Abstract
Extracellular vesicles (EVs), derived from cell membranes, demonstrate the potential to be excellent therapeutics and drug carriers. Although EVs are promising, the process to develop high-quality and scalable EVs for their translation is demanding. Within this research, we analyzed the production of EVs, their purification and their post-bioengineering, and we also discussed the biomedical applications of EVs. We focus on the developments of methods in producing EVs including biological, physical, and chemical approaches. Furthermore, we discuss the challenges and the opportunities that arose when we translated EVs in clinic. With the advancements in nanotechnology and immunology, genetically engineering EVs is a new frontier in developing new therapeutics in order to tailor to individuals and different disease stages in treatments of cancer and inflammatory diseases.
Keywords: Extracellular vesicles, Microvesicles, Biomimetic Vesicles, Exosomes, Nitrogen cavitation
1. Introduction
Nanotechnology has been exploited in pharmaceutical industries for several decades in order to improve the efficacy of drug administration [1–4]. For example, liposomes[5] and polymeric nanoparticles [6, 7] have been used to encapsulate drugs for improving their solubility and bioavailability. Targeting ligands (e.g. antibodies and peptides) are conjugated to the surface of nanoparticles[6, 8] to allow the delivery of nanoparticles to specific cell types in diseased tissues. Although there are advances in synthetic chemistry and bioengineering for design of targeted drug delivery systems[6, 7, 9, 10], the simple bio-functionalization of nanoparticles could not avoid the rapid clearance by immune systems. The human body is a complex system, thus it is challenging that pre-designed nanoparticles can efficiently deliver therapeutics to disease lesions[11–13].
Cells in the body constantly release cell membrane compartments to deliver signaling molecules (such as proteins or DNA) to adjacent cells or in distant organs. These compartments are called extracellular vesicles (EVs). EVs have been utilized in diagnosis[14], and drug delivery[15, 16] due to their exceptional biocompability, intrinsically targeting specific molecules and maintaining long circulation times [17].
According to ISEV (The international Society for Extracellular Vesicles), EVs are broadly defined as naturally released particles, which are comprised of the lipid bilayer membrane[18]. Specifically, EVs are spherical membrane compartments, which contain lipids, proteins and various nucleic acid species from their parent cells, acting as critical mediators between cells that regulate both physiological and pathological conditions in the body [19, 20]. Based on their biogenesis and sizes, EVs are categorized into three types: exosomes, microvesicles, and apoptotic bodies. Exosomes are produced from endosomal compartments in the cells. The sizes of exosomes are 30 to 100 nm in diameter. Microvesicles are directly generated through outward budding and fission of the cell plasma membrane with sizes that range between 50–2000 nm. When cells are dying, the cell membrane is disrupted to form apoptotic bodies with the sizes of 50 to 5000 nm [21, 22]. Due to the size similarity in EVs, it is challenging to separate the subpopulations of EVs [21]. The biomarkers of EVs may be used to differentiate between the three types of EVs based on their biogenesis.
Both eukaryotic and prokaryotic cells can produce EVs[23], but prokaryotic cells (such as gram-positive and gram-negative bacteria) generate a unique structure in which bacteria bud their outer membrane to form nanosized vesicles, called the outer membrane vesicles (OMVs). OMVs have been developed for drug delivery and vaccination[24, 25]. In this review, our focus is to discuss the current developments in EVs. The EVs were first observed in culture media. The generation of EVs using cell culture techniques remains challenging to translate EVs in the clinic due to their low production and reproducibility[26]. Therefore, it is urgent to develop new and innovative technologies to generate high-quality, reproducible EVs that could be scaled up for the advancement of biomedical applications.
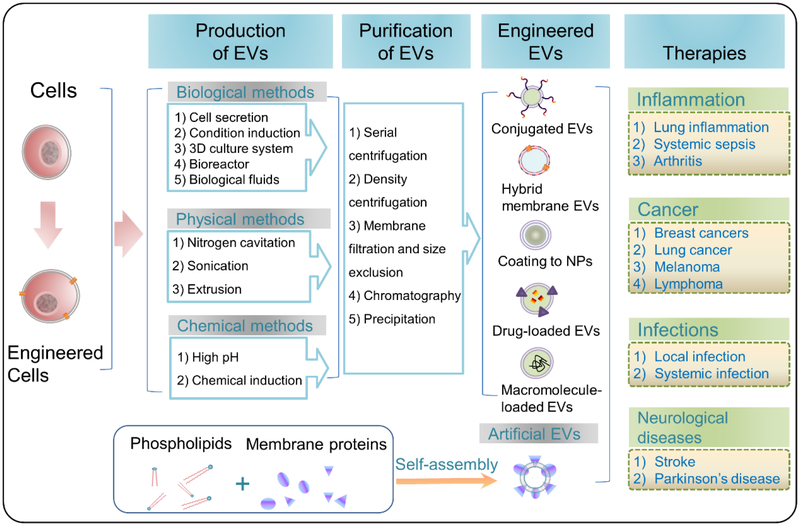
Herein, we will review the current technologies and approaches used in the production of EVs for drug delivery. We also discuss what types of cells have been utilized to generate EVs, and summarize their biomedical applications in a wide range of disease models. Finally, we address the current challenges in technologies and methods in the translation of EVs and the future directions for EVs-based personalized and precision nanomedicine. Fig. 1 is the summary of the current status on EVs technologies and their biomedical applications.
Fig. 1.
Schematic illustration of approaches and technologies used in the generation of EVs, their purification and their bioengineering for a wide range of biomedical applications. In addition, artificial EVs are self-assembled using phospholipids and cell membrane proteins.
2. Generation of EVs
EVs were first discovered in reticulocytes in the early 1980s. The progress in the instruments of ultracentrifugation and electron microscopes enabled to visualize the structures of EVs[27]. Since then, a wide range of cells showed to secrete EVs, such as tumor cells and immune cells. The recent findings showed that EVs can transport signaling molecules (proteins and nucleic acid contents) to regulate physiological functions. Thus, EVs may be a novel drug delivery platform. However, there are many limits in the current technologies used in the production of EVs. The natural secretion of EVs is a very slow process and the compositions and structures of EVs are heterogeneous. Therefore, it is difficult to translate EVs to the clinic, and demanding to develop other methods to generate EVs. The following summarizes three major approaches: 1) biological methods; 2) physical methods; 3) chemical methods.
2.1. EVs Produced by Biological Methods
2.1.1. Cell Secretion
When cells grow in culture media, they spontaneously release EVs. Using this approach, EVs can be generated from a wide range of cells: human primary bronchial epithelial cell[28], epithelial cells[29], EBV(Epstein-Barr virus)-immortalized B cells[30], HUVECs (Human umbilical vein endothelial cells)[31], leukocytes[32], platelets[33], stem cells[34], cancer cells[35] and even neural cells[36]. Secretion of EVs is a slow and costly process. To scale up the production of EVs, it is required to increase culture media volumes. The purification of EVs requires to concentrate the culture media.
2.1.2. Environmental Induction
Under certain culture conditions, cells can speed up the release of EVs and their compositions are strongly dependent on culture conditions. The radiation or serum starvation can promote the secretion of EVs containing unique surface markers. Kucharzewska et al. [37]reported that TSAP6, a p53-regulated gene product, triggered the exosome release from lung cancer cells after γ-radiation. Aharon et al.[38] reported that monocyte cells (THP-1) after the treatment by serum starvation or the stimulation by endotoxin and calcium ionophore A23187 resulted in the release of microvesicles that expressed several exosome markers, such as Tsg 101, leaflet phospholipids, and monocyte markers (CD18, CD14).
In case of in vivo studies, some disease conditions can dramatically increase the concentrations of EVs in the body fluids[38, 39]. Baran et al[39] found that the number of microvesicles in cancer patients was significantly elevated in all stages compared to that of all healthy persons, and the microvesicles possessed membrane proteins of CCR6 and HER-2/neu as the biomarkers.
Although it is interesting to study the secretion of EVs in disease conditions for diagnosis [40], the increased production of EVs and their upregulated targeting proteins may allow to exploit these EVs for development of EVs-based drug delivery systems. A discussion on the body fluids that are collected to isolate EVs for drug delivery is discussed in the following sections.
2.1.3. 3D Culture System
To solve the low yield of EVs in 2D cell culture systems, 3D cell culture systems or hollow fiber culture systems have been established [41–43]. Rocha et al. [43] established a 3D cell culture microwell array for EV production and compared EVs derived from gastric cancer cells in a 2D culture system to those made in a 3D culture system by analyzing RNA and proteins. They found that the 3D culture system was more efficient to produce EVs than the 2D culture system. Interestingly, the upregulation of microRNA and downregulation of some proteins were observed in the 3D system, suggesting that the cellular architecture plays a central role in regulating cellular functions. The data may imply that the 3D architecture in vivo could generate the unique EVs compared to those in vitro.
