ABSTRACT
Background
Few studies have assessed the associations of ceramides and sphingomyelins (SMs) with diabetes in humans.
Objective
We assessed associations of 15 circulating ceramides and SM species with incident diabetes in 2 studies.
Methods
The analysis included 435 American-Indian participants from the Strong Heart Study (nested case-control design for analyses; mean age: 57 y; 34% male; median time until diabetes 4.3 y for cases) and 1902 participants from the Strong Heart Family Study (prospective design for analyses; mean age: 37 y; 39% male; median 12.5 y of follow-up). Sphingolipid species were measured using stored plasma samples by sequential LC and MS. Using logistic regression and parametric survival models within studies, and an inverse-variance-weighted meta-analysis across studies, we examined associations of 15 ceramides and SM species with incident diabetes.
Results
There were 446 cases of incident diabetes across the studies. Higher circulating concentrations of ceramides containing stearic acid (Cer-18), arachidic acid (Cer-20), and behenic acid (Cer-22) were each associated with a higher risk of diabetes. The RRs for incident diabetes per 1 SD of each log ceramide species (μM) were 1.22 (95% CI: 1.09, 1.37) for Cer-18, 1.18 (95% CI: 1.06, 1.31) for Cer-20, and 1.20 (95% CI: 1.08, 1.32) for Cer-22. Although the magnitude of the risk estimates for the association of ceramides containing lignoceric acid (Cer-24) with diabetes was similar to those for Cer-18, Cer-20, and Cer-22 (RR = 1.13; 95% CI: 1.01, 1.26), the association was not statistically significant after correction for multiple testing (P = 0.007). Ceramides carrying palmitic acid (Cer-16), SMs, glucosyl-ceramides, or a lactosyl-ceramide were not associated with diabetes risk.
Conclusions
Higher concentrations of circulating Cer-18, Cer-20, and Cer-22 were associated with a higher risk of developing diabetes in 2 studies of American-Indian adults. This trial was registered at clinicaltrials.gov as NCT00005134.
Keywords: sphingolipids, ceramides, sphingomyelin, diabetes, American Indians
Introduction
Type 2 diabetes and related complications are leading causes of morbidity and mortality among American Indians (AIs). AIs are 2.5 times more likely to have diagnosed diabetes than non-Hispanic whites of similar age (1). Strategies to prevent diabetes have had varying levels of success in AI communities over the past few decades (2–4), and identifying novel and potentially modifiable factors that influence diabetes may provide insights to inform future diabetes prevention efforts.
Ceramides and sphingomyelins (SMs) have been implicated in several biological processes related to diabetes in cell and animal studies, including high levels of impaired insulin signaling and glucose transport, insulin resistance, inflammation, oxidative stress, and apoptosis in β-cells (5–12). In animal studies, inhibition of de novo sphingolipid synthesis improves insulin sensitivity and glucose homeostasis, major risk factors for diabetes, and reduces lipotoxic responses associated with metabolic disorders such as obesity (10, 11, 13–16).
Most published studies have examined total ceramide and SM concentrations with diabetes-related phenotypes, but previous studies highlight the unique metabolic and biological processes of individual species (17, 18). In animal and in vitro models, ceramides containing fatty acid 16:0 induced apoptosis (17), whereas ceramides containing fatty acids 20:0 and 22:0 (18) did not induce apoptosis—suggesting that the associations of ceramides and SMs with health outcomes may differ by species. Further, sphingolipids are components of plasma phospholipid fatty acids, and previous work indicates that higher concentrations of plasma phospholipid SFAs 20:0, 22:0, and 24:0 are associated with a lower risk of diabetes, whereas higher concentrations of SFA 16:0 are associated with a higher risk (19–21). Although circulating fatty acids are a major component of sphingolipids, few studies have directly examined the relation of specific sphingolipid species with diabetes. A better understanding of the association of ceramide and SM species with health outcomes is important since sphingolipids are modifiable through known drug therapies (22), and possibly diet (23–26).
The purpose of this analysis was to examine associations of 15 circulating ceramides and SM species with incident diabetes among AIs who participated in the Strong Heart Study (SHS) and Strong Heart Family Study (SHFS). We hypothesized that sphingolipids with palmitic acid (i.e. 16:0) would be associated with a higher risk of diabetes, whereas sphingolipids with very long-chain SFAs (i.e. 20:0, 22:0, and 24:0) would be associated with a lower risk of diabetes.
