had started receiving any study-specific supplement or treatment. With identical care between the study arms before discharge, it appears this difference in mortality rates between treatment arms in the CM group was a chance finding. Data from these children were included in baseline analyses, because they contribute to the accurate description of this study group in its entirety at baseline. There were no differences in sociodemographic or laboratory findings between children who died before discharge and those who did not.
An additional limitation is that although iron status markers including hemoglobin returned to normal values among children in the CM group and were unaffected by treatment arm, we cannot conclude that supplemental iron was unnecessary in this group without a no-iron control group, which was not ethically permissible. We also did not collect dietary data, so although we assume dietary iron was equivalent between the treatment arms in each study group, we cannot substantiate this assumption.
Blood transfusion is the required standard of care for children with SMA, so all children with SMA received blood transfusions. Transfusion would increase iron stores in all children with SMA, owing to the rich iron content of hemoglobin, but because all children with SMA received a blood transfusion, it would not affect outcomes between immediate and delayed treatment in this study. The effect of immediate compared with delayed iron treatment in children with SMA in the absence of blood transfusion cannot be studied, because it is unethical to withhold blood transfusion from children with SMA.
Finally, malaria incidence in the cohort was lower than anticipated, mirroring a general decrease in malaria incidence in the Kampala area during the study period. The study therefore had power to detect only large differences in overall malaria incidence. Children with SMA showed a trend toward a decrease in severe malaria that required admission (IRR: 0.39; 95% CI: 0.14, 1.12), but owing to the even lower incidence of severe malaria, only decreases of >70% would be detectable with the study sample size. Future studies with a larger sample size are needed to clarify the risk of subsequent severe malaria with immediate compared with delayed iron treatment in SMA.
In conclusion, the current study demonstrates that delaying the start of iron supplementation until 28 d after antimalarial treatment in children with SMA and iron deficiency is associated with better long-term iron status, no increase in malaria incidence, and a potential decrease in subsequent severe malaria incidence. The study findings also demonstrate that children with SMA have persistently increased ferritin and lower hemoglobin concentrations 12 mo after discharge compared with children with CM. Together, the findings suggest that children with SMA maintain chronic inflammation after discharge that may lead to persistently lower hemoglobin concentrations and that delayed iron treatment may be of benefit in these children.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SEC: drafted the initial manuscript, was responsible for Institutional Review Board application, statistical analyses, and interpretation of the data; SEC and CCJ: conceptualized and designed the study; ROO and CCJ: directed the conduct of the study; ROO, MKG, and CCJ: interpreted the data; SEC and ASS: were responsible for conduct of the study and data collection; MKG: helped to conceptualize and design the study; and all authors: agree to be accountable for all aspects of the work and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 1U01HD064698-01 (to CCJ), National Institute of Neurological Disorders and Stroke grant R01 NS055349 (to CCJ), and Fogarty International Center grant D43 NS078280 (to CCJ).
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available upon publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to Debbie Bennett (e-mail: debabenn@iu.edu).
SEC and ROO contributed equally to this work.
Abbreviations used: CC, community children; CM, cerebral malaria; CRP, C-reactive protein; IRR, incidence rate ratio; SMA, severe malarial anemia; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin.