2.1.4. Bioreactor System
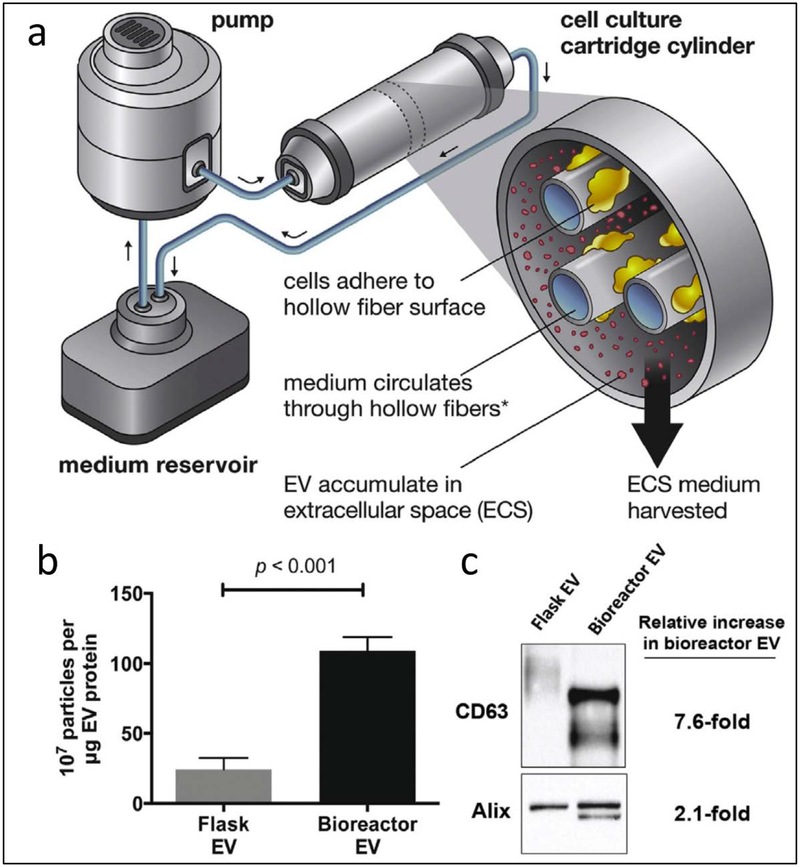
To scale up the production of EVs in a cell culture system, a bioreactor system was proposed to continuously generate EVs. Watson et al. [41] developed a hollow-fiber bioreactor comprised of a pump, a medium reservoir and a cell culture cartridge cylinder (Fig. 2a). HEK293 cells were grown on hollow fiber surfaces and the medium circulated through the hollow fibers. The result showed that the production of EVs using a bioreactor system was 5 times higher than that made using flask cell culture (Fig. 2b). CD63 and Alix are the markers of EVs released from the multivesicular body and plasma membrane. The bioreactor system more efficiently produced EVs compared to the conventional cell culture after the analysis of Western blots of the biomarkers of CD63 and Alix (Fig. 2c). The bioreactor system demonstrates a potential tool to scale up the production of EVs in the clinic.
Fig. 2.
Bioreactor system for improved production of EVs. (a) Schematic of a hollow fiber bioreactor system comprised of a pump, a medium reservoir and a cell culture cartridge cylinder. (b) HEK293 cells cultured in a hollow fiber bioreactor produced EVs with 5 fold higher than using tissue culture flasks. Data presented as mean ± SEM and statistical significance was compared by ANOVA. (c) Western blot analysis showed 7.6-fold and 2.1-fold increase in EV-associated markers of EVs made by hollow-fiber bioreactor compared to that using tissue culture flask. CD63 and Alix are markers for EVs. Adapted from Watson et al. 2016 [41].
2.1.5. Biological Fluid Isolation
Recent studies show that the body fluid samples contain a lot of EVs because cells constantly release EVs into the bloodstream. EVs have been found in tears[44], urine[45], salivary[46], blood [47–50] and even milk[51], which are strongly correlated with physiological and pathological conditions. For example, cancer patients highly secrete the unique EVs that possess the tumor cell markers. Because the pathological conditions promote the expression of disease proteins in EVs, the collection of biological fluids may be used to enrich these EVs for targeted drug delivery [48].
2.2. EVs Produced by Physical Methods:
The central component of EVs is made of cell membrane and the cell plasma membrane-formed vesicles are dominant in EVs[15, 16]. EVs made using natural secretion approaches have the low yield, the heterogeneity in their compositions and the less reproducibility [16]. It is urgent to develop new and novel methods to solve these problems. The recent physical methods to make EVs with nitrogen cavitation, membrane porous extrusion and sonication will be discussed. These methods rely on the physical forces to break cell membrane, allowing cytosol contents to be released from a cell. The disrupted cell membrane then reform to small vesicles. This physical method may have the advantage to make a large amount of EVs used in clinic.
2.2.1. Nitrogen Cavitation
Cavitation is a physics term meaning that a rapid pressure change in a liquid causes the formation of many small vapor-filled cavities. When these cavities (so-called “bubbles” or “voids”) collapse, they can generate an intense shock forcing objects to break. Nitrogen cavitation means that nitrogen gas is chosen to generate the pressure to form cavitation forces. Nitrogen cavitation was first used to generate EVs from white blood cells in the Wang’s group[15, 16]. This process allows a cell suspension to take place in a cavitation chamber and nitrogen to be filled, which increases the pressure, diffusing nitrogen inside the cells. When the pressure suddenly dropped, nitrogen came out to form bubbles inside the cells, generating a strong force to break them. Cytosol contents were quickly released from the cells, and the vesicular structures of the cell membrane were formed.
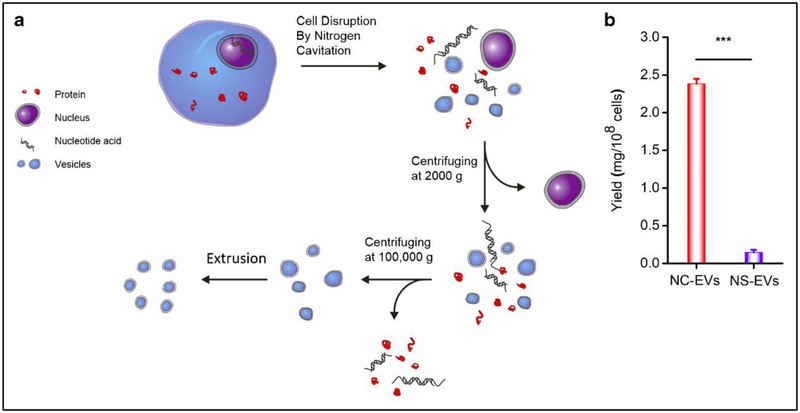
To prove this concept, Gao et al. [15] performed nitrogen cavitation to generate neutrophil-derived membrane nanovesicles and studied their biological compositions (Fig. 3a). They found that 50–75% of cell plasma membrane formed the vesicles with diameters of 180–200 nm, suggesting that nitrogen cavitation is a novel method to generate the high-purity of EVs from neutrophils. Furthermore, they compared the production yield of EVs using nitrogen cavitation to that using the natural secretion approach [16] (Fig. 3b). They found that the yield of EVs made by nitrogen cavitation increased by 16 folds. They also performed the Western blots and proteomics of EVs, finding that the nitrogen cavitation approach generates the pure plasma membrane-formed vesicles without cellular organelles (such as ER, lysosomes, mitochondria). These studies show that nitrogen cavitation is a new method to produce EVs from a wide range of cell lines and it is ready to scale up their production.
Fig. 3.
Generation of EVs by nitrogen cavitation. (a) Scheme of a preparation process of EVs by nitrogen cavitation and their purification. Adapted from Gao et al. 2016 [15]. (b) Yield of EVs generated by nitrogen cavitation (NC-EVs) and by natural secretion (NS-EVs), and quantified by the amount of proteins in EVs. Adapted from Gao et al. 2017 [16].
2.2.2. Extrusion via Porous Membrane
Extrusion is a process that allows soft materials to maneuver through a porous membrane to make nanoparticles such as liposomes, emulsion nanoparticles, nanofibers, and nanotubes[52, 53]. Jang et al. [54] used a serial porous membrane with the pore sizes of 10 to 1000 nm to break monocytes to form nanoscale vesicles, which were loaded with chemotherapeutics. The results showed that EVs made by the extrusion approach were similar to naturally secreted exosomes. Interestingly, the yield of EVs using extrusion was 100 times higher than that using culture secretion. Although they showed high production rate of EVs, the authors did not analyze the biological compositions of EVs produced by extrusion method. The extrusion approach cannot eliminate intracellular components, which can cause toxicity when these EVs are used in clinic.
2.2.3. Sonication
Sonication is a commonly used tool for the preparation of liposomes [55], and it was also exploited for the EV preparation. Thamphiwatana et al.[56] developed macrophage derived biomimetic nanoparticles using the sonication approach. In their studies, the mouse macrophages were disrupted under sonication to form nanosized vesicles. The nanovesicles showed the improved treatment of sepsis in the mouse model.
2.3. EVs Produced by Chemical Methods
2.3.1. High pH Solution Induced Formation of EVs
Chemical agents may be used to peel the cell membrane, such as alkaline solution can dissolve the cell membrane. After the cell membrane suspension was neutralized, the membrane components may self-assembly to form EVs under sonication. Using the method, Go et al. [57] prepared extracellular vesicle–mimetic ghost nanovesicles from human U937 monocytes and the vesicles were loaded with dexamethasone. The authors showed that the nanovesicles had similar physical features when compared with naturally released EVs. Interestingly there was a 200‐fold increase in production of EVs compared to that using the cell culture method. Furthermore, they showed that the nanovesicles did not contain intracellular compartments, such as cytosolic proteins and nucleic acids. The nanovesicles was capable to mitigate OMV‐induced SIRS (systemic inflammatory response syndrome).
