ABSTRACT
Background
Phytoestrogens are plant-derived hormonally active compounds found in soy, cruciferous vegetables, nuts, and seeds. Although phytoestrogens have been associated with altered endogenous hormonal activity, luteal phase deficiency, and reduced endometrial decidualization, the literature reporting examinations of phytoestrogen intake and fertility presents mixed findings.
Objectives
We sought to evaluate prospectively the association between dietary phytoestrogen intake (isoflavones, lignans, and coumestans) and fecundability, the per-cycle probability of conception, in 2 cohorts of women planning pregnancy.
Methods
Pregnancy Study Online (PRESTO) and Snart Foraeldre (SF) are parallel web-based preconception cohort studies of women from North America and Denmark, respectively, who are trying to conceive. Participants complete an online baseline questionnaire on sociodemographic, lifestyle, and medical factors. We ascertained intake of individual phytoestrogens from validated FFQs. We measured fecundability using data on menstruation and pregnancy status from bimonthly follow-up questionnaires. We analyzed data from 4880 PRESTO and 2898 SF female study participants who had been attempting conception for ≤6 cycles at study entry. We used proportional probabilities regression models to estimate fecundability ratios (FRs) and 95% CIs.
Results
Phytoestrogen intake varied across cohorts, yet was associated with higher socioeconomic status and healthier behaviors in both cohorts. After adjustment for potential confounders, phytoestrogen intake was not substantially associated with fecundability in either cohort. We observed some evidence of improved fecundability with increasing isoflavone intake among women age ≥30 years in PRESTO (FR: 1.12; 95% CI: 0.94, 1.34, for comparison of ≥90th with <25th percentile intake) and SF (corresponding FR: 1.19; 95% CI: 0.92, 1.55). Lignan intake was associated with slightly increased fecundability in SF (FR for comparison of 75th to 90th with <25th percentile: 1.10; 95% CI: 0.96, 1.26), but decreased fecundability in PRESTO (FR for comparison of ≥90th with <25th percentile: 0.83; 95% CI: 0.72, 0.97).
Conclusions
We did not observe strong associations between phytoestrogen intake and prospectively-measured fecundability among North American or Danish pregnancy planners.
Keywords: fecundability, isoflavones, lignans, phytoestrogens, preconception, time to pregnancy
Introduction
Phytoestrogens, which are plant-derived compounds, include isoflavones (found in soy products), lignans (found in nuts, seeds, and cruciferous vegetables), and coumestans (found in sprouts, split peas, and beans). Phytoestrogens are structurally similar to estrogens, can bind to estrogen receptors α and β, and exhibit both estrogenic and anti-estrogenic effects in vitro (1, 2). Phytoestrogen intake has been associated with decreased risks of several hormone-dependent diseases, including breast (3, 4) and endometrial cancers (5, 6), and improved bone mineral density and strength among postmenopausal women (7–14). Therefore, it is biologically plausible that dietary phytoestrogens could affect endogenous hormonal activity and fertility.
The literature on the association between phytoestrogens and fertility, however, is limited and markedly mixed. Reduced fertility has been observed among captive cheetahs fed soy-based diets (15) and sheep grazing on red clover, which is high in isoflavones (16). An in vitro study of human endometrial stromal cells demonstrated that high levels of the isoflavones daidzein and genistein interfere with the decidualization process (17). Epidemiologic and clinical studies indicate that phytoestrogens may inhibit ovulation by reducing circulating levels of luteinizing hormone (18). In a prospective cohort study of healthy, regularly-menstruating women, isoflavone intake was associated with increased risk of luteal phase deficiency (19), but not with sporadic anovulation or sex hormone concentrations (20). Conversely, isoflavone intake has been associated with decreased markers of inflammation (21), which could be beneficial for fertility (22). Likewise, in 2 randomized trials of women undergoing fertility treatment, oral phytoestrogen supplementation resulted in higher rates of implantation and clinical pregnancy compared with standard treatment (23, 24). In a prospective cohort study of women undergoing assisted reproductive technology, higher intake of soy isoflavones showed a positive dose–response relation with live birth rates (25). The only study to assess the association between phytoestrogens and fecundability, the per-cycle probability of conception, found that higher preconception urinary concentrations of lignans, but not isoflavones, was associated with shorter time to pregnancy (26).
In the present study, we examine the prospective association between self-reported dietary intake of isoflavones, lignans, and coumestans and fecundability in 2 cohorts of pregnancy planners, one from North America and the other from Denmark.
Subjects and Methods
Study populations
Pregnancy Study Online (PRESTO) and Snart Foraeldre (SF) are ongoing internet-based preconception cohort studies of pregnancy planners from the United States and Canada (PRESTO) and Denmark (SF). Details of the study methods have been described elsewhere (27, 28). Briefly, eligible women were 21–45 years old (PRESTO) or 18–45 years old (SF), in a stable relationship with a male partner, and attempting to conceive without fertility treatment at enrollment. Participation involves completion of a baseline questionnaire on demographic, lifestyle, behavioral, medical and reproductive factors, as well as brief follow-up questionnaires every 8 weeks for up to 12 months or until reported conception, whichever occurs first. Ten days after baseline, women are invited to complete a validated study-specific food-frequency questionnaire (FFQ): the National Cancer Institute (NCI) Diet History Questionnaire II (29) in PRESTO and an FFQ designed specifically for the cohort in SF (30).
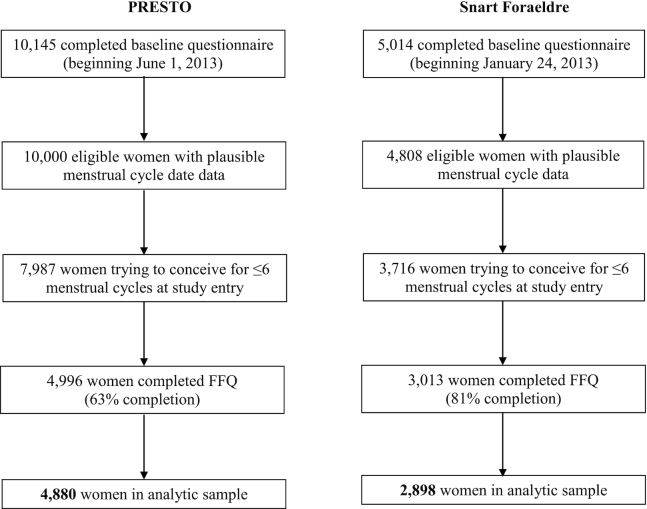
Enrollment began in June 2013 for PRESTO and June 2011 for SF (although the SF FFQ was not implemented until February 2013). Women enrolled in the study through February 2019 are included in the present analysis. We excluded women who had implausible baseline last menstrual period (LMP) dates, who had been attempting conception for at least 6 cycles at study entry and who did not complete the FFQ. We additionally excluded women with implausible energy intake (<600 or >3800 kcal/d) and those who skipped more than 12 items on the FFQ (Figure 1). The final analytic sample included 4880 PRESTO women and 2898 SF women.
FIGURE 1.
Flowchart of analytic sample, PRESTO and Snart Foraeldre cohorts.
The Institutional Review Board at Boston University Medical Center and the Danish Data Protection Agency approved this study. This study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Boston Medical Center Institutional Review Board and the Danish Data Protection Agency. Informed consent was obtained online from all subjects.
Assessment of phytoestrogen intake
On the PRESTO FFQ, we ascertained information on 277 individual food items. Questions were asked in reference to average intake during the last year. We used the nutrient composition of the individual food items to calculate phytoestrogen intake. Specifically, we used the NCI Diet*Calc software version 1.5.0 to calculate intake of isoflavones (daidzein, genistein, glycitein, formononetin, and biochanin-A) and coumestans (coumestrol) (31). Because the NCI nutrient composition database did not include lignans, we created an ad hoc database using published values on lignan content of foods in Western diets (32) to calculate intake of matairesinol, lariciresinol, pinoresinol, and secoisolariciresinol. We did not have information on lignan content for 11 of the 277 food items (rice milk, milkshakes/sodas, whole-milk yogurt, low-fat yogurt, creamed soups, avocado/guacamole, mango, other fruits, other juice, calcium-fortified other juice, and tomato/vegetable juice). We adjusted phytoestrogen intake for total energy intake using the nutrient residual method (33).