References
- 1. Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MKet al.. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133–43. [DOI] [PubMed] [Google Scholar]
- 2. Gwamaka M, Kurtis JD, Sorenson BE, Holte S, Morrison R, Mutabingwa TK, Fried M, Duffy PE. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis. 2012;54(8):1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Veenemans J, Milligan P, Prentice AM, Schouten LRA, Inja N, van der Heijden AC, de Boer LCC, Jansen EJS, Koopmans AE, Enthoven WTMet al.. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8(11):e1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Georgieff MK, Ramel SE, Cusick SE. Nutritional influences on early brain development. Acta Paediatr. 2018;107:1310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cusick SE, Georgieff MK. The role of nutrition in brain development: the golden opportunity of the “first 1000 days.”. J Peds. 2016;175:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull. 2007;28(4 Suppl):S621–7. [DOI] [PubMed] [Google Scholar]
- 7. Desai MR, Mei JV, Kariuki SK, Wannemuehler KA, Phillips-Howard PA, Nahlen BL, Kager PA, Vulule JM, ter Kuile FO. Randomized, controlled trial of daily iron supplementation and intermittent sulfadoxine-pyramethamine for the treatment of mild childhood anemia in western Kenya. J Infect Dis. 2003;187(4):658–66. [DOI] [PubMed] [Google Scholar]
- 8. Nwanyanwu OC, Ziba C, Kazembe PN, Gamadzi G, Gandwe J, Redd SC. The effect of oral iron therapy during treatment for Plasmodium falciparum malaria with sulphadoxine-pyramethamine on Malawian children under 5 years of age. Ann Trop Med Parasitol. 1996;90(6):589–95. [DOI] [PubMed] [Google Scholar]
- 9. Verhoef H, West CE, Nzyuko SM, de Vogel S, van der Valk R, Wanga MA, Kuijsten A, Veenemans J, Kolk FJ. Intermittent administration of iron and sulfadoxine-pyramethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet. 2002;360(9337):908–14. [DOI] [PubMed] [Google Scholar]
- 10. de Mast Q, Nadjm B, Reyburn H, Kemna EHJM, Amos B, Laarakkers CMM, Silalye S, Verhoef H, Sauerwein RW, Swinkels DWet al.. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis. 2009;199(2):253–62. [DOI] [PubMed] [Google Scholar]
- 11. de Mast Q, van Dongen-Lases EC, Swinkels DW, Nieman A-E, Roestenberg M, Druilhe P, Arens TA, Luty AJ, Hermsen CC, Sauerwein RWet al.. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol. 2009;145(5):657–64. [DOI] [PubMed] [Google Scholar]
- 12. Prentice AM. Iron metabolism, malaria, and other infections: what is all the fuss about?. J Nutr. 2008;138(12):2537–41. [DOI] [PubMed] [Google Scholar]
- 13. Doherty CP, Cox SE, Fulford AJ, Austin S, Hilmers DC, Abrams SA, Prentice AM. Iron incorporation and post-malaria anaemia. PLoS One. 2008;7:e2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howard CT, McKapo US, Quakyi IA, Bosompem KM, Addison EA, Sun K, Sullivan D, Semba RD. Relationship of hepcidin with parasitemia and anemia among patients with uncomplicated Plasmodium falciparum malaria in Ghana. Am J Trop Med Hyg. 2007;77(4):623–6. [PubMed] [Google Scholar]
- 15. World Health Organization. Vitamin and Mineral Information System (VMNIS): WHO Global Database on Anaemia. [Internet]. Geneva, Switzerland: WHO; [cited 10 December, 2019]. Available from: https://www.who.int/vmnis/anaemia/en/. [Google Scholar]
- 16. Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna Aet al.. Malaria in Uganda: challenges to control on the long road to elimination. I. Epidemiology and current control efforts. Acta Trop. 2012;121(3):184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teshome EM, Prentice AM, Demir AY, Andang'o PEA, Verhoef H. Diagnostic utility of zinc protoporphyrin to detect iron deficiency in Kenyan preschool children: a community-based survey. BMC Hematol. 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caldwell BM, Bradley RH. Home Inventory Administration Manual. 3rd ed. Little Rock, AR: University of Arkansas; 2001. [Google Scholar]
- 19. World Health Organization. Assessing the iron status of populations: including literature reviews: report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April 2004. 2nd ed. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 20. Bangirana P, John CC, Idro R, Opoka RO, Byarugaba J, Jurek AM, Boivin MJ. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One. 2009;4(11):e7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, Armitage AE, Drakesmith H. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;199:1922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cusick SE, Opoka RO, Abrams SA, John CC, Georgieff MK, Mupere E. Delaying iron therapy until 28 days after antimalarial treatment is associated with greater iron incorporation and equivalent hematologic recovery after 56 days in children: a randomized controlled trial. J Nutr. 2016;146(9):1769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cusick SE, Opoka RO, Ssemata AS, Georgieff MK, John CC. Comparison of iron status 28 d after provision of antimalarial treatment with iron therapy compared with antimalarial treatment alone in Ugandan children with severe malaria. Am J Clin Nutr. 2016;103(3):919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Opoka RO, Hamre KES, Brand N, Bangirana P, Idro R, John CC. High postdischarge morbidity in Ugandan children with severe malarial anemia or cerebral malaria. J Pediatric Infect Dis Soc. 2017;6(3):e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg. 2001;64(1–2 Suppl):57–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.