2.3.2. Chemically-induced Formation of EVs
Chemical agents can cause the cell membrane blebbing to form plasma membrane-derived vesicles. Recently, Ingato et al. [58] proposed to use sulfhydryl-blocking agents to peel cell membrane and they found that this chemical approach can generate the EVs using DTT (dithiothreitol) and PFA (paraformaldehyde) in 2 h at 10 times higher than those using natural secretion in 48 h. Their studies showed that chemical methods could be a novel approach for the rapid and large-scale generation of EVs in the future. In addition, saturated fatty acid can stimulate renal tubular epithelial cells to allow the cells to secrete EVs[59].
2.4. Artificial Biomimetic EVs
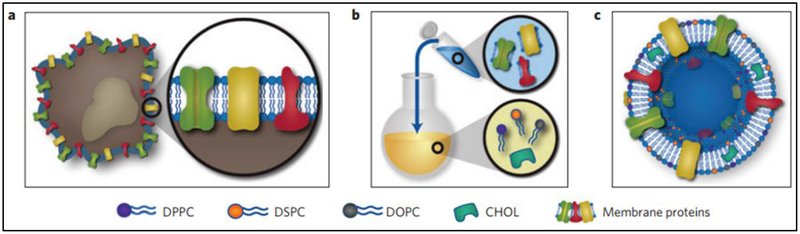
Cell-derived EVs are promising for the next generation of nanomedicines, but it remains unknown whether current technologies enable EVs to move to the clinic because of limited cell resources and unknown immunotoxicity. Inspired with the structure of EVs, a top-down synthetic strategy to make EVs-like nanoparticles was proposed. In the study [60], the authors incorporate leukocyte plasma membrane proteins into lipid-formed nanovesicles (Fig. 4). Leukosomes, proteolipid vesicles, were formulated similar to that of liposomes, but they have the tissue targeting capability. For examples, these leukosomes preferentially targeted inflamed vasculature, improving the delivery of dexamethasone to inflamed tissues. This study may offer another option to generate EVs-like vesicles in order to help solve the translation barriers for EVs.
Fig. 4.
Leukosome synthesis and formulation. (a) Extraction of proteolipid material from murine J774 macrophages. (b) Protein enrichment of the phospholipid film. (c) Vesicular formulation of leukosomes. DPPC, 1, 2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, 1, 2-distearoyl-sn-glycero-3-phosphocholine; DOPC, 1, 2-dioleoyl-sn-glycero-3-phosphocholine; CHOL, cholesterol. Adapted from Molinaro et al. 2016 [60].
3. Purification of EVs
EVs are secreted from culture media, which make their compositions complex and heterogeneous. It is important to develop novel approaches to isolate EVs and concentrate them. Several approaches and methods have been reported, such as differential centrifugation, density gradient centrifugation, chemical precipitation, microbead capture technology, size exclusion chromatography, and membrane filtration[61]. Here, we review several approaches in purifying EVs.
3.1. Serial Centrifugation
After culture media containing naturally secreted EVs are collected, the differential centrifugation is used to eliminate dead cells, cell debris and some organelles. To obtain EVs, the high-speed centrifugation is used at 100 000 g to obtain a pellet of EVs. Due to the large centrifugation force, it is likely that pelleted EVs form a lump, which may be difficult to resuspend. Overall, the timing and the speed of centrifugation are critical [16].
3.2. Density Gradient Centrifugation
Density gradient centrifugation is a method in which the components of a sample are separated based on their density. Iwai et al. [46] performed a step gradient media comprised of sucrose (2.0, 1.6, 1.18 and 0.8 M), iohexol (50, 40, 30, and 20%) and iodixanol solution (47, 37, 28 and 18%), and subsequently the sample was centrifuged at 160,000×g for 17 h at 4°C to isolate EVs. The results showed that this technique can produce high-quality EVs based on the analysis of markers of EVs using Western blots and other tools. The problem of this method is costly and time-consuming.
3.3. Membrane Filtration and Size Exclusion.
Heinemann et al.[62] developed a three-step protocol to isolate exosomes from either cell culture media or large volumes of biofluid. This method was comprised of dead-end pre-filtration, tangential flow filtration (TFF) and low-pressure track-etched membrane filtration. TFF is a pressure-driven separation process to concentrate a suspension. When the fluids flow across the membrane surface, the solvent and small objects pass through the membrane, but larger particles stay, and then they can be concentrated. The study showed that this method can efficiently purify exosomes and remove cell debris and other organelles. Blans et al. [63] successfully isolated EVs from human body fluids using the size-exclusion chromatography.
3.4. Other Methods:
Affinity chromatography is exploited to isolate EVs. The concept is based on the binding ligands highly expressed on EVs to enrich them. Several affinity methods have been established to isolate EVs, such as using antibodies to target membrane proteins (e.g. CD63)[64]. The annexins can bind to phosphatidylserine on EVs [65], allowing them to be used for purification of EVs. Moreover, proteoglycans[16, 66] can interact with concanavalin A or heparin, so the coating of concanavalin A was used to isolate the EVs with the high expression of proteoglycans.
The precipitation[67] is an uncommon method used for the purification of EVs. EVs have negative charges, which allow positively charged protamine that may bind to the EVs to isolate them. Adding of polyethylene glycol (PEG) can induce the precipitation of EVs, thus this method has been used to isolate EVs. While the precipitation approaches were efficient to isolate EVs, it may be difficult to separate the precipitation inducers from EVs. In addition, magnetic nanowire[68] and microfluidic device[69] have also been developed to purify EVs.
In general, serial centrifugations and density gradient centrifugations may be good methods to purify EVs [70, 71], but the times and costs are the barrier to apply those technologies in the clinic. In contrast, the membrane filtration and size exclusion possess a narrow range of size distribution in EVs and may be likely translational. Currently, purifying EVs is a challenging topic in EVs, but it is the most important research area.
4. Engineering EVs
Post modifications of EVs are important for targeted drug delivery and imaging. In this section, we will discuss several strategies and approaches to engineer EVs for their biomedical applications.
4.1. Ligand-linked EVs
The process to increase circulation time and enhance the targeting of diseased tissues was to link targeting molecules to EVs in order to improve the properties of EVs in vivo. However, binding chemicals to EVs is not a good approach since this causes the change in the structures of EVs. EVs are made of a lipid bilayer, therefore inserting targeting ligand molecules into a bilayer of EVs makes it an easy method for biofunctionalization of EVs. Cholesterol[53] or long chain fatty acid[72] are designed to hook targeted molecules to the surface of EVs. For example, Kooijmans et al.[72] demonstrated that epidermal growth factor receptor (EGFR) conjugated with phospholipid-polyethylene glycol (PEG) complexes can be incorporated into the bilayer of EVs. The results showed that these modified EVs can improve their circulation and the targeting capacity to tumor tissues.
4.2. Hybrid Membrane of EVs
EVs as endogenous communication systems attract strategies for improved drug delivery, but the loading of therapeutics and imaging agents to EVs still remains a barrier in exploiting EVs as drug delivery systems. Fusion of functionalized liposomes with EVs combines the advantages of liposomes and EVs, so-called hybrid EVs. The recent study[73] demonstrated this concept, and the proposed fusion strategy enables the efficient loading of therapeutics to EVs. Moreover, this allows EVs to merge the current mature liposome technology in pharmaceutical industries.
4.3. Coating of EVs to NPs
The proposition of encapsulating nanoparticles with cell-membrane-derived nanovesicles or EVs is to increase drug loading inside the nanovesicles, which improves the ability to target nanoparticle delivery systems. This strategy shows a broad range of biomedical applications of EVs[74]. For example, Illes et al. [75] coated Hela cell-derived exosomes to metal-organic framework NPs by the fusion approach. Thamphiwatana et al. [56] reported a core-shell structure comprised of poly (lactic-co-glycolic acid) (PLGA) NP as a core and EVs derived from mouse macrophages (cell line J774) as a shell, called Mø-NPs. The results showed that Mø-NPs possess antigens with the same orientation as their parent cells, which can act as macrophage decoys to neutralize endotoxins. Furthermore, Mø-NPs can sequester proinflammatory cytokines, which inhibit the progression of sepsis. Mø-NPs in a mouse Escherichia coli bacteremia model that shows a reduction in the proinflammatory cytokine levels and bacterial dissemination, thereby liberating the mouse from bacterial infections.
Other nanomaterials: gold[76], gelatin[77], silica[78], and iron oxide nanoparticles[14], are coated by EVs. In addition, nanogel[79], nanomotors[80] and nanosheets[78] are loaded in the EVs to improve nanoparticle biological properties. The studies show that coating EVs to nanoparticles may be a novel approach to biofunctionalizing nanoparticles in targeted drug delivery.
4.4. Drug-loaded EVs
Like liposomes, EVs can be loaded with therapeutics via the active or passive approaches for their biomedical applications [81]. Here, we categorize them into endogenous loading, biological, physical and chemical approaches, and summarize the current approaches to loading cargos in Table 1.
Table 1.
