Abstract
Objective
To examine the relationship of weight change during early, mid, and late pregnancy with the development of a hypertensive disorder of pregnancy (HDP).
Study design
These data are from a prospective cohort study of nulliparous women with live singleton pregnancies. “Early” weight change was defined as the difference between self-reported pre-pregnancy weight and weight at the first visit (between 6–13 weeks’ gestation); “mid” weight change was defined as the weight change between the first and second visits (between 16–21 weeks’ gestation); “late” weight change was defined as the weight change between the second and third visits (between 22–29 weeks’ gestation). Weight change in each time period was further characterized as inadequate, adequate, or excessive based on the Institute of Medicine’s (IOM’s) trimester-specific weekly weight gain goals based on pre-pregnancy body mass index. Multivariable Poisson regression was performed to adjust for potential confounders.
Main outcome measure
Development of any hypertensive disorder of pregnancy
Results
Of 8,296 women, 1,564 (18.9%) developed a HDP. Weight gain in excess of the IOM recommendations during the latter two time periods was significantly associated with HDP. Specifically, trimester-specific excessive weight gain was associated with increased risk of developing HDP in the mid period (aIRR 1.16, 95% CI 1.01–1.35) as well as in the late period (aIRR = 1.19, 95% CI = 1.02–1.40). The weight gain preceded the onset of clinically apparent disease.
Conclusions
Excessive weight gain as early as the early second trimester was associated with increased risks of development of HDP.
Keywords: hypertensive disorders, pregnancy, preeclampsia, gestational weight gain
Introduction
Approximately 5–9% of all pregnancies in the United States are complicated by hypertensive disorders of pregnancy (HDP), which include gestational hypertension, preeclampsia, superimposed preeclampsia (SIPE), hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome, and eclampsia [1]. The prevalence of HDP has increased over time [2], and HDPs are a leading cause of maternal mortality [3], stillbirth [4], and medically-indicated preterm delivery [5]. They are also associated with an increased risk of long-term cardiovascular morbidity and mortality for women [6].
Weight change during pregnancy is associated with the risk of HDP. Pre-pregnancy [7] and gestational weight loss [8] are associated with a lower chance of HDP in obese women. Conversely, both pre-pregnancy obesity [9] and gestational weight gain in excess of the Institute of Medicine’s recommendations [10] are associated with increased risk of developing HDP. However, most of these studies examined total gestational weight gain [11]. Less is known about whether weight gain at different time points in pregnancy confers differential risk. Gestational weight gain late in pregnancy is associated with increased maternal fat mass [12], whereas excess gestational weight gain is associated with placental maternal vascular malperfusion [13], a risk factor for preeclampsia. One study showed that, especially among women who were of normal weight, excess weight gain prior to 25 weeks’ gestation was associated with a higher prevalence of HDP, especially at term [14]. In addition, it is uncertain whether weight gain is a cause or a consequence of a hypertensive disorder, as the increased capillary permeability and decreased plasma oncotic pressure associated with preeclampsia is associated with fluid retention, often leading to rapid weight gain [15].
Thus, our objective was to examine the relationships between weight change during three different time periods in pregnancy and development of HDP in a cohort of nulliparous women.
Methods
The Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-To-Be (NuMoM2b) is a prospective cohort study in which nulliparous women with singleton pregnancies were enrolled from hospitals associated with eight geographically-diverse clinical centers. Women were eligible for enrollment if they had a live singleton pregnancy, had no previous pregnancy that progressed beyond 20 weeks’ gestation, and were between 6+0 and 13+6 weeks of gestation at recruitment. Exclusion criteria included maternal age younger than 13 years, history of three of more spontaneous abortions, current pregnancy complicated by suspected fatal fetal malformation or known fetal aneuploidy, assisted reproduction with a donor oocyte, multifetal reduction, or plan to terminate the pregnancy. Women were further excluded if they were already participating in an intervention study anticipated to influence pertinent maternal or fetal outcomes, were previously enrolled in this study, or were unable to provide informed consent. Each site’s local governing institutional review board approved the study and all women provided written informed consent prior to participation. Specifically, at Northwestern University, the affiliation of the primary author of this paper, protocol number STU00030933 was approved on 10/1/2010. Full details of the study protocol previously have been published [16].