Methods
Study design
We used plasma samples stored at –80°C from the SHS and SHFS cohorts to measure sphingolipids and to examine the associations of sphingolipids with incident diabetes. In the SHS, a case-control design was used to preserve existing stored blood specimens/optimize study resources. In the SHFS, a longitudinal design was used, and sphingolipids of all participants were measured using plasma samples at baseline. Analyses examined the associations of each sphingolipid with risk of incident diabetes in the SHS and SHFS separately, and then risk estimates from the 2 cohorts were combined in meta-analyses.
Setting
The SHS and SHFS are 2 longitudinal cohorts designed to assess risk factors for cardiovascular diseases in 12 AI communities in Arizona, North and South Dakota, and Oklahoma. Details of the study designs, survey methods, and laboratory techniques of the SHS and the SHFS have been reported previously (27, 28). In brief, the SHS included 4549 participants aged between 45 and 74 y who participated in 3 study exams: 1989–1991 (Phase 1), 1993–1995 (Phase 2), and 1998–1999 (Phase 3). The SHFS comprised 2780 participants aged between 14 and 93 y from 91 families (i.e. family members of original SHS participants). SHFS participants completed 2 examinations over a period of 11 y: 2001–2003 (Phase 4) and 2007–2009 (Phase 5). Procedures followed were in accordance with the ethical standards of the responsible Institutional Review Board from each Indian Health Service region. All communities approved the study, and written informed consent was obtained from all participants at each exam.
Study sample
SHS
From participants without prevalent diabetes at the Phase 2 exam, we randomly selected a sample of 146 participants who developed diabetes within 15 y of the Phase 2 exam as cases (median time until diabetes = 4.3 y; range: 0.3–15 y). For each case, we randomly selected 2 age- and gender-matched controls who did not develop diabetes during the same follow-up time (n = 289) using risk-set sampling. Specifically, for those cases whose diabetes was identified at the Phase 3 exam, controls were chosen from those without diabetes at the Phase 3 exam. Later cases of diabetes were matched to controls who participated in Phase 6 (2010–2017)and did not have a diabetes diagnosis in the 15 y after the Phase 2 exam. In total, there were 435 participants from the SHS in the analytic sample (Figure 1).
FIGURE 1.
Participant flow chart for analytic sample in the Strong Heart Study and the Strong Heart Family Study.
SHFS
Among the 2713 participants with available sphingolipid measures at the Phase 4 exam, we excluded participants with prevalent diabetes (n = 510) and those without follow-up (n = 274). Additionally, participants missing data for measures of baseline glucose (n = 6), waist circumference (n = 14), and education (n = 7) were excluded. In total, there were 1902 participants from the SHFS in the analytic sample (Figure 1). Median time of follow-up was 12.5 y (range: 3.0–15.6 y).
Data collection
Each SHS and SHFS examination included a standardized personal interview, physical examination, and laboratory work-up. The data collection procedures were identical at all SHS and SHFS exams except for physical activity and diabetes ascertainment, and have been described in detail previously (27, 28).
Measurement of sphingolipids
SMs and ceramides containing distinct SFAs acylated to the sphingoid backbone, including palmitic acid [16:0 (16 carbons, 0 double bonds)], stearic acid (18:0), arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0), were measured using stored plasma samples from the SHS and the SHFS by sequential LC/MS. Details of the laboratory methods and quality control procedures have been reported in detail previously (29). In brief, all specimens from the SHFS were received and randomized prior to laboratory measurements. All specimens from SHFS and the SHS were handled identically. Cases and controls from SHS were assayed in the same batch. The lab technicians were blinded to case-control status. The CVs were computed over the entire study period by the assay lab. We determined the CV (SD divided by the mean) for a quality control sample that was run (precipitated and analyzed by LC-MS/MS) in duplicate in each batch. One replicate was injected onto the LC-MS/MS instrument near the beginning of the run and one replicate towards the end, in a random position in each batch. The quality control sample was an independent pool of EDTA-anticoagulated plasma.
In total, 22 sphingolipid species were measured. For the current investigation, we restricted analyses to the 15 sphingolipid species with CV ≤20%. This included 6 ceramide species [i.e. ceramide with 16:0 (Cer-16), ceramide with 18:0 (Cer-18), ceramide with 20:0 (Cer-20), ceramide with 22:0 (Cer-22), and a composite concentration of Cer-24 computed as the sum of the concentrations of 2 species of ceramides with 24:0 having the distinct “d181” and “d182” sphingoid backbones]; 6 SM species (i.e. SM-14, SM-16, SM-18, SM-20, SM-22, SM-24); 3 glucosyl-ceramides (GluCer) (i.e. GluCer-16, GluCer-22, and GluCer-24); and 1 lactosyl-ceramide (LacCer) (i.e. LacCer-16).