We created a similar ad hoc phytoestrogen database for the SF data, using the PRESTO database and a phytoestrogen database of the National Food Composition Database Fineli® as the reference (34). For each of the 293 individual food items ascertained on the Danish FFQ, we assigned individual phytoestrogen values. For foods that were represented in the PRESTO database, we assigned the phytoestrogen content values from the PRESTO database. When needed, we applied a correction factor to adjust the values from prepared foods in PRESTO to raw foods of the Danish food list. The phytoestrogen content of Danish breads and muesli was calculated based on Danish recipes and the phytoestrogen content of flour, seeds, etc. was obtained from the Finnish database. For the remaining foods that were not in the PRESTO database, we allocated values from the Finnish database. A few foods were not present in either database; for these foods, we assigned values from a similar food.
Assessment of fecundability
On the follow-up questionnaires in both cohorts, we collected information on pregnancy status and intervening pregnancy losses. We collected information on menstrual cycle regularity, typical cycle length, and LMP dates at baseline and over follow-up. For women with irregular cycles, we estimated typical cycle length using LMP dates over follow-up and information on the number of menstrual cycles per year. We calculated study time, in discrete cycles, using the following formula: (cycles trying to conceive at study entry) + [(LMP date from most recent follow-up questionnaire − date of baseline questionnaire)/cycle length] + 1.
Assessment of covariates
We collected covariate data from the baseline questionnaires, follow-up questionnaires, and FFQs. Covariates of interest included demographics [age, race/ethnicity (PRESTO only), education, income, and employment), lifestyle and behavioral factors (height, weight, physical activity, and cigarette smoking history), dietary factors [alcohol, caffeine, and sugar-sweetened soda intake; total energy intake; multivitamin and folic acid use; diet quality [measured by the 2010 Healthy Eating Index (HEI) score in PRESTO (35, 36) and the Nutrient Rich Diet (NRD) 15.3 score in SF (37); and intake of dietary fiber, saturated fat, fruits, and vegetables, calculated from the FFQ], and reproductive factors [intercourse frequency, doing something to improve chances of conception (e.g., timing intercourse, ovulation testing, monitoring cervical mucus quality), parity, and last method of contraception]. We did not ascertain racial/ethnic information in SF because the population is overwhelmingly non-Hispanic white.
Data analysis
Because phytoestrogen intake was calculated differently in the 2 cohorts, we conducted analyses in parallel. We used life-table methods to calculate the proportion of women who conceived during follow-up. We measured Spearman correlation coefficients between the individual log-transformed phytoestrogen intakes. We used the nutrient residual method to standardize individual phytoestrogen intake to the mean energy intake in each cohort (33).
Women contributed menstrual cycles at risk to the analysis from cohort entry until pregnancy, initiation of fertility treatment, cessation of pregnancy attempt, loss to follow-up, or 12 cycles, whichever came first. We examined the association between phytoestrogen intake and fecundability using a proportional probabilities regression model (38). This model estimates fecundability ratios (FR; the per-cycle probability of conception in exposed compared with unexposed women) and 95% CIs. The model includes binary indicators of cycle number at risk to account for the decline in baseline fecundability over time. We used the Andersen–Gill data structure to account for left truncation by allowing for delayed entry into the risk set (i.e., when women enter the study after already attempting conception for several menstrual cycles) (39, 40). We analyzed each phytoestrogen variable individually in relation to fecundability; we also created summary variables for total isoflavones and lignans and analyzed these groups in relation to fecundability. We categorized individual and grouped phytoestrogens, creating cut points at the 25th, 50th, 75th, and 90th percentiles. We also used restricted cubic splines to examine nonlinear associations (41, 42).
We selected potential confounders based on a literature review and consideration of the strength and direction of causal relations between variables. Final models were adjusted for total energy intake (continuous), age (<25, 25–29, 30–34, ≥35 y), race/ethnicity [non-Hispanic white, non-Hispanic black, Asian, Hispanic, or other/mixed race (PRESTO only)], household income (<$50,000, $50,000–$99,999, $100,000–$149,999, or ≥$150,000 USD/y in PRESTO; <25,000, 25,000–39,999, 40,000–64,999, ≥65,000 DKK/mo in SF), education (≤12, 12–15, 16, and ≥17 y), BMI (kg/m2) (<25, 25–29, 30–34, ≥35 kg/m2), cigarette smoking history (never, former, current occasional, current regular), sugar-sweetened soda intake (0, 1, 2–6, ≥7 drinks/wk), daily multivitamin or folic acid use (yes, no), intercourse frequency (<1, 1, 2–3, ≥4 times/wk), doing something to improve chances of conception (yes, no), last method of contraception (hormonal, withdrawal/rhythm, or barrier methods), and HEI score (PRESTO) or NRD score (SF). We also mutually adjusted finals models for other types of phytoestrogens (for example, final models for daidzein, an isoflavone, were adjusted for total lignan and coumestrol intakes).
We stratified final multivariable models by attempt time at study entry (<3 compared with 3–6 cycles) to assess the possibility of reverse causation (i.e., taking longer to conceive prompting dietary changes). We also stratified by female BMI (<25 compared with ≥25 kg/m2) and female age (<30 compared with ≥30 y) to assess whether associations were stronger among overweight or older women, subgroups of women at greater risk of infertility. We ran additional sensitivity analyses restricted to women with regular cycles and women with no history of polycystic ovary syndrome, as these women may change their diet while trying to conceive.
We used a Markov chain Monte Carlo multiple imputation method to impute missing data on outcome and covariates. We generated 5 imputation datasets and combined point estimates and SEs. Women who did not complete any follow-up questionnaires (n = 53 in PRESTO; n = 201 in SF) were assigned 1 cycle of observation and imputed their pregnancy status. Covariate missingness ranged from 0% (age) to 4% (income) in PRESTO and from 0% (age) to 10% (income) in SF. Among women who completed the FFQ, there were no missing phytoestrogen values. All statistical analyses were conducted using SAS version 9.4 (43).
Results
During follow-up, 73.7% of PRESTO women conceived (accounting for censoring). Eleven percent of the women initiated fertility treatment, 3.2% stopped trying to conceive, 6.6% were lost to follow-up, 10.3% were censored at 12 cycles, and 4.4% were still actively participating in the study. In SF, 82.8% of women conceived (accounting for censoring). Women who did not conceive either initiated fertility treatment (6.5%), stopped trying to conceive (3.4%), were lost to follow-up (14.0%), were censored at 12 cycles (9.4%), or were still actively participating in the study (0.8%).
The mean age of participants at baseline was 30.1 ± 4.0 years for PRESTO and 29.1 ± 3.8 years for SF. The racial/ethnic composition of PRESTO was 86.8% non-Hispanic white, 1.6% non-Hispanic black, 1.8% Asian, 5.5% Hispanic, and 4.4% mixed or other race/ethnicity. More than 40% of women in both cohorts had graduate education. Eighteen percent of women had an annual household income of at least $150,000 in PRESTO, compared with 32.2% in SF. Women in PRESTO were heavier (mean BMI = 27.2 kg/m2) than SF women (mean BMI = 24.1 kg/m2) and less physically active (35.3 metabolic equivalents of task hours/week in PRESTO compared with 63.8 in SF). Current cigarette smoking and alcohol use were similar in both cohorts.
Median isoflavone intake was higher in PRESTO than in SF (1060 µg/d and 482 µg/d, respectively). The majority of isoflavone intake in PRESTO was from soy-based meat substitute consumption, whereas intake of these isoflavones in SF was driven by soy milk and other foods (e.g., tea, coffee; Table 1). Sources of biochanin-A and formononetin were similar in both cohorts. Lignan intake was higher in SF (median intake = 430 µg/d) than in PRESTO (median intake = 124 µg/d). Cruciferous vegetables and nuts and seeds contributed to intake in both cohorts, but in SF, a large contribution to lignan intake came from seedy breads. Food contributions to and intakes of coumestrol were similar in both cohorts. Daidzein and genistein were highly correlated in both cohorts (Supplemental Table 1). Biochanin-A and formononetin were not strongly correlated with other phytoestrogens in either cohort. Coumestrol was associated with pinoresinol, but not other phytoestrogens. In PRESTO, individual lignans were moderately correlated with each other, whereas in SF, only lariciresinol and pinoresinol were correlated.