Data were collected by trained research personnel during three study visits, scheduled to occur between 6+0 and 13+6 weeks’ gestation (visit 1), 16+0 and 21+6 weeks’ gestation (visit 2), and 22+0 and 29+6 weeks’ gestation (visit 3). At least 30 days after delivery, trained and certified chart abstractors reviewed the medical records of all participants and recorded final birth outcomes.
The exposure of interest for this study was weight change in each of three time periods. “Early” weight change was defined as the difference between self-reported pre-pregnancy weight and weight at the first study visit; “mid” weight change was defined as the weight change between the first and second study visits; “late” weight change was defined as the weight change between the second and third study visits. In order to assist with clinical translation of these results, we further characterized weight change in each time period as follows: as the time between visits varied among women, we standardized weight change across visits by calculating a ‘per-week’ value for each time period, then categorized the rates according to the Institute of Medicine’s (IOM) recommendations for weekly weight gain per trimester [17] based on BMI at the first visit. Thus, the weekly rate was categorized as ‘inadequate’, ‘appropriate’, or ‘excessive’ for each time period, using the IOM’s first trimester goal weight gain of 1.1–4.4 pounds total for all women to define ‘appropriate’ for the ‘early’ period, and the second/third trimester weekly weight gain values for both the ‘mid’ and ‘late’ time periods. This approach of creating weight change categories, as opposed to modeling weight change as a linear continuous variable, has been utilized in several other studies examining the association of weight change and pregnancy outcomes, and may aid in clinical translation [18–21].
Women were included only if they had complete data on weight change, timing of visits, pregnancy outcomes, and a body mass index (BMI; weight in kilograms/meter2) value from the initial study visit. We excluded women with a fetal death. We also excluded women who gained or lost more than 4 standard deviations of the mean in one of any of the three time periods in order to guard against implausible values.
The outcome of interest was any new-onset HDP during the pregnancy. Women were considered to have a hypertensive disorder if they had antepartum gestational hypertension, or antepartum, intrapartum, or postpartum (up to 14 days) preeclampsia, eclampsia, or superimposed preeclampsia. In order to define the occurrence of these conditions, we used the 2013 American College of Obstetricians and Gynecologists’ (ACOG’s) Task Force on Hypertension in Pregnancy definitions of hypertensive disease [15]. We chose to analyze all hypertensive disorders of pregnancy together as one outcome, rather than splitting into separate diagnoses (eclampsia, preeclampsia, etc.) as there is often overlap in terms of diagnosis, and clinically they are managed largely in the same way.
We also assessed a number of potential confounding factors. These variables included race/ethnicity (self-reported as non – Hispanic white, non – Hispanic black, Hispanic, Asian, or other), maternal age at first visit (years), maternal BMI at visit 1, maternal education (less than high school, high school graduate, some college, associate’s or technical degree, college graduate, education beyond college), insurance (private, public, or self-pay), gravidity (one, two, three or more), smoking status (defined as having smoked any tobacco product in the three months prior to pregnancy), and maternal diabetes (none, pre-existing, or gestational). Potential confounding variables were retained in multivariable models if they were associated with either weight change or with HDP at a p value of < 0.10. The reference group in these analyses were women whose gestational weight gain was appropriate according to IOM standards.
In sensitivity analysis, we repeated all regressions limiting the sample to women with term deliveries (≥ 37 weeks’ gestation) in an effort to study only women who had the outcome remote from the final measured weight change. We also repeated all regressions limiting the sample to women whose diagnosis of a hypertensive disorder occurred following the final weight measurement, to address concerns with previous studies regarding reverse causality.
For bivariable analyses, we used Spearman’s rho for correlation analyses between two continuous variables, student t tests or one-way ANOVA for comparison of continuous with categorical variables, and chi square tests for comparison of two categorical variables. We used the Hotelling T multivariable test of means to compare weight change across the three different time periods. Finally, we used Poisson regression for multivariable analyses. All analyses were carried out in STATA release 15.0 (StataSoft Corp., College Station, TX). All statistical tests were two-tailed and considered significant at the p < 0.05 level.