Diabetes ascertainment
In the SHS, cases of diabetes were based on the 1999 WHO definition, including participants with follow-up fasting plasma glucose ≥126 mg/dL or 2-h oral-glucose-tolerance test (OGTT) ≥200 mg/dL at the study exams. Additionally, any participant taking insulin or oral antidiabetic medications or with evidence of a physician-diagnosis of diabetes from annual medical record surveillance and review was considered to have diabetes (30). In the SHFS, incident diabetes was defined based on all of the criteria above, (except for OGTT since that measure was not available) through the end of Phase 5 (2007–2009). After Phase 5 and through December 2017, cases of diabetes in the SHFS were ascertained through phone interview and confirmed by documentation in medical records. Since type 1 diabetes is rare in AI populations, we assumed all new occurrences of diabetes in the SHS and the SHFS were type 2.
SHS
Conditional logistic regression was used to evaluate the associations of plasma sphingolipids and incident diabetes, with 2 controls matched to each case based on age (±5 y), sex, and site. There were 3 models fit to examine associations of sphingolipids with incident diabetes. Model 1 (a minimally adjusted model) included a term for age to account for residual confounding within matched sets. Model 2 (primary model) additionally adjusted for a priori confounders, including education (years), smoking (never, former, current), physical activity (metabolic equivalent task hours per week), BMI (log-transformed), and waist circumference. In Model 3 (exploratory model), we additionally adjusted for LDL cholesterol to assess if LDL cholesterol concentrations mediate or confound the relation of each sphingolipid of interest with incident diabetes. Observed associations were estimated by ORs for diabetes per 1 SD in log sphingolipid species concentration (μM).
SHFS
Parametric survival models with a Weibull distribution were used to examine the associations of each sphingolipid with incident diabetes. These models were selected for the analysis to account for interval-censored incident diabetes. The SHFS comprised extended families, so robust SE estimates that account for clustering of risk factors among family members were used.
Similar to analyses in the SHS, we fitted 3 models to examine the associations of sphingolipid and incident diabetes in the SHFS. Model 1 (a minimally adjusted model) adjusted for age, sex, and site. Model 2 (primary model) included further adjustments for education (years), smoking (never, former, current), physical activity (steps per day), BMI (log-transformed), and waist circumference. Model 3 (exploratory model) additionally included adjustment for LDL cholesterol since sphingolipids are associated with LDL. Results include estimated HRs for diabetes per 1 SD in log sphingolipid species concentration (μM).
As associations of sphingolipid species with incident diabetes may differ by age, sex, or BMI, we examined potential effect modification of these factors with each sphingolipid species of interest on incident diabetes in exploratory analyses in both the SHS and the SHFS. To evaluate effect modification, a multiplicative term was created individually for sex, age, and BMI, with each sphingolipid, and included in the primary model (Model 2). Wald tests were used to determine the statistical significance of the interaction terms.
In the SHS and the SHFS analyses, multiple imputations were used (20 replicates) to address occasional missing values for physical activity (n = 16 in the SHS and n = 157 in the SHFS) using information on age, sex, site, BMI, and waist circumference. Imputations were executed in the multivariate imputation by chained equations (MICE) package in R (version 3.4.0) using the Fully Conditional Expectation method with predictive mean matching methods, as described previously (29, 31, 32).
Meta-analysis
The prespecified standardized analyses (described above) for the SHS and the SHFS were performed. The ORs (SHS), HRs (SHFS), and corresponding SEs for the associations from 3 prespecified multivariable models from each study were then compiled, and meta-analyses were performed using inverse-variance-weighted fixed-effects methods in STATA 13.1 (Stata Corporation). Inverse-variance-weighted fixed-effects meta-analyses approximates results that would be obtained if the data from all studies could be analyzed together with adjustment for study (33). A Bonferroni correction was used to adjust for multiple comparisons; the significance threshold of 0.0033 (0.05/15 sphingolipid species) was used. As the meta-analysis combined results from studies that reported ORs (SHS) and HRs (SHFS), for the purposes of this article, we decided to call the meta-analyzed parameter RR —which is a slightly more generic term than OR or HR.
Results
Baseline characteristics of SHS and SHFS participants are described in Table 1. On average, SHS participants were older than SHFS participants (57 y compared with 37 y), and ∼34–39% of participants from each cohort were male. In both cohorts, participants reported an average of 12 y of education, mean BMI was 31 kg/m2, and 37–38% of participants reported current smoking. The distribution of circulating concentrations of sphingolipids species are shown in Supplemental Table 1. In both the SHS and the SHFS, circulating concentrations of sphingolipid species were correlated, but the observed correlations were slightly lower in the SHS than in the SHFS (Supplemental Tables 2and3).