TABLE 1.
Top individual contributors to phytoestrogen intake among female pregnancy planners in the PRESTO (n = 4880) and SF (n = 2898) cohorts1
| Rank by intake2 | Food item | Intake, mg/d | Rank by concentration3 | Food item | Concentration, µg/g |
|---|---|---|---|---|---|
| PRESTO | |||||
| Daidzein | |||||
| 1 | Soy-based meat substitutes | 181 ± 615 | 1 | Soy milk | 133 |
| 2 | Coffee | 180 ± 219 | 2 | Soy-based meat substitutes | 65.0 |
| 3 | Meal replacement bars & liquids | 102 ± 225 | 3 | Meal replacement bars & liquids | 15.4 |
| Genistein | |||||
| 1 | Soy-based meat substitutes | 254 ± 872 | 1 | Soy milk | 179 |
| 2 | Meal replacement bars & liquids | 176 ± 379 | 2 | Soy-based meat substitutes | 92.0 |
| 3 | Soy milk | 91.1 ± 1130 | 3 | Meal replacement bars & liquids | 27.0 |
| Glycitein | |||||
| 1 | Soy-based meat substitutes | 43.5 ± 139 | 1 | Soy milk | 17.4 |
| 2 | Meal replacement bars & liquids | 33.0 ± 67.2 | 2 | Soy-based meat substitutes | 14.0 |
| 3 | Soy milk | 10.3 ± 122 | 3 | Meal replacement bars & liquids | 4.40 |
| Biochanin-A | |||||
| 1 | Peas | 55.0 ± 95.5 | 1 | Peas | 11.0 |
| 2 | Chicken mixtures | 9.44 ± 20.6 | 2 | Beef stews/pot pies | 1.16 |
| 3 | Beef stews/pot pies | 7.97 ± 17.0 | 3 | Chili | 0.91 |
| Formononetin | |||||
| 1 | Beans | 7.54 ± 7.27 | 1 | Bean soups | 0.33 |
| 2 | Bean soups | 6.40 ± 8.46 | 2 | Beans | 0.32 |
| 3 | Other vegetables | 1.01 ± 3.11 | 3 | Nuts & seeds | 0.06 |
| Matairesinol | |||||
| 1 | Sweet potatoes | 0.95 ± 1.74 | 1 | Sweet potatoes | 0.17 |
| 2 | Onions | 0.57 ± 0.56 | 2 | Onions | 0.09 |
| 3 | Coffee | 0.45 ± 0.49 | 3 | Oranges, tangelos | 0.02 |
| Lariciresinol | |||||
| 1 | Broccoli | 9.39 ± 11.1 | 1 | Winter squash | 0.91 |
| 2 | Nuts & seeds | 4.30 ± 6.51 | 2 | Broccoli | 0.82 |
| 3 | Coffee | 3.92 ± 2.80 | 3 | Dried apricot | 0.62 |
| Pinoresinol | |||||
| 1 | Tea | 10.411.6 | 1 | Dried apricot | 1.90 |
| 2 | Peaches, nectarines, plums | 1.91 ± 4.65 | 2 | Coleslaw | 0.44 |
| 3 | Strawberries | 1.65 ± 2.65 | 3 | Cabbage/sauerkraut | 0.44 |
| Secoisolariciresinol | |||||
| 1 | Coffee | 19.0 ± 23.1 | 1 | Dried apricot | 1.48 |
| 2 | Tea | 9.85 ± 11.0 | 2 | Nuts & seeds | 0.47 |
| 3 | Nuts & seeds | 5.77 ± 8.74 | 3 | Green beans | 0.31 |
| Coumestrol | |||||
| 1 | Tea | 52.6 ± 99.8 | 1 | Egg rolls | 1.46 |
| 2 | Citrus juice | 13.1 ± 27.4 | 2 | Pasta | 0.67 |
| 3 | Pasta | 5.72 ± 11.1 | 3 | Grapefruit | 0.50 |
| SF | |||||
| Daidzein | |||||
| 1 | Soy milk | 519 ± 2420 | 1 | Soy milk | 60.4 |
| 2 | Coffee | 117 ± 134 | 2 | Beans | 9.55 |
| 3 | Beans | 30.8 ± 45.6 | 3 | Fruit juice | 5.80 |
| Genistein | |||||
| 1 | Soy milk | 729 ± 3410 | 1 | Soy milk | 84.9 |
| 2 | Tea | 48.8 ± 78.3 | 2 | Beans | 10.2 |
| 3 | Beans | 32.8 ± 48.5 | 3 | Low-fat cheese | 2.86 |
| Glycitein | |||||
| 1 | Beans | 8.34 ± 12.34 | 1 | Beans | 2.58 |
| 2 | Cheese | 0.51 ± 0.96 | 2 | Low-fat cheese | 0.32 |
| 3 | Ice cream | 0.04 ± 0.03 | 3 | Ice cream | 0.01 |
| Biochanin-A | |||||
| 1 | Peas | 95.6 ± 74.4 | 1 | Peas | 13.3 |
| 2 | Beans | 3.37 ± 4.99 | 2 | Beans | 1.05 |
| 3 | Nuts & seeds | 0.09 ± 0.09 | 3 | Nuts & seeds | 0.05 |
| Formononetin | |||||
| 1 | Beans | 1.08 ± 1.60 | 1 | Muesli | 1.35 |
| 2 | Other vegetables | 0.43 ± 0.42 | 2 | Bean sprouts | 0.43 |
| 3 | Rye bread | 0.37 ± 0.29 | 3 | Beans | 0.33 |
| Matairesinol | |||||
| 1 | Rye bread | 25.1 ± 19.1 | 1 | Flat bread | 0.61 |
| 2 | Onions | 1.01 ± 0.63 | 2 | Crisp bread | 0.57 |
| 3 | Wheat bread | 0.92 ± 0.80 | 3 | Rye bread | 0.46 |
| Lariciresinol | |||||
| 1 | Cabbage | 12.5 ± 10.2 | 1 | Squash | 0.91 |
| 2 | Squash | 5.13 ± 5.74 | 2 | Broccoli | 0.82 |
| 3 | Broccoli | 3.16 ± 3.44 | 3 | Dried apricot | 0.62 |
| Pinoresinol | |||||
| 1 | Cabbage | 17.1 ± 14.0 | 1 | Dried apricot | 1.90 |
| 2 | Tea | 6.00 ± 9.62 | 2 | Cabbage | 0.44 |
| 3 | Peach/nectarine | 2.51 ± 3.06 | 3 | Peach/nectarine | 0.37 |
| Secoisolariciresinol | |||||
| 1 | Wheat bread | 212 ± 166 | 1 | Wheat bread | 66.4 |
| 2 | Muesli | 91 ± 145 | 2 | Flat bread | 65.8 |
| 3 | Rye bread | 65.6 ± 53.8 | 3 | Farmer's bread | 65.7 |
| Coumestrol | |||||
| 1 | Tea | 43.0 ± 69.1 | 1 | Grapefruit | 0.50 |
| 2 | Fruit juice | 10.1 ± 13.1 | 2 | Orange juice | 0.44 |
| 3 | Other vegetables | 1.38 ± 1.37 | 3 | Bean sprouts | 0.36 |
PRESTO, Pregnancy Study Online; SF, Snart Foraeldre.
Phytoestrogen intake (mean ± SD mg/d) among women in the PRESTO and SF cohorts.
Phytoestrogen concentration (µg/g) of individual foods in the Snart Foraeldre and PRESTO phytoestrogen databases.
In PRESTO, women with high isoflavone and lignan intake were older, of higher socioeconomic status, more likely to be non-Hispanic white or Asian, and less likely to be non-Hispanic black or Hispanic (Table 2). They also practiced more healthful behaviors, including having lower BMI, higher levels of physical activity, being less likely to smoke cigarettes, drank fewer sugar-sweetened sodas, and were more likely to take daily multivitamins or folic acid. They drank more alcohol and caffeinated beverages, but these contributed to phytoestrogen intake. Isoflavone and lignan intakes were also positively associated with more healthful diets, including overall diet quality, lower saturated fat intake, and higher dietary fiber intake. Coumestrol intake was not strongly associated with demographic, behavioral, dietary, or reproductive variables (Supplemental Table 2). In SF, patterns were generally similar, but weaker, with some exceptions (e.g., physical activity was inversely related to phytoestrogen intake; Table 2).
