Abstract
Purpose
Discrepancies between clinicians’ assessment of chemotherapy-induced peripheral neuropathy (CIPN) and patient-reported outcomes (PRO) have been described, though the underlying reasons are unknown. Our objective was to identify potential patient-specific factors associated with under-describing of CIPN to clinicians in women with non-metastatic breast cancer treated with paclitaxel.
Methods
Patients enrolled in an observational study (n = 60) completed weekly CIPN PRO using the EORTC CIPN20. Clinician-documented CIPN using the NCI CTCAE were abstracted from the electronic medical record and paired with CIPN20 data at weeks 7 and 10. Patients were classified as under-describers if their CIPN20 was above the 80th percentile of the CIPN20 distribution for that CTCAE grade from an independent clinical trial (N08CA). Demographics, Assessment of Survivor Concerns (ASC), Trust in Oncologist Scale (TiOS), and health literacy assessment were collected post-treatment via survey. Repeated measures cumulative logistic regression models were used to identify factors associated with under-describing CIPN.
Results
Forty-two women completed the survey (response rate 70%). Three and 9 patients were categorized as under-describers at weeks 7 and 10, respectively. Women who were not working (OR = 9.00, 95%CI 1.06–76.15), had lower income (OR = 7.04, 95%CI 1.5–32.99), and displayed higher trust in their oncologist’s competence (OR = 1.29, 95%CI 1.03–1.62 for a 0.1-unit increase in score) were more likely to under-describe CIPN symptoms.
Conclusions
This preliminary study identified non-working status, low income and trust in oncologist’s competence as potential factors influencing under-description of CIPN to the clinical team. Further work is needed to clarify these relationships and test additional factors.
Keywords: Breast cancer, Chemotherapy-induced peripheral neuropathy, Patient reported outcome measures, Taxoids
Highlights
-
•
This pilot study examined factors associated with under-describing of neuropathy.
-
•
Patient-reported and clinician-documented neuropathy severity were compared.
-
•
Non-working status and low income were associated with neuropathy under-describing.
-
•
Trust in oncologist’s competence was associated with neuropathy under-describing.
-
•
Recording patient-clinician interactions would confirm under-describing behavior.
1. Introduction
Taxanes are one of several classes of chemotherapeutic agents available for the approximately 260,000 patients diagnosed with breast cancer annually [1]. One common side effect from taxanes is chemotherapy-induced peripheral neuropathy (CIPN), occurring in 20–60% of patients [2,3]. CIPN significantly affects patients’ quality of life [4,5], even after completion of treatment [6]. Due to the lack of effective agents for preventing or treating CIPN, chemotherapy treatment must be decreased, delayed or discontinued in approximately 25% of patients receiving paclitaxel for treatment of non-metastatic breast cancer [7] to prevent progression of moderate symptoms to severe, potentially irreversible, CIPN [[8], [9], [10]]. The decision to disrupt treatment is made collaboratively between clinicians and patients by weighing the risks of further symptom progression and subsequent impact on quality of life against the risk of reduced treatment effectiveness [11,12].
Within clinical trials, CIPN severity is assessed by clinicians using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) scale, based on patients’ description of how symptoms interfere with their activities of daily living [13]. The CTCAE has been criticized for its lack of validity, reliability, sensitivity, floor effects, and inadequate distinction of subtle differences in CIPN severity [[14], [15], [16], [17], [18]]. Unlike other side effects, for which objective thresholds in clinical parameters can be easily established to grade severity (e.g., anemia), CIPN assessment is primarily subjective and thus prone to discrepancies between clinicians and patients [[19], [20], [21]]. Specifically, in one study, severe CIPN was reported by 19% of patients but only 12% of their doctors [19]. Similarly, Bennet et al. found that 60% of patients reported CIPN that interfered with activities of daily living, but only 10% of their clinicians documented severe CIPN in the electronic medical record (EMR) [20]. As a result, patient-reported outcomes (PROs), defined as direct reporting of health information by patients without clinician interpretation [22], have gained increasing relevance in oncology, both in clinical trials [23] and clinical practice [24]. PROs provide an opportunity for patients to directly assess and report their subjective side effects [25].
One of the most frequently used PROs to measure CIPN is the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Chemotherapy Induced Peripheral Neuropathy (CIPN20) scale [26]. The psychometric characteristics of the CIPN20 have been extensively investigated by multiple authors [17,[26], [27], [28]] and were demonstrated to be more sensitive and reliable than the CTCAE grading for CIPN symptoms [17,26]. In an analysis of CIPN20 and CTCAE using data from two phase III clinical trials, there was a strong association (p < 0.0001) between CIPN20 score and CTCAE grade [29]. However, there was both substantial variability in CIPN20 scores within each CTCAE grade and overlap in CIPN20 scores between CTCAE grades [29].
The underlying reasons for the discrepancies in CIPN severity assessment between clinicians and patients are unknown, but are hypothesized to be due to one or more of the following: 1) miscommunication of symptoms by patients to their oncologist during appointments, or 2) characterization of symptoms by oncologists as less severe based on their clinical experience and comparison against previous cases [30]. Regarding the first hypothesis, patients receiving paclitaxel for non-metastatic breast cancer are often primarily concerned with treatment effectiveness and may be unaware of the risk of irreversible CIPN [31]. This may cause patients to intentionally under-describe CIPN symptoms to their oncologists to avoid unwanted treatment disruptions, which may account for the lower rates of CIPN in CTCAE data compared with matched PRO data. We are not aware of any previous work focusing on patient-related factors associated with CIPN PRO variability within CTCAE grades or discrepancies between patient-reported and clinician-documented CIPN severity. Therefore, the aim of this study was to identify potential patient-specific factors associated with apparent under-describing of CIPN to clinicians in women with non-metastatic breast cancer who received treatment with paclitaxel. Given the wealth of literature supporting the validity and reliability of the CIPN20 measure, in this analysis we assume that the PRO is a better indicator of CIPN symptoms than the CTCAE and that any discrepancy between them is indicative of patient under- or over-describing CIPN to the clinical team during appointments.