Strategies for loading cargos and targeted ligands in EVs
| Loading stages | No. | Method | Cargo | Ref. |
|---|---|---|---|---|
| Source cells | 1 | Condition induction | Membrane ligands | [33, 82] |
| 2 | Transfection | Membrane ligand, siRNA | [8, 83] | |
| 3 | Donor cell metabolism | Link molecule, siRNA | [84, 85] | |
| EVs | 1 | Electroporation | DNA, siRNA, chemical | [86–88] |
| 2 | Sonication | Chemical, siRNA | [89, 90] | |
| 3 | Incubation | Chemical | [91] | |
| 4 | Freeze and thaw cycles | Enzyme | [92] | |
| 5 | Saponin treatment | Chemical | [88] | |
| 6 | Extrusion | Chemical | [88] | |
| 7 | Fusion | Macromolecule, chemical | [73] | |
| 8 | Lyophilization | Chemical | [88] | |
| 9 | Chemical conjugation | Link molecule, antibody, enzyme, diagnostic agent | [84] | |
| 10 | Dialysis | Chemical | [88] | |
| 11 | Remote loading | Chemical | [16, 93, 94] |
4.4.1. Endogenous Loading
EVs can contain many signaling molecules from donor cells during the cell secretion, such as proteins, RNA[95], and DNA[96]. These endogenous biomolecules act as bioactive cargos to transport signals to adjacent cells or in distant organs. The studies show that these EVs play many roles in cell differentiation[97], metastasis and angiogenesis[98] and immune regulation[99]. EVs may also be utilized as biomarkers for diagnosis [100]. This is a spontaneous process, therefore it is difficult to control the loading contents and efficiency.
4.4.2. Biological Methods
With advances in biotechnology, some membrane proteins can be expressed in the cells, and these proteins on EVs can be used in therapies, such as using cell transfection techniques [8, 96]. Zhang et al. [8] reported cellular nanovesicles (NVs) expressed with PD-1 receptors on their membrane to enhance antitumor responses by disrupting PD-1/PD-L1 immune checkpoints. Furthermore, the authors loaded 1-methyl-tryptophan, an inhibitor of indoleamine 2,3-dioxygenase, into PD-1 engineered NVs to synergistically enhance the anti-immunity. These nanovesicles increased CD8+ cell infiltration in tumor microenvironments to kill tumor cells.
Delivery of therapeutic macromolecules via EVs is a promising strategy in drug delivery [101–104]. For example, Kojima et al. [104] proposed a genetic device (so-called Exosomal transfer into cells (EXOtic) device) that can engineer the production of designer exosomes using cells. This device can package specific mRNA in exosomes and deliver them to the targeted cells. The authors designed catalase mRNA and packaged them in exosomes. In a Parkinson’s disease mouse model, they showed that their exosomes can attenuate neurotoxicity and neuroinflammation.
4.4.3. Physical Methods
Similar to that of liposomes, therapeutics can be loaded on the surface of EVs or in a bilayer of EVs. Energy and forces are needed to incorporate therapeutics in EVs. Thus, sonication[89], electroporation[86, 87], saponin treatment[88], and incubation at the high temperature [88] are applied to load drug cargos into EVs. For instance, Gao et al. [105] designed a peptide, CP05 that can specifically bind to CD63 highly expressed on exosomes. Using this peptide, the authors can link therapeutics to the exosomal surface. For example, dystrophin splice–correcting phosphorodiamidate morpholino oligomer (PMO, a muscle-targeting peptide) can be linked to the exosomes via CP05 to treat Duchenne muscular dystrophy. The study shows that design of exosomal anchor peptides enables effective functionalization of exosomes for drug delivery. In addition, the bilayer of EVs may incorporate hydrophobic molecules. Gao et al. [15] loaded the EV with 5-(p-Fluorophenyl)-2-ureido-thiophene-3-carboxamide (TPCA-1) an anti-inflammatory drug, which resulted with a loading of 2% efficiency.
4.4.4. Chemical Methods
Chemical conjugation is a promising approach to modify EVs for therapeutic loading. Wang M. et al. [84] proposed a general strategy to conjugate chemicals to proteins on EVs. In this study they conjugated azides to newly synthesized proteins or glycan/glycoproteins on exosomes during the formation of exosomes. Through a bioorthogonal click chemistry, the azides link a wide range of drugs and proteins. This strategy provides a new platform for chemically remodeling EVs.
It is challenging to load hydrophilic drugs inside EVs since the membrane is the barrier for their membrane permeability. Gao [16] proposed a remote loading of hydrophilic drugs based on the pH gradient between inside and outside of EVs. In this study, they prepared EVs in the alkaline buffer to produce an inside and outside pH gradient. A weak acid drug (piceatannol used for anti-inflammation therapy) can penetrate the membrane of EVs allowing an accumulation inside the EVs. This approach resulted in a loaded piceatannol inside EVs, which increased the efficiency 3 times more compared to that of a method without the use of a pH gradient.
Engineering EVs is critical to their biomedical applications. For example, encapsulating drug-loaded polymer nanoparticles inside EVs is a novel method in order to increase therapies of EVs. Liposomes have been used in the clinic. Combining liposomes and EVs may enable the rapid translation of EVs technologies in the clinic using hybrid membrane structures of liposome/EVs. The remotely loading of hydrophilic therapeutics inside EVs based on the pH gradient is a new direction to encapsulate a wide range of clinical drugs inside EVs, and it may be ready to translate them in the clinic. Together, post-engineering of EVs will increase the capability of EVs in drug loading and targeted delivery, thus enabling them in biomedical applications.
5. Biomedical Applications
Since EVs possess many unique characteristics for targeted drug delivery and diagnosis, they have shown a wide range of biomedical applications. Here, we select several diseases that have been intensively studied, such as cancer and infectious diseases. Table 2 is the summary of current disease models that were treated with the use of EVs.
Table 2.
Summary of Biomedical Applications of EVs
| Types | Source | Target | Diseases | Ref. |
|---|---|---|---|---|
| Natural EVs | Monocyte/macrophage | Neuron | Parkinson’s disease | [92] |
| Monocyte/macrophage | Cancer cell | Tumor | [54, 89] | |
| HEK293 -HetIL-15 | Cancer cell | Tumor | [41] | |
| MSCs | Endothelium | Lung inflammation and injuries | [106] | |
| HEK293 -CD3-anti-EGFR | Cancer and T cells | Tumor | [13] | |
| Biomimetic EV | ||||
| Neutrophil | Endothelium | Inflammation, sepsis, arthritis, ischemia/reperfusion | [16, 91, 107, 108] | |
| Neutrophil | Cancer cell | Tumor, Metastasis | [109] | |
| Monocyte/macrophage | Endothelium | Inflammation, sepsis | [57, 78] | |
| Monocyte/macrophage | Endotoxins, cytokines | Inflammation, sepsis | [56] | |
| T cell | HIV | Infection | [110] | |
| Red blood cell | Cancer cell | Tumor | [94] | |
| Red blood cell | Bacteria | infection | [79] | |
| Platelet | Cancer cell, bacteria | Infection, tumor | [93] | |
| Platelet | Macrophage | Immune thrombocytopenia | [111] | |
| Platelet, Neuro cells | Cancer cell | Cancer | [72] | |
| Epithelial cell | Bacteria | infection | [112] | |
| HEK293 -PD-1 | Cancer cell | Tumor | [8] | |
| Artificial EVs | None | Endothelium | inflammation | [60] |
5.1. Anti-inflammation Therapies
Inflammation is the immune response to bacterial and viral infections, and tissue injury to protect the body homeostasis[113]. Sometimes, overreactive inflammation responses can lead to many acute and chronic inflammatory diseases [114, 115]. The vascular inflammation is one of features of inflammation responses that activate white blood cells to migrate to infectious sites. For example, endothelium lining the lumen of blood vessels upregulates intercellular adhesion molecule 1 (ICAM-1) to bind to neutrophils (a type of white blood cells) via integrin β2 on the neutrophils[116–119]. Inspired with the unique interaction between cells during inflammation response, Gao et al. [15] designed nanovesicles derived from activated neutrophils using nitrogen cavitation. In addition, another study by the same group[16] show that these nanovesicles are similar to EVs in size and structure, and the yield of EVs produced using nitrogen cavitation was much higher. They also demonstrated that these EVs made from neutrophils can specifically target inflamed lung vasculature and mitigate inflammation response to prevent acute lung injury. The studies reveal a new strategy to develop targeted EVs in order to treat inflammatory diseases.
5.2. Cancer Therapies
EVs have been applied to treat cancer. For example, Zhang et al.[94] reported a therapeutic usage of red blood cell (RBC) membrane formed EVs on a 4T1 breast cancer model. After remote-loading of doxorubicin (Dox), the authors achieved a significant therapeutic efficacy where 33% of the mice receiving nanoparticles had complete regressions and remained tumor-free. Additionally, the application of PD-1-integrated biomimetic EVs[8] resulted in the similar outcomes. In this study, engineered biomimetic EVs derived from PD-1 transfected HEK293 cells presented PD-1 receptors on their membrane, with enhanced antitumor responses by disrupting the PD-1/PD-L1 immune inhibitory axis. The EVs exhibited a long circulation and could bind to the PD-L1 on melanoma cancer cells.