TABLE 2.
Baseline characteristics of female pregnancy planners by dietary phytoestrogen intake in the PRESTO and SF cohorts1
| PRESTO (n = 4880) | SF (n = 2898) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoflavone intake,2 µg/d | Lignan intake,3 µg/d | Isoflavone intake,2 µg/d | Lignan intake,3 µg/d | |||||||||
| Characteristic | <705 | 1062–1786 | ≥3085 | <88 | 124–167 | ≥210 | <302 | 484–790 | ≥2101 | <338 | 431–548 | ≥687 |
| Number of women | 1220 | 1221 | 488 | 1229 | 1242 | 488 | 727 | 729 | 284 | 736 | 716 | 289 |
| Age at baseline, y | 29.5 ± 4.1 | 30.2 ± 3.9 | 30.7 ± 3.8 | 28.9 ± 3.9 | 30.5 ± 3.8 | 31.0 ± 3.9 | 28.1 ± 4.0 | 29.7 ± 3.6 | 29.4 ± 3.6 | 28.6 ± 3.9 | 29.2 ± 3.7 | 29.5 ± 4.2 |
| White, non-Hispanic, % | 83.6 | 88.7 | 86.4 | 84.6 | 87.9 | 86.7 | — | — | — | — | — | — |
| Black, non-Hispanic, % | 3.5 | 0.9 | 0.2 | 2.2 | 1.1 | 1.2 | — | — | — | — | — | — |
| Hispanic, % | 7.0 | 5.0 | 5.9 | 6.8 | 5.0 | 5.4 | — | — | — | — | — | — |
| Asian, % | 0.5 | 1.9 | 3.0 | 1.2 | 1.9 | 3.1 | — | — | — | — | — | — |
| Household income <$50,000/y, % | 23.3 | 15.5 | 11.7 | 24.1 | 13.1 | 15.4 | 11.3 | 12.8 | 10.1 | 12.2 | 10.7 | 14.3 |
| Less than college degree, % | 29.8 | 20.4 | 15.7 | 30.8 | 19.1 | 16.4 | 29.8 | 12.8 | 19.3 | 25.7 | 16.6 | 15.7 |
| BMI, kg/m2 | 28.7 ± 8.0 | 26.7 ± 7.0 | 26.0 ± 6.0 | 29.2 ± 8.0 | 26.6 ± 7.1 | 25.7 ± 6.0 | 25.4 ± 5.9 | 23.6 ± 4.1 | 23.4 ± 4.6 | 24.8 ± 5.6 | 23.9 ± 4.5 | 23.5 ± 5.0 |
| Physical activity, MET-h/wk | 30.5 ± 25.2 | 36.4 ± 23.8 | 39.6 ± 28.2 | 27.4 ± 22.6 | 37.7 ± 25.1 | 45.2 ± 31.0 | 67.5 ± 75.5 | 64.4 ± 72.9 | 60.5 ± 62.3 | 67.2 ± 81.3 | 63.1 ± 71.7 | 59.2 ± 58.6 |
| Current regular cigarette smoker, % | 7.2 | 3.8 | 2.5 | 5.1 | 4.1 | 3.6 | 4.9 | 8.6 | 2.7 | 6.8 | 5.5 | 2.8 |
| Total energy intake, kcal/d | 1560 ± 565 | 1590 ± 539 | 1540 ± 486 | 1550 ± 520 | 1610 ± 533 | 1510 ± 503 | 1870 ± 528 | 1830 ± 477 | 1860 ± 502 | 1890 ± 555 | 1850 ± 497 | 1810 ± 514 |
| Alcohol, drinks/wk | 2.7 ± 4.1 | 3.3 ± 3.7 | 3.5 ± 4.2 | 2.3 ± 4.0 | 3.6 ± 5.3 | 3.8 ± 4.3 | 1.9 ± 2.8 | 2.6 ± 2.7 | 2.3 ± 2.5 | 2.2 ± 2.6 | 2.5 ± 2.7 | 2.2 ± 2.7 |
| Caffeine, mg/d | 94 ± 149 | 145 ± 127 | 132 ± 123 | 96 ± 158 | 136 ± 114 | 172 ± 167 | 56.6 ± 74 | 225 ± 181 | 164 ± 157 | 133 ± 161 | 173 ± 171 | 165 ± 155 |
| Sugar-sweetened soda intake, drinks/wk | 2.1 ± 4.1 | 0.7 ± 1.8 | 0.3 ± 0.9 | 2.5 ± 5.1 | 0.7 ± 2.1 | 0.41.1 | 1.4 ± 2.3 | 0.6 ± 1.4 | 0.6 ± 1.1 | 1.3 ± 2.2 | 0.7 ± 1.5 | 0.6 ± 1.6 |
| Daily multivitamin/folic acid use, % | 79.8 | 83.9 | 87.1 | 80.0 | 87.7 | 86.6 | 69.5 | 71.5 | 74.5 | 68.8 | 72.3 | 71.3 |
| NRD score | — | — | — | — | — | — | 1000 ± 93 | 1050 ± 67 | 1050 ± 58 | 1000 ± 97 | 1050 ± 68 | 1030 ± 66 |
| HEI 2010 score | 61.1 ± 11.9 | 67.2 ± 9.7 | 72.3 ± 8.5 | 57.2 ± 9.9 | 69.5 ± 9.6 | 71.7 ± 9.7 | — | — | — | — | — | — |
| Dietary fiber intake, mg/d | 13.4 ± 4.6 | 15.2 ± 4.1 | 18.8 ± 5.9 | 11.9 ± 3.2 | 16.0 ± 4.3 | 19.1 ± 6.5 | 21.0 ± 4.9 | 23.5 ± 4.9 | 25.2 ± 6.1 | 19.8 ± 4.6 | 24.0 ± 5.0 | 24.5 ± 5.6 |
| Saturated fat intake, g/d | 21.7 ± 4.9 | 20.9 ± 3.9 | 18.1 ± 4.0 | 22.0 ± 4.4 | 20.4 ± 4.0 | 18.8v4.7 | 30.5 ± 5.4 | 30.0 ± 5.3 | 28.9 ± 5.6 | 31.7 ± 6.3 | 29.5 ± 4.7 | 29.3 ± 5.3 |
| Fruit intake, g/d | 185 ± 220 | 157 ± 118 | 182 ± 152 | 122 ± 158 | 186 ± 147 | 215 ± 185 | 115 ± 71 | 130 ± 71 | 149 ± 80 | 111 ± 69 | 138 ± 78 | 131 ± 75 |
| Vegetable intake, g/d | 150 ± 106 | 169 ± 97 | 194 ± 110 | 109 ± 63 | 183 ± 92 | 235 ± 132 | 225 ± 114 | 322 ± 159 | 448 ± 200 | 254 ± 151 | 331 ± 161 | 318 ± 161 |
| Parous, % | 35.4 | 30.1 | 21.8 | 39.0 | 28.2 | 24.9 | 41.9 | 30.4 | 28.2 | 33.8 | 34.7 | 35.2 |
| Intercourse frequency <1/wk, % | 19.8 | 21.4 | 22.9 | 21.5 | 20.3 | 19.9 | 22.3 | 15.8 | 21.5 | 21.8 | 15.5 | 16.5 |
| Doing something to improve chances, % | 74.0 | 76.2 | 79.0 | 76.4 | 77.1 | 76.5 | 73.0 | 74.9 | 76.4 | 71.2 | 74.3 | 72.8 |
| Hormonal last method of contraception, % | 39.5 | 39.0 | 34.6 | 42.3 | 39.1 | 32.4 | 65.0 | 53.5 | 49.5 | 62.7 | 56.9 | 52.7 |
Values are means ± SDs for continuous variables and percentages for binary variables. With the exception of age, all variables are age standardized to the cohort at baseline. Phytoestrogen intake is adjusted for total energy intake using the nutrient residual method. Categories presented are <25th percentile, 50th–75th percentile, and ≥90th percentile (lowest, middle, and highest of 5 categories). FR, fecundability ratio; HEI, Healthy Eating Index; NRD, nutrient rich density; PRESTO, Pregnancy Study Online; SF, Snart Foraeldre.