2. Methods
This was a pilot retrospective study nested within a prospective observational study which aimed to identify clinical and genetic predictors of paclitaxel-induced peripheral neuropathy [32]. The prospective study enrolled 60 women, aged 18 years or older, with early stage breast cancer scheduled to receive weekly paclitaxel 80 mg/m2 infusions for 12 weeks at the University of Michigan Rogel Comprehensive Cancer Center. Institutional Review Board approval was obtained for both studies.
2.1. Prospective collection of patient-reported CIPN
Each patient completed the CIPN20 prior to initiating paclitaxel and weekly throughout treatment. CIPN20 was collected for research purposes only and patients were instructed that this information would not be shared with their clinical team. The CIPN20 scale contains 20 items grouped in three subscales: sensory (9 items), motor (8 items) and autonomic (3 items). Responses are provided on a 4-point Likert scale (1 = not at all, 2 = a little, 3 = quite a bit, 4 = very much). The total score is the sum of the scores of the individual questions and ranges from 20 to 80, with higher scores reflecting more severe CIPN symptoms. In this study, the last CIPN20 item was excluded because it pertained to males, resulting in a possible score range of 19–76 (hereafter, CIPN19) [26]. Permission to use the scale was obtained prior to data collection. Psychometric testing during scale development obtained Cronbach alpha coefficients for the sensory, motor and autonomic subscales of 0.82, 0.73 and 0.76, respectively [26]. Strong correlation coefficient scores supported test-retest reliability: sensory (r = 0.836), motor (r = 0.844), and autonomic (r = 0.726) [27]. The sensory and motor scales exhibited moderate-to-high responsiveness to change (Cohen’s d = 0.82 and 0.48, respectively) [17]. Subsequent exploratory factor analysis suggested a more reliable and valid two-factor structure of upper and lower extremity subscales, instead of the original three-factor structure [17]. Nevertheless, confirmatory factor analysis did not support either structure of the scale, recommending that all items be summed to obtain a final score [28]. This was the approach used in this study.
2.2. Retrospective collection of clinician-documented CIPN
After completion of the prospective study, clinician-documented CIPN was retrospectively abstracted from the EMR. Patients were typically evaluated in clinic prior to treatment initiation and after 4, 7, 10, and 12 weeks of treatment. Complete visit notes for weeks 7, 10, and 12 were reviewed by one of the research team members and all data pertaining to neuropathy were abstracted verbatim from the EMR. Notes at the week 4 visit were not reviewed due to the low rates of CIPN at this early time point in treatment. Quality control of the data extraction process was performed on a random sample of 10% of patients by a second researcher to ensure accuracy and consistency. If the note included a CTCAE grade of CIPN, the patient was assigned that grade. If the note included only textual descriptions of CIPN symptoms, rather than a grade, two independent researchers who were not involved with data extraction and who were blinded to the patient’s CIPN19 data assigned a grade based on the CTCAE v4.0 criteria [13]. Discrepancies in CTCAE grade assignment were reconciled by a senior member of the research team with expertise in CIPN. A CTCAE grade 0 was assigned for all visits that took place but for which a specific mention of CIPN symptoms was not found in the clinical note.
2.3. Classification as under-describer or over-describer
Paired patient-reported (CIPN19) and clinician-documented (CTCAE) CIPN data collected at weeks 7, 10, and 12 were compared. If a subject had missing CIPN19 data, scores from the previous week were used. If no data were available from the previous week, CIPN19 scores were coded as missing data. Due to the substantial variability of CIPN19 scores within each CTCAE grade reported by Le-Rademacher et al. [29], and the absence of established thresholds for translating PRO to CTCAE, we assumed that scores in the highest percentile of the CIPN19 distribution within each CTCAE grade would correspond to apparent under-describing of CIPN by patients to their clinicians, whereas over-describers would be in the lowest percentile and regular-describers in the center of the distribution. The over-describer category does not apply to CTCAE Grade 0, as a low score simply means absence of CIPN. Despite this, all categories are presented for all CTCAE grades for consistency purposes (Appendices I, II, III). As an external standard, we used the CIPN19 score distribution per CTCAE grade of the N08CA trial reported by Le-Rademacher et al. [29], which analyzed data from 164 patients with ovarian, lung and other cancers receiving a paclitaxel and carboplatin regimen. This distribution was used to set percentile thresholds to classify our patients as under-describers (CIPN19 ≥ 80th percentile), over-describers (CIPN19 ≤ 20th percentile) or regular-describers (Appendices I, II, III) for each time point for which they had matched PRO and CTCAE CIPN data.
2.4. Collection of patient factors that may predict CIPN describing behavior
Women who completed the prospective study were re-contacted (median = 4.9, range 0.1–14.3 months) to complete a questionnaire including socio-demographic, clinical and treatment characteristics (age, race, education level, marital status, employment status, annual household income, language spoken at home, and number of daily prescription medications), and constructs postulated to be associated with under-describing of CIPN to clinicians, namely: fear of cancer recurrence, trust in oncologist, and health literacy. We hypothesized that under-describing was associated with high fear of cancer recurrence, low trust in the oncologist, and low health literacy. Constructs were assessed using the validated scales detailed below.
The Assessment of Survivor Concerns (ASC) scale comprises 5-items grouped in two domains: cancer worry, described by three questions exploring fear of cancer recurrence (score range 3–12), and health worry, including two questions pertaining to worry about health in general (score range 2–8). Responses are provided on a 4-point scale (1 = not at all, 2 = a little bit, 3 = somewhat, 4 = very much) and published evidence supports the measure’s reliability and validity [33].