5.3. Infectious Diseases
Inspired by the natural pathogen–host interactions and adhesion, Angsantikul et al. [112] reported the development of a novel targeted nanotherapeutic for the treatment of Helicobacter pylori infection. They collected plasma membrane of gastric epithelial cells (e.g., AGS cells) to obtain EVs and loaded antibiotic-encapsulated polymeric NPs to EVs. The biomimetic nanoparticles (denoted AGS-NPs) possessed the same surface antigens from the AGS cells allowing them to interact with H. pylori bacteria. The results showed that the complex EVs can effectively treat infections from Helicobacter pylori, demonstrating the potential to develop targeted drug delivery to treat a wide range of pathogens.
5.4. Neurological Diseases
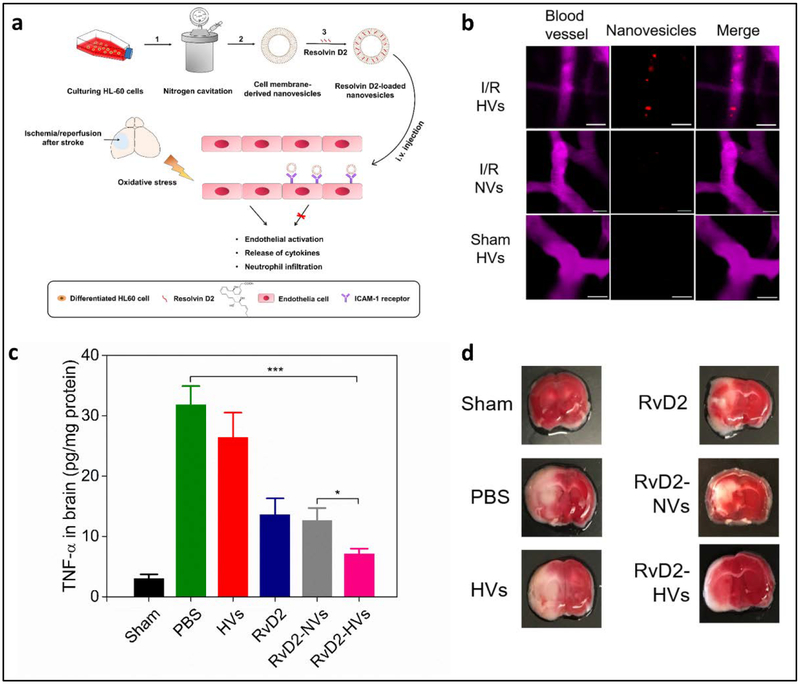
Neurological diseases are the huge burden due to ageing, and it is needed to develop novel technologies to treat them, such as stroke and Parkinson’s disease. Ischemic stroke is an acute and severe brain disease, leading to disability and death[120, 121]. In clinic, reperfusion of blood to ischemic brain is the only option to reverse brain damage after a stroke. Reperfusion processes cause an inflammation response that further damages the brain. During the reperfusion, brain endothelium strongly interacts with neutrophils to cause brain injury. Dong et al. [108] proposed an idea that neutrophil membrane-derived nanovesicles may deliver anti-inflammation agent (such as Resolvin D2 (RvD2)) to inflamed cerebral endothelium to prevent ischemia/reperfusion to lead to the brain damage (Fig. 5a). In this study, they demonstrated that nanovesicles can specifically bind to blood vessel in the ischemic brain using intravital microscopy (Fig. 5b). When they loaded RvD2 to nanovesicles and delivered them to a mouse that had a stroke, they observed that nanovesicles dramatically decreased the inflammation response (such as TNF-α) as shown in Fig. 5c. In addition, the histology of the brain (Fig. 5d) showed a reduction in the infarct volume after the mice were treated with RvD2-loaded nanovesicles. This study offers a new strategy in the inhibition of neuroinflammation using neutrophil-derived nanovesicles for stroke therapies.
Fig. 5.
Neutrophil membrane-derived nanovesicles target inflamed cerebral endothelium to treat ischemic/reperfusion injury. (a) Scheme of the preparation process and the administration of nanovesicles into animals. (b) Intravital images of fluorescently labeled-nanovesicles (red) binding to ischemic brain endothelium (pink) instead of normal brain endothelium. (c) Resolvin D2 loaded-nanovesicles (RvD2-EVs) significantly reduced the TNF-α level in brain tissue, indicating that they reduced the local inflammation. (d) Brain sections histology after several treatments. RvD2-EVs significantly improved the infarct volume on mice. *p<0.05 and ***p<0.001. Reprinted with permission from [108]. Copyright (2019) American Chemical Society.
6. Challenges and Perspectives in Their Translation
6.1. Scalability
While a substantial progress on EVs has been seen in the past decades, there are several barriers in translating EVs-based therapies. First, it is needed to develop reproducible and scalable approaches to generate clinic-grade EVs [122]. In order to improve the generation of natural EV, culture conditions such as hypoxia[40], increased calcium concentrations in medium[123, 124] and serum starvation [38] should be analyze because these may increase the production of EVs. In addition, the continuous culture system has shown the high yield of EVs by 40 times compared to traditional culture flask settings [41]. Gao et al. [16] have developed a nitrogen cavitation approach to generate neutrophil-derived cell membrane nanovesicles, which resulted in the reproduction of EVs that were 16-fold higher than naturally secreted EVs[16]. In addition, Gho et al. [54, 57] utilized extrusion and sonication to achieve the highest production of EVs by 100–200 folds compared to naturally secreted EVs. However, the authors did not study the compositions of their EVs, and the purity of EVs was unknown. The self-assembly of phospholipid and membrane proteins can form EVs-like nanoparticles[60]. This self-assembly process cannot warrant the surface orientation of membrane proteins and isolated membrane proteins may not maintain conformations after they leave the cell membrane. To meet the basic requirements of biopharmaceuticals (such as repeatability, efficiency and costs), the bioreactor system and nitrogen cavitation approach may hold the most promising perspectives in the commercialization of EVs.
6.2. Stability
Another problem is the instability of membrane proteins in EVs. Since most proteins are very sensitive to the environments (such as pH, temperature and buffers), the conditions of generation of EVs and their drug loading are critical [125, 126]. Unlike liposomes, some buffers aggregate EVs, which can be avoided if chosen an appropriate buffer for either generation of EVs or loading of drugs to EVs. The long-term storage of EVs is a factor for their biomedical applications because proteins on EVs may lose their functions [127].
6.3. Targeting Ability
The third issue in clinical applications is the targeting ability of EVs. Native EVs generally possess the capacity to target certain tissues, such as the brain and muscle etc. In the work[15, 16], the authors differentiated the HL60 cells to upregulate the expression of integrin β2, a ligand of activated endothelium, which increase the targeting ability of EVs that are derived from HL-60 cells. To increase tissue targeting, EVs can be modified using physical, chemical and biological methods as discussed in this manuscript. It is not clear whether these modifications can cause off-targeting and increased clearance.
6.4. Heterogeneous Compositions of EVs
The current most studies on EVs focused on developing approaches to generate EVs and their new biomedical applications, but few studies investigated how the compositions and structures of EVs affect their applications in clinic. No matter using biological, chemical or physical methods to produce EV, the intracellular components from their parent cells may remain in the EVs. Do they cause immunotoxicity? How do they affect the functions of EVs in terms of therapies? The recent studies [15, 16] tried to address this gap in the field of EVs by comparing the protein profiles of naturally secreted EVs and nitrogen cavitated EVs. The finding shows that nitrogen cavitation approach can dramatically remove intracellular components (such as DNA and organelles), and the EVs maintain all surface proteins of their parent cells. It is imperative to develop systemic approaches to study the biological compositions of EVs that are made by using physical, chemical and biological methods.
7. Conclusion
The research on EVs in the past decades shows that unique features of EVs are transforming the traditional nanoparticle-based drug delivery. EVs have supreme biocompatibility, long circulation times, low immunogenicity, and most importantly parent cell features that make them novel drug carriers. Different to the conventional drug carriers, EVs act similar to that of bioactive drugs. These features show EVs may be novel drug carriers and therapeutics.
The three major strategies to the generation of EVs are biological methods, physical methods and chemical methods. To scale up and reproduce EVs, nitrogen cavitation, sonication and extrusion approaches have demonstrated the potential in EVs translation. To increase therapeutic outcomes, many post-engineered EV approaches have been developed. Remotely loading of therapeutics inside EVs, genetic modifications of EVs and hybrid membrane components of EVs have demonstrated great opportunities to improve therapeutic outcomes in a wide range of diseases, such as cancer, infectious diseases and neurological diseases.
Although EVs have shown the translational potential to develop personalized and precision nanomedicines, the reproducibility and scalability of production of EVs should be addressed. In particular, it is required to develop innovative strategies for the production of EVs. In addition, the rigorous proteomics analysis should be developed to determine biological compositions of EVs, and different approaches to produce EVs will be analyzed to address whether the compositions of EVs are dependent on EVs production methods. Investigating the trafficking of EVs and their biodistribution is critical to understand how interactions of EVs conduct within the body. To do so, it is needed to develop advanced imaging systems, such as intravital microscopy and in vivo imaging[23, 118, 128].
In summary, this review summarizes the current status on strategies and approaches to produce EVs, and their biomedical applications. With advances in nanotechnology and immunology, genetically engineering EVs may be a new frontier in developing therapeutics tailored to individuals and disease stages, further improving treatments of cancer and inflammatory diseases.
Key nouns:
Natural EVs are naturally secreted from cells
Artificial EVs are self-assembled by membrane lipids and proteins.