Results
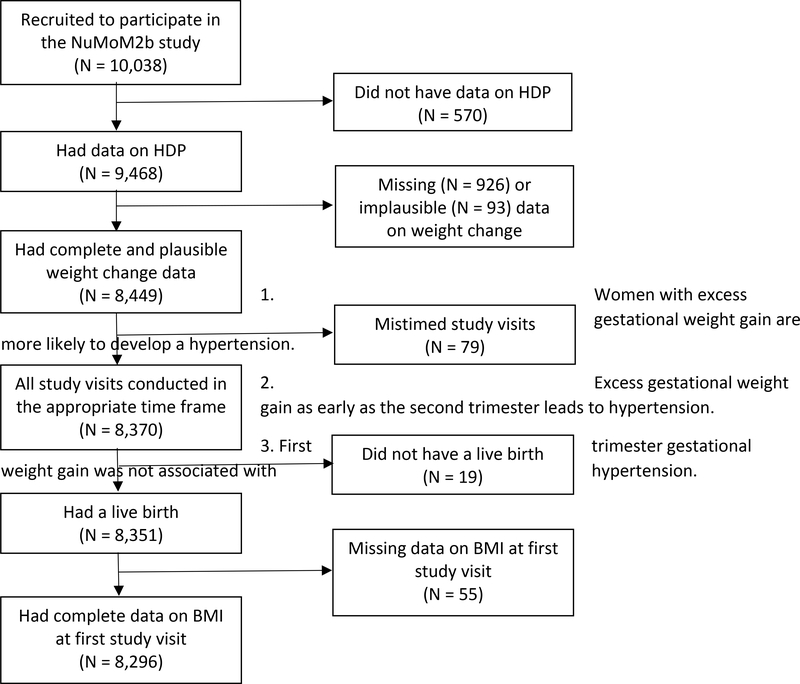
Of the 10,038 women enrolled in the NuMoM2b study, 570 were missing data on presence of HDP and were excluded from this analysis. Another 55 were missing data on initial BMI, 93 had implausible data on weight change for at least one of the three time periods, 926 were missing data on weight gain, and 79 were excluded as at least one of their study visits did not fall within the prescribed time frame. Nineteen women were excluded due to stillbirth. The final population for this analysis included 8,296 women (Figure 1). Women with missing data on HDP had similar weight change as women with complete data on hypertensive disorders in each of the three time periods (p = 0.23, 0.08, and 0.09 for the early, mid, and late periods, respectively).
Figure 1:
Study sample
Mean weight change differed across the three time periods: 4.5 ± 7.2 pounds in the early period, 5.4 ± 4.9 pounds in the mid period, and 10.5 ± 5.8 pounds in the late period (p < 0.001). Many factors were associated with weight change in each of the three time periods, including maternal age, race and ethnicity, educational attainment, diabetes, and smoking (Table 1). Initial visit BMI was negatively associated with weight change in the mid and late time periods (Spearman’s rho = −0.16, p < 0.001 for the mid period, Spearman’s rho = −0.07, p < 0.001 for the late period).