The Trust in Oncologist Scale (TiOS), originally developed in Dutch [34] and cross-culturally adapted into English [35], is an 18-item questionnaire assessing patients’ trust in their oncologist in four domains: competence, fidelity, honesty, and caring. Responses are provided on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the total score is calculated by adding responses to each item and dividing by the number of items (range 1–5). Similar scores can be calculated for each domain. Higher scores indicate more trust in oncologists. The English version presented strong internal consistency (Cronbach alpha = 0.94) and construct validity was demonstrated by significant correlation with satisfaction with oncologists (rs = 0.62), willingness to recommend oncologists to others (rs = 0.59), number of previous visits with oncologists (rs = 0.21), and trust in health care (rs = 0.33) [35]. Confirmatory factor analysis did not confirm the four-dimensional theoretical structure, but rather one dimension. Nevertheless, due to the pilot nature of this study, we included domain scores in the analyses.
Health literacy was estimated using three questions previously shown to be effective in detecting inadequate health literacy: (1) I have problems learning about my medical condition because of the difficulty in understanding written information; (2) I am confident in filling out medical forms by myself; and (3) I often have someone help me read hospital materials [36,37]. Responses were provided on a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree) and added to yield a final score between 3 and 15. Question number 2 was reverse coded to match the direction of the scale in the other questions. Health literacy scores between 0 and 10 were considered ‘adequate’ while scores >10 denote ‘inadequate’ health literacy. Health literacy was used as a continuous variable in all analyses.
The scales were combined into a single questionnaire and delivered via Qualtrics (Copyright© 2018 Qualtrics, Provo, UT). Women were invited to participate via e-mail or patient portal and contacted up to three times. All women provided written informed consent prior to study initiation.
2.5. Data analyses
Visit data for weeks 7, 10 and 12 were summarized using frequencies and percentages. Mean and standard deviation (SD) of CIPN19 scores were calculated and box and whisker plots were constructed to present the range of CIPN19 scores per CTCAE grade.
Characteristics of patients categorized as under-, regular- and over-describers were summarized using mean and standard deviation (SD) for continuous variables (age, number of medications and survey scores), and frequency and percentages for categorical variables (race, education level, marital status, employment status, annual household income, and language spoken at home). Data were summarized for visits in weeks 7 and 10, but not for week 12 due to the high number of missing values. Due to small sample size, Kruskal-Wallis (for continuous variables) and Fisher’s exact tests (for categorical variables) were used to compare characteristics and survey scores among under-, regular-, and over-describers.
Repeated measures cumulative logistic regression models were used to identify patient-factors (i.e., demographics or survey responses) associated with increased probability of under-describing versus regular- and over-describing. Generalized estimating equations were used to account for correlations among observations for the same individual. Several models were tested, and final model selection was based on quasi-AIC (QIC). Independent variables in the final model included: age, number of medications, education level, employment status, household income, marital status, health literacy, confidence in the oncologist and survivor concerns. Race and language spoken at home were not included due to limited frequencies in one of the categories. Additionally, the model was adjusted for CTCAE grade. Data analysis was performed with SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA) and statistical significance set at p < 0.05.
3. Results
3.1. Retrospective collection of clinician-documented CIPN
Of the 180 potential visits (weeks 7, 10 and 12 visits for 60 participants), sixty (33.3%) did not occur, 2 in week 7 and 5 in week 10 due to early treatment discontinuation, and 53 in week 12 due to clinician’s decision to not schedule a visit on the last day of treatment. Of the remaining 120 visits, 110 (91.7%) explicitly mentioned CIPN symptoms, of which 29 (26.4%) used a specific CTCAE grade and 81 (73.6%) provided textual descriptions of symptoms, without assigning a specific grade (Fig. 1).
Fig. 1.
Neuropathy symptom descriptions documented in the electronic medical record for visits in weeks 7, 10 and 12.
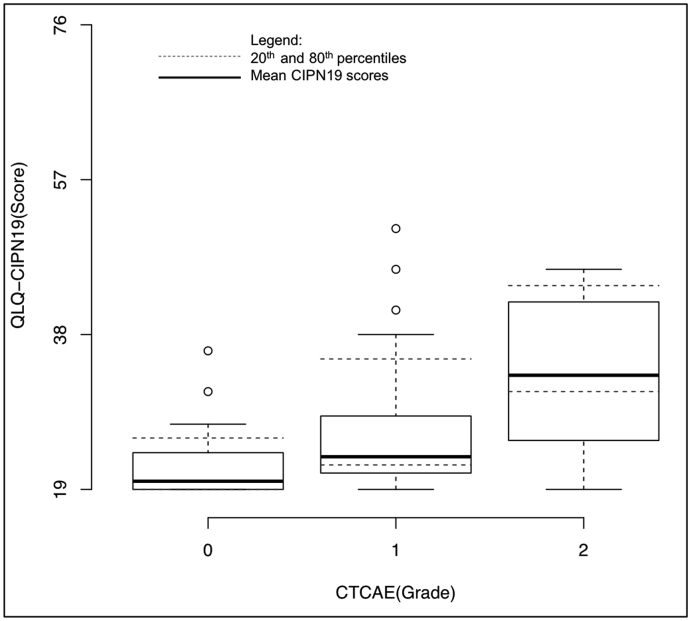
For patients with clinician-graded CTCAE grade 0 neuropathy, CIPN19 scores ranged from 19 to 36 [mean (SD) = 21.7 (3.79)], 19–51 for grade 1 [mean (SD) = 25.9 (6.55)], and 19–46 for grade 2 [mean (SD) = 33.7 (8.86)] (Fig. 2). There were no occurrences of CTCAE grades 3 or 4.