Engineered EVs are modification of cells or EVs for biomedical applications.
Acknowledgements:
This work was supported by NIH grant (R01EB027078) to Z. W. We thank Mindy Lee in the College of Pharmacy and Pharmaceutical Sciences at Washington State University for editing the manuscript.
Abbreviations:
- EVs
extracellular vesicles
- HUVECs
human umbilical vein endothelial cells
- 3D
three-dimension
- TFF
tangential flow filtration
- DNA
Deoxyribonucleic acid
- RNA
ribonucleic acid
- siRNA
small interfering RNA
- NPs
nanoparticles
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-ligand 1
- MSCs
mesenchymal stem cells
- HIV
human immunodeficiency virus
- PMO
phosphorodiamidate morpholino oligomer
- HEK
Human embryonic kidney
- Dox
Doxorubicin
- ICAM-1
Intercellular adhesion molecule 1
- RBC
Red blood cell
- DTT
dithiothreitol
- PFA
paraformaldehyde
- EBV
Epstein-Barr virus
- OMV
outer membrane vesicle
- SIRS
systemic inflammatory response syndrome
- DPPC
1, 2-dipalmitoyl-sn-glycero-3-phosphocholine
- DSPC
1, 2-distearoyl-sn-glycero-3-phosphocholine
- DOPC
1, 2-dioleoyl-sn-glycero-3-phosphocholine
- PEG
polyethylene glycol
Footnotes
Disclosure of potential conflicts of interest:
No potential conflicts of interest are disclosed.
References
- [1].Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB, Delivery of nanoparticle: complexed drugs across the vascular endothelial barrier via caveolae, IUBMB Life 63(8) (2011) 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang AZ, Langer R, Farokhzad OC, Nanoparticle delivery of cancer drugs, Annu Rev Med 63 (2012) 185–98. [DOI] [PubMed] [Google Scholar]
- [3].Mitragotri S, Anderson DG, Chen X, Chow EK, Ho D, Kabanov AV, Karp JM, Kataoka K, Mirkin CA, Petrosko SH, Shi J, Stevens MM, Sun S, Teoh S, Venkatraman SS, Xia Y, Wang S, Gu Z, Xu C, Accelerating the Translation of Nanomaterials in Biomedicine, ACS Nano 9(7) (2015) 6644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mitragotri S, Burke PA, Langer R, Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies, Nat Rev Drug Discov 13(9) (2014) 655–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang J, Zhang L, Gao HL, Liu YY, Zhang QY, Ran R, Zhang ZR, He Q, Co-delivery of doxorubicin and P-gp inhibitor by a reduction-sensitive liposome to overcome multidrug resistance, enhance anti-tumor efficiency and reduce toxicity, Drug Deliv 23(4) (2016) 1130–1143. [DOI] [PubMed] [Google Scholar]
- [6].Zhang CY, Gao J, Wang Z, Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management, Advanced materials 30(43) (2018) e1803618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang CY, Lin W, Gao J, Shi X, Davaritouchaee M, Nielsen AE, Mancini RJ, Wang Z, pH-Responsive Nanoparticles Targeted to Lungs for Improved Therapy of Acute Lung Inflammation/Injury, ACS applied materials & interfaces 11(18) (2019) 16380–16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang XD, Wang C, Wang JQ, Hu QY, Langworthy B, Ye YQ, Sun WJ, Lin J, Wang TF, Fine J, Cheng H, Dotti G, Huang P, Gu Z, PD-1 Blockade Cellular Vesicles for Cancer Immunotherapy, Adv Mater 30(22) (2018). [DOI] [PubMed] [Google Scholar]
- [9].Wang Z, Tiruppathi C, Minshall RD, Malik AB, Size and dynamics of caveolae studied using nanoparticles in living endothelial cells, ACS Nano 3(12) (2009) 4110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shi J, Schellinger JG, Pun SH, Engineering biodegradable and multifunctional peptide-based polymers for gene delivery, J Biol Eng 7(1) (2013) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, Chen YY, MacMillan P, Chan WCW, Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors, Acs Nano 12(8) (2018) 8423–8435. [DOI] [PubMed] [Google Scholar]
- [12].Hu CMJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao WW, Zhang K, Chien S, Zhang LF, Nanoparticle biointerfacing by platelet membrane cloaking, Nature 526(7571) (2015) 118–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y, Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity, J Am Chem Soc 140(48) (2018) 16413–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Silva AKA, Di Corato R, Pellegrino T, Chat S, Pugliese G, Luciani N, Gazeau F, Wilhelm C, Cell-derived vesicles as a bioplatform for the encapsulation of theranostic nanomaterials, Nanoscale 5(23) (2013) 11374–11384. [DOI] [PubMed] [Google Scholar]
- [15].Gao J, Chu DF, Wang ZJ, Cell membrane-formed nanovesicles for disease-targeted delivery, J Control Release 224 (2016) 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gao J, Wang SH, Wang ZJ, High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy, Biomaterials 135 (2017) 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vader P, Mol EA, Pasterkamp G, Schiffelers RM, Extracellular vesicles for drug delivery, Adv Drug Deliver Rev 106 (2016) 148–156. [DOI] [PubMed] [Google Scholar]
- [18].Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK, Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J Extracell Vesicles 7(1) (2018) 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vader P, Breakefield XO, Wood MJ, Extracellular vesicles: emerging targets for cancer therapy, Trends in molecular medicine 20(7) (2014) 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raposo G, Stoorvogel W, Extracellular vesicles: Exosomes, microvesicles, and friends, J Cell Biol 200(4) (2013) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akers JC, Gonda D, Kim R, Carter BS, Chen CC, Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies, J Neuro-Oncol 113(1) (2013) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Juan T, Furthauer M, Biogenesis and function of ESCRT-dependent extracellular vesicles, Semin Cell Dev Biol 74 (2018) 66–77. [DOI] [PubMed] [Google Scholar]
- [23].Wang SH, Dong XY, Gao J, Wang ZJ, Targeting Inflammatory Vasculature by Extracellular Vesicles, Aaps J 20(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang SH, Gao J, Wang ZJ, Outer membrane vesicles for vaccination and targeted drug delivery, Wires Nanomed Nanobi 11(2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang SH, Gao J, Li M, Wang LG, Wang ZJ, A facile approach for development of a vaccine made of bacterial double-layered membrane vesicles (DMVs), Biomaterials 187 (2018) 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM, Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment, Biotechnology advances 36(8) (2018) 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Peebles DA, Hochberg A, Clarke TD, Analysis of manual reticulocyte counting, American journal of clinical pathology 76(5) (1981) 713–7. [DOI] [PubMed] [Google Scholar]
- [28].Downey N, McGarvey LP, Martin SL, Crilly A, Lockhart JC, Fulton C, Reilhill J, Lundy FT, Isolation and Characterisation of Exosomes from Human Primary Bronchial Epithelial Cells, Irish J Med Sci 187 (2018) S252–S252. [Google Scholar]
- [29].Sheller S, Urrabaz-Garza R, Kechichian T, Saade G, Menon R, Isolation and characterization of amnion epithelial cell-derived exosomes, Am J Obstet Gynecol 214(1) (2016) S417–S418. [Google Scholar]
- [30].Khan G, Ahmed W, Isolation and Characterization of Exosomes Released by EBV-Immortalized Cells, Methods Mol Biol 1532 (2017) 147–158. [DOI] [PubMed] [Google Scholar]
- [31].Zhang YZ, Liu F, Song CG, Cao XL, Zhang YF, Wu HN, Guo CJ, Li YQ, Zheng QJ, Zheng MH, Han H, Exosomes derived from human umbilical vein endothelial cells promote neural stem cell expansion while maintain their stemness in culture, Biochem Biophys Res Commun 495(1) (2018) 892–898. [DOI] [PubMed] [Google Scholar]
- [32].Hong CW, Extracellular Vesicles of Neutrophils, Immune Netw 18(6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li JN, Tan M, Xiang QQ, Zhou Z, Yan HB, Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response, Thromb Res 154 (2017) 96–105. [DOI] [PubMed] [Google Scholar]
- [34].Ma T, Fu B, Yang X, Xiao Y, Pan M, Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/beta-catenin signaling in cutaneous wound healing, J Cell Biochem (2019). [DOI] [PubMed] [Google Scholar]
- [35].Xu H, Jiao X, Wu Y, Li S, Cao L, Dong L, Exosomes derived from PM2.5treated lung cancer cells promote the growth of lung cancer via the Wnt3a/betacatenin pathway, Oncol Rep 41(2) (2019) 1180–1188. [DOI] [PubMed] [Google Scholar]
- [36].Deng MY, Xiao H, Peng HL, Yuan H, Xu YX, Zhang GS, Tang JG, Hu ZP, Preservation of neuronal functions by exosomes derived from different human neural cell types under ischemic conditions, Eur J Neurosci 47(2) (2018) 150–157. [DOI] [PubMed] [Google Scholar]
- [37].Kucharzewska P, Belting M, Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress, J Extracell Vesicles 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aharon A, Tamari T, Brenner B, Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells, Thromb Haemostasis 100(5) (2008) 878–885. [DOI] [PubMed] [Google Scholar]