Table 1:
Cohort characteristics
| Variablea | Overall cohort (N = 8,296) | Early-period weight changeb | P valuec | Mid-period weight changeb | P valuec | Late-period weight changeb | P valuec |
|---|---|---|---|---|---|---|---|
| Visit 1 BMI (kg/m2) | 26.1 ± 6.1 | 0.19d | < 0.001 | −0.16d | < 0.001 | −0.07d | < 0.001 |
| Age (years) | 27.2 ± 5.6 | 0.10d | < 0.001 | 0.15d | < 0.001 | −0.10d | < 0.001 |
| Race/ethnicity: | 0.05 | < 0.001 | < 0.001 | ||||
| Non – Hispanic white | 5,260 (63.4) | 4.6 ± 6.2 | 5.6 ± 4.7 | 10.9 ± 5.6 | |||
| Non – Hispanic black | 1,042 (12.6) | 3.9 ± 9.7 | 4.9 ± 5.7 | 9.5 ± 6.6 | |||
| Hispanic | 1,247 (15.0) | 4.4 ± 8.3 | 5.2 ± 4.9 | 10.0 ± 5.8 | |||
| Asian | 335 (4.0) | 4.7 ± 5.4 | 5.7 ± 4.2 | 9.6 ± 4.8 | |||
| Other race | 413 (5.0) | 4.2 ± 8.4 | 5.3 ± 5.2 | 10.7 ± 6.1 | |||
| Education: | 0.02 | < 0.001 | < 0.001 | ||||
| Less than high school | 592 (7.1) | 3.6 ± 9.7 | 4.4 ± 4.9 | 10.7 ± 6.4 | |||
| High school grad | 898 (10.8) | 4.3 ± 9.4 | 5.1 ± 5.6 | 11.0 ± 6.3 | |||
| Some college | 1,564 (18.9) | 4.4 ± 8.1 | 4.8 ± 5.2 | 10.7 ± 6.3 | |||
| Associate/technical degree | 847 (10.2) | 4.4 ± 7.4 | 5.1 ± 4.9 | 10.9 ± 6.0 | |||
| College graduate | 2,397 (28.9) | 4.6 ± 6.0 | 5.7 ± 4.6 | 10.4 ± 5.5 | |||
| Education beyond college | 1,998 (24.1) | 4.7 ± 5.3 | 6.2 ± 4.4 | 10.0 ± 5.1 | |||
| Insurance status: | 0.54 | < 0.001 | 0.31 | ||||
| Public | 2,134 (26.6) | 4.4 ± 9.4 | 5.0 ± 5.4 | 10.6 ± 6.4 | |||
| Other (including military) | 143 (1.8) | 4.1 ± 6.7 | 5.3 ± 5.0 | 10.4 ± 5.4 | |||
| Private | 5,742 (71.6) | 4.5 ± 6.2 | 5.6 ± 4.7 | 10.4 ± 5.5 | |||
| Gravidity: | 0.01 | 0.02 | 0.04 | ||||
| One | 6,242 (75.2) | 4.3 ± 7.1 | 5.4 ± 4.9 | 10.6 ± 5.8 | |||
| Two | 1,553 (18.7) | 4.7 ± 7.2 | 5.5 ± 4.9 | 10.4 ± 5.9 | |||
| Three or more | 501 (6.0) | 5.3 ± 7.6 | 6.0 ± 4.8 | 9.9 ± 5.7 | |||
| Smoked within 3 months of pregnancy: | < 0.001 | < 0.001 | 0.002 | ||||
| Smoked | 1,439 (17.4) | 5.4 ± 8.7 | 6.0 ± 5.4 | 10.9 ± 6.3 | |||
| Did not smoke | 6,854 (82.7) | 4.3 ± 6.8 | 5.3 ± 4.8 | 10.4 ± 5.7 | |||
| Diabetes: | < 0.001 | 0.02 | < 0.001 | ||||
| None | 7,832 (94.4) | 4.4 ± 7.1 | 5.5 ± 4.9 | 10.6 ± 5.8 | |||
| Pre-existing | 119 (1.4) | 5.8 ± 9.0 | 4.2 ± 5.1 | 8.6 ± 6.4 | |||
| Gestational | 345 (4.2) | 5.9 ± 8.6 | 5.4 ± 5.1 | 9.3 ± 5.8 |
Data presented mean ± standard deviation for continuous variables, N(%) for categorical variables
Data presented as Spearman’s correlation coefficient between weight change for continuous variables, weight change mean ± standard deviation for categorical variables
P value for Spearman’s correlation coefficient for continuous variables, one-way ANOVA for categorical variables.
Spearman’s correlation coefficient
Among women in the study population, 1,564 (18.9%) developed a HDP. Of these, 851 (54.4%) developed gestational hypertension, 667 (42.6%) developed preeclampsia, 41 (2.6%) developed superimposed preeclampsia, and 5 (0.3%) developed eclampsia. Onset of hypertension occurred at a mean of 24.3 ± 4.7 weeks following the early weight change measurement, 17.4 ± 4.8 weeks following the mid weight change measurement, and 8.7 ± 4.8 weeks following the late weight change measurement.