Fig. 2.
Box and whisker plot of the distribution of CIPN19 scores per CTCAE grade and illustrating the threshold for the 20th (over-describers) and 80th percentiles (under-describers) of the distribution (dotted line).
3.2. Predictors of discrepancies between patient-reported and clinician-documented CIPN
Of the 60 women who completed the prospective trial, 42 (70%) completed the post-treatment survey. Women were on average 55.3 (SD = 10.8) years-old and most were white, married, spoke English at home, had at least a college degree, worked, and had a household income greater than $50,000 (Table 1). After determining the 20th and 80th percentiles of the CIPN19 distribution per CTCAE grade, 3, 25, and 13 patients were categorized as under-, regular-, and over-describers at the week 7 visit, while 9, 17, and 14 patients were categorized as under-, regular-, and over-describers at the week 10 visit (Appendices II, III).
Table 1.
Patient demographic and clinical characteristics and bivariate analyses across different levels of symptom describing.
| Characteristic | All patientsa (n = 42) |
Week 7 Visitb |
Week 10 Visitc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Under-describers (n = 3) |
Regular-describer (n = 25) |
Over-describer (n = 13) |
P-value | Under-describers (n = 9) |
Regular-describers (n = 17) |
Over-describers (n = 14) |
P-value | ||
| Age mean (SD) | 55.3 (10.8) | 43.7 (12.1) | 55.9 (9.6) | 56.8 (12.1) | 0.179 | 49.9 (13.4) | 57.9 (9.4) | 55.1 (11.4) | 0.262 |
| # daily prescription medications mean (SD) | 1.8 (0.8) | 2.3 (0.6) | 1.8 (0.8) | 1.7 (0.9) | 0.417 | 2.2 (0.7) | 1.8 (0.9) | 1.5 (0.8) | 0.105 |
| Race n (%) | |||||||||
| White/Caucasian | 38 (93) | 3 (100) | 21 (88) | 13 (100) | 0.637 | 7 (88) | 15 (88) | 14 (100) | 0.414 |
| Other | 3 (7) | 0 | 3 (12) | 0 | 1 (12) | 2 (12) | 0 | ||
| Level of Education n (%) | |||||||||
| Less than College | 8 (20) | 1 (33) | 4 (17) | 3 (23) | 0.576 | 1 (12) | 4 (24) | 3 (21) | >0.999 |
| College or more | 33 (80) | 2 (67) | 20 (83) | 10 (77) | 7 (88) | 13 (76) | 11 (79) | ||
| Employment status n (%) | |||||||||
| Working | 25 (61) | 1 (33) | 13 (54) | 10 (77) | 0.242 | 3 (37) | 9 (53) | 11 (79) | 0.150 |
| Not working | 16 (39) | 2 (67) | 11 (46) | 3 (23) | 5 (63) | 8 (47) | 3 (21) | ||
| Household Income n (%) | |||||||||
| Less than $50,000 | 9 (24) | 1 (33) | 7 (32) | 1 (8) | 0.272 | 4 (57) | 4 (25) | 1 (7) | 0.033 |
| More than $50,000 | 29 (76) | 2 (67) | 15 (68) | 12 (92) | 3 (43) | 12 (75) | 13 (93) | ||
| Marital Status n (%) | |||||||||
| Married/partnered | 29 (71) | 2 (67) | 17 (71) | 9 (69) | >0.999 | 5 (63) | 11 (65) | 12 (86) | 0.423 |
| Not married/partnered | 12 (29) | 1 (33) | 7 (29) | 4 (31) | 3 (37) | 6 (35) | 2 (14) | ||
| Spoken language at home n (%) | |||||||||
| English | 38 (93) | 3 (100) | 22 (92) | 12 (92) | >0.999 | 8 (100) | 15 (88) | 13 (93) | >0.999 |
| Other | 3 (7) | 0 | 2 (8) | 1 (8) | 0 | 2 (12) | 1 (7) | ||
| ASC Cancer worry mean (SD) | 8.8 (2.6) | 8.7 (3.5) | 9.0 (2.7) | 8.4 (2.5) | 0.757 | 9.2 (2.7) | 8.9 (2.8) | 8.7 (2.6) | 0.881 |
| ASC Health worry mean (SD) | 5.2 (2.0) | 6.0 (2.0) | 5.3 (2.0) | 5.1 (2.1) | 0.753 | 5.9 (2.0) | 5.2 (2.1) | 5.1 (2.0) | 0.674 |
| Health literacy mean (SD) | 5.0 (2.4) | 8.0 (2.6) | 4.3 (2.0) | 5.9 (2.6) | 0.016 | 5.8 (2.6) | 4.9 (2.7) | 5.0 (2.3) | 0.644 |
| TiOS Competence mean (SD) | 4.5 (0.6) | 4.4 (0.5) | 4.5 (0.7) | 4.4 (0.5) | 0.608 | 4.6 (0.5) | 4.5 (0.6) | 4.3 (0.7) | 0.565 |
| TiOS Fidelity mean (SD) | 4.5 (0.5) | 4.5 (0.5) | 4.6 (0.6) | 4.5 (0.5) | 0.708 | 4.7 (0.5) | 4.6 (0.5) | 4.4 (0.6) | 0.620 |
| TiOS Honesty mean (SD) | 4.5 (0.5) | 4.3 (0.6) | 4.5 (0.5) | 4.4 (0.5) | 0.671 | 4.5 (0.5) | 4.5 (0.5) | 4.4 (0.5) | 0.672 |
| TiOS Caring mean (SD) | 4.4 (0.5) | 4.2 (0.8) | 4.4 (0.5) | 4.4 (0.5) | 0.908 | 4.6 (0.5) | 4.4 (0.5) | 4.4 (0.5) | 0.694 |
| TiOS Total mean (SD) | 4.5 (0.5) | 4.4 (0.6) | 4.5 (0.6) | 4.4 (0.5) | 0.812 | 4.6 (0.5) | 4.5 (0.5) | 4.4 (0.6) | 0.734 |
One missing value.