- [39].Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, Czupryna A, Szczepanik A, Zembala M, Circulating tumour-derived microvesicles in plasma of gastric cancer patients, Cancer immunology, immunotherapy : CII 59(6) (2010) 841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M, Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development, P Natl Acad Sci USA 110(18) (2013) 7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, Felber BK, Chen XY, Gursel I, Pavlakis GN, Efficient production and enhanced tumor delivery of engineered extracellular vesicles, Biomaterials 105 (2016) 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guo QS, Xia B, Moshiach S, Xu CF, Jiang YD, Chen YJ, Sun Y, Lahti JM, Zhang XA, The microenvironmental determinants for kidney epithelial cyst morphogenesis, Eur J Cell Biol 87(4) (2008) 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rocha S, Carvalho J, Oliveira P, Voglstaetter M, Schvartz D, Thomsen AR, Walter N, Khanduri R, Sanchez JC, Keller A, Oliveira C, Nazarenko I, 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles, Advanced science 6(4) (2019) 1800948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rolfo CD, Giallombardo M, Castiglia M, Chacartegui JJ, Provencio M, Alessandro R, Carreca AP, Roca P, Oliver J, Bover I, Van Meerbeeck JP, Russo A, Peeters M, Pauwels P, Exosomes isolation and characterization in non small cell lung carcinoma patients: Proof of concept study., J Clin Oncol 33(15) (2015). [Google Scholar]
- [45].Merchant ML, Powell DW, Wilkey DW, Cummins TD, Deegens JK, Rood IM, McAfee KJ, Fleischer C, Klein E, Klein JB, Microfiltration isolation of human urinary exosomes for characterization by MS, Proteom Clin Appl 4(1) (2010) 84–96. [DOI] [PubMed] [Google Scholar]
- [46].Iwai K, Minamisawa T, Suga K, Yajima Y, Shiba K, Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations, J Extracell Vesicles 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tamkovich SN, Yunusova NV, Stakheeva MN, Somov AK, Frolova AE, Kiryushina NA, Afanasyev SG, Grigor’eva AE, Laktionov PP, Kondakova IV, Isolation and Characterization of Exosomes from Blood Plasma of Breast Cancer and Colorectal Cancer Patients, Biochem Mosc-Suppl S 11(3) (2017) 291–295. [DOI] [PubMed] [Google Scholar]
- [48].Orozco-Romero MD, Borja-Urby R, Ponce-Castaneda MV, Duran-Figueroa NV, Isolation and cellular characterization of exosomes from plasma for their use as diagnostic biomarkers, Acta Bioquim Clin L 50(4) (2016) 783–790. [Google Scholar]
- [49].Nadarajan P, Chong SG, Kane R, Cooke G, Fabre A, Keane MP, Isolation and Characterization of Serum Exosomes in Non Small Cell Lung Cancer, Irish J Med Sci 186 (2017) S404–S405. [Google Scholar]
- [50].Taverna S, Giallombardo M, Gil-Bazo I, Carreca AP, Castiglia M, Chacartegui J, Araujo A, Alessandro R, Pauwels P, Peeters M, Rolfo C, Exosomes isolation and characterization in serum is feasible in non-small cell lung cancer patients: critical analysis of evidence and potential role in clinical practice, Oncotarget 7(19) (2016) 28748–28760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nordgren TM, Heires AJ, Zempleni J, Swanson BJ, Wichman C, Romberger DJ, Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice, J Nutr Biochem 64 (2019) 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guo P, Huang J, Zhao YP, Martin CR, Zare RN, Moses MA, Nanomaterial Preparation by Extrusion through Nanoporous Membranes, Small 14(18) (2018). [DOI] [PubMed] [Google Scholar]
- [53].Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, Fang Y, Xia Y, Cheng G, He X, Zheng SY, Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery, Cancer Res 78(3) (2018) 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS, Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors, Acs Nano 7(9) (2013) 7698–7710. [DOI] [PubMed] [Google Scholar]
- [55].Mendez R, Banerjee S, Sonication-Based Basic Protocol for Liposome Synthesis, Methods Mol Biol 1609 (2017) 255–260. [DOI] [PubMed] [Google Scholar]
- [56].Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang QZ, Olson J, Luk BT, Zhang S, Fang RH, Gao WW, Nizet V, Zhang LF, Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management, P Natl Acad Sci USA 114(43) (2017) 11488–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Go G, Lee J, Choi DS, Kim SS, Gho YS, Extracellular Vesicle-Mimetic Ghost Nanovesicles for Delivering Anti-Inflammatory Drugs to Mitigate Gram-Negative Bacterial Outer Membrane Vesicle-Induced Systemic Inflammatory Response Syndrome, Advanced healthcare materials 8(4) (2019) e1801082. [DOI] [PubMed] [Google Scholar]
- [58].Ingato D, Edson JA, Zakharian M, Kwon YJ, Cancer Cell-Derived, Drug-Loaded Nanovesicles Induced by Sulfhydryl-Blocking for Effective and Safe Cancer Therapy, Acs Nano 12(9) (2018) 9568–9577. [DOI] [PubMed] [Google Scholar]
- [59].Cobbs A, Chen X, Zhang Y, George J, Huang MB, Bond V, Thompson W, Zhao X, Saturated fatty acid stimulates production of extracellular vesicles by renal tubular epithelial cells, Mol Cell Biochem 458(1–2) (2019) 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Furman NET, Wang X, Parodi A, Tasciotti E, Biomimetic proteolipid vesicles for targeting inflamed tissues, Nat Mater 15(9) (2016) 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Heinemann ML, Vykoukal J, Sequential Filtration: A Gentle Method for the Isolation of Functional Extracellular Vesicles, Extracellular Vesicles: Methods and Protocols 1660 (2017) 33–41. [DOI] [PubMed] [Google Scholar]
- [62].Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA, Alt E, Vykoukal J, Benchtop isolation and characterization of functional exosomes by sequential filtration, J Chromatogr A 1371 (2014) 125–35. [DOI] [PubMed] [Google Scholar]
- [63].Blans K, Hansen MS, Sorensen LV, Hvam ML, Howard KA, Moller A, Wiking L, Larsen LB, Rasmussen JT, Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography, J Extracell Vesicles 6(1) (2017) 1294340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lozano-Andres E, Libregts SF, Toribio V, Royo F, Morales S, Lopez-Martin S, Vales-Gomez M, Reyburn HT, Falcon-Perez JM, Wauben MH, Soto M, Yanez-Mo M, Tetraspanin-decorated extracellular vesicle-mimetics as a novel adaptable reference material, J Extracell Vesicles 8(1) (2019) 1573052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yoshida T, Ishidome T, Hanayama R, High Purity Isolation and Sensitive Quantification of Extracellular Vesicles Using Affinity to TIM4, Current protocols in cell biology 77 (2017) 3 45 1–3 45 18. [DOI] [PubMed] [Google Scholar]
- [66].Reiter K, Aguilar PP, Wetter V, Steppert P, Tover A, Jungbauer A, Separation of virus-like particles and extracellular vesicles by flow-through and heparin affinity chromatography, J Chromatogr A 1588 (2019) 77–84. [DOI] [PubMed] [Google Scholar]
- [67].Deregibus MC, Figliolini F, D’Antico S, Manzini PM, Pasquino C, De Lena M, Tetta C, Brizzi MF, Camussi G, Charge-based precipitation of extracellular vesicles, Int J Mol Med 38(5) (2016) 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lim J, Choi M, Lee H, Kim YH, Han JY, Lee ES, Cho Y, Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires, J Nanobiotechnol 17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S, Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes, Lab Chip 14(11) (2014) 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ, A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods, Methods Mol Biol 1295 (2015) 179–209. [DOI] [PubMed] [Google Scholar]
- [71].Cumba Garcia LM, Peterson TE, Cepeda MA, Johnson AJ, Parney IF, Isolation and Analysis of Plasma-Derived Exosomes in Patients With Glioma, Frontiers in oncology 9 (2019) 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens M, Heijnen HFG, van Bergen En Henegouwen PMP, Vader P, Schiffelers RM, PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time, J Control Release 224 (2016) 77–85. [DOI] [PubMed] [Google Scholar]
- [73].Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D, Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems, Acs Nano 12(7) (2018) 6830–6842. [DOI] [PubMed] [Google Scholar]
- [74].Fang RH, Kroll AV, Gao WW, Zhang LF, Cell Membrane Coating Nanotechnology, Adv Mater 30(23) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Illes B, Hirschle P, Baenert S, Cauda V, Wuttke S, Engelke H, Exosome-Coated Metal-Organic Framework Nanoparticles: An Efficient Drug Delivery Platform, Chem Mater 29(19) (2017) 8042–8046. [Google Scholar]
- [76].Gao WW, Hu CMJ, Fang RH, Luk BT, Su J, Zhang LF, Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes, Adv Mater 25(26) (2013) 3549–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Li LL, Xu JH, Qi GB, Zhao XZ, Yu FQ, Wang H, Core-Shell Supramolecular Gelatin Nanoparticles for Adaptive and “On-Demand” Antibiotic Delivery, Acs Nano 8(5) (2014) 4975–4983. [DOI] [PubMed] [Google Scholar]
- [78].Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Vittoria Enzo M, Isenhart L, Ferrari M, Tasciotti E, Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions, Nat Nanotechnol 8(1) (2013) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang Y, Zhang JH, Chen WS, Angsantikul P, Spiekermann KA, Fang RH, Gao WW, Zhang LF, Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection, J Control Release 263 (2017) 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].de Avila BEF, Gao WW, Karshalev E, Zhang LF, Wang J, Cell-Like Micromotors, Accounts Chem Res 51(9) (2018) 1901–1910. [DOI] [PubMed] [Google Scholar]
- [81].Ingato D, Lee JU, Sim SJ, Kwon YJ, Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery, J Control Release 241 (2016) 174–185. [DOI] [PubMed] [Google Scholar]