In bivariable analyses, the development of HDP was significantly associated with the amount of weight change in each of the three time periods (Table 2). In multivariable analyses, this association remained significant for excessive weight gain in the mid and late periods (mid period: adjusted incidence rate ratio [aIRR] = 1.16, 95% confidence interval (CI) = 1.01–1.35; late period: aIRR = 1.19, 95% CI = 1.02–1.40). A higher initial BMI as well as pre-existing and gestational diabetes also were significantly associated with HDP in multivariable models (aIRR 1.05, 95% CI 1.04–1.06 for BMI, aIRR 1.46, 95% CI 1.08–1.97 for pre-existing diabetes, and aIRR 1.28, 95% CI 1.04–1.58 for gestational diabetes). Limiting our sample to the 7,674 women with term deliveries did not substantively change our results (Table 3). Similarly, limiting our sample to the 8,188 women whose diagnosis of an HDP occurred following the final weight measurement did not change our findings in terms of significance or direction (Table 4).
Table 2:
Association of weight change with hypertensive disorders of pregnancy, with weight change standardized to Institute of Medicine trimester-specific guidelines based on pre-pregnancy body mass index
| Variable | Hypertensive disorder of pregnancy (N = 1,564) | No Hypertensive disorder of pregnancy (N = 6,732) | P valuea | Unadjusted incidence rate ratio | 95 % confidence interval | Adjusted incidence rate ratiob | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Early weight change (IOM category): | 0.004 | ||||||
| Inadequate | 417 (26.7) | 2,030 (30.2) | 0.93 | 0.81 – 1.07 | 0.89 | 0.77 – 1.03 | |
| Adequate | 358 (22.9) | 1,600 (23.8) | (ref) | (ref) | |||
| Excessive | 789 (50.5) | 3,102 (46.1) | 1.11 | 0.98 – 1.26 | 0.95 | 0.83 – 1.08 | |
| Mid weight change (IOM category): | < 0.001 | ||||||
| Inadequate | 587 (37.5) | 2,794 (41.5) | 1.06 | 0.91 – 1.23 | 1.03 | 0.89 – 1.21 | |
| Adequate | 243 (15.5) | 1,244 (18.5) | (ref) | (ref) | |||
| Excessive | 734 (46.9) | 2,694 (40.0) | 1.31 | 1.13 – 1.51 | 1.16 | 1.01 – 1.35 | |
| Late weight change (IOM category): | < 0.001 | ||||||
| Inadequate | 184 (11.8) | 1,091 (16.2) | 0.94 | 0.77 – 1.16 | 0.80 | 0.64 – 0.99 | |
| Adequate | 177 (11.3) | 979 (14.5) | (ref) | (ref) | |||
| Excessive | 1,203 (76.9) | 4,662 (69.3) | 1.34 | 1.14 – 1.57 | 1.20 | 1.02 – 1.40 |
P value for t tests for continuous variables, chi square tests for categorical variables.
In addition to the factors listed here, multivariable models also adjusted for maternal BMI at first visit, maternal age at first visit, maternal race, maternal education, insurance status, gravidity, smoking within 3 months of pregnancy, and maternal diabetes, all of which were significant at the p < 0.10 level with either weight gain or hypertensive disorders in bivariable analyses, but not in multivariable analyses. These incidence rate ratios also account for fixed effects by study site and subsite.
Table 3:
Association of weight change with hypertensive disorders of pregnancy, with sample limited to women with term deliveries
| Variable | Hypertensive disorder of pregnancy (N = 1,348) | No Hypertensive disorder of pregnancy (N = 6,326) | P valuea | Unadjusted incidence rate ratio | 95 % confidence interval | Adjusted incidence rate ratiob | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Early weight change (IOM category): | 0.05 | ||||||
| Inadequate | 365 (27.1) | 1,902 (30.1) | 0.93 | 0.80 – 1.08 | 0.88 | 0.75 – 1.03 | |
| Adequate | 314 (23.3) | 1,491 (23.6) | (ref) | (ref) | |||
| Excessive | 669 (49.6) | 2,933 (46.4) | 1.07 | 0.93 – 1.22 | 0.91 | 0.79 – 1.05 | |
| Mid weight change (IOM category): | < 0.001 | ||||||
| Inadequate | 489 (36.3) | 2,621 (41.4) | 1.01 | 0.86 – 1.19 | 1.00 | 0.85 – 1.18 | |
| Adequate | 218 (16.2) | 1,183 (18.7) | (ref) | (ref) | |||
| Excessive | 641 (47.6) | 2,522 (39.9) | 1.30 | 1.12 – 1.52 | 1.17 | 1.00 – 1.37 | |
| Late weight change (IOM category): | < 0.001 | ||||||
| Inadequate | 148 (11.0) | 1,018 (16.1) | 0.89 | 0.71 – 1.12 | 0.76 | 0.60 – 0.96 | |
| Adequate | 151 (11.2) | 907 (14.3) | (ref) | (ref) | |||
| Excessive | 1,049 (77.8) | 4,401 (69.6) | 1.35 | 1.14 – 1.60 | 1.19 | 1.01 – 1.42 |
P value for t tests for continuous variables, chi square tests for categorical variables.