One missing value.
Two missing values.
In the bivariate analysis, patients who under-described CIPN symptoms at week 7 had lower health literacy [mean (SD) = 8.0 (2.6)] than regular- [mean (SD) = 4.3 (2.0)] or over-describers [mean (SD) = 5.9 (2.6)] (p = 0.016); however, this association was not found for discrepancies at week 10 (p = 0.644) (Table 1). No significant associations between CIPN describing category and the other demographic and clinical characteristics were found.
In the logistic regression, employment status, income, and the TiOS competence scale were associated with the probability of being an under-describer (Table 2). When adjusting for CTCAE grade, the odds of being an under-describer were 9.00 times (95%CI 1.06, 76.15) greater in women who were not working and 7.04 times (95%CI 1.5, 32.99) greater in women who had incomes <$50,000. The odds of being an under-describer were 1.29 (95%CI 1.03, 1.62) times greater for each 0.1-unit increase in the patient’s trust in their oncologist’s competence (a 0.1-unit increase corresponding to a 16.7% of a standard deviation for TiOS competence). In a post-hoc analysis, we constructed a single model with income and working status, as these variables are highly correlated, and found evidence that this association is more likely to be due to income (OR = 6.46, 95%CI 1.73, 24.13, p = 0.006).
Table 2.
Cumulative logistic regression with repeated measures predicting under-describing.
| Predictor | Estimate | SE | P value | Estimate CI | OR | OR 95% CI |
|---|---|---|---|---|---|---|
| Age | −0.05 | 0.05 | 0.249 | (-0.14, 0.04) | 0.95 | (0.87, 1.04) |
| Number of medications | 0.07 | 0.44 | 0.870 | (-0.78, 0.93) | 1.07 | (0.46, 2.52) |
| ASC Cancer worry | −0.38 | 0.22 | 0.083 | (-0.81, 0.05) | 0.68 | (0.44, 1.05) |
| ASC Health worry | 0.54 | 0.29 | 0.065 | (-0.03, 1.11) | 1.71 | (0.97, 3.04) |
| Health Literacy | 0.02 | 0.1 | 0.861 | (-0.18, 0.22) | 1.02 | (0.83, 1.24) |
| TiOS Competencea | 0.25 | 0.12 | 0.029 | (0.03, 0.48) | 1.29 | (1.03, 1.62) |
| TiOS Fidelitya | −0.18 | 0.15 | 0.213 | (-0.47, 0.11) | 0.83 | (0.63, 1.11) |
| TiOS Honestya | −0.08 | 0.10 | 0.383 | (-0.27, 0.10) | 0.92 | (0.77, 1.11) |
| TiOS Caringa | −0.06 | 0.09 | 0.509 | (-0.24, 0.12) | 0.94 | (0.79, 1.13) |
| Education (ref: less than college) Higher than college |
0.65 | 0.82 | 0.427 | (-0.95, 2.25) | 1.91 | (0.39, 9.51) |
| Employment (ref: Working) not working | 2.20 | 1.09 | 0.044 | (0.06, 4.33) | 9.00 | (1.06, 76.15) |
| Income (ref: Over $50,000) less than $50,000 | 1.95 | 0.79 | 0.013 | (0.41, 3.5) | 7.04 | (1.5, 32.99) |
| Marital Status (ref: Married) Not married |
0.55 | 0.64 | 0.387 | (-0.7, 1.8) | 1.74 | (0.5, 6.05) |
CI, confidence interval.
OR, odds ratio.
SE, standard error.
OR interpretation should be for every 0.1-unit increase instead of 1-unit increase in the score of each TiOS subscale.
4. Discussion
Divergence between patient- and physician-assessed symptom severity has been widely reported in oncology [30,38,39] and other medical specialties [40,41]. To understand factors associated with this discrepancy, we categorized patients as under-, over-, or regular-describers of CIPN to clinicians by comparing their PRO, assumed to be indicative of actual CIPN, with clinician-documented CTCAE, assumed to reflect the CIPN described to the clinician during treatment with paclitaxel. Our findings indicated that women who were not working, had lower income, and displayed higher trust in their oncologist’s competence were more likely to under-describe CIPN symptoms.
Few studies have investigated predictors of discrepancies between patient and clinician symptom assessments, and none have identified employment status, income, or trust in their clinician as contributory variables. Agreement between oral mucositis reported by patients using a checklist and documented by physicians in the EMR was higher in older patients, but was not associated with education, comorbid conditions, or disease characteristics [38]. In a study conducted within ophthalmology, non-documentation of symptoms in the EMR was more common in return visit patients compared with new patients, otherwise discrepancy between patients and physicians’ assessment of blurry vision, discomfort, or redness were not associated with any patient (age, sex, diagnosis) or clinician/clinic (years in practice, clinic volume or presence of a medical scribe) characteristics [40].
In our logistic regression model, women who were not working and had lower income were more likely to have under-described CIPN symptoms to their clinicians. We speculate that women who work may fully convey their CIPN to avoid severe symptoms that could interfere with their job performance. Furthermore, patients who had higher trust in their oncologist’s competence were more likely to under-describe symptoms. This finding contradicts our a priori hypothesis and previous literature showing that patients with higher trust in their primary and specialty care physician are more likely to voice their treatment preferences during appointments [42]. It is possible that women who trust their oncologist’s competence defer to them in determining what topics to discuss during appointments and are less willing to volunteer CIPN if not specifically asked. In addition to patient perceptions, clinician-oriented communication patterns, with oncologists dominating the conversation, interrupting, and asking close-ended questions negatively impacts symptom reporting [43]. Open communication styles can help elicit symptoms, but others advocate transition from a communication-oriented model to using systematic assessment (e.g., PROs) [44]. Lower health literacy was associated with more under-describing behavior in the bivariate analysis (at week 7), but not in the regression model. Health literacy is known to correlate with education and income [45,46], likely explaining the lack of association for health literacy when accounting for income in the regression model. Properly investigating the independent effects of health literacy and income would likely require a much larger study with greater heterogeneity in both factors and possibly using lengthier instruments that can more sensitively detect relevant individual differences in health literacy.