TABLE 1.
Characteristics of the Strong Heart Study and the Strong Heart Family Study participants at the time of plasma sphingolipids assessment1
| SHS (n = 435) | SHFS (n = 1902) | |
|---|---|---|
| Age, y | 57 ± 7 | 37 ± 16 |
| Male, % | 34 | 39 |
| Education, y | 12 ± 3 | 12 ± 2 |
| BMI, kg/m2 | 31 ± 6 | 31 ± 7 |
| Waist circumference, cm | ||
| Male | 103 ± 12 | 101 ± 18 |
| Female | 104 ± 15 | 99 ± 17 |
| Smoking, % | ||
| Never | 29 | 41 |
| Ever | 32 | 21 |
| Current | 37 | 38 |
| Physical activity | ||
| MET hours per week | 92 ± 84 | — |
| Steps per day | — | 6316 ± 3987 |
| Serum cholesterol | ||
| Total cholesterol, mg/dL | 199 ± 38 | 181 ± 36 |
| HDL cholesterol, mg/dL | ||
| Male | 38 ± 13 | 49 ± 14 |
| Female | 45 ± 16 | 54 ± 15 |
| LDL cholesterol, mg/dL | 127 ± 34 | 100 ± 30 |
| LDL/HDL cholesterol, ratio | 3.3 ± 1.3 | 2.1 ± 0.9 |
| Triglycerides, mg/dL | 146 ± 92 | 149 ± 94 |
| Fasting insulin, μU/mL | 17 ± 13 | 16 ± 16 |
| Fasting plasma glucose, mg/dL | 103 ± 11 | 94 ± 10 |
Values are mean ± SD or %. MET, metabolic equivalent task; SHFS, Strong Heart Family Study; SHS, Strong Heart Study.
For this analysis, we had data available on 446 cases of incident diabetes [a subset of 146 cases from the SHS and all incident cases (n = 300) from the SHFS]. Higher circulating concentrations of Cer-18, Cer-20, and Cer-22 were each associated with a higher risk of incident diabetes after adjustment for age, sex, site, education, smoking, physical activity, BMI, and waist circumference (Figure 2). Although the magnitude of the risk estimate for the association of Cer-24 with incident diabetes was similar to those for Cer-18, Cer-20, and Cer-22 with incident diabetes, the RR for Cer-24 with incident diabetes was not statistically significant at the prespecified threshold for multiple testing (P = 0.007). A minimally adjusted model (Supplemental Figure 1) or adjusting for LDL cholesterol did not meaningfully change reported RRs (Supplemental Figure 2).
FIGURE 2.

Forest plots of prospective associations of plasma ceramides with incident diabetes in the Strong Heart Study and the Strong Heart Family Study. RRs and 95% CIs per 1 SD of each log ceramide species (μM) are represented by a filled diamond and horizontal line for each cohort, and by a hollow diamond for the overall pooled results. The shading around the filled diamond reflects the relative weight of the study in the meta-analysis. n = 435 for SHS and n = 1902 for SHFS. Cohort-specific associations were assessed in models adjusted for age, sex, site, education, physical activity, smoking, BMI, and waist circumference. Models denoted with “*” means P < 0.0033. Cer, ceramide; SHFS, Strong Heart Family Study; SHS, Strong Heart Study.
SM species, glucosyl-ceramides, and lactosyl-ceramides were not associated with diabetes risk—although higher concentrations of circulating LC-16 showed marginal negative association with diabetes risk before correction for multiple testing (Figures 3 and 4). Similar to the analyses for ceramides, a minimally adjusted model or additional adjustment for LDL cholesterol (Supplemental Figures 3–6) did not produce materially different results. Models that assessed sphingolipids categorically (quartiles) produced similar estimates (Supplemental Figures 7–9).
FIGURE 3.

Forest plots of prospective associations of plasma sphingomyelin species with incident diabetes in the Strong Heart Study and the Strong Heart Family Study. RRs and 95% CIs per 1 SD of each log sphingomyelin species (μM) are represented by a filled diamond and horizontal line for each cohort, and by a hollow diamond for the overall pooled results. The shading around the filled diamond reflects the relative weight of the study in the meta-analysis. n = 435 for SHS and n = 1902 for SHFS. Cohort-specific associations were assessed in models adjusted for age, sex, site, education, physical activity, smoking, BMI, and waist circumference. SHFS, Strong Heart Family Study; SHS, Strong Heart Study; SM, sphingomyelin.
FIGURE 4.