In addition to the factors listed here, multivariable models also adjusted for maternal BMI at first visit, maternal age at first visit, maternal race, maternal education, insurance status, gravidity, smoking within 3 months of pregnancy, and maternal diabetes, all of which were significant at the p < 0.10 level with either weight gain or hypertensive disorders in bivariable analyses, but not in multivariable analyses. These incidence rate ratios also account for fixed effects by study site and subsite.
Table 4:
Association of weight change with hypertensive disorders of pregnancy, with sample limited to women whose diagnosis of a hypertensive disorder occurred following all study visits.
| Variable | Hypertensive disorder of pregnancy (N = 1,456) | No Hypertensive disorder of pregnancy (N = 6,732) | P valuea | Unadjusted incidence rate ratio | 95 % confidence interval | Adjusted incidence rate ratiob | 95% confidence interval |
|---|---|---|---|---|---|---|---|
| Early weight change (IOM category): | 0.01 | ||||||
| Inadequate | 389 (26.7) | 2,030 (30.2) | 0.92 | 0.80 – 1.07 | 0.89 | 0.77 – 1.04 | |
| Adequate | 337 (23.2) | 1,600 (23.8) | (ref) | (ref) | |||
| Excessive | 730 (50.1) | 3,102 (46.1) | 1.09 | 0.63 – 1.25 | 0.95 | 0.83 – 1.08 | |
| Mid weight change (IOM category): | < 0.001 | ||||||
| Inadequate | 544 (37.4) | 2,794 (41.5) | 1.04 | 0.89 – 1.21 | 1.02 | 0.87 – 1.19 | |
| Adequate | 232 (15.9) | 1,244 (18.5) | (ref) | (ref) | |||
| Excessive | 680 (46.7) | 2,694 (40.0) | 1.28 | 1.10 – 1.49 | 1.14 | 1.01 – 1.33 | |
| Late weight change (IOM category): | < 0.001 | ||||||
| Inadequate | 167 (11.5) | 1,091 (16.2) | 0.93 | 0.75 – 1.15 | 0.79 | 0.63 – 0.99 | |
| Adequate | 163 (11.2) | 979 (14.5) | (ref) | (ref) | |||
| Excessive | 1,126 (77.3) | 4,662 (69.3) | 1.36 | 1.16 – 1.61 | 1.21 | 1.02 – 1.43 |
P value for t tests for continuous variables, chi square tests for categorical variables.
In addition to the factors listed here, multivariable models also adjusted for maternal BMI at first visit, maternal age at first visit, maternal race, maternal education, insurance status, gravidity, smoking within 3 months of pregnancy, and maternal diabetes, all of which were significant at the p < 0.10 level with either weight gain or hypertensive disorders in bivariable analyses, but not in multivariable analyses. These incidence rate ratios also account for fixed effects by study site and subsite.
Discussion
We demonstrated that gestational weight gain between 16–22 weeks’ gestation and between 22–30 weeks’ gestation that was in excess of the Institute of Medicine’s trimester specific recommendations was associated with increased risk of HDP among nulliparous women. Conversely, gestational weight gain between 6–13 weeks’ gestation was not associated with risk of HDP. Pre-pregnancy weight also has been shown to be a risk factor for HDP, independent of gestational weight gain [7]. Our study also shows an increased risk of HDP with higher initial BMI, even after adjustment for gestational weight gain.