A higher CIPN PRO than CTCAE could mean different scenarios: 1) the patient under-described symptoms to the clinician; 2) the patient exaggerated symptom severity on the PRO measure; 3) the patient has a different interpretation compared to the clinician of what constitutes “severe” CIPN; or 4) the patient accurately described CIPN but the clinician did not adequately document in the EMR. Thus, our definition of under- (and over-) describing may not correspond to actual behavior. Confirmation of which behavior occurred would require recording the patient-clinician interaction or interviewing patients after treatment. Further, our thresholds for defining describer categories were based on the distribution from another clinical trial and our results may have changed had we used our own distribution or alternative thresholds.
This pilot study was an initial attempt to identify factors associated with under-describing CIPN. This behavior could result in inappropriately continuing treatment after the development of moderate-to-severe CIPN, leading to permanent damage, diminished quality of life [4,5] and increased risk of falls [47]. Avoiding irreversible CIPN is particularly important in patients with non-metastatic breast cancer who have overall good prognosis, even in the absence of taxane treatment. Discovering characteristics associated with the tendency to under-describe CIPN could enable targeting of efforts to promote complete symptom disclosure, such as the use of PROs.
This study has limitations. First, survey data were collected post-treatment, in some cases several months later; thus, responses may be affected by recall bias and no causal relationships can be established. This is especially relevant for variables that are prone to change over time, such as trust in the oncologist and survivor concerns; hence, interpretation of these results should be cautious. Second, lack of CIPN documentation in the EMR was considered to be CTCTAE grade 0, which may not be accurate and could lead to overestimation of the number of under-describers. Third, the study was conducted at a single institution with a limited number of clinicians, and with a very homogenous sample of patients with regard to race (White) and education level (high), and a high proportion of individuals with adequate health literacy. Even though this pilot study did not aim at generalizability, the findings could have differed had we used a more diverse sample. Fourth, 74% of our CTCAE grades were assigned retrospectively based on textual descriptions in the EMR. We ameliorated this limitation by having two blinded researchers independently assign CTCAE grades. Finally, we analyzed time points independently without accounting for overlap between patients who may have under-described CIPN in week 7 but not in week 10, and vice-versa. The results of this preliminary analysis should be considered hypothesis generating.
Concluding, this pilot study suggests that women who were not working, had lower income, and displayed higher trust in their oncologist’s competence were more likely to under-describe CIPN symptoms to their clinicians, as indicated by lower CIPN severity documented in the EMR than reported directly on the CIPN20 scale. Future work should confirm under-describing by recording patient-clinician interactions or interviewing patients to understand if, and why, they withhold symptoms.
Funding source
Financial support was received from the National Center for Advancing Translational Sciences under award number KL2TR000434 and 2UL1TR000433 (PI: Hertz), and the Michigan Institute for Clinical & Health Research (MICHR) Pilot Grant Program Seed Grants at the University of Michigan under award number U050294. Study sponsors had no role in the study design, collection, analysis or interpretation of data.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Michigan Institutional Review Board reference number HUM00086259 and University of Michigan Rogel Comprehensive Cancer Center reference number 2014.002, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Declaration of competing interest
Syverson owns stock in Pfizer Inc. and AbbVie Inc. Loprinzi received personal fees from Pled Pharma, Metys, Disarm Therapeutics, and Asahi Kasei. Farris is a consultant and received remuneration from Quio Technologies. All other authors declare that they have no conflict of interest.
Acknowledgements
We thank all the patients who accepted to participate in this study. We also thank Dr. Adam Sima and Dr. Le Kang from the Biostatistics Consulting Lab at Virginia Commonwealth University for providing supervision to the doctoral student who was a part of this study (Ms. Jin Liu).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.02.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.DeSantis C., Ma J., Bryan L., Jemal A. Breast cancer statistics, 2013. CA A Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Pereira S., Fontes F., Sonin T., Dias T., Fragoso M., Castro-Lopes J.M., Lunet N. Neurological complications of breast cancer: a prospective cohort study. Breast. 2015;24(5):582–587. doi: 10.1016/j.breast.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Reyes-Gibby C.C., Morrow P.K., Buzdar A., Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain. 2009;10(11):1146–1150. doi: 10.1016/j.jpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckhoff L., Knoop A., Jensen M.B., Ewertz M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Canc. 2015;51(3):292–300. doi: 10.1016/j.ejca.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Beijers A., Mols F., Dercksen W., Driessen C., Vreugdenhil G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. 2014;12(11):401–406. doi: 10.12788/jcso.0086. [DOI] [PubMed] [Google Scholar]