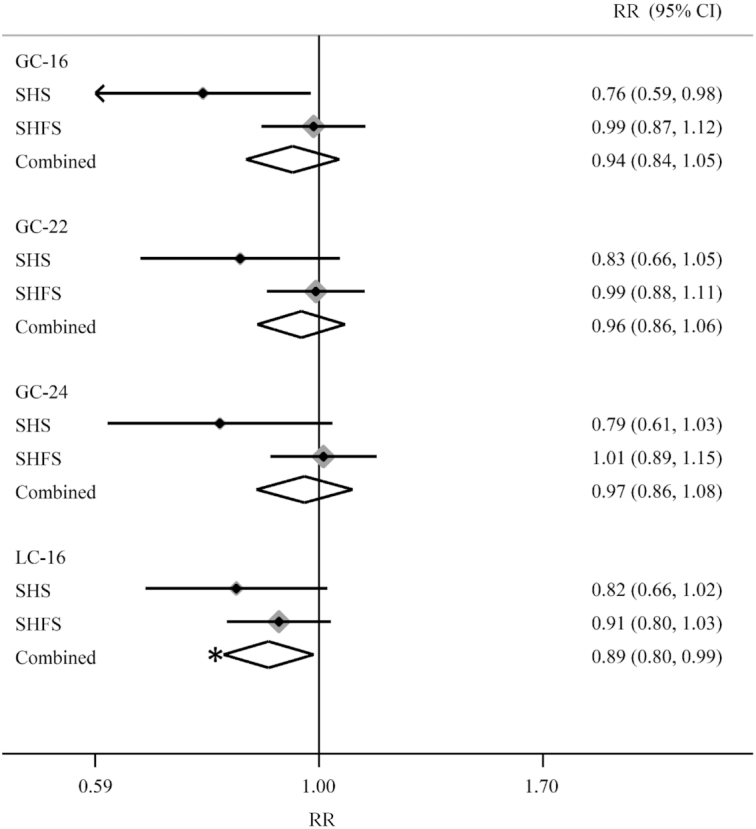
Forest plots of prospective associations of plasma glucosyl-ceramides and lactosyl-ceramides with incident diabetes in the Strong Heart Study and the Strong Heart Family Study. RRs and 95% CIs per 1 SD of each log glucosyl-ceramides species (μM) are represented by a filled diamond and horizontal line for each cohort, and by a hollow diamond for the overall pooled results. The shading around the filled diamond reflects the relative weight of the study in the meta-analysis. n = 435 for SHS and n = 1902 for SHFS. Cohort-specific associations were assessed in models adjusted for age, sex, site, education, physical activity, smoking, BMI, and waist circumference. Models denoted with “*” means P < 0.0033. GC, glucosyl-ceramide; LC, lactosyl-ceramide; SHFS, Strong Heart Family Study; SHS, Strong Heart Study.
We observed little evidence of effect modification for each sphingolipid species with age (no P-interaction < 0.05), sex (smallest P-interaction = 0.02 for Cer-22), or BMI (smallest P-interaction = 0.02 for Cer-24) on risk of incident diabetes after adjustment for multiple comparisons. Sensitivity analyses that further adjusted for family history of diabetes in the SHS (this information was not available in the SHFS) produced similar results (data not shown).
Discussion
In this meta-analysis that included 2 studies with participants from 12 AI communities throughout the USA (total n = 2337), higher concentrations of circulating ceramides carrying fatty acids 18:0, 20:0, and 22:0 (i.e. Cer-18, Cer-20, and Cer-22) were each associated with a higher risk of developing diabetes. On the other hand, circulating SMs, glucosyl-ceramides, or a lactosyl-ceramide did not appear to be associated with diabetes risk. These findings suggest that the relations of circulating sphingolipids with diabetes risk differ by sphingolipid species. These results were not consistent with our original hypothesis; we did not observe differential association of the relation of ceramides and SMs carrying palmitic acid (i.e. 16:0) versus ceramides and SMs carrying very long-chain SFAs (i.e. 20:0, 22:0, and 24:0). As we have previously observed support for our hypothesis with vascular-related outcomes [e.g. higher concentrations of ceramides and SMs containing 16:0 and 18:0 were associated with a higher risk of heart failure, whereas higher concentrations of ceramides and SMs containing 20:0, 22:0, and 24:0 were associated with a lower risk (34)], the biological mechanisms that drive associations of sphingolipids with vascular-related outcomes are likely different to mechanisms influencing diabetes risk.