Gestational weight gain previously has been associated with increased risk of HDP [22, 23]. One key question that impedes interpretation of results from earlier studies, however, is whether that association is a consequence of reverse causality. That is, does weight gain precede the development of HDP, or does it actually occur after the disease, which is characterized by increased capillary permeability and extra-vascular extravasation of fluid. Our data indicate that the extent of weight gain is indeed a risk factor for the subsequent development of HDP, as all of the weight gain studied in this sample preceded 30 weeks’ gestation, and thus typically preceded development of an overt HDP by many weeks. These results are congruent with those of Ruhstaller et. al. [24], who found that excess weight gain prior to 28 weeks’ gestation was associated with a higher risk of HDP in women with chronic hypertension; Hutcheon et. al., who measured gestational weight gain through 25 weeks in Sweden [14]; and of MacDonald-Wallis et. al., who examined gestational weight gain through 18 weeks in the United Kingdom [25]. Our study generalizes these previous findings in a cohort of nulliparous women in the United States. Most clinical guidelines recommend monitoring women in pregnancy for rapid weight gain as a manifestation of fluid retention and possible development of a HDP [15]; these data do not contradict these guidelines.
Pre-pregnancy weight and gestational weight gain are both potentially modifiable risk factors for HDP. Several studies have evaluated interventions aimed at reducing GWG, including dietary counseling [26] and exercise [27], although as yet there is no weight-modification method that has been shown consistently to lead to better pregnancy outcomes, including decreased risk of HDP. Randomized trials of higher-risk groups of women, such as those with gestational diabetes, suggest that even relatively small and late reductions in the amount of weight gain are associated with a lower risk of HDP [28]. Our data support that weight gain may be a modifiable factor in the development of HDP in a general population of women. Further studies and trials may help to determine whether particular interventions may both contribute to optimizing weight gain and/or lowering the risk for HDP.
This analysis has strengths, including a racially and geographically diverse cohort of nulliparous women from the United States, multiple objective measurements of weight change at different points in time, and prospectively-collected and detailed data. This paper also has limitations that should be acknowledged. Women with missing data may differ from women with complete data in ways that biases results. While the NuMoM2b cohort is diverse and was selected to represent the population of nulliparous pregnant women in the United States, these results may still not be generalizable to all nulliparous pregnant women. Also, this study cannot be generalized to women not included, such as multiparous women. There may be other confounding variables for which we could not account. Finally, as this is an observational study, all findings are correlational only.
In conclusion, we demonstrated that gestational weight gain in excess of the IOM guidelines as early as the second trimester was associated with increased risk of development of HDP, adding evidence that indicates weight gain precedes, rather than follows, the onset of clinically-apparent HDP.
Acknowledgments
FUNDING: Support for the NuMoM2b study was provided by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development: U10 HD063036, RTI International; U10 HD063072, Case Western Reserve University; U10 HD063047, Columbia University; U10 HD063037, Indiana University; U10HD063041, University of Pittsburgh; U10 HD063020, Northwestern University; U10 HD063046, University of California Irvine; U10 HD063048, University of Pennsylvania; and U10 HD063053, University of Utah. In addition, support was provided by respective Clinical and Translational Science Institutes to Indiana University (UL1TR001108) and University of California Irvine (UL1TR000153). The funding sources had no role in the study design, the collection/interpretation/analysis of these data, or the decision to submit this manuscript for publication.
Footnotes
CONFLICT OF INTEREST: Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Centers for Disease Control and Prevention. Data on selected pregnancy complications in the United States: hypertensive disorders, 1993–2014. 2017.