Sum of daidzein, genistein, glycitein, biochanin-A, and formononetin.
Sum of matairesinol, lariciresinol, pinoresinol, and secoisolariciresinol.
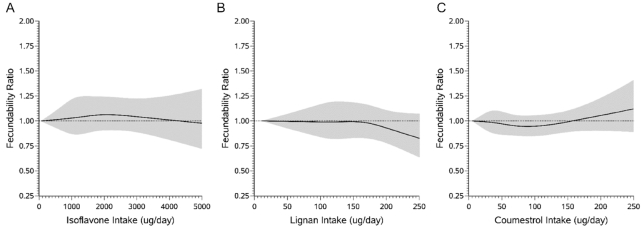
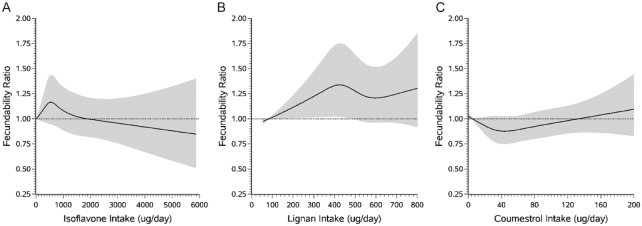
Total isoflavone intake was not strongly associated with fecundability in either cohort (Table 3; Figures 2 and 3). In SF, formononetin was associated with reduced fecundability. We did not observe a similar inverse association in PRESTO, but the range of formononetin exposure was substantially narrower.
TABLE 3.
Female dietary phytoestrogen intake and fecundability among pregnancy planners in the PRESTO and SF cohorts1
| PRESTO (n = 4880) | SF (n = 2898) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure,2 µg/d | Cycles, n | Pregnancies, n | Crude FR3 (95% CI) | Adjusted FR4 (95% CI) | Exposure,2 µg/d | Cycles, n | Pregnancies, n | Crude FR3 (95% CI) | Adjusted FR4 (95% CI) |
| Total isoflavones | |||||||||
| <705 | 5290 | 695 | Ref | Ref | <302 | 2821 | 442 | Ref | Ref |
| 705–1061 | 4993 | 773 | 1.11 (1.01–1.22) | 1.03 (0.93–1.13) | 302–483 | 2651 | 521 | 1.21 (1.08–1.36) | 1.16 (1.03–1.31) |
| 1062–1786 | 4841 | 807 | 1.19 (1.08–1.31) | 1.09 (0.99–1.20) | 484–790 | 2507 | 494 | 1.22 (1.09–1.37) | 1.13 (1.00–1.28) |
| 1787–3084 | 2890 | 484 | 1.17 (1.06–1.30) | 1.06 (0.95–1.19) | 791–2100 | 1605 | 281 | 1.10 (0.96–1.26) | 1.03 (0.89–1.19) |
| ≥3085 | 1885 | 318 | 1.19 (1.06–1.35) | 1.06 (0.93–1.20) | ≥2101 | 1089 | 174 | 1.04 (0.88–1.21) | 0.97 (0.82–1.14) |
| Daidzein | |||||||||
| <272 | 5200 | 723 | Ref | Ref | <101 | 2756 | 466 | Ref | Ref |
| 272–462 | 5014 | 749 | 1.04 (0.95–1.14) | 0.96 (0.88–1.06) | 101–234 | 2638 | 482 | 1.07 (0.96–1.20) | 1.02 (0.91–1.15) |
| 463–781 | 4937 | 804 | 1.11 (1.02–1.22) | 1.03 (0.94–1.14) | 235–433 | 2629 | 506 | 1.11 (0.99–1.24) | 1.04 (0.92–1.17) |
| 782–1265 | 2881 | 482 | 1.13 (1.01–1.25) | 1.04 (0.93–1.16) | 434–914 | 1569 | 279 | 1.04 (0.91–1.19) | 0.98 (0.85–1.13) |
| ≥1265 | 1867 | 319 | 1.15 (1.02–1.29) | 1.03 (0.90–1.17) | ≥915 | 1081 | 179 | 0.99 (0.85–1.16) | 0.93 (0.79–1.09) |
| Genistein | |||||||||
| <270 | 5167 | 706 | Ref | Ref | <51 | 2609 | 462 | Ref | Ref |
| 270–436 | 5022 | 766 | 1.07 (0.97–1.17) | 1.00 (0.91–1.10) | 51–100 | 2755 | 484 | 1.00 (0.89–1.12) | 0.96 (0.85–1.08) |
| 437–841 | 4918 | 797 | 1.13 (1.03–1.23) | 1.02 (0.93–1.12) | 101–246 | 2623 | 502 | 1.08 (0.96–1.20) | 0.99 (0.86–1.13) |
| 842–1582 | 2929 | 488 | 1.14 (1.02–1.26) | 1.01 (0.90–1.12) | 247–1050 | 1597 | 291 | 1.04 (0.91–1.18) | 0.96 (0.82–1.12) |
| ≥1582 | 1863 | 320 | 1.18 (1.05–1.33) | 1.04 (0.91–1.18) | ≥1051 | 1089 | 173 | 0.93 (0.79–1.08) | 0.86 (0.72–1.02) |
| Glycitein | |||||||||
| <27 | 5107 | 724 | Ref | Ref | <4 | 2799 | 493 | Ref | Ref |
| 27–49 | 5047 | 732 | 0.99 (0.90–1.09) | 0.93 (0.84–1.02) | 4–7 | 2644 | 488 | 1.05 (0.94–1.17) | 0.99 (0.88–1.11) |
| 50–111 | 5019 | 816 | 1.09 (1.00–1.19) | 0.97 (0.88–1.07) | 8–13 | 2700 | 486 | 1.03 (0.92–1.15) | 0.92 (0.82–1.04) |
| 112–226 | 2865 | 486 | 1.13 (1.02–1.25) | 1.00 (0.89–1.11) | 14–22 | 1475 | 271 | 1.04 (0.91–1.18) | 0.93 (0.80–1.07) |
| ≥227 | 1861 | 319 | 1.13 (1.00–1.27) | 0.99 (0.88–1.12) | ≥23 | 1055 | 174 | 0.94 (0.81–1.10) | 0.85 (0.72–1.00) |
| Biochanin-A | |||||||||
| <26 | 4856 | 718 | Ref | Ref | <61 | 2711 | 492 | Ref | Ref |
| 26–53 | 5095 | 792 | 1.03 (0.94–1.13) | 0.98 (0.89–1.08) | 61–87 | 2738 | 462 | 0.95 (0.85–1.06) | 0.92 (0.82–1.03) |
| 54–97 | 5010 | 797 | 1.05 (0.95–1.15) | 0.99 (0.90–1.09) | 88–133 | 2594 | 490 | 1.04 (0.93–1.16) | 1.01 (0.90–1.13) |
| 98–172 | 2888 | 474 | 1.08 (0.98–1.20) | 1.07 (0.96–1.19) | 134–206 | 1572 | 287 | 1.00 (0.87–1.13) | 0.95 (0.83–1.08) |
| ≥173 | 2050 | 296 | 0.97 (0.86–1.10) | 0.95 (0.84–1.07) | ≥207 | 1058 | 181 | 0.95 (0.81–1.10) | 0.87 (0.75–1.02) |
| Formononetin | |||||||||
| <9.8 | 4654 | 694 | Ref | Ref | <2.4 | 2600 | 479 | Ref | Ref |
| 9.8–10.0 | 4794 | 734 | 1.01 (0.90–1.13) | 0.94 (0.84–1.05) | 2.4–4.5 | 2570 | 475 | 1.01 (0.90–1.13) | 0.97 (0.86–1.09) |
| 10.0–10.4 | 5271 | 821 | 1.01 (0.89–1.16) | 0.96 (0.84–1.09) | 4.6–10.3 | 2707 | 478 | 0.97 (0.86–1.08) | 0.91 (0.81–1.02) |
| 10.5–11.0 | 3037 | 476 | 1.04 (0.87–1.23) | 0.99 (0.84–1.18) | 10.4–25.7 | 1712 | 293 | 0.94 (0.83–1.08) | 0.87 (0.76–1.00) |
| ≥11.1 | 2143 | 352 | 1.09 (0.96–1.24) | 0.99 (0.87–1.13) | ≥25.8 | 1084 | 187 | 0.95 (0.82–1.11) | 0.87 (0.74–1.02) |
| Total lignans | |||||||||
| <88 | 5157 | 710 | Ref | Ref | <338 | 2786 | 458 | Ref | Ref |
| 88–123 | 4929 | 769 | 1.10 (1.00–1.21) | 0.99 (0.89–1.09) | 338–430 | 2601 | 492 | 1.16 (1.04–1.30) | 1.13 (1.00–1.27) |
| 124–167 | 4966 | 812 | 1.14 (1.04–1.25) | 0.95 (0.86–1.06) | 431–548 | 2644 | 472 | 1.09 (0.97–1.23) | 1.04 (0.92–1.17) |