Several studies in animals and in vitro indicate that ceramides play a role in the development of diabetes (10, 35); however, the mechanisms involved in these processes are complex (36). Ceramides inhibit several insulin intermediates, including insulin receptor substrate, protein kinase B (Akt), and glucose transporter type 4 (Glut-4) (36). Ceramides have also been shown to promote β-cell apoptosis and dysfunction by triggering the extrinsic apoptotic pathway, the mitochondrial release of cytochrome c and generation of free radicals, and endoplasmic reticulum stress (36). Obese, insulin-resistant rodents treated with myriocin, a drug that inhibits serine-palmitoyl transferase (i.e. a key enzyme in the metabolism of ceramides and SMs), had lowered concentrations of ceramides in the liver, muscle, and circulation (13, 15), weight loss (15), improvements in insulin sensitivity and glucose tolerance (11, 14, 15) in the liver and muscle, and were less likely to develop diabetes during a 6-wk follow-up (11). Findings from in vitro studies indicate that exposing cell cultures to 16:0 increased ceramides and inhibited Akt/protein kinase B (Akt/PKB)—a key regulator in insulin-mediated glucose transport (10, 35), with possibly greater effects in the context of high concentrations of circulating glucose (37). The addition of ceramide analogs to cultured cells, muscles, adipocytes, and hepatocytes has also been shown to inhibit insulin-mediated glucose uptake and activation of Akt/PKB (38). Lowering ceramides by inhibiting enzymes needed for ceramide synthesis, including serine-palmitoyl transferase (i.e. the enzyme that myriocin targets) negated the effects of 16:0 on impaired insulin-mediated glucose uptake and transport (5). Findings from these studies support the results reported herein, suggesting that higher concentrations of ceramide species are associated with a higher risk of incident diabetes.
Only a handful of studies have assessed the associations of ceramides and diabetes-related biomarkers in humans. In 2 small studies (n= <64), individuals with type 2 diabetes had higher circulating concentrations of Cer-18 and Cer-20, but not Cer-22 (39) or Cer-24 (40), than individuals without diabetes (39, 40). In untargeted analyses of 135 lipid species from 2 case-control studies (n = <300), higher concentrations of plasma dihydroceramide 18:0 [d(18:0)], a precursor to ceramides, was associated with a higher risk of developing diabetes (41). In a large study in Finland (n = 8045) that assessed the associations of 4 ceramides (i.e. Cer-16, Cer-18, Cer-24, and Cer-24:1) with diabetes risk, higher concentrations of Cer-18, Cer-20, Cer-22, and Cer-24:1, and the ratios of Cer-18/Cer-16, Cer-18/Cer-24, and Cer-18/Cer-24:1, were each associated with a higher risk of incident diabetes (42). Although we did not include analyses of ceramide ratios with diabetes risk in our prespecified analysis plan, in post hoc meta-analyses, we also observed an association of Cer-18/Cer-16 with incident diabetes (RR = 1.71; 95% CI: 1.17, 2.50) in the SHS and the SHFS, whereas the RR for Cer-18/Cer-24 with incident diabetes was of borderline significance (RR = 1.87; 95% CI: 0.97, 3.58). The results of these studies complement the findings in this report, suggesting that ceramides are involved in processes related to both: 1) the development of diabetes and 2) the diagnosed diabetes phenotype.
Insulin resistance is an early marker of diabetes risk, and previous work in the SHFS indicated that higher concentrations of Cer-16, Cer-20, and Cer-22 are associated with higher fasting insulin concentrations among AIs without diabetes—suggesting a role of circulating ceramides in prediabetes (29). Specifically, a 2-fold higher concentration of each ceramide was associated with 11–16% higher fasting plasma insulin and HOMA-IR (29). On the other hand, in that analysis, higher concentrations of SM-18, SM-20, and SM-22 were each associated with lower fasting insulin concentrations, HOMA-IR, and HOMA-β—but only among participants with normal BMI. We did not observe interaction of any of the SMs with BMI on risk of diabetes in the present analysis. This may be due to limited power to assess interaction of SMs with diabetes. Alternatively, this may suggest that the impact of BMI on the relations of SMs and metabolic dysfunction may occur early in the trajectory to diabetes.
To our knowledge, no studies have assessed the influence of foods or nutrients on specific sphingolipid species. However, sphingolipids comprise circulating fatty acids—and dietary factors influence circulating concentrations of some fatty acids, and it is plausible that consumption of foods known to impact circulating fatty acids may also impact concentrations of specific sphingolipid species. In particular, previous work has shown that dietary intake of nuts influences circulating concentrations of very long-chain SFAs 20:0 and 22:0 (26). In the diet, SFA 18:0 may be derived from animal products, including beef and hard cheeses, and shea butter, cocoa butter, and chocolate (43–45). SFAs 16:0 and 18:0 are also end products of de novo lipogenesis, which is driven by high doses of rapidly digested carbohydrates as well as fructose (46–48).