- [82].Akbar N, Digby JE, Cahill TJ, Tavare AN, Corbin AL, Saluja S, Dawkins S, Edgar L, Rawlings N, Ziberna K, McNeill E, Johnson E, Aljabali AA, Dragovic RA, Rohling M, Belgard TG, Udalova IA, Greaves DR, Channon KM, Riley PR, Anthony DC, Choudhury RP, Study O, Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction, Jci Insight 2(17) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kosaka N, Iguchi H, Yoshioka Y, Hagiwara K, Takeshita F, Ochiya T, Competitive interactions of cancer cells and normal cells via secretory microRNAs, The Journal of biological chemistry 287(2) (2012) 1397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang M, Altinoglu S, Takeda YS, Xu QB, Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery, Plos One 10(11) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T, Secretory mechanisms and intercellular transfer of microRNAs in living cells, The Journal of biological chemistry 285(23) (2010) 17442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lamichhane TN, Raiker RS, Jay SM, Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery, Mol Pharmaceut 12(10) (2015) 3650–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P, Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles, J Control Release 172(1) (2013) 229–238. [DOI] [PubMed] [Google Scholar]
- [88].Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM, Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins, J Control Release 205 (2015) 35–44. [DOI] [PubMed] [Google Scholar]
- [89].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine : nanotechnology, biology, and medicine 12(3) (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM, Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication, Cell Mol Bioeng 9(3) (2016) 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gao J, Wang XX, Zhang JS, Guo RL, Preparation of heat-treated PAN/SiO2 hybrid hollow fiber membrane contactor for acetylene absorption, Sep Purif Technol 159 (2016) 116–123. [Google Scholar]
- [92].Haney MJ, Klyachko NL, Zhaoa YL, Gupta R, Plotnikova EG, He ZJ, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV, Exosomes as drug delivery vehicles for Parkinson’s disease therapy, J Control Release 207 (2015) 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ying M, Zhuang J, Wei X, Zhang X, Zhang Y, Jiang Y, Dehaini D, Chen M, Gu S, Gao W, Lu W, Fang RH, Zhang L, Remote-Loaded Platelet Vesicles for Disease-Targeted Delivery of Therapeutics, Advanced functional materials 28(22) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhang XX, Angsantikul P, Ying M, Zhuang J, Zhang QZ, Wei XL, Jiang Y, Zhang Y, Dehaini D, Chen MC, Chen YJ, Gao WW, Fang RH, Zhang LF, Remote Loading of Small-Molecule Therapeutics into Cholesterol-Enriched Cell-Membrane-Derived Vesicles, Angew Chem Int Edit 56(45) (2017) 14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y, Hou KZ, Jiang YH, Yang XH, Liu YP, Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation, Digest Liver Dis 41(12) (2009) 875–880. [DOI] [PubMed] [Google Scholar]
- [96].Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH, Differential fates of biomolecules delivered to target cells via extracellular vesicles, Proc Natl Acad Sci U S A 112(12) (2015) E1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan JD, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang YB, Bromberg J, Lyden D, Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET, Nature medicine 18(6) (2012) 883–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Janowska-Wieczorek A, Wysoczynski M, Marquez L, Ratajczak MZ, Microvesicles and exosomes derived from activated platelets induce metastasis and angiogenesis, Exp Hematol 32(7) (2004) 95–95. [DOI] [PubMed] [Google Scholar]
- [99].Clayton A, Harris CL, Court J, Mason MD, Morgan BP, Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59, Eur J Immunol 33(2) (2003) 522–531. [DOI] [PubMed] [Google Scholar]
- [100].Szabo G, Momen-Heravi F, Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets, Nat Rev Gastro Hepat 14(8) (2017) 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadiere B, Amigorena S, Thery C, Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses, Cancer Res 68(4) (2008) 1228–35. [DOI] [PubMed] [Google Scholar]
- [102].Wang QY, Yu JJ, Kadungure T, Beyene J, Zhang H, Lu Q, ARMMs as a versatile platform for intracellular delivery of macromolecules, Nat Commun 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Manfredi F, Di Bonito P, Arenaccio C, Anticoli S, Federico M, Incorporation of Heterologous Proteins in Engineered Exosomes, Lentiviral Vectors and Exosomes as Gene and Protein Delivery Tools 1448 (2016) 249–260. [DOI] [PubMed] [Google Scholar]
- [104].Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, Auslander S, Tan KR, Fussenegger M, Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment, Nat Commun 9(1) (2018) 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q, Moulton HM, Seow Y, Yin H, Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy, Sci Transl Med 10(444) (2018). [DOI] [PubMed] [Google Scholar]
- [106].Potter DR, Miyazawa BY, Gibb SL, Deng X, Togaratti PP, Croze RH, Srivastava AK, Trivedi A, Matthay M, Holcomb JB, Schreiber MA, Pati S, Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma, The journal of trauma and acute care surgery 84(2) (2018) 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Zhang QZ, Dehaini D, Zhang Y, Zhou JL, Chen XY, Zhang LF, Fang RH, Gao WW, Zhang LF, Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis, Nat Nanotechnol 13(12) (2018) 1182–+. [DOI] [PubMed] [Google Scholar]
- [108].Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z, Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke, ACS nano 13(2) (2019) 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kang T, Zhu QQ, Wei D, Feng JX, Yao JH, Jiang TZ, Song QX, Wei XB, Chen HZ, Gao XL, Chen J, Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis, Acs Nano 11(2) (2017) 1397–1411. [DOI] [PubMed] [Google Scholar]
- [110].Wei XL, Zhang G, Ran DN, Krishnan N, Fang RH, Gao WW, Spector SA, Zhang LF, T-Cell-Mimicking Nanoparticles Can Neutralize HIV Infectivity, Adv Mater 30(45) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wei XL, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, Zhou JR, Kim HW, Gao WW, Lu WY, Zhang LF, Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia, Biomaterials 111 (2016) 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Angsantikul P, Thamphiwatana S, Zhang Q, Spiekermann K, Zhuang J, Fang RH, Gao W, Obonyo M, Zhang L, Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection, Advanced therapeutics 1(2) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Medzhitov R, Origin and physiological roles of inflammation, Nature 454(7203) (2008) 428–35. [DOI] [PubMed] [Google Scholar]
- [114].Dinarello CA, Anti-inflammatory Agents: Present and Future, Cell 140(6) (2010) 935–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tabas I, Glass CK, Anti-inflammatory therapy in chronic disease: challenges and opportunities, Science 339(6116) (2013) 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chu D, Gao J, Wang Z, Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection, ACS Nano 9(12) (2015) 11800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chu D, Zhao Q, Yu J, Zhang F, Zhang H, Wang Z, Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy, Adv Healthc Mater 5(9) (2016) 1088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang ZJ, Imaging Nanotherapeutics in Inflamed Vasculature by Intravital Microscopy, Theranostics 6(13) (2016) 2431–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wang Z, Li J, Cho J, Malik AB, Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils, Nat Nanotechnol 9(3) (2014) 204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Iadecola C, Anrather J, The immunology of stroke: from mechanisms to translation, Nat Med 17(7) (2011) 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shichita T, Sakaguchi R, Suzuki M, Yoshimura A, Post-ischemic inflammation in the brain, Front Immunol 3 (2012) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Jiang XC, Gao JQ, Exosomes as novel bio-carriers for gene and drug delivery, Int J Pharm 521(1–2) (2017) 167–175. [DOI] [PubMed] [Google Scholar]
- [123].Constantinescu P, Wang B, Kovacevic K, Jalilian I, Bosman GJCGM, Wiley JS, Sluyter R, P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells, Bba-Biomembranes 1798(9) (2010) 1797–1804. [DOI] [PubMed] [Google Scholar]
- [124].Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G, Dubyak GR, P2X7 Receptor-Stimulated Secretion of MHC Class II-Containing Exosomes Requires the ASC/NLRP3 Inflammasome but Is Independent of Caspase-1, J Immunol 182(8) (2009) 5052–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zbacnik TJ, Holcomb RE, Katayama DS, Murphy BM, Payne RW, Coccaro RC, Evans GJ, Matsuura JE, Henry CS, Manning MC, Role of Buffers in Protein Formulations, J Pharm Sci-Us 106(3) (2017) 713–733. [DOI] [PubMed] [Google Scholar]
- [126].Bischof JC, He XM, Thermal stability of proteins, Ann Ny Acad Sci 1066 (2005) 12–33. [DOI] [PubMed] [Google Scholar]
- [127].Song JG, Lee SH, Han HK, Biophysical evaluation of aminoclay as an effective protectant for protein stabilization during freeze-drying and storage, Int J Nanomed 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Chu DF, Dong XY, Zhao Q, Gu JK, Wang ZJ, Photosensitization Priming of Tumor Microenvironments Improves Delivery of Nanotherapeutics via Neutrophil Infiltration, Adv Mater 29(27) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]