| 168–209 | 2796 | 477 | 1.19 (1.07–1.32) | 0.97 (0.85–1.09) | 549–686 | 1573 | 299 | 1.15 (1.01–1.31) | 1.10 (0.96–1.26) |
| ≥210 | 2051 | 309 | 1.08 (0.96–1.22) | 0.83 (0.72–0.97) | ≥687 | 1069 | 191 | 1.06 (0.91–1.23) | 1.03 (0.88–1.21) |
| Matairesinol | |||||||||
| <2.1 | 5441 | 718 | Ref | Ref | <17 | 2697 | 473 | Ref | Ref |
| 2.1–3.0 | 4779 | 760 | 1.17 (1.06–1.28) | 1.09 (0.99–1.20) | 17–25 | 2626 | 485 | 1.08 (0.97–1.21) | 1.07 (0.95–1.20) |
| 3.1–4.2 | 4795 | 774 | 1.17 (1.07–1.29) | 1.04 (0.94–1.15) | 26–40 | 2667 | 462 | 1.03 (0.92–1.16) | 1.01 (0.90–1.14) |
| 4.3–5.8 | 2961 | 499 | 1.23 (1.11–1.37) | 1.06 (0.94–1.19) | 41–56 | 1591 | 283 | 1.03 (0.90–1.18) | 0.99 (0.87–1.14) |
| ≥5.9 | 1923 | 326 | 1.22 (1.09–1.38) | 1.04 (0.91–1.18) | ≥57 | 1092 | 209 | 1.11 (0.96–1.28) | 1.09 (0.94–1.26) |
| Lariciresinol | |||||||||
| <28.4 | 5206 | 679 | Ref | Ref | <23 | 2812 | 435 | Ref | Ref |
| 29.4–40.4 | 5097 | 760 | 1.10 (1.00–1.21) | 1.00 (0.90–1.10) | 23–34 | 2786 | 512 | 1.15 (1.03–1.30) | 1.10 (0.97–1.25) |
| 40.5–55.4 | 4661 | 840 | 1.29 (1.17–1.41) | 1.09 (0.98–1.22) | 35–48 | 2586 | 480 | 1.17 (1.04–1.32) | 1.10 (0.95–1.26) |
| 55.5–71.0 | 2931 | 486 | 1.21 (1.08–1.34) | 1.03 (0.91–1.17) | 49–63 | 1349 | 283 | 1.29 (1.13–1.47) | 1.19 (1.02–1.39) |
| ≥71.1 | 2004 | 312 | 1.16 (1.02–1.31) | 0.95 (0.82–1.09) | ≥64 | 1140 | 202 | 1.13 (0.97–1.31) | 1.04 (0.87–1.24) |
| Pinoresinol | |||||||||
| <10.3 | 5053 | 720 | Ref | Ref | <18 | 2774 | 432 | Ref | Ref |
| 10.3–17.0 | 4910 | 773 | 1.07 (0.98–1.17) | 0.98 (0.89–1.08) | 18–28 | 2857 | 509 | 1.11 (0.99–1.25) | 1.06 (0.94–1.20) |
| 17.1–27.6 | 4957 | 805 | 1.11 (1.01–1.22) | 0.99 (0.89–1.09) | 29–41 | 2419 | 470 | 1.20 (1.07–1.35) | 1.14 (1.00–1.29) |
| 27.7–45.9 | 2935 | 471 | 1.11 (1.00–1.23) | 0.97 (0.85–1.10) | 42–59 | 1563 | 292 | 1.18 (1.03–1.34) | 1.06 (0.91–1.24) |
| ≥46.0 | 2044 | 308 | 1.05 (0.93–1.18) | 0.85 (0.72–1.00) | ≥60 | 1060 | 209 | 1.22 (1.06–1.42) | 1.14 (0.95–1.37) |
| Secoisolariciresinol | |||||||||
| <37.6 | 5084 | 708 | Ref | Ref | <244 | 2671 | 456 | Ref | Ref |
| 37.7–56.8 | 4906 | 796 | 1.13 (1.03–1.24) | 1.01 (0.91–1.11) | 245–322 | 2641 | 488 | 1.09 (0.97–1.22) | 1.09 (0.97–1.22) |
| 56.9–81.0 | 5057 | 774 | 1.07 (0.98–1.18) | 0.90 (0.81–1.00) | 323–440 | 2724 | 484 | 1.05 (0.94–1.18) | 1.01 (0.90–1.14) |
| 81.1–104.6 | 2799 | 490 | 1.19 (1.07–1.32) | 0.99 (0.88–1.12) | 441–574 | 1554 | 291 | 1.09 (0.96–1.25) | 1.07 (0.94–1.23) |
| ≥104.7 | 2053 | 309 | 1.05 (0.93–1.19) | 0.84 (0.73–0.96) | ≥575 | 1083 | 193 | 1.03 (0.89–1.20) | 1.03 (0.88–1.20) |
| Coumestrol | |||||||||
| <16 | 4819 | 774 | Ref | Ref | <17 | 2426 | 447 | Ref | Ref |
| 16–32 | 4989 | 775 | 0.96 (0.88–1.05) | 0.94 (0.86–1.03) | 17–31 | 2834 | 492 | 0.95 (0.84–1.06) | 0.91 (0.81–1.03) |
| 33–72 | 5064 | 752 | 0.95 (0.86–1.04) | 0.94 (0.86–1.04) | 32–66 | 2854 | 482 | 0.94 (0.84–1.05) | 0.92 (0.81–1.03) |
| 73–156 | 3054 | 451 | 0.94 (0.85–1.04) | 0.94 (0.85–1.05) | 67–137 | 1500 | 289 | 1.02 (0.89–1.16) | 0.99 (0.86–1.13) |
| ≥157 | 1973 | 325 | 1.03 (0.91–1.16) | 1.08 (0.95–1.22) | ≥138 | 1059 | 202 | 1.02 (0.88–1.19) | 1.00 (0.85–1.17) |
FR, fecundability ratio; HEI, Healthy Eating Index; NRD, nutrient rich density; PRESTO, Pregnancy Study Online; Ref, reference; SF, Snart Foraeldre.
Exposure categories are delineated by the <25th, 25–50th, 50–75th, 75–90th, and ≥90th percentiles.
Adjusted for total energy intake.
Adjusted for total energy intake, age, race/ethnicity (PRESTO), income, education, BMI, smoking, sugar-sweetened soda intake, multivitamin/folic acid use, intercourse frequency, doing something to improve chances of conception, HEI score (PRESTO), and NRD score (SF). Models are also mutually adjusted for other classes of phytoestrogens (i.e., daidzein models are adjusted for total lignans and coumestrol).
FIGURE 2.
Association between female dietary phytoestrogen intake and fecundability, fit using restricted cubic splines, among 4880 pregnancy planners in the PRESTO cohort. The solid line represents the FR and the shaded area represents the 95% CI. The reference level for the FR is the lowest level of intake in the cohort. The curves are adjusted for total energy intake, age, race/ethnicity, income, education, BMI, smoking, sugar-sweetened soda intake, multivitamin/folic acid use, intercourse frequency, doing something to improve chances of conception, and HEI score; models are also mutually adjusted for other classes of phytoestrogens. The splines are trimmed at the 95th percentile and have 4 knot points each at the 25th, 50th, 75th, and 90th percentiles. FR, fecundability ratio; HEI, Healthy Eating Index; PRESTO, Pregnancy Study Online.