This study has many strengths. The SHS and the SHFS are large multi-site prospective studies of risk factors for cardiovascular disease in an underserved population of AIs. The availability of detailed data on demographic, behavioral, and health factors maximized our capacity to control for potential confounders. This study also has limitations. Although many sphingolipids were measured with good precision, it was not possible to examine the associations of all ceramide species carrying SFAs (e.g. lactosyl- and glycosyl-ceramides) due to measurement error associated with the laboratory assay. We adjusted for several demographic and behavioral factors that may be related to both circulating sphingolipids and diabetes risk, but residual confounding due to poorly measured or unmeasured factors (e.g. diet, plasma branched-chain amino acids, diacylglycerol) is possible. Finally, circulating sphingolipid concentrations are related to diet, metabolism, and genetics, and the generalizability of these findings to other populations is unknown.
In conclusion, these findings suggest that higher concentrations of circulating ceramides carrying different SFAs (i.e. Cer-18, Cer-20, and Cer-22) are associated with a higher risk of diabetes in 2 large cohorts of AI men and women over a wide age range. Replication of these findings in other studies is needed to better understand if lowering circulating ceramides may be a plausible target for diabetes prevention efforts.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—AMF, PNJ, BM, BVH, JU, DSS, IBK, NS, and RNL: designed research; AH: conducted research; BVH and JU: provided essential reagents or materials; PNJ, BM, CY, and CS: analyzed data or performed statistical analysis; AMF, PNJ, and RNL: wrote the manuscript; AMF and RNL: had primary responsibility for the final content; and all authors read and approved the final version of the manuscript.
Notes
This research was supported by R01DK103657 and P30 DK035816 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). The Strong Heart Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services, under contract numbers 75N92019D00027, 75N92019D00028, 75N92019D00029, and 75N92019D00030. The study was previously supported by research grants: R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319 and by cooperative agreements: U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521.
Author disclosures: The authors report no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental Tables 1–3 and Supplemental Figures 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: AI, American Indian; Akt, protein kinase B; Cer, ceramide; OGTT, oral-glucose-tolerance test; SHFS, Strong Heart Family Study; SHS, Strong Heart Study; SM, sphingomyelin.
References
- 1. CDC. Diabetes prevalence among American Indians and Alaska Natives and the overall population – United States, 1994–2002. Morb Mortal Wkly Rep. 2003;52(30):702–4. [PubMed] [Google Scholar]
- 2. Jiang L, Manson SM, Beals J, Henderson WG, Huang H, Acton KJ, Roubideaux Y. Translating the diabetes prevention program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians diabetes prevention demonstration project. Diabetes Care. 2013;36(7):2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang L, Chang J, Beals J, Bullock A, Manson SM. Neighborhood characteristics and lifestyle intervention outcomes: results from the Special Diabetes Program for Indians. Prev Med. 2018;111:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee ET, Welty TK, Cowan LD, Wang W, Rhoades DA, Devereux R, Go O, Fabsitz R, Howard BV. Incidence of diabetes in American Indians of three geographic areas: the Strong Heart Study. Diabetes Care. 2002;25(1):49–54. [DOI] [PubMed] [Google Scholar]
- 5. Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278(12):10297–303. [DOI] [PubMed] [Google Scholar]
- 6. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR et al.. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5):1858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu W, Ross J, Geng T, Brice SE, Cowart LA. Differential regulation of dihydroceramide desaturase by palmitate versus monounsaturated fatty acids: implications for insulin resistance. J Biol Chem. 2011;286(19):16596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382(Pt 2):619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watson ML, Coghlan M, Hundal HS. Modulating serine palmitoyl transferase (SPT) expression and activity unveils a crucial role in lipid-induced insulin resistance in rat skeletal muscle cells. Biochem J. 2009;417(3):791–801. [DOI] [PubMed] [Google Scholar]
- 10. Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585–94. [DOI] [PubMed] [Google Scholar]
- 11. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A et al.. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–79. [DOI] [PubMed] [Google Scholar]
- 12. Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J Biol Chem. 2005;280(20):20148–53. [DOI] [PubMed] [Google Scholar]