- 6.Seretny M., Currie G.L., Sena E.S., Ramnarine S., Grant R., MacLeod M.R., Colvin L., Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Speck R.M., Sammel M.D., Farrar J.T., Hennessy S., Mao J.J., Stineman M.G., DeMichele A. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract. 2013;9(5):e234–e240. doi: 10.1200/JOP.2012.000863. [DOI] [PubMed] [Google Scholar]
- 8.Bhatnagar B., Gilmore S., Goloubeva O., Pelser C., Medeiros M., Chumsri S., Tkaczuk K., Edelman M., Bao T. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. SpringerPlus. 2014;3:366. doi: 10.1186/2193-1801-3-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Canc. 2008;44(11):1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Vahdat L., Papadopoulos K., Lange D., Leuin S., Kaufman E., Donovan D., Frederick D., Bagiella E., Tiersten A., Nichols G., Garrett T., Savage D., Antman K., Hesdorffer C.S., Balmaceda C. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Canc Res. 2001;7(5):1192–1197. [PubMed] [Google Scholar]
- 11.de Morree E.S., Vogelzang N.J., Petrylak D.P., Budnik N., Wiechno P.J., Sternberg C.N., Doner K., Bellmunt J., Burke J.M., Ochoa de Olza M., Choudhury A., Gschwend J.E., Kopyltsov E., Flechon A., van As N., Houede N., Barton D., Fandi A., Jungnelius U., Li S., Li J.S., de Wit R. Association of survival benefit with docetaxel in prostate cancer and total number of cycles administered: a post hoc analysis of the mainsail study. JAMA Oncol. 2017;3(1):68–75. doi: 10.1001/jamaoncol.2016.3000. [DOI] [PubMed] [Google Scholar]
- 12.Loibl S., Skacel T., Nekljudova V., Luck H.J., Schwenkglenks M., Brodowicz T., Zielinski C., von Minckwitz G. Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Canc. 2011;11:131. doi: 10.1186/1471-2407-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute . 2010. Common Terminology criteria for Adverse Events (CTCAE) [Google Scholar]
- 14.Trotti A., Colevas A.D., Setser A., Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25(32):5121–5127. doi: 10.1200/JCO.2007.12.4784. [DOI] [PubMed] [Google Scholar]
- 15.Bruner D.W., Bryan C.J., Aaronson N., Blackmore C.C., Brundage M., Cella D., Ganz P.A., Gotay C., Hinds P.S., Kornblith A.B., Movsas B., Sloan J., Wenzel L., Whalen G., National Cancer I. Issues and challenges with integrating patient-reported outcomes in clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007;25(32):5051–5057. doi: 10.1200/JCO.2007.11.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavaletti G., Frigeni B., Lanzani F., Mattavelli L., Susani E., Alberti P., Cortinovis D., Bidoli P. Chemotherapy-Induced Peripheral Neurotoxicity assessment: a critical revision of the currently available tools. Eur J Canc. 2010;46(3):479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Lavoie Smith E.M., Barton D.L., Qin R., Steen P.D., Aaronson N.K., Loprinzi C.L. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European organization for research and treatment of cancer QLQ-CIPN20 questionnaire. Qual Life Res. 2013;22(10):2787–2799. doi: 10.1007/s11136-013-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basch E., Jia X., Heller G., Barz A., Sit L., Fruscione M., Appawu M., Iasonos A., Atkinson T., Goldfarb S., Culkin A., Kris M.G., Schrag D. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirillo M., Venturini M., Ciccarelli L., Coati F., Bortolami O., Verlato G. Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol. 2009;20(12):1929–1935. doi: 10.1093/annonc/mdp287. [DOI] [PubMed] [Google Scholar]
- 20.Bennett B.K., Park S.B., Lin C.S., Friedlander M.L., Kiernan M.C., Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Support Care Canc. 2012;20(11):2959–2967. doi: 10.1007/s00520-012-1428-5. [DOI] [PubMed] [Google Scholar]
- 21.Shimozuma K., Ohashi Y., Takeuchi A., Aranishi T., Morita S., Kuroi K., Ohsumi S., Makino H., Mukai H., Katsumata N., Sunada Y., Watanabe T., Hausheer F.H. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Canc. 2009;17(12):1483–1491. doi: 10.1007/s00520-009-0613-7. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcome. 2006;4:79. doi: 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvert M., Kyte D., Mercieca-Bebber R., Slade A., Chan A.W., King M.T., the S.-P.R.O.G., Hunn A., Bottomley A., Regnault A., Chan A.W., Ells C., O’Connor D., Revicki D., Patrick D., Altman D., Basch E., Velikova G., Price G., Draper H., Blazeby J., Scott J., Coast J., Norquist J., Brown J., Haywood K., Johnson L.L., Campbell L., Frank L., von Hildebrand M., Brundage M., Palmer M., Kluetz P., Stephens R., Golub R.M., Mitchell S., Groves T. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. J Am Med Assoc. 2018;319(5):483–494. doi: 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 24.Basch E., Barbera L., Kerrigan C.L., Velikova G. Implementation of patient-reported outcomes in routine medical care. Am Soc Clin Oncol Educ Book. 2018;38:122–134. doi: 10.1200/EDBK_200383. [DOI] [PubMed] [Google Scholar]
- 25.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postma T.J., Aaronson N.K., Heimans J.J., Muller M.J., Hildebrand J.G., Delattre J.Y., Hoang-Xuan K., Lanteri-Minet M., Grant R., Huddart R., Moynihan C., Maher J., Lucey R. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Canc. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Cavaletti G., Cornblath D.R., Merkies I.S., Postma T.J., Rossi E., Frigeni B., Alberti P., Bruna J., Velasco R., Argyriou A.A., Kalofonos H.P., Psimaras D., Ricard D., Pace A., Galie E., Briani C., Dalla Torre C., Faber C.G., Lalisang R.I., Boogerd W., Brandsma D., Koeppen S., Hense J., Storey D., Kerrigan S., Schenone A., Fabbri S., Valsecchi M.G. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol. 2013;24(2):454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieffer J.M., Postma T.J., van de Poll-Franse L., Mols F., Heimans J.J., Cavaletti G., Aaronson N.K., Group C.I.-P. Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20) Qual Life Res. 2017;26(11):2999–3010. doi: 10.1007/s11136-017-1626-1. [DOI] [PubMed] [Google Scholar]