- [2].Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–306. [DOI] [PubMed] [Google Scholar]
- [3].Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hirst JE, Villar J, Victora CG, Papageorghiou AT, Finkton D, Barros FC, et al. The antepartum stillbirth syndrome: risk factors and pregnancy conditions identified from the INTERGROWTH-21(st) Project. BJOG. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Canoy D, Cairns BJ, Balkwill A, Wright FL, Khalil A, Beral V, et al. Hypertension in pregnancy and risk of coronary heart disease and stroke: A prospective study in a large UK cohort. Int J Cardiol. 2016;222:1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adane AA, Mishra GD, Tooth LR. Adult Pre-pregnancy Weight Change and Risk of Developing Hypertensive Disorders in Pregnancy. Paediatr Perinat Epidemiol. 2017;31(3):167–75. [DOI] [PubMed] [Google Scholar]
- [8].Beyerlein A, Schiessl B, Lack N, von Kries R. Associations of gestational weight loss with birth-related outcome: a retrospective cohort study. BJOG. 2011;118(1):55–61. [DOI] [PubMed] [Google Scholar]
- [9].Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol. 2015;125(1):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ren M, Li H, Cai W, Niu X, Ji W, Zhang Z, et al. Excessive gestational weight gain in accordance with the IOM criteria and the risk of hypertensive disorders of pregnancy: a meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kominiarek MA, Saade G, Mele L, Bailit J, Reddy UM, Wapner RJ, et al. Association Between Gestational Weight Gain and Perinatal Outcomes. Obstet Gynecol. 2018;132(4):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Widen EM, Factor-Litvak PR, Gallagher D, Paxton A, Pierson RN Jr., Heymsfield SB, et al. The Pattern of Gestational Weight Gain is Associated with Changes in Maternal Body Composition and Neonatal Size. Matern Child Health J. 2015;19(10):2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scifres CM, Parks WT, Feghali M, Caritis SN, Catov JM. Placental maternal vascular malperfusion and adverse pregnancy outcomes in gestational diabetes mellitus. Placenta. 2017;49:10–5. [DOI] [PubMed] [Google Scholar]
- [14].Hutcheon JA, Stephansson O, Cnattingius S, Bodnr LM, Wikstrom AK, Johansson K. Pregnancy weight gain before diagnosis and risk of pre eclampsia: A population-based cohort study in nulliparous women. Hypertension. 2018. August;72(2):433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].American College of Obstetrians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- [16].Haas DM, Parker CB, Wing DA, Parry S, Grobman WA, Mercer BM, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b). Am J Obstet Gynecol. 2015;212(4):539 e1–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Collection: Reports funded by National Institutes of Health; Washington (DC)2009. [PubMed] [Google Scholar]
- [18].Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol. 2013;208(3):205 e1–7. [DOI] [PubMed] [Google Scholar]
- [19].Bogaerts A, Van den Bergh BR, Ameye L, Witters I, Martens E, Timmerman D, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol. 2013;122(5):999–1009. [DOI] [PubMed] [Google Scholar]
- [20].Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, Smulian JC. Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol. 2007;110(6):1319–25. [DOI] [PubMed] [Google Scholar]
- [21].McBain RD, Dekker GA, Clifton VL, Mol BW, Grzeskowiak LE. Impact of inter-pregnancy BMI change on perinatal outcomes: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2016;205:98–104. [DOI] [PubMed] [Google Scholar]
- [22].Heude B, Thiebaugeorges O, Goua V, Forhan A, Kaminski M,Foliguet B, et al. Pre-pregnancy body mass index and weight gain during pregnancy: relations with gestational diabetes and hypertension, and birth outcomes. Matern Child Health J. 2012;16(2):355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fortner RT, Pekow P, Solomon CG, Markenson G, Chasan-Taber L. Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. Am J Obstet Gynecol. 2009;200(2):167 e1–7. [DOI] [PubMed] [Google Scholar]
- [24].Ruhstaller KE, Bastek JA, Thomas A, McElrath TF, Parry SI, Durnwald CP. The Effect of Early Excessive Weight Gain on the Development of Hypertension in Pregnancy. Am J Perinatol. 2016;33(12):1205–10. [DOI] [PubMed] [Google Scholar]
- [25].Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2013;209(4):327 e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Di Carlo C, Iannotti G, Sparice S, Chiacchio MP, Greco E, Tommaselli GA, et al. The role of a personalized dietary intervention in managing gestational weight gain: a prospective, controlled study in a low-risk antenatal population. Arch Gynecol Obstet. 2014;289(4):765–70. [DOI] [PubMed] [Google Scholar]
- [27].Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol. 2011;204(5):402 e1–7. [DOI] [PubMed] [Google Scholar]
- [28].Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]