- 13. Frangioudakis G, Garrard J, Raddatz K, Nadler JL, Mitchell TW, Schmitz-Peiffer C. Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology. 2010;151(9):4187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ussher JR, Koves TR, Cadete VJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM et al.. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59(10):2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297(1):E211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Z, Zhang H, Liu J, Liang CP, Li Y, Li Y, Teitelman G, Beyer T, Bui HH, Peake DA et al.. Reducing plasma membrane sphingomyelin increases insulin sensitivity. Mol Cell Biol. 2011;31(20):4205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. [DOI] [PubMed] [Google Scholar]
- 18. Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science (New York, NY). 2008;322(5898):110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB, Song X, Djousse L, Siscovick DS, McKnight B et al.. Plasma phospholipid very-long-chain SFAs and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101(5):1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Am J Clin Nutr. 2011;93(1):127–42. [DOI] [PubMed] [Google Scholar]
- 22. Rahmaniyan M, Curley RW Jr., Obeid LM, Hannun YA, Kraveka JM. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem. 2011;286(28):24754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwab US, Maliranta HM, Sarkkinen ES, Savolainen MJ, Kesaniemi YA, Uusitupa MI. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. 1996;45(2):143–9. [DOI] [PubMed] [Google Scholar]
- 24. Iggman D, Riserus U.. Role of different dietary saturated fatty acids for cardiometabolic risk. Clin Lipidol. 2011;6(2):209–23. [Google Scholar]
- 25. Fretts AM, Mozaffarian D, Siscovick DS, King IB, McKnight B, Psaty BM, Rimm EB, Sitlani C, Sacks FM, Song X et al.. Associations of plasma phospholipid SFAs with total and cause-specific mortality in older adults differ according to SFA chain length. J Nutr. 2016;146(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133(4):1060–3. [DOI] [PubMed] [Google Scholar]
- 27. Lee E, Welty T, Fabsitz R, Cowan L, Ngoc A, Oopik A, Cucchiara A, Savage P, Howard B. The Strong Heart Study: a study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–55. [DOI] [PubMed] [Google Scholar]
- 28. North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, Fabsitz RR, Roman MJ, MacCluer JW. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the Strong Heart Family Study. Am J Epidemiol. 2003;157(4):303–14. [DOI] [PubMed] [Google Scholar]
- 29. Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, Fretts AM, Umans JG, Howard BV, Sitlani CM, Siscovick DS et al.. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the Strong Heart Family Study. Diabetes. 2018;67(8):1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization. Definition, diagnosis, and classification of diabetes mellitus and its complications: a report of a WHO Consultation. Geneva: WHO; 1999:1–59. Available from: https://apps.who.int/iris/handle/10665/66040 [Google Scholar]
- 31. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 32. Van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 33. Lin DY, Zeng D.. Meta-analysis of genome-wide association studies: no efficiency gain in using individual participant data. Genet Epidemiol. 2010;34(1):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lemaitre RN, Jensen PN, Hoofnagle A, McKnight B, Fretts AM, King IB, Siscovick DS, Psaty BM, Heckbert SR, Mozaffarian D et al.. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ Heart Fail. 2019;12(7):e005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274(34):24202–10. [DOI] [PubMed] [Google Scholar]
- 36. Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Veret J, Coant N, Berdyshev EV, Skobeleva A, Therville N, Bailbe D, Gorshkova I, Natarajan V, Portha B, Le Stunff H. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem J. 2011;438(1):177–89. [DOI] [PubMed] [Google Scholar]
- 38. Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nut Rev. 2007;65(6 Pt 2):S39–46. [DOI] [PubMed] [Google Scholar]
- 39. Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS, Perreault L. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab. 2015;309(4):E398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wigger L, Cruciani-Guglielmacci C, Nicolas A, Denom J, Fernandez N, Fumeron F, Marques-Vidal P, Ktorza A, Kramer W, Schulte A et al.. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Reports. 2017;18(9):2269–79. [DOI] [PubMed] [Google Scholar]
- 42. Hilvo M, Salonurmi T, Havulinna AS, Kauhanen D, Pedersen ER, Tell GS, Meyer K, Teeriniemi AM, Laatikainen T, Jousilahti P et al.. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia. 2018;61(6):1424–34. [DOI] [PubMed] [Google Scholar]
- 43. Kris-Etherton PM, Mustad VA. Chocolate feeding studies: a novel approach for evaluating the plasma lipid effects of stearic acid. Am J Clin Nutr. 1994;60(6 Suppl):1029S–36S. [DOI] [PubMed] [Google Scholar]
- 44. Tholstrup T. Influence of stearic acid on hemostatic risk factors in humans. Lipids. 2005;40(12):1229–35. [DOI] [PubMed] [Google Scholar]
- 45. Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70(6):1001–8. [DOI] [PubMed] [Google Scholar]
- 46. Volk BM, Kunces LJ, Freidenreich DJ, Kupchak BR, Saenz C, Artistizabal JC, Fernandez ML, Bruno RS, Maresh CM, Kraemer WJ et al.. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One. 2014;9(11):e113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97(9):2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000;225(3):191–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