- 29.Le-Rademacher J., Kanwar R., Seisler D., Pachman D.R., Qin R., Abyzov A., Ruddy K.J., Banck M.S., Lavoie Smith E.M., Dorsey S.G., Aaronson N.K., Sloan J., Loprinzi C.L., Beutler A.S. Patient-reported (EORTC QLQ-CIPN20) versus physician-reported (CTCAE) quantification of oxaliplatin- and paclitaxel/carboplatin-induced peripheral neuropathy in NCCTG/Alliance clinical trials. Support Care Canc. 2017;25(11):3537–3544. doi: 10.1007/s00520-017-3780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson T.M., Rogak L.J., Heon N., Ryan S.J., Shaw M., Stark L.P., Bennett A.V., Basch E., Li Y. Exploring differences in adverse symptom event grading thresholds between clinicians and patients in the clinical trial setting. J Canc Res Clin Oncol. 2017;143(4):735–743. doi: 10.1007/s00432-016-2335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertz D.L., Krumbach E., Nobles B., Erickson S. San antonio breast cancer symposium, san antonio, TX. 2017. Farris KB the role of patient perceptions in under reporting chemotherapy induced peripheral neuropathy (CIPN) Cancer Res 2018;78(4 Suppl): Abstract nr P4-11-06. [Google Scholar]
- 32.Hertz D.L., Kidwell K.M., Vangipuram K., Li F., Pai M.P., Burness M., Griggs J.J., Schott A.F., Van Poznak C., Hayes D.F., Lavoie Smith E.M., Henry N.L. Paclitaxel plasma concentration after the first infusion predicts treatment-limiting peripheral neuropathy. Clin Canc Res. 2018;24(15):3602–3610. doi: 10.1158/1078-0432.CCR-18-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotay C.C., Pagano I.S. Assessment of Survivor Concerns (ASC): a newly proposed brief questionnaire. Health Qual Life Outcome. 2007;5:15. doi: 10.1186/1477-7525-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillen M.A., Koning C.C., Wilmink J.W., Klinkenbijl J.H., Eddes E.H., Kallimanis-King B.L., de Haes J.C., Smets E.M. Assessing cancer patients’ trust in their oncologist: development and validation of the Trust in Oncologist Scale (TiOS) Support Care Canc. 2012;20(8):1787–1795. doi: 10.1007/s00520-011-1276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillen M.A., Butow P.N., Tattersall M.H., Hruby G., Boyle F.M., Vardy J., Kallimanis-King B.L., de Haes H.C., Smets E.M. Validation of the English version of the trust in oncologist scale (TiOS) Patient Educ Counsel. 2013;91(1):25–28. doi: 10.1016/j.pec.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Peterson P.N., Shetterly S.M., Clarke C.L., Bekelman D.B., Chan P.S., Allen L.A., Matlock D.D., Magid D.J., Masoudi F.A. Health literacy and outcomes among patients with heart failure. J Am Med Assoc. 2011;305(16):1695–1701. doi: 10.1001/jama.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chew L.D., Bradley K.A., Boyko E.J. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 38.Sikorskii A., Wyatt G., Tamkus D., Victorson D., Rahbar M.H., Ahn S. Concordance between patient reports of cancer-related symptoms and medical records documentation. J Pain Symptom Manag. 2012;44(3):362–372. doi: 10.1016/j.jpainsymman.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falchook A.D., Green R., Knowles M.E., Amdur R.J., Mendenhall W., Hayes D.N., Grilley-Olson J.E., Weiss J., Reeve B.B., Mitchell S.A., Basch E.M., Chera B.S. Comparison of patient- and practitioner-reported toxic effects associated with chemoradiotherapy for head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2016;142(6):517–523. doi: 10.1001/jamaoto.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valikodath N.G., Newman Casey P.A., Lee P.P., Musch D.C., Niziol L.M., Woodward M.A. Agreement of ocular symptom reporting between patient-reported outcomes and medical records. JAMA Ophthalmol. 2017;135(3):225–231. doi: 10.1001/jamaophthalmol.2016.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pakhomov S.V., Jacobsen S.J., Chute C.G., Roger V.L. Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manag Care. 2008;14(8):530–539. [PMC free article] [PubMed] [Google Scholar]
- 42.Bell R.A., Kravitz R.L., Thom D., Krupat E., Azari R. Unsaid but not forgotten: patients’ unvoiced desires in office visits. Arch Intern Med. 2001;161(16):1977–1984. doi: 10.1001/archinte.161.16.1977. [DOI] [PubMed] [Google Scholar]
- 43.Berry D.L., Wilkie D.J., Thomas C.R., Jr., Fortner P. Clinicians communicating with patients experiencing cancer pain. Canc Invest. 2003;21(3):374–381. doi: 10.1081/cnv-120018228. [DOI] [PubMed] [Google Scholar]
- 44.Bent S., Padula A., Avins A.L. Brief communication: better ways to question patients about adverse medical events: a randomized, controlled trial. Ann Intern Med. 2006;144(4):257–261. doi: 10.7326/0003-4819-144-4-200602210-00007. [DOI] [PubMed] [Google Scholar]
- 45.Cutilli C.C., Bennett I.M. Understanding the health literacy of America: results of the national assessment of adult literacy. Orthop Nurs. 2009;28(1):27–32. doi: 10.1097/01.NOR.0000345852.22122.d6. quiz 33-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin H.S., Johnson M., Mendelsohn A.L., Abrams M.A., Sanders L.M., Dreyer B.P. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124(Suppl 3):S289–S298. doi: 10.1542/peds.2009-1162E. [DOI] [PubMed] [Google Scholar]
- 47.Bao T., Basal C., Seluzicki C., Li S.Q., Seidman A.D., Mao J.J. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Canc Res Treat. 2016;159(2):327–333. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.