FIGURE 3.
Association between female dietary phytoestrogen intake and fecundability, fit using restricted cubic splines, among 2898 pregnancy planners in the Snart Foraeldre cohort. The solid line represents the FR and the shaded area represents the 95% confidence interval. The reference level for the FR is the lowest level of intake in the cohort. The curves are adjusted for total energy intake, age, income, education, BMI, smoking, sugar-sweetened soda intake, multivitamin/folic acid use, intercourse frequency, doing something to improve chances of conception, and Nutrient Rich Density score; models are also mutually-adjusted for other classes of phytoestrogens. The splines are trimmed at the 95th percentile and have four knot points each at the 25th, 50th, 75th, and 90th percentiles. FR, fecundability ratio.
In SF, we observed some evidence of an association between total lignan intake and improved fecundability (Table 3, Figure 3); fecundability increased with increasing lignan intake from 50 to 400 µg/d, with little additional increase in fecundability at intakes of >400 µg/d (Figure 3). In PRESTO, we observed the opposite associations, with high lignan intake of lignan being associated with reduced fecundability. Coumestrol was not appreciably associated with fecundability in either cohort (Table 3; Figures 2 and 3).
Results were similar across strata of attempt time at study entry (Supplemental Table 3). The association between lignan intake and reduced fecundability in PRESTO was only evident among normal-weight women (Supplemental Table 4); there was no evidence of effect measure modification by BMI in SF. We also observed some evidence that total isoflavone intake was associated with improved fecundability among women age ≥30 y in both cohorts (Supplemental Table 5), but not among women age <30 y.
Results were similar to those of the main analysis when restricting to women with regular menstrual cycles and when restricting to women with no polycystic ovary syndrome diagnosis (data not shown).
Discussion
In these 2 preconception cohorts of pregnancy planners from North America and Denmark, we did not observe strong associations between dietary phytoestrogen intake in the past year and fecundability. We did observe some evidence of an inverse association between formononetin intake and fecundability in SF, but not in PRESTO.
Our findings are not consistent with those of the single other preconception cohort study that examined the association between phytoestrogens and fecundability. In the LIFE (Longitudinal Investigation of Fertility and the Environment) study, phytoestrogens were measured in the preconception urine of 501 couples from Texas and Michigan who were planning a pregnancy (26). An increase of 1 natural log unit in female urinary concentrations of the lignans enterodiol and enterolactone was associated with 13% and 12% improved fecundability, respectively. Although the study design of the LIFE study is similar to that of PRESTO and SF, our exposure assessment was different. We ascertained phytoestrogen intake over the past year using FFQs, whereas the LIFE investigators measured urinary concentrations of phytoestrogens. Because phytoestrogens have short half-lives [approximately 4 h for enterodiol and 12 h for enterolactone (44)], urinary concentrations capture phytoestrogen intake in a short time window.
Our findings are consistent with results from the EARTH (Environment and Reproductive Health) study, a cohort of 315 women seeking fertility treatment at Massachusetts General Hospital (25), where pretreatment soy isoflavone intake, ascertained via FFQ, was associated with higher probability of implantation, clinical pregnancy, and live birth. Although we observed little overall association between isoflavone intake and fecundability in either cohort, higher isoflavone intake was associated with improved fecundability among women ≥30 years old. The mean age of female EARTH participants was 36 ± 4 years, and it is likely that the large majority of women were ≥30 years old. Specific causes of infertility vary by age. Thus, the observed age interaction may signify that isoflavones influence risk of specific types of subfertility that affect older reproductive-aged women, such as ovulatory infertility. However, there was no clear dose–response relation in either cohort, and our results could reflect chance variation.
Due to important differences in diet and sources of phytoestrogens in North America and Denmark, we used separate FFQs and phytoestrogen databases in the 2 cohorts. Inconsistent associations with fecundability across cohorts may be due to differential ascertainment of phytoestrogen food sources. For example, lignan intake in SF was driven by bread; in PRESTO, we asked about bread as white bread or nonwhite bread, and we did not collect information on multigrain or other seedy breads that may have high lignan content. Therefore, breads were not a main contributor to lignan intake in PRESTO.
Some variation in results across cohorts may also stem from use of different exposure categories, which was necessary due to the differences in range of phytoestrogens. For example, in SF, but not PRESTO, we observed a dose–response relation between formononetin intake and reduced fecundability. The range of formononetin intake was substantially narrower in PRESTO than in SF, making comparison across cohorts difficult. Likewise, because lignan intake was much higher in SF compared with PRESTO, the highest total lignan category in PRESTO was ≥210 µg/d, and the lowest total lignan category in SF was <388 µg/d. In addition, our phytoestrogen exposure assessment likely ranks individuals within each cohort appropriately by phytoestrogen intake, but the absolute values may not reflect true intake.
Misclassification of the timing and extent of phytoestrogen intake was unavoidable. First, use of an FFQ relies on accurate reporting of average food intake over the past year by participants. Second, particularly for compounds like phytoestrogens, which are found in a wide range of foods, calculation of phytoestrogen intake relies on assumptions about the inclusion of phytoestrogen-containing foods (e.g., flax seeds) in mixed recipes (e.g., granola bars). Because our FFQs were not designed specifically to assess phytoestrogen intake, we did not ask detailed questions about phytoestrogen-containing foods and thus had to rely on assumptions about intake (for example, the type of bread consumed in PRESTO). Lastly, the phytoestrogen content of phytoestrogen-containing foods is derived from databases that have measured phytoestrogen content in food samples, which may not be consistent across products. The databases contain a limited number of food samples, and some of the values are over a decade old. In addition, there are several phytoestrogens present in foods that were not in the databases. Because participants completed the FFQ at (or close to) the beginning of their pregnancy attempt time, any miclassification is likely nondifferential with respect to fecundability.
Many phytoestrogen-containing foods (e.g., nuts, seeds, cruciferous vegetables, soy products) are consumed as part of a healthful diet. Therefore, confounding by socioeconomic status and lifestyle is likely. As expected, we observed substantial attenuation of our results after controlling for a wide range of sociodemographic, lifestyle, reproductive, and dietary factors. We do not expect that residual confounding accounts for our relatively null findings, as unmeasured confounding by socioeconomic status or healthy lifestyle would likely result in an observed positive association between phytoestrogen intake and fecundability.
Given the strong relation between phytoestrogen intake, healthy lifestyle, and socioconomic status, it is plausible that differential loss to follow-up may bias our results. In PRESTO, we observed higher probability of loss to follow-up among women in the lowest, compared with those in the highest, category of isoflavones (9.9% compared with 2.5%), lignans (9.8% compared with 3.9%), and coumestrol (6.2% compared with 5.0%); differences in SF were smaller (16.6% compared with 17.3% for isoflavones, 16.7% compared with 11.4% for lignans, and 15.3% compared with 13.2% for coumestrol). If loss to follow-up is related to lower fecundability, then differential loss to follow-up would result in the underrepresentation of low phytoestrogen consumers with lower fecundability in the analytic cohort, which could lead to a spurious inverse association.
In conclusion, we found only slight associations between phytoestrogen intake and fecundability. Unlike previous research, this study enrolled women during the preconception period, thereby reducing potential for reverse causation, and controlled for a wide range of sociodemographic, lifestyle, and dietary variables. However, nondifferential misclassification due to imperfect measurement of phytoestrogen intake may have affected our results.
Supplementary Material
Acknowledgements
We are grateful to Michael Bairos and Anders Riis for their contributions to this work.
The authors’ responsibilities were as follows— EEH, EMM, KJR, LAW: designed the research; AKW, EEH, EMM, ET, SKW, SEM, LV, AL, KLT, KJR, LAW: conducted the research; LV, AL: provided essential materials (Finnish Phyto-oestrogen database); AKW: analyzed the data; AKW: wrote the paper; AKW: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21-HD072326, R01-HD086742, and R01-HD060680).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: EARTH, Environment and Reproductive Health; FR, fecundability ratio; HEI, Healthy Eating Index; LIFE, Longitudinal Investigation of Fertility and the Environment; LMP, last menstrual period; NCI, National Cancer Institute; NRD, nutrient rich density; PRESTO, Pregnancy Study Online; SF, Snart Foraeldre.
References
- 1. Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006;101(4-5):246–53. [DOI] [PubMed] [Google Scholar]
- 2. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. [DOI] [PubMed] [Google Scholar]
- 3. Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr. 2010;92(1):141–53. [DOI] [PubMed] [Google Scholar]
- 4. Rienks J, Barbaresko J, Nothlings U. Association of isoflavone biomarkers with risk of chronic disease and mortality: a systematic review and meta-analysis of observational studies. Nutr Rev. 2017;75(8):616–41. [DOI] [PubMed] [Google Scholar]
- 5. Bandera EV, Williams MG, Sima C, Bayuga S, Pulick K, Wilcox H, Soslow R, Zauber AG, Olson SH. Phytoestrogen consumption and endometrial cancer risk: a population-based case-control study in New Jersey. Cancer Causes Control. 2009;20(7):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. Phytoestrogen intake and endometrial cancer risk. J Natl Cancer Inst. 2003;95(15):1158–64. [DOI] [PubMed] [Google Scholar]
- 7. Shedd-Wise KM, Alekel DL, Hofmann H, Hanson KB, Schiferl DJ, Hanson LN, Van Loan MD. The soy isoflavones for reducing bone loss study: 3-yr effects on pQCT bone mineral density and strength measures in postmenopausal women. J Clin Densitom. 2011;14(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdi F, Alimoradi Z, Haqi P, Mahdizad F. Effects of phytoestrogens on bone mineral density during the menopause transition: a systematic review of randomized, controlled trials. Climacteric. 2016;19(6):535–45. [DOI] [PubMed] [Google Scholar]
- 9. Ho SC, Woo J, Lam S, Chen Y, Sham A, Lau J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporos Int. 2003;14(10):835–42. [DOI] [PubMed] [Google Scholar]
- 10. Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001;86(11):5217–21. [DOI] [PubMed] [Google Scholar]
- 11. Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97(1):109–15. [DOI] [PubMed] [Google Scholar]
- 12. Horiuchi T, Onouchi T, Takahashi M, Ito H, Orimo H. Effect of soy protein on bone metabolism in postmenopausal Japanese women. Osteoporos Int. 2000;11(8):721–4. [DOI] [PubMed] [Google Scholar]
- 13. Ikeda Y, Iki M, Morita A, Kajita E, Kagamimori S, Kagawa Y, Yoneshima H. Intake of fermented soybeans, natto, is associated with reduced bone loss in postmenopausal women: Japanese Population-Based Osteoporosis (JPOS) Study. J Nutr. 2006;136(5):1323–8. [DOI] [PubMed] [Google Scholar]
- 14. Zhang X, Shu XO, Li H, Yang G, Li Q, Gao YT, Zheng W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165(16):1890–5. [DOI] [PubMed] [Google Scholar]
- 15. Setchell KD, Gosselin SJ, Welsh MB, Johnston JO, Balistreri WF, Kramer LW, Dresser BL, Tarr MJ. Dietary estrogens–a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93(2):225–33. [DOI] [PubMed] [Google Scholar]
- 16. Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Br Vet J. 1946;102:348–52. [DOI] [PubMed] [Google Scholar]
- 17. Salsano S, Perez-Deben S, Quinonero A, Gonzalez-Martin R, Dominguez F. Phytoestrogen exposure alters endometrial stromal cells and interferes with decidualization signaling. Fertil Steril. 2019;112(5):947–58..e3. [DOI] [PubMed] [Google Scholar]
- 18. Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15(4):423–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andrews MA, Schliep KC, Wactawski-Wende J, Stanford JB, Zarek SM, Radin RG, Sjaarda LA, Perkins NJ, Kalwerisky RA, Hammoud AO et al.. Dietary factors and luteal phase deficiency in healthy eumenorrheic women. Hum Reprod. 2015;30(8):1942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filiberto AC, Mumford SL, Pollack AZ, Zhang C, Yeung EH, Schliep KC, Perkins NJ, Wactawski-Wende J, Schisterman EF. Usual dietary isoflavone intake and reproductive function across the menstrual cycle. Fertil Steril. 2013;100(6):1727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Filiberto AC, Mumford SL, Pollack AZ, Zhang C, Yeung EH, Perkins NJ, Wactawski-Wende J, Schisterman EF. Habitual dietary isoflavone intake is associated with decreased C-reactive protein concentrations among healthy premenopausal women. J Nutr. 2013;143(6):900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radin RG, Sjaarda LA, Silver RM, Nobles CJ, Mumford SL, Perkins NJ, Wilcox BD, Pollack AZ, Schliep KC, Plowden TC et al.. C-Reactive protein in relation to fecundability and anovulation among eumenorrheic women. Fertil Steril. 2018;109(2):232–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shahin AY, Ismail AM, Zahran KM, Makhlouf AM. Adding phytoestrogens to clomiphene induction in unexplained infertility patients–a randomized trial. Reprod Biomed Online. 2008;16(4):580–8. [DOI] [PubMed] [Google Scholar]
- 24. Unfer V, Casini ML, Gerli S, Costabile L, Mignosa M, Di Renzo GC. Phytoestrogens may improve the pregnancy rate in in vitro fertilization-embryo transfer cycles: a prospective, controlled, randomized trial. Fertil Steril. 2004;82(6):1509–13. [DOI] [PubMed] [Google Scholar]
- 25. Vanegas JC, Afeiche MC, Gaskins AJ, Minguez-Alarcon L, Williams PL, Wright DL, Toth TL, Hauser R, Chavarro JE. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil Steril. 2015;103(3):749–55..e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mumford SL, Sundaram R, Schisterman EF, Sweeney AM, Barr DB, Rybak ME, Maisog JM, Parker DL, Pfeiffer CM, Louis GM. Higher urinary lignan concentrations in women but not men are positively associated with shorter time to pregnancy. J Nutr. 2014;144(3):352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA et al.. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol. 2015;29(4):360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wise LA, Wesselink AK, Mikkelsen EM, Cueto H, Hahn KA, Rothman KJ, Tucker KL, Sorensen HT, Hatch EE. Dairy intake and fecundability in 2 preconception cohort studies. Am J Clin Nutr. 2017;105(1):100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the eating at America's table study. Am J Epidemiol. 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 30. Knudsen VK, Hatch EE, Cueto H, Tucker KL, Wise L, Christensen T, Mikkelsen EM. Relative validity of a semi-quantitative, web-based FFQ used in the ‘Snart Foraeldre’ cohort - a Danish study of diet and fertility. Public Health Nutr. 2016;19(6):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, Schatzkin A. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;152(3):279–86. [DOI] [PubMed] [Google Scholar]
- 32. Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer. 2006;54(2):184–201. [DOI] [PubMed] [Google Scholar]
- 33. Willett W. Nutritional Epidemiology. 2nd edition New York, NY (USA): Oxford University Press; 1998. [Google Scholar]
- 34. Valsta LM, Kilkkinen A, Mazur W, Nurmi T, Lampi AM, Ovaskainen ML, Korhonen T, Adlercreutz H, Pietinen P. Phyto-oestrogen database of foods and average intake in Finland. Br J Nutr. 2003;89(Suppl 1):S31–8. [DOI] [PubMed] [Google Scholar]
- 35. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Drewnowski A. Defining nutrient density: development and validation of the nutrient rich foods index. J Am Coll Nutr. 2009;28(4):421S–6S. [DOI] [PubMed] [Google Scholar]
- 38. Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129(5):1072–8. [DOI] [PubMed] [Google Scholar]
- 39. Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007;165(4):444–52. [DOI] [PubMed] [Google Scholar]
- 40. Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013;27(5):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Durrleman S, Simon R. Flexible regression models with cubic splines. Statist Med. 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 42. Li R, Hertzmark E, Spiegelman D. The SAS GLMCURV9 Macro. Boston, MA: Channing Laboratory; 2008. [Google Scholar]
- 43. SAS. SAS Institute Inc. 2014. SAS/STAT® 9.4 User's Guide. Cary, NC. Cary, NC: SAS Institute; 2014. [Google Scholar]
- 44. Kuijsten A, Arts IC, Vree TB, Hollman PC. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr. 2005;135(4):